Abstract
Background
Random biopsy esophageal surveillance can be subject to sampling errors, resulting in diagnostic uncertainty. Optical frequency domain imaging (OFDI) is a high-speed, three-dimensional endoscopic microscopy technique. When deployed through a balloon-centering catheter, OFDI can automatically image the entire distal esophagus (6.0 cm length) in approximately 2 minutes.
Objective
To test a new platform for guided biopsy that allows the operator to select target regions of interest on an OFDI dataset, and then use a laser to mark the esophagus at corresponding locations. The specific goals include determining the optimal laser parameters, testing the accuracy of the laser marking process, evaluating the endoscopic visibility of the laser marks, and assessing the amount of mucosal damage produced by the laser.
Design
Experimental study conducted in five swine in vivo.
Setting
Massachusetts General Hospital.
Main Outcome Measurements
Success rate, including endoscopic visibility of laser marks and accuracy of the laser marking process for selected target sites, and extent of the thermal damage caused by the laser marks.
Results
All of the laser-induced marks were visible by endoscopy. Target locations were correctly marked with a success rate of 97.07% (95% CI, 89.8%-99.7%). Thermal damage was limited to the superficial layers of the mucosa and was observed to partially heal within 2 days.
Limitations
An animal study with artificially placed targets to simulate pathology.
Conclusions
The study demonstrates that laser marking of esophageal sites identified in comprehensive OFDI datasets is feasible and can be performed with sufficient accuracy, precision, and visibility to guide biopsy in vivo.
Surveillance for dysplasia and intramucosal carcinoma in Barrett's patients is accomplished by random, four-quadrant endoscopic biopsy of the involved esophageal segment. Because the random biopsy procedure only evaluates a small fraction of the involved tissue, this procedure can have a high sampling error. Optical coherence tomography (OCT) is an optical imaging modality that provides cross-sectional images of the esophageal mucosa with a resolution (∼10 μm) that permits accurate detection and diagnosis of esophageal pathology.1-4 Due to limitations in image acquisition speed and catheter design, OCT in the esophagus was previously limited to point sampling, where only small areas of mucosa were imaged at any given time. A second-generation form of OCT, termed optical frequency domain imaging (OFDI), is capable of imaging at much higher acquisition rates (100-fold increase), while still preserving the image quality of OCT.5,6 When implemented, by using a balloon-centering catheter with helically scanning optics, OFDI enables a comprehensive microscopy of a 6-cm segment of the distal esophagus in 2 minutes in vivo.7,8
This capability to image the entire distal esophagus at microscopic resolution opens up the possibility of using this technology to guide biopsy. We have developed an OFDI guided biopsy platform that uses a laser to create marks in the superficial esophageal mucosa at sites that correspond to regions of interest in the image dataset. Because these marks are visible by endoscopy, the tissue in the vicinity of the marks may be subsequently biopsied by the endoscopist. Figure 1 presents the principle of operation of the proposed image-guided biopsy platform.
Figure 1.
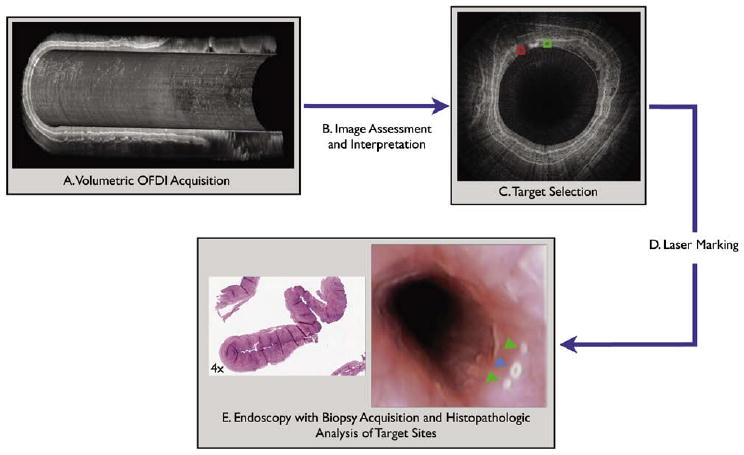
Flow chart outlining the proposed steps required for performing a guided biopsy by using optical microscopy with laser marking capabilities, including (A) comprehensive microscopic image of the esophagus; (B) image data assessment and interpretation; (C) target region of interested selection; (D) computer-controlled laser marking at corresponding sites, which creates superficial white marks around the targets; and (E) endoscopic biopsy at the marked sites and histopathologic analysis. OFDI, optical frequency domain imaging system.
