Abstract
Four experiments examined the effects of varying the CS-US interval (and US density) on learning in an appetitive magazine approach task with rats. Learning was assessed with conditioned response (CR) measures, as well as measures of sensory-specific stimulus-outcome associations (Pavlovian-instrumental transfer, potentiated feeding, and US devaluation). The results from these studies indicate that there exists an inverse relation between CS-US interval and magazine approach CRs, but that sensory-specific stimulus-outcome associations are established over a wide range of relatively long, but not short, CS-US intervals. These data suggest that simple CR measures provide us with different information about what is learned than measures of the specific stimulus-outcome association, and that time is a more critical variable for the former than latter component of learning.
Keywords: CS-US interval, S-O associations, Pavlovian – Instrumental Transfer, Motivational processes, Timing processes
Pavlovian conditioning is commonly described as the formation of an association between the internal representations of conditioned and unconditioned stimuli (CS and US, respectively). A growing body of evidence supports the view that as a result of learning, the CS comes to activate different neural representations of the US that are fairly complex and distributed over several central nervous system structures (e.g., Balleine, 2005; Holland, 1997; Schoenbaum, Chiba, & Gallagher, 1999). Key to a psychological analysis is a characterization of these complex US representations in terms of the multiple attributes of the real world events, i.e., the reinforcers, about which these representations are presumed to code.
A considerable amount of behavioral work has suggested that specific sensory features of the US as well as more general “motivational” features are coded by these neural representations. One popular theory of Pavlovian conditioning, AESOP theory (Wagner and Brandon, 1989), explicitly acknowledges that Pavlovian conditioning consists of separately learned associations involving sensory or motivational features of the US. Strong support for this view comes from studies that have dissociated the neural structures mediating these two classes of learning. For example, Balleine and his colleagues using a Pavlovian-to-instrumental transfer design (PIT) have shown that Pavlovian cues can exert both specific and general control over instrumental responses, and that these different effects depend upon different neural substrates (Corbit & Balleine, 2005). In one study, two instrumental responses were each reinforced with different reinforcers (US1 and US2, respectively). In addition, Pavlovian training was conducted off-baseline with three different CSs in which CS1 was paired with US1, CS2 with US2, and CS3 with a third reinforcer, US3. When the three different CSs were tested for their effects on instrumental responding, CS1 and CS2 exerted reinforcer-specific control over instrumental performance, selectively elevating only the response with which the CS shared a US, whereas CS3 non-selectively energized both instrumental responses. It is tempting to conclude from this result that the first two CSs displayed selective transfer of control through the activation of sensory-specific representations of their associated USs, and that the third CS displayed nonselective transfer of control through the activation of a more general motivational representation of the US. This distinction gained further support from the finding that lesions of the basolateral amygdala abolished the selective transfer shown by CS1 and CS2, but not the general transfer shown by CS3, and that lesions of the central nucleus of the amygdala abolished the general but not selective transfer.
Although this distinction between general and specific reinforcer representations receives empirical support from these studies, the variables that influence learning of these different reinforcer components have not been studied extensively. One variable thought for some time to be critical is the CS-US interval. Konorski (1967) speculated that learning about the general motivational properties of reinforcement occurs rapidly and optimally to long duration cues, whereas learning about sensory-specific properties of reinforcement is slower to develop and optimally occurs with short duration cues. These speculations have gained some support from findings that heart rate conditioning in rabbits develops rapidly and is optimal with relatively long CS-US intervals, whereas, nictitating membrane response (NMR) conditioning in these animals develops more slowly and is optimal with relatively short CS-US intervals (e.g., for a review see Lennartz and Weinberger, 1992; but for a critique see Kehoe and Macrae, 1994). While these results are consistent with Konorski's claims, to accept them as supportive evidence requires that NMR conditioning reflects sensory-based learning and heart rate conditioning reflects motivational learning. An alternative possibility is that these two different response systems are simply differentially sensitive to CS-US interval, without making any additional assumptions about the content of the learning differing in the two cases (e.g., Holland, 1980).
Since the Pavlovian-to-instrumental tranfer test has been useful in making distinctions between learning about sensory and motivational properties of reinforcement, the present studies used this technique to assess the role of the CS-US interval. In the first two experiments we varied the CS-US interval between 20 s and 180 s. The third experiment varied the rate of US occurrence within the CS, while training and testing with a common CS duration. The fourth experiment explored 2-s and 20-s CS-US intervals with several different measures of sensory-specific learning (PIT, potentiated feeding, and selective reinforcer devaluation).
Experiment 1
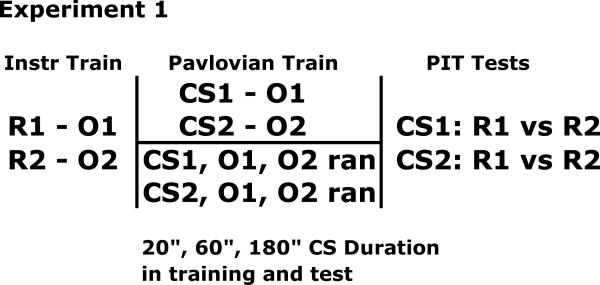
In Experiment 1 we systematically varied the CS-US interval between groups and examined the effects of this variation on PIT. The design of the experiment is shown in Figure 1. Initially, all subjects were trained to make lever press and chain pull responses for different reinforcing outcomes (R1-US1, R2-US2). This training was followed by a Pavlovian conditioning phase in which different stimuli were paired with different reinforcing outcomes (CS1-US1, CS2-US2), but different groups were trained with different CS-US intervals (20 s, 60 s, or 180 s). Three control groups were additionally run to determine what unconditioned effects the CSs might have on instrumental performance. These groups received both US1 and US2 randomly during the intertrial intervals. Finally, the effects of Pavlovian training upon instrumental responding were examined in choice tests. Learning about sensory and motivational properties of the US can both, in principal, be observed in the PIT test. Sensory-specific learning would be assessed with the observation of outcome specific PIT, and motivational learning would be assessed by non-specific PIT. The former effect would be revealed by greater response rates when the CS and response were both reinforced previously with the same versus different outcomes, and the latter effect would be revealed by the observation that a CS non-selectively elevates the response with which it does not share an outcome relative to the pre CS period. On the basis of Konorski's (1967) views it might be anticipated that selective PIT would be greatest with short CS-US intervals and decline in magnitude with long CS-US intervals, and that non-selective PIT would be most likely to occur with long CS-US intervals.
Figure 1.
Design for Experiment 1.
Method
Subjects
Subjects were 48, experimentally naïve, male Sprague-Dawley rats (supplied by Charles River Breeders). Their weights varied between 337 and 432 g at the beginning of the experiment. The rats were housed individually in a colony room that was on a 16 hr light/8 hr dark cycle, and they were maintained at 85% of their ad lib body weights by daily supplemental feedings (given following the final experimental session of the day). Subjects were run in the experiment during the light phase of their light/dark cycle.
Apparatus
The apparatus consisted of two sets of eight identical standard conditioning chambers, each of which was housed in a sound- and light-resistant shell. The conditioning chambers measured 30.5 cm × 24.0 cm × 25.0 cm. Two end walls were constructed of aluminum, and the sidewalls as well as the ceiling were made from clear Plexiglas. The floor consisted of 0.60-cm diameter stainless steel rods spaced 2.0 cm apart. In the center of one end wall 1.2 cm above the grid floor was a recessed food magazine measuring 3.0 × 3.6 × 2.0 cm (length × width × depth). Two 45-mg pellets (P.J. Noyes Co., Formula A) were dropped onto the magazine floor when this US was scheduled. A 0.1 ml droplet of a 20% sucrose solution (w/v) was delivered through a gravity-feed valve (ASCO Red-Hat valve) directly into a well located on the floor of the food magazine when this US was scheduled. On the inner walls of the recessed magazine were an infrared detector and emitter enabling the automatic recording of head movements inside the magazine. These were located 0.9 cm above the magazine floor and 0.8 cm recessed from the front wall. Located 3.0 cm to the right of the magazine and 8.0 cm above the floor was a lever (4 cm in width). This lever protruded into the chamber at all times, but access to the lever was prevented during magazine training, Pavlovian training, and instrumental training with the alternate response by a sheet metal covering. Located approximately 3 cm to the left of the magazine and about 3 cm away from the front wall was a chain suspended through the ceiling from a microswitch mounted on the outer part of the ceiling. This chain was withdrawn from the chamber during magazine training, Pavlovian training, and instrumental training with the lever. A 6-W light bulb was mounted on the bottom of the sidewall of the outer chamber, below and behind the rear wall of the conditioning chamber. When activated, this light bulb flashed with approximately equal on-off pulse durations at a frequency of approximately 2/s. A speaker was mounted 22 cm behind the front wall of the conditioning chamber (where the food magazine was located), and was used to present a white noise stimulus (produced by a Grason-Stadler white noise generator). The white noise measured 12 dB above a background level of 78 dB (C weighting). The chamber was dark except when the visual stimulus was presented. A fan attached to the outer shell provided for cross-ventilation within the shell as well as background noise. All experimental events were controlled and recorded automatically by a Pentium-based PC and interfacing equipment (Alpha Products) located in the same room.
Procedure
The rats were initially magazine trained with pellet and sucrose USs. On each of two days, one magazine training session with one US was followed immediately by a second session with the other US. The order was counterbalanced across days. In each session, 20 USs of one kind were delivered according to a variable time 60-sec schedule. Sucrose (0.1 ml of a 20% solution) and pellet (two 45 mg pellets) USs were delivered to the same food magazine.
Instrumental training
On the day following magazine training, all rats were taught with continuous reinforcement schedules first to press the lever for one outcome and then to pull the chain for the other outcome. This training ceased after each response separately was reinforced 50 times. For half of the rats, lever press responses were reinforced by pellets and chain pull responses by sucrose, and for the remaining rats these response-outcome contingencies were reversed. Instrumental training continued with variable-interval (VI) schedules of reinforcement for a total of 7 sessions. The average inter-reinforcement interval was gradually increased across sessions and on successive days averaged 10 s, 15 s, 15 s, 30 s and 60 s in sessions 5 through 7. The rats were given two 20-min sessions per day, one for the lever and one for the chain. Only one manipulandum was present in a session, and the order of training with lever and chain was balanced across days.
Pavlovian Conditioning
Rats in the experimental groups received delay conditioning with the Flash (F) and Noise (N) stimuli over the next 24 sessions. The chain was withdrawn from the chamber and the lever made inaccessible by the sheet metal covering during these sessions. Each conditioning session was 50 min in duration and included two reinforced presentations each of N and F. The CS-US intervals, measured from CS onset to US onset, on both trial types were 20 s, 60 s, or 180 s, respectively, for the three experimental groups. Four different trial sequences (i.e., FNNF, NFFN, NNFF, FFNN) were used irregularly across sessions. Each of these used a different combination of intertrial intervals, but the exact times of US occurrence were the same in each group. Thus, the time between trials differed in each group due to the different CS durations, but the overall session time was held constant. The intertrial intervals (ITI) varied between 7.75 and 15.75 min with a mean of 11.75 min in the group trained with 20 s CS-US intervals. The mean ITIs for groups trained with 60 s and 180 s CS-US intervals, respectively, were 11.08 and 9.08 min. The animals were removed from the chambers 100 s after the final trial. For half of the subjects, N was paired with pellets and F with sucrose, whereas the reverse was true for the remaining subjects. These two sub-groups were arranged orthogonally to the earlier instrumental response-outcome counterbalancing.
Subjects in three random contingency control groups also were trained over 24 sessions. Subjects in these groups were trained with CS durations of 20 s, 60 s, or 180 s, respectively. These groups were trained with the same number of CS and US presentations as in their corresponding experimental groups, however, the USs were presented randomly during the intertrial intervals (i.e., at any time that the CSs were not presented).
Pavlovian to Instrumental Transfer Test
Two instrumental retraining sessions (with a VI 60 s schedule) with each response were given to the rats on the two days following Pavlovian conditioning. An 8-min extinction session occurred on the following day, in which both response manipulanda were available. This session was run in order to familiarize the rats with the choice procedure, as well as to lower the overall levels of instrumental responding prior to the transfer test sessions.
The first transfer test session occurred on the following day. In this test lever and chain were concurrently available during the entire session, but no reinforcements could be earned. The test session lasted for 36 min, and consisted of alternating periods of equal duration of stimulus off and stimulus on. These durations were 20 s, 60 s, and 180 s, respectively, for the groups trained with 20-s, 60-s, and 180-s CS-US intervals. The total number of test trials differed in each group in such a way to hold constant the total amount of stimulus time (9 min) across each group. This meant that each CS was presented 27, 9, and 3 times, respectively, in the groups trained with 20-s, 60-s, and 180-s stimuli. For subjects trained with 20-s stimuli, the following sequence was used: NFFNFNNFNFNFFNFNNFNFFNFNFNNFNFFNNFNFFNNFFNFNNFFNNFNFFN. The sequences in groups trained with 60-s and 180-s stimuli were identical, respectively, to the first 18 and 6 trials in this sequence.
