Abstract
Background
Knowledge on the action of thyroid hormone (TH) is augmented by the study of tissue responses to TH in vitro. In order to support the growth of cells in vitro, calf serum (CS) is usually added to the medium to provide necessary nutrients and growth factors. However, the content of endogenous TH in the CS may obfuscate changes with small doses of TH. We therefore compared the use of TH-depleted medium, either by resin treatment (stripped-CS) or by the use of CS from a thyroidectomized calf (TxCS) for gene expression studies.
Methods
We describe the method for preparing a thyroidectomized calf, harvesting the blood and preparing the serum. We utilized methimazole in conjunction with the thyroidectomy to prevent TH synthesis in the event of regrowth of the thyroid remnant.
Results
Total triiodothyronine (T3) and thyroxine concentrations in TxCS were low at <30 ng/dL and <1 μg/dL, respectively. We compared the effect of T3 on basic transcription element-binding protein (BTEB)1 and stanniocalcin (STC)-1 mRNA expression in human fibroblasts from a normal individual and a subject with resistance to TH (RTH) cultured in stripped-CS to TxCS and demonstrated that with stripped-CS and TxCS differences in the BTEB1 and STC-1 expression of normal and RTH fibroblasts could be detected.
Conclusions
Both stripped-CS and TxCS are suitable to detect subtle differences in TH responsiveness between normal and RTH human skin fibroblasts, yet TxCS is not as costly as stripped-CS and relatively easy to prepare.
Introduction
Thyroid hormone (TH) is required for normal growth, development, and metabolism of vertebrate systems. Studies on the action of TH on human tissue in vitro have advanced our understanding of the mechanisms involved in TH action. In order to measure the responses of cells to TH in vitro l-3,3′,5-triiodothyronine (LT3) is added to the culture medium containing bovine serum (calf serum [CS]) and analysis is made by comparing the response with and without LT3. However, due to the presence of endogenous TH in CS, it is impossible to observe effects within the physiological levels of TH. Therefore it is necessary to use measurements obtained with TH-depleted CS as a baseline and then measure changes following treatment with TH. There are two general methods for obtaining CS depleted in TH, namely the use of serum from a thyroidectomized calf (Tx-CS) or CS stripped of TH by passing through charcoal or anion exchange resin to remove the TH (stripped-CS). In our experience, recent lots of commercially available Tx-CS contained unacceptably high amounts of TH. Stripped-CS is not only depleted in other substances, including growth factors that may otherwise not support optimal tissue response to TH, but also sustain large losses in the process of TH removal.
We describe a method for preparing Tx-CS with low TH by thyroidectomy of a weaned calf followed by treatment with an antithyroid drug. This method can be performed in any Animal Resource Center and is relatively inexpensive. Normal human skin fibroblasts and fibroblasts from a subject with resistance to thyroid hormone (RTH) were cultured in medium with Tx-CS or stripped-CS prior to TH treatment to test which serum is better suited for experiments using physiological TH concentrations. Data demonstrate that Tx-CS and stripped-CS are equally suitable to detect differences in TH responsiveness in normal and RTH skin fibroblasts.
Materials and Methods
Thyroidectomy of calf
A 3-week-old weaned male Holstein calf was obtained from a commercial milking farm, supplied by a United States Department of Agriculture licensed dealer. At this age the calf was able to drink a soy-based commercial calf formula out of a bucket and eat solid food. The calf weighed 46 kg on arrival, gained 17 kg by the time of harvest 22 days later, so it was reasoned that levels of growth factors should be reasonably high.
Seven days after arrival at our facility, the calf was pre-anesthetized with ketamine (4.4 mg/kg i.v.) and xylazine (0.1 mg/kg i.v.) then inhalation anesthesia via a mask with isoflurane. The calf was immediately intubated with a 10-mm endotracheal tube and maintained on isoflurane (1.0–2.1%, inhaled concentration). A 10-mm orogastric tube was placed to prevent gas accumulation in the rumen. Intraoperative monitoring included measurement of heart rate, respiratory rate, electrocardiography (ECG), saturation of peripheral oxyhemoglobin (spO2), end-tidal carbon dioxide (etCO2), tidal volumes, noninvasive blood pressure, and body temperature (Datex Ohmeda Cardiocap, II, Datex Ohmeda Inc., Madison, WI).