Several of these steps have been demonstrated in previous studies of the OFDI for esophageal imaging. Comprehensive three-dimensional microscopy of the distal esophagus by using OFDI in vivo was demonstrated in both swine8 and patient7 studies (step A), and the accuracy of OCT/OFDI for detecting esophageal pathology relevant to Barrett's esophagus has been demonstrated in multiple studies1,2,4 (steps B and C). The goal of this article is to investigate the accuracy and safety of marking target sites identified on the OFDI datasets by using small laser-induced injuries that are visible by endoscopy (step D).
Materials and Methods
OFDI guided biopsy system
Imaging
The technical details of our clinical balloon-based OFDI systems have been described previously.6,7 In short, the OFDI system generates axial depth profiles by measuring the delay of the source signal as it is reflected by subsurface structures. Volumetric OFDI images are obtained by scanning the imaging beam over the tissue surface in two dimensions. The OFDI system consists of a wavelength swept source centered at 1310 nm with a tuning range of 143 nm resulting in a 6-micron axial resolution in tissue. Cross-sectional esophageal images were acquired at a frame rate of 10 frames/second, with 4096 A-lines per frame. An 18-mm diameter, balloon-centering catheter was used in this study, with the imaging parameters previously described, and resulted in a transverse image resolution of 13.8 μm. Helical pullback scanning was conducted through the balloon-centering catheter at a pullback rate of 0.5 mm/second, resulting in a longitudinal image-image spacing of 50 μm. With these parameters, comprehensive volumetric imaging of a 6-cm segment of distal esophagus was captured in 2 minutes.
Laser marking
We have modified the OFDI system to provide tissue irradiation at 1480 nm through the same optical probe used for imaging. This wavelength was selected due to the relatively high absorption of water at this wavelength and because it could be coupled through the same rotary junction and catheter without significant power loss. A schematic of the optical layout of the OFDI laser marking system is provided in Figure 2. The marking laser light and the OFDI imaging light were combined by using a wavelength division multiplexer prior to the fiber optic rotary junction. The marking laser power was set to provide 380 mW at the tissue surface with a beam spot size of 60 μm. A digital signal output from the OFDI system console was used to turn the marking laser on and off.
Figure 2.
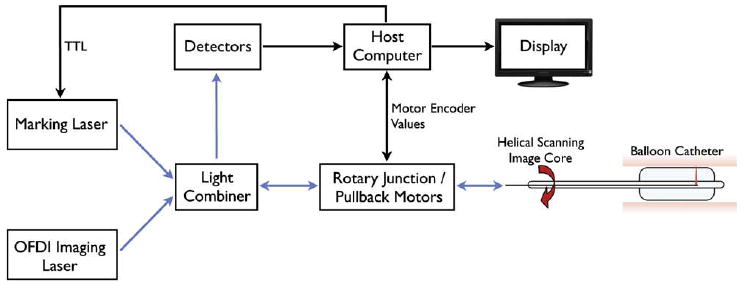
Schematic of the optical frequency domain imaging (OFDI) system with guided biopsy. Both the marking laser and the OFDI imaging source are launched into the sample arm of the OFDI interferometer and are transmitted the tissue surface through the optical rotary junction and imaging catheter. TTL, •••.
