Abstract
While it is well established that exercise can improve cognitive performance, it is unclear how long these benefits endure after exercise has ended. Accordingly, the effects of voluntary exercise on cognitive function and brain-derived neurotrophic factor (BDNF) protein levels, a major player in the mechanisms governing the dynamics of memory formation and storage, were assessed immediately after a 3-week running period, or after a 1-week or 2-week delay following the exercise period. All exercised mice showed improved performance on the radial arm water maze relative to sedentary animals. Unexpectedly, fastest acquisition (fewest errors and shortest latency) occurred in animals trained following a 1-week delay, while best memory performance in the probe trial was observed in those trained immediately after the exercise period. Assessment of the time course of hippocampal BDNF availability following exercise revealed significant elevations of BDNF immediately after the exercise period (186% of sedentary levels) and at 1 and 2 weeks after exercise ended, with levels returning to baseline by 3–4 weeks. BDNF protein levels showed a positive correlation with cognitive improvement in radial water maze training and with memory performance on day 4, supporting the idea that BDNF availability contributes to the time-dependent cognitive benefits of exercise revealed in this study. Overall, this novel approach assessing the temporal endurance of cognitive and biochemical effects of exercise unveils new concepts in the exercise-learning field, and reveals that beneficial effects of exercise on brain plasticity continue to evolve even after exercise has ended.
Keywords: Hippocampus, physical activity, BDNF, radial-arm water maze, spatial, mouse, detraining
INTRODUCTION
Human and animal studies demonstrate that exercise participation is a powerful behavioral intervention to improve cognitive function and brain health. In particular, human studies have demonstrated robust effects of exercise in the aged population, where higher physical activity is associated with improved cognitive scores on multiple aspects of cognition including executive function (Yaffe et al., 2001, Colcombe and Kramer, 2003, Colcombe et al., 2004, Weuve et al., 2004), as well as with reduced incidence of dementia (Abbott et al., 2004, Larson et al., 2006), attenuation of age-related loss of brain perfusion, reduced age-dependent brain atrophy (Rogers et al., 1990, Colcombe et al., 2003), and even with increased brain volume in select cortical regions (Erickson and Kramer, 2008). Consistent with research in humans, rodent studies demonstrate that exercise can facilitate acquisition and/or retention in various hippocampal-dependent tasks including the Morris water maze (Vaynman et al., 2004, van Praag et al., 2005), radial arm maze (Schweitzer et al., 2006), radial arm water maze (Nichol et al., 2007, Khabour et al., 2009), passive avoidance (Radak et al., 2006), active avoidance (Greenwood et al., 2007), and object recognition (O’Callaghan et al., 2007). Not all studies have consistently demonstrated improvements in both acquisition and retention, suggesting that exercise effects on different aspects of cognition may depend on factors such as the duration of exercise exposure, type of exercise undertaken (eg forced vs voluntary), task difficulty, or other variables that have not yet been defined.
While it is well established that exercise can improve acquisition and/or retention of a cognitive task, it is unclear how long these benefits endure after exercise has ended. One study has demonstrated that beneficial effects of exercise on memory (passive avoidance task) are reversible, and are lost by 6 weeks after exercise participation has ended (detraining) (Radak et al., 2006). However, a more defined timecourse of exercise benefits with detraining has not been examined. It will be important to determine when cognitive benefits are most robust relative to the exercise period, and if the recency of exercise participation differentially modulates various aspects of cognitive performance (eg, acquisition/consolidation vs retention/recall). The current study will address these questions.
In addition to confirming exercise-induced benefits on cognitive function, animal studies have provided much insight to effects of exercise on brain health and function on a biological level. Exercise has multi-dimensional effects on brain function, including activating brain plasticity mechanisms, increasing neurogenesis and vascularization (Black et al., 1990, van Praag et al., 2005, Ding et al., 2006c), building synaptic structure (Farmer et al., 2004, Eadie et al., 2005, Vaynman et al., 2006), increasing brain metabolic capacity, and augmenting antioxidant defenses. For example, exercise induces enzymes in the Krebs cycle, increases availability of the electron transport chain components, and upregulates ATP synthesis in the brain (Ding et al., 2006b, Cui et al., 2007, Kirchner et al., 2008, Opii et al., 2008).
One of the net effects of exercise is to prime the molecular mechanisms responsible for encoding (van Praag et al., 1999, Farmer et al., 2004, O’Callaghan et al., 2007), resulting in a lowered threshold for acquisition and facilitating other aspects of synaptic plasticity. Correspondingly, a number of plasticity-related molecules are induced in the hippocampus in response to exercise, including intracellular kinase signaling systems (CaMKII, MPKI and MAPKII), transcription factors (CREB), neurotransmitter systems that mediate synaptic excitability (glutamatergic and GABAergic signaling), plasticity-related growth factors (brain-derived neurotrophic factor (BDNF)), and synaptic vesicle trafficking molecules (syntaxin, synaptotagmin, synapsin I) (Tong et al., 2001, Molteni et al., 2002). Interestingly, recent data investigating exercise-dependent regulation of plasticity-related molecules has revealed different temporal patterns of induction of genes and signaling pathways (Molteni et al., 2002). For example, Ser-133 phosphorylation and activation of CREB in the hippocampus by exercise is rapid (already after 1 day of wheel running) while p42 and p44 activation of MAPK signaling is delayed relative to CREB, but remains detectable longer, even after 1 month of exercise. The temporal dependence for induction of the various signaling systems and genes by exercise likely has implications for brain function, and for the relative benefits gained from short-term or sustained exercise intervention.