A second transfer test session was conducted after two additional instrumental retraining sessions with each response. This second test session was conducted exactly as the first one.
Statistical Analysis
Standard analysis of variance (ANOVA) techniques were used to evaluate the data. Where appropriate, the post-hoc methods of Rodger (1974) were used to further evaluate simple main effects and interactions.
Results
Instrumental training was successful. On the final day of instrumental VI 60 training the mean instrumental response rate was 14.7 responses per min. The average response rate for pellets was higher than for sucrose (16.9 versus 12.5 responses per minute), but responding on the chain and lever manipulanda did not differ (14.9 and 14.5 responses per minute).
Pavlovian Magazine Approach
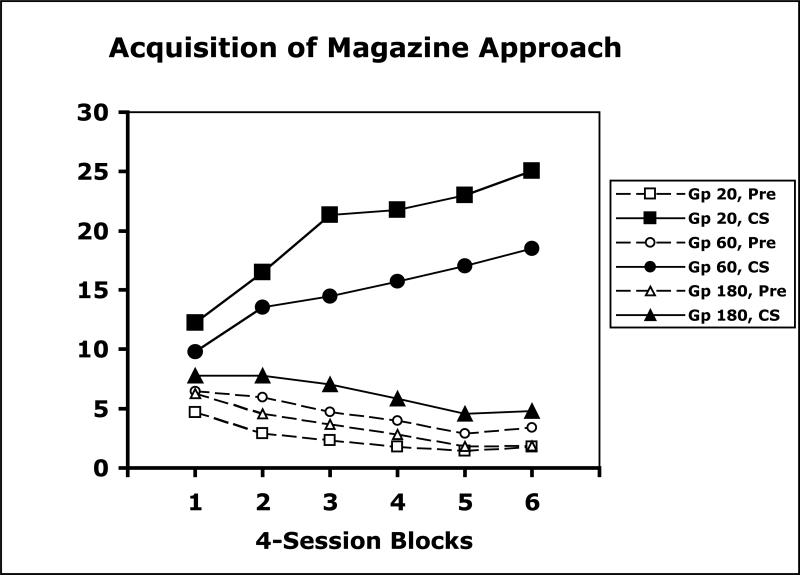
The data from the Pavlovian acquisition phase were examined initially by comparing across the experimental groups the overall rates of magazine approach CRs during the CSs as well as during a 60-s Pre CS period (Figure 2). The amount of magazine approach CRs was inversely related to the CS-US interval, however, responding during the CSs exceeded Pre CS response levels in each of the three experimental groups. On the final 4-session block of training, these three groups displayed different levels of magazine responding to the CSs, F(2,21)=25.51. Post-hoc tests following the procedures recommended by Rodger (1974) revealed that each group differed from one another. The three groups did not differ in Pre CS responding, F(2,21)=2.62, p=.10. All 24 subjects in these groups responded more in the presence of the CS than during the Pre CS period.
Figure 2.
Mean magazine responses per minute over successive blocks of training during CS and Pre CS periods for separate groups in Experiment 1 trained with 20-s, 60-s, or 180-s CS-US intervals.
The random control groups displayed either comparable or reduced levels of magazine responding during the CS (5.8, 4.4, 3.6 responses per minute, respectively for groups trained with 20, 60, and 180-s CSs) versus the Pre CS periods (5.3, 4.9, 6.2 responses per minute). All 8 of the subjects in the random group trained with 180-s CSs displayed lower levels of magazine entry during the CS (3.6 responses per minute) relative to the Pre CS period (6.2 responses per minute). These data suggest that in spite of rather large differences in overall responding, learning occurred in each of the groups that received CS-US pairings.
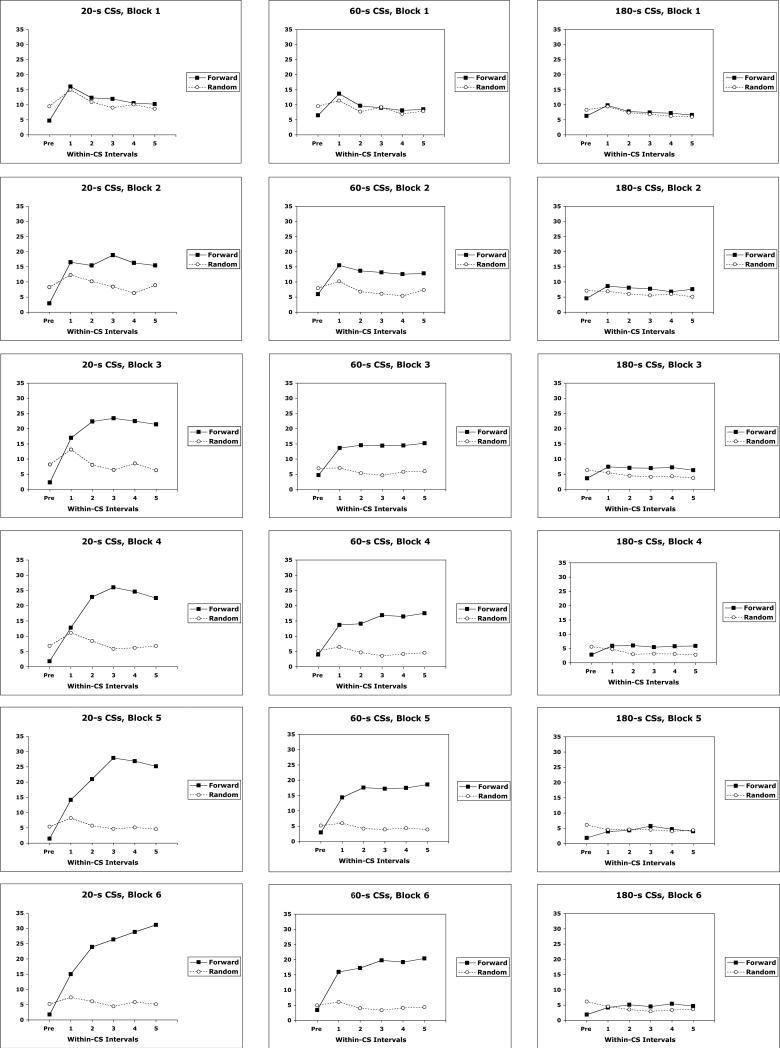
The temporal distribution of magazine approach CRs was also examined. Figure 3 displays magazine CRs for the paired and random groups distributed across successive fifths of the CS, and over successive 4-session blocks of training. Groups trained with different CS durations are shown in different columns, and data from different points in training are shown in different rows. The data show that by the 2nd or 3rd block of training differences emerged between the groups given paired and random CS-US presentations. Furthermore, the data show clearly timed CRs in the experimental groups trained with 20 s and 60 s CS-US intervals by the end of training (bottom row). However, these clearly timed CR functions underwent a transition from flat, non-timed CR functions early in training to peaked, timed CR functions late in training. This can be seen by comparing the data in Blocks 2 and 6 in groups trained with 20-s and 60-s CSs.
Figure 3.
Mean magazine responses across successive quintiles of the CS period for groups trained with forward CS-US pairings (Forward) and random CS-US presentations (Random). Groups trained with 20-s, 60-s, and 180-s stimuli are shown in separate columns, and data from different blocks of training are shown across different rows.
These data were analyzed with a Contingency (positive/random) × CS Duration (20/60/180) × Block (1-6) × Within-CS Interval (1-5) ANOVA. The 4-way interaction was significant in this analysis, F(40,840) = 1.60, p=0.01, as were most of the other main effects or interactions. To investigate the source of this 4-way interaction, separate Contingency × Within-CS Interval ANOVAs were performed at each block of training, and these were performed separately for the groups trained with a common CS duration. Separate error terms were used for groups trained with different CS durations since these groups greatly differed in their error variance (MSerror = 20.76, 5.659, and 1.244 in groups trained, respectively, with 20 s, 60 s, and 180 s CS durations). These analyses revealed Contingency main effects from Block 2-6 in groups trained with 20 s (smallest F(1,14) = 6.83) and 60 s CS (smallest F(1,14) = 5.60) durations, and in Blocks 3-4 in the groups trained with a 180 s CS duration (smallest F(1,14) = 4.70). This indicates that conditioning occurred in each experimental group by the 2nd or 3rd block of training. Furthermore, this analysis also revealed that significant Contingency × Within-CS Interval interactions occurred in Blocks 3-6 in groups trained with 20 s CS durations (smallest F(4,336) = 5.10), Blocks 4-6 in groups trained with 60 s CS durations (smallest F(4,336) = 4.13, and in Block 6 in groups trained with 180 s CS durations (F(4,336) = 2.56). This indicates that whereas responding was relatively flat across the CS in the random control groups, responding increased across the CS interval in the experimental groups.
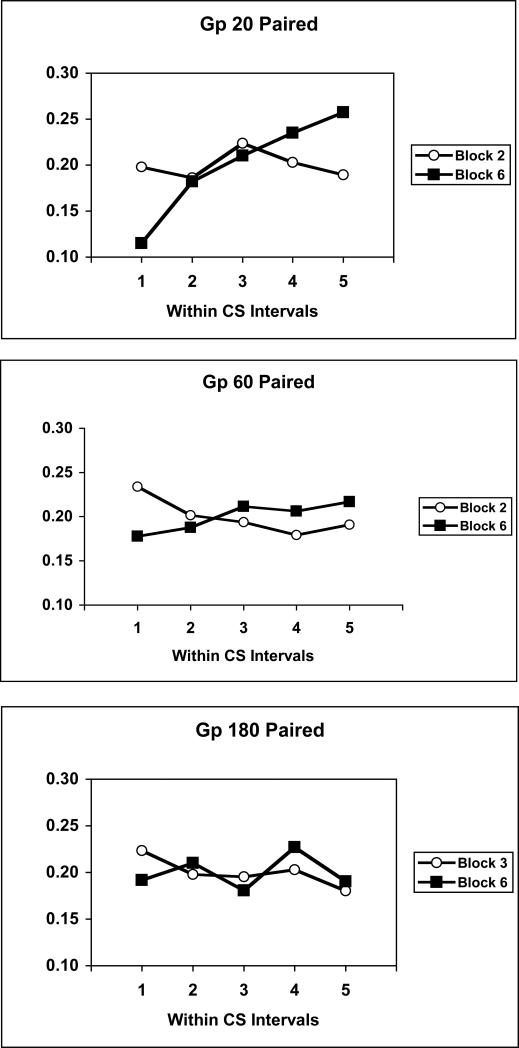
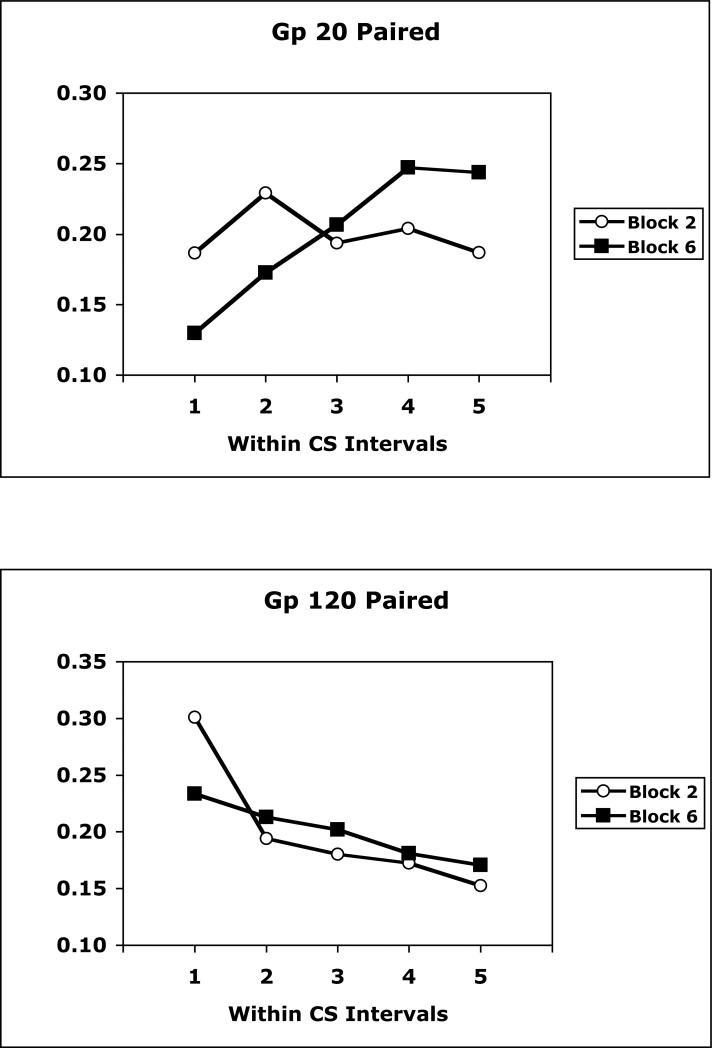
Because there were large differences in overall magazine approach responding in groups trained with different CS-US intervals, the within-CS distribution of responding was assessed in another way - by transforming the magazine rate data to a relative rate measure. This measure consisted of expressing the rate of responding in any within-CS interval as a proportion of total responding across the CS for a given subject. Figure 4 illustrates mean relative responding across the CS in Blocks 2 and 6 for experimental groups trained with 20 s and 60 s CS-US intervals, and in Blocks 3 and 6 for the group trained with a 180 s CS-US interval. These blocks were chosen because they involve comparisons between blocks in which conditioning first emerged and the final block of training. Whereas responding was relatively flat, or decreased somewhat across the CS when conditioning first emerged, responding tended to accelerate across the CS late in training. This pattern was most obvious in the group trained with a 20 s CS-US interval, was seen to a lesser degree in the group trained with a 60 s CS-US interval, but was not seen in the group trained with a 180 s CS-US interval.