OFDI guided biopsy procedure
To demonstrate the OFDI-guided laser marking process, we first performed OFDI imaging with the balloon-centering catheter. During helical scanning, electronic signals (motor counter values) from the pullback and rotational motor controllers, that corresponded to the longitudinal and rotational position of the catheter, were digitized and associated with each A-line in the image dataset. The acquired OFDI image data was displayed on a computer monitor at a rate of approximately 5 frames/second. This display rate was sufficient to detect targets in real time. With identification of a target region of interest, the longitudinal pullback of the imaging catheter was stopped. Circumferential OFDI imaging continued, but the rotational speed of the catheter was reduced to approximately 1 Hz to increase positional accuracy. Then, the longitudinal position of the inner catheter optics was adjusted so that the target was within the field of view. This step was implemented to compensate for possible movement of the balloon relative to the esophagus as a result of peristalsis, as was previously described in our ongoing clinical studies.7 Other motion artifacts due to physiological functions, such as respiration, were not observed. To determine the coordinates for laser marking, the user clicked the image on either side of the target (ie, red box in Fig. 1C). Using the counter values and knowledge of the A-line where the box was placed on the image, the computer sent signals to the rotational motor controller to stop scanning and to position the catheter optics so that they would direct the laser to the indicated position on the esophagus. Once the catheter was in position, a button on the screen was pressed and the computer sent signals to activate the marking laser. The catheter was then repositioned to the opposite side of the target (ie, green box in Fig. 1C) and a second mark was made in the same manner. This process was repeated for each target.
Animal studies
A total of five Yorkshire swine (48 to 52 kgs) were imaged in this study, both to determine the required laser marking parameters (n = 2) and to determine the success rate of the laser marking guided biopsy imaging platform (n = 3). This study protocol was approved by and carried out in accordance with the regulations set forth by the Massachusetts General Hospital Subcommittee on Research Animal Care. The swine were anesthetized, intubated, and ventilated by using standard methods. An upper endoscopy procedure (Pentax EG-2930 endoscope; Pentax EPM-3300 video endoscopy system) was then performed on each of the swine, at which time an endotracheal tube was placed to facilitate ease of insertion and removal of the OFDI balloon catheter. In our ongoing clinical studies, we position the balloon catheter at the gastroesophageal junction, using a rapid exchange guidewire provision at the distal tip of the catheter.7 In the current swine study, however, the balloon catheter was placed in the esophagus parallel to the endoscope. Once positioned in the desired location, the endoscope was removed, the balloon was inflated, and the experimental procedure commenced. The balloon catheter acted to dilate the esophagus and help stabilize the imaging probe with respect to the esophageal wall. The stabilization imparted by the balloon was sufficient for imaging/marking and other methods, such as pharmaceutical agents, which were not required to further reduce motion caused by peristalsis. At the completion of the laser marking procedure, the catheter was withdrawn, and the endoscope was introduced to evaluate the visibility of the laser marking process. Two of the five swine were sacrificed 48 hours after the marking procedure to determine the tissue response to the laser injury. All other swine were sacrificed immediately after the imaging procedure.
Experimental procedure
Determining optimal exposure parameters
The initial step of this study was to determine the optimal exposure duration, considering both clear endoscopic visualization of the laser marks and minimization of tissue damage. The OFDI balloon catheter was inserted and inflated proximal to the gastroesophageal junction. Four-quadrant 380 mW laser markings were made on the esophageal mucosa of two swine in increasing exposure durations (0.5, 1, 2, 5, and 10 seconds) at 1-cm intervals within the 6-cm imaging window of the balloon catheter. At the completion of the laser marking, the balloon catheter was removed and the video endoscopy of the laser markings was recorded.
