Abstract
Due to the random generation of T cell antigen receptors, a large fraction of developing T cells have the potential to recognize self-determinants. To prevent this self-reactive T cell repertoire from mediating autoimmunity, the immune system utilizes several mechanisms to induce tolerance to self. The majority of self-reactive T cells undergo negative selection (i.e., apoptosis) during development if their antigen receptors have high affinity for MHC-self-peptide complexes present in the thymus. Nonetheless, some T cells recognize self-epitopes that are not present in the thymus, and will thus reach maturation and migrate to peripheral lymphoid organs were they can be subject to a number of peripheral tolerance mechanisms such as deletion, inactivation (i.e., anergy) or suppression. While peripheral tolerization of naive (i.e., antigen-inexperienced) T cells has been studied extensively, there are potential situations in which self-reactive T cells might first encounter immunogenic forms of antigen (deriving from pathogens or vaccines) and thus be programmed to develop effector and memory functions. This article will review recent studies that have explored the potential of effector and memory T cells to undergo peripheral tolerization, as well as potential implications of these findings for autoimmunity and tumor-immunity.
Keywords: T Cell Tolerance, Effector T Cells, Memory T Cells, Autoimmunity, Tumor-Immunity, Cytokines
INTRODUCTION
As a result of the random process of V-D-J gene recombination that generates a diverse repertoire of T cell antigen receptors (TCRs) which enables the T cell repertoire to respond to a wide variety of pathogenic challenges (reviewed in [1]), many T cells express potentially self-reactive TCRs. In fact, given the high degree of complexity of the host genome compared to that of most pathogens, the immune system likely generates many more T cells with the potential to recognize self-determinants compared to the number of T cells that will ultimately be required to respond to infections. Thus, it is not surprising that there are multiple mechanisms that induce tolerance to self-antigens at different stages of T cell development. The majority of potentially self-reactive T cells undergo negative selection during development in the thymus, where immature T cells expressing TCRs that recognize MHC molecules presenting self-epitopes at high affinity/avidity undergo apoptosis [2–5]. This form of central tolerance eliminates developing T cells that recognize ubiquitously expressed antigens [2, 3, 5], blood-borne antigens [6], as well as some epitopes deriving from proteins that serve specialized functions in non-lymphoid tissues [7].
Although central tolerance is highly efficient, some T cells will develop specificity for self-antigens that are not present in the thymus, and thus the immune system utilizes several peripheral tolerization mechanisms to prevent these T cells from becoming inappropriately activated once they have matured and migrated to secondary lymphoid organs. Self-reactive T cells in the periphery are sometimes ignorant of their cognate self-epitopes, retaining a naïve phenotype and potential to be primed [8, 9]. More often than not, however, self-reactive T cells become subject to one of several active peripheral tolerance mechanisms. The most definitive form of peripheral tolerance involves deletion or death, which can be programmed by members of the TNF/NGF superfamily of receptors such as CD95 (Fas) as well as other molecules like TNF and DR [10, 11] or by depletion of survival factors like IL-2 (i.e., growth factor withdrawal) [12]. Self-reactive T cells can also undergo functional inactivation (also referred to as adaptive tolerance or anergy), in which they become hyporesponsive to antigenic stimulation [13]. Ultimately, inactivation may simply represent an intermediate point in a pathway leading to deletion, since inactivation and deletion are often observed in the same systems [14–16]. Inactivation/deletion can be induced by bone marrow-derived antigen presenting cells (APCs) [17, 18], most probably dendritic cells (DCs) [19, 20], which under steady-state conditions acquire self-antigens from parenchymal tissues and subsequently present them to naïve T cells in the draining lymph nodes. This interaction induces naïve T cells to undergo an initial proliferative response, followed by the development of non-responsiveness [21, 22] or deletion [18, 23]. In contrast to when DCs acquire pathogen-derived antigens where the presence of invariant pathogen-derived inflammatory factors (i.e., pathogen-associated molecular patterns or PAMPs) induce the expression of costimulatory molecules that endow DCs with the ability to prime cognate naïve T cells to develop effector and memory functions, DCs that acquire self-antigens under non-inflammatory conditions express sub-optimal levels of costimulation and thus tolerize rather than prime cognate naïve T cells [24–29]. Peripheral tolerance can also be mediated in trans by T regulatory cells (Tregs) that dampen the function of autoreactive T cells. Tregs can exert their function in a contact-dependent manner (sometimes via the production of TGF-β), and are classically defined as CD4+CD25+ (although other phenotypes can also express regulatory function using other mechanisms) [30, 31].
While peripheral tolerization of naïve self-reactive T cells has been studied extensively, the potential of effector and memory self-reactive T cells to undergo peripheral tolerization has been less well studied. Although it had been demonstrated that effector/memory T cells can be tolerized through intravenous administration of high doses of soluble cognate antigens [32–35], it had generally been thought that effector/memory T cells are inherently more resistant to undergoing peripheral tolerization than naïve T cells in part because the former have less stringent costimulatory requirements for undergoing activation in vitro [36–41], and sub-optimal costimulation appears to be a critical parameter in conferring tolerogenic antigen presenting function [26–29]. Thus, while the approach of using high dose exogenous soluble autoantigens to tolerize autoreactive effector/memory T cells was established as a potential avenue to treat autoimmune diseases (reviewed in [42]), it wasn’t clear whether effector/memory T cells would be susceptible to undergoing tolerization in response to physiological forms of tolerizing antigens such as self-antigens that might be expressed at relatively low levels.