Figure 4.
Mean relative magazine response rates across successive quintiles of the CS period in Blocks 2 or 3 and 6 of training for groups trained with 20-s, 60-s, or 180-s CS-US intervals.
The data were analyzed with separate Within-CS Interval × Block ANOVAs performed on each group using a pooled error term. This analysis revealed significant interactions in groups trained with a 20 s, F(4,84) = 6.99, or 60 s, F(4,84) = 2.76, but not a 180 s CS-US interval, as well as a main effect of Within-CS Interval only in the group trained with a 20 s CS-US interval, F(4,84) = 5.71.
Pavlovian to Instrumental Transfer
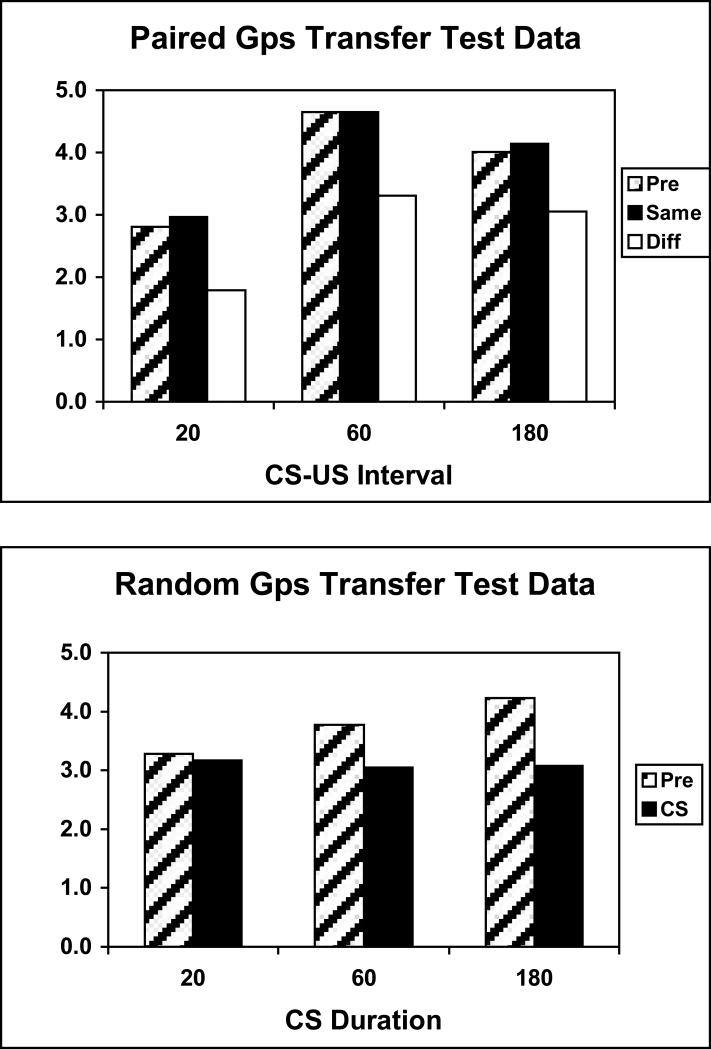
The data of most interest came from the PIT tests. The data have been averaged over both tests because there were no differences across the two. Figure 5 shows mean levels of instrumental responding in each paired group (top) and each random group (bottom). For the paired groups, the Pre CS response rates are shown as well as response rates during the CS broken down by whether the CS and response were reinforced previously with the same or different USs. Overall responding during the Pre CS and CS periods are displayed in the three random controls.
Figure 5.
Data for the Paired and Random groups from the PIT tests of Experiment 1. Mean instrumental responding for the Paired groups is shown separately during the Pre stimulus periods, and during the stimuli when the stimulus and response were reinforced previously with the same or different US. Mean instrumental responding for the Random groups is shown during the pre stimulus and stimulus periods collapsed across responses.
The data clearly show that the CSs exerted US-specific control over instrumental responding in these tests, biasing choice in favor of the response with which the CS shared a US. This selective transfer was comparable in groups trained with 20-s, 60-s, and 180-s CS-US intervals, and it showed up as a selective lowering of the response reinforced with a different US from the CS. A Group (20, 60, 180) × Response (Same/Diff) ANOVA performed on these data revealed significant main effects of Group, F(2,21)=7.52, and Response, F(1,21)=8.62, but no significant Group × Response interaction, F(2,21)=.04.
The random contingency groups’ data was analyzed by comparing overall levels of responding during the CS and in the Pre CS periods. Overall, the CSs tended to reduce instrumental responding, and they did so in increasing amounts with longer CS durations. A Group (Ran 20, 60, 180) × Period (Pre CS, CS) ANOVA performed on these data revealed a significant main effect of Period, F(1,21)=28.39, and a significant Group × Period interaction, F(2,21)=6.09. Separate 1-way ANOVAs comparing Pre CS to CS responding in each random control group using a pooled error term revealed that the CSs reduced responding in the Random 60 and Random 180 groups, Fs(1,21)=11.47 and 28.85, respectively.
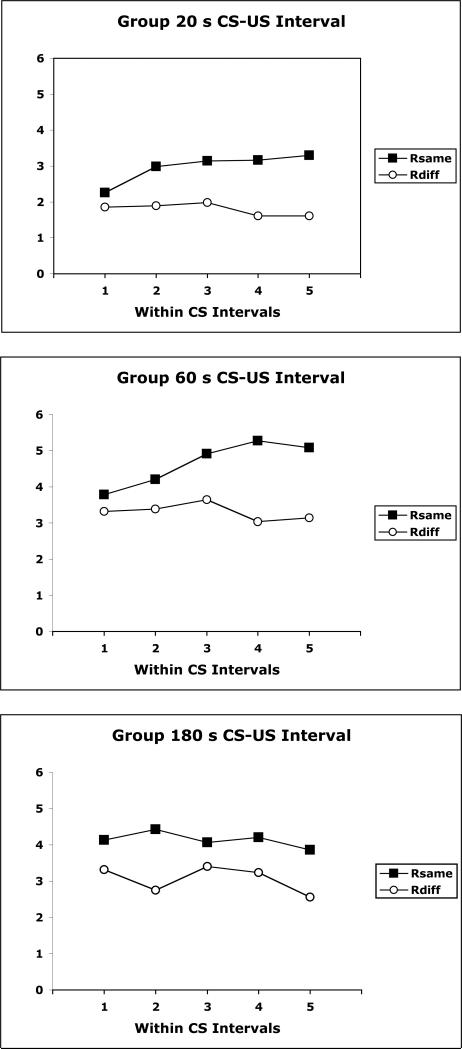
Temporally specific PIT effects were also examined. Figure 6 displays for each paired group the effects of the CSs on Same and Different responses over successive 5ths of the CS interval averaged over the two tests. It is evident that greater US-selective transfer occurred towards the end of the CS interval in the groups trained with 20-s and 60-s CS-US intervals. This temporal control was not so evident in the group trained with 180-s CS-US interval. These data were analyzed with a Group (20, 60, 180) × Response (Same/Diff) × Interval (1-5) ANOVA. This analysis revealed significant main effects of Group, F(2,21)=7.49 and Response, F(1,21)=8.67, and also a significant Response × Interval interaction, F(4,84)=3.16.
Figure 6.
Data from the PIT tests of Experiment 1 presented over successive quintiles of the CS for groups trained with 20-s, 60-s, and 180-s CS-US intervals. Data are shown for the responses previously reinforced by the same or different US as that signaled by the CS.
Discussion
The data from Experiment 1 indicate that wide variations in CS-US interval produce large differences in acquisition of magazine approach CRs. In spite of these large differences in the abilities of CSs trained at different CS-US intervals to evoke CRs, these stimuli produced comparable US-specific effects in Pavlovian to instrumental transfer tests. The hypothesis that sensory learning, as revealed by selective PIT, would decrease with increasing CS-US intervals and be replaced by more general, motivational learning was not supported by these data. Indeed, there was no evidence in this experiment that the stimuli had acquired associations involving the motivational components of the US. Pavlovian to instrumental transfer in this experiment was manifest only in terms of a selective influence on instrumental performance. In other words, the stimuli failed to generally elevate responding, as would be expected if the rats learned about the general motivational properties of the US. Instead, after training at different CS-US intervals, the stimuli were equally capable at selectively biasing choice in favor of the instrumental response with which they had earlier shared a US in training. Moreover, this effect showed up as a selective lowering of the different outcome instrumental response relative to baseline levels of responding, not as a selective elevation of the same outcome response.
Another feature of the present data worth considering is the temporal specificity of this learning. During Pavlovian training, magazine approach CRs clearly displayed temporal specificity ultimately peaking towards the end of the CS interval close in time to the actual time of the US. However, CRs did not begin at this point. Early in training when the paired and random groups first differed, CRs in the paired groups were uniformly distributed across the CS. As training progressed, the CR timing functions in groups trained with 20 s or 60 s CS-US intervals changed their shape from a uniformly flat function to one that was peaked towards the appropriate time. This result is not consistent with those reported by Balsam and his colleagues (e.g., Drew et al., 2005) who showed that when CRs first began to occur, they appeared late in the CS. The reason for this discrepancy will be taken up in the general discussion.
It is of additional interest to point out that the selective PIT effect we observed here was also shown to be temporally specific, but that temporally specific PIT was not required for US-selective PIT. Only two of the three paired groups displayed good temporal control of US-selective PIT. The 180-s CS-US interval group did not display good temporal control of PIT, but they displayed US-selective PIT that was comparable overall to the other groups. This points to a distinction between the temporal and US-selective features of learning.
One potential problem with the present results concerns the method of testing. In this study, the CSs were tested in each group at their training durations. Although the three groups were exposed to each CS for a total of 9 min during the tests, different numbers of trials and different amounts of time between trials occurred in the three groups. It is possible that these differences in test conditions may have obscured any potential differences in selective PIT that may have been produced by different CS-US intervals in training. The next experiment explored this possibility.
Experiment 2
Whereas Experiment 1 examined CS-US interval effects on PIT using a test procedure that confounded conditions of testing with the conditions of training, the present study avoided this problem. The design of the experiment appears in Figure 7. There were two groups of rats trained with either 20-s or 120-s CS-US intervals. Both of these groups were then tested with 20-s and 120-s CS durations. The group trained with 180-s CS-US intervals in Experiment 1 was replaced with a 120-s CS-US interval group in this experiment to encourage somewhat higher CR levels during acquisition. There were several other differences in procedure introduced in the present study. Subjects were given Pavlovian training prior to instrumental training, they were given more instrumental training sessions, and the PIT test was conducted with each response separately rather than involving a choice. Holland (2004) found that general PIT was more likely to occur after the response was more extensively trained. The other procedural differences from Experiment 1 were introduced to more closely match the procedures used by Holland (2004).
Figure 7.
Design for Experiment 2.
Method
Subjects
Subjects were 32, experimentally naïve, male Sprague-Dawley rats (supplied by Charles River Breeders). Their weights varied between 407 and 505 g at the beginning of the experiment. The rats were housed and maintained at 85% of their free feeding weight as in Experiment 1 throughout the experiment.
Apparatus
The same apparatus was used as in Experiment 1.
Procedure
The rats were initially magazine trained with pellet and sucrose USs over two days as in Experiment 1.
Pavlovian conditioning
The rats were given Pavlovian training with two CS-US pairs over the next 24 sessions. These sessions were conducted exactly as in Experiment 1 with the exception that one group was trained with 20-s CS-US intervals, and a second group was trained with 120-s CS-US intervals. Random control groups were not run in this experiment.
Instrumental training
Starting on the day following Pavlovian conditioning, all rats were given lever press and chain pull training. Each response was reinforced on a continuous reinforcement schedule until it met a 50 reinforcements criterion. Half of the rats were trained with Lever Press – Pellet and Chain Pull – Sucrose contingencies, while the remaining rats were trained with the reverse contingencies. These squads were arranged to be orthogonal with respect to their Pavlovian training.
