Abstract
Background
Whether thyroid cancer is more aggressive in radiation-exposed patients is not resolved. The frequency of aggressive features in post-Chernobyl patients suggests this may be the case. Our aim was to address this question by re-examining the pattern of risk factors for recurrence of thyroid cancers found in a cohort exposed to external radiation.
Methods
The study population was drawn from a cohort of 4296 people, followed since 1974, who were treated before the age of 16 with conventional external radiation for benign conditions of the head and neck between 1939 and the early 1960s. The study group consisted of 390 patients who had surgically verified thyroid cancer. Potential risk factors for recurrence were evaluated by proportional hazards analysis.
Results
Fifty patients had recurrences an average of 8.7 years after diagnosis while the other 340 patients were followed for an average of 19.7 years. The sooner after radiation exposure the cancer occurred, the more likely it was to recur (hazard ratio, 0.96/year; 95% confidence interval [CI] 0.91–0.99). Taking into account the effect of the onset of screening in 1974, the features predictive of recurrence were younger age at the initial diagnosis (hazard ratio, 0.95/year; 95% CI, 0.91–0.99) and the size of the thyroid cancer (hazard ratio, 1.2/cm; 95% CI, 1.0–1.6).
Conclusion
Although not based on a direct comparison, we conclude that thyroid cancers following external radiation exposure are not, on average, more aggressive than other thyroid cancers. The similarity of risk factors for recurrence suggests that they should be treated and followed in the same way as non–radiation-induced thyroid cancers.
Introduction
While there is a great deal known about radiation as a cause of thyroid cancer, much less is known about how radiation-related thyroid cancers behave and should be treated. We first addressed this question in 1986 using the clinical findings in a cohort of 4296 radiated-exposed individuals (1). At that time there were 296 cases of thyroid cancer in the cohort. A distinctive finding was the high frequency of multicentricity, but the baseline and treatment factors related to recurrence were very similar to those for thyroid cancer patients in the general population. Based on this and the findings of others we recommended that patients with radiation-related thyroid cancer should be treated in the same way as unexposed patients with thyroid cancer (2–8).
The thyroid cancer cases following the Chernobyl disaster suggest that this conclusion may not hold in all circumstances. The histological characteristics of the thyroid cancers found in patients living near Chernobyl had aggressive features and many cases required multiple surgical and radioactive iodine treatments (9–11). However, it is not known to what extent these features are related to the patients' age and environment, in addition to the radiation exposure.
The principal aim of the present study was to reevaluate the behavior of the thyroid cancers, by examining which risk factors are related to recurrence, in our cohort with the larger number of cases and the longer follow-up that have accrued since our last analysis. We then compared these factors to those reported in other, larger studies of sporadic cases.
Methods
Study subjects
The study cohort consists of 4296 individuals who were treated before the age of 16 with conventional external radiation for benign conditions of the head and neck at Michael Reese Hospital in Chicago between 1939 and the early 1960s. Information on whether they had thyroid surgery was available for 3126 (72.8%) of them. Of these, 1112 had thyroid surgery and 390 (12.5% of the 3126) had thyroid cancer. These 390 individuals are the subjects of this study. The study was reviewed and approved by the University of Illinois at Chicago Institutional Review Board.
Follow-up
Information about the subjects was obtained by sending out questionnaires between September 2006 and June 2007. The information requested included a detailed history of diagnostic thyroid investigations including ultrasounds, scans, and fine-needle aspirations and their results; thyroid medications; radioactive iodine treatments; and all thyroid surgeries after the initial one for cancer. Forty-one of the subjects had died before the most recent survey was conducted.
Recurrences of cancer were defined as surgery with one or more malignant nodules or metastases demonstrated by radioactive iodine therapy–related imaging. The criteria for demonstrating a recurrence with radioactive iodine were uptake outside the thyroid bed, uptake in a previously unaffected area, or uptake in the thyroid bed at least 1 year after therapy with ≥100 mCi of radioactive iodine. Surgery within 6 months of the original surgery was not considered a recurrence. No attempt was made to distinguish between persistent and recurrent disease.
The time to recurrence was calculated using the date on which a new nodule was removed by surgery or, if there was no surgery, the date of radioactive iodine therapy. For subjects with more than one recurrence, only the earliest was considered.
Statistical analysis
The time trends for the cases of thyroid cancer and the recurrences were calculated by Kaplan–Meier analysis. To investigate the factors affecting the rate of recurrence, univariate and multivariate analyses were performed by Cox analysis, as implemented with the Epicure (HiroSoft International Corp., Seattle, WA) and SPSS (SPSS Inc., Chicago, IL) programs. Significance was assessed by determining whether or not the 95% Wald confidence interval for the hazard ratio included 1.0.