THE POTENTIAL PHYSIOLOGICAL RELEVANCE OF EFFECTOR/MEMORY T CELL PERIPHERAL TOLERIZATION
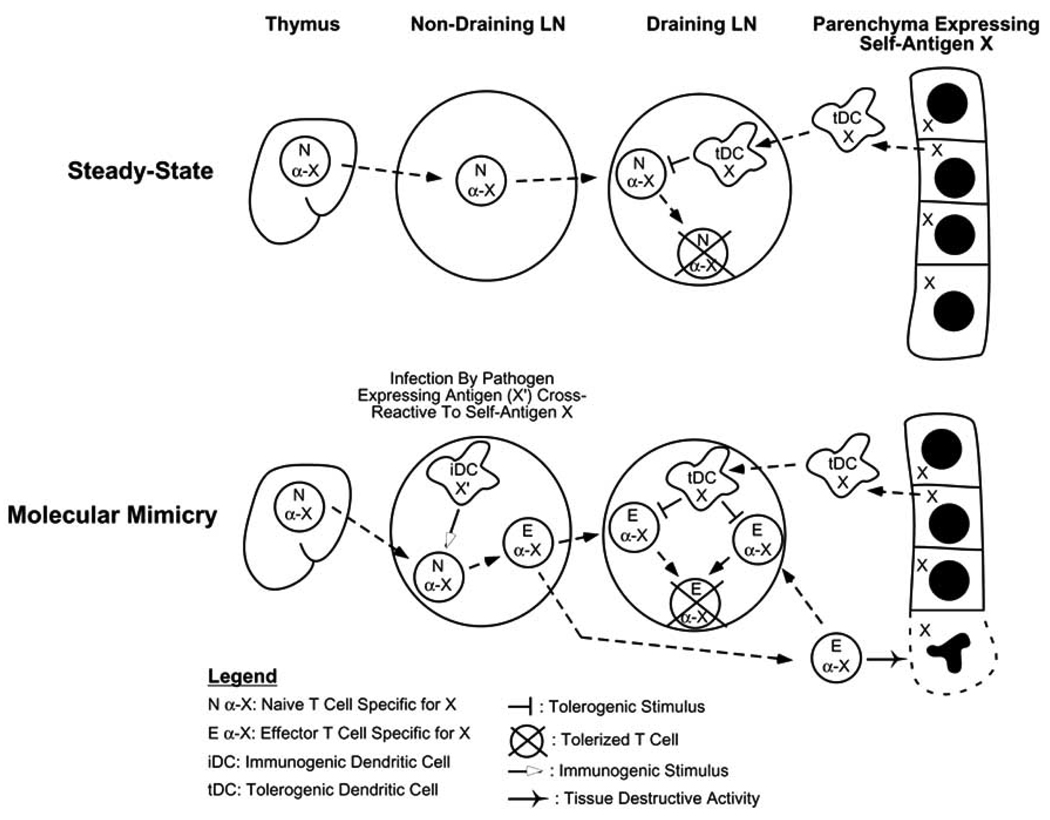
The question of whether effector/memory T cells are susceptible to tolerization under physiological conditions is potentially relevant in several contexts. Some pathogen-derived antigens are similar in structure to certain self-antigens, and if cognate naïve T cells first encounter these antigens in the context of an infection (possibly because they recently completed maturation in the thymus and have not yet encountered cognate peripheral self-antigen) they might be primed to develop effector/memory functions and thus mediate autoimmunity. This scenario, referred to as molecular mimicry, is supported by studies in both mice [8, 43–45] and humans [46–49]. As illustrated in (Fig. 1), if these autoreactive effector/memory T cells were susceptible to undergoing tolerization following encounter with cognate self-antigen, the extent of autoimmune damage might be minimized.
Fig. 1.
Hypothetical scenario in which peripheral tolerization of effector T cells limits the extent of autoimmune damage that ensues following molecular mimicry. Under normal steady-state conditions, naïve T cells specific for the parenchymal self-antigen X will undergo tolerization when they reach the draining LN where they encounter tolerogenic DCs (tDC) presenting X. Molecular mimicry can lead to autoimmune damage of parenchymal tissues expressing X if cognate naïve T cells first encounter immunogenic DCs (iDC) that present a cross-reactive pathogen-derived antigen (X’). Since X will still be presented by tolerogenic DCs in the draining LN, tolerization will occur simultaneous to tissue damage, thus shortening the duration of the effector phase of the autoimmune response.