Evaluating the success rate of the laser-marking method
After determining the optimal marking of laser exposure durations, the success rate of the guided biopsy platform was evaluated in three swine. The first targets were generated. The purpose of the marks was to act as targets to simulate areas of possible esophageal pathology that may be found in the clinical setting. The OFDI balloon catheter was inflated proximal to the gastroesophageal junction and a number of laser marks (between 5 and 16) were placed randomly through the balloon by manually translating the catheter pullback position and selecting random rotational angles. A total of 68 targets were made in the three swine.
After the generation of the targets, the imaging core was advanced, engaged, and OFDI imaging commenced. During OFDI, the targets were located and marked according to the procedures previously described. Because the entire esophagus was used for this experiment, with a length that greatly exceeded the balloon's length (6 cm), the balloon catheter was deflated, repositioned, and reinflated at a different location in the esophagus, and the experimental procedure was repeated. Once all of the targets were identified and marked, comprehensive esophageal OFDI imaging was once again performed. Because the marks and targets can be clearly seen by OFDI (Fig. 3), quantitative parameters related to marking efficacy, including the presence or absence of two marks around the target, and the mean distance between the target and marks, were measured from the OFDI dataset acquired after laser marking. After the final OFDI imaging, the catheter was removed, and the video endoscopy was conducted. For each target, the number of visible marks was recorded by the endoscopist (N.S.N). For each target, the marking was determined to be successful only if both marks were (1) adjacent to, and on either side of, the targets, (2) within 4 mm of the targets, and (3) visible by endoscopy. The success rate was defined as the ratio between the number of targets and marking sets that satisfied the three criteria above and the total number of targets.
Figure 3.
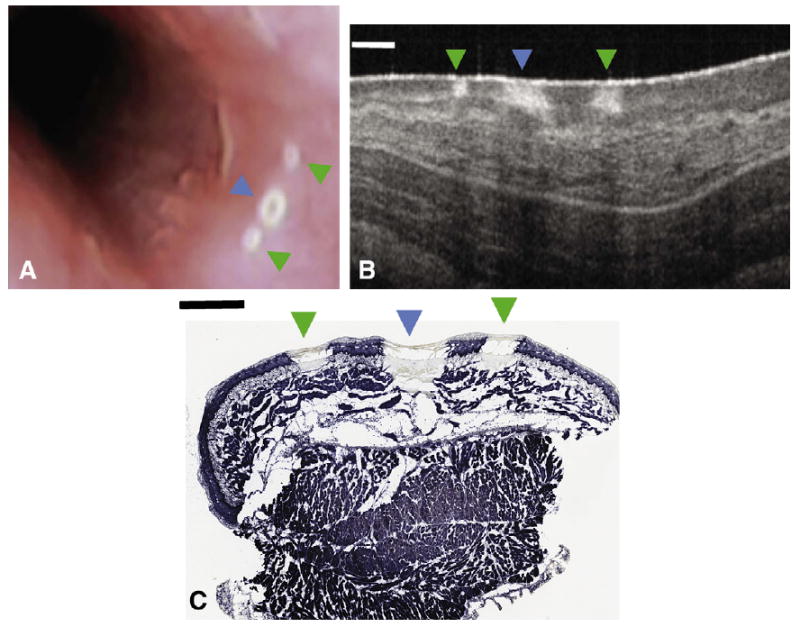
A video-endoscopy image (A) and cross-sectional optical frequency domain image (B) obtained in vivo, and the corresponding nitro-blue tetrazolium chloride stained histology slide (C) of the target mark (blue arrowhead) surrounded by two guiding marks (green arrowheads). Thermally damaged tissue is apparent through a lack of blue staining. Scale bars represent 1 mm.
Histology processing and analysis
After necropsy the entire esophagus was prosected and frozen, and 20-micron tissue sections were cut every 60 microns through each of the laser markings. The sections were stained by using nitro-blue tetrazolium chloride to differentiate thermally damaged tissue from viable tissue.9 The maximum injury depth was measured on the slide as a percentage of the full thickness of the mucosa measured from the epithelium for each of the laser marks. A total of 13 sets of markings (where a set consists of 1 target and 2 marks) were assessed at day 0; 12 sets were assessed at day 2.
