Abstract
Uterine leiomyoma are common, benign tumors that are enriched in extracellular matrix. The tumors are characterized by a disoriented and loosely packed collagen fibril structure similar to other diseases with disrupted Transforming growth factor β (TGF-β) signaling. Here we characterized TGF-β3 signaling and the expression patterns of the critical extracellular matrix component versican in leiomyoma and myometrial tissue and cell culture. We also demonstrate the regulation of the versican variants by TGF-β3. Using leiomyoma and matched myometrium from 15 patients, messenger RNA (mRNA) from leiomyoma and myometrium was analyzed by semiquantitative real time reverse transcription–polymerase chain reaction (RT-PCR), while protein analysis was done by western blot. Transforming growth factor β3 transcripts were increased 4-fold in leiomyoma versus matched myometrium. Phosphorylated-TGF-β RII and phosphorylated-Smad 2/3 complex were greater in leiomyoma as documented by Western blot. The inhibitor Smad7 transcripts were decreased 0.44-fold. The glycosaminoglycan (GAG)-rich versican variants were elevated in leiomyoma versus myometrial tissue: specifically V0 (4.27 ± 1.12) and V1 (2.01 ± 0.27). Treatment of leiomyoma and myometrial cells with TGF-β3 increased GAG-rich versican variant expression 7 to 12 fold. Neutralizing TGF-β3 antibody decreased the expression of the GAG-rich versican variants 2 to 8 fold in leiomyoma cells. Taken together, the aberrant production of excessive and disorganized extracellular matrix that defines the leiomyoma phenotype involves the activation of the TGF-β signaling pathway and excessive production of GAG-rich versican variants.
Keywords: Versican variants, TGF-β3, leiomyomas, immortalized cells, extracellular matrix
INTRODUCTION
Uterine leiomyomata are tumors enriched in extracellular matrix (ECM) proteins that contribute to the symptomatic bulk of these tumors. We previously reported that leiomyomas exhibit not only increased levels of ECM gene expression by microarray analysis, but also that the ECM collagen fibril structure and orientation were disoriented and loosely packed in a nonparallel manner when compared to matched myometrium via electron microscopy.1,2 The dysregulated accumulation of the ECM represents an imbalance between synthesis and dissolution, and like other fibroproliferative conditions such as keloids, may represent disorders of wound healing.3,4 Leiomyomas represent an excellent model for the study of disease processes involving disrupted ECM.
Versican is a versatile ECM proteoglycan that contributes to the structural properties of the matrix through its ability to bind hyaluronan and the glycosaminoglycan (GAG) chondroitin sulfate, thereby influencing cell growth, migration, and differentiation.5–8 In addition to its interaction with hyaluronan, versican interacts with a number of other ECM molecules such as tenascin,9 fibulins,10,11 fibrillin,12 and fibronectin,13 as well as cell surface proteins including selectins,14,15 integrin β1,16 CD44,17 and epidermal growth factor (EGF) receptor.18 Through these interactions, Zheng and colleagues demonstrated increased tumor growth via versican’s interactions of the EGF-binding domain with fibronectin and vascular endothelial growth factor (VEGF).19 Furthermore, versican expression was predictive of tumor growth or relapse in patients with a diagnosis of organ-confined prostate cancer20 and in women with node-negative breast cancer.21 These results support the role of versican as an important structure in matrix organization while influencing tumor growth and proliferation through its versatile binding domains.
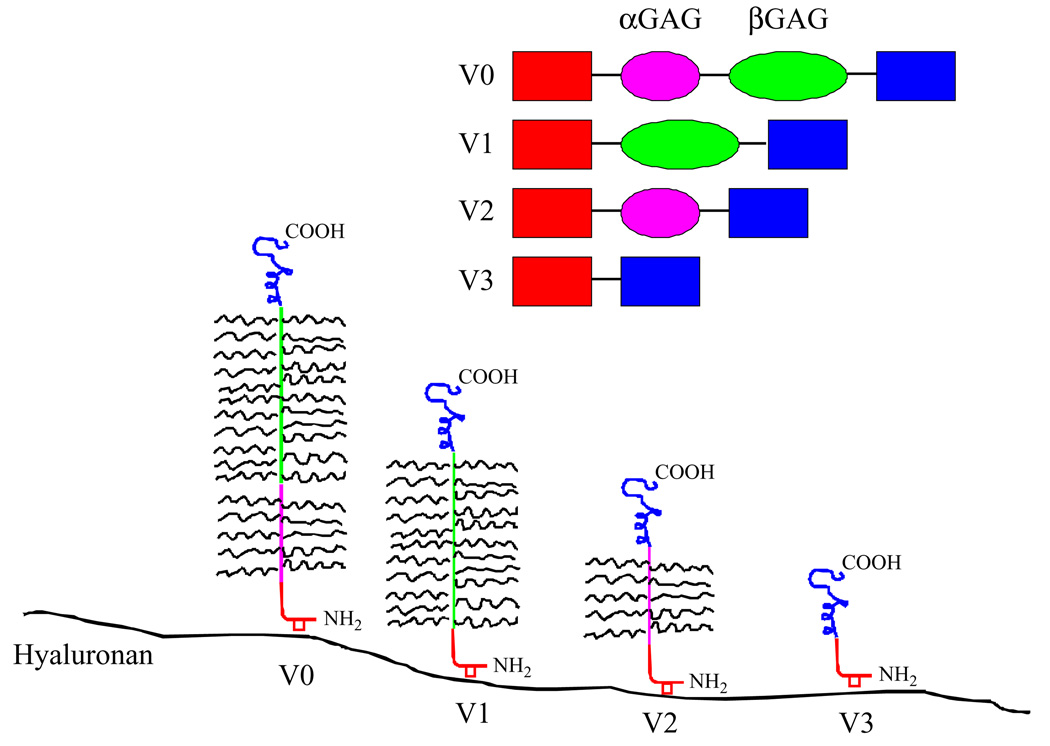
Versican has 4 different isoforms that mediate the different functions in the ECM. The isoforms contain different combinations of 2 GAG-binding domains (GAGα and GAGβ) within the middle portion of the core protein.5 Differential RNA splicing of GAGα and GAGβ results in the 4 versican variants: V0 has both of these domains, V1 has GAGβ only, V2 has GAGα only, and V3 is devoid of GAG motifs (Figure 1).5,22 The wide range of interactions mediated by versican is thought to result from differential expression of the GAG domains and from properties conferred by the N- and C-terminal domains.23–25
Figure 1.
A model of different isoforms generated by alternative splicing of the messenger RNA (mRNA) transcript for versican. Adapted with permission from Curr Opin Cell Biol. 2002;14(5):617–623.
In humans, versican variant expression has been noted in over 20 tissue types,26 and particularly in tissues that undergo remodeling. Differential expression of the versican variants was demonstrated in response to repetitive trauma in tendons24 and was suggested to contribute to changes in matrix structure and function in such ten-donopathies. Differential versican variant expression is associated with developmental events including organo-genesis, limb bud formation, cartilage development, and mesenchymal condensation.27 Versican has also been implicated in tissues undergoing rapid remodeling, such as the uterus during pregnancy where versican achieves its highest concentration in the ripe cervix immediately after vaginal delivery.28,29 Thus, a growing body of evidence has emphasized the association between the differential expression of the versican variants and connective tissue remodeling. In leiomyomas, the process of wound healing is altered,3 which may in part be explained by differential expression of the versican variants.