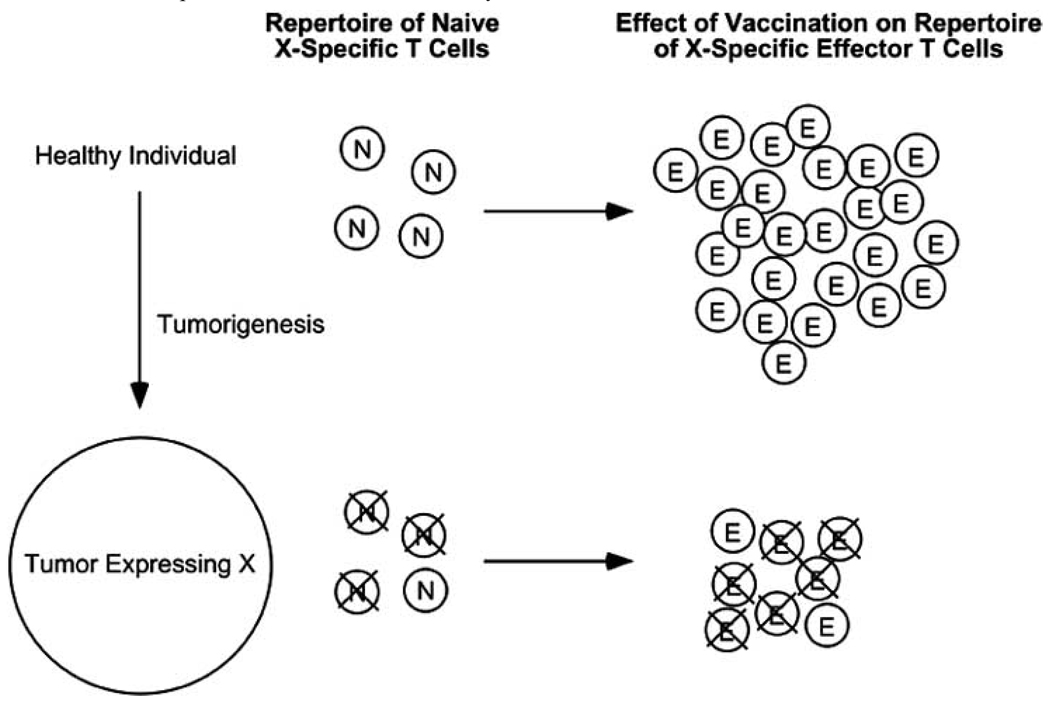
The potential of the immune system to tolerize effector/memory T cells might also be relevant to tumor-immunity. Since the majority of human cancers result from environmental and hereditary factors rather than from oncogenic viruses (papillomaviruses causing cervical tumors and Epstein-Barr viruses that cause lymphomas being notable exceptions), therapies aimed at programming the immune system to destroy tumors are in essence attempting to induce autoimmune responses directed towards tumors. Thus, tolerance mechanisms that normally function to prevent the development of autoimmunity directed towards healthy tissues could potentially impede the development of effective anti-tumor-immunity (reviewed in [50]). Tolerance is likely to be the most pronounced for tumor-associated antigens that are expressed on both tumors and normal tissues (i.e., differentiation antigens), since the T cell tolerance induction machinery will have had access to these antigens long before tumors develop [51, 52]. Nonetheless, peripheral T cell tolerance can also develop towards antigens expressed exclusively on tumors [53], interestingly via the same tolerance induction pathway [54] that is utilized for normal self-antigens [17] (i.e., indirect presentation by bone marrow-derived APCs). Although this type of tolerization is not always absolute, tumor-reactive T cells that manage to avoid tolerization tend to express TCRs that have relatively low avidities for their cognate tumor-associated epitopes, and thus have a lesser potential to mediate tumor-immunity in response to vaccination [52, 55, 56]. These observations have contributed to the notion that T cell tolerance can impair tumor-immunity by shrinking the repertoire of tumor-reactive T cells that can be activated through vaccination. However, if tumor-reactive effector/memory T cells that could be primed through vaccination were susceptible to undergoing peripheral tolerization, this could represent an additional level at which tolerance negatively impacts tumor-immunity (Fig. 2).
Fig. 2.
Hypothetical scenario in which the tolerization of both naïve and effector T cells impedes vaccine-induced tumor-immunity. Tolerogenic DCs presenting tumor antigen X induce the tolerization of a significant fraction of the naïve X-specific T cell repertoire. Following vaccination to X, X-specific naïve T cells that initially avoided tolerization are able to expand and differentiate into effectors, however, they will remain susceptible to tolerization due to the continued presentation of X by tolerogenic DCs.
EFFECTOR/MEMORY T CELLS ARE SUSCEPTIBLE TO PERIPHERAL TOLERIZATION
To assess the response of memory CD8 cells to self-antigen, Kreuwel et al. adoptively transferred TCR transgenic memory CD8 cells specific for an influenza hemagglutinin (HA) epitope into transgenic recipient mice that express HA as self-antigen in pancreatic islet β cells [57]. Interestingly, although the HA-specific memory CD8 cells initially underwent a proliferative response, they eventually lost the capacity to respond to vaccination and mediate diabetes via autoimmune destruction of the islet β cells. Furthermore, the kinetics of tolerization followed a similar time course as naïve counterparts, indicating the naïve and memory CD8 cells are equally susceptible to undergoing peripheral tolerization in response to self-antigen.
Also using a TCR transgenic adoptive transfer system, studies from our own laboratory have demonstrated that T helper 1 (Th1) effector CD4 cells are also highly susceptible to undergoing peripheral tolerization in response to self-antigen. In our basic experimental paradigm, clonotypic Th1 effector CD4 cells (marked by the ability to express high levels of the cytokines IFN-γ, TNF-α and IL-2) are first generated by adoptively transferring naïve TCR transgenic CD4 cells specific for influenza HA into non-transgenic recipient mice that have been infected with an HA-expressing recombinant vaccinia virus. Subsequently, the Th1 clonotypic CD4 effectors are recovered from the vaccinia-infected primary recipients and re-transferred into transgenic secondary recipients that express HA as a self-antigen in a variety of parenchymal tissues. Although the retransferred clonotypic Th1 effectors undergo an initial proliferative response upon encountering self-HA, they ultimately lose the ability to express cytokines and to undergo further division in response to antigenic stimulation (reminiscent of the response of naïve CD4 cells to self-antigen [21, 22]). Additionally, this loss of function occurred in transgenic secondary recipients that expressed either high or low levels of self-HA, and could be mediated by bone marrow-derived APCs that indirectly present parenchymally-derived HA [58]. Taken together, these results indicate that Th1 effector CD4 cells undergo tolerization in response to parenchymal self-antigen under the same physiological conditions as naïve counterparts, further supporting the conclusion that effector/memory T cells are not inherently resistant to tolerization.
