Abstract
The non-natural amino acid p-cyanophenylalanine (PheCN) has recently emerged as a useful fluorescent probe of proteins; however, its photophysical properties have not been systematically examined. Herein, we measure the fluorescence quantum yield and the fluorescence lifetime of PheCN in a series of solvents. It is found that the fluorescence lifetime of PheCN shows a linear dependence on the Kamlet-Taft parameter α of the protic solvents used, indicating that the solute-solvent hydrogen bonding interactions mediate the non-radiative decay rate. Thus, results of this study provide a basis for quantitative application of PheCN fluorescence in protein conformational studies.
1. Introduction
Recently, p-cyanophenylalanine (PheCN) has emerged as a useful spectroscopic probe of protein structure and dynamics. For example, its C≡N stretching frequency is sensitive to environment [1–3] and thus has been used to probe the orientation and hydration status of individual sidechains of a peptide that is bound to model membranes [4,5], as well as the structure of amyloid fibers [6]. In addition, the fluorescence quantum yield of PheCN in water is about 5 times larger than that of phenylalanine (Phe) [7] and decreases upon dehydration, making it an attractive fluorescent probe of protein conformational changes [8,9]. What is more, its utility as a spectroscopic probe is further increased by its ability to excite tryptophan (Trp) fluorescence via the mechanism of fluorescence energy transfer (FRET) [8,10–13]. Finally, and perhaps most importantly, the major advantage of using PheCN in protein conformational studies is that as a non-natural amino acid, it minimally perturbs the physical properties of the native protein in question because of its small size, especially when it replaces either a Phe or Tyr (tyrosine) residue in the native sequence.
While previous studies have established the utility of PheCN as a valuable fluorescent probe, many details of its photophysics are not known, which, in many cases, limits its application to a qualitative context. Herein, we measure the fluorescence lifetime and quantum yield of PheCN in a variety of solvents, attempting to understand how environmental factors, such as hydrogen bonding and polarity, determine its fluorescence properties. We find that its fluorescence lifetimes measured in a series of aprotic solvents show no correlation with the polarity of the solvent, while for the protic solvents a good correlation exists between the lifetime and the Kamlet-Taft solvatochromatic parameter α [14] of the solvent. Taken together, these results indicate that the fluorescence lifetime of PheCN is sensitive to hydrogen bonding and is especially useful for probing the local hydration/dehydration status of proteins [15,16].
2. Experimental section
2.1. Materials
p-Cyanophenylalanine (>99%) was purchased from BACHEM (Torrance, CA). All solvents are of spectrophotometric or fluorometric grade and were purchased from either Acros (acetonitrile, formamide, 1, 2-propane diol, tetraydrofuran, formic acid) or Fisher Scientific (1-butanol, 2-propanol, 1-propanol, methanol, ethanol). All solvents were used as received, except for acetonitrile and tetrahydrofuran, which were dried with magnesium sulfate and distilled onto molecular sieves (4A) under argon pressure to remove water. In addition, Millipore water was used to prepare all aqueous solutions. All solution samples were prepared by directly dissolving an appropriate amount of lyophilized PheCN solid in the targeted solvent. For solvents wherein the solubility of the amino acid is low, the sample solution was further sonicated for 5 minutes and allowed to settle for several hours before use.
2.2. Absorption and steady-state fluorescence measurements
The absorption and fluorescence spectra were collected at room temperature on a Perkin Elmer Lambda 25 UV/Vis spectrometer and a Jobin Yvon Horiba Fluorolog 3.10 spectrofluorometer, respectively, using a 1 cm quartz cuvette. For fluorescence measurements, the spectral resolution was 1 nm (for both excitation and emission) and the integration time was 1s/nm.
Quantum yields (Φ) were calculated using the following equation [17]:
| (1) |
Where I, Abs, and η stand for the integrated fluorescence intensity, the absorbance of PheCN at the excitation wavelength of 280 nm, and the refractive index of the solvent, respectively. The quantum yield of PheCN in water, measured previously [7], served as the reference. The anti-log factor is the “inner-filter” correction that accounts for attenuation of the excitation beam due to optical densities of the sample and reference [18].