Statistics
Mean and standard deviation values are reported for the distribution of the laser markings with respect to the targets. The performance of the laser marking technique was evaluated based on the visibility of the marks at endoscopy and the positioning of the marks relative to the target. Two-sided confidence intervals were calculated for the laser marking accuracy at a 95% level of confidence. Depth of injury measurements, computed as a percentage of mucosal thickness, were calculated for the nitro-blue tetrazolium chloride histology and mean and standard deviation values are reported.
Results
Optimal exposure parameters
Even though marks generated by the laser at all of the exposure durations were visible on endoscopy, some of the marks produced at 0.5-second and 1.0-second exposure times required close scrutiny to locate. We found that 2-second marks were immediately identified in call cases and selected this exposure duration for the remainder of the study. Although not previously tested, 8-second marks were chosen for the targets. We determined that 8-second markings would provide greater contrast from the 2-second and 5-second exposures, while reducing the injury size and depth resulting from 10-second exposures. Figure 3 shows a video endoscopy view of the 2-second marks surrounding the target.
Success rate of the laser-marking method
All of the laser-induced marks (n = 68×2) were visible by endoscopy. Of the 68 marking pairs, 66 sets had both marks placed within 4 mm of the target. Figure 4 shows the distribution of the laser marks in the order acquired. Excluding the two outliers that were caused by a system software calculation error, which was subsequently corrected, the average distance of the marks from the center of the targets, measured on the OFDI images, was -1.90 ± 0.47 mm for the first set of marks located counter-clockwise to the target and +2.50 ± 0.55 mm for the second set of marks located clockwise to the target. The overall success rate of the laser marking was 97.06% (95% CI, 89.8%-99.7%).
Figure 4.
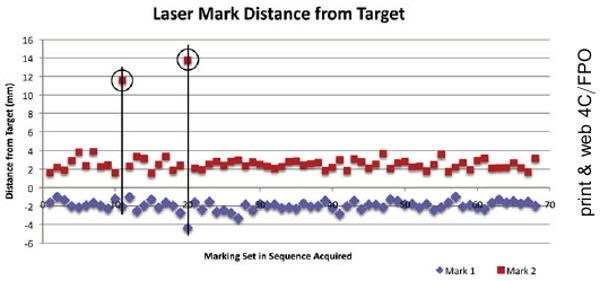
A scatter plot showing the distance of the guiding marks from the target mark position, as determined on the optical frequency domain imaging dataset. The two outliers falling outside the 4-mm range are highlighted (black circle and line).
Safety of the laser marking method
The anatomic depth of thermal injury was calculated on 13 sets of markings (1 set consisting of 2×2-second, and 1×8-second exposures) as described in the Materials and Methods section. The depth of injury for the 2-second marks and the 8-second targets extended to 27.03% ± 4.83% and 38.98% ± 7.15% of the mucosal thickness, respectively, with the majority of damage limited to the epithelium and lamina propria layers. Significant healing was observed in all of the laser markings at day 2 with regeneration of the epithelium and remodeling of the lamina propria and muscularis mucosal layers (Fig. 5).
Figure 5.