BDNF in particular is emerging as a central player in hippocampal plasticity, and is thought to be a key molecule mediating benefits of exercise on cognition (for reviews, see (Cotman and Berchtold, 2002, Cotman et al., 2007). BDNF gene and protein expression are rapidly upregulated in response to exercise, within a few days of exercise onset, with robust and sustained increases most apparent in the hippocampus (Cotman and Berchtold, 2002). The importance of BDNF signaling in exercise-dependent improvements in cognitive performance has been demonstrated by use of blocking antibodies to TrkB, the receptor for BDNF. Specifically, intra-hippocampal injection of an anti-TrkB antibody to block BDNF signaling attenuates beneficial effects of exercise on hippocampal-dependent learning, blocking improvements in both acquisition and retention of a spatial learning task (Vaynman et al., 2004). In addition, blocking BDNF signaling with anti-TrkB attenuates the induction of synaptic proteins (synaptophysin, synapsin) in the hippocampus in response to exercise (Vaynman et al., 2006), indicating that BDNF signaling likely initiates a cascade of downstream events that are important in mediating the effects of exercise on cognitive function.
In this study, we investigate whether exercise-induced benefits to cognitive function on a hippocampal-dependent task endure for some time after exercise has ended, and whether benefits to task performance may be related to the availability of BDNF protein in the hippocampus. Thus, we assessed effects of voluntary exercise on acquisition and retention using the radial arm water maze (RWM), and investigated if the effects of exercise on cognitive performance were altered by the introduction of a 1 or 2 week delay period between the end of exercise and behavioral testing. The RWM is a hybrid of the dry radial arm maze and the Morris water maze and tests reference memory, as mice need to learn the fixed location of an escape platform based on extramaze spatial cues. In parallel with behavioral measures, hippocampal BDNF protein levels were assessed to evaluate the timecourse of stability of exercise-induced BDNF after exercise has ended, and to assess the relationship between BDNF level and cognitive task performance.
EXPERIMENTAL PROCEDURES
Animals
Young adult male C57bl/6 mice (Jackson Labs), 2 months of age at experiment onset, were individually housed with ad libitum access to food and water, in a 12-h light/dark vivarium. Each cage of the exercising animals was individually equipped with a running wheel (Minimitter, OR, USA) that occupied roughly half of the cage. Running activity was voluntary and the nightly distance run was monitored by computer software (VitalView, Minimtter Co., OR, USA). All exercising animals had access to running wheels for 3 weeks. For 2 groups of exercised animals, wheels were removed at the end of the 3-week period, and animals underwent subsequent cognitive training or assessment of BDNF levels at 1-week (EX/delay-1) and 2 weeks (EX/delay-2) following the end of exercise. For animals that underwent cognitive training immediately at the end of the 3 week exercise period (EX group), running wheels were left in the cages during the 3 days of cognitive training in order to avoid introducing potential stress due to an altered home cage environment. Sedentary animals were housed in standard cages with no running wheels. Initially, when mice first have access to running wheels, they run approximately 1–2 km per night, and progressively increase their running distance to an average of 3–5 km per night by about the 5th night of running, and maintain this level of activity on subsequent nights. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) for the University of California at Irvine, CA, USA, and were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the suffering and the number of animals used.
Radial Arm Water Maze
The RWM is a hybrid of the Morris water maze and the radial arm maze, with the advantage that no food deprivation needs to be introduced, as immersion in water provides motivation to learn the cognitive task. An added benefit is that errors can be scored, as in the radial arm maze, rather than relying solely on latency to escape, or proximity to the escape platform. The RWM task took place in a circular water tank (1 meter diameter), and consisted of 6 arms of transparent plexiglass (10 cm wide, 33 cm long, 15 cm high) that radiated outward from a central region at an angle of 60 degrees between arms (figure 1), following the design of the Morgan laboratory (College of Medicine, Tampa Florida) (Alamed et al., 2006) and as used previously (Nichol et al., 2009). Water (at room temperature, ~ 23°C), made opaque with a non-toxic powdered paint, was filled to a depth of 9 cm such that the mice were prevented from touching the floor of the pool, and the maze arms extended 6 cm above the water, preventing the mice from escaping onto the edges of the maze arms. A hidden plexiglass escape platform (10 cm wide, 8 cm long, 8 cm high), was located at the end of one of the maze arms, submerged ~1 cm below the surface of the water, and remained in this fixed location throughout all training trials for a given mouse. Locations of the escape platform were balanced across groups, using 4 different escape arms. At the start of a trial, the mouse was placed at the end of one of the arms facing the wall and was allowed 60 seconds to explore the maze arms and locate the platform. If the mouse did not find the platform within 60 seconds, it was guided to the correct arm and onto the platform. The mouse remained on the platform for 20 seconds to get spatial bearing relative to the room cues. Spatial cues of different sizes and patterns were affixed to each of the testing room walls to provide ample spatial information for the animals to learn the escape platform location. After each trial, each mouse was lightly towel dried and returned to its home cage, which was placed on a heating pad to prevent hypothermia. Four groups of animals were tested: sedentary (SED; n=15), Exercise (EX; n=13), Exercise + 1-week delay (EX/Delay-1; n=8), Exercise + 2 weeks delay (EX/Delay-2; n=7). Each animal underwent 10 trials/day in blocks of 5 trials, for 3 days. The start location varied across the 5 trials, using each arm (with the exception of the target arm) once, in random order. The order of the start arms was different each day. Animals were tested in sets of 4, with an intertrial interval of approximately 5 minutes. The interblock interval was approximately 2–3 hrs. All behavioral testing took place between 8 AM 3 PM. Animals were trained in the same order each day, such that behavioral testing occurred at similar times across days for a given animal. Data recorded from the training trials were errors (the number of arm entries before finding the platform), latency to find the escape platform, and failures (trials where the platform was not located within 60 seconds). On the 4th day, a 60-second probe trial was given in the absence of an escape platform to test memory for the platform location. The probe trial was video-recorded and analyzed for duration of time spend searching in each arm.
