Abstract
Monogenic deficiency diseases provide unique opportunities to define the contributions of individual molecules to human physiology and to identify pathologies arising from their dysfunction. Here we describe a deficiency disease in two human siblings that presented with severe bleeding, frequent infections and osteopetrosis at an early age. These symptoms are consistent with but more severe than those reported for people with leukocyte adhesion deficiency III (LAD-III). Mechanistically, these symptoms arose from an inability to activate the integrins expressed on hematopoietic cells, including platelets and leukocytes. Immortalized lymphocyte cell lines isolated from the two individuals showed integrin activation defects. Several proteins previously implicated in integrin activation, including Ras-associated protein-1 (RAP1)1 and calcium and diacylglycerol-regulated guanine nucleotide exchange factor-1 (CALDAG-GEF1)2, were present and functional in these cell lines. The genetic basis for this disease was traced to a point mutation in the coding region of the KINDLIN3 (official gene symbol FERMT3) gene3. When wild-type KINDLIN-3 was expressed in the immortalized lymphocytes, their integrins became responsive to activation signals. These results identify a genetic disease that severely compromises the health of the affected individuals and establish an essential role of KINDLIN-3 in integrin activation in humans. Furthermore, allogeneic bone marrow transplantation was shown to alleviate the symptoms of the disease.
Kindlin-3 is one of the three-member kindlin family of intracellular proteins that are linked to the actin cytoskeleton3. The family is evolutionarily conserved with an ortholog, UNC-112, found in Caenorhabditis elegans4. Each kindlin contains a C-terminal FERM domain that is most similar to that of talin, another cytoskeletal protein involved in integrin regulation. Kindlins and talin bind to nonoverlapping sites in the cytoplasmic tails of integrins5. Kindler disease, associated with a deficiency of Kindlin-1, has multiple symptoms, including skin blistering and poikiloderma6. Kindlin-2 deficiency is embryonically lethal in zebrafish and mice but has not been described in humans7. Kindlin-3 expression is restricted to hematopoietic cells. Kindlin-3–deficient mice die by day 7 after birth, bleed, and have an absence of platelet aggregation and altered erythrocyte shape8,9. Only very limited information is available on the functions of kindlins. Kindlin-2 has been reported to influence integrin-mediated strengthening of cell adhesion5,10 and to cooperate with talin in inducing integrin activation11.
The two siblings described in this report, a boy (subject 1) and a girl (subject 2), presented with recurrent clinical bleeding and infections by 2 weeks of age. Both subjects had normal platelet counts, hemoglobin levels and peripheral blood cell morphology. Leukocyte counts ranged from 35 × 109 to 70 × 109 cells per liter, with equal numbers of neutrophils and lymphocytes. There was no family history of recurrent infections and bleeding (see Supplementary Note online for details). Subject 1 developed mucosal bleeding and lymphadenitis by 2 weeks of age. After this age, mucosal bleeding, requiring erythrocyte transfusions, continued, and the subject experienced additional bouts of infections. At the time of referral at 7 months of age, his height and weight were at the third percentile, and hepatosplenomegaly was present. Osteopetrosis was observed, confirmed by skeletal survey, and continued to progress. Infections and multiple episodes of bleeding continued, including an intracranial hemorrhage after minimal head trauma. Subject 2 developed mucocutaneous bleeding at 2 weeks of age. She also had hepatosplenomegaly A skeletal survey showed osteopetrosis. Multiple episodes of mucocutaneous bleeding, which responded to platelet infusions, continued from this age. She experienced intracranial hemorrhage at 4 months of age.
Most abnormalities of blood cell functions (as described below) were documented in primary and immortalized cells (Supplementary Figs. 1 and 2 online) from both subjects, but their small blood volumes limited repeated assays. Bleeding was the most troublesome symptom, and the subjects’ platelets showed no aggregation response to various agonists, including ADP, thrombin and phorbol 12-myristate 13-acetate (PMA) (Fig. 1a). The absence of a response to PMA is particularly noteworthy, as PMA does aggregate platelets from subjects with leukocyte adhesion deficiency I variant (LAD-Iv) or LAD-III, who have a similar but milder phenotype12,13. A marked defect in platelet adhesion to fibrinogen was observed; the subjects’ cells attached but failed to develop lamellapodia or spread (Fig. 1b). Soluble fibrinogen binding to integrin αIIbβ3, a hallmark of its activation status14, was also markedly defective with the subjects’ platelets, as measured by radiolabeled ligand binding (Fig. 1c) and FACS (Fig. 1d). Suppressed fibrinogen binding is a defining feature of Glanzmann’s thrombasthenia, which is typically associated with an absence of functional αIIbβ3 (ref 15). However, expression of the αIIb and β3 integrin subunits was normal (Fig. 1d). Instead, activation of αIIbβ3 integrin was the specific response absent in the subjects’ platelets. ADP or thrombin treatment did not lead to a significant increase in binding of the activation-specific monoclonal antibody (mAb) to αIIbβ3 integrin, PAC-1 (ref. 16) (Fig. 1d). Thrombin did induce P-selectin surface expression, indicating that platelet responses were not globally impaired (Fig. 1d). Thus, bleeding in the subjects can be attributed to failure of αIIbβ3 integrin to activate.