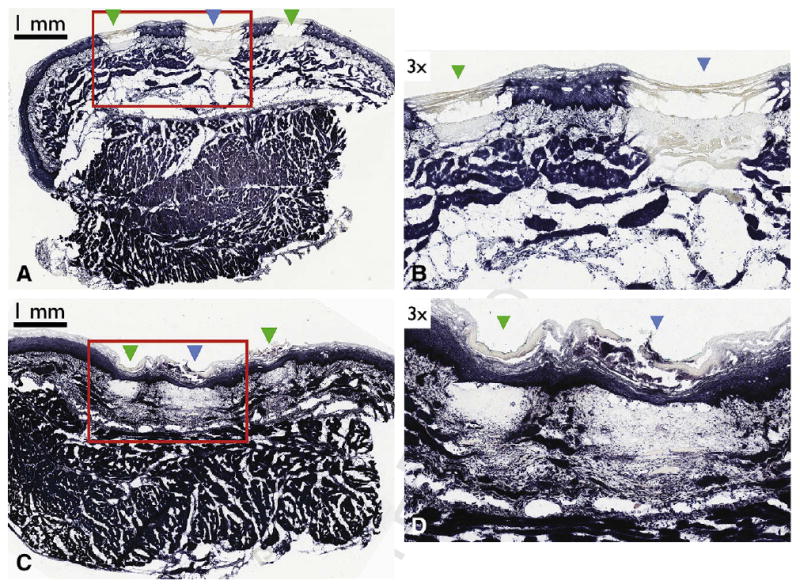
Nitro-blue tetrazolium chloride histology demonstrating depth of injury and healing of the laser-induced targets and marks. (A) Histopathology obtained from a swine sacrificed immediately after laser marking depicting both the target mark (blue arrowhead) surrounded by two guiding marks (green arrowheads). (B) Expanded view of (A). (C) Histopathology obtained from a swine sacrificed at day 2, depicting both the target mark (blue arrowhead) surrounded by two guiding marks (green arrowheads). (D) Expanded view of C. Scale bars represent 1 mm.
Discussion
In this study, we have shown that laser marking is a safe and efficacious method for guiding endoscopic biopsy based on OFDI image data. This guided biopsy method has the potential to increase the diagnostic accuracy of current surveillance protocols, to guide alternative interventional treatments, such as the delineation of margins for mucosal resection, and to allow direct registration of the endoscopic microscopy images with histopathology.
In this study, we identified targets on the fly and marked them in real time. We have also conceived of another mode of operation, which may be more useful clinically, when the image targets are human disease as opposed to artificially generated targets. For this mode of operation, the three-dimensional dataset can be acquired in its entirety, all target regions of interest selected on the computer screen, and then marked en masse. The technology described here is fully capable of operating in this mode.
Although previous studies have demonstrated that OCT is capable of diagnosing esophageal pathology with good accuracy,1-4 in the clinical setting, images of regions that are likely to contain the most severe disease will need to be identified in the GI suite. The development and implementation of automated and semi-automated detection and diagnosis algorithms may additionally help the treating physician to mine the large volumetric OFDI data-sets, thereby reducing the time required for intraprocedural evaluation of the images.10
One criterion that we used to determine the success rate of the guided biopsy platform was the visibility of the laser markings at endoscopy. In this study, the targets were made with laser exposure durations of 8 seconds, producing markings that were more prominent at endoscopy than the 2-second marks used for guiding biopsy. As such, the endoscopy reader may have been biased in determining the visibility of the laser markings by first perceiving the target. Future clinical studies aimed at diagnosing and marking esophageal pathology will be less affected by this interpretation bias.
This study was conducted in normal squamous epithelium. Due to the increased vascularity of columnar epithelium that is typical of specialized intestinal metaplasia, we anticipate that laser marking will be effective for target delineation in Barrett's esophagus. To provide evidence in support of this assumption, we have created 2-second marks on swine duodenum ex vivo (Fig. 6). As can be seen in this figure, 2-second laser marks are clearly apparent in the swine duodenal tissue; however, we have yet to test this marking procedure in Barrett's mucosa. Optimizing the laser parameters for human disease and testing OFDI-guided biopsy in patients is a subject of future research. If we find that this laser does not produce visible marks in human Barrett's esophagus, we can investigate the use of other marking wavelengths that may generate greater video endoscopic contrast. Alternatively, we can register endoscopy to OFDI images by using anatomical and/or artificial landmarks placed on the esophagus (ie, India Ink tattoos) prior to imaging.