The baseline variables that were tested as potential risk factors for recurrence of thyroid cancer were sex (male vs. female), radiation dose to the thyroid (gray, Gy), age at time of radiation exposure (years), age at time of diagnosis of first thyroid cancer (years), and latency (years). Latency was defined as the time between radiation exposure and first surgery.
The findings at the time of first surgery that were tested as potential risk factors for recurrence were the number of nodules (as an integer), the number of lobes involved (one vs. two), tumor histology (papillary vs. follicular, including two cases of Hürthle cell cancer), capsular invasion (present vs. absent), vascular invasion (present vs. absent), cervical lymph node involvement (present vs. absent), and size of the largest cancer nodule (in centimeters, as a continuous variable). Previously, we reviewed the early-occurring follicular cancers to determine if they were, in fact, follicular-variant papillary cancers, but the materials were not available to do this in every case. In some cases the largest focus of thyroid cancer was in a lymph node. When a lymph node was the largest focus of cancer and no focus was found within the gland, we considered it unifocal and unilateral.
To determine if any aspect of treatment affected the rate of recurrences, we tested the extent of the initial surgery, post-operative ablative radioactive-iodine therapy, and post-operative suppressive thyroid hormone therapy. The extent of thyroidectomy was defined for each lobe as total, subtotal, or nodulectomy and was assigned a value of 1.00, 0.50, or 0.25, respectively. Based on these criteria the fraction of thyroid tissue removed was calculated as the sum of right and left lobes divided by 2. Thyroid hormone treatment was expressed as a continuous variable, the fraction of time on therapy between the time of first surgery and time of recurrence or date of last follow-up. The type and dose of thyroid hormone were available in almost all cases. However, these were not used in the analysis as the reason for giving treatment and the thyrotropin (TSH) levels aimed for and attained were not available. Radioactive iodine ablation was defined as the use of ≥29 mCi within 1 year of the surgery.
To determine the effect of extensive publicity and more rigorous screening programs that began in 1974, we grouped and compared the subjects according to those who had more clinically evident thyroid cancer (1973 and earlier) and those whose cancer was discovered mostly as a result of more rigorous screening programs (1974 and later). Complementary log-log plots (also called one minus log vs. time plots) for these two groups were calculated and compared using the SPSS program (12).
Results
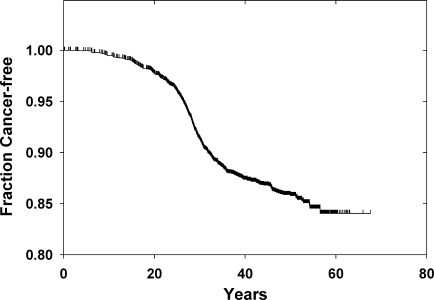
In the cohort there were 390 cases of thyroid cancer, 124 diagnosed before 1974 and 266 cases in 1974 or later. The number of new cases appears to abate about 30 years after radiation exposure (Fig. 1). Of the 349 living subjects at the time of the latest follow-up, 101 returned the survey. For all but nine of the rest, follow-up of at least 1 year had been obtained previously.
FIG. 1.
Thyroid cancer from time of exposure in the cohort. Shown is a Kaplan–Meier plot starting at the time of radiation exposure and extending to the time of thyroid cancer or last vital status.
Recurrence
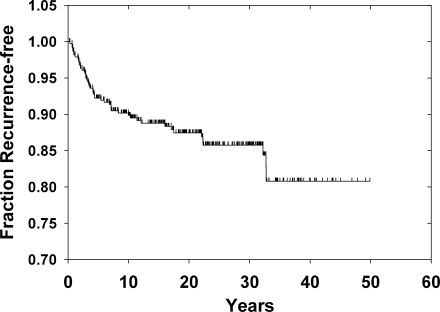
In the 390 thyroid cancer cases, 50 (12.8%) had recurrences between 0.67 and 53.3 years after the initial surgery (Fig. 2). The average time to recurrence was 8.7 years. As seen in Figure 2, the distribution was skewed to earlier times and the median time to recurrence was 4.1 years. For the 340 without recurrence the follow-up times were normally distributed (mean 19.7 ± 11.4 [SD] years, median 19.2 years). In total, there were 7147 person-years of follow-up accrued after the diagnosis of thyroid cancer.
FIG. 2.