Figure 1.
Analysis of platelet activation in subjects. (a) Aggregation curves of platelets from subject 1 stimulated by PMA (100 nM), ADP (10 μM) or thrombin (1 U ml−1). Aggregation of platelets from a healthy volunteer in response to ADP is shown for comparison. (b) Platelets from subject 2 and a healthy control were isolated as described in the Supplementary Methods, treated with 100 nM PMA (top) or 1 U ml−1 thrombin (bottom) and added to fibrinogen-coated cover slips for 30 min. Representative fluorescence images of adherent platelets stained with Texas Red–phalloidin (top) or antibody to β3 integrin labeled with FITC (bottom) are shown. Note the presence of platelet aggregates in control, but not in the subject 2’s sample. Scale bar, 10 μm. (c) Time curves of 125I-fibrinogen binding to platelets from subject 1 and control platelets in response to thrombin. Nonspecific binding was determined in the presence of a 50-fold excess of unlabeled fibrinogen (Supplementary Methods). (d) Integrin inside-out activation but not integrin expression was deficient in subjects’ platelets. Platelets were stimulated with 20 μM ADP, 1 U ml−1 thrombin or 100 nM PMA or were left unstimulated. For fibrinogen binding, FITC-labeled fibrinogen was added to the platelet suspension in the presence and absence of PMA, and the mean fluorescence intensity (MFI) was determined by FACS. Levels of PAC-1 binding, P-selectin, and αIIb and β3 integrin subunits on control and subjects’ platelets were also assessed by FACS analysis. β3 integrin data incorporate measurements for both subjects; the other data are from subject 1. The difference between subjects and control was not significant (NS). Fold increases over control (unstimulated control platelets) are shown. (The data represent means ± s.d., n = 3, **P < 0.01, *P < 0.05).
Individuals with LAD-I lack β2 integrins on their leukocytes, rendering them susceptible to frequent infections17–19. Expression of the leukocyte integrins αLβ2 and αMβ2 (Supplementary Fig. 1b online) and other membrane markers (CD32, CD47, CD4, CD19, CD16 and CD36) were normal in the subjects (data not shown). However, polymorphonuclear leukocytes (PMNs) from the subjects failed to adhere to fibrinogen (Fig. 2a) when stimulated with PMA, a response mediated by activated αMβ2 integrin. PMN adhesion to denatured ovalbumin, also mediated by αMβ2 integrin but not requiring activation, did occur (Fig. 2a); however, the subjects’ PMNs failed to spread on this substrate (Supplementary Fig. 3b online). Neutrophil inhibitory factor, a specific, high-affinity and activation-independent ligand of αMβ2 integrin20, bound well to both subjects’ PMNs (Supplementary Fig. 1a), but αMβ2-mediated PMN aggregation induced by PMA (Fig. 2b) or formyl-methionyl-leucyl-phenylalanine (fMLP) (Fig. 2c) was suppressed, confirming a selective impairment of activation-dependent functions of αMβ2 integrin. Superoxide production upon PMA stimulation was >60% that in control cells from healthy volunteers, indicating that phagocyte oxidase function was intact, and 20% of control when the cells were stimulated with opsonized zymosan, consistent with defective function of αMβ2 integrin (Fig. 2d).
Figure 2.