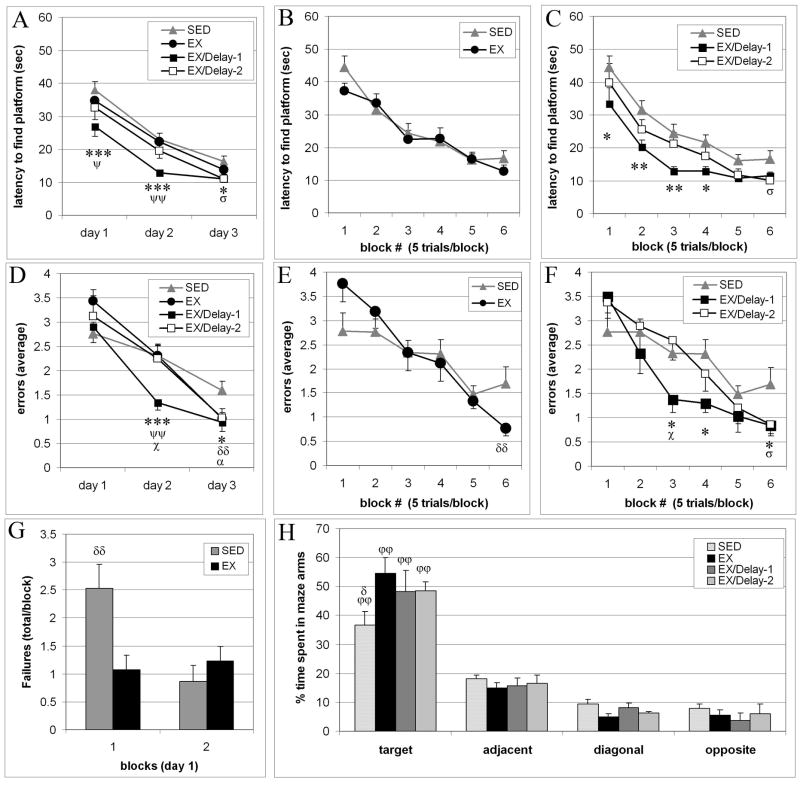
Figure 1.
Exercise effects on acquisition and retention of the radial water maze. Latency and errors by day (A,D) or by blocks of 5 trials (B,C, E, F) after 3 weeks of voluntary wheel running exercise with no delay (EX), a 1-week delay (EX/delay-1) or a 2-week delay (EX/delay-2) between exercise and cognitive training. Latency or errors by day (averaged over 2 blocks of 5 trials per day) reveals that animals with exercise exposure learn the task faster than sedentary animals (A, D). Latency and errors by block of 5 trials (B,E) reveals that EX and SED groups have similar latencies to find the platform (B) while the EX group ultimately makes fewer errors on block 6 (E). Latency and errors by block of 5 trials (C, F) reveals that fastest acquisition was observed when a 1-week delay was introduced between exercise and cognitive training while after 2 weeks delay, acquisition speed was intermediate between the performance of SED and EX/Delay-1 groups. Failure to find the escape platform was more frequent in SED than EX animals in block 1 of training (G). Probe trial performance reveals that all animals learned the task, spending more time in the target arm where the platform had been located, relative to the other maze arms (H). Exercised animals showed greater preference for the target arm relative to SED animals, with strongest memory in animals with no delay between exercise and cognitive training. Percentage of time in each arm was calculated as the ratio of “time in arm” divided by “time spent in all arms”. All data are averages ± sem. (SED vs EX: δ p ≤ 0.05, δδ p ≤ 0.01. SED vs EX/Delay-1: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.005. SED vs EX/Delay-2: σ p ≤ 0.05. EX vs EX/Delay-1: Ψp ≤ 0.05, ΨΨp ≤ 0.01. EX/Delay-1 vs EX/Delay-2: χp ≤ 0.05. Target arm vs non-target arms: ϕϕ p ≤ 0.0001)).
BDNF protein
Hippocampal BDNF protein levels after exercise, and the timecourse of protein decay at 1, 2, 3 and 4 weeks after the end of exercise, were assessed in separate groups of mice from those that underwent cognitive testing. Separate groups of mice was used so that BDNF protein levels at the beginning of behavioral testing could be established without confounding effects on BDNF levels by the behavioral testing itself. Group sizes were as follows: Sed (n=27), EX (n=17), EX/Delay-1 (n=6), EX/Delay-2 (n=5), EX/Delay-3 (n=6), EX/Delay-4 (n=5). Animals were sacrificed by decapitation between 8–10 AM, with animals across all groups sacrificed in a given session. Brains were rapidly removed, hippocampi dissected, and frozen on dry ice. Tissue was stored at −70°C until processing for protein using the E-Max ELISA kit (Promega, WI, USA) as described previously (Berchtold et al., 2005). Briefly, tissue was homogenized in lysis buffer (18ul/mg tissue) containing 137 mM NaCl, 20mM Tris-HCl (pH 8.0), 1% NP40, 10% glycerol, and a cocktail of proteinase inhibitors (complete mini EDTA-free proteinase inhibitors, Roche, 1 tablet/10 ml). Homogenized samples were diluted in 2 volumes DPBS buffer (0.2g KCl, 8.0g NaCl, 0.2g KH2PO4, 1.15 g NA2HPO4, 654 ul 1M MgCl2, 905 ul 1M CaCl2) and centrifuged for 3 minutes at 14,000 r.p.m. at 4°C. Supernatant was collected and a bicinchoninic acid (BCA) assay run to determine protein concentrations, according to manufacturer s recommendations (Pierce Chemical, Rockford, Illinois). Protein concentrations were adjusted to 2 ug/ul with lysis buffer, followed by dilution to 1.5 ug/ul with block and sample buffer (B&S buffer, supplied with ELISA kit). Before samples were loaded, 96-well flat-bottomed Immulon-2 plates (DYNEX Technologies) were incubated overnight at 4°C with carbonate-coating buffer containing anti-BDNF monoclonal antibody. Samples were diluted 1:2 in B&S buffer, loaded onto the plate (100 ul/well) and incubated for 2 h at room temperature with shaking. A standard curve was established using serial dilutions of known amounts of BDNF ranging from 0–500 pg/ml, diluted in B&S buffer. Plates were washed 5X with TBST (20mM Tris-HCl, 150 mM NaCl, 0.05% Tween-20), followed by incubation with anti-human BDNF polyclonal antibody, 5 washes with TBST, and 1 h incubation with horseradish peroxidase. Enzyme solution (1:1 TMB and peroxidase substrate) was prepared 1 h in advance and subsequently incubated on the plate for 10 min. After the samples turned blue, the reaction was stopped with phosphoric acid and absorbance was read at 450 nm using a plate reader. The R-value for the standard curve was consistently ≥0.99. All sample values were in the linear range of the standard curve, with values from sedentary animals falling in the range of 25–45 pg/ml (~3.46 ± 0.9 ng/mg tissue). Samples were run in triplicate and BDNF standards in duplicate on 96 well plates, with 27 samples run on a given plate. Because not all 45 samples could be run on one plate, each plate was run with standards and sedentary samples serving as internal plate controls. Data obtained from each plate were normalized to the sedentary samples run on the given plate, and data expressed as percentage of sedentary were combined across plates.