Instrumental VI training began on the following day and continued for 14 sessions. The average value of the VI during the first three sessions was 10 s, 30 s, and 30 s. Thereafter, the rats were trained on a VI 60 s schedule.
Pavlovian to Instrumental Transfer Tests
One Pavlovian retraining session was given on the next day, and this was followed by the first set of PIT tests run over the next 2 days. On each of these days all subjects were given 2, 32 min sessions, one with Lever and one with Chain in that order. On the first of these days, subjects were tested with either 20-s CSs or 120-s CSs (counterbalanced). On the second of these days, subjects were tested with the CSs presented at the duration they had not experienced in Test 1. For the 120-s tests, the session consisted of alternating presentations of 2-min stimulus off and 2-min stimulus on periods where the stimuli were presented in an NFFNFNNF sequence. The 20-s CS tests were run identically, except that the duration of the stimuli was reduced to 20 s. This meant that 2-min stimulus off periods alternated with 20-s stimulus on and then 100 s post stimulus periods. Thus, in the 120-s CS tests, there was a cumulative total of 8 min of stimulus on time for each stimulus in the session, whereas in the 20-s CS tests there was a cumulative total of 80 s stimulus on time in the 32 min session.
This first set of tests was followed over the next 4 days by 2 instrumental retraining sessions and then 2 more Pavlovian retraining sessions. A second set of PIT tests was given over the next 2 days. These tests were conducted like the first set of PIT tests except that (1) Chain was tested first on each day and Lever second, and (2) the order of testing subjects with 20-s and 120-s CSs over days was counterbalanced with respect to the order to which they were exposed in the first set of tests.
Results
Pavlovian training proceeded as expected. By the end of training, subjects were responding to the 20-s CSs at a higher level than the 120-s CSs (26.2 and 7.4 responses per minute, respectively). Pre CS response rates were lower than CS response rates in both groups (2.0 and 2.3 responses per minute, respectively). In addition, the temporal distribution of magazine approach CRs followed a similar pattern across the conditioning phase to that seen in Experiment 1 (Figures 2 and 3). To examine this more thoroughly Figure 8 presents the relative response rates occurring across successive portions of the CS in groups trained with 20 s and 120 s CSs during blocks 2 and 6 of training. The distribution of responding across the CS changed from a flat one early to an accelerating one later in training in the group trained with 20 s CSs, whereas responding generally decreased across the CSs, though somewhat more sharply early in training, in the group trained with 120 s CSs.
Figure 8.
Mean relative magazine response rates across successive quintiles of the CS period in Blocks 2 and 6 of training for groups trained with 20-s or 120-s CS-US intervals.
These data were analyzed with separate Block × Within CS Interval ANOVAs performed on each group using a pooled error term. The Block × Within CS Interval interaction was significant in group 20, F;4,56 = 6.09, as well as in group 120, F;4,56 = 3.01. Post-hoc contrasts following the methods of Rodger (1974) revealed that relative response rates on block 2 of training in group 20 s subjects were somewhat higher in the second portion of the CS but were equal in all other portions, ps < .05. Proportionally less responding occurred early in the CS in these subjects, while more responding occurred late in the CS in block 6, ps < .05. Subjects trained with 120 s CSs generally decreased their responding across the CS in blocks 2 and 6, though responding in the first portion of the CS was greater in block 2 than in block 6, ps < .05. This presumably reflects the reduction across training of any unconditioned effects of the stimuli manifest at the beginning of the CSs.
Instrumental training also proceeded as expected. On the final day of instrumental VI 60 training the mean instrumental response rate was 21.1 responses per min. The average response rate for pellets was higher than for sucrose (28.2 versus 14.1 responses per minute), but responding on the chain and lever manipulanda were similar (22.6 and 19.6 responses per minute).
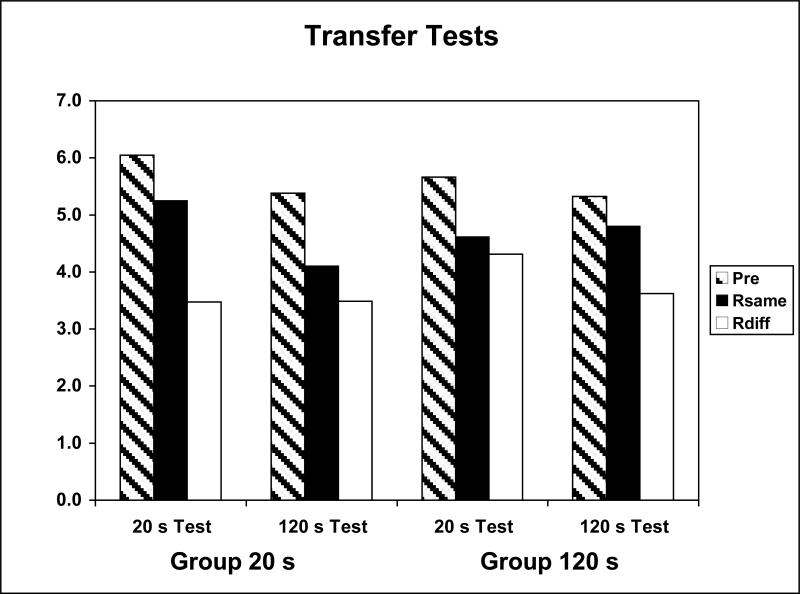
The data of most interest came from the PIT tests, and these are displayed in Figure 9, which shows the session data averaged over the 4 tests. The data are from the entire CS and are broken down in terms of Same, Different, and Pre CS response rates. Overall, both groups displayed reinforcer-specific PIT, but this effect was somewhat greater when the stimuli were tested at their training durations. In other words the group trained with a 20-s CS-US interval showed larger selective PIT when tested with 20-s CSs than when tested with 120-s CSs, but the reverse was true for the group trained with a 120-s CS-US interval. Once again, this selective transfer showed up in terms of a selective lowering of Different responses relative to the Pre CS period.
Figure 9.
Data from the PIT tests of Experiment 2 for the groups trained with 20-s and 120-s CS-US intervals. Mean instrumental responding is shown for both 20-s and 120-s tests during pre stimulus periods and during the stimuli when the stimulus and response were reinforced previously with the same or different US.
These data were analyzed with a Group (20 s, 120 s) × Test CS Duration (20 s, 120 s) × Response (Same, Diff) ANOVA on the data averaged over the 4 test sessions. This analysis revealed a significant main effect of Response, F(1,14)=6.97, as well as a significant Group × Test CS Duration × Response interaction, F(1,14)=8.09.
Discussion
The data from the present study agree with those from Experiment 1 in showing that comparable levels of US-specific PIT occurred after the stimuli were trained with short (20 s) and long (120 s) CS-US intervals. The data further support this conclusion by showing these effects when the stimuli were tested under comparable testing conditions. There was some generalization decrement, however, revealed in these tests by the fact that each training group performed somewhat poorer when tested with CS durations that differed from those they had experienced during training. Nevertheless, these data suggest that the results obtained in Experiment 1 are not likely due to the possibility that some test CS durations are better than others at revealing sensory specific learning.
The present data, unlike Holland's (2004), provided little evidence that the CSs generally facilitated instrumental responding. One possible reason for this discrepancy is that training with multiple instrumental reinforcers may render the PIT test especially sensitive to assessing sensory-specific components of Pavlovian learning. This explanation gains some support from Holland's (2004) finding that Pavlovian CSs non-selectively energized instrumental responding when just one instrumental reinforcer was used, whereas this non-selective PIT effect was greatly attenuated in a group trained with two instrumental reinforcers. In addition, there were several other procedural differences that could contribute to this difference.
Experiment 3
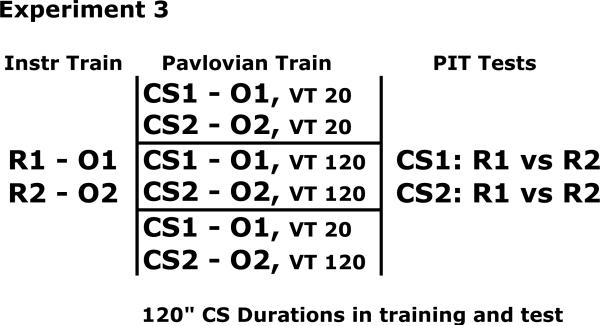
In each of the previous two experiments we observed that the degree to which CSs exerted US-specific control over instrumental responses in a transfer test was unaffected by wide variations in CS-US interval. In Experiment 1 we obtained this result using a test procedure that confounded CS duration during testing with CS duration during training, while in Experiment 2 the result was obtained when the CSs were tested under more comparable conditions. The present experiment used a different strategy to examine the role of the “proximity” of CS and US in determining sensory-specific learning. In Experiment 3 we varied the density of US occurrence within the CS. This manipulation can be thought of as being similar to CS-US interval manipulations. Some authors have assumed that the critical variable in a conditioning experiment is the rate of US occurrence (e.g., Gallistel and Gibbon, 2000; Gibbon and Balsam, 1981). From this perspective, CS-US interval manipulations are interpreted in terms of manipulations of US rate. Short CS-US intervals correspond to a higher rate of US occurrence within the CS compared to long CS-US intervals. For the same reason that one might expect shorter CS-US intervals to result in superior sensory-specific learning about the US, one might also expect that CSs associated with higher US densities should result in more sensory-specific learning about the US as well. The present study examined this possibility using both a between-group and within-group experimental design (see Figure 10).
Figure 10.
Design for Experiment 3.
Two separate groups of subjects were trained with 2 CS-US pairs where each CS was 120 s in duration. In different groups of subjects the US was presented within the CS at a rate of 1/120 s or 6/120 s. This corresponds to the different rates of US occurrence used in Experiment 2 that compared CS-US intervals of 120 s and 20 s. A third group of subjects was trained with a US density of 1/120 s in one CS-US pair, and 6/120 s in the other CS-US pair. During the PIT test, all subjects were tested in the same way with 120-s CSs durations. Thus, the present study avoided the problems associated with different test procedures used in Experiment 1 or differential generalization between training and test as observed in Experiment 2. Furthermore, this experiment allows us to examine the notion that CS-US proximity may influence the content of learning by varying US density as an alternative to varying CS-US interval. If more sensory-specific learning occurs with CSs associated with higher US densities, then these stimuli should exert greater selective PIT in the present study.
Method
Subjects
Subjects were 48, experimentally naïve, male Sprague-Dawley rats (supplied by Charles River Breeders). Their weights varied between 362 and 465 g at the beginning of the experiment. The rats were housed and maintained at 85% of their free feeding weight as in Experiment 1 throughout the experiment.
Apparatus
The same apparatus was used as in Experiment 1. In addition, another auditory stimulus was used. This stimulus was a 1500 Hz auditory stimulus (T) presented at an intensity that was 5 dB above baseline levels.
Procedure
The rats were initially magazine trained with pellet and sucrose USs over two days as in Experiment 1.
Instrumental training
Initially, all subjects were given continuous reinforcement training as in the previous experiments and this was followed by 10 sessions of VI training with each response with each response-reinforcement pair counterbalanced as in Experiments 1 and 2. The VI values during the first three sessions were 10 s, 30 s, and 30 s. Thereafter, the rats were trained on a VI 60 s schedule.
Pavlovian conditioning
All rats were given Pavlovian training with two CS-US pairs over the next 10 sessions. Each of these sessions was 56 min long and contained 4 trials of each of 2 CSs. Four different trial sequences with different intertrial intervals were used across days. The ITI varied randomly between 3 and 7 min, with a mean of 5 min. The subjects were pseudorandomly assigned to 3 different groups with n's of 16. Subjects in Group VT 20 s and VT 120 s were trained with USs randomly occurring within each CS according to, respectively, a VT 20 s or VT 120 s schedule of US delivery, with the constraint that 6 USs and 1 US, respectively, occurred on each trial in the two groups. Subjects in Group Mixed were trained with USs occurring in one CS according to a VT 20 s schedule, and in the other CS according to a VT 120 s schedule. N and F stimuli were used in Groups VT 20 s and VT 120 s, and these were fully counterbalanced across the different CS-US and response-US assignments, but Group Mixed was trained with T and F stimuli. On Day 1 of training subjects in Group Mixed were inadvertently run with T instead of N, and, consequently, this stimulus was used throughout the study in place of N.
Pavlovian to Instrumental Transfer Tests
All subjects were given instrumental VI 60 s retraining sessions with Lever and Chain over the next two days. This was followed on the next day by an 8-min extinction test in which both Lever and Chain manipulanda were concurrently available. The first PIT test occurred on the next day and was similar to the test of the 120-s CSs in Experiment 2. Briefly, each CS was presented 4 times in an FNNFNFFN sequence (or FTTFTFFT for Group Mixed), and 2 min periods in which no stimulus was presented alternated with 2 min periods with one or the other CS present. One additional instrumental retraining session occurred on the next day and a second PIT test occurred on the next session that was run identically to Test 1 with the exception that the trial sequence was NFFNFNNF (or TFFTFTTF in Group Mixed).