2.3. Fluorescence lifetime measurements
Fluorescence lifetimes were measured at room temperature using a time-correlated single photon counting (TCSPC) system, the details of which have been described elsewhere [19]. Briefly, the 275 nm excitation pulses were derived from the third harmonic of a femtosecond Ti:Sapphire laser (Coherent Chameleon). A subtractive double grating monochromator was used to eliminate the residual excitation light and also to allow selective detection of the fluorescence emission of PheCN at 310 nm using an MCP-PMT detector (Hamamatsu R2809U). Time-to-amplitude conversion was achieved via a TCSPC board (Becker & Hickl, SPC-730). Data fitting was carried out in FLUOFIT (Picoquant GmbH) by convoluting an appropriate decay function with the experimentally determined instrument response function.
3. Results and discussion
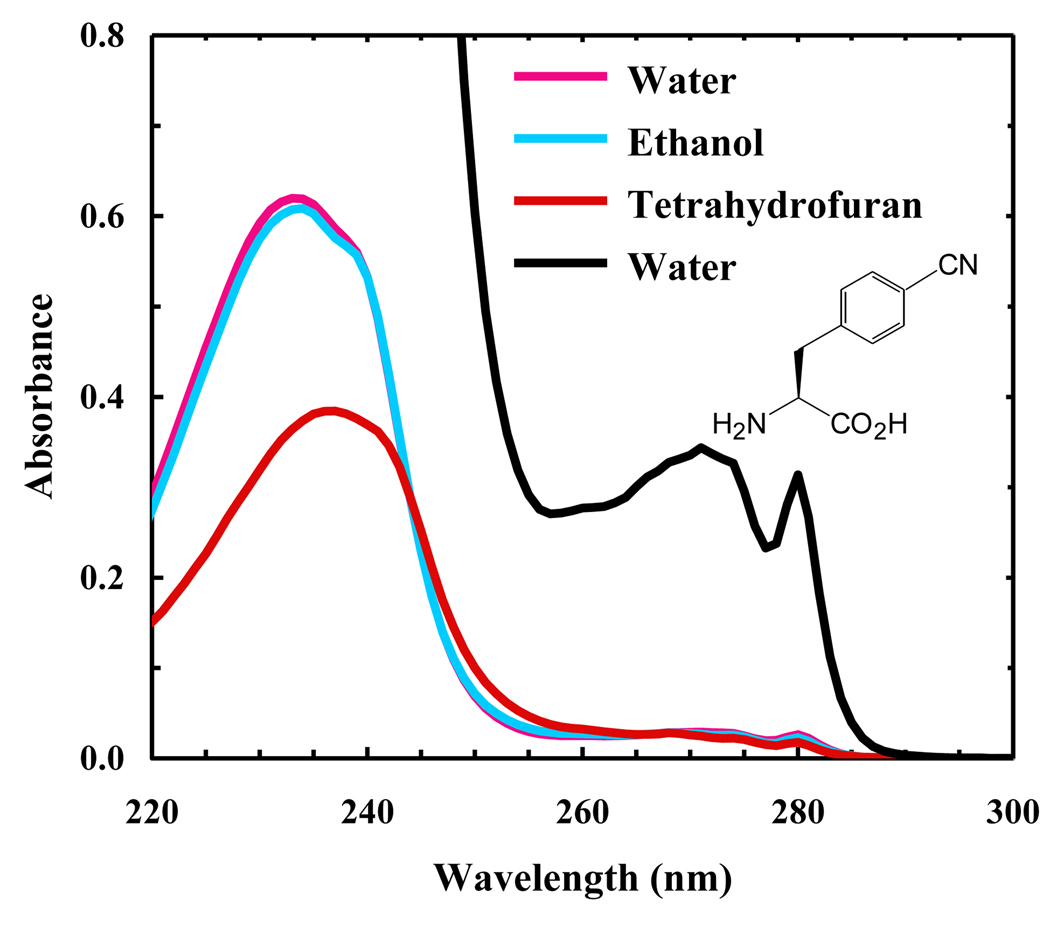
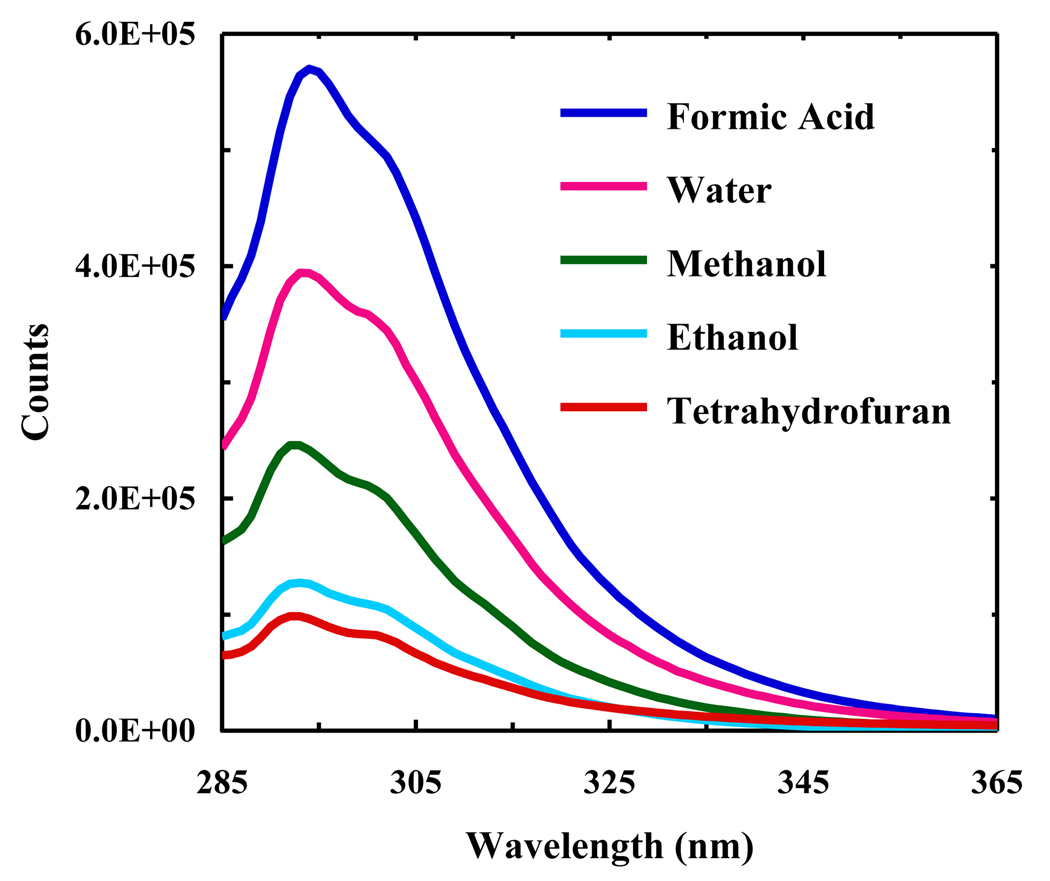
As shown (Figure 1), the absorption spectrum of PheCN in water is dominated by two broad bands centered at around 240 and 280 nm, respectively. For benzene and benzonitrile the corresponding bands have been attributed to two π*←π transitions [20]. In the case of benzonitrile, it has been shown that the electronic transition dipole moment to the first excited singlet state (1Lb) is along the minor axis of the substituted benzene ring, whereas the transition moment to the second excited singlet state (1La) is along the major axis [20,21]. We believe that these assignments should also hold true for PheCN. As indicated (Figure 2), the fluorescence spectrum of PheCN peaks at around 295 nm and is independent of the excitation wavelength (e.g., 240 or 280 nm), suggesting that the fluorescence emission occurs from the lowest excited singlet state, a feature that is common to polyatomic organic molecules [22]. In addition, the profiles of both the absorption (in the region above 260 nm) and fluorescence spectra of PheCN are relatively insensitive to solvent (Figure 1 and 2), which is in agreement with previous studies indicating that, for benzonitrile, the dipole moment of the ground state is very similar to that of the first excited state [23,24].
Figure 1.
Representative absorption spectra of PheCN in different solvents, as indicated. The PheCN concentration was approximately 25 µM. The black line represents the absorption spectrum of PheCN in water at a higher solute concentration (~370 µM). Also shown in the figure is the molecular structure of PheCN.