Recurrences among the 390 cases of thyroid cancer. Shown is a Kaplan–Meier plot starting on the date of the first surgery for thyroid cancer and extending to the time of recurrence or last vital status.
Thirty recurrences were confirmed by surgery and pathological analysis. In six there was cancer in the thyroid bed and lymph nodes, in 15 the recurrence was limited to one or more lymph nodes, in six the recurrence was confined to the thyroid, and in three the information was not available. There was one surgical case that also had pulmonary metastases. Twenty were considered recurrent on the basis of radioactive iodine therapy–related imaging. Four of these had pulmonary metastases (for a total of five cases with lung involvement). Ten had cervical uptake outside of the thyroid bed and five had persistent uptake confined to the region of the thyroid gland. In one, the location of the uptake was not available.
Baseline characteristics related to recurrence
The earlier a thyroid cancer occurred, the more likely it was to recur. The time of occurrence could be expressed either as the age of diagnosis or as the latency between exposure and diagnosis. The hazard ratio for recurrence was 0.94/year (95% CI, 0.91–0.97) for the former and 0.94/year (95% CI, 0.91–0.97) for the latter. We could not determine whether age or latency was more important as the two measures were too highly correlated to each other to analyze simultaneously.
Since 1974 marked the year when screening began and all members of the cohort were likely to have received additional medical attention, we stratified the risk analyses according to the year cancer was diagnosed. There were 124 cases diagnosed before 1974 and 266 cases diagnosed in 1974 or later. There was a marked difference in the recurrence rates for cases before 1974 compared to 1974 and later, 24.2% versus 7.5% (hazard ratio, 0.39; 95% CI, 0.22–0.71). The relationship between the years of diagnosis and recurrence were as follows: 19 cases were diagnosed before 1974 and recurred before 1974, 11 cases were diagnosed before 1974 and recurred in 1974 or later, and 20 cases were diagnosed in or after 1974.
Age at exposure and dose are the most clearly documented risk factors for developing radiation-related thyroid cancer, but neither influenced the chance of recurrence (Table 1). Sex has not been shown to be a risk factor for developing radiation-related thyroid cancer and, in this cohort, it was not a risk factor for recurrence (Table 1). In contrast, age at the diagnosis of cancer was significantly related to the chance of recurrence, with a decline throughout the entire range of ages (hazard ratio 0.95/year; 95% CI, 0.91–0.99).
Table 1.
Proportional Hazards Analysis of Baseline Factors and the Risk of Cancer Recurrence
| Risk factor | No. of patients | No. of recurrences (%) | Hazard ratio (95% CI)a |
|---|---|---|---|
| Sex | 0.74 (0.42–1.30) | ||
| Male | 207 | 27 (13.0) | |
| Female | 183 | 23 (12.6) | |
| Age at Rx therapy (y) | 0.96 (0.86–1.08) | ||
| <5 | 291 | 38 (13.1) | |
| 5–10 | 84 | 11 (13.2) | |
| 10–15 | 15 | 1 (6.7) | |
| Thyroid radiation dose (Gy)b | 1.18 (0.70–2.00) | ||
| <0.5 | 88 | 8 (9.0) | |
| 0.51–1.0 | 243 | 35 (14.4) | |
| 1.01–2.0 | 21 | 3 (14.3) | |
| >2.0 | 5 | 2 (40.0) | |
| Age at first thyroid cancer diagnosis (y) | 0.95 (0.91–0.99) | ||
| <10 | 3 | 2 (67) | |
| 11–20 | 39 | 16 (41) | |
| 21–30 | 122 | 12 (9.8) | |
| 31–40 | 160 | 16 (10) | |
| 41–50 | 45 | 3 (6.7) | |
| >50 | 21 | 1 (4.8) |
By Cox analysis, stratified according to those who had thyroid cancer in 1973 and earlier and those whose cancer was discovered in 1974 and later. All but sex were continuous variables.
Thyroid doses could not be estimated for 33 subjects.
CI, confidence interval.
Cancer characteristics related to recurrence
The frequencies of the pathology characteristics of the thyroid cancers in this cohort and the hazard ratios for recurrence for each characteristic are shown in Table 2. All of the characteristics associated with higher risk in nonradiation cases (multifocality, bilaterality, larger size, more cancer foci, and lymph node metastases) were associated with hazard ratios above 1.0, but only larger size was significant.
Table 2.
