Abstract
The cellular organelles we know as mitochondria are thought to have originated as symbiotic bacteria. Indeed, the two use common mechanisms to trigger innate immune responses to injury and infection, respectively.
Serious physical injury, or trauma, is a major cause of morbidity and mortality worldwide1. Patients who survive the initial trauma, through medical and surgical interventions, often remain critically ill. One life-threatening post-traumatic complication is the systemic inflammatory response syndrome (SIRS), which is characterized by shock and compromised function of several organs. Clinically, symptoms of post-traumatic SIRS include fever, increased heart rate and low blood pressure (shock) — complications that also occur during the systemic inflammatory response to severe infection, known as sepsis. The molecular mechanism that underlies the development of SIRS after trauma and the cause of the similarity between post-traumatic and septic SIRS have been poorly understood. In this issue, Zhang et al.2 report on one of the pathways that triggers trauma-associated SIRS and link it to pathways implicated in sepsis-associated SIRS (see page 104).
Given the similarities between post-traumatic SIRS and the host response to overwhelming sepsis, it was previously proposed that SIRS develops after trauma as a result of bacterial translocation from the bowel to the blood (due to lowered blood pressure and the subsequent poor perfusion of the bowel). However, this theory was later disproved3. More recent theories posit that the innate immune system and its pattern-recognition receptors are the main components of the common molecular pathway leading to SIRS in both infectious and non-infectious settings4.
In patients with severe infection, the innate immune system responds to a set of evolutionarily conserved molecules known as pathogen-associated molecular patterns (PAMPs), which are expressed by a variety of pathogens. Native molecules released after tissue injury — and therefore called damage-associated molecular patterns (DAMPs) — can act through signalling pathways shared with PAMPs, initiating a similar innate immune response even in the absence of microbial infection5. Recent work has demonstrated that some DAMPs — including HMGB1 and S100 proteins — are rapidly released into the blood of severely injured patients, and elevated levels of HMGB1 in the blood have been linked to the development of organ failure after trauma6. Nonetheless, the evolutionary links between the signalling pathways that are triggered by PAMPs and DAMPs have not been clearly identified.
Mitochondria provide a potential link between internal and external triggers of the innate immune response, particularly given the evidence that these organelles originated from bacteria and initially lived as intracellular symbionts in eukaryotic cells (such as those of plants and animals). Indeed, mitochondrial DNA and bacterial DNA share many similar structural motifs7. Zhang et al.2 therefore hypothesized that intra-mitochondrial components, including mitochondrial DNA, may act as DAMPs, triggering the same pathways that respond to PAMPs and perhaps partly explaining the similarities between the immune response to trauma and to overwhelming infection.
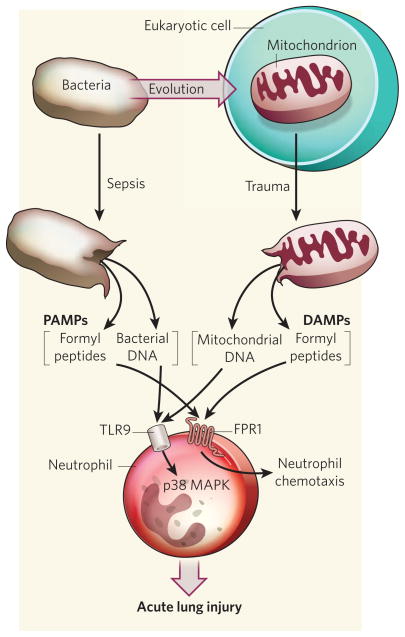
The authors report that severe trauma in humans causes a rapid release of mitochondrial DNA into the circulating blood, probably secondary to the tissue necrosis caused by the extensive force of the injury. Moreover, they show that mitochondrial DAMPs such as formyl peptide attract neutrophils, a type of white blood cell, as part of the initial response of the innate immune system. These molecular stimuli also activate neutrophils through formyl peptide receptor-1 on their surface, and promote a neutrophil-mediated inflammatory response through release of the immune mediators MMP-8 and IL-8 and phosphorylation of several MAP kinase enzymes (Fig. 1).
Figure 1. PAMPs, DAMPs and the inflammatory response.
Zhang et al.2 find that, like bacterial DNA released following sepsis, mitochondrial DNA released by severe trauma can also act through toll-like receptor-9 (TLR9) to activate neutrophils through activation of p38 MAP kinase (MAPK) enzyme. Similarly, formylated peptides released from bacteria and mitochondria in these settings attract neutrophils by the process of chemotaxis to sites of inflammation and injury through formyl peptide receptor-1 (FPR1). In both cases, the outcome may be acute lung injury, as part of the systemic inflammatory response syndrome (SIRS). DAMPs, damage-associated molecular patterns; PAMPs, pathogen-associated molecular patterns.
Mitochondrial DNA could also activate p38 MAP kinase by binding to toll-like receptor-9 (TLR9) on the surface of neutrophils — an interaction that could be blocked using DNA sequences that bind to the portions of mitochondrial DNA that closely resemble bacterial DNA. But perhaps the most important observation Zhang et al. make is that intravenous injection of mitochondrial DAMPs into rats causes acute lung injury, which is a major cause of respiratory failure in critically ill patients8. Acute lung injury is characterized by protein-rich pulmonary oedema (swelling), accumulation of large numbers of neutrophils in the lungs and high concentrations of pro-inflammatory cytokines, including IL-6 and tumour-necrosis factor-α. Although the lung injury induced by DAMPs was not severe, it may act as a priming stimulus that then promotes additional lung injury after exposure to further inflammatory stimuli, such as blood transfusion or infection, which are common after trauma9.
Zhang and colleagues’ data elegantly demonstrate how molecular motifs conserved between bacteria and mitochondria may explain some of the similarities in the innate immune responses to external and internal danger signals, and in patients with SIRS caused by injury or infection. Their study also raises fresh questions.
For one, what other mitochondrial DAMPs — apart from mitochondrial DNA and formyl peptides — trigger the innate immune response to trauma? Also, does the quantity of mitochondrial DAMPs released after trauma directly affect important clinical outcomes, including mortality and the requirement for life-sustaining therapies, such as mechanical ventilation for respiratory failure and dialysis for renal failure? Do similar cellular pathways mediate the manifestations of other forms of serious injury, such as burns or severe hypovolaemic shock? Will inhibition of these pathways after injury be beneficial for survival or to prevent organ failure after trauma?
The optimal balance between an appropriate and an excessive immune response following trauma or severe sepsis is still not known. Nonetheless, by unravelling the missing link between the innate immune responses to microbial and traumatic injury, the findings of this study2 should provide clues to help solve the remaining puzzles.
Contributor Information
Carolyn S. Calfee, Email: carolyn.calfee@ucsf.edu.
Michael A. Matthay, Email: michael.matthay@ucsf.edu.
References
- 1.Mathers CD, Loncar D. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Q, et al. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore FA, et al. J Trauma. 1996;40:501–512. doi: 10.1097/00005373-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki A, Medzhitov R. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mollen KP, et al. Shock. 2006;26:430–437. doi: 10.1097/01.shk.0000228797.41044.08. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MJ, et al. Crit Care. 2009;13:R174. doi: 10.1186/cc8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray MW, Burger G, Lang BF. Genome Biol. 2001;2:reviews1018.1–1018.5. doi: 10.1186/gb-2001-2-6-reviews1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ware LB, Matthay MA. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 9.Looney MR, et al. J Clin Invest. 2009;119:3450–3461. doi: 10.1172/JCI38432. [DOI] [PMC free article] [PubMed] [Google Scholar]