Total thyroidectomy was performed through a standard midventral neck incision. After dissection of skin, subcutaneous tissue, and muscle layers, the thyroid gland lobes were identified on either side of the midcervical trachea. Through a combination of blunt and sharp dissection, preserving blood vessels and nerves in the area, the entire thyroid gland was removed. The parathyroid glands were identified and preserved. Closure was with 3/0 polydioxanon suture under the skin, and 2/0 nylon in the skin. Skin sutures were removed 10–14 days postoperatively.
The calf was given soy milk and feed ad lib. Methimazole (Tapazole®; King Pharmaceuticals, Bristol, TN), 20 mg every 12 hours, was administered by hand with the soy milk from the day after thyroidectomy until 24 hours prior to the final bleeding. The methimazole was administered in order to assure low TH levels in case of regrowth of any thyroid remnant left after surgery.
All procedures were approved by the University of Chicago Institutional Animal Care and Use Committee. The University of Chicago Animal Resources Center is an Association for Assessment and Accreditation of Laboratory Animal Care approved facility.
Phlebotomy and measurement of thyroid hormone levels
We obtained a 5-mL blood sample weekly after surgery from the anterior jugular vein by restraining the calf, to check for levels of total triiodothyronine (TT3) and total thyroxine (TT4). Levels were undetectable (TT3 < 30 ng/dL and TT4 < 1 μg/dL) 23 days post-thyroidectomy while on methimazole. The calf weighed 63 kg at the time of final bleed. The calf was pre-anesthetized with 4.4 mg/kg ketamine i.v. and 0.1 mg/kg diazepam i.v., intubated as before, and maintained on isoflurane anesthesia. Blood was initially harvested through the jugular vein. After spontaneous blood flow through the jugular significantly decreased (after 1135 mL), a series of epinephrine injections of 0.5 mg were administered every 5 minutes for 30 minutes to stimulate cardiac pumping activity to obtain an additional 755 mL of blood. After the epinephrine treatment no longer increased blood loss, open cardiac puncture was able to obtain an additional 130 mL of blood and after the heart chambers were emptied a femoral vein cutdown removed the 40 mL of remaining blood. Blood was directly collected in sterile plastic 250-mL centrifuge bottles. The blood was allowed to clot overnight at 4°C. The clot was rimmed out and separated from the serum, filtered with 45-μm millipore filters, and stored in 50-mL sterile culture tubes for further evaluation. Serum separated from blood collected after injection of epinephrine was kept separately.
Serum TT4 and TT3 were measured by chemiluminescence immunoassay (Roche Boehringer Mannheim, Indianapolis, IN). Free (F)T4I and FT3I were calculated as the product of TT4 and TT3, respectively, and the T4 resin uptake ratio. Thyrotropin (TSH) was not measured due to lack of specific bovine TSH antibody.
Cell culture and treatments
Human skin was obtained by punch biopsy from a normal individual (Normal) and a patient with RTH, heterozygous for a dominant negative TRβ mutation (A317T). The study was approved by the Institutional Review Board of the University of Chicago. Primary cultures of fibroblasts were grown in Dulbecco's modified Eagle medium (Life Technologies Inc., Gaithersburg, MD) supplemented with 10% fetal bovine serum (FCS) as previously described in detail (1). At confluency, the medium was replaced with one containing TH-depleted CS. In separate culture dishes, the CS used was from either a thyroidectomized calf (Tx-CS) or stripped of TH by treatment of FCS with anion exchange resin (Aldrich Amberlite® IRA-400 Cl; Sigma, St. Louis, MO) (stripped-CS). Forty-eight hours later, LT3 was added to a final concentration of 5 × 10−10 and 2 × 10−9 M. Based on the FT3 concentration in the medium containing 10% FCS, measured by equilibrium dialysis, these two T3 doses correspond to a third to half physiological and 3–5 times physiological, respectively. All experiments were carried out at early cell passages (≤10). For DNA and RNA measurement, cells were grown in medium containing 10% of either FCS or Tx-CS. After 4 days the cells were harvested and DNA and RNA were measured.