Figure 2.
Representative fluorescence spectra of PheCN in different solvents, as indicated. The PheCN concentration was approximately 370 µM and the excitation wavelength was 275 nm.
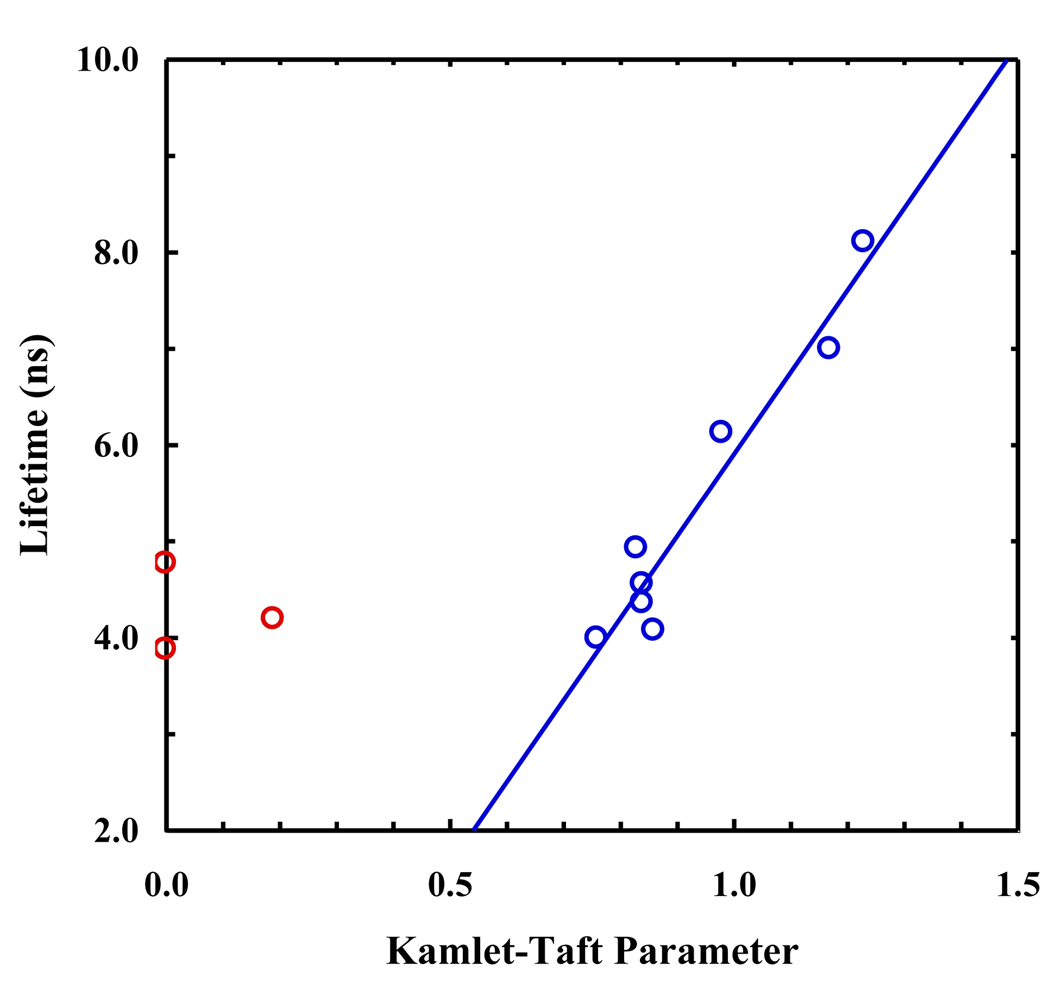
As shown (Figure 3 and Table 1), the fluorescence decay kinetics of PheCN in protic solvents fit reasonably well to a single-exponential function (i.e., χ2 < 1.2) and the resulting fluorescence lifetimes (τ) are in the range of 5–8 ns. However, for those data obtained in acetonitrile, tetrahydrofuran, formamide, and 1,2-propane diol, an additional component with a lifetime of about 1 ns and a relative intensity of 7–16% is required to fit the data satisfactorily. Since PheCN has lower solubility in these solvents when in the form of a free amino acid, we tentatively attribute this fast phase to the presence of PheCN aggregates wherein the fluorescence decay is expected to become faster due to self-quenching. This assignment is corroborated by the fact that the fluorescence decay kinetics of PheCN in a mixture of tetrahydrofuran (THF) and water (9:1, v/v) can be adequately described by a single-exponential function with a lifetime of 4.4 ns. As indicated (Figure 4), the fluorescence lifetimes of PheCN measured in those protic solvents show a linear dependence on the Kamlet-Taft α parameter [14]. Since the latter is an empirical measurement of the hydrogen-bond donating ability of the solvent, this result suggests that the fluorescence lifetime of PheCN is mediated by the strength of the hydrogen bond formed between the solvent and C≡N group. This picture is consistent with the recent infrared study of Aschaffenburg et al. [25] which showed that the C≡N stretching vibration of benzonitrile is also linearly dependent on the Kamlet-Taft α parameter of the solvent, although a good fit could only be achieved by considering a weaker but significant linear contribution from the solvent polarity/polarizability parameter π*. Thus, fluorescence lifetime measurements could potentially be very useful in providing a more quantitative assessment of the local hydration status of PheCN in a protein context in comparison to infrared measurement of the stretching frequency of the C≡N group.