Impaired function of αMβ2, α4β1 and αVβ3 integrins on subjects’ cells. (a) Adhesion of neutrophils isolated from subject 1 and a healthy control to fibrinogen and denatured ovalbumin in the presence or absence of 200 nM PMA. Specificity of adhesion was determined in the presence of an excess of αMβ2 ligand, neutrophil inhibitory factor (NIF). Adhesion of control resting neutrophils to ovalbumin was assigned a value of 100%. Data shown represent means ± s.d. (n = 3, ** P < 0.01). (b,c) Subject 1’s lymphocytes and neutrophils did not aggregate upon stimulation. Normal and subject lymphocytes (b) and neutrophils (c) were stimulated with 0.16 μM PMA and 1 μM fMLP, respectively. Representative photographs of lymphocytes were taken 25 min after stimulation (b). Scale bar, 100 μm. Neutrophil aggregation was measured 10 min after stimulation (c). (Means ± s.d.; n = 3, ** P < 0.01). (d) Neutrophil oxidative burst was impaired in subject 1. Superoxide release from neutrophils stimulated with PMA or opsonized zymosan (OZ) particles was measured as described in the Supplementary Methods (data represent means ± s.d.; n = 3, ** P < 0.01, * P < 0.05). (e) Subject 1’s lymphocytes, unlike control cells, did not adhere to intercellular adhesion molecule-1 in response to PMA (n = 3, ** P < 0.01). (f) Expression of the β2 integrin activation epitope on subject 1’s (left) and control (right) lymphocytes. Cells were stimulated with PMA (solid line) and analyzed for binding of mAb 24 by FACS analysis. The dotted line represents staining with an isotype-matched control mAb. (g) Binding of activation-dependent ligand WOW-1 Fab to lymphocytes from subject 2 and control cells was measured by FACS analysis in the presence or absence of PMA (200 nM). The data represent means ± s.d., n = 3, ** P < 0.01. (h) Peripheral blood mononuclear cells were isolated from the blood of a healthy control, the two subjects and their parents and immortalized. Adhesion of immortalized cells to fibrinogen in the presence or absence of 200 nM PMA, 1 mM EDTA and 200 nM RGD peptide is shown, as indicated. The data represent means ± s.d., n = 5, ** P < 0.01.
Lymphocytes from the subjects also showed defects in integrin activation. Adhesion to the D1D2 domains of intercellular adhesion molecule-1, which is mediated by activated αLβ2 integrin, was suppressed (Fig. 2e). When lymphocytes were stimulated with PMA, they failed to interact with mAb 24 (Fig. 2f), which reacts selectively with activated β2 integrins. Integrins other than the β2 subfamily also failed to activate on leukocytes from the subjects; the activation-specific mAb WOW-1 that binds to integrins αVβ3 and αVβ5 did not bind the subjects’ PMNs (Fig. 2g).
Because of the frequency and severity of bleeding, both subjects underwent allogeneic bone marrow transplantation (BMT). Before BMT, Epstein-Barr virus–transformed immortalized cell lines were established from lymphocytes from both subjects, their parents and a healthy control individual. As determined by FACS, integrins β1, β2 and β3 were expressed at similar levels on all of the immortalized cell lines (Supplementary Fig. 1b). The defects in integrin activation shown by the subjects’ lymphocytes were recapitulated in the cell lines: the subjects’ cells did not adhere to fibronectin or fibrinogen, with or without PMA stimulation, whereas adhesion of control cells and cells from parents to both substrates was stimulated by PMA (Fig. 2h and Supplementary Fig. 2a,b online). This adhesion was integrin mediated on the basis of its sensitivity to EDTA and to an RGD peptide (Fig. 2h and Supplementary Fig. 2a). Similar results were observed in soluble ligand binding assays (Supplementary Fig. 3c). Dithiothreitol treatment did lead to integrin activation in the subjects’ cell lines; that is, the cells became adherent to fibronectin (Supplementary Fig. 2d) and bound soluble fibrinogen (Supplementary Fig. 2e). Thus, the subjects’ cells expressed integrins that were not activated by agonist stimulation, known as inside-out signaling, but were functional if inside-out signaling was bypassed.
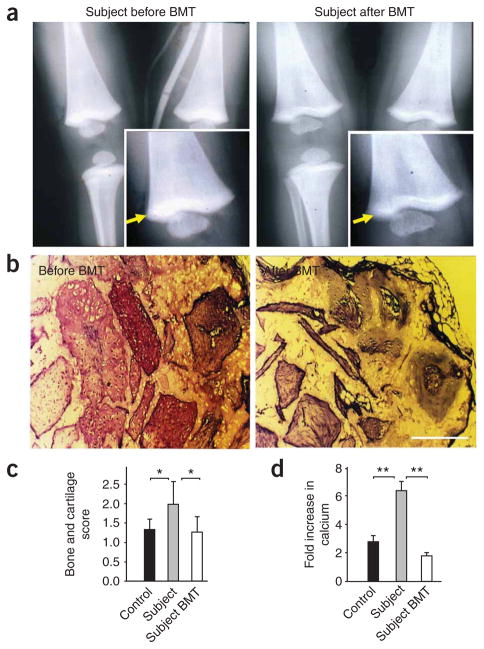
Both subjects showed early (at around 5 months of age) osteopetrosis, as evidenced by X-rays of long bones, especially in the area proximal to the growth plate (Fig. 3a). The misbalance between bone forming and bone resorptive activity of the subjects’ cells was traced to abnormalities of bone marrow–derived mesenchymal stem cells. The subjects’ mesenchymal stem cells produced substantially higher amounts of bone and cartilage when loaded into ceramic cubes and implanted into immunocompromised mice than did control cells (Fig. 3b,c). Scoring for bone growth revealed obvious differences between the marrow cells from subject 1 and those harvested from a healthy donor (Fig. 3b,c). Calcium accumulation stimulated by osteogenic compounds was substantially higher in subjects’ cells than in control cells (Fig. 3d). The osteopetrosis observed in these subjects suggests that integrin activation may be involved in osteogenesis as well as in bone resorption17,21, a hypothesis that warrants further study.