Data Analysis
Errors and latency to find the platform were averaged across the 5 trials/block and were analyzed using repeated measures ANOVA across all 6 training blocks, and by 1-way ANOVA for each training block or day across groups, followed by Fisher test for post hoc analysis. Failures were assessed only on day 1 (blocks 1 and 2), using 1 way ANOVA across groups. For the probe trial, time spent in the distal 2/3 of each arm was recorded. Values for time spent in the 2 arms adjacent to the target arm, or the 2 diagonal arms, were averaged. Percentage time in each arm (target, adjacent, diagonal, opposite) was calculated as the ratio of “time in arm” divided by “time spent in all arms”. Probe trial data were analyzed by repeated measures ANOVA across arms, and by 1-way ANOVA for the target arm followed by Fisher test for post hoc analysis. BDNF protein values were obtained in triplicate for each sample, and triplicates were averaged to obtain one value per sample. Data were normalized relative to sedentary controls and analyzed by one-way ANOVA, followed by Fisher test for post hoc analysis (Statview 5.0.1). To assess potential relationships between BDNF protein and cognitive performance, group average values for BDNF protein were plotted versus (1) the change in errors over days (day 1 minus day 3 errors), (2) the change in errors over blocks (block 1 minus block 6 errors), and (3) the percentage time spent in the target arm in the probe trial on day 4. BDNF protein values were used as the independent variable, predicting cognitive performance. Because a relationship between BDNF protein and performance would not necessarily be expected to be linear, particularly as BDNF levels become elevated, log and polynomial regression lines were fitted to the data points, and were tested for significance using F-tests.
RESULTS
RWM
To determine if exercise influenced the acquisition of the RWM task relative to sedentary animals, and to determine if the effects of exercise were altered by the introduction of a delay period between the end of exercise and cognitive training on the RWM task, latency to reach the platform location and errors committed during acquisition were compared across treatment groups. In addition, a probe trial was conducted at the end of training to evaluate the strength of the memory for the platform location, and to evaluate how this was modulated by exercise experience.
Shortest latency to platform seen in animals with a 1 or 2 week delay between exercise and cognitive testing
All groups learned the task, as evidenced by consecutively shorter latencies across days (repeated measures ANOVA across days 1–3 (F(3,2)=149.53, p<0.0001). Repeated measures ANOVA across days 1–3 indicated a significant effect of group (F(3,39)=4.016, p=0.0139), with post-hoc analysis revealing significant differences between animals that underwent cognitive training after 1 week delay (EX/delay-1) versus SED (p<.0025) and versus EX (p=0.0318). Analysis of latency by day (Figure 1A) revealed a significant group effect on day 1 (F(3,84)=3.043, p=0.033), day 2 (F(3,84)=5.036, p=0.003), and day 3 (F(3,84)=3.298, p=0.024). Post hoc analysis revealed significant differences between SED and EX/delay-1 animals on day 1 (p=0.005), day 2 (p=0.005), and day 3 (p=0.048) and between SED and EX/delay-2 animals on day 3 (p=0.05). In addition, there were significant differences between EX and EX/delay-1 animals on day 1 (p=0.033) and day 2 (p=0.007), but not on day 3 (Figure 1A).
Repeated measures analysis of latency by block revealed a significant effect of group (repeated measures ANOVA across blocks 1–6: (F(3,39)=4.016, p=0.0139), with post-hoc analysis revealing significantly shorter latency in EX/delay-1 (p=0.0001) and EX/delay-2 (P=0.043) relative to sedentary controls. EX/delay-1 animals also showed significantly shorter latencies across trials relative to EX (p=0.006). Analysis of latency by block revealed no differences between SED and EX (Figure 1B). In contrast, post-hoc analysis revealed that EX/delay-1 animals had significantly shorter latencies than SED animals already on day 1 (block 1, p=0.05; block 2, p=0.007), maintained significantly shorter latencies vs SED throughout training on day 2 (block 3, p=0.008; block 4, p=0.028), and showed a trend for significant shorter latencies vs SED on day 3 (block 5, p=0.069, block 6, p=0.085) (Figure 1C). EX/Delay-1 animals also had shorter latencies than EX animals on block 2 (p=0.002), block 3 (p=0.028), and block 4 (p=0.018). EX/delay-2 animals showed significantly shorter latency than SED animals only on block 6 (p=0.042) (Figure 1C) and no significant differences from EX animals.
Overall, animals that were trained on the cognitive task 1 or 2 weeks after exercising showed the shortest latency to the platform across training blocks, an effect that was particularly notable in the 1-week delay group.