One of the most interesting aspects of the Th1 effector CD4 cell tolerization pathway is that different functions are lost with different kinetics: the ability to express the effector cytokines IFN-γ and TNF-α are dramatically reduced within 24 hours of exposure to self-HA, while the ability to express non-effector functions such as IL-2 production and proliferation are lost only after several days of self-antigen exposure [59]. In addition to indicating that the Th1 effector CD4 cell tolerization process is complex, this observation likely has physiological relevance since IFN-γ and TNF-α can both play critical roles in mediating autoimmunity [60–63] and tumor-immunity [64–68]. Thus, effectors that can produce IL-2 and proliferate but not express IFN-γ or TNF-α would probably not be very effective in mediating either tumor-immunity or autoimmune pathology.
EXPLORING THE RELATIONSHIP BETWEEN EFFECTOR/MEMORY T CELL TOLERIZATION AND TUMOR-IMMUNITY AND AUTOIMMUNITY
Although the finding that effector/memory T cells are highly susceptible to undergoing peripheral tolerization is potentially important for understanding both tumor-immunity as well as molecular mimicry scenarios that lead to autoimmunity, it also raises important questions. For example, the clonotypic TCR transgenic adoptive transfer systems that have been used to demonstrate that effector/memory T cells are susceptible to undergoing tolerization are somewhat analogous to adoptive immunotherapy approaches for treating cancer in which autologous tumor-reactive effector T cells are expanded ex vivo prior to being injected back into cancer patients [69], and since tumor antigens can be presented in a tolerogenic manner and effector T cells are susceptible to undergoing tolerization, it might be predicted that tolerance should diminish the efficacy of adoptive immunotherapy. Nonetheless, recent clinical trials have demonstrated that adoptive immunotherapy can induce significant tumor regressions [70, 71], raising the question of whether tolerance is having an impact. It is interesting to note, however, that these trials used the cytotoxic drug cyclophosphamide (Cytoxan or CY) to condition patients prior to receiving tumor-reactive effector T cells and/or exogenous IL-2 thereafter. CY and IL-2 can also augment adoptive immunotherapy in mouse models, and although the precise mechanisms by which these drugs work is not precisely understood, it has been proposed that they might have a variety of effects such as eliminating regulatory cells [72, 73], eliciting T cell growth factors [74] or type I interferons [75], and enhancing the engraftment of adoptively transferred tumor-reactive effector T cells [76] by creating space [77–79]. Given the potential for tumor-associated antigens to be presented in a tolerogenic manner as well as the susceptibility of adoptively transferred effector T cells to undergoing tolerization, we used our effector CD4 cell tolerization model described in the previous section to explore the idea that CY and IL-2 might have an additional effect which is to impede the tolerization of adoptively transferred effector T cells [80]. Although the tolerizing antigen in this system derives from healthy tissues rather than from tumors, we reasoned that it would be an appropriate system to test this possibility for the following reasons. First, adoptive immunotherapy strategies generally target differentiation antigens that are expressed not only on tumors but also on the normal tissues from which the tumors derive [70, 71], and therefore the bulk of potentially tolerogenic antigen likely derives from normal tissues. Furthermore, the pathways by which normal self-antigens and tumor-antigens are presented to induce T cell tolerance appear to be similar (i.e., indirect presentation by bone marrow-derived APCs [17, 54]). Finally, in our system the model self-antigen HA can be expressed at high levels in several organs, leading to rapid and profound tolerization of adoptively transferred HA-specific effector CD4 cells [58, 59], and thus this system represents a stringent test for the ability of CY and IL-2 to impede the tolerization of adoptively transferred effector T cells. CY + IL-2 treatment not only enhanced the proliferation and accumulation of adoptively transferred effector CD4 cells encountering cognate self-antigen (as would have been predicted from previous studies), but these T cells also exhibited a delay in the rate in which they lost the ability to express the effector cytokines IFN-γ and TNF-α. CY was more effective than IL-2 in impeding tolerization, although the combination of both drugs was the most effective. Interestingly, the ability of CY and IL-2 to rescue effector cytokine expression potential was the greatest in mice that expressed the highest levels of self-antigen [80], in contrast to normal conditions where greater levels of self-antigen expression result in more rapid and profound T cell tolerization [81]. Although we do not currently understand the mechanistic basis for this reversal, this effect would likely be beneficial in the context of adoptive immunotherapy to treat cancer since patients expressing higher levels of the tolerizing/targeted tumor-associated antigens would be predicted to experience the greatest rescue of T cell function.