The TGF-βs are multifunctional peptides that exhibit diverse biological actions, including stimulation or inhibition of cell growth and differentiation, as well as regulation of ECM production.30,31 Transforming growth factor β signaling regulates the structure and function in diseases characterized by aberrant ECM production, including leiomyomas,32–34 glomerulonephritis,35 pulmonary fibrosis,36 liver cirrhosis,37 scleroderma,38 and keloids.39,40 The dominant isoform in the cells of mesenchymal origin, including smooth muscle cells, is TGF-β3.41 The messenger RNAs (mRNAs) and proteins for TGF-β1, -β2, and -β3 as well as their receptors have been detected in human myometrium and leiomyoma.42 Our laboratory and others have demonstrated that TGF-β3 is 3- to 5-fold overexpressed in leiomyoma versus myometrium,2,32,43 while no significant difference was observed in TGF-β1 mRNA abundance.44 Furthermore, the increase of TGF-β3 expression in leiomyoma versus myometrium correlated with disoriented collagen fibrils and ultrastructural abnormalities of the ECM.3 We therefore hypothesized that TGF-β3 would modulate the versican variant expression, ultimately contributing to the dysregulated ECM seen in leiomyomas.
Our gene profiling studies suggested that both total versican expression and TGF-β3 transcripts were overexpressed in leiomyoma tissue compared to matched myometrium.2,45 In the current study, we characterized the TGF-β3 signaling pathway noting differences with prior studies and specifically demonstrate the differential expression of the splice variants of versican in leiomyoma tissue and cell culture compared to matched myometrium. We further demonstrate that TGF-β3 regulates the versican variant expression in leiomyoma and myometrial cell culture.
MATERIALS AND METHODS
Tissue Collection and Demographics
All materials and methods for collection of leiomyoma and myometrium and RNA isolation were conducted as previously described.46 After obtaining informed consent, 15 women underwent hysterectomy at the National Naval Medical Center in Bethesda, Maryland. The surgical indication was symptomatic leiomyomas. Inclusion criteria allowed all patients undergoing an abdominal or vaginal hysterectomy for symptomatic leiomyomata uteri who were willing to participate in the study. All leiomyomata were reviewed by a pathologist and none contained leiomyosarcoma or myxoid pathology and all were noted to be benign leiomyomata. The only patients excluded were patients who did not have leiomyomata identified in the pathological specimen. Investigational review board approval for tissue collection and analysis was obtained at the National Institutes of Health, the Uniformed Services University of the Health Sciences, and the National Naval Medical Center in Bethesda, Maryland. Leiomyoma and myometrial tissue samples 3 to 10 mm3 in size were harvested. For some leiomyomas, this represented the entire specimen. From larger leiomyomas, tissue was taken at random from the center, middle, and peripheral locations. Patient and leiomyoma characteristics for confirmatory studies are described in Table 1. Each patient provided 1 leiomyoma and matched myometrium.
Table 1.
Patients and Leiomyoma Characterization
| Patient Number |
Race | Fibroid Size (cm) |
Location |
|---|---|---|---|
| 1 | Afro-American | 4.0 × 3.5 | Subserosal |
| 2 | Caucasian | 5.0 × 4.0 | Submucosal |
| 3 | Afro-American | 3.8 × 3.5 × 2.8 | Submucosal |
| 4 | Afro-American | 7.0 × 7.0 | Subserosal |
| 5 | Caucasian | 5.0 × 4.0 | Subserosal |
| 6 | Afro-American | 6.5 × 7.5 | Submucosal |
| 7 | Afro-American | 3.5 × 2.3 × 3.0 | Subserosal |
| 8 | Unknown | 7.5 × 5.0 × 4.0 | Intramural |
| 9 | Afro-American | 2.0 × 1.3 × 1.4 | Submucosal |
| 10 | Afro-American | 2.0 × 1.3 × 1.4 | Submucosal |
| 11 | Caucasian | 5.5 × 4.0 × 5.0 | Intramural |
| 12 | Asian | 4.0 × 2.5 × 3.0 | Subserosal |
| 13 | Caucasian | 6.0 × 4.0 × 3.0 | Intramural |
| 14 | Afro-American | 6.0 × 6.0 × 4.0 | Subserosal |
| 15 | Afro-American | 4.0 × 4.0 × 4.0 | Subserosal |
Tissue destined for RNA isolation was minced and immediately placed in RNAlater (Ambion, Inc, Austin, Texas). Samples were either processed immediately or were placed at 4°C overnight and then stored at −80°C. Tissues for protein isolation were cut in 5-mm sections and frozen immediately at −80°C.
In each case, normal myometrial tissue was harvested as a control from an adjacent region of the uterus, but care was taken to avoid the leiomyoma pseudocapsule. The location and size of the leiomyomata evaluated are available in Table 1.
RNA Isolation
RNA isolation was performed according to a standard protocol.46 Briefly, tissue (either fibroid or myometrial) was removed from the RNAlater (Ambion) solution and reminced. This tissue was then placed in TRIzol reagent (Invitrogen, Carlsbad, California) at a quantity of 1 mL per 100 mg of tissue. Tissue homogenization was performed with a Tissue Tearor model 985–370 (Biospec Products, Inc, Bartlesville, Oklahoma). The tissue homogenate was left at room temperature for 5 minutes and then mixed with 0.2 mL of choloroform per 1 mL of TRIzol reagent used. This mixture was kept at room temperature for 5 minutes and then centrifuged at 12 000 rpm for 15 minutes at 4°C. The aqueous layer was transferred and mixed with 0.5 mL of isopropanol per 1 mL of TRIzol reagent used. This mixture was left at room temperature for 10 minutes and then was centrifuged at 12 000 rpm for 10 minutes at 4°C. The liquid was decanted, the pellet dried, and then washed with 1.5 mL of 75% ethanol per 1 mL of TRIzol reagent used and centrifuged at 7500 rpm for 5 minutes at 4°C. The liquid was decanted and the pellet resuspended in diethylpyrocarbonate (DEPC)-treated sterile water. To ensure that there was no contaminating DNA present, all RNA samples were treated with DNAse-I enzyme using the Turbo DNA-free kit in accordance with the manufacturer’s protocol (Ambion). RNA integrity and concentration were confirmed by agarose electrophoresis and spectrophotometry measurements using the A260/A280 ratio.
Immortalized Cell Culture
Leiomyoma and myometrial tissue were minced into 1-to 2-mm pieces. Primary cell cultures were generated and immortalized using recombinant retrovirus containing the E6/E7 open reading frames of human papillomavirus (HPV) type 16.47,48 The immortalized cell lines were confirmed to be derived either from myometrium or leiomyoma using the specific biomarker gene array as described previously.45
Transforming Growth Factor β3 and Anti-TGF-β3 Treatment
The effect of exogenously added TGF-β3 on versican variant expression was tested using myometrial and leiomyoma-immortalized cells that had been allowed to grow to 80% confluence in culture dishes with DMEM (Dulbecco’s minimal essential medium) with 10% fetal bovine serum (FBS) that contained antibiotics and antimycotics at 37°C in a humidified atmosphere (5% CO2 in air). After 3 to 4 days of culture, the cells were washed with phosphate-buffered saline (PBS), and the medium was changed to DMEM with serum-free media with antibiotics and antimycotics for 48 hours. Transforming growth factor β3 (Sigma-Aldrich, Inc, St Louis, Missouri) was then added to myometrial and leiomyoma cells for 24 hours at the following concentrations: 0, 0.1, and 1.0 ng/mL in DMEM in serum-free media. Transforming growth factor β3 neutralizing antibody (Sigma-Aldrich) was also added to myometrial and leiomyoma cells at the following concentrations: 0, 0.1, and 1.0 µg/mL in DMEM in serum-free media to examine the autocrine effect of TGF-β3 on versican expression. These concentrations and chosen treatment times were based on prior studies using primary leiomyoma and myometrial cell cultures.32,43,49,50
Primer and Probe Design
Total RNA (either leiomyoma or myometrium) was first diluted to yield 10 ng per µL stock concentrations. Primers and probes used for versican mRNA were described previously24 and confirmed with BLASTn searches (www.ncbi.nih.gov/BLAST). BLASTn searches revealed no significant similarity to other sequences. The probes were chosen to span exon splice junctions. All RNA samples were assayed a minimum of 3 times on the same plate at 5 ng/well and each target assay was performed at least 3 times with similar results. The values obtained for versican mRNA expression were normalized with the internal housekeeping gene 18S mRNA expression and the relative expression was determined against corresponding myometrium or untreated cells.