Fewest errors in exercising animals
Repeated measures ANOVA across days revealed that all groups showed a significant decrease in errors across days, indicating that all groups learned the task (F(3,2)=63.374, p<0.0001), with a significant interaction between groups across days (F(3,6)=2.337, p=0.039). Analysis of errors by day (2 blocks/day) revealed that there were significant differences in error rates amongst the groups which were apparent already on day 2 (F(3,84)=3.316, p=0.023) and remained present on day 3 (F(3,84)=3.316, p=0.02). Post hoc analysis identified significantly fewer errors on day 2 in EX/delay-1 vs SED (p=0.004), EX/delay-1 vs EX (p=0.008), and EX/delay-1 vs EX/Delay-2 (p=0.023) groups (Figure 1D). In addition, post hoc analysis revealed that all exercise groups committed significantly fewer errors than SED animals on day 3 (SED vs EX, p=0.009; SED vs EX/Delay-1, p=0.013; SED vs EX/Delay-2, p=0.04) (Figure 1D). Post-hoc analysis of errors by block additionally showed significantly fewer errors by EX/Delay-1 animals relative to EX animals on block 3 (p=0.046) and a trend for decreased errors on block 4 (p=.069), fewer errors in Ex/Delay-1 relative to SED animals on block 3 (p=0.064), block 4 (p=0.043) and block 6 (p=0.038), and fewer errors in Ex/Delay-1 animals relative to EX/Delay-2 animals on block 3 (p=0.046). All exercising groups committed significantly fewer errors than SED animals on block 6 (SED vs EX, p=0.011; SED vs. EX/Delay-1, p=0.043; SED vs. EX/Delay-2, p=0.05) (Figure 1E,F).
Interestingly, post-hoc analysis of errors by block revealed a non-significant trend for more errors on block 1 in EX animals compared to SED (p=0.078), but this performance trend was reversed by block 6, where EX animals committed significantly fewer errors than SED animals (p=0.01), indicating better performance in EX animals by the end of training (Figure 1E). Detailed analysis of the differences between EX and SED animals on block 1 revealed that the greater number of errors by the exercise group on block 1 was attributable to a fundamentally different response of EX and SED animals to the novel experience of the water maze. Specifically, while EX animals vigorously explored the maze and entered many arms searching for a possible escape, SED animals tended to “freeze” and swim in circles in the center of the maze without making a decision to enter the maze arms. Notably, analysis of the “failure” rate (failure to locate the escape platform in a 60 second trial) revealed that SED animals had significantly more failures than EX animals selectively in block 1 (p=0.014)(Figure 1G).
Overall, animals that exercised made fewer errors than sedentary animals across training. Notably, across all treatment groups, a 1-week delay between exercise and cognitive training resulted in accelerated acquisition of the RWM task.
Exercised animals show better retention in the probe trial
To assess the strength of the memory for the escape platform location (e.g., the target arm), a 60 second probe trial was administered on day 4 of behavioral testing, in the absence of the escape platform. Repeated measures ANOVA across all maze arm options (target, adjacent, diagonal, opposite) revealed a significant preference for the target arm across groups (F(3,3)=99.648, p<0.0001) (Figure 1H), with a trend for a group effect (F(3,41)=2.3, p=0.092), and an interaction between maze arm and group (F(3,9)=1.988, p=0.046). In addition, there was a tendency for all three exercise groups to spend a greater percentage of time searching in the target arm, compared with the percentage of time spent by the SED group. Of all the groups, the EX group spent the greatest proportion of time in the target arm. (Figure 1H). The EX group spent significantly more time in the target arm than did the SED group (p=0.013), while there was no significant difference in the percentage time spent in the target arm between EX versus EX/Delay-1 (p=0.44) or versus EX/Delay-2 (p=0.478) (Figure 1H). While there was no significant difference between the three exercise groups, the effect size was greatest in animals with no delay before cognitive testing (47.9%) and was somewhat smaller after a delay of 1 week (30.8%) or 2 weeks (31.4%). Retrospective power analysis indicates that the difference in time spent in the target arm (between EX vs EX/delay-1 and EX/delay-2) would likely be significant with increased sample sizes of ~35 animals/group. These data suggest that the recency of the exercise stimulus impacts the strength of retention/recall. Overall, consistent with the trends shown for superior acquisition (eg, fewer errors and shorter latency) of the RWM task by exercising animals, exercised animals also showed enhanced memory for the location of the escape platform.
BDNF protein remains elevated after exercise ends
We next investigated how exercise and subsequent delays after exercise affect availability of BDNF protein in the hippocampus, because BDNF is thought to be a central molecule involved in hippocampal-dependent learning, and because BDNF availability is increased following exercise. BDNF protein levels were evaluated in a new set of SED, EX, EX/delay-1, and EX/delay-2 animals that had not undergone cognitive training in the RWM, as cognitive training itself also modulates BDNF availability. In addition, for a more complete timecourse of BDNF protein stability after exercise, BDNF protein was additionally evaluated at 3 and 4 weeks delay after the end of exercise.
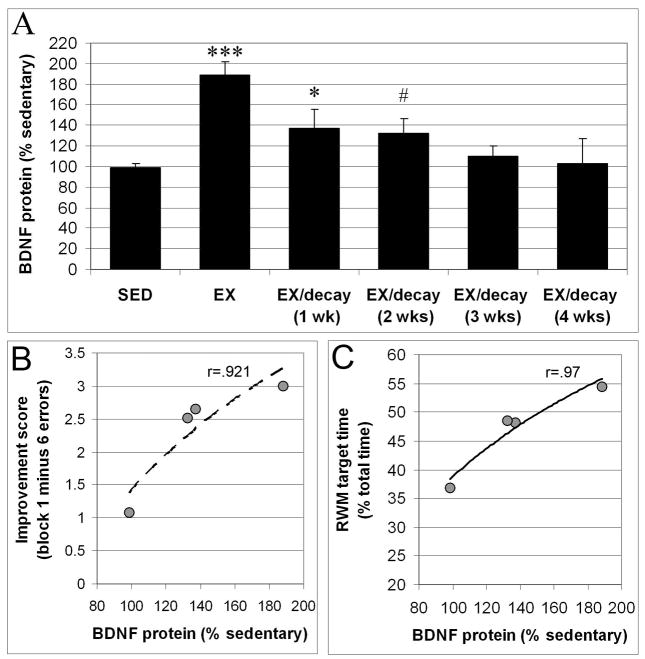
Comparison of BDNF protein levels across groups revealed significant differences between groups (F(5,60)=12.032, p<0.0001). Post-hoc analysis revealed that BDNF levels were significantly elevated over sedentary levels (Figure 2A) in the EX (188% of SED levels, p<0.0001) and EX/delay-1 (137% of SED levels, p=0.031) groups, with a trend toward significance in the SED vs. EX/delay-2 comparison (133% of SED levels, p=0.075). BDNF protein was no longer significantly elevated at 3 and 4 weeks after the end of exercise (respectively, 109% and 103% of SED levels). Thus, while BDNF protein remained elevated for some time after exercise ended, BDNF was not maintained at the same level of induction as seen immediately at the end of the 3-week exercise period. BDNF levels were significantly reduced from peak levels already at 1-week after the end of exercise (p=0.008) and at all longer timepoints (2 wks, p=0.007; 3 and 4 wks, p<0.0001) (Figure 2A).