Figure 6.
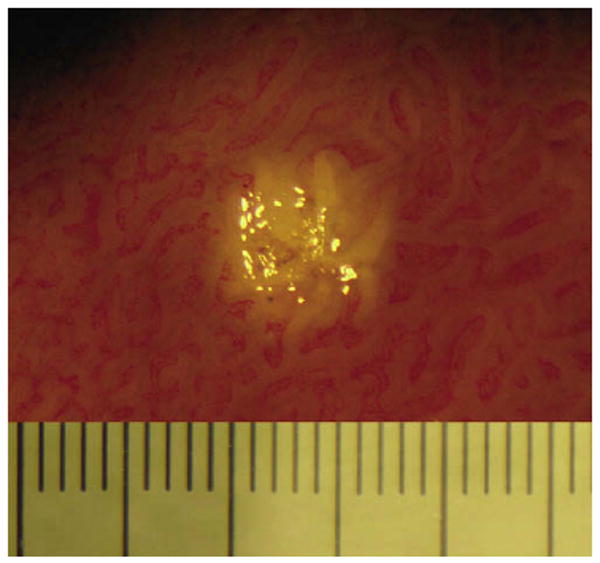
Photograph of swine duodenum depicting a 2-second laser mark performed approximately 1-hour post sacrifice. Large tick marks represent 0.5 mm.
We have previously reported on the use of a 2.5-cm diameter balloon catheter in our clinical studies, however, due to the smaller esophageal diameter of swine, a 1.8-cm diameter balloon was used here. This balloon diameter caused considerable dilation of the esophagus, reducing motion artifacts due to peristalsis and eliminating regions without contact (ie, artifacts that have been observed in our previous clinical studies). Based on an assessment of the datasets acquired in our ongoing clinical studies in which we have imaged approximately 100 patients with our balloon catheter, we do not anticipate that physiological motion, including that caused by peristalsis or respiration, will significantly affect our marking accuracy. To date we have not observed motion artifacts from respiration, however, if this motion becomes problematic we may consider the use of larger or variable diameter balloon catheters to further stabilize the esophageal wall with respect to the balloon catheter. The compression of the balloon, however, may affect diagnostic criteria,11 and may impact the generation of laser marks. These questions will be addressed in future clinical studies designed to test the efficacy of the guided biopsy procedure in patients.
The anatomic depth of the thermal injuries caused by the 2-second laser exposures was measured to extend to 27.03% of the full thickness of the mucosa, with partial healing observed at day 2. Given that the histologic depth of biopsies obtained by using standard forceps has been reported to extend to the muscularis mucosa,12 we anticipate that the risk to the patient undergoing this procedure will be low and not significantly greater than that of standard endoscopic practice.
The overarching goal of this research is to provide endoscopists with a method for guiding biopsies to decrease the sampling errors associated with surveillance. To this end, in addition to being able to accurately mark targets, the technique must be cost-effective and must be capable of being performed within an acceptable procedural time. In our studies, the time required to identify a target and place two marks surrounding the target did not exceed 2 minutes/target. Under the assumption that this technique will decrease the amount of biopsies required, we therefore believe that this entire procedure can be conducted without unduly extending the procedure time. The time required to identify disease targets has not yet been established; however, this is a topic for future studies. A cost-effectiveness analysis is also beyond the scope of this article, but we note that the use of guided biopsies should decrease the expense of surveillance by minimizing the number of biopsies obtained and by providing a more accurate diagnosis, which will result in more appropriate and timely patient management.
Although future clinical studies are required to ultimately determine the clinical viability of this technology, we have demonstrated that OFDI-guided biopsy with laser marking is feasible in the swine esophagus. OFDI-guided biopsy may prove to be a powerful diagnostic tool and, when used as an alternative to conventional random biopsy in the surveillance of Barrett's esophagus, may work to increase the biopsy yield and reduce the total number of biopsies acquired.