In addition to providing new insights into the mechanisms by which CY and IL-2 might augment the efficacy of adoptive immunotherapy strategies to treat cancer, our study might also help to understanding the relationship between molecular mimicry scenarios that lead to autoimmunity and effector T cell tolerization. As speculated earlier, effector T cell tolerization might represent a contingency mechanism to limit the extent of autoimmune damage that results from molecular mimicry. Given that effector/memory T cells are highly susceptible to undergoing peripheral tolerization, it is not obvious why molecular mimicry-induced autoimmunity is not always repressed. One possibility is that the extent of ensuing autoimmune pathology is the result of a dynamic balance between the rate at which autoreactive effectors can inflict tissue damage relative to their rate of tolerization. Thus, when the expression of the relevant self-antigen is limited to discrete anatomical locations (e.g., the pancreatic islets [8, 9]), tolerogenic presentation of that antigen will be restricted to the corresponding draining lymph node [82, 83]. This will likely result in a relatively slow rate of tolerization due to the time required for all of the autoreactive effectors to migrate through the draining lymph node, and a greater likelihood for the development of overt autoimmune symptoms. In contrast, when the relevant self-antigen is more widely expressed (such as in our system), the rate of tolerization will be more rapid, and therefore the likelihood of inflicting substantial tissue damage will be less. Consistent with this hypothesis, we found in our system that delaying the rate of effector CD4 cell tolerization via treatment with CY + IL-2 resulted in autoimmunity in a significant fraction of mice that expressed the highest levels of self-antigen [80]. Taken together, these observations suggest that the effector T cell tolerization pathway can limit (but not completely prevent) molecular mimicry-induced autoimmune pathology.
WHAT ARE THE MECHANISMS THAT MEDIATE EFFECTOR/MEMORY T CELL TOLERIZATION?
Given the potential importance of effector/memory T cell tolerization to both autoimmunity and tumor-immunity, it will be important to dissect the underlying mechanisms so that strategies can be developed to manipulate these pathways for therapeutic benefit. As summarized in Table 1, there appear to be both similarities and differences in the pathways by which naïve and effector/memory T cells undergo tolerization.
Table 1.
Parameters that Regulate Tolerance in Naïve Versus Effector/Memory T Cells
| Naïve T Cells | Effector/Memory T Cells | |
|---|---|---|
| Tolerance can be induced by APCs in lymphoid organs. |
+ | + |
| Initial proliferative phase precedes development of fully tolerant state. |
+ | + |
| Tolerance can be induced in parenchymal tissues. | – | ? |
| Functions lost during tolerization. | 1) Proliferation and IL-2 production in response to antigenic stimulation. 2) The potential to differentiate into effector and memory cells. |
1) Proliferation and IL-2 production in response to antigenic stimulation. 2) The ability to express effector and memory functions. |
| Examples of costimulatory molecules that might play a role in regulating tolerance versus immunity. |
CD40, OX40 | ? |
Tolerization of both naïve and effector/memory T cells can be initiated by bone marrow-derived APCs that indirectly present parenchymally-derived self-antigen under steady-state conditions [17, 18, 22, 58], and both naïve [18, 21–23] and effector/memory [57–59] T cells undergo an initial proliferative phase prior to reaching a fully non-responsive state. Additionally, the level of self-antigen expression (i.e., antigen density) appears to have a similar influence on the tolerization of both naïve and effector/memory T cells. Higher levels of self-antigen expression result in a more robust proliferative response following initial antigen encounter and/or a more rapid rate of tolerization in the case of both naïve [21, 81, 84] and effector/memory T cells [57, 58, 81], although tolerization represents the ultimate outcome of self-antigen recognition in all cases.
There are also likely to be differences in the mechanisms that regulate tolerization in naïve versus effector/memory T cells. For example, since effector/memory T cells migrate more efficiently through non-lymphoid tissues compared to naïve T cells [85, 86], immunomodulatory factors located in non-lymphoid tissues are more likely to influence effector/memory T cell tolerization. Furthermore, since Th1 effector CD4 cell tolerization involves a loss in the ability to express effector cytokines such as IFN-γ [59], in contrast to naïve CD4 cell tolerization in which the ability to express IFN-γ is not significantly gained or lost [22], there are likely to be differences in the intrinsic intracellular signaling pathways that mediate tolerance between naïve and effector/memory T cells. At present little is known regarding possible differences in the costimulatory signals that regulate tolerance in naïve versus effector/memory T cells. As discussed earlier, it is generally thought that tolerization of naïve T cells is mediated by steady-state DCs that, due to their lack of exposure to inflammatory signals, express sub-optimal levels of various costimulatory ligands. Experiments in which the administration of costimulatory agonists are able to functionally rescue naïve T cells encountering tolerizing forms of antigen have identified several costimulatory ligands whose absence on DCs could potentially contribute to tolerogenic capacity (e.g., CD40 [27, 87–91] and OX40L [92–94]). Interestingly, the in vitro observation that effector/memory T cells have less stringent costimulatory requirements for undergoing activation compared to naïve T cells [36–41] contributed to the notion that effector/memory T cells would be more resistant to undergoing tolerization. Nonetheless, given that effector/memory T cells are highly susceptible to tolerization [57, 58], as well as the ability of bone marrow-derived APCs to tolerize these cells under steady-state conditions [58], it would seem reasonable to speculate that sub-optimal costimulation also plays a role in effector/memory T cell tolerization. In the future it will be interesting to assess whether the same or different costimulatory pathways regulate tolerization in naïve versus effector/memory T cells.
ACKNOWLEDGEMENTS
A.J.A. was supported by National Institutes of Health Grants AI49813, AI57441 and CA109339 as well as Research Scholar Grant # RSG-02-235-01-LIB from the American Cancer Society.