Figure 2.
BDNF protein levels are elevated after 3 weeks of exercise, progressively decline after exercise ends, and correlate with performance in the radial water maze. While reduced from peak levels at the end of exercise, BDNF protein levels remain elevated over sedentary levels for several weeks after exercise has ended (A). Regression analysis reveals that higher BDNF protein levels correlate with better “improvement scores” (errors(block 1) – errors(block 6)) (B) and with longer time searching in the target arm in the memory probe trial on day 4 (C). Log curves were fitted to the regression data. Values shown are averages ± sem (significance vs SED: * p≤0.05, *** p≤0.0001, # p=0.075).
BDNF protein and relation to cognitive performance
The timepoints at which BDNF protein was measured in this second set of animals, e.g., immediately after the end of exercise and after 1 and 2 weeks delay, corresponded to the timepoints where cognitive testing was initiated in the first group of animals. We next evaluated how BDNF availability at the onset of cognitive testing correlated with improvement across training days and performance in the memory probe trial on day 4. Log regression lines were fit to the data points, revealing a strong relationships between BDNF levels and improvement score over days (errors(day 1) - errors(day 3)) (r(4)=.948, p=.05) or blocks (errors(block 1) - errors(block 6)) (r(4)=.921, p=.0789)(Figure 2B), and between BDNF availability with preference for the target arm in the probe trial on day 4 of testing (r(4)=.97, p=0.02)(Figure 2C). Interestingly, for the relation between BDNF protein level and improvement score (by blocks), the improvement score showed a steep response to small increases in BDNF (e.g., in the range of 100–140% of sedentary BDNF levels), and then appeared to flatten out with more elevated BDNF levels (Figure 2B), suggestive of a ceiling effect. Indeed, the mathematical model best fitting to the data is a second-degree polynomial, yielding a correlation coefficient r=1, significant at p=0.0038. Overall, these data suggest that BDNF availability at the onset of cognitive testing is related to the overall acquisition of a new cognitive task, and may be particularly important in determining the strength of recall in memory probe trials.
DISCUSSION
This study investigated in mice if voluntary exercise experience influenced the acquisition of the RWM, a hippocampal-dependent cognitive task, and if the effects of exercise on cognitive performance were altered by the introduction of a delay period between the end of exercise and cognitive training. In parallel, hippocampal BDNF protein levels were assessed to evaluate the time-course of stability of exercise-induced BDNF after exercise has ended, and to assess the relationship between BDNF level and cognitive task.
Exercise and subsequent delays improve cognitive performance
Overall, all exercised groups showed improved performance on the RWM relative to sedentary animals, with faster acquisition as well as stronger memory performance during the probe trial. Unexpectedly, introduction of a delay between exercise and cognitive training improved acquisition of the task over the benefits observed when cognitive training occurred immediately after the 3-week exercise exposure. This effect was particularly notable in animals with a 1-week delay between exercise and cognitive training, which showed the fastest acquisition rate of all groups, although all exercised groups reached the same performance criteria on day 3 of training. Consistent with the trends for superior task acquisition by exercising animals, exercised animals also showed enhanced memory for the location of the escape platform relative to sedentary animals, with slight weakening of the memory in animals that had a delay between exercise and cognitive training. These results demonstrate that the cognitive benefits of exercise endure for several weeks after exercise has ended, and further suggest that beneficial effects of exercise on brain plasticity continue to evolve for a time after exercise has ended, with potentially different benefits for acquisition/consolidation versus memory/retrieval. It should be noted that the EX group had continued access to running wheels during cognitive testing, in order to avoid potentially introducing stress associated with a changed environment immediately prior to cognitive testing. It is possible that concurrent exercise in the cognitive testing period could have produced some acute effects on cognition, which slowed task acquisition but resulted in stronger memory performance in these animals.
Effects of exercise on synaptic molecular pathways have a temporal dependence
It is well established that exercise is a powerful stimulus that regulates numerous molecular and signaling endpoints in the brain, providing multiple pathways by which exercise can modulate cognitive function and brain health (Tong et al., 2001, Molteni et al., 2002, Ding et al., 2006b, Cotman et al., 2007, Opii et al., 2008, Stranahan et al., 2008). Investigation of the response of plasticity-related genes in the hippocampus has revealed various temporal patterns of gene induction in response to exercise (Tong et al., 2001, Molteni et al., 2002). For example, while CREB and CaMKII gene expression is already induced by 3 days of exercise, induction of MAPKI and MAPKII gene expression is slower, rising significantly only after 7 days of exercise (Molteni et al., 2002). In parallel, CREB phosphorylation and activation by exercise is rapid (already after 1 day of exercise) while activation of MAPK signaling is delayed relative to CREB, but remains detectable longer, even after 1 month of exercise (Shen et al., 2001).
Similar to the temporal pattern of gene induction in response to exercise, our data reveal that a time-dependent pattern of molecular response is present after exercise ends. We demonstrate in mice that BDNF protein shows robust upregulation at the end of the exercise period, then declines somewhat but remains elevated over sedentary levels for several weeks, and eventually returns to baseline levels 3–4 weeks after exercise ends. These data are in agreement with our previous findings in rats that BDNF protein remains elevated after exercise ends (Berchtold et al., 2005). Emerging data strongly indicate that BDNF is a major player in the mechanisms governing the dynamics of memory formation and storage (reviewed in (Bekinschtein et al., 2008a)). It is possible that the modest decline in BDNF availability at 1 and 2 weeks after exercise is related to the somewhat smaller effect size in the probe trial performance for delay animals, relative to animals that underwent cognitive training when BDNF was at peak levels. Indeed, the pattern of BDNF availability at the onset of cognitive testing across the 4 treatment groups corresponded closely to the improvement in performance across acquisition days well as performance in the memory probe trial on the last day of cognitive testing. These data suggest that BDNF availability at the onset of cognitive testing is related to acquisition of a new cognitive task and may be particularly important in determining the strength of recall in memory probe trials.