Figure 3.
Osteopetrosis in subjects was rescued by BMT. (a) Subject 1’s X-ray before (left) and 3 months after (right) BMT. Arrow indicates higher bone density near the growth plate. (b) Bone and cartilage formation by subject 1’s bone marrow–derived mesenchymal stem cells before and after BMT. Bone marrow samples were isolated and cultured as described in the Supplementary Methods. Cells were loaded into porous ceramic cubes coated with fibronectin and implanted into immunocompromised mice. The cubes were harvested, processed and stained with Mallory-Heidenhain at 6 weeks after implantation as described in the Supplementary Methods. Scale bar, 250 μm. (c) Ceramic cubes loaded with control cells, subject 1’s cells and subject 1’s cells after BMT were processed as in b. Bone and cartilage formation was scored as described in the Supplementary Methods. The scoring system was as follows: 1, 1–25%; 2, 26–50%; 3, 50–75% of bone and cartilage. Data are means ± s.d., n = 8, * P < 0.05. (d) Calcium accumulation by bone marrow cells from healthy controls (at least two different sources of bone marrow were used in four experiments), subject 1 and subject 1 after BMT was measured as described in the Methods; fold increase upon addition of osteogenic supplement is shown. The data represent means ± s.d., n = 4, ** P < 0.01).
BMT resolved all of the overt clinical problems in both subjects. The macroscopic bone density defects corrected over time (Fig. 3a). Bone and cartilage production by the mesenchymal stem cells that we implanted into immunocompromised mice and calcium accumulation in cell cultures also reverted to control levels after BMT (Fig. 3b–d). Also responding to BMT were platelet and PMN functions, as evidenced by fibrinogen binding to platelets (Supplementary Fig. 3a) and PMN spreading on denatured ovalbumin (Supplementary Fig. 3b).
In searching for the molecular defect in the subjects, we considered several candidates implicated in integrin activation by western blotting and functional assays18. Talin, filamin and vinculin (Supplementary Fig. 4a online) were present in the subjects’ platelets and cell lines in amounts similar to those in control samples. The protein kinase C pathway was functional, as indicated by increased L-selectin shedding upon PMA stimulation (Supplementary Fig. 4b). Signaling molecules extracellular signal–regulated kinase, p38 and RAP1A were present, as determined by western blotting, and were activated by PMA (Supplementary Figs. 4c and 5a online). Given the substantial body of data implicating RAP1 and its effectors RAPL (regulator for cell adhesion and polarization enriched in lymphoid tissue) and RAP1-interacting adaptor molecule in integrin activation19,22, we sequenced their complementary DNAs from subject cells but found no deviations from the sequence available in GenBank (data not shown). In contrast to cells from individuals with LAD-III23, RAP1 activation was present and even at elevated levels in the subjects’ cells (Supplementary Fig. 5a). The amount of CALDAG-GEF1 (encoded by RASGRP2), also implicated in LAD-Iv and LAD-III, was indistinguishable from that in control cells at both the protein and the mRNA levels (Supplementary Fig. 5b,c).
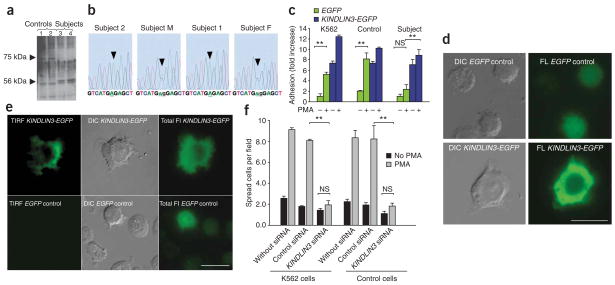
With recent data implicating Kindlin-3 in the activation of αIIbβ3 integrin9, we performed western blotting to measure KINDLIN-3 abundance in the immortalized cell lines. The KINDLIN-3 band at 75 kDa in extracts of the control and parental cells was absent in cells from both subjects (Fig. 4a). Instead, a band of 56 kDa was detected in the subjects’ but not in control cells (Fig. 4a). RT-PCR with specific primers confirmed the presence of KINDLIN3 messenger RNA in control and subject cells (data not shown). To identify the 75-kDa band that was missing in the subjects’ cells, we purified the fraction containing this protein. The isolated protein was analyzed by mass spectrometry at two independent facilities and was identified by >40 peptides as KINDLIN-3, and it was also recognized by antibody to KINDLIN-3 (Supplementary Fig. 6a,b online).
Figure 4.