Results
Instrumental training proceeded uneventfully. On the final day of instrumental VI 60 training the mean instrumental response rate was 15.3 responses per min. The average response rate for pellets was higher than for sucrose (20.2 versus 10.5 responses per minute), and responding on the Lever was slightly greater than responding on the Chain (16.0 and 14.6 responses per minute, respectively).
Magazine approach responding during the Pavlovian conditioning phase will not be presented because there was no attempt in this study to assess magazine approach responses during the CSs prior to US delivery, which could have occurred at any time within the CS. However, magazine approach responses during the CSs in the transfer tests revealed differences as a function of US density. Magazine approach CRs did not differ during the Pre CS periods in Groups VT 20 and VT 120, however, there were more CRs during the CSs in Group VT 20 (12.7 responses per minute) than in Group VT 120 (8.7), F(1,30)=8.95. Similarly, in Group Mixed there were more CRs during the VT 20 CS (11.7 responses per minute) than during the VT 120 CS (7.8), F(1,15)=7.43.
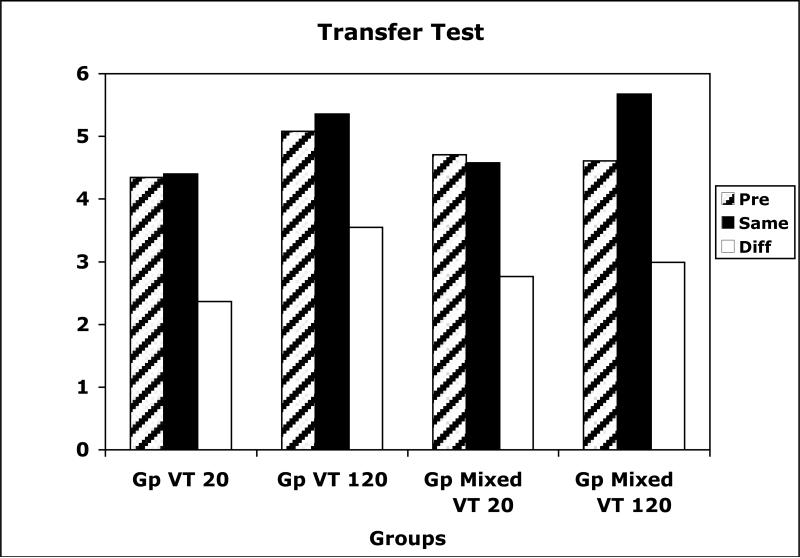
The instrumental response data during the PIT test are presented in Figure 11. The data show that the CSs exerted US-specific control whether they were associated with a high or low US density, and, once again, the effect showed up as a selective lowering of Diff responses relative to the Pre CS period. A US Density × Response between groups ANOVA revealed significant main effects of US Density, F(1,30) = 5.98, and Response, F(1,30) = 25.14, but no interaction. Similary, a US Density × Response within groups ANOVA performed on Group Mixed only revealed a significant main effect of Response, F(1,15) = 23.67. There were no differences, either between groups or within groups, in Pre CS response rates.
Figure 11.
Data from the PIT tests of Experiment 3 for groups trained with USs presented during the CSs according to a variable time 20-s schedule (VT 20), a variable time 120-s schedule (VT 120), or both (Gp Mixed). Mean instrumental responses are shown separately during the pre stimulus period, and during the stimuli according to whether the stimulus and response were reinforced previously with the same or different US.
Discussion
The results from the present study agree with those found in Experiments 1 and 2. A 6-fold difference in US density had no effect on US-specific PIT. These results were not compromised by differences in testing procedures across the different US density conditions. Furthermore, because the same CS durations were used in training and in testing, there were no generalization decrement problems introduced by changing the CS duration, as may have been the case in Experiment 2. It is conceivable, however, that Group VT 20 may have undergone more generalization decrement during the PIT test than Group VT 120 because the transition from high density USs during training sessions to no USs in extinction test sessions may have been more obvious to Group VT 20 subjects. If this were so, then the amount of selective PIT observed in this group may have underestimated the strength of learning. This analysis is challenged, however, by the observation that there was no difference in the amount of selective PIT between VT 20 and VT 120 conditions in Group Mixed.
Before accepting the conclusion supported by these results that CS-US proximity (conceptualized in terms of US rate or CS-US interval) plays little role in sensory-specific learning, it may be argued that even greater CS-US proximity than was used here may reveal such effects. This possibility was examined in the next experiment.
Experiment 4
In Experiments 1 through 3 the most proximal condition was a 20-s CS-US interval and a US density of 1/20 s. It is possible that an even shorter CS-US interval may produce more selective learning than would be found with longer intervals. One reason why we chose a 20-s CS-US interval as our “short” CS-US interval condition was to increase the stability of our PIT measure. Shorter duration CSs do not permit for much sampling time across the CS in which to assess transfer. Holland and Gallagher (2003), however, observed that general PIT was obtained with a shorter CS-US interval when responding was monitored in Post CS periods. These authors did not use an experimental design that would permit for an assessment of sensory-specific PIT, but it is possible that such effects could be assessed in this way with very short CS-US intervals. The present experiment (see Figure 12), therefore, compared two groups trained with 2 or 20-s CS-US intervals, and assessed PIT in both CS and Post CS intervals. Both groups were also tested with each cue duration.
Figure 12.
Design for Experiment 4.
In addition, the present study examined sensory-specific learning in two other ways. Following an earlier observation of Weingarten (1983) that CSs can potentiate feeding in sated rats, Holland and his colleagues found that CSs not only potentiated feeding in rats sated on their home cage chow (Holland & Gallagher, 2003), but did so in a US-specific manner (Galarce, Crombag, & Holland, 2006; Petrovich, Ross, Gallagher, & Holland, 2006). A CS increased sated rats’ intake of the food US previously paired with that CS, but not intake of other foods. A second aim of the present study was, therefore, to examine the ability of CSs trained with 2 s or 20-s CS-US intervals to selectively potentiate feeding. Each of two CSs was presented in separate test sessions while subjects were consuming one or the other US.
Following the PIT and potentiated feeding (PF) tests, subjects were given selective US devaluation tests to further assess any differences in sensory-specific learning in CSs trained with 2 or 20-s CS-US intervals. For this test, one of the USs was devalued, through pairings with illness, and the effect of this devaluation was assessed on conditioned magazine approach responses evoked by the CSs whose associated US was devalued or not. The observation of selective devaluation effects has been taken as evidence that the CS has associated with the sensory specific qualities of the US (for an early review see Delamater and LoLordo, 1991). This follows from the assumption that sensory features of the US undergo devaluation, and decreased responding to the CS in test is mediated by its activation of those now devalued sensory features of the US (see Rozeboom, 1958). The present study, therefore, examined potential differences between 2 and 20-s CS-US intervals at supporting sensory-specific learning with three separate test procedures that have been used to assess such learning.
Method
Subjects
The subjects were 24 male experimentally naïve Long-Evans rats (supplied by Charles River Breeders, Raleigh, NC). Their weights varied between 336 and 387 g (20 rats) or 472 and 580 g (4 rats) at the beginning of the experiment. The rats were housed individually in a colony room that was on a 12/12 light/dark cycle. Except during the satiation and potentiated feeding test phases, they were maintained at 85% of their free feeding weight as in Experiments 1-3. Experimental sessions were conducted during the light phase of the rats’ light/dark cycle.
Apparatus
The apparatus consisted of eight conditioning chambers, similar to those used in the previous experiments. Each chamber measured 22.9 × 20.3 × 20.3 cm, and was composed of aluminum front and back walls and clear acrylic side walls and top. An infrared activity monitor (Coulbourn Instruments, Allentown, PA, USA) and a panel of infrared lights used to illuminate the chamber for video recording were placed on the top of each chamber. An illuminated clear acrylic liquid cup, with a capacity of about 2 ml, was placed behind a 6 × 6 cm square hole in the center of the front wall. Infrared phototransistors within the liquid cup recess, 1.0 cm from the floor and 0.3 cm from the front wall, were used to detect head entries and time spent in the cup. The primary measure of Pavlovian conditioning was the time spent in the liquid cup during CS presentations, expressed as a percentage of the CS-US interval. Responding during the 5-s periods before each CS was also recorded. Liquids were delivered to the cup through 33-gauge channels connected to Coulbourn syringe pumps by PE-100 tubing. An aluminum lever (2.0 × 2.0 cm) was mounted on each side of the food cup, centered between the cup and the side walls; throughout the Pavlovian training sessions, they were covered with aluminum boxes (3.0 × 2.0 × 3.0 cm). A speaker, which was used to present auditory cues, was placed on the back wall of a sound-resistant shell which enclosed each experimental chamber. The auditory cues were a white noise (80dB) and a 1,500-Hz, 80-dB tone. A television camera was placed 18 cm above the speaker to record the rat's behavior, and a second camera was placed under the transparent food cup, to record consummatory responses. Images from each camera were digitized, and were recorded and displayed in real time on video monitors, each of which showed 4 chambers or food cups. Video data are not presented in this article. The shell with the speaker and camera was enclosed in another sound-resistant shell, on which a fan was mounted. This fan provided ventilation and constant background noise (68 dB).
Procedure
The rats were initially magazine trained with maltodextrin (4%, w/v) and sucrose (4%, w/v) USs over four days. One of these USs was given on days 1 and 3 and the other US was given on days 2 and 4. Each session was 64 min long and included 16, 0.2-ml deliveries of the appropriate US, each lasting 1 s.
Pavlovian conditioning
There were 10 Pavlovian conditioning sessions, each lasting 32 min. Each session contained 4 reinforced T and 4 reinforced N trials. CS and US identity were fully counterbalanced. In Group 2 (n=12), the CS duration was 3 s and the US was delivered in the final 1 s of the CS creating a CS-US interval (onset to onset) of 2 s. Group 20 (n=12) was trained similarly except that the CS was 21 s in duration with the US overlapping in the final 1 s of the CS.
Instrumental training
All rats were then given continuous reinforcement training with the left and right levers, as in the previous experiments. The different response-US combinations were counterbalanced and orthogonal to the Pavlovian contingencies. There were a total of 12, 30-min VI training sessions, 6 with each lever. As in the previous experiments, the two responses were trained in separate sessions. For each lever, the value of the VI was 30 s for the first 2 sessions and 60 s for the remaining 4 sessions.
After Pavlovian and instrumental training, subjects were given PIT, PF, and US Devaluation tests. The order of PIT and PF tests was counterbalanced across subjects, and the US Devaluation test occurred at the end of the experiment.
Pavlovian to Instrumental Transfer Tests
One Pavlovian reminder session was given prior to the first PIT test. For rats that received the PF test first, one instrumental reminder session was also given for each lever. There were 2 PIT test sessions, in which both levers were simultaneously available. Each PIT test was 30 min long and consisted of 4 T and 4 N trials. In Test 1, stimulus durations corresponded to the original training CS-US intervals, 2 s for Gp 2 and 20 s for Gp 20. In test 2, the test durations were reversed; Gp 2 was tested with 20 s CSs and Gp 20 was tested with 2 s CSs. Between Tests 1 and 2, all subjects were given one Pavlovian retraining session (with Levers covered) and then one VI 60 retraining session with each Lever.
Potentiated Feeding Tests
One Pavlovian reminder session was given prior to satiation and the PF tests. After the reminder session, all rats were given unlimited access to their home cage chow and water for 10 days prior to the first PF test session. Three 10-min PF test sessions followed over the next three days. In these sessions, consumption of one of the USs (counterbalanced within each group) was assessed in the presence of the Same, Different, or no CS, in separate sessions. The first 5 min of each session was a pretest period, and the final 5 min comprised the test period. In the test period, one of the CSs (counterbalanced) was presented continuously (5 min) in the first session and the other CS was presented in the third session; no CS was presented in test period in the second session. Previous experiments (e.g., Holland, Petrovich, & Gallagher, 2002, Holland & Gallagher, 2003) showed that placement in the chamber alone produces substantial eating; the purpose of the 5-min pretest interval before each test was to reduce the contribution of that effect to consumption observed in the test intervals. Each session began with 1.7 ml of one of the USs present in the liquid cup. The liquid level in each cup was maintained by two experimenters, blind to the experimental conditions, who each observed 4 liquid cups on a monitor located in an adjacent room. Whenever the liquid level in the cup went below the marked initial level, the experimenter activated an additional 0.2-ml delivery to that cup. The measure of consumption was the number of deliveries needed to maintain the liquid level. After the final PF test, the rats were returned to a food restriction regime over the next 5 days, to reduce their body weights to 85% of their free feeding weights.