REFERENCES
- 1.Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004;4:123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 2.Kappler J, Roehm M, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 3.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 4.Sebzda E, Wallace VA, Mayer J, Yeung RS, Mak TW, Ohashi PS. Positive and negative thymocyte selection induced by different concentrations of a single peptide. Science. 1994;263:1615–1618. doi: 10.1126/science.8128249. [DOI] [PubMed] [Google Scholar]
- 5.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 6.Bogen B, Dembic Z, Weiss S. Clonal deletion of specific thymocytes by an immunoglobulin idiotype. EMBO J. 1993;12:357–363. doi: 10.1002/j.1460-2075.1993.tb05664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson MS, Venanzi ES, Klein L, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 8.Ohashi PS, Oehen S, Buerki K, et al. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 9.Oldstone MB, Nerenberg M, Southern P, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65:319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 10.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 11.Krueger A, Fas SC, Baumann S, Krammer PH. The role of CD95 in the regulation of peripheral T-cell apoptosis. Immunol Rev. 2003;193:58–69. doi: 10.1034/j.1600-065x.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 12.Duke RC, Cohen JJ. IL-2 addiction: withdrawal of growth factor activates a suicide program in dependent T cells. Lymphokine Res. 1986;5:289–299. [PubMed] [Google Scholar]
- 13.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 14.Rocha B, von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 15.Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald HR, Bascherieri S, Lees RK. Clonal expansion precedes anergy and death of VB8+ peripheral T cells responding to staphlococcal enterotoxin B in vivo. Eur J Immunol. 1991;21:1963–1966. doi: 10.1002/eji.1830210827. [DOI] [PubMed] [Google Scholar]
- 17.Adler AJ, Marsh DW, Yochum GS, et al. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen presenting cells. J Exp Med. 1998;187:1555–1564. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurts C, Kosaka H, Carbone FR, Miller JFAP, Heath WR. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J Ex Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurts C, Cannarile M, Klebba I, Brocker T. Dendritic cells are sufficient to cross-present self-antigens to CD8 T cells in vivo. J Immunol. 2001;166:1439–1442. doi: 10.4049/jimmunol.166.3.1439. [DOI] [PubMed] [Google Scholar]
- 20.Belz GT, Behrens GM, Smith CM, et al. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J Exp Med. 2002;196:1099–1104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adler AJ, Huang CT, Yochum GS, Marsh DW, Pardoll DM. In vivo CD4+ T cell tolerance induction versus priming is independent of the rate and number of cell divisions. J Immunol. 2000;164:649–655. doi: 10.4049/jimmunol.164.2.649. [DOI] [PubMed] [Google Scholar]
- 22.Higgins AD, Mihalyo MA, McGary PW, Adler AJ. CD4 cell priming and tolerization are differentially programmed by APCs upon initial engagement. J Immunol. 2002;168:5573–5581. doi: 10.4049/jimmunol.168.11.5573. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez J, Aung S, Redmond WL, Sherman LA. Phenotypic and functional analysis of CD8(+) T cells undergoing peripheral deletion in response to cross-presentation of self-antigen. J Exp Med. 2001;194:707–717. doi: 10.1084/jem.194.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finkelman FD, Lees A, Birnbaum R, Gause WC, Morris SC. Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J Immunol. 1996;157:1406–1414. [PubMed] [Google Scholar]
- 25.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 26.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 27.Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenkins MK, Khoruts A, Ingulli E, et al. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- 29.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 30.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 31.Shevach EM. Certified professionals: CD4(+)CD25(+) suppressor T cells. J Exp Med. 2001;193:F41–F46. doi: 10.1084/jem.193.11.f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Critchfield JM, Racke MK, Zuniga-Pflucker JC, et al. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science. 1994;263:1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- 33.Brocke S, Gijbels K, Allegretta M, et al. Treatment of experimental encephalomyelitis with a peptide analogue of myelin basic protein. Nature. 1996;379:343–346. doi: 10.1038/379343a0. [DOI] [PubMed] [Google Scholar]
- 34.Tisch R, Wang B, Serreze DV. Induction of glutamic acid decarboxylase 65-specific Th2 cells and suppression of autoimmune diabetes at late stages of disease is epitope dependent. J Immunol. 1999;163:1178–1187. [PubMed] [Google Scholar]
- 35.Hayashi N, Liu D, Min B, Ben-Sasson SZ, Paul WE. Antigen challenge leads to in vivo activation and elimination of highly polarized TH1 memory T cells. Proc Natl Acad Sci USA. 2002;99:6187–6191. doi: 10.1073/pnas.092129899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horgan KJ, Van Seventer GA, Shimizu Y, Shaw S. Hyporesponsiveness of "naive" (CD45RA+) human T cells to multiple receptor-mediated stimuli but augmentation of responses by co-stimuli. Eur J Immunol. 1990;20:1111–1118. doi: 10.1002/eji.1830200525. [DOI] [PubMed] [Google Scholar]