Figure 3.
A representative fluorescence decay curve (blue) of PheCN in 2-propanol obtained with an excitation wavelength of 275 nm. The red line represents the best fit of these data to a single-exponential function convoluted with the instrument response function. Also shown in the top panel are the residuals of the fit.
Table 1.
Photophysical properties of p-cyanophenylalanine (PheCN) in different solvents. The Kamlet-Taft α parameters are adopted from reference 14.
| Solvent | α | Components | % | τ(ns)a | QYb | kr (s−1) | knr (s−1) | χ2 |
|---|---|---|---|---|---|---|---|---|
| tetrahydrofuran | 0 | 2 | 93 | 3.9 | 3.1 × 10−2 | 7.9 × 106 | 2.5 × 108 | 1.26 |
| 7 | 0.4 | |||||||
| acetonitrile | 0.19 | 2 | 85 | 4.2 | 2.6 × 10−2 | 6.2 × 106 | 2.3 × 108 | 1.02 |
| 15 | 1.1 | |||||||
| formamide | 0 | 2 | 94 | 4.8 | 8.3 × 10−2 | 1.7 × 107 | 1.9 × 108 | 1.07 |
| 6 | 0.5 | |||||||
| 1,2 propane diol | 0.83 | 2 | 84 | 4.9 | 1.0 × 10−1 | 2.1 × 107 | 1.8 × 108 | 1.10 |
| 16 | 1.0 | |||||||
| 2-propanol | 0.76 | 1 | 100 | 4.0 | 7.1 × 10−2 | 1.8 × 107 | 2.3 × 108 | 1.16 |
| 1-propanol | 0.84 | 1 | 100 | 4.4 | 7.4 × 10−2 | 1.7 × 107 | 2.1 × 108 | 1.16 |
| 1-butanol | 0.84 | 1 | 100 | 4.6 | 7.9 × 10−2 | 1.7 × 107 | 2.0 × 108 | 1.14 |
| ethanol | 0.86 | 1 | 100 | 4.1 | 3.4 × 10−2 | 8.5 × 106 | 2.4 × 108 | 1.14 |
| methanol | 0.98 | 1 | 100 | 6.1 | 7.6 × 10−2 | 1.2 × 107 | 1.5 × 108 | 1.13 |
| water | 1.17 | 1 | 100 | 7.0 | 1.1 × 10−1 | 1.6 × 107 | 1.3 × 108 | 1.04 |
| formic acid | 1.23 | 1 | 100 | 8.1 | 1.7 × 10−1 | 2.1 × 107 | 1.0 × 108 | 1.05 |
The percent error in these values was estimated to be about ±5%.
The percent error in these values was estimated to be about ±10%.