Isolation and reverse transcription of RNA
The medium was removed and the cell layer was washed twice with Hank's buffered saline solution (Invitrogen, Carlsbad, CA). Total RNA was extracted using phenol/guanidine isothiocyanate (TRIZOL; Invitrogen, Carlsbad, CA). RNA was reverse transcribed with the SuperscriptTM III RNase H Reverse Transcriptase Kit (Invitrogen) using 2 μg of total RNA and 100 ng of random hexamers.
Real-time PCR
Quantification of mRNAs was performed in an ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA), using SYBR Green I as detector dye. The reaction mixtures contained 12.5 μL iTaq SYBR Green Supermix with ROX (BioRad, Hercules, CA), 0.3 μmol/L of each primer, 10 ng template cDNA, and nuclease-free water to a final volume of 25 μL. The oligonucleotide primers for basic transcription element-binding protein (BTEB)1 were designed to cross from exon 2 to exon 3: forward primer 5′-CTC CCA TCT CAA AGC CCA TTA C-3′ and reverse primer 5′-TGA GCG GGA GAA CTT TTT AAG G-3′. Primers for stanniocalcin 1 (STC-1) were designed to cross from exon 1 to exon 3: forward primer 5′-TGT GAG CCC CAG GAA ATC C-3′ and reverse primer 5′-TTC CTG CAC CTC AGC AAT CA-3′. The reaction conditions were 95°C for 2 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 45 seconds. Gene expression was calculated relative to that in untreated cells and normalized for ubiquitin-conjugating enzyme E2D2 (UBE2D2) (2), measured under the same conditions, using the 2−ΔΔCT method (3).
Results
Thyroidectomy of calf
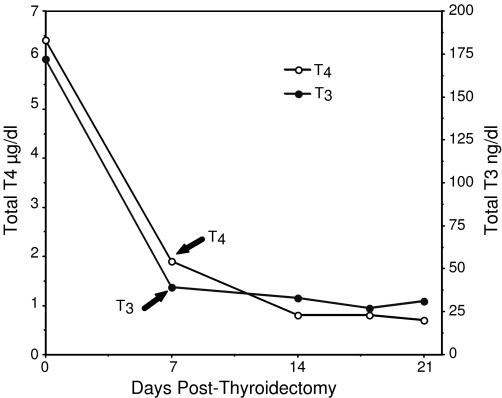
Initial TT4 and TT3 in the calf's serum were 6.4 μg/dL and 172 ng/dL, respectively, and decreased to 27% and 29% of baseline values, respectively, 7 days post thyroidectomy with methimazole treatment. Nadir for TT4 (<1) and TT3 (<30 ng/dl) concentrations was reached at 3 weeks (Fig. 1). The TT4 and TT3 concentrations in the final processed TxCS were <1 and 31 ng/dL. TSH was not measured due to the lack of specific antibodies for bovine TSH. The TH concentrations in stripped-CS (values before stripping in parentheses) were TT3 43 ng/dL (128 ng/dL) and TT4 1.2 μg/dL (10.4 μg/dL). The TT4 and TT3 concentrations in stripped-CS and TxCS therefore were almost comparable.
FIG. 1.
Thyroid hormone levels (total thyroxine [TT4], open circles; total triiodothyronine [TT3], closed circles) in calf sera before and after thyroidectomy. Calf was given methimazole (Tapazole®), 20 mg twice a day until 24 hours prior to the final bleeding.