US Devaluation and Test
One Pavlovian reminder session preceded this phase. Over the next 4 sessions, all rats were trained with a discriminative flavor aversion learning procedure. On days 1 and 3, one of the former USs (sucrose or maltodextrin) was presented on each rat's home cages in a bottle for 20 min, followed immediately by an intraperitoneal injection of LiCl (10 mg/kg, 0.3 M). On days 2 and 4 the other US was presented in a similar fashion but with no injections. Thus, half of the rats learned an aversion to sucrose and the remaining to maltodextrin, counterbalanced with respect to all prior training contingencies. On the next day, all rats received a 32-min US Devaluation Test, in which the N and T CSs were presented 4 times each. Half of the subjects in each group were tested with the CSs presented at durations that matched their training CS-US intervals (2 or 20 s), and the remaining subjects were tested with 10-s CS durations. In all cases, responding was recorded for 20 s after CS onset. In previous experiments, we have observed maximum sensitivity to devaluation during time intervals immediately prior to or after the normal time of US delivery (e.g., Holland & Straub, 1979; Pickens et al., 2005). Thus, our primary measure of post-devaluation responding in the rats that were tested with their original cue durations was responding during the 5-s interval beginning 15 s after CS onset in Group 20 and during the 5-s interval beginning with CS onset in Group 2. Likewise, in the rats that were tested with 10-s cue durations, our primary measure was responding during the second 5-s interval in Group 20 and the first 5-s interval in Group 2. Thus, our primary measure was a 5-s sample of behavior in all rats. Responding in the other intervals is also reported, but less extensively. After the completion of the post-devaluation test, each rat received 20-min presentations of each of the two USs in their home cages (one US each day, in counterbalanced order, over the next 2 days) to assess the discriminated flavor aversion.
Results
Pavlovian and instrumental conditioning
Initial Pavlovian and instrumental conditioning proceeded as would be expected. Throughout Pavlovian training, Gp 2 rats displayed higher levels of magazine approach responding during the CSs than Group 20 rats. On the final day of conditioning, rats in Gp 2 spent an average of 61.7 ± 2.4% of their time with their head in the magazine during the 2-sec CSs, compared to 42.2 ± 3.0% during the last 2 s of the 20-s CSs in Group 20. Pre-CS responding was 5.9 ± 1.5% in Group 2 and 5.8 ± 1.8% in Group 20. Responding to the noise was greater than responding to the tone, but the two USs were equally effective as reinforcers. A Group (2 or 20) × Cue (noise or tone) × US (sucrose or maltodextrin) ANOVA showed a significant effect of Group, F(1, 20 ) = 29.06, and Cue, F(1, 20) = 14.21; other Fs < 1. Instrumental lever pressing was acquired similarly in both groups; on the final training session, the rates of lever pressing in Groups 2 and 20 were 6.7 ± 0.6 and 6.4 ± 1.0 responses/min, respectively. A Group × Lever (left or right) × US ANOVA showed no significant effects or interactions, Fs < 1.
PIT tests
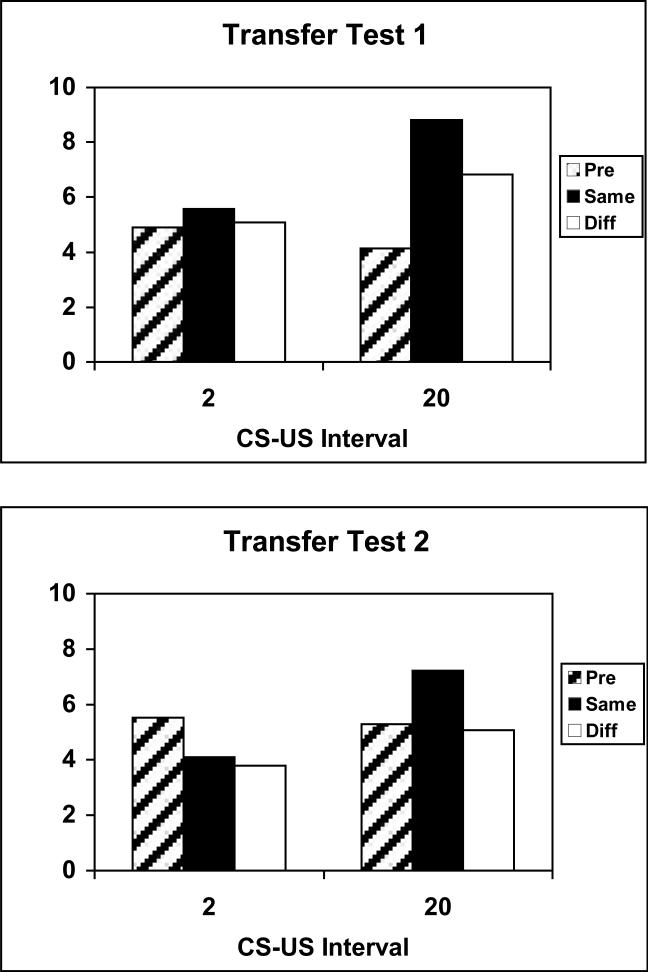
As in Experiments 1 and 2, rats trained with 20-s CS-US intervals showed selective PIT in Test 1: responding on each lever was more frequent in the presence of the same CS, which signaled the reinforcer normally earned by responses to that lever, than in the presence of the different CS. Unlike in the previous experiments, we also observed nonselective PIT: both CSs elevated lever pressing above baseline. By contrast, rats in Group 2 showed no evidence for PIT, selective or general. Figure 13 (top portion) shows the results of Test 1, in which Group 20 was tested with 20-s CSs and Group 2 was tested with 2-s CSs. As in previous studies with short-duration CSs (Holland & Gallagher, 2003), we sampled post-CS periods as well as CS periods in Group 2. The data shown in Figure 13 include responding during the entire 20-s CS interval in Group 20, and in the 20-s period that began with the 2-s CS in Group 2. Analyses that sampled only the first 10-s after CS onset in both groups showed comparable patterns of differences and statistical significance.
Figure 13.
Data from the PIT tests of Experiment 4 for groups trained with 20-s or 2-s CS-US intervals. Mean instrumental responses (+/- standard error) are shown separately during the pre stimulus period, and during the stimuli according to whether the stimulus and response were reinforced previously with the same or different US. Panel A shows data from the test sessions in which the stimuli were tested at their training durations in each group. Panel B shows data from the second set of test sessions in which the stimuli were tested at the other groups training durations.
Initial ANOVAs that included test order (before or after the PF test), lever, CS, and US showed no significant main effects or interactions for those variables, so they were omitted from subsequent analyses. A Group × Cue (baseline, same, or different) ANOVA showed a significant main effect of Cue, F(2, 44) = 16.88, and a significant Group × Cue interaction, F(2, 44) = 10.33. Individual comparisons using the Tukey honestly significant difference (HSD) procedure showed that in Group 20, the response rate during the same CS was significantly higher than during the different CS and pre-CS periods, and responding during the different CS was greater than responding in the pre-CS periods. By contrast, in Group 2 none of these comparisons was reliable.
Figure 13 (bottom portion) also shows the results of Test 2, in which the rats in Group 20 were tested with 2-s CSs and the rats in Group 2 were tested with 20-s CSs. As in Test 1, a 20-s sampling period, beginning with CS onset, was used in each group. The rats in Group 20 maintained selective PIT, but there was no evidence for general PIT. That is, responding was greater during the same CS than during the different CS, but only the same CS elevated responding over pre-CS levels. As in Test 1, there was no evidence for either selective or general PIT in Group 2; indeed, the CSs tended to suppress responding relative to pre-CS levels, although this suppression was significant only for the different CS.
A Group × Cue ANOVA showed a reliable effect of cue, F(2, 44) = 4.48, and a significant Group × Cue interaction, F(2, 44) = 7.54. Individual comparisons (Tukey HSD) showed that in Group 20, the response rate during the same CS was significantly higher than during the different CS and pre-CS periods, but responding during the different CS did not differ from pre-CS responding. By contrast, in Group 2, pre-CS responding was significantly greater than responding during the different CS. Pre-CS and same CS responding did not differ significantly.
Potentiated feeding tests
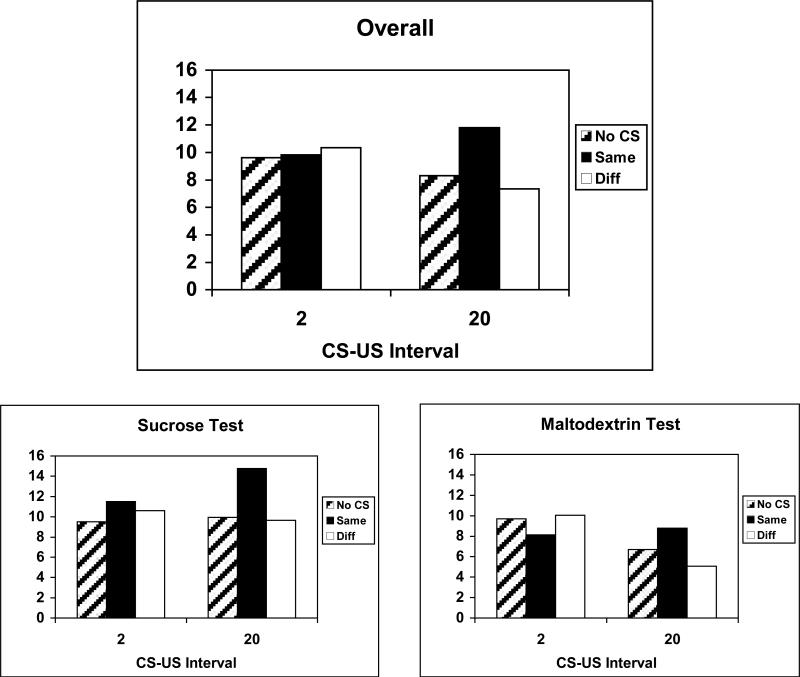
Figure 14 shows consumption during the PF tests, conducted while the rats were sated on lab chow. The left portion of Figure 10 shows consumption of all rats, and the middle and right portions of Figure 10 show separately the consumption of rats tested with sucrose and maltodextrin, respectively. Relative to consumption in the no-CS condition, only Group 20 showed CS-potentiated feeding. Furthermore, in that group, the CS-potentiated feeding was highly specific: consumption was only potentiated by the same CS, which had previously signaled the available test US.
Figure 14.
Data from the potentiated feeding tests in Experiment 4, shown separately for the groups trained with 20-s or 2-s CS-US intervals. Mean number of US deliveries (+/- standard error) is shown for the test sessions in which no CS was presented and in sessions in which the CS signaled the same or different US than was actually delivered. Data are shown collapsed across US identity, as well as for each US (sucrose or maltodextrin).
Initial ANOVAs that included test order (before or after PIT tests) and CS identity (tone or noise) showed no significant main effects or interaction with those factors. A Group (2 or 20) × Test US (sucrose or maltodextrin) × Test Cue (same, different, or no CS) ANOVA showed significant main effects of Test US, F(1, 20) = 12.04, and Test Cue, F(2, 40) = 5.98, and a significant Group × Test Cue interaction, F(2, 40) = 7.81. In addition, the Group × Test US, F(1, 20) = 3.95, p = 0.061, and Test US × Test Cue, F(2, 40) = 3.20, p = 0.051, interactions were marginally significant. In Group 20, the simple main effects of both Test US (consumption was greater in sucrose tests than maltodextrin tests), F(1, 10) = 30.83, and Cue, F(2, 20) = 15.77, were significant, but their interaction was not, F(2, 20) = 1.36. Individual comparisons (Tukey HSD) showed that in Group 20, sucrose consumption was greater during the same CS test than during either the different CS or no-CS tests, which did not differ from each other. Maltodextrin consumption was significantly greater in the same CS test than in the different CS test, but the remaining differences were not significant. Within Group 2, there were no significant simple main effects or interactions of Test US and Test Cue, Fs < 1.93.
Conditioned flavor aversion training and devaluation test
The day before conditioned flavor aversion training with one of the USs, all rats received a single Pavlovian reminder training session. In that session, responding in Group 2 to the CS paired with the to-be-devalued US was 61.2 ± 2.7% and responding to the other CS was 60.8 ± 2.6%. Responding in Group 20 to those cues was 49.0 ±3.4% and 45.4 ± 5.0%. Pre-CS responding was 9.4 ± 2.0% and 10.6 ± 2.1% in Groups 2 and 20, respectively.
Discriminative conditioned taste aversion training in the home cages proceeded rapidly and did not differ across groups or liquid identity (sucrose or maltodextrin). On the first trial, the rats consumed 15.8 ± 0.7 ml of the test liquid, and on the final home cage test trials they drank 3.1 ± 0.2 ml of the liquid that had been paired with LiCl and 13.8 ± 0.7 ml of the unpaired liquid. A Group × Fluid × Contingency (paired or unpaired) × Trial ANOVA showed significant main effects of Contingency, F(1, 20) = 74.00, and Trial, F(2, 40) = 53.52, and a significant Contingency × Trial interaction, F(2, 40) = 423.83. No other effects or interactions were reliable, Fs < 1.55.