Reverse Transcription Polymerase Analysis
The primers were then generated on a 3900 High-Throughput DNA Synthesizer (Applied Biosystems, Foster City, California). Their composition and concentrations were verified and determined by measuring optical density with a standard spectrophotometer. These were added to the reaction mix at 300 nmol/L concentration. iScript One-Step reverse transcription–polymerase chain reaction (RT-PCR) kit for Probes (Bio-Rad, Hercules, California) was used with either the Gene Amp PCR System 9700 (Applied Biosystems) or the MyCycler Ther-mocyler (BioRAD, Hercules, California) for thermal cycling. Reverse transcription was performed at 50°C for 30 minutes, followed by 94°C for 2 minutes. The samples then underwent 40 cycles at the following temperatures: 94°C for 15 seconds, 50°C to 65°C (depending on the optimal annealing temperatures of the designed primers) for 30 seconds, and 72°C for 1 minute 30 seconds. Primers for glyceraldehyde-3-phosphate (GAPDH) or 18S ribosomal RNA (rRNA) were included as an internal control to verify that a similar amount of starting template was loaded in each well. Leiomyoma and myometrium samples were then electrophoresed side-by-side at the same starting concentration on a 2% agarose gel with ethydium bromide for endpoint RT-PCR analysis. For real-time RT-PCR, data analysis was performed with Bio-RAD iCycler software, version 3.1.
Protein Isolation
Uterine leiomyoma and myometrium were frozen at ‒80°C for storage. Protein isolation and lysate preparation were described previously.51 Briefly, the tissues were minced and placed (150 mg wet tissue per mL) in a 1-mol/L Tris-HCl (pH 8.0) buffer containing NP40, 5 mol/L NaCl, NaF, sodium orthovanadate, Complete Protease inhibitor tablet from Roche Diagnostics (Mannheim, Germany), and 0.5 mol/L EDTA. Protein was extracted overnight at 4°C with gentle tumbling. After 15 to 30 seconds of homogenization and 2 hours of tumbling, the homogenates were centrifuged at 14 000g for 20 minutes. In the supernatant, protein was measured with the BCA kit from Pierce (Rockford, Illinois) using BSA as a standard.
Western Blot
NuPage Bis-Tris 4% to 12% gels from Novex (San Diego, California) and Rainbow molecular weight markers from Amersham were used; 50 µg of protein were loaded per lane. Polyclonal antibodies to TGF-β RII, p-TGF-β RII, and p-Smad 2/3 from Santa Cruz Biotechnology (Santa Cruz, California) and polyclonal antibody to Versican V0/V1 from Affinity BioReagents (Rockford, Illinois) were used. Equal lane loading was confirmed by a β-actin antibody from Santa Cruz Biotechnology. Secondary antibodies and the SuperSignal West Pico chemiluminescent kit were used from Pierce Biotechnology (Rockford, Illinois). Three replicates of each experiment were performed.
Statistical Analysis
Statistical analysis for the real-time RT-PCR data was performed using a 2-tailed Student t test.52 The data are presented as mean of differential expression among all patients with 95% confidence intervals. The immunohistochemistry data was analyzed using the ImageJ 1.34n software (share-ware, http://www.uhnresearch.ca/facilities/wcif/imagej/) to analyze quantity of pixels in the myometrial cells in comparison to the fibroid cells. Five random sections of the immunohistochemical fixed slides were digitally photographed for the myometrium and fibroid samples of each different protein. Using the ImageJ software, random cells on each section of the slide underwent histogram analysis and the mean number of pixels was recorded. The P value was generated using a standard 2-tailed Student t test, and considered significant if <.05. Wilcoxon sign rank tests were used for nonparametric data. Western blot data was analyzed using the ImageJ software to calculate the number of pixels in each band and comparing myometrium to leiomyoma.
RESULTS
Transforming Growth Factor β3 Expression by Human Leiomyoma Tissue
Using quantitative end-point RT-PCR and also quantitative real-time RT-PCR, we confirmed that TGF-β3 mRNA was elevated 4-fold in surgical specimens of uterine leiomyoma in comparison with patient-matched myometrium (Figure 2A and B). Transforming growth factor β1, another isoform of TGF-β, revealed no significant elevation (results not shown), which was consistent with previous findings.44 Our results also demonstrated no statistically significant difference in TGF-β RIII, Smad 2, Smad 3, Smad 4, and Smad 6 mRNA expression (Figure 2B). TGF-β RI and TGF-β RII mRNA were both significantly decreased in uterine leiomyomas (P ≤ .001, and P ≤ .01, respectively; Figure 2A and B). Smad7, primarily responsible for inhibition of TGF-β intracellular signaling activity, exhibited a significant 2-fold decrease of mRNA in uterine leiomyoma (P = .042; Figure 2A and B). These results, in aggregate, demonstrated that template encoding 2 primary receptors of TGF-β signaling were decreased in uterine leiomyoma tissues and that other components of the TGF-β cell signaling pathway transcript expression were unchanged in leiomyomata relative to myometrium, while the inhibitory Smad, Smad 7 was decreased in leiomyomas. Of note, no differences in gene or protein expression were noted between different ethnic groups; however, the study was not appropriately powered to address this question.
Figure 2.
Expression of transforming growth factor β (TGF-β) ligands, TGF-β receptors, and Smad second messenger system. A, Semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR) expression for TGF-β3, TGF-β RI, TGF-β1 RII, and Smad 7. Lane 1 = molecular size standard (STD; 300 base pair marker listed), lane 2 = no template control (C), lane 3 = myometrial tissue transcript gene expression (M), and lane 4 = leiomyoma tissue transcript gene expression (L). B, Relative fold expression differences in leiomyoma versus myometrial tissue by real-time quantitative RT-PCR for the following genes: TGF-β3 (P≤.01), TGF-β RI (P≤.001), TGF-β RII (P≤.001), Smad 2 (NS), Smad 3 (NS), Smad 4 (NS), Smad 6 (NS), and Smad 7 (P = .042). C, Representative Western blot analysis demonstrating overexpression of both phosphorylated TGF-β RII and phosphorylated Smad 3 with largely unchanged expression of unphosphorylated TGF-β-RII.
Activation of the TGF-β cell signaling pathway requires interaction of ligand with TGF-β RII, phosphorylation of TGF-β RII, interaction of this complex with TGF-β RI, and activation of the Smad pathway by phosphorylation of the Smad 2/3 complex.53 We have found that TGF-β RII and Smad 2/3 complex were phosphorylated to a greater extent in leiomyomata compared with myometrium (Figure 2C). The increased levels of phosphorylated TGF-β RII occurred in the background of almost equal levels of total or unphosphorylated TGF-RII (Figure 2C) and decreased transcript expression (Figure 2B). Collectively, these results suggested that leiomyomata samples contain elevated levels of ligand (TGF-β3), increased activated TGF-β RII, increased Smad 2/3 phosphorylation, and decreased Smad7 expression strongly suggesting greater activation of the TGF-β signaling pathway.