In order to provide a better understanding of why the fluorescence lifetime of PheCN shows a linear dependence on the Kamlet-Taft α parameter of those protic solvents, we further measured its fluorescence quantum yields (Φ) and consequently determined the radiative (kr) and non-radiative (knr) rate constants for each solvent using the following relationships:
| (2) |
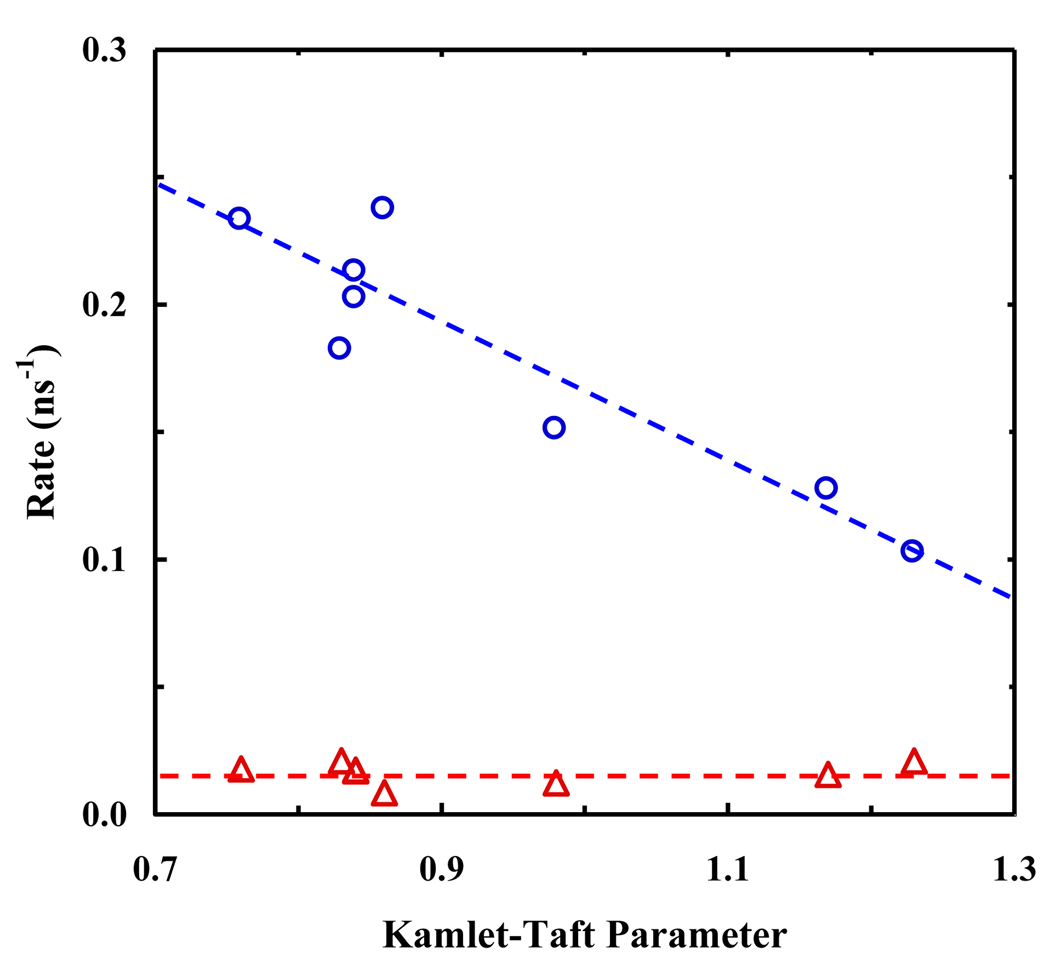
where τ is the fluorescence lifetime. As shown (Table 1), the fluorescence quantum yield (QY) of PheCN tends to increase with increasing hydrogen bonding ability of the solvent. As a result, knr also shows a clear dependence on the Kamlet-Taft α parameter where a larger α corresponds to a slower non-radiative decay rate, while kr is much less sensitive to solvent (Figure 5). These results are consistent with the observation that the absorption and emission spectral profiles and the molar absorptivity of PheCN at wavelengths longer than 260 nm are insensitive to solvent, which may indicate that it is mainly the non-radiative decay channels that are affected by the solute-solvent interactions [26,27]. However, the observed trend for knr herein is somewhat unusual as a stronger interaction with solvent is often expected to result in a faster non-radiative decay rate of the excited state, as observed for cyano substituted indolines [28].