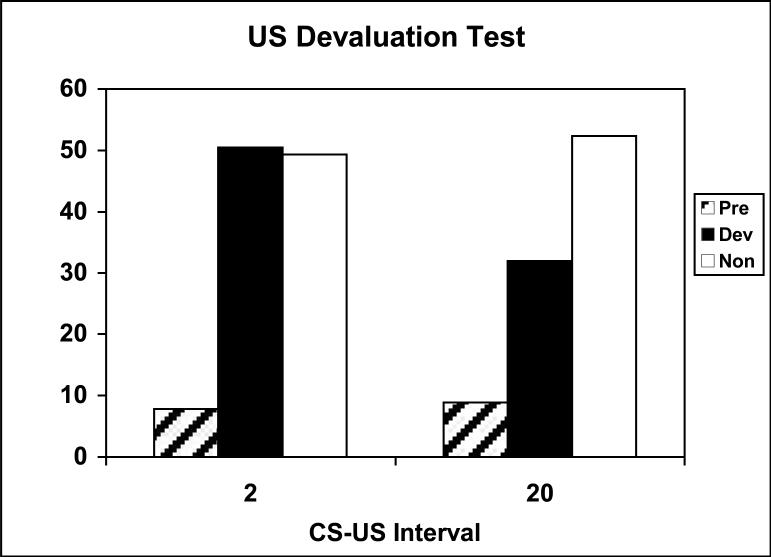
Figure 15 shows the results of the devaluation test of responding to the two CSs, conducted in extinction. The rats in Group 20 showed a selective devaluation effect, whereas the rats in Group 2 did not. (Note that our devaluation procedure did not permit assessing the occurrence of a general, nonselective devaluation effect.)
Figure 15.
Results from the US devaluation test in Experiment 4, shown separately for groups trained with 20-s or 2-s CS-US intervals. Mean % time in food cup (+/- standard error) is shown during pre stimulus and stimulus periods when the associated US was devalued or non-devalued (see Methods for details).
Initial analysis of the data showed no difference in responding attributable to the test procedure (10-s cues or original cue durations), so subsequent analyses collapsed across that variable. A Group (2 or 20) × Devalued US (sucrose or maltodextrin) × CS identity (tone or noise) × Test Cue (signal for the devalued or nondevalued US) ANOVA showed a significant effect of Test Cue, F(1, 16) = 14.32, and a significant Group × Test Cue interaction, F(1, 16) = 17.91. Individual comparisons (Tukey HSD tests) showed that responding to the devalued CS in Group 20 was significantly lower than responding in every other condition.
This ANOVA also revealed a significant CS identity × Test Cue interaction, F(1, 16) = 12.68, reflecting a larger devaluation effect with the tone CS. Examination of responding in the Pavlovian reminder training session indicated that this difference reflected a significant preexisting bias in responding, F(1, 16) = 33.80. A reanalysis of the test data, using performance in the reminder session as a covariate, gave results comparable to the original analysis: there was a significant effect of Test Cue, F(1, 15) = 14.31, and a significant Group × Test Cue interaction, F(1, 15) = 17.79, with no other significant effects or interactions. Individual comparisons within this analysis also yielded the same conclusions as in the original analysis.
Finally, it is worth noting that our conclusions do not depend on the particular data sampling procedure we adopted for the data shown in Figure 15 (see methods). Among the rats tested with their original cue durations, test responding during the 2-s CS period alone in Group 2 was 62.7 ± 2.3% during the signal for the devalued US, and 61.8 ± 6.2% during the other CS; responding in the intervals beyond the first 5 s after CS onset did not differ significantly from pre-CS responding in that group. Similarly, in Group 20, responding during the signal for the devalued US was 15.2 ± 5.7%, 18.1 ±4.0%, 32.1 ± 7.0% and 35.2 ±6.4% during the first 2-s, first 5-s, second 5-s and third 5-s periods after CS onset, respectively; responding in those same periods for the other (nondevalued) CS was 29.1 ± 9.8%, 35.5 ± 6.2%, 48.3 ± 8.8%, and 48.3 ± 8.4%. Thus, regardless of sampling period, the rats in Group 20 showed selective devaluation and the rats in Group 2 did not.
Discussion
Contrary to expectations based on Konorski's (1967) views, rats trained with short (2-s) CS-US intervals showed less evidence for sensory-specific learning than rats trained with longer (20-s) CS-US intervals. Despite showing greater conditioning (as indexed by magazine behavior in training), the rats trained with 2-s CS-US intervals failed to show sensory specific PIT, potentiated feeding, or devaluation effects. By contrast, each of these effects was highly selective in rats trained with 20-s intervals.
As in Experiments 1-2, with a 20-s CS-US interval, PIT was selective: the same CS enhanced instrumental response rates more than the different CS. Unlike in Experiments 1-2, the different CS also elevated responding over baseline levels. This later observation may be interpreted as evidence for a general PIT, as described by Corbit and Balleine (2005) and Holland (2004). Alternately, it could be argued that the facilitatory effects of the different CS in Experiment 4 reflected greater generalization between the two CSs, the two responses, or the two USs than occurred in the previous experiments. However, it is notable that Holland (2004) found a general PIT effect, using lever and chain responses, and food pellet and liquid sucrose USs, as in Experiments 1-3 of the present report. Although we cannot specify the basis of this difference, it may be important that the baseline instrumental response rates were somewhat lower in Experiment 4 and most of Holland's (2004) experiments than in Experiments 1-3.
Rats trained with 20-s CS-US intervals also showed selective potentiation of feeding while satiated on lab chow. Although a CS paired with the US that was available in the test enhanced consumption of that US, a CS that had been paired with the other US did not. This observation indicates that cue-potentiated feeding is not based solely on conditioning of a general state of “hunger”. Instead, it may reflect conditioning of specific appetites (Booth, 1985), or the enhancement of the palatability of the food by the CS. Using taste reactivity measures, Delamater, LoLordo, and Berridge (1986) demonstrated that auditory CSs can increase fluid palatability, and Holland (1990) showed that this effect is partly related to the ability of the CS to evoke a sensory-specific US representation. Thus, it is possible that by activating a specific US representation, a CS may enhance the palatability of a particular food item.
The results of the devaluation test in Group 20 confirmed previous observations of sensory specific devaluation with Pavlovian conditioning procedures (e.g. Colwill & Motzkin, 1994; Holland, 1988, 1990). Although the design of this experiment did not permit a controlled evaluation of a more general devaluation effect, it is worth noting that comparison of magazine response levels before and after taste aversion training reveals no evidence of decreased responding to the CS whose US partner was not devalued. Thus, as with potentiated feeding, the devaluation effect was highly US-specific.
The rats trained with 2-s CS-US intervals not only failed to show selectivity in any of the tests of Experiment 4, but also provided no evidence for general motivational learning, as indexed by these tests. Although there was some evidence from the performance of the rats in Group 20 that PIT may be more difficult to observe with short CS presentations in testing, the rats in Group 2 showed no evidence for PIT with 2-s test CS presentations, and suppression of responding when tested with longer CSs. Likewise, the CSs had no effect on consumption in the PF tests, when the CS was presented continuously. Finally, magazine responding during the 2-s CS presentations alone was not reduced in the devaluation test, relative to responding in those periods in the Pavlovian retraining session given before taste aversion learning, for either of the CSs.
General Discussion
The data from the present set of studies consistently indicate that sensory-specific associations in Pavlovian magazine approach conditioning occur over a wide range of CS-US intervals and US densities. These results were obtained using Pavlovian to instrumental transfer, potentiated feeding, and US devaluation tests to assess sensory-specific learning. The results clearly indicate that the degree of US-specific transfer exerted by CSs trained with CS-US intervals varying between 20 and 180 sec did not differ, nor did CSs associated with US densities between 1/20 sec and 1/120 sec, but that stimuli trained with a 20-sec CS-US interval produced superior selective PIT, PF, and US devaluation effects than did stimuli trained with a 2 sec CS-US interval. In contrast, another measure of learning, magazine approach CRs, was inversely related to CS-US interval across the range of intervals studied here.
These data are surprising in view of traditional ways of thinking about how CS-US interval and US density manipulations influence the content of learning. We examine, here, the implications of these data for two different theoretical approaches to Pavlovian conditioning: Konorski's framework (1967), and Wagner and Brandon's AESOP theory (1989).
As noted in the general introduction, Konorski (1967) distinguished between learning about highly specific and more general components of reinforcement. This distinction arose from earlier considerations regarding the stimuli that control different classes of behavior, i.e., preparatory and consummatory responses (e.g., Craig, 1918). Konorski assumed that highly specific sensory components of the US, be they activated by the US itself or associatively by the CS, are responsible for eliciting consummatory responses. On the other hand, Konorski assumed that more diffuse affective or motivational states control the production of more general preparatory responses, and that associations with these features of the US enable the CS to conditionally evoke preparatory CRs. According to this scheme, Konorski additionally assumed that short duration CSs would more readily associate with the highly specific sensory features capable of eliciting consummatory CRs, and that long duration CSs would more readily associate with the general motivational features of the US capable of eliciting preparatory CRs. Konorski reached this conclusion by noting that the experience of specific sensations accompanying US presentation is highly phasic. Through the law of similarity short duration CSs should readily associate with these stimuli. Long duration CSs, on the other hand, should have an advantage in entering into associations with the temporally more enduring motivational states evoked by potent USs for the same reason.
On the basis of these considerations we anticipated that sensory-specific PIT would be greatest with short CS-US intervals and a high US density, while general PIT would be greatest with long CS-US intervals and a low US density. The present data were not consistent with these expectations. The degree of sensory-specific PIT was similar across a wide range of CS-US intervals and US densities, and with a very short CS-US interval no sensory-specific PIT was obtained. Furthermore, there was little evidence in the present experiments to support Konorski's assertion that learning about general motivational properties of the US should be especially likely with longer CS-US intervals. We found no evidence for general PIT across a range of intervals from 20 to 180 s in Experiments 1-3 and with 2-s intervals in Experiment 4. Only in the first test of rats trained with 20-s intervals in Experiment 4 was there any evidence that a CS elevated the rate of an instrumental response with which it did not share an outcome. Recent evidence from Balleine's lab suggests that a better procedure to assess learning about general features of the US may be one in which transfer is assessed with a third CS that was previously associated with a third US not used to support instrumental responding (Corbit & Balleine, 2005). It remains possible that with this or other procedures that may be more sensitive to general PIT, one might find changes in the amount of general transfer across different CS-US intervals and US densities.
One reason why our conclusion may be questioned has to do with the status of the response measures we employed. In particular, it may be argued that our various measures all could be categorized as preparatory responses. Konorski assumed that sensory-specific reinforcer representations were important in the control of consummatory responses, and that motivational components of the reinforcer were important in the control of preparatory responses. If our various response measures are best construed as preparatory then, from Konorski's perspective, it is difficult to see why we should have observed sensory-specific effects in any of our tests. However, it may be the case that other measures of sensory-specific associations that are more consummatory in nature would produce a different pattern of results than was observed here.
A somewhat related approach to Konorski's is the AESOP theory proposed by Wagner and Brandon (1989). This theory also distinguishes between learning about sensory and motivational properties of the US, and like its predecessor, SOP theory (Wagner, 1981), AESOP assumes that excitatory conditioning is dependent upon the amount of joint processing given to the CS and US in a primary activation state (called A1). AESOP goes on to assume that sensory and motivational properties of the US are processed and learned about independently, with the additional proviso that the processing given to the motivational properties of the US decays more slowly than processing of the sensory properties of the US.
It is instructive to consider how this model explains the role of CS-US interval in delay conditioning (see Wagner, 1981). Briefly, very short and very long CS-US intervals are thought to result in poor conditioning because the amount of A1 processing given to the CS is non-optimal in these cases when the US occurs. If the US were to occur when A1 processing of the CS is at its peak, then optimal conditioning will occur. Since the CS-US interval function is thought to depend solely on CS processing dynamics, then it is not obvious how this model can explain why different response measures display different sensitivities to CS-US interval (e.g., Schneiderman, 1972).
In the present studies we also found that different measures of learning resulted in different sensitivities to CS-US interval. Our measures of sensory-specific learning revealed that 2-s CS-US intervals resulted in no sensory-specific learning, but that longer intervals ranging from 20 s to 180 s did result in sensory-specific learning. On the other hand, conditioned magazine approach CRs revealed exactly the opposite sensitivity to CS-US interval. What are we to make of these differences in terms of AESOP theory? One could assume that a 2-s CS-US interval produces no detectable amount of sensory-specific learning in PIT, PF, and devaluation tests because the CS does not receive optimal A1 processing by the time the US occurs. However, this solution would not anticipate why the 2 s CS-US interval produced the greatest amount of magazine approach CRs.
Since AESOP allows for the processing dynamics of different components of the US to differ, then it seems possible that optimal processing of different components of the US might occur at different times. This could result in different non-monotonic CS-US interval functions for different components of the US, and could, in principle, make sense of our observations that PIT, PF, and US devaluation effects were directly related while magazine entry CRs were inversely related to CS-US interval. On the other hand, since our measures of sensory-specific learning revealed better evidence of such learning with CS-US intervals greater than 2”, then optimal sensory processing of the US would have to be assumed to occur later than the optimal processing of those (presumably non-sensory) features of the US that support magazine entry CRs. This assumption is at odds with AESOP's view that sensory processing occurs rapidly, while motivational processing occurs slowly.