Versican Variants in Human Leiomyoma Tissue
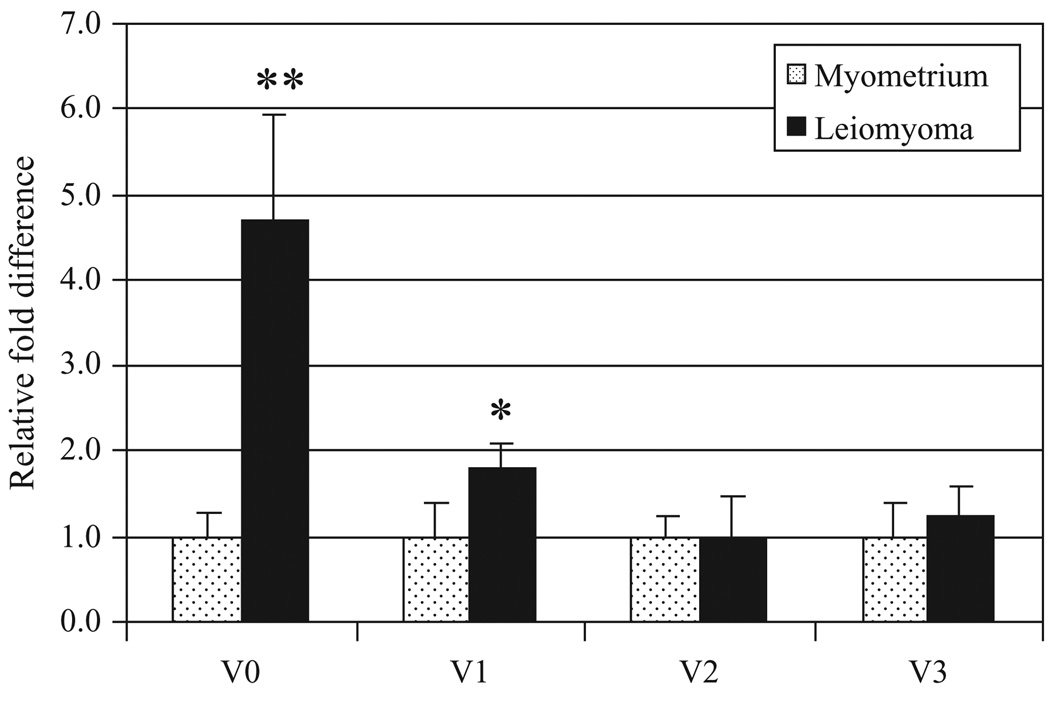
We initially demonstrated increased protein expression of the versican variants V0/V1 in our immortalized leiomyoma cells compared with our myometrial cells using a polyclonal antibody (Figure 2C). Using primers specific for the mRNA of versican glycosaminoglycan splice variants, we found that templates for all 4 variants were present in both leiomyoma and myometrial tissue and that their expression was altered in leiomyoma using quantitative real-time PCR (Figure 3). Overall, V0 showed increased gene expression in leiomyoma versus myometrium (median = 4.27 ± 1.12), while V1 demonstrated more modest elevation (V1: median = 2.01 ± 0.27), and V2 and V3 expression were comparable between leiomyoma and myometrium (V2: median = 1.01 ± 0.55; and V3: median = 1.24 ± 0.29).
Figure 3.
Versican variant expression in leiomyoma versus myometrial tissue. Median values noted in pink squares and individual values noted in blue diamonds. V0: 4.27 ± 1.12; V1: 2.01 ± 0.27; V2: 1.01 ± 0.55; V3: 1.24 ± 0.29. * P = .05, ** P< .001.
Versican Variant Expression in Cell Culture After Treatment With TGF-β3 or Anti-TGF-β3
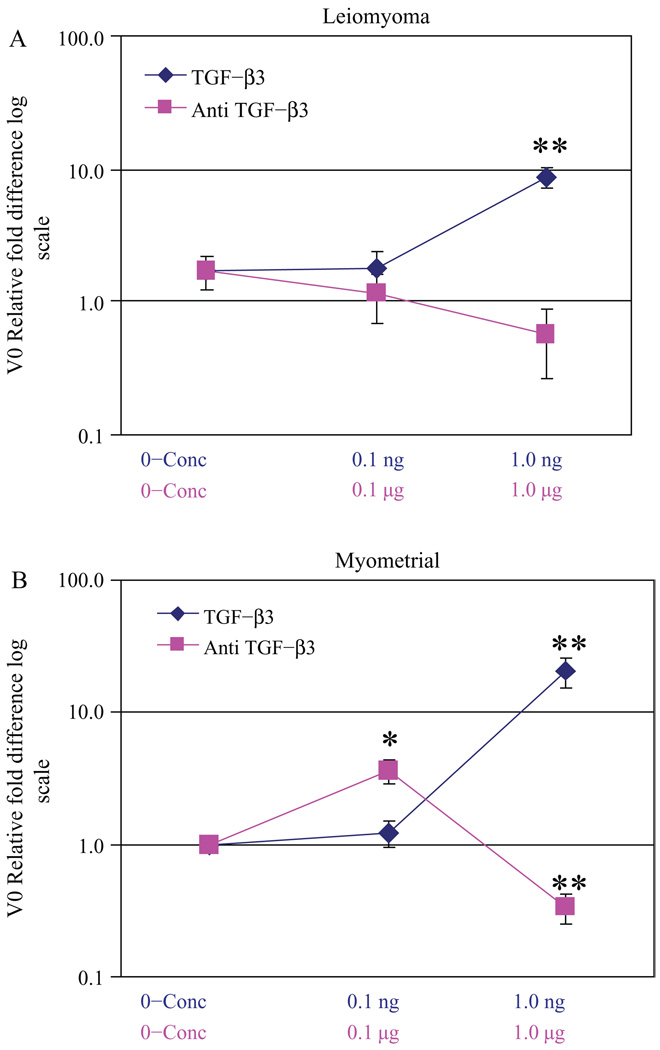
We treated our immortalized leiomyoma and myometrial cells with TGF-β3 or α-TGF-β3 antibody to determine whether there was an effect of TGF-β3 on versican variant V0 mRNA expression (Figure 4). We estimated the dose of treatment of TGF-β3 and α-TGF-β3 antibody based on previous studies using TGF-β1.32,43,50 Untreated myometrial cells were used as a normative reference point for the aberrant leiomyoma cells and also for the TGF-β3-treated myometrial and leiomyoma cell lines. With TGF-β3 treatment, variant V0 showed increased expression in both leiomyoma and myometrial cells versus untreated myometrial cells (Figure 4A and B). This increase was most pronounced with myometrial cells. With the addition of α-TGF-β3 antibody, leiomyoma cells exhibited a more pronounced decrease in relative fold expression of V0 compared with myometrial cells, thus suggesting endogenous TGF-β3 expression and function in leiomyoma cells (Figure 4A and B). We interpret the findings to indicate that TGF-β3 regulated versican variant V0 in both leiomyoma and myometrial cells.
Figure 4.
Relative fold difference of versican variant V0 expression in transforming growth factor β3 (TGF-β3) and α-TGF-β3 antibody-treated cells: leiomyoma cells treated with TGF-β3 and α-TGF-β3 antibody versus untreated myometrial cells (A) and myometrial cells treated with TGF-β3 and αi-TGF-β3 antibody versus untreated myometrial cells (B), * P< .01 and ** P< .001.