Subjects have a point mutation in KINDLIN3. (a) Western blot for KINDLIN-3 in subjects’ and control lymphocytes. The positions of full-length KINDLIN-3 and its short form are indicated with arrows. Lanes 1 and 2 show samples of immortalized cell lines from different healthy individuals; lanes 3 and 4 show subjects 1 and 2, respectively. (b) Chromatograms of genomic PCR sequence. Color-coded nucleotide sequence is shown at the bottom. Analyses of subjects 1 and 2 as well as their parents (M and F) are shown. Arrow indicates the point mutation. (c) KINDLIN3 expression rescues adhesion and spreading defects in subject 1’s cells. Cells from subject 1, a healthy control and the K562 cell line were transfected with KINDLIN3-EGFP or EGFP vector control as indicated, and equal amounts of cells were plated on a fibrinogen-coated plate as described in the Supplementary Methods. Adherent cells were counted in six random fields. The data represent means ± s.d., n = 3, ** P < 0.01. (d) Expression of KINDLIN3 rescues the defect in spreading on fibronectin in subject 1’s cells. Microphotographs of EGFP- or KINDLIN3-EGFP–transfected subject 1’s cells are shown. Left, Nomarski microscopy (DIC, differential interference contrast); right, fluorescence microscopy (FL). Scale bar, 10 μm. (e) TIRF analysis of KINDLIN3-EGFP–expressing subject 1’s cells adhered to fibrinogen-coated plates. Cells were transfected with EGFP or KINDLIN3-EGFP as indicated. Cells were visualized using TIRF (left), Nomarski (center) or fluorescence (right) microscopy. Scale bar, 10 μm. (f) Knockdown of KINDLIN3 in control cells recapitulates the subjects’ cells’ adhesion and spreading defects. K562 lymphocytes or control cell lines were transfected with KINDLIN3–specific siRNA, control siRNA or remained untransfected as indicated. Adhesion assays were performed as in d. The data represent means ± s.d., n = 3, ** P < 0.01.
Focusing on KINDLIN3 mRNA, we sequenced RT-PCR products from both subjects and found a point mutation creating a premature stop codon; codon 16, TGG (tryptophan), was converted to TGA (stop) (Fig. 4b). The sequence of the second exon of a genomic PCR product verified the G→A mutation in the subjects’ DNA and heterozygosity for the mutation in both parents (Fig. 4b and Supplementary Fig. 6c). A unique BspHI restriction site is predicted to arise from the mutation, and this was borne out in the subjects and their parents by genomic PCR followed by BspHI restriction digestion (Supplementary Fig. 6d). A potential explanation for the KINDLIN-356-kDa fragment in the subjects is the presence of an alternative downstream ATG transcription initiation site at codon 181 (Supplementary Fig. 6c).
In rescue experiments, KINDLIN-3 expression in transformed lymphocytes from the subjects induced cell adhesion to levels observed in control cells (Fig. 4c,d and Supplementary Movies 1 and 2 online). The subjects’ cells without transfection showed virtually no adhesion to both fibrinogen and fibronectin and the few attached cells remained round (Fig. 4d and Supplementary Movies 1 and 2). After transfection of subject 1’s cells with KINDLIN3 cDNA, the cells not only adhered but also ruffled, followed by full spreading (Fig. 4d). Total internal reflection fluorescence (TIRF) imaging of cells from subject 1 transfected with KINDLIN3-EGFP revealed KINDLIN-3 at contact sites of the adherent cells with the substrate. In contrast, cells from subject 1 cells transfected with a control vector did not establish any contacts with the substrate (Fig. 4e). Moreover, RNA interference–based knockdown of KINDLIN3 in the control cells from healthy individuals and in the K562 lymphoblastoid cell line ablated responsiveness to PMA, a hallmark feature of the subjects’ cells (Fig. 4f).
In this study, we identify the cellular and molecular basis of the severe bleeding, frequent infections and osteopetrosis in a pair of siblings. Platelet, leukocyte and bone marrow mesenchymal cell responses were all abnormal and arose from loss of full-length KINDLIN-3, which resulted in an inability to activate integrins expressed on hematopoietic cells. We propose that this disease be designated integrin activation deficiency disease (IADD), reflecting the defect underlying the symptoms. IADD may be identical to LAD-Iv or LAD-III13,24; however, the designation LAD does not capture the primary symptom in our subjects, bleeding, and other symptoms also appear to be more severe. Furthermore, in cells from the two individuals we studied, we did not observe an absence of CALDAG-GEF1 expression and of RAP1 activation as previously noted in cells from subjects with LAD-III and LAD-Iv. In addition, in contrast to our results, in cells from subjects with LAD-III and LAD-Iv, integrins were capable of being activated in response to PMA12,13. Nevertheless, in a recent publication, subjects with LAD-III were described as also having a mutation in KINDLIN325, further clouding understanding of the defects in these individuals.