Proportional Hazards Analysis of Cancer Characteristics and the Risk of Cancer Recurrence
| Risk factor | No. of patients | No. of recurrences (%) | Hazard ratio(95% CI)a |
|---|---|---|---|
| Focalityb | 1.80 (0.98–3.29) | ||
| Unifocal | 175 | 17 (9.7) | |
| Multifocal | 194 | 31 (16.0) | |
| No. of lobes involvedb | 1.56 (0.83–2.92) | ||
| 1 | 280 | 33 (11.8) | |
| 2 | 87 | 14 (16.1) | |
| No. of cancer nodulesc | 1.33 (0.98–1.80) | ||
| 1 | 175 | 17 (9.7) | |
| 2 | 131 | 18 (13.7) | |
| ≥3 | 63 | 13 (20.6) | |
| Largest cancer nodule (cm)d | 1.27 (1.01–1.60) | ||
| ≤10 | 167 | 14 (8.4) | |
| 10–20 | 114 | 16 (14.0) | |
| >20 | 44 | 10 (22.7) | |
| Lymph node metastasesb | 1.66 (0.90–3.04) | ||
| No | 252 | 24 (9.5) | |
| Yes | 118 | 23 (19.5) | |
| Tissue invasionc | 0.88 (0.59–1.31) | ||
| Major | 37 | 4 (10.8) | |
| Minor | 64 | 14 (21.9) | |
| None | 270 | 30 (11.1) | |
| Vessel invasionb | |||
| Present | 42 | 11 (26.2) | |
| Absent | 326 | 37 (11.3) | 0.58 (0.29–1.16) |
| No. of benign nodulesc | |||
| 0 | 132 | 28 (21.2) | |
| 1 | 91 | 9 (9.9) | |
| 2 | 79 | 7 (8.7) | |
| ≥3 | 67 | 4 (6.0) | 0.77 (0.56–1.06) |
| Histologyb | |||
| Papillary | 345 | 46 (13.3) | |
| Follicular | 29 | 3 (10.3) | 0.81 (0.25–2.60) |
By Cox analysis, stratified according to those who had thyroid cancer in 1973 and earlier and those whose cancer was discovered in 1974 and later.
Bilevel variable.
Multilevel variable.
Continuous variable.
There was no unambiguous way to analyze the size of the largest focus as a risk factor for recurrence because in 34 cases the largest focus was in a lymph node. Including cancer foci in lymph nodes, the size of the largest malignant focus (in centimeters) was significant (p < 0.05), with a lower bound of the 95% CI of 1.01. Secondary analyses were as follows: (a) excluding the cases where the largest focus was in a lymph node, (b) including the cases, using the largest focus found in the thyroid, but excluding those with no cancer found in the thyroid, and (c) including the cases using largest focus in the thyroid and 1 mm (“microscopic”) for the cases where no focus was found in the thyroid. The point estimates of the hazard ratio and confidence intervals were virtually the same as shown in Table 2, with lower bounds of 0.97, 0.99, and 0.95, respectively, but p values >0.05. When the size of the largest focus and age at cancer diagnosis were both included in the stratified model, neither was significant.
Focality and the number of cancer foci were of borderline significance with lower bounds of 0.98, and the other factors were not significant. Finally, the number of benign nodules accompanying the cancers was not related to the frequency of recurrence. When reduced to bilevel variables the number of cancer nodules (1 vs. > 1), the number of benign nodules (none vs. ≥ 1), and tissue invasion (none vs. minor or major) were still not related to recurrence.
It was surprising that lymph node metastases were not statistically predictive of recurrence, so we performed several secondary analyses. If we omitted stratification by the year of cancer occurrence, lymph node metastases were significantly related to recurrence (hazard ratio 2.03, 95% CI, 1.14–3.62). However, when we controlled for whether surgery was before or after 1974 or for the age at which the cancer occurred, lymph node metastases were not significantly predictive of recurrence (the estimates of the hazard and confidence intervals were nearly identical to those in the stratified model shown in Table 2).
Therapeutic factors related to recurrence
The extent of surgery, radioactive iodine ablation and thyroid hormone treatment were not predictive of recurrence (Table 3). Ablation was used for more advanced cases; cases where the biggest cancer focus was larger (1.60 cm vs. 1.27 cm, p < 0.05) and lymph node spread at time of diagnosis was more common (66.2% vs. 33.8%, p < 0.05). When these factors were included in the stratified model, ablation was still associated with a nonsignificant recurrence risk, but with a smaller magnitude.
Table 3.