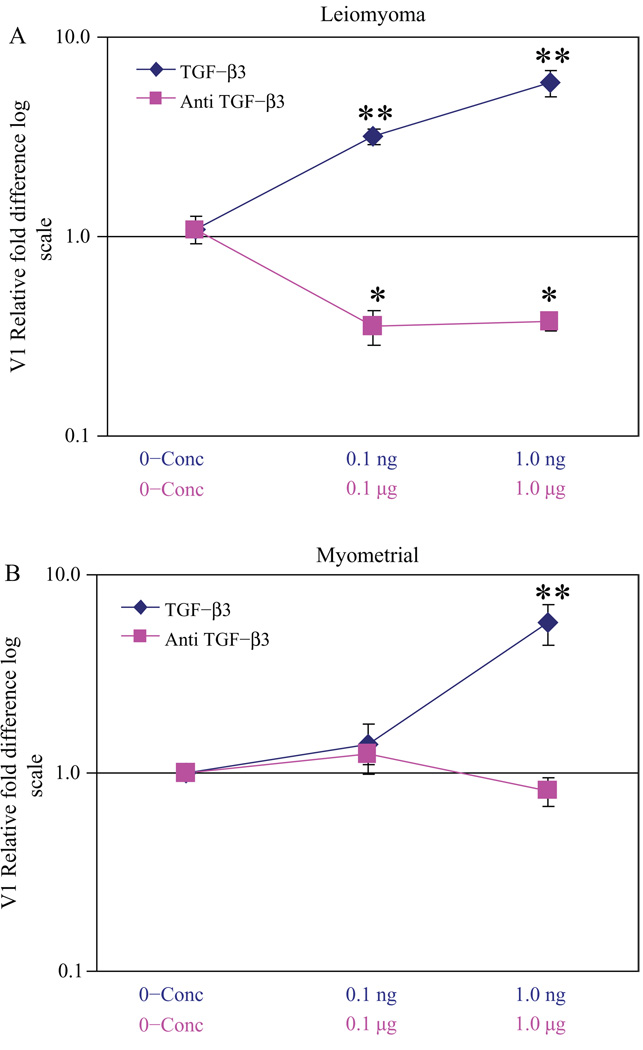
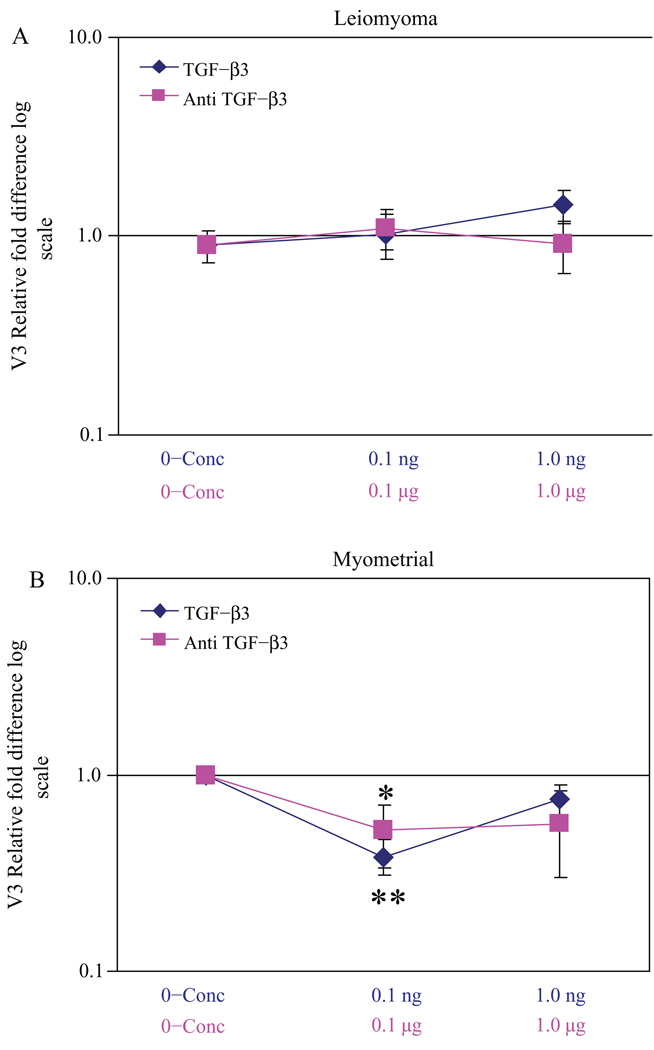
TGF-β3 also was noted to regulate versican variant V1 expression (Figure 5A and B). A more substantial decreased expression of V1 was noted in leiomyoma cells with Anti-TGF-β3 treatment compared to myometrial cells treated similarly (Figure 5A and B). These results indicated that variants found to be overexpressed in leiomyomas (Figure 3) were regulated by TGF-β3. In contrast, V3, which lacks any glycosaminoglycan side chains, exhibited minimal change in expression in leiomyoma cells with the addition of either TGF-β3 or α-TGF-β3 antibody (Figure 6A). A smaller decreased expression of V3 was noted in myometrial cells treated with TGF-β3, suggesting that the more GAG-rich variants were over-expressed with TGF-β3 treatment (Figure 6B). V2 expression was similar to V3 in the presence of TGF-β3 or α-TGF-β3 antibody (data not shown).
Figure 5.
Relative fold difference of versican variant V1 expression in transforming growth factor β3 (TGF-β3) and α-TGF-β3 antibody-treated cells: leiomyoma cells treated with TGF-β3 and α-TGF-β3 antibody versus untreated myometrial cells (A) and myometrial cells treated with TGF-β3 and α-TGF-β3 antibody versus untreated myometrial cells (B), *P< .01 and ** P< .001.
Figure 6.
Relative fold difference of versican variant V3 expression in transforming growth factor β3 (TGF-β3) and α-TGF-β3 antibody treated cells: leiomyoma cells treated with TGF-β3 and α-TGF-β3 antibody versus untreated myometrial cells (A) and myometrial cells treated with TGF-β3 and α-TGF-β3 antibody versus untreated myometrial cells (B), * P< .05 and ** P< .001.
DISCUSSION
The versican variants are important proteoglycans that influence the ECM through both their unique structure and also through their different functional protein-binding domains.23 Versican’s interactions with other proteins modulate the organization of the ECM and promote tumor growth and cellular proliferation. We demonstrated that the GAG-rich versican variant transcripts (V0 and V1) were overexpressed in leiomyoma tissue and immortalized cells further supporting the importance of glycosaminoglycans in the dysregulated ECM of leiomyomas. We also demonstrated that the TGF-β3 pathway was activated and that TGF-β3 notably regulated the expression of the GAG-rich versican variants V0 and V1.
The increased expression of versican variants V0 and V1 noted in leiomyomas may contribute to both the disorganization of the ECM and to the increased stiffness of these tumors. In leiomyomas, we have recently noted a 3-fold increased stiffness in leiomyoma tissue versus matched myometrium that corresponded to increased sulfated glycosaminoglycan and collagen content.54 This builds upon previous work that demonstrated increased levels of total glycosaminoglycans (heparan sulfate, keratin sulfate, chondroitin sulfate, and heparin) in leiomyomas versus myometrium.55–57 Both Berto et al and Wolanska et al reported higher levels of chondroitin sulphate glycosylaminoglycan in leiomyomas. Furthermore, the increased mechanical stress of these bulky tumors has been demonstrated to activate the solid-state Rho-GEF signaling pathway and may further contribute to tumor growth58 and more specifically to leiomyogenesis.54
With increased amounts of glycosaminoglycans, tumor bulk55 was increased in leiomyomas. Orientation of ECM matrix mediates tension-dependent cell migration,59 and alterations in orientation that influence matrix rigidity has been shown to influence cell growth, viability, differentiation, and motility.58,60 Thus, versican’s role in contributing to an increased and dysregulated ECM may stimulate growth via a tension-mediated pathway.
Versican exerts its various properties through its interactions with many other proteins beyond the glycosylaminoglycans that are noted to be altered in leiomyomas. Studies have shown versican to interact with fibronectin13 as well as collagen type I both of which are altered in leiomyomas compared with myometrium.43,61 Furthermore, through its C-terminal domain, versican binds both11,12 fibrillin-1and fibulin-2, which are modular ECM proteins that are expressed in extracellular fibrils, basement membranes, and elastic fibers. Fibulin-1C has been implicated in leiomyoma formation62 and its expression may interestingly be mediated through versican.