Regardless of the problems these data pose for AESOP theory, the differences we found with PIT, PF, and US devaluation measures, on the one hand, and magazine approach CRs, on the other, suggest that at least two aspects of learning are being assessed. It seems clear that PIT, PF, and US devaluation tests all assess sensory-specific learning, but the component of learning assessed by magazine approach CRs is less clear. To be sure, the US devaluation test does rely on differences in magazine CRs under conditions in which the associated US is devalued or not. However, simple magazine approach seems multi-determined because CS-US interval affected our two sets of measures differently. Perhaps, as others have suggested, magazine approach CRs quite sensitively assess learning about the temporal features of the US (e.g., see Kirkpatrick and Church, 2000). This could help explain why this measure of learning revealed an inverse function with CS-US interval. It has been long known that interval timing is more accurate with shorter than longer intervals to be timed (e.g., for a review see Lejeune and Wearden, in press). Greater timing accuracy may result in more CRs, as has sometimes been assumed in timing theories (e.g., Gibbon and Balsam, 1981).
One additional aspect of the present results is relevant to the point that separate learning processes may underlie our magazine CR and PIT, PF, and US devaluation measures. In Experiment 1 we reported that accurate timing of magazine approach CRs changed across the acquisition phase (see Figure 2). Early in training responding was relatively uniform across the entire CS, but later it became peaked towards the end of the CS (when the US ultimately occurred). This pattern of timed CRs is not always obtained. Several investigators have found that when CRs begin to occur early in training, they do so near the time at which the US also occurs (Balsam, Drew, and Yang, 2002; Drew, Zupan, Cooke, Couvillon, & Balsam, 2005; Holland, 2000; Kirkpatrick and Church, 2000). With further training, the tendency for CRs to occur at this time increases without the general shape of the curve changing. The present data, in contrast, suggest that the shape of the curve does change from relatively flat to peaked.
Some have suggested that learning to time the occurrence of the US is primary, and learning about the qualitative (and other) features of the US is secondary to this (e.g., Kirkpatrick and Church, 1998; Balsam, Drew, and Yang, 2002). The present data suggest, in contrast, that there is some non-temporal aspect of learning that occurs (when the temporal distribution is flat) that precedes learning to time the US occurrence. Because our PIT effects were largely independent of the level of magazine approach CRs, it is tempting to conclude that learning about the sensory qualities of the US may be this non-temporal aspect of learning. In support of this distinction, while in Experiment 1 training with 20 and 60-sec CS-US intervals resulted in temporal control over the selective PIT effect, training with a 180-sec CS-US interval produced selective PIT that was not obviously timed (see Figure 4). This suggests that a CS can come to activate a detailed representation of its associated US in the absence of clear temporal control.
It seems possible that temporal control emerges at different rates depending on the CS-US interval. In experiment 1 we found especially sharp temporal control of conditioned magazine approach CRs when a 20 s CS-US interval was used, less sharp temporal control with a 60 s CS-US interval, and very little temporal control with a 180 s CS-US interval. Since all three of these groups were given the same number of conditioning trials, it is possible that sharper temporal control would have developed with more conditioning trials given to groups trained with 60 s and 180 s CS-US intervals. Under these conditions temporally-specific, selective PIT may be found even when a 180 s CS-US interval is used in training. Such a result would imply that at the very least learning about sensory and temporal properties of the US occur at different rates, with sensory-based learning requiring fewer trials. Whether this means that sensory and temporal learning are dissociable processes will require further analysis.
One final point concerns an apparent discrepancy in the results we obtained in the PIT tests of Experiments 1-3 (carried out in ARD's lab), on the one hand, and Experiment 4 (carried out in PCH's lab) on the other. Selective PIT was revealed in the first 3 experiments as a selective lowering of the response associated with a different US from that signaled by the CS, whereas, in the final experiment selective PIT was revealed as a greater enhancement above pre CS levels of the response associated with the same US as that signaled by the CS. It is important to note that it is the difference in responding when the response was reinforced by the same versus different US from that signaled by the CS that is critical in determining if the CS has associated with a sensory-specific representation of the US. Where these two responses fall with reference to the baseline level of responding is irrelevant with respect to sensory-specific learning. We do not have a clear understanding as to why under some conditions the effect shows up as a selective elevation, while under other conditions it shows up as a selective depression of responding, but this may have to do with relatively trivial differences in experimental chamber configurations that promote different levels of competing magazine approach responses. Nonetheless, comparisons between responding in the presence of different stimuli are more informative than comparisons between responding in the presence versus absence of stimuli for the simple reason that any non-specific effects the stimuli might exert on performance can be equated in the former type of comparison. A non-associative control condition would be required to determine if non-specific elevation of instrumental responding in the presence of a CS is associatively based, but this sort of control is rarely employed.
In summary, the results from the present studies were highly consistent in showing that sensory specific learning occurs across a wide range of CS-US intervals and US densities, and that contrary to traditional thinking, such learning fails to occur with very short CS-US intervals. These findings were documented in a Pavlovian magazine approach conditioning paradigm that investigated sensory specific learning using three different measures (PIT, PF, and US devaluation tests). The results from these tests highlight the importance of US-specific processes in Pavlovian conditioning and show only a limited role for CS-US interval. In contrast, our measure of overt CRs, magazine approach, was extremely sensitive to CS-US interval, showing an inverse relation. This suggests that different learning processes can be revealed with different measures. Whether or not other variables shown to strongly influence conditioned responding also have little impact on sensory-specific learning remains an open question.
Acknowledgements
This research was supported by NIMH (065947) and PSC-CUNY (67378) grants awarded to ARD as well as NIMH grant (065879) awarded to PCH. The authors gratefully acknowledge the assistance of Marianna Ostrovskaya, Megan DiNenna, and Vanessa McKenna in collecting the data reported here.
References
- Balleine BW. Neural bases of food-seeking: Affect, arousal, and reward in corticostriatolimbic circuits. Physiology & Behavior. 2005;86:717–730. doi: 10.1016/j.physbeh.2005.08.061. [DOI] [PubMed] [Google Scholar]
- Balsam PD, Drew MR, Yang C. Timing at the start of associative learning. Learning and Motivation. 2002;33:141–155. [Google Scholar]
- Booth DA. Food-conditioned eating preferences and aversions with interoceptive elements: conditioned appetites and satieties. Annals of the New York Academy of Sciences. 1985;443:22–37. doi: 10.1111/j.1749-6632.1985.tb27061.x. [DOI] [PubMed] [Google Scholar]
- Colwill RM, Motzkin DK. Encoding of the unconditioned stimulus in Pavlovian conditioning. Animal Learning & Behavior. 1994;22:384–394. [Google Scholar]
- Corbit LH, Balleine BW. Double dissociation of basolateral and cental amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer. Journal of Neuroscience. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig W. Appetites and aversions as constituents of instincts. Biological Bulletin. 1918;34:91–107. [Google Scholar]
- Delamater AR, LoLordo VM. Event revaluation procedures and associative structures in Pavlovian conditioning. In: Dachowski L, Flaherty CF, editors. Current topics in animal learning: Brain, emotion, and cognition. Lawrence Erlbaum Associates, Inc.; Hillsdale, NJ: 1991. pp. 55–94. [Google Scholar]
- Delamater AR, LoLordo VM, Berridge KC. Control of fluid palatability by exteroceptive Pavlovian signals. Journal of Experimental Psychology: Animal Behavior Processes. 1986;12:143–152. [PubMed] [Google Scholar]
- Drew MR, Zupan B, Cooke A, Couvillon PA, Balsam PD. Temporal control of conditioned responding in goldfish. Journal of Experimental Psychology: Animal Behavior Processes. 2005;31:31–39. doi: 10.1037/0097-7403.31.1.31. [DOI] [PubMed] [Google Scholar]
- Galarce EM, Crombag HS, Holland PC. Reinforcer-specificity of appetitive and consummatory behavior of rats after Pavlovian conditioning with food reinforcers. 2006. Submitted for publication. [DOI] [PMC free article] [PubMed]
- Gallistel CR, Gibbon J. Time, rate, and conditioning. Psychological Review. 2000;107:289–344. doi: 10.1037/0033-295x.107.2.289. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Balsam P. Spreading association in time. In: Locurto CM, Terrace HS, Gibbon J, editors. Autoshaping and conditioning theory. Academic Press; New York: 1981. pp. 219–253. [Google Scholar]
- Holland PC. CS US interval as a determinant of the form of Pavlovian appetitive conditioned responses. Journal of Experimental Psychology: Animal Behavior Processes. 1980;6:155–174. [PubMed] [Google Scholar]
- Holland PC. Excitation and inhibition in unblocking. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:261–279. [PubMed] [Google Scholar]
- Holland PC. Event representation in Pavlovian conditioning: Image and action. Cognition. 1990;37:105–131. doi: 10.1016/0010-0277(90)90020-k. [DOI] [PubMed] [Google Scholar]
- Holland PC. Brain mechanisms for changes in processing of conditioned stimuli in Pavlovian conditioning: Implications for behavior theory. Animal Learning & Behavior. 1997;25:373–399. [Google Scholar]
- Holland PC. Trial and intertrial durations in appetitive conditioning in rats. Animal Learning & Behavior. 2000;28:121–135. [Google Scholar]
- Holland PC. Relations between Pavlovian-instrumental transfer and reinforcer devaluation. Journal of Experimental Psychology: Animal Behavior Processes. 2004;30:104–117. doi: 10.1037/0097-7403.30.2.104. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on CS-potentiated feeding and Pavlovian-instrumental transfer. European Journal of Neuroscience. 2003;17:1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- Holland PC, Petrovitch GD, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiology & Behavior. 2002;76:117–129. doi: 10.1016/s0031-9384(02)00688-1. [DOI] [PubMed] [Google Scholar]
- Holland PC, Straub JJ. Differential effects of two ways of devaluing the unconditioned stimulus after Pavlovian appetitive conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1979;5:65–78. doi: 10.1037//0097-7403.5.1.65. [DOI] [PubMed] [Google Scholar]
- Kehoe EJ, McCrea RA. Classical conditioning of the rabbit nictitating membrane can be fast or slow: Implications for Lennartz and Weinberger's (1992) two-factor theory. Psychobiology. 1994;22:1–4. [Google Scholar]
- Kirkpatrick K, Church RM. Are separate theories of timing and conditioning necessary? Behavioral Processes. 1998;44:163–182. doi: 10.1016/s0376-6357(98)00047-3. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick K, Church RM. Independent effects of stimulus and cycle duration in conditioning: The role of timing processes. Animal Learning & Behavior. 2000;28:373–388. [Google Scholar]
- Konorski J. Integrative activity of the brain. University of Chicago Press; Chicago: 1967. [Google Scholar]
- Lejeune H, Wearden JH. Scalar properties in animal timing: Conformity and violations. Quarterly Journal of Experimental Psychology. doi: 10.1080/17470210600784649. In Press. [DOI] [PubMed] [Google Scholar]
- Lennartz RC, Weinberger NM. Analysis of response systems in Pavlovian conditioning reveals rapidly versus slowly acquired conditioned responses: Support for two factors, implications for behavior and neurobiology. Psychobiology. 1992;20:93–119. [Google Scholar]
- Petrovich GD, Ross CA, Gallagher M, Holland PC. Learned contextual cue potentiates eating in rats. Physiology & Behavior. 2006 doi: 10.1016/j.physbeh.2006.09.031. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Saddoris MP, Gallagher M, Holland PC. Orbitofrontal lesions impair use of cue-outcome associations in a devaluation task. Behavioral Neuroscience. 2005;119:317–322. doi: 10.1037/0735-7044.119.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger RS. Multiple contrasts, factors, error rate, and power. British Journal of Mathematical & Statistical Psychology. 1974;27:179–198. [Google Scholar]
- Rozeboom WW. “What is learned?”: An empirical enigma. Psychological Review. 1958;65:22–33. doi: 10.1037/h0045256. [DOI] [PubMed] [Google Scholar]
- Schneiderman N. Response system divergencies in aversive classical conditioning. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current theory and Research. Appleton-Century-Crofts; New York: 1972. pp. 341–346. [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J Neurosci. 1999;19(5):1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AR. SOP: A model of automatic memory processing in animal behavior. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Erlbaum; Hillsdale, N. J.: 1981. pp. 5–47. [Google Scholar]
- Wagner AR, Brandon SE. Evolution of a structured connectionist model of Pavlovian conditioning (AESOP). In: Klein SB, Mower RR, editors. Contemporary learning theories: Pavlovian conditioning and the status of traditional learning theory. Erlbaum; Hillsdale, N. J.: 1989. pp. 149–189. [Google Scholar]
- Weingarten HP. Conditioned cues elicit feeding in sated rats: A role for learning in meal initiation. Science. 1983;220:431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]