Cell growth in Tx-CS containing medium
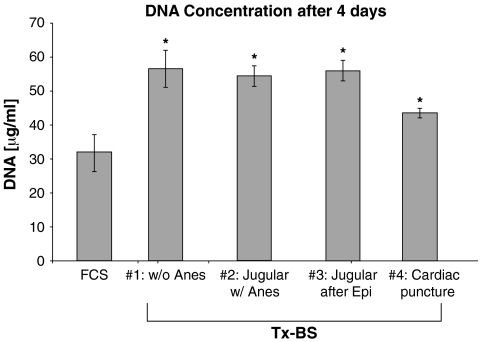
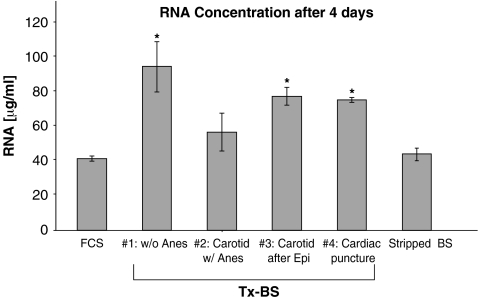
Cell growth was assessed in normal fibroblasts by measurement of total DNA (Fig. 2) and RNA (Fig. 3) concentrations in the dish at the end of a 4-day incubation. The concentrations of DNA (32 ± 6 μg/mL versus 56 ± 6 μg/mL, ANOVA p = 0.007) and RNA (41 ± 1 μg/mL versus 95 ± 14 μg/mL, ANOVA p = 0.004) were higher in the TxCS compared to the stock FCS. There was no apparent effect of the anesthetic, nor the use of epinephrine to improve cardiac output towards the end of the exsanguination on DNA and RNA concentrations with the different batches of serum harvested (Figs. 2 and 3).
FIG. 2.
DNA concentration in the tissue culture dish after 4 days of culture with untreated fetal bovine serum (FCS) or serum from a thyroidectomized calf (Tx-CS). #1, performed during weekly blood draw from a jugular vein using gentle restraint to the obtain the blood, without anesthesia (Anes); #2, jugular vein blood during anesthesia; #3, jugular blood collected during six doses of epinephrine (Epi) to stimulate cardiac contractions; #4, following cardiac puncture. Each point represents three different dishes and DNA concentrations were determined in duplicate. Normal skin fibroblasts were used. *, ANOVA p < 0.004 and p significant at 5% using Fisher's test.
FIG. 3.
RNA concentration in the tissue culture dish after 4 days of culture with untreated FCS, Tx-CS, and stripped-CS. #1, performed during weekly blood draw from jugular vein using gentle restraint to the obtain the blood, without anesthesia; #2, jugular vein blood during anesthesia; #3, jugular vein blood collected during six doses of epinephrine to stimulate cardiac contractions; #3, following cardiac puncture. Each point represents three different dishes and RNA concentrations were determined in duplicate. Normal skin fibroblasts were used. *, ANOVA p < 0.007 and p significant at 5% using Fisher's test.
BTEB1 and STC-1 expression in normal and RTH fibroblasts in TxCS and stripped-FCS containing medium
BTEB1 has been previously reported to be a thyroid hormone receptor (TR) β dependent gene that is up-regulated in human skin fibroblasts by TH (4). We found STC-1 to be another TH-responsive gene in human fibroblasts, probably induced by TH through activation of PI3K (5). We examined the expression of BTEB1 and STC-1 in normal and RTH fibroblasts cultured in medium with TxCS, stripped-CS, or FCS.
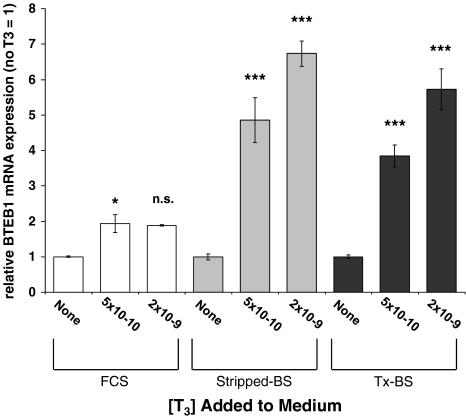
When cultured with untreated FCS, only a small effect of TH is seen on BTEB1 expression in normal fibroblasts with less than twofold stimulation after incubation with LT3, 5 × 10−10 (1.9; p < 0.05) or 2 × 10−9 M (1.9; NS) (Fig. 4, open bars). However, compared to the expression in the same medium in the absence of TH, there was a significant increase in BTEB1 expression after the addition of LT3 in the same two doses to stripped-CS (4.9-fold; p < 0.005 and 6.7-fold; p < 0.001) and TxCS (3.8-fold; p < 0.005 and 5.7-fold; p < 0.005) (Fig. 4, gray and black bars). Not only was the BTEB1 induction significantly higher than in cells grown in FCS containing medium, in addition a dose response was preserved with increasing BTEB1 expression with increasing LT3 dose.