After treating the immortalized leiomyoma and myometrial cells with TGF-β3, we noted an increased expression of the GAG-rich V0 and V1. This increase in V0 and V1 corresponds to the aberrant deposition of ECM noted in leiomyomas. The large number of GAG subunits found in V0 and V1 and also other proteoglycans collectively serve as a repository that contain essential growth factors, which further stimulate leiomyoma proliferation and ECM deposition via autocrine and paracrine mechanisms.63,64 Notably, with the treatment of the neutralizing antibody α-TGF-β3, V0 and V1 expression in leiomyoma cells decreased to a greater extent compared with myometrial cells. This corresponds to previous data suggesting a 3- to 5-fold overexpression of TGF-β3 in leiomyoma versus myometrium.2,32,43 Our data suggest that TGF-β3 regulates the versican variant expression in conjunction with the disrupted ECM in leiomyogenesis.
Versican is one of several ECM molecules that accumulate in lesions of atherosclerosis and re-stenosis and is thought to be partly mediated by increased levels of the GAG-deficient isoform, V3.65,66 By overexpressing the GAG deficient V3, the arterial smooth muscle phenotype is dramatically altered with a noted increase in cell adhesion.67 Interestingly, when we treated our cells with TGF-β3, V3 levels were unaltered in both leiomyoma and myometrial cells, suggesting that TGF-β3 treatment favors the bulky GAG-rich versican variants that are hydrophilic (Figures 4 and 5). This corresponds with the high water content noted in the outer myometrium where leiomyomas are commonly found,68 further supporting the importance of the dysregulated GAG-rich ECM noted in leiomyomas.
Altered expression of TGF-β3 leads to several pathological disorders including tissue fibrosis.39,69 Many investigators have demonstrated that TGF-β3 functions via the Smad pathway in various tissue types31,70–72 and also in leiomyoma tissue and cells.50,73 Our results demonstrate that the essential elements of the TGF-β signaling pathway were altered in surgical specimens of leiomyoma versus patient-matched myometrium and that the TGF-β-Smad-dependent pathway was activated. The active phosphorylated forms of both Smad3 and the TGF-β RII were increased relative to matched myometrium in the setting of largely equivalent levels of unphosphorylated total TGF-β RII. Furthermore, we demonstrated decreased levels of the inhibitory Smad7 in untreated leiomyomas versus myometrium.
Transforming growth factor β3 regulates ECM protein expression in leiomyoma cells and tissue. Arici and Sozen showed that TGF-β3 stimulated the ECM protein fibronectin43 while the immunoneutralizing antibody for TGF-β3 has been demonstrated to decrease collagen type I and III production in leiomyoma and myometrial cells.32 The expression of fibromodulin, an important collagen-binding ECM protein rich in oligosaccharides, has been demonstrated to be regulated by TGF-β in myometrial and leiomyoma cells.49 These effects of TGF-β on fibromodulin were reversed with following pretreatment with Smad3 siRNA, thus supporting the mechanistic importance of the Smad-mediated signaling pathway for aberrant ECM protein expression in leiomyoma and myometrial cells treated with TGF-β. Versican’s altered expression by TGF-β3 may be regulated in similar manner.
However, the impact of TGF-β3 treatment on the versican variants has not been analyzed in leiomyomas or myometrium. Follow up microarray analysis of leiomyomas and myometrial tissue has further implicated TGF-β3’s close relationship to a number of ECM genes including various collagens, proteoglycans, elastin, and versican.3,74 Total versican expression was originally demonstrated to be overexpressed by microarray analysis in both leiomyoma versus matched myometrial tissue51 and also in primary leiomyoma cell culture compared to matched myometrial cell culture.33 The upregulated total versican results were recently confirmed in both tissue and primary cell culture in leiomyomas by RT-PCR and protein analysis.45 The studies examining total versican in leiomyoma tissue and cell culture also demonstrated concurrent alterations of the TGF-β3 signaling pathway.
In conclusion, our study uniquely demonstrated the differential gene expression of the versican variants in leiomyoma and matched myometrium tissue. We further demonstrated that the active TGF-β3 signaling pathway directly affects the versican variant expression in leiomyoma and myometrial cells, favoring the GAG-rich variants. Our work suggests that production of essential components of the aberrant ECM in leiomyomas may further contribute to their disordered tumor growth.
ACKNOWLEDGMENT
This study was supported by the Berlex Foundation and by the intramural research program of the Reproductive Biology and Medicine Branch, the Eunice Kennedy Shriver National Institute for Child Health and Human Development, NIH.
Footnotes
The work was performed at the Department of Obstetrics and Gynecology, Uniformed Services University of the Health Sciences, Bethesda, Maryland.
For reprints and permissions queries, please visit SAGE’s Web site at http://www.sagepub.com/journalsPermissions.nav
REFERENCES
- 1.Leppert PC, Baginski T, Prupas C, Catherino WH, Pletcher S, Segars JH. Comparative ultrastructure of collagen fibrils in uterine leiomyomas and normal myometrium. Fertil Steril. 2004;82 suppl 3:1182–1187. doi: 10.1016/j.fertnstert.2004.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catherino WH, Leppert PC, Stenmark MH, et al. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes Chromosomes Cancer. 2004;40(3):204–217. doi: 10.1002/gcc.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leppert PC, Catherino WH, Segars JH. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol. 2006;195(2):415–420. doi: 10.1016/j.ajog.2005.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart EA, Nowak RA. New concepts in the treatment of uterine leiomyomas. Obstet Gynecol. 1998;92(4 pt 1):624–627. doi: 10.1016/s0029-7844(98)00243-9. [DOI] [PubMed] [Google Scholar]
- 5.Dours-Zimmermann MT, Zimmermann DR. A novel glycosaminoglycan attachment domain identified in two alternative splice variants of human versican. J Biol Chem. 1994;269(52):32992–32998. [PubMed] [Google Scholar]
- 6.LeBaron RG, Zimmermann DR, Ruoslahti E. Hyaluronate binding properties of versican. J Biol Chem. 1992;267(14):10003–10010. [PubMed] [Google Scholar]
- 7.Zimmermann DR, Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989;8(10):2975–2981. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evanko SP, Johnson PY, Braun KR, Underhill CB, Dudhia J, Wight TN. Platelet-derived growth factor stimulates the formation of versican-hyaluronan aggregates and pericellular matrix expansion in arterial smooth muscle cells. Arch Biochem Biophys. 2001;394(1):29–38. doi: 10.1006/abbi.2001.2507. [DOI] [PubMed] [Google Scholar]
- 9.Aspberg A, Miura R, Bourdoulous S, et al. The C-type lectin domains of lecticans, a family of aggregating chondroitin sulfate proteoglycans, bind tenascin-R by protein-protein interactions independent of carbohydrate moiety. Proc Natl Acad Sci U S A. 1997;94(19):10116–10121. doi: 10.1073/pnas.94.19.10116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aspberg A, Adam S, Kostka G, Timpl R, Heinegard D. Fibulin-1 is a ligand for the C-type lectin domains of aggrecan and versican. J Biol Chem. 1999;274(29):20444–20449. doi: 10.1074/jbc.274.29.20444. [DOI] [PubMed] [Google Scholar]