Figure 5.
The radiative and non-radiative decay rate constants of PheCN versus solvent Kamlet-Taft α parameter. The dashed lines are to guide the eyes.
A previous study [20] has suggested the existence of a bent conformation of benzonitrile in the gas phase with a “dark” S1 state that is nearly isoenergetic with the 1Lb state of the linear conformation. If such a state also exists in PheCN in the solution phase, it could serve as a major non-radiative decay channel of the excited state population. As a result, hindrance of the bending motion of the molecule via hydrogen bonding interactions between the C≡N group and solvent would limit access to this state, which in turn would decrease the non-radiative decay rate of the excited state, as observed.
Moreover, the photophysics of PheCN appear to be very different from those of Trp and Tyr. It has been shown that the fluorescence lifetimes of free Trp and Tyr depend on the protonation state of the carboxylic acid group of the amino acids as charge transfer from the aromatic ring to the protonated carboxyl or amide groups is one of the major non-radiative decay processes for these naturally occurring fluorescent amino acids [29–31]. However, we find that the fluorescence lifetime of PheCN in water is insensitive to pH (e.g., t = 7.0 ± 0.2 ns at both pH 2 and 7), suggesting that charge transfer to the carboxyl group is unlikely to be a major decay channel of the fluorescent state of PheCN. In addition, we find that the fluorescence of PheCN in a peptide environment (i.e., GKFCNTV in water) decays exponentially with a lifetime of 6.8 ± 0.2 ns, indicating that the amide group is also unlikely to have a significant contribution to the quenching of PheCN fluorescence. Taken together, these results further substantiate the notion that the fluorescence lifetime of PheCN in protic solvents is mediated by the hydrogen bonding interactions between the C≡N group and solvent molecules.
4. Conclusion
In summary, the fluorescence lifetime of PheCN shows a linear dependence on the Kamlet-Taft hydrogen bond donating (α) parameter of the protic solvents used in the current study. In addition, it is found that this linear relationship is largely determined by the solvent dependence of the non-radiative decay rate of the excited state. Taken together, these results suggest that the solute-solvent hydrogen bonding interactions play an important role in mediating the fluorescence lifetime of PheCN, a mechanism that is different from that proposed to describe the photophysics of its structural analogue, Tyr. Thus, in comparison with the C≡N stretching vibration, we believe that measurements of the fluorescence lifetime of PheCN will be able to provide a more quantitative description regarding the local hydration status of proteins.
Figure 4.
Fluorescence lifetime of PheCN versus the solvent Kamlet-Taft α parameter. The blue open circles represent protic solvents, while the red open circles represent aprotic solvents. The line is to guide the eyes.
Acknowledgements
We gratefully acknowledge financial support from the National Institutes of Health (GM-065978 and RR-001348).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Getahun Z, Huang CY, Wang T, De Leon B, DeGrado WF, Gai F. J. Am. Chem. Soc. 2003;125:405. doi: 10.1021/ja0285262. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee S, Chowdhury P, DeGrado WF, Gai F. Langmuir. 2007;23:11174. doi: 10.1021/la701686g. [DOI] [PubMed] [Google Scholar]