Allogeneic BMT seemed to alleviate all major symptoms of IADD and restored hematopoietic cell functions. The apparent restriction of KINDLIN-3 expression to hematopoietic cells is consistent with the defects in blood and bone marrow cell–mediated responses observed in these individuals. However, the frequency and severity of bleeding suggests possible additional defects in individuals with IADD, perhaps in vascular cell properties. In this regard, it is noteworthy that mice deficient in αV integrins develop intracranial bleeding, a symptom that was observed in both subjects with IADD. IADD resembles many but not all of the defects reported in Kindlin3-deficient mice. The defects in platelet aggregation and bleeding are similar9. Indeed, in this issue of Nature Medicine, Moser et al.26 report defects in leukocyte function in Kindlin3-deficient mice. We did not observe the abnormalities in erythrocyte shape reported for the Kindlin3−/− mouse8. Finally, we emphasize that current understanding of the functions of KINDLIN-3 is very limited27. Although we assign the primary defects in the subjects with IADD to an absence of integrin activation, kindlin family members might link other receptors to the actin cytoskeleton, and the severe symptoms of IADD may arise from yet undescribed functions of KINDLIN-3.
METHODS
Clinical samples
We conducted the studies in accordance with the ethical standards of the Institutional Review Board at University Hospitals of Cleveland and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all individuals. Further details of the subjects’ histories are provided in the Supplementary Note. All animal studies were performed according protocols approved by Institutional Animal Care and Use Committees of Case Western Reserve University and Cleveland Clinic.
Cell lines and kindlin rescue experiments
We transformed peripheral blood mononuclear cells from the subjects, their parents and an unrelated control subject with Epstein-Barr virus (B95-8 strain from American Type Culture Collection, ATCC) supernatant to create immortalized cell lines. We cultured the immortalized cells in RPMI1640 1× medium supplemented with 10% heat-inactivated FBS, L-glutamine (2 mM), penicillin-streptomycin (100 U) (all from Invitrogen), and MEM nonessential amino acids from Sigma. We maintained MEG01 and K562 cells (ATCC) in medium of the same composition. The details of functional assays of primary cells isolated from subjects and of the immortalized cells are provided in the Supplementary Methods online. To express KINDLIN-3 in the cell lines from the subjects and to test the rescue of function, we transfected the cells with a KINDLIN3-EGFP fusion construct. This construct was created using the EGFP-C2 vector (BD Biosciences) and an EcoRI- and KpnI-cut PCR product; this PCR product was generated from IMAGE clone 6709978 (obtained from ATCC) used as a template with a set of two primers with EcoRI and KpnI restriction sites for in-frame EGFP fusion: 5′-TTAAGAATTCATGGCGGGGATGAAGACAGC-3′ and 5′-ATATGGTACCTCAGAAGGCCTCATGGCCCC-3′. We performed transfection using protocol T16 and nucleofection solution V from Amaxa, Inc. Twenty-four to 48 h after transfection, we preincubated 2 × 106 cells per ml with 2% BSA and left them untreated or stimulated them with 500 nM PMA for 90 min in microtiter plates coated with 10 μl ml−1 of fibronectin or 40 μg ml−1 of fibrinogen. We washed the wells twice with RPMI 1640 without FBS. We fixed adherent cells with 4% formaldehyde in PBS and counted EGFP-positive cells in four random fields.
Genomic PCR and reverse transcription PCR
We performed genomic PCR with direct 5′-GCAGCAGCCGCAGCCATGGCGGGGATGAAG-3′ and reverse 5′-GATCTGCTCCACAATCTTCAGGAGCACCCC-3′ primers corresponding to nucleotides 74–103 and 218–247 of GenBank entry NM_031471. We performed CALDAGGEF1 semiquantitative RT-PCR with two primers sets: the first set for CALDAGGEF1 (GenBank entry NM_153819), 5′-GAACTTCAACACGCTGATGGCAGTGGTCGG-3′ and 5′-AAGAGCTGCTTCATCTTGGCCCCGTTGAGC-3′, and the second set for GAPDH (GenBank entry NM_002046), 5′-ACAGTCCATGCCATCACTGCCACCCAGAAG-3′ and 5′-GGTCAGGTCCACCACTGACACGTTGGCAGT-3′. RT-PCR with 200 ng total RNA was stopped at cycles 16–35; linear accumulation of PCR products detectable by ethidium bromide was evident for cycles 18–30. PCR products at cycle 28 are shown for both parental and subject samples. We performed one-step RT-PCR on total RNA with gene-specific primers according to the Qiagen’s protocol.