- 37.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 38.Sagerstrom CG, Kerr EM, Allison JP, Davis MM. Activation and differentiation requirements of primary T cells in vitro. Proc Natl Acad Sci USA. 1993;90:8987–8991. doi: 10.1073/pnas.90.19.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dubey C, Croft M, Swain SL. Naive and effector CD4 T cells differ in their requirements for T cell receptor versus costimulatory signals. J Immunol. 1996;157:3280–3289. [PubMed] [Google Scholar]
- 40.Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J Immunol. 1994;152:2675–2685. [PubMed] [Google Scholar]
- 41.Pihlgren M, Dubois PM, Tomkowiak M, Sjogren T, Marvel J. Resting memory CD8+ T cells are hyperreactive to antigenic challenge in vitro. J Exp Med. 1996;184:2141–2151. doi: 10.1084/jem.184.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liblau R, Tisch R, Bercovici N, McDevitt H. Systemic antigen in the treatment of T-cell-mediated autoimmune diseases. Immunol Today. 1997;18:599–604. doi: 10.1016/s0167-5699(97)01171-7. [DOI] [PubMed] [Google Scholar]
- 43.Ohteki T, Hessel A, Bachmann MF, et al. Identification of a cross-reactive self ligand in virus-mediated autoimmunity. Eur J Immunol. 1999;29:2886–2896. doi: 10.1002/(SICI)1521-4141(199909)29:09<2886::AID-IMMU2886>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 44.Bachmaier K, Neu N, de la Maza LM, Pal S, Hessel A, Penninger JM. Chlamydia infections and heart disease linked through antigenic mimicry. Science. 1999;283:1335–1339. doi: 10.1126/science.283.5406.1335. [DOI] [PubMed] [Google Scholar]
- 45.Panoutsakopoulou V, Sanchirico ME, Huster KM, et al. Analysis of the relationship between viral infection and autoimmune disease. Immunity. 2001;15:137–147. doi: 10.1016/s1074-7613(01)00172-8. [DOI] [PubMed] [Google Scholar]
- 46.Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gross DM, Forsthuber T, Tary-Lehmann M, et al. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science. 1998;281:703–706. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- 48.Lang HL, Jacobsen H, Ikemizu S, et al. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat Immunol. 2002;3:940–943. doi: 10.1038/ni835. [DOI] [PubMed] [Google Scholar]
- 49.Levin MC, Lee SM, Kalume F, et al. Autoimmunity due to molecular mimicry as a cause of neurological disease. Nat Med. 2002;8:509–513. doi: 10.1038/nm0502-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 51.Hu J, Kindsvogel W, Busby S, Bailey MC, Shi Y, Greenberg PD. An evaluation of the potential to use tumor-associated antigens as targets for antitumor T cell therapy using transgenic mice expressing a retroviral tumor antigen in normal lymphoid tissues. J Exp Med. 1993;177:1681–1690. doi: 10.1084/jem.177.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morgan DJ, Kreuwel HT, Fleck S, Levitsky HI, Pardoll DM, Sherman LA. Activation of low avidity CTL specific for a self epitope results in tumor rejection but not autoimmunity. J Immunol. 1998;160:643–651. [PubMed] [Google Scholar]
- 53.Stavely-O'Carroll K, Sotomayor E, Montgomery J, et al. Induction of antigen-specific T cell anergy: an early event in the course of tumor progression. Proc Natl Acad Sci USA. 1998;95:1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sotomayor EM, Borrello I, Rattis FM, et al. Cross-presentation of tumor antigens by bone marrow-derived antigen-presenting cells is the dominant mechanism in the induction of T-cell tolerance during B-cell lymphoma progression. Blood. 2001;98:1070–1077. doi: 10.1182/blood.v98.4.1070. [DOI] [PubMed] [Google Scholar]
- 55.Theobald M, Biggs J, Hernandez J, Lustgarten J, Labadie C, Sherman LA. Tolerance to p53 by A2.1-restricted cytotoxic T lymphocytes. J Exp Med. 1997;185:833–841. doi: 10.1084/jem.185.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cordaro TA, de Visser KE, Tirion FH, Schumacher TN, Kruisbeek AM. Can the low-avidity self-specific T cell repertoire be exploited for tumor rejection? J Immunol. 2002;168:651–660. doi: 10.4049/jimmunol.168.2.651. [DOI] [PubMed] [Google Scholar]
- 57.Kreuwel HT, Aung S, Silao C, Sherman LA. Memory CD8(+) T cells undergo peripheral tolerance. Immunity. 2002;17:73–81. doi: 10.1016/s1074-7613(02)00337-0. [DOI] [PubMed] [Google Scholar]
- 58.Higgins AD, Mihalyo MA, Adler AJ. Effector CD4 cells are tolerized upon exposure to parenchymal self-antigen. J Immunol. 2002;169:3622–3629. doi: 10.4049/jimmunol.169.7.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Long M, Higgins AD, Mihalyo MA, Adler AJ. Effector CD4 cell tolerization is mediated through functional inactivation and involves preferential impairment of TNF-alpha and IFN-gamma expression potentials. Cell Immunol. 2003;224:114–121. doi: 10.1016/j.cellimm.2003.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang XD, Tisch R, Singer SM, et al. Effect of tumor necrosis factor alpha on insulin-dependent diabetes mellitus in NOD mice. I. The early development of autoimmunity and the diabetogenic process. J Exp Med. 1994;180:995–1004. doi: 10.1084/jem.180.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor GA, Carballo E, Lee DM, et al. A pathogenetic role for TNF alpha in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity. 1996;4:445–454. doi: 10.1016/s1074-7613(00)80411-2. [DOI] [PubMed] [Google Scholar]
- 62.von Herrath MG, Oldstone MB. Interferon-gamma is essential for destruction of beta cells and development of insulin-dependent diabetes mellitus. J Exp Med. 1997;185:531–539. doi: 10.1084/jem.185.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seewaldt S, Thomas HE, Ejrnaes M, et al. Virus-induced autoimmune diabetes: most beta-cells die through inflammatory cytokines and not perforin from autoreactive (anti-viral) cytotoxic T-lymphocytes. Diabetes. 2000;49:1801–1809. doi: 10.2337/diabetes.49.11.1801. [DOI] [PubMed] [Google Scholar]