Acknowledgments
This work was supported by the National Institutes of Health (grant no. R01CA103769), and by the Center for Integration of Medicine and Innovative Technology (development of the imaging platform). The authors would like to thank William Puricelli, Joseph Gardecki, and Alexander Chau for their assistance in this study.
Abbreviations
- OFDI
optical frequency domain imaging
- OCT
optical coherence tomography
Footnotes
Disclosure: The following author disclosed financial relationships relevant to this publication: Brett E. Bouma received a research grant from Olympus Corporation. All other authors disclosed no financial relationships relevant to this publication.
References
- 1.Evans JA, Poneros JM, Bouma BE, et al. Optical coherence tomography to identify intramucosal carcinoma and high-grade dysplasia in Barrett's esophagus. Clin Gastroenterol Hepatol. 2006;4:38–43. doi: 10.1053/S1542-3565(05)00746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans JA, Poneros JM, Bouma BE, et al. Identifying intestinal metaplasia at the squamocolumnar junction using optical coherence tomography. Gastrointest Endosc. 2007;65:50–6. doi: 10.1016/j.gie.2006.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isenberg G, Sivak MV, Jr, Chak A, et al. Accuracy of endoscopic optical coherence tomography in the detection of dysplasia in Barrett's esophagus: a prospective, double-blinded study. Gastrointest Endosc. 2005;62:825–31. doi: 10.1016/j.gie.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 4.Poneros JM, Brand S, Bouma BE, Tearney GJ, Compton CC, Nishioka NS. Diagnosis of specialized intestical metaplasia by optical coherence tomography. Gastroenterology. 2001;120:7–12. doi: 10.1053/gast.2001.20911. [DOI] [PubMed] [Google Scholar]
- 5.Yun SH, Tearney GJ, de Boer JF, Iftimia N, Bouma BE. High-speed optical frequency-domain imaging. Opt Express. 2003;11:2953–63. doi: 10.1364/oe.11.002953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yun SH, Tearney GJ, Vakoc BJ, et al. Comprehensive volumetric optical microscopy in vivo. Nat Med. 2006;12:1429–33. doi: 10.1038/nm1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suter MJ, Vakoc BJ, Shishkov YP, et al. Comprehensive microscopy of the esophagus in human patients with optical frequency domain imaging. Gastrointest Endosc. 2008;68:745–53. doi: 10.1016/j.gie.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vakoc BJ, Shishkov M, Yun SH, et al. Comprehensive esophageal microscopy by using optical frequency-domain imaging. Gastrointest Endosc. 2007;65:898–905. doi: 10.1016/j.gie.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neumann RA, Knobler RM, Pieczkowski F, Gebhart W. Enzyme histochemical analysis of cell viability after argon laser-induced coagulation necrosis of the skin. J Am Acad Dermatol. 1991;25:991–8. doi: 10.1016/0190-9622(91)70296-e. [DOI] [PubMed] [Google Scholar]
- 10.Qi X, Sivak MV, Isenberg G, Willis JE, Rollins AM. Computer-aided diagnosis of dysplasia in Barrett's esophagus using endoscopic optical coherence tomography. J Biomed Opt. 2006;11:044010. doi: 10.1117/1.2337314. [DOI] [PubMed] [Google Scholar]
- 11.Westphal V, Rollins AM, Willis J, Sivak MV, Jr, Izatt JA. Correlation of endoscopic opitcal coherence tomography with histology in the lower-GI tract. Gastrointest Endosc. 2005;61:537–54. doi: 10.1016/s0016-5107(05)00084-2. [DOI] [PubMed] [Google Scholar]
- 12.Yang R, Naritoku W, Laine L. Prospective, randomized comparison of disposable and reusable biopsy forceps in gastrointestinal endoscopy. Gastrointest Endosc. 1994;40:671–4. [PubMed] [Google Scholar]