FIG. 4.
Effect of l-triiodothyronine (LT3) on expression of BTEB1 in normal fibroblasts cultured with untreated FCS, stripped-CS, and Tx-CS. The values are reported as relative to the activity of basic transcription element-binding protein (BTEB) in each medium without LT3. *, ANOVA p < 0.05; ***, ANOVA p < 0.001.
Although fibroblasts are responsive to TH, the responses can be subtle or mild. In order to determine whether Tx-CS or stripped-CS would best demonstrate the differences in gene expression, we tested the response to TH on normal and RTH fibroblasts in the two TH-depleted media.
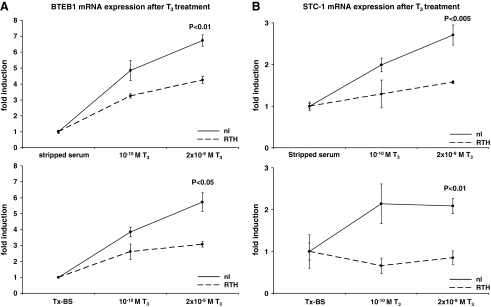
Addition of 2 × 10−9 M T3 led to increases in BTEB1 expression in both media. The increase was significantly higher in the normal than in the RTH fibroblasts grown in stripped-CS with 6.7- and 4.25-fold, respectively (p < 0.01) (Fig. 5A). Similarly, when grown in medium containing Tx-CS, BTEB1 was significantly more induced in normal fibroblasts than in the fibroblasts from the subject with RTH (5.7- vs. 3.0-fold; p < 0.05) (Fig. 5A).
FIG. 5.
Effect of LT3 on expression of (A) BTEB1 and (B) STC-1 in fibroblasts from a normal subject (solid line) and one with resistance to thyroid hormone (RTH, dashed line) cultured with untreated stripped-CS and Tx-CS. The values are reported as fold induction relative to the respective FCS without added LT3.
For STC-1, the same pattern of expression could be observed. TH treatment led to an increase in STC-1 expression in both types of media in normal fibroblasts. The increase was significantly higher in normal fibroblasts than in RTH fibroblasts in Tx-CS (2.7- vs. 1.6-fold; p < 0.005) as well as in stripped-CS (2.1- vs. 0.9-fold; p < 0.01) (Fig. 5B).
Taken together these data show that stripped-CS and Tx-CS are effective in supporting cultured human skin fibroblast. In a study examining the effect of this protocol on TH-induced gene expression in fibroblasts from a normal subject and one with RTH, both media equally revealed the differences in responses.
Discussion
Laboratories that investigate the in vitro effects of TH have used either stripped-CS or Tx-CS to support cell growth. We have found variable results when using the resin purification procedure per Samuels et al. (6). Additionally there is a volume loss of up to 50% when using this procedure, significantly increasing the cost. On the other hand, there were no satisfactory commercial sources of thyroidectomized calf serum that was sufficiently TH depleted. We found that even with expert and complete thyroidectomy, if the animal is not placed on antithyroid medication, the thyroid hormone levels can rise again due to regrowth of thyroid tissue from very small remnants. Furthermore, most researchers in an academic setting may think that performing the thyroidectomy on site would be near to impossible.
We evaluated the cost and ease of preparing our own stock of thyroidectomized bovine serum. With the assistance of two large animal veterinarians (coauthors C.W. and M.N.) we were able to write a protocol for approval by the Institutional Animal Care and Use Committee. The calf was purchased for $300 from a local milk farm by a licensed vendor. The animal was housed in a holding area as is used for pigs at our institution at a cost of $5 per day. The procedure was completed in 30 days. At the time of exsanguination we obtained 2225 mL of whole blood, nearly 55% total blood volume calculated for a calf of 63 kg. This resulted in 775 mL of serum that could be used for in vitro experiments at an estimated cost, based on University of Chicago pricing, of $1000. To obtain an equal amount of stripped-CS, (with an estimated 50% loss during preparation) would cost $1800.