- 3.Lindquist BA, Furse KE, Corcelli SA. Phys. Chem. Chem. Phys. 2009;11:8119. doi: 10.1039/b908588b. [DOI] [PubMed] [Google Scholar]
- 4.Tucker MJ, Getahun Z, Nanda V, DeGrado WF, Gai F. J. Am. Chem. Soc. 2004;126:5078. doi: 10.1021/ja032015d. [DOI] [PubMed] [Google Scholar]
- 5.Strzalka J, Liu J, Tronin A, Churbanova IY, Johansson JS, Blasie JK. Biophys. J. 2009;96:4164. doi: 10.1016/j.bpj.2009.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marek P, Gupta R, Raleigh DP. Chembiochem. 2008;9:1372. doi: 10.1002/cbic.200800052. [DOI] [PubMed] [Google Scholar]
- 7.Tucker MJ, Oyola R, Gai F. Biopolymers. 2006;83:571. doi: 10.1002/bip.20587. [DOI] [PubMed] [Google Scholar]
- 8.Tucker MJ, Oyola R, Gai F. J. Phys. Chem. B. 2005;109:4788. doi: 10.1021/jp044347q. [DOI] [PubMed] [Google Scholar]
- 9.Tang J, Yin H, Qiu J, Tucker MJ, DeGrado WF, Gai F. J. Am. Chem. Soc. 2009;131:3816. doi: 10.1021/ja809007f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glasscock JM, Zhu YJ, Chowdhury P, Tang J, Gai F. Biochemistry. 2008;47:11070. doi: 10.1021/bi8012406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waegele MM, Tucker MJ, Gai F. Chem. Phys. Lett. 2009;478:249–253. doi: 10.1016/j.cplett.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyake-Stoner SJ, Miller AM, Hammill JT, Peeler JC, Hess KR, Mehl RA, Brewer SH. Biochemistry. 2009;48:5953. doi: 10.1021/bi900426d. [DOI] [PubMed] [Google Scholar]
- 13.Taskent-Sezgin H, Chung J, Patsalo V, Miyake-Stoner SJ, Miller AM, Brewer SH, Mehl RA, Green DF, Raleigh DP, Carrico I. Biochemistry. 2009;48:9040. doi: 10.1021/bi900938z. [DOI] [PubMed] [Google Scholar]
- 14.Marcus Y. Chem. Soc. Rev. 1993;22:409. [Google Scholar]
- 15.Zhang L, Yang Y, Kao Y, Wang L, Zhong D. J. Am. Chem. Soc. 2009;131:10677. doi: 10.1021/ja902918p. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Wang L, Kao Y, Qiu W, Yang Y, Okobiah O, Zhong D. Proc. Natl. Acad. Sci. USA. 2007;104:18461. doi: 10.1073/pnas.0707647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams ATR, Winfield SA, Miller JN. Analyst. 1983;108:1067. [Google Scholar]
- 18.Weinryb I, Steiner RF. Biochemistry. 1970;9:135. doi: 10.1021/bi00803a018. [DOI] [PubMed] [Google Scholar]
- 19.Hill PA, Wei Q, Troxler T, Dmochowski IJ. J. Am. Chem. Soc. 2009;131:3069. doi: 10.1021/ja8100566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis FD, Holman B. J. Phys. Chem. 1980;84:2326. [Google Scholar]
- 21.Hara K, Suzuki H, Rettig W. Chem. Phys. Lett. 1988;145:269. [Google Scholar]
- 22.Bixon M, Jortner J. J. Chem. Phys. 1968;48:715. [Google Scholar]
- 23.Kawski A, Kuklinski B, Bojarski P. Chem. Phys. Lett. 2006;419:309. [Google Scholar]
- 24.Siglow K, Neusser HJ. J. Phys. Chem. A. 2001;105:7823. [Google Scholar]
- 25.Aschaffenburg DJ, Moog RS. J. Phys. Chem. B. 2009;113:12736. doi: 10.1021/jp905802a. [DOI] [PubMed] [Google Scholar]
- 26.Strickler SJ, Berg RA. J. Chem. Phys. 1962;37:814. [Google Scholar]
- 27.Mahaney M, Huber JR. Chem. Phys. Lett. 1984;105:395. [Google Scholar]
- 28.Pal K, Kallay M, Koehler G, Zhang H, Bitter I, Kubinyi M, Vidoczy T, Grabner G. Chemphyschem. 2007;8:2627. doi: 10.1002/cphc.200700479. [DOI] [PubMed] [Google Scholar]
- 29.Petrich JW, Chang MC, Mcdonald DB, Fleming GR. J. Am. Chem. Soc. 1983;105:3824. [Google Scholar]
- 30.Wiczk W, Rzeska A, Lukomska J, Stachowiak K, Karolczak J, Malicka J, Lankiewicz L. Chem. Phys. Lett. 2001;341:99. [Google Scholar]
- 31.Guzow K, Szabelski M, Rzeska A, Karolczak J, Sulowska H, Wiczk W. Chem. Phys. Lett. 2002;362:519. [Google Scholar]