In addition to BDNF, it is probable that other molecules and signaling pathways regulated in the hippocampus by exercise undergo distinct temporal patterns of decay after exercise ends. Little is known about the post-exercise stability of endpoints other than BDNF. It has previously been reported that hippocampal BDNF and NGF protein levels are below control levels 6 weeks after exercise has ended (8 wks swimming, 5x/wk) (Radak et al., 2006), and that exercise-dependent stimulation of neurogenesis has ceased 3 weeks after exercise has ended (Kitamura et al., 2003), but more detailed timecourses of these endpoints have not been assessed. Because BDNF may play a role in exercise-dependent effects on genesis and survival of new neurons in the hippocampus, it will be of interest to determine how rapidly exercise effects on neurogenesis decline after exercise ends. In addition, because enhanced neurogenesis may be involved in exercise benefits to cognition, it will be of interest to evaluate how the timecourse of decay for neurogenesis correlates with exercise effects on different aspects of cognition. While the current study did not investigate neurogenesis, the profile of decay for neurogenesis will need to be defined in future studies in order to address these important questions. Overall, it is likely that the profiles of decay of the various endpoints regulated by exercise define the relative cognitive gains that persist after exercise ends, such as the greater benefits to acquisition than to recall observed in this study.
Benefits of a delay may differentially affect mechanisms for acquisition/consolidation vs. recall
A recent concept emerging from the learning and memory field is that different molecular mechanisms mediate the acquisition, consolidation, storage and recall phases of cognition. For example, CREB activity is essential to consolidation of learning, but not to recall (Guzowski and McGaugh, 1997). Interestingly, enabling plasticity mechanisms involved in acquisition may actively interfere with recall, as suggested by a recent study demonstrating that chronic enhancement of hippocampal CREB activity interfered with the retrieval of spatial information (Viosca et al., 2009). In addition to CREB, BDNF is implicated as a critical factor in multiple phases of cognition, including acquisition, memory consolidation/storage, and memory retrieval, based on multiple studies where BDNF availability or signaling were blocked, via hippocampal or ventricular infusion of antisense oligonucleotides to BDNF or function blocking antibodies (Mizuno et al., 2000, Bekinschtein et al., 2008a, Bekinschtein et al., 2008b, Slipczuk et al., 2009).
The concept that different molecular mechanisms have unique contributions to the various phases of cognition has also been observed in studies assessing signaling mechanisms mediating exercise benefits to cognition. For example, blocking hippocampal insulin-like growth factor (IGF) signaling during exercise has no detrimental effect on post-exercise acquisition of the morris water maze (MWM), but prevents exercise-enhancement of recall in the probe trial (Ding et al., 2006a). In contrast, blocking hippocampal BDNF signaling during exercise impairs both post-exercise acquisition as well as recall in this same task (Vaynman et al., 2004). Interestingly, blocking hippocampal CamKII signaling during exercise actually results in faster MWM task acquisition than normally seen with exercise, but prevents the exercise-dependent improvement in recall in the probe trial (Vaynman et al., 2007). As the field evolves, additional molecules and signaling pathways with unique roles in specific phases of learning and memory and exercise-enhancement of cognition will undoubtedly be discovered.
The emerging evidence that different phases of learning and memory have different molecular events associated with them may help explain the phenomenon we observed in this study. Namely, introducing a delay between exercise and cognitive training preferentially facilitated acquisition of the RWM task beyond the improvement normally seen with exercise, but did not further improve memory performance in the probe trial. These data suggest that the molecular mechanisms associated with acquisition and recall evolve differently during the delay period. Plasticity mechanisms important in acquisition/consolidation appear to undergo a potential maturing and optimization in the delay period after exercise, while the mechanisms important in modulating the strength of recall are relatively unaffected, or undergo a slight decay.
Introduction of a delay between exercise and cognitive testing unveils new concepts in the exercise-learning field
Assessment of the effects of exercise on cognitive function with a delay after exercise is a novel approach that has not previously been described in the literature. Importantly, this approach introduces new concepts into the exercise-learning field, in particular with respect to the benefits of exercise on different aspects of cognitive function and how plasticity mechanisms activated by exercise evolve after exercise ends. Further, the concept that plasticity mechanisms evolve after exercise ends and can preferentially enhance unique aspects of memory formation is particularly exciting, given the accumulating evidence that molecular events taking place hours or days after learning are proving to be critical in memory storage/retrieval. It will be important to next elucidate how the various genes and molecular pathways regulated by exercise respond temporally after exercise ends, to define their relationship to exercise-enhancement of cognition, and in particular, to determine the roles of each signaling system in the different phases of learning and memory. Ultimately, application of this approach provides the opportunity to investigate the temporal windows of the effects of exercise on brain function at the behavioral, cellular, and molecular levels.
Acknowledgments
Funding for this research was provided by RO1 AG034667-01 to C.W.C from the N.I.A..