- 11.Olin AI, Morgelin M, Sasaki T, Timpl R, Heinegard D, Aspberg A. The proteoglycans aggrecan and Versican form networks with fibulin-2 through their lectin domain binding. J Biol Chem. 2001;276(2):1253–1261. doi: 10.1074/jbc.M006783200. [DOI] [PubMed] [Google Scholar]
- 12.Isogai Z, Aspberg A, Keene DR, Ono RN, Reinhardt DP, Sakai LY. Versican interacts with fibrillin-1 and links extracellular microfibrils to other connective tissue networks. J Biol Chem. 2002;277(6):4565–4572. doi: 10.1074/jbc.M110583200. [DOI] [PubMed] [Google Scholar]
- 13.Yamagata M, Yamada KM, Yoneda M, Suzuki S, Kimata K. Chondroitin sulfate proteoglycan (PG-M-like proteoglycan) is involved in the binding of hyaluronic acid to cellular fibronectin. J Biol Chem. 1986;261(29):13526–13535. [PubMed] [Google Scholar]
- 14.Kawashima H, Hirose M, Hirose J, Nagakubo D, Plaas AH, Miyasaka M. Binding of a large chondroitin sulfate/dermatan sulfate proteoglycan, versican, to L-selectin, P-selectin, and CD44. J Biol Chem. 2000;275(45):35448–35456. doi: 10.1074/jbc.M003387200. [DOI] [PubMed] [Google Scholar]
- 15.Kawashima H, Atarashi K, Hirose M, et al. Oversulfated chondroitin/dermatan sulfates containing GlcAbeta1/IdoAalpha1– 3GalNAc(4,6-O-disulfate) interact with L- and P-selectin and chemokines. J Biol Chem. 2002;277(15):12921–12930. doi: 10.1074/jbc.M200396200. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Chen L, Zheng PS, Yang BB. beta 1-Integrin-mediated glioma cell adhesion and free radical-induced apoptosis are regulated by binding to a C-terminal domain of PG-M/versican. J Biol Chem. 2002;277(14):12294–12301. doi: 10.1074/jbc.M110748200. [DOI] [PubMed] [Google Scholar]
- 17.Bajorath J, Greenfield B, Munro SB, Day AJ, Aruffo A. Identification of CD44 residues important for hyaluronan binding and delineation of the binding site. J Biol Chem. 1998;273(1):338–343. doi: 10.1074/jbc.273.1.338. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Sheng W, Chen L, et al. Versican V1 isoform induces neuronal differentiation and promotes neurite outgrowth. Mol Biol Cell. 2004;15(5):2093–2104. doi: 10.1091/mbc.E03-09-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng PS, Wen J, Ang LC, et al. Versican/PG-M G3 domain promotes tumor growth and angiogenesis. FASEB J. 2004;18(6):754–756. doi: 10.1096/fj.03-0545fje. [DOI] [PubMed] [Google Scholar]
- 20.Ricciardelli C, Quinn DI, Raymond WA, et al. Elevated levels of peritumoral chondroitin sulfate are predictive of poor prognosis in patients treated by radical prostatectomy for early-stage prostate cancer. Cancer Res. 1999;59(10):2324–2328. [PubMed] [Google Scholar]
- 21.Ricciardelli C, Brooks JH, Suwiwat S, et al. Regulation of stromal versican expression by breast cancer cells and importance to relapse-free survival in patients with node-negative primary breast cancer. Clin Cancer Res. 2002;8(4):1054–1060. [PubMed] [Google Scholar]
- 22.Naso MF, Zimmermann DR, Iozzo RV. Characterization of the complete genomic structure of the human versican gene and functional analysis of its promoter. J Biol Chem. 1994;269(52):32999–33008. [PubMed] [Google Scholar]
- 23.Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol. 2002;14(5):617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- 24.Corps AN, Robinson AH, Movin T, et al. Versican splice variant messenger RNA expression in normal human Achilles tendon and tendinopathies. Rheumatology (Oxford) 2004;43(8):969–972. doi: 10.1093/rheumatology/keh222. [DOI] [PubMed] [Google Scholar]
- 25.Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15(7):483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- 26.Cattaruzza S, Schiappacassi M, Ljungberg-Rose A, et al. Distribution of PG-M/versican variants in human tissues and de novo expression of isoform V3 upon endothelial cell activation, migration, and neoangiogenesis in vitro. J Biol Chem. 2002;277(49):47626–47635. doi: 10.1074/jbc.M206521200. [DOI] [PubMed] [Google Scholar]
- 27.Kamiya N, Watanabe H, Habuchi H, et al. Versican/PG-M regulates chondrogenesis as an extracellular matrix molecule crucial for mesenchymal condensation. J Biol Chem. 2006;281(4):2390–2400. doi: 10.1074/jbc.M509341200. [DOI] [PubMed] [Google Scholar]
- 28.Westergren-Thorsson G, Norman M, Bjornsson S, et al. Differential expressions of mRNA for proteoglycans, collagens and transforming growth factor-beta in the human cervix during pregnancy and involution. Biochim Biophys Acta. 1998;1406(2):203–213. doi: 10.1016/s0925-4439(98)00005-2. [DOI] [PubMed] [Google Scholar]
- 29.Ryu KY, Mahendroo M. Regulated changes in crosslinks between versican and hyaluronan may facilitate cervical ripening. Reprod Sci. 2007;14 Suppl 1:68. [Google Scholar]
- 30.Massague J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- 31.He W, Dorn DC, Erdjument-Bromage H, Tempst P, Moore MA, Massague J. Hematopoiesis controlled by distinct TIF1gamma and Smad4 branches of the TGFbeta pathway. Cell. 2006;125(5):929–941. doi: 10.1016/j.cell.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 32.Lee BS, Nowak RA. Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-beta 3 (TGF beta 3) and altered responses to the antiproliferative effects of TGF beta. J Clin Endocrinol Metab. 2001;86(2):913–920. doi: 10.1210/jcem.86.2.7237. [DOI] [PubMed] [Google Scholar]
- 33.Luo X, Ding L, Xu J, Chegini N. Gene expression profiling of leiomyoma and myometrial smooth muscle cells in response to transforming growth factor-beta. Endocrinology. 2005;146(3):1097–1118. doi: 10.1210/en.2004-1377. [DOI] [PubMed] [Google Scholar]
- 34.Stewart EA, Disalvo D, Sharif NA, Sultana N, Margolin SB. Pirfenidone for the treatment of uterine leiomyomas: pilot study data. J Soc Gynecolog Invest. 1999;6:229A. [Google Scholar]
- 35.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69(2):213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 36.Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293(3):L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 37.Yata Y, Gotwals P, Koteliansky V, Rockey DC. Dose-dependent inhibition of hepatic fibrosis in mice by a TGF-beta soluble receptor: implications for antifibrotic therapy. Hepatology. 2002;35(5):1022–1030. doi: 10.1053/jhep.2002.32673. [DOI] [PubMed] [Google Scholar]
- 38.Verrecchia F, Mauviel A, Farge D. Transforming growth factor-beta signaling through the Smad proteins: role in systemic sclerosis. Autoimmun Rev. 2006;5(8):563–569. doi: 10.1016/j.autrev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6(7):506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 40.Bonniaud P, Margetts PJ, Ask K, Flanders K, Gauldie J, Kolb M. TGF-beta and Smad3 signaling link inflammation to chronic fibrogenesis. J Immunol. 2005;175(8):5390–5395. doi: 10.4049/jimmunol.175.8.5390. [DOI] [PubMed] [Google Scholar]
- 41.Derynck R, Lindquist PB, Lee A, et al. A new type of transforming growth factor-beta, TGF-beta 3. EMBO J. 1988;7(12):3737–3743. doi: 10.1002/j.1460-2075.1988.tb03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang XM, Dou Q, Zhao Y, McLean F, Davis J, Chegini N. The expression of transforming growth factor-beta s and TGF-beta receptor mRNA and protein and the effect of TGF-beta s on human myometrial smooth muscle cells in vitro. Mol Hum Reprod. 1997;3(3):233–240. doi: 10.1093/molehr/3.3.233. [DOI] [PubMed] [Google Scholar]