- 64.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qin Z, Blankenstein T. CD4+ T cell--mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN gamma receptor expression by nonhematopoietic cells. Immunity. 2000;12:677–686. doi: 10.1016/s1074-7613(00)80218-6. [DOI] [PubMed] [Google Scholar]
- 66.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 67.Poehlein CH, Hu HM, Yamada J, et al. TNF plays an essential role in tumor regression after adoptive transfer of perforin/IFN-gamma double knockout effector T cells. J Immunol. 2003;170:2004–2013. doi: 10.4049/jimmunol.170.4.2004. [DOI] [PubMed] [Google Scholar]
- 68.Winter H, Hu HM, McClain K, Urba WJ, Fox BA. Immunotherapy of melanoma: a dichotomy in the requirement for IFN-gamma in vaccine-induced antitumor-immunity versus adoptive immunotherapy. J Immunol. 2001;166:7370–7380. doi: 10.4049/jimmunol.166.12.7370. [DOI] [PubMed] [Google Scholar]
- 69.Yee C, Riddell SR, Greenberg PD. Prospects for adoptive T cell therapy. Curr Opin Immunol. 1997;9:702–708. doi: 10.1016/s0952-7915(97)80052-0. [DOI] [PubMed] [Google Scholar]
- 70.Yee C, Thompson JA, Byrd D, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoover SK, Barrett SK, Turk TM, Lee TC, Bear HD. Cyclophosphamide and abrogation of tumor-induced suppressor T cell activity. Cancer Immunol Immunother. 1990;31:121–127. doi: 10.1007/BF01742376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Proietti E, Greco G, Garrone B, et al. Importance of cyclophosphamide-induced bystander effect on T cells for a successful tumor eradication in response to adoptive immunotherapy in mice. J Clin Invest. 1998;101:429–441. doi: 10.1172/JCI1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schiavoni G, Mattei F, Di Pucchio T, et al. Cyclophosphamide induces type I interferon and augments the number of CD44(hi) T lymphocytes in mice: implications for strategies of chemoimmunotherapy of cancer. Blood. 2000;95:2024–2030. [PubMed] [Google Scholar]
- 76.Greenberg PD, Cheever MA. Treatment of disseminated leukemia with cyclophosphamide and immune cells: tumor-immunity reflects long-term persistence of tumor-specific donor T cells. J Immunol. 1984;133:3401–3407. [PubMed] [Google Scholar]
- 77.Maine GN, Mule JJ. Making room for T cells. J Clin Invest. 2002;110:157–159. doi: 10.1172/JCI16166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dummer W, Niethammer AG, Baccala R, et al. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185–192. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu HM, Poehlein CH, Urba WJ, Fox BA. Development of antitumor immune responses in reconstituted lymphopenic hosts. Cancer Res. 2002;62:3914–3919. [PubMed] [Google Scholar]
- 80.Mihalyo MA, Doody AD, McAleer JP, et al. In vivo cyclophosphamide and IL-2 treatment impedes self-antigen-induced effector CD4 cell tolerization: implications for adoptive immunotherapy. J Immunol. 2004;172:5338–5345. doi: 10.4049/jimmunol.172.9.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh NJ, Schwartz RH. The Strength of Persistent Antigenic Stimulation Modulates Adaptive Tolerance in Peripheral CD4+ T Cells. J Exp Med. 2003;198:1107–1117. doi: 10.1084/jem.20030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kurts C, Heath WR, Carbone FR, Allison J, Miller JF, Kosaka H. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morgan DJ, Kurts C, Kreuwel HT, Holst KL, Heath WR, Sherman LA. Ontogeny of T cell tolerance to peripherally expressed antigens. Proc Natl Acad Sci USA. 1999;96:3854–3858. doi: 10.1073/pnas.96.7.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morgan DJ, Kreuwel HTC, Sherman LA. Antigen concentration and precursor frequency determine the rate of CD8+ T cell tolerance to peripherally-expressed antigens. J Immunol. 1999;163:723–727. [PubMed] [Google Scholar]
- 85.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 86.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 87.Sotomayor EM, Borrello I, Tubb E, et al. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat Med. 1999;5:780–787. doi: 10.1038/10503. [DOI] [PubMed] [Google Scholar]
- 88.Diehl L, den Boer AT, Schoenberger SP, et al. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat Med. 1999;5:774–779. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 89.Maxwell JR, Campbell JD, Kim CH, Vella AT. CD40 activation boosts T cell immunity in vivo by enhancing T cell clonal expansion and delaying peripheral T cell deletion. J Immunol. 1999;162:2024–2034. [PubMed] [Google Scholar]
- 90.Lefrancois L, Altman JD, Williams K, Olson S. Soluble antigen and CD40 triggering are sufficient to induce primary and memory cytotoxic T cells. J Immunol. 2000;164:725–732. doi: 10.4049/jimmunol.164.2.725. [DOI] [PubMed] [Google Scholar]
- 91.Garza KM, Chan SM, Suri R, et al. Role of antigen-presenting cells in mediating tolerance and autoimmunity. J Exp Med. 2000;191:2021–2027. doi: 10.1084/jem.191.11.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maxwell JR, Weinberg A, Prell RA, Vella AT. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J Immunol. 2000;164:107–112. doi: 10.4049/jimmunol.164.1.107. [DOI] [PubMed] [Google Scholar]
- 93.Bansal-Pakala P, Jember AG, Croft M. Signaling through OX40 (CD134) breaks peripheral T-cell tolerance. Nat Med. 2001;7:907–912. doi: 10.1038/90942. [DOI] [PubMed] [Google Scholar]
- 94.Lathrop SK, Huddleston CA, Dullforce PA, Montfort MJ, Weinberg AD, Parker DC. A signal through OX40 (CD134) allows anergic, autoreactive T cells to acquire effector cell functions. J Immunol. 2004;172:6735–6743. doi: 10.4049/jimmunol.172.11.6735. [DOI] [PubMed] [Google Scholar]