Transient expression and knockdown of KINDLIN-3
We performed small interfering RNA (siRNA)-mediated knockdown with Dharmacon ON-TARGETplus siRNA of the sequences: 5′-CCGAAUUGUACACGAGUAU-3′, 5′-UGGAGCAGAUCAAUCGCAA-3′, 5′-ACUACAGCUUCUUCGAUUU-3′ and 5′-ACUACAAGAGCCAGGACGA-3′. Control (scrambled sequence) siRNA was from the same supplier. Specific but not control siRNA decreased expression of KINDLIN-3 by more than 75% (data not shown). To monitor the efficiency of transfections, we used siGLO in 1:10 ratio with siRNAs in experiments done with GeneSilencer reagent (both from Genlantis).
Kindlin-3 isolation and mass spectroscopy
We lysed MEG01 cells (5 × 107) in 25 ml of lysis buffer (150 mM NaCl, 20 mM HEPES KOH pH 7.6 and 1% NP-40) supplemented with protease inhibitor cocktail (Roche). We removed insoluble material by centrifugation, diluted the supernatant with three volumes of 5 mM NaCl, 20 mM HEPES and 0.1% NP-40 and loaded it on HiTrap SP FF 5-ml columns (GE Life Sciences) equilibrated in the same buffer but with 40 mM NaCl. We eluted proteins with steps of 100, 150, 175, 200 and 250 mM NaCl. We diluted the 200 mM NaCl eluate two times with 5 mM NaCl, 20 mM HEPES and 0.01% NP-40 and loaded it on Heparin HP 5-ml columns (GE Life Sciences) equilibrated with 100 mM NaCl. We eluted the proteins with a 300–500 mM NaCl gradient in 3-ml fractions. We applied the fraction containing a prominent 75-kDa band on SDS-PAGE on a PD-10 column equilibrated with 50 mM NaCl in HEPES buffer, pH 7.0 without detergent. We incubated the eluate at 22 °C for 40 min, collected the precipitated protein by centrifugation, dissolved it in Laemmli sample buffer and separated it by SDS-PAGE on a 10% gel. We excised the 75-kDa band, digested it with trypsin and subjected the tryptic peptides to mass-spectroscopy at the Cleveland Clinic and Roswell Park Cancer Institute proteomic facilities.
Statistical analyses
The data are shown as means ± s.e.m. We used an unpaired t test for comparisons between two groups. P values < 0.05 were considered to be significant.
Supplementary Material
Acknowledgments
We thank Roswell Park Cancer Institute Proteomics Resources, Lerner Research Institute Proteomics and Imaging Cores (J. Drazba) and Stranad Fellows I. Byzov and R. Swaninger for help with the project, A. Graybiel (Massachusetts Institute of Technology) for providing antibodies to CALDAG-GEF1 and L. Parise (University of North Carolina) for providing the GST-RALGDS fusion cDNA construct. This study was supported by HL073311 and HL071625 US National Institutes of Health grants.
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
AUTHOR CONTRIBUTIONS
N.L.M. identified the Kindlin-3 mutation, performed molecular biology and protein biochemistry studies and wrote the manuscript; L.Z. contributed to study design and experiments on primary leukocytes from subjects; J.C. performed assays with EGFP-Kindlin-3 rescue and siRNA-mediated KINDLIN3 knockdown and western blotting; A.C. performed microscopy studies and FACS analysis; O.R. performed cell culture work and molecular biology; Y.-Q.M. performed molecular biology and Kindlin-3–specific antibody preparation; E.A.P. performed platelet studies; M.T. performed neutrophil analysis; D.P.L. and A.I.C. performed osteogenesis assays; S.B.S. originally described the subjects, designed clinical studies and wrote the manuscript; E.F.P. designed the studies, interpreted the results and wrote the manuscript; T.V.B. performed experiments with platelets and leukocytes, designed the general strategy, interpreted data and wrote the manuscript.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Hattori M, Minato N. Rap1 GTPase: functions, regulation, and malignancy. J Biochem. 2003;134:479–484. doi: 10.1093/jb/mvg180. [DOI] [PubMed] [Google Scholar]
- 2.Crittenden JR, et al. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat Med. 2004;10:982–986. doi: 10.1038/nm1098. [DOI] [PubMed] [Google Scholar]
- 3.Ussar S, Wang HV, Linder S, Fassler R, Moser M. The Kindlins: subcellular localization and expression during murine development. Exp Cell Res. 2006;312:3142–3151. doi: 10.1016/j.yexcr.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 4.Rogalski TM, Mullen GP, Gilbert MM, Williams BD, Moerman DG. The UNC-112 gene in Caenorhabditis elegans encodes a novel component of cell-matrix adhesion structures required for integrin localization in the muscle cell membrane. J Cell Biol. 2000;150:253–264. doi: 10.1083/jcb.150.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi X, et al. The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. J Biol Chem. 2007;282:20455–20466. doi: 10.1074/jbc.M611680200. [DOI] [PubMed] [Google Scholar]