It has previously been shown that a pituitary cell line was able to respond equally well to TH when cultured in either stripped-CS or Tx-BS (6). However, it was not known whether skin fibroblasts, a readily available tissue from patients, behaved similarly. There are potential pitfalls with the use of either type of serum. In stripped-CS, certain unidentified growth factors could also be bound by the resin. In the Tx-CS, growth factors are thought to be present in a normal amount, but there could be effects of anesthesia and methimazole. We found no apparent effect of anesthesia or epinephrine on the RNA or DNA concentration. We bled the calf 24 hours after methimazole was held. The half life of methimazole is less than 5 hours. However, measurement of methimazole is not readily available and the possibility of an effect from some remaining methimazole cannot be completely ruled out. It is also possible Tx-CS may be depleted in factors other than thyroid hormone that might be important for normal physiology in vitro. An alternative approach for preparation of TH-free serum, although less practical, could be affinity chromotography with high-affinity T3 antibodies.
When compared, we found that both types of serum allowed study of TH responsiveness of genes in human skin fibroblasts. RTH subjects have reduced tissue responsiveness to TH (7). In order to study this in vitro, the culture conditions should be optimized so that subtle differences in responsive genes can be observed (8). We have demonstrated that in skin fibroblasts, the use of both Tx-CS and stripped-CS allowed to demonstrate differences in TH responsiveness in the BTEB1 and STC-1 genes.
In conclusion, while the use of stripped-CS is acceptable, preparation of Tx-CS is within the capabilities of most Academic Medical Centers with experienced and skilled large animal veterinarians and is more cost efficient.
Acknowledgments
We acknowledge the expert assistance of the Animal Health Technicians in the Carlson Clinic: Caroline Miskell, CVT, Karin Peterson, CVT, and Margaret Bruner, CVT. This study was supported by NIH Grants DK17050, DK 58258, RR18372 and RR00055, IFORES Grant University of Duisburg-Essen, and Seymour J. Abrams Center for Thyroid Research.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Murata Y. Ceccarelli P. Refetoff S. Horwitz AL. Matsui N. Thyroid hormone inhibits fibronectin synthesis by cultured human skin fibroblasts. J Clin Endocrinol Metab. 1987;64:334–339. doi: 10.1210/jcem-64-2-334. [DOI] [PubMed] [Google Scholar]
- 2.Hamalainen N. Pette D. Myosin and SERCA isoform expression in denervated slow-twitch muscle of euthyroid and hyperthyroid rabbits. J Muscle Res Cell Motil. 2001;22:453–457. doi: 10.1023/a:1014543507149. [DOI] [PubMed] [Google Scholar]
- 3.Livak KJ. Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 4.Moeller LC. Dumitrescu AM. Walker RL. Meltzer PS. Refetoff S. Thyroid hormone responsive genes in cultured human fibroblasts. J Clin Endocrinol Metab. 2005;90:936–943. doi: 10.1210/jc.2004-1768. [DOI] [PubMed] [Google Scholar]
- 5.Moeller LC. Ulanowski NE. Refetoff S. Mann K. Janssen OE. Stanniocalcin 1 is induced by thyroid hormone via cytosolic action of the thyroid hormone receptor β (Abstract) The Endocrine Society's 88th Annual Meeting. 2006.
- 6.Samuels HH. Stanley F. Casanova J. Depletion of l-3,5,3'-triiodothyronine and l-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology. 1979;105:80–85. doi: 10.1210/endo-105-1-80. [DOI] [PubMed] [Google Scholar]
- 7.Refetoff S. Weiss RE. Resistance to thyroid hormone. In: Thakker T, editor. Molecular Genetics of Endocrine Disorders. Chapman & Hill; London: 1997. pp. 85–122. [Google Scholar]
- 8.Sobieszczyk S. Refetoff S. Sobieszczyk S, Refetoff S 1987 Abnormal response of fibronectin messenger RNA to triiodothyronine in fibroblasts from patients with generalized resistance to thyroid hormone (Abstract T-24). 63rd Annual Meeting of the American Thyroid Association; Montreal, Quebec: American Thyroid Association; 1987. [Google Scholar]