ABBREVIATIONS
- BDNF
brain-derived neurotrophic factor
- CaMKII
Ca2+/calmodulin-dependent kinase
- CREB
cAMP response element binding protein
- IGF
insulin-like growth factor
- MAPK
mitogen activated protein kinase
- MWM
morris water maze
- RWM
radial arm water maze
- trkB
tyrosine kinase B receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. Jama. 2004;292:1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- Alamed J, Wilcock DM, Diamond DM, Gordon MN, Morgan D. Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nature protocols. 2006;1:1671–1679. doi: 10.1038/nprot.2006.275. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Izquierdo I, Medina JH. BDNF and memory formation and storage. Neuroscientist. 2008a;14:147–156. doi: 10.1177/1073858407305850. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, Izquierdo I, Medina JH. BDNF is essential to promote persistence of long-term memory storage. Proceedings of the National Academy of Sciences of the United States of America. 2008b;105:2711–2716. doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C, Berchtold N, Christie L. Exercise builds brain health: An interplay of central and peripheral factors. Trends Neurosci. 2007;30:464072. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:292–298. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Cui L, Hofer T, Rani A, Leeuwenburgh C, Foster TC. Comparison of lifelong and late life exercise on oxidative stress in the cerebellum. Neurobiology of aging. 2007 doi: 10.1016/j.neurobiolaging.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006a;140:823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Souda P, Whitelegge JP, Gomez-Pinilla F. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. The European journal of neuroscience. 2006b;24:1265–1276. doi: 10.1111/j.1460-9568.2006.05026.x. [DOI] [PubMed] [Google Scholar]
- Ding YH, Li J, Zhou Y, Rafols JA, Clark JC, Ding Y. Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Current neurovascular research. 2006c;3:15–23. doi: 10.2174/156720206775541787. [DOI] [PubMed] [Google Scholar]
- Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. The Journal of comparative neurology. 2005;486:39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Erickson K, Kramer AF. Exercise effects on cognitive and neural plasticity in older adults. British journal of sports medicine. 2008 doi: 10.1136/bjsm.2008.052498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Dorey AA, Fleshner M. Therapeutic effects of exercise: wheel running reverses stress-induced interference with shuttle box escape. Behavioral neuroscience. 2007;121:992–1000. doi: 10.1037/0735-7044.121.5.992. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McGaugh JL. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabour OF, Alzoubi KH, Alomari MA, Alzubi MA. Changes in spatial memory and BDNF expression to concurrent dietary restriction and voluntary exercise. Hippocampus. 2009 doi: 10.1002/hipo.20657. [DOI] [PubMed] [Google Scholar]
- Kirchner L, Chen WQ, Afjehi-Sadat L, Viidik A, Skalicky M, Hoger H, Lubec G. Hippocampal metabolic proteins are modulated in voluntary and treadmill exercise rats. Experimental neurology. 2008;212:145–151. doi: 10.1016/j.expneurol.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Mishina M, Sugiyama H. Enhancement of neurogenesis by running wheel exercises is suppressed in mice lacking NMDA receptor epsilon 1 subunit. Neurosci Res. 2003;47:55–63. doi: 10.1016/s0168-0102(03)00171-8. [DOI] [PubMed] [Google Scholar]
- Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. Journal of Neuroscience. 2000;201:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. The European journal of neuroscience. 2002;16:1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW. Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. 2009;5:287–294. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol KE, Parachikova AI, Cotman CW. Three weeks of running wheel exposure improves cognitive performance in the aged Tg2576 mouse. Behavioural brain research. 2007;184:124–132. doi: 10.1016/j.bbr.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan RM, Ohle R, Kelly AM. The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behavioural brain research. 2007;176:362–366. doi: 10.1016/j.bbr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Opii WO, Joshi G, Head E, Milgram NW, Muggenburg BA, Klein JB, Pierce WM, Cotman CW, Butterfield DA. Proteomic identification of brain proteins in the canine model of human aging following a long-term treatment with antioxidants and a program of behavioral enrichment: relevance to Alzheimer’s disease. Neurobiology of aging. 2008;29:51–70. doi: 10.1016/j.neurobiolaging.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radak Z, Toldy A, Szabo Z, Siamilis S, Nyakas C, Silye G, Jakus J, Goto S. The effects of training and detraining on memory, neurotrophins and oxidative stress markers in rat brain. Neurochemistry international. 2006;49:387–392. doi: 10.1016/j.neuint.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Rogers RL, Meyer JS, Mortel KF. After reaching retirement age physical activity sustains cerebral perfusion and cognition. J Am Geriatr Soc. 1990;38:123–128. doi: 10.1111/j.1532-5415.1990.tb03472.x. [DOI] [PubMed] [Google Scholar]
- Schweitzer NB, Alessio HM, Berry SD, Roeske K, Hagerman AE. Exercise-induced changes in cardiac gene expression and its relation to spatial maze performance. Neurochemistry international. 2006;48:9–16. doi: 10.1016/j.neuint.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Shen H, Tong L, Balazs R, Cotman CW. Physical activity elicits sustained activation of the cyclic AMP response element-binding protein and mitogen-activated protein kinase in the rat hippocampus. Neuroscience. 2001;107:219–229. doi: 10.1016/s0306-4522(01)00315-3. [DOI] [PubMed] [Google Scholar]
- Slipczuk L, Bekinschtein P, Katche C, Cammarota M, Izquierdo I, Medina JH. BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS ONE. 2009;4:e6007. doi: 10.1371/journal.pone.0006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Becker KG, Zhang Y, Maudsley S, Martin B, Cutler RG, Mattson MP. Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice. Neurobiology of aging. 2008 doi: 10.1016/j.neurobiolaging.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Shen H, Perreau V, Balazs R, Cotman CW. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiology of disease. 2001;8:1046–1056. doi: 10.1006/nbdi.2001.0427. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. PNAS. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. The European journal of neuroscience. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. The select action of hippocampal calcium calmodulin protein kinase II in mediating exercise-enhanced cognitive function. Neuroscience. 2007;144:825–833. doi: 10.1016/j.neuroscience.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman SS, Ying Z, Yin D, Gomez-Pinilla F. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain research. 2006;1070:124–130. doi: 10.1016/j.brainres.2005.11.062. [DOI] [PubMed] [Google Scholar]
- Viosca J, Malleret G, Bourtchouladze R, Benito E, Vronskava S, Kandel ER, Barco A. Chronic enhancement of CREB activity in the hippocampus interferes with the retrieval of spatial information. Learning & memory (Cold Spring Harbor, NY) 2009;16:198–209. doi: 10.1101/lm.1220309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. Jama. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Archives of internal medicine. 2001;161:1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]