- 6.Siegel DH, et al. Loss of kindlin-1, a human homolog of the Caenorhabditis elegans actin-extracellular-matrix linker protein UNC-112, causes Kindler syndrome. Am J Hum Genet. 2003;73:174–187. doi: 10.1086/376609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowling JJ, et al. Kindlin-2 is an essential component of intercalated discs and is required for vertebrate cardiac structure and function. Circ Res. 2008;102:423–431. doi: 10.1161/CIRCRESAHA.107.161489. [DOI] [PubMed] [Google Scholar]
- 8.Kruger M, et al. SILAC mouse for quantitative proteomics uncovers kindlin-3 as an essential factor for red blood cell function. Cell. 2008;134:353–364. doi: 10.1016/j.cell.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 9.Moser M, Nieswandt B, Ussar S, Pozgajova M, Fassler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14:325–330. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- 10.Dowling JJ, Vreede AP, Kim S, Golden J, Feldman EL. Kindlin-2 is required for myocyte elongation and is essential for myogenesis. BMC Cell Biol. 2008;9:36. doi: 10.1186/1471-2121-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma YQ, Qin J, Wu C, Plow EF. Kindlin-2 (Mig-2): a co-activator of beta3 integrins. J Cell Biol. 2008;181:439–446. doi: 10.1083/jcb.200710196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alon R, et al. A novel genetic leukocyte adhesion deficiency in subsecond triggering of integrin avidity by endothelial chemokines results in impaired leukocyte arrest on vascular endothelium under shear flow. Blood. 2003;101:4437–4445. doi: 10.1182/blood-2002-11-3427. [DOI] [PubMed] [Google Scholar]
- 13.Kuijpers TW, et al. Natural history and early diagnosis of LAD-1/variant syndrome. Blood. 2007;109:3529–3537. doi: 10.1182/blood-2006-05-021402. [DOI] [PubMed] [Google Scholar]
- 14.Quinn MJ, Byzova TV, Qin J, Topol EJ, Plow EF. Integrin αIIbβ3 and its antagonism. Arterioscler Thromb Vasc Biol. 2003;23:945–952. doi: 10.1161/01.ATV.0000066686.46338.F1. [DOI] [PubMed] [Google Scholar]
- 15.Newman PJ, Seligsohn U, Lyman S, Coller BS. The molecular genetic basis of Glanzmann thrombasthenia in the Iraqi-Jewish and Arab populations in Israel. Proc Natl Acad Sci USA. 1991;88:3160–3164. doi: 10.1073/pnas.88.8.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shattil SJ, Hoxie JA, Cunningham M, Brass LF. Changes in the platelet membrane glycoprotein IIb. IIIa complex during platelet activation. J Biol Chem. 1985;260:11107–11114. [PubMed] [Google Scholar]
- 17.Feng X, et al. A Glanzmann’s mutation in β3 integrin specifically impairs osteoclast function. J Clin Invest. 2001;107:1137–1144. doi: 10.1172/JCI12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasirer-Friede A, Kahn ML, Shattil SJ. Platelet integrins and immunoreceptors. Immunol Rev. 2007;218:247–264. doi: 10.1111/j.1600-065X.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- 19.Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol. 2005;17:509–516. doi: 10.1016/j.ceb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Moyle M, et al. A hookworm glycoprotein that inhibits neutrophil function is a ligand of the integrin CD11b/CD18. J Biol Chem. 1994;269:10008–10015. [PubMed] [Google Scholar]
- 21.Miura Y, et al. Defective osteogenesis of the stromal stem cells predisposes CD18-null mice to osteoporosis. Proc Natl Acad Sci USA. 2005;102:14022–14027. doi: 10.1073/pnas.0409397102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5:546–559. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 23.Kinashi T, et al. LAD-III, a leukocyte adhesion deficiency syndrome associated with defective Rap1 activation and impaired stabilization of integrin bonds. Blood. 2004;103:1033–1036. doi: 10.1182/blood-2003-07-2499. [DOI] [PubMed] [Google Scholar]
- 24.Alon R, Etzioni A. LAD-III a novel group of leukocyte integrin activation deficiencies. Trends Immunol. 2003;24:561–566. doi: 10.1016/j.it.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Mory A, et al. Kindlin-3: a new gene involved in the pathogenesis of LAD-III. Blood. 2008;112:2591. doi: 10.1182/blood-2008-06-163162. [DOI] [PubMed] [Google Scholar]
- 26.Moser M, et al. Kindlin-3 is for required for β2 integrin–mediated leukocyte adhesion to endothelial cells. Nat Med. 2009 February 22; doi: 10.1038/nm.1921. advance online publication. [DOI] [PubMed] [Google Scholar]
- 27.Larjava H, Plow EF, Wu C. Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. EMBO Rep. 2008;9:1203–1208. doi: 10.1038/embor.2008.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.