- 43.Arici A, Sozen I. Transforming growth factor-beta3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril. 2000;73(5):1006–1011. doi: 10.1016/s0015-0282(00)00418-0. [DOI] [PubMed] [Google Scholar]
- 44.Vollenhoven BJ, Herington AC, Healy DL. Epidermal growth factor and transforming growth factor-beta in uterine fibroids and myometrium. Gynecol Obstet Invest. 1995;40(2):120–124. doi: 10.1159/000292319. [DOI] [PubMed] [Google Scholar]
- 45.Malik M, Catherino WH. Novel method to characterize primary cultures of leiomyoma and myometrium with the use of confirmatory biomarker gene arrays. Fertil Steril. 2007;87(5):1166–1172. doi: 10.1016/j.fertnstert.2006.08.111. [DOI] [PubMed] [Google Scholar]
- 46.Catherino WH, Prupas C, Tsibris JC, et al. Strategy for elucidating differentially expressed genes in leiomyomata identified by microarray technology. Fertil Steril. 2003;80(2):282–290. doi: 10.1016/s0015-0282(03)00953-1. [DOI] [PubMed] [Google Scholar]
- 47.Malik M, Webb J, Catherino WH. Retinoic acid treatment of human leiomyoma cells transformed the cell phenotype to one strongly resembling myometrial cells. Clin Endocrinol (Oxf) 2008;69(3):462–470. doi: 10.1111/j.1365-2265.2008.03207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rhim JS. Generation of immortal human prostate cell lines for the study of prostate cancer. Methods Mol Med. 2003;81:69–77. doi: 10.1385/1-59259-372-0:69. [DOI] [PubMed] [Google Scholar]
- 49.Levens E, Luo X, Ding L, Williams RS, Chegini N. Fibromo-dulin is expressed in leiomyoma and myometrium and regulated by gonadotropin-releasing hormone analogue therapy and TGF-beta through Smad and MAPK-mediated signalling. Mol Hum Reprod. 2005;11(7):489–494. doi: 10.1093/molehr/gah187. [DOI] [PubMed] [Google Scholar]
- 50.Xu J, Luo X, Chegini N. Differential expression, regulation, and induction of Smads, transforming growth factor-beta signal transduction pathway in leiomyoma, and myometrial smooth muscle cells and alteration by gonadotropin-releasing hormone analog. J Clin Endocrinol Metab. 2003;88(3):1350–1361. doi: 10.1210/jc.2002-021325. [DOI] [PubMed] [Google Scholar]
- 51.Tsibris JC, Segars J, Coppola D, et al. Insights from gene arrays on the development and growth regulation of uterine leiomyomata. Fertil Steril. 2002;78(1):114–121. doi: 10.1016/s0015-0282(02)03191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fleisch MC, Maxwell CA, Barcellos-Hoff MH. The pleiotropic roles of transforming growth factor beta in homeostasis and carcinogenesis of endocrine organs. Endocr Relat Cancer. 2006;13:379–400. doi: 10.1677/erc.1.01112. [DOI] [PubMed] [Google Scholar]
- 54.Rogers R, Norian J, Malik M, et al. Mechanical homeostasis is altered in uterine leiomyoma. Am J Obstet Gynecol. 2008;198(4):474. doi: 10.1016/j.ajog.2007.11.057. e471–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolanska M, Sobolewski K, Drozdzewicz M, Bankowski E. Extracellular matrix components in uterine leiomyoma and their alteration during the tumour growth. Mol Cell Biochem. 1998;189(1–2):145–152. doi: 10.1023/a:1006914301565. [DOI] [PubMed] [Google Scholar]
- 56.Mitropoulou TN, Theocharis AD, Stagiannis KD, Karamanos NK. Identification, quantification and fine structural characterization of glycosaminoglycans from uterine leiomyoma and normal myometrium. Biochimie. 2001;83(6):529–536. doi: 10.1016/s0300-9084(01)01281-0. [DOI] [PubMed] [Google Scholar]
- 57.Berto AG, Sampaio LO, Franco CR, Cesar RM, Jr, Michelacci YM. A comparative analysis of structure and spatial distribution of decorin in human leiomyoma and normal myometrium. Biochim Biophys Acta. 2003;1619(1):98–112. doi: 10.1016/s0304-4165(02)00446-4. [DOI] [PubMed] [Google Scholar]
- 58.Paszek MJ, Zahir N, Johnson KR, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 59.Keller R, Davidson LA, Shook DR. How we are shaped: the biomechanics of gastrulation. Differentiation. 2003;71(3):171–205. doi: 10.1046/j.1432-0436.2003.710301.x. [DOI] [PubMed] [Google Scholar]
- 60.Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166(6):877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab. 1994;79(3):900–906. doi: 10.1210/jcem.79.3.8077380. [DOI] [PubMed] [Google Scholar]
- 62.Luo X, Ding L, Chegini N. CCNs, fibulin-1C and S100A4 expression in leiomyoma and myometrium: inverse association with TGF-beta and regulation by TGF-beta in leiomyoma and myometrial smooth muscle cells. Mol Hum Reprod. 2006;12(4):245–256. doi: 10.1093/molehr/gal015. [DOI] [PubMed] [Google Scholar]
- 63.Sozen I, Arici A. Interactions of cytokines, growth factors, and the extracellular matrix in the cellular biology of uterine leiomyomata. Fertil Steril. 2002;78(1):1–12. doi: 10.1016/s0015-0282(02)03154-0. [DOI] [PubMed] [Google Scholar]
- 64.Sozen I, Arici A. Cellular biology of myomas: interaction of sex steroids with cytokines and growth factors. Obstet Gynecol Clin North Am. 2006;33(1):41–58. doi: 10.1016/j.ogc.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 65.Wight TN, Merrilees MJ. Proteoglycans in atherosclerosis and restenosis: key roles for versican. Circ Res. 2004;94(4):1158–1167. doi: 10.1161/01.RES.0000126921.29919.51. [DOI] [PubMed] [Google Scholar]
- 66.Huang R, Merrilees MJ, Braun K, et al. Inhibition of versican synthesis by antisense alters smooth muscle cell phenotype and induces elastic fiber formation in vitro and in neointima after vessel injury. Circ Res. 2006;98(3):370–377. doi: 10.1161/01.RES.0000202051.28319.c8. [DOI] [PubMed] [Google Scholar]
- 67.Lemire JM, Merrilees MJ, Braun KR, Wight TN. Overex-pression of the V3 variant of versican alters arterial smooth muscle cell adhesion, migration, and proliferation in vitro. J Cell Physiol. 2002;190(1):38–45. doi: 10.1002/jcp.10043. [DOI] [PubMed] [Google Scholar]
- 68.McCarthy S, Scott G, Majumdar S, et al. Uterine junctional zone: MR study of water content and relaxation properties. Radiology. 1989;171(1):241–243. doi: 10.1148/radiology.171.1.2928531. [DOI] [PubMed] [Google Scholar]
- 69.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342(18):1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 70.Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-beta signal transduction. J Cell Sci. 2001;114(pt 24):4359–4369. doi: 10.1242/jcs.114.24.4359. [DOI] [PubMed] [Google Scholar]
- 71.Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19(8):1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.ten Dijke P, Hill CS. New insights into TGF-beta-Smad signalling. Trends Biochem Sci. 2004;29(5):265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 73.Chegini N, Luo X, Ding L, Ripley D. The expression of Smads and transforming growth factor beta receptors in leiomyoma and myometrium and the effect of gonadotropin releasing hormone analogue therapy. Mol Cell Endocrinol. 2003;209(1–2):9–16. doi: 10.1016/j.mce.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 74.Arslan AA, Gold LI, Mittal K, et al. Gene expression studies provide clues to the pathogenesis of uterine leiomyoma: new evidence and a systematic review. Hum Reprod. 2005;20(4):852–863. doi: 10.1093/humrep/deh698. [DOI] [PubMed] [Google Scholar]