Proportional Hazards Analysis of Treatment Modalities and the Risk of Cancer Recurrence
| Risk factor | No. of patients | No. of recurrences (%) | Hazard ratio (95% CI)a |
|---|---|---|---|
| Surgery fraction | 0.90 (0.30–2.77) | ||
| ≤0.5 | 106 | 16 (15.1) | |
| >0.5 | 284 | 33 (11.6) | |
| Radioactive iodine ablation | 1.30 (0.72–2.33) | ||
| No | 259 | 32 (12.4) | |
| Yes | 131 | 18 (13.7) | |
| Thyroid hormone replacement | 0.74 (0.50–1.11) | ||
| None | 30 | 9 (30.0) | |
| Partial | 22 | 12 (9.1) | |
| Full | 334 | 39 (11.7) |
By Cox analysis, stratified according to those who had thyroid cancer in 1973 and earlier and those whose cancer was discovered in 1974 and later. Surgery and thyroid hormone replacement were analyzed as continuous variables.
Discussion
In 1986 we reported on the clinical behavior of 296 cases of radiation-related thyroid cancer (1). On the basis of an analysis of risk factors for recurrence we concluded that radiation-related thyroid cancer and non–radiation-related cancer behaved similarly. In the current study, follow-up has nearly doubled, to a median duration of 19 years, the longest being 53 years. In addition, nearly 100 new thyroid cancer cases were identified and are included in this report. Although the radiation effect is waning, Fig. 1 shows that cases continue to occur (13). The findings and our conclusion, that thyroid cancer following external radiation exposure is not more aggressive than other thyroid cancers, remain largely unchanged.
As in the previous study, the age at thyroid cancer diagnosis and latency remain the most important risk factors for recurrence (1). However, we cannot definitively distinguish between age and latency because these two factors are very highly correlated. This finding extends one we made before, that short latency is associated with an increased risk of recurrence of small (<10 mm) thyroid cancers, many detected by screening irradiated individuals (14). The age-dependent pattern of younger age being associated with a higher risk of recurrence has also been observed in patients with non–radiation-induced papillary thyroid cancer (15,16). Therefore, this factor may be a characteristic of radiation, a result of young age, or both.
The cancers diagnosed before 1974 had a much higher frequency of recurrence. The initiation of screening in 1974 of the entire cohort, including those with cancer, could have resulted in the enhanced detection of recurrences. However, since approximately two thirds of the recurrences in the early cases occurred before 1974, the advent of screening in 1974 could not account completely for this frequency. To take into account the onset of screening in 1974, we stratified the risk analyses. Retrospectively, we found that stratification was not required as the complementary log-log versus time plots were parallel (data not shown). If date of surgery was treated as a risk factor, the magnitudes of the other risk estimates were not appreciably changed. The same risk factors retained significance and the confidence intervals were little altered, confirming that either approach was valid.
Neither lymph node metastases, extent of surgery, radioactive iodine ablation, nor thyroid hormone treatment were predictive of recurrence though, in at least some studies, all of them have been found to be prognostic factors for recurrence in patients with spontaneous papillary thyroid cancer (15,17–22). They may not show up in our analyses for two reasons. First, even though this is one of the largest cohorts of radiation-induced thyroid cancers, the number of cases and recurrences may not be large enough to see these effects. In contrast, for example, the benefit of total thyroidectomy required an analysis of 52,173 patients (22). Second, the effect of age may be so strong that other factors may be difficult to detect.
Even though it is not significant, the risk of recurrence is estimated to be greater in patients receiving radioactive iodine ablation therapy and, therefore, requires comment. This is due, in part, to the use of ablation in more advanced cases. Also, selection bias may play a role in this. It is likely that patients who are treated with radioactive iodine have more follow-up isotope scans, ultrasounds, and thyroglobulin measurements, and are therefore more likely to have recurrences diagnosed.
The decreasing response rate to questionnaires in recent years may have affected the findings. If so, this most likely would have exaggerated poorer clinical outcomes. At some time, nearly all of the individuals with thyroid cancer have been in touch with the program. Knowing about the accumulated experience of the program, we believe that patients with persistent concerns were more likely to stay in contact than those with less reason to be concerned. It is possible, but unlikely, that thyroid cancer–related deaths occurred in the subjects of this report, without our being aware of them. We do not have matched controls because, beginning in 1974 the exposed patients were subjected to intensive case finding (screening) and no adequate control for this is available. Thus, we rely on the nature of the risk factors for recurrence to reach our conclusions.
Other studies have largely arrived at the same conclusion we have, that thyroid cancer following external radiation exposure is not more aggressive than other thyroid cancers (2–4,6,7,23). Lundgren (23) reported a study, very likely free of bias, using the comprehensive health records of Sweden. This study used a nested case–control design, comparing radiation exposure in 601 patients who died of differentiated thyroid cancer at least 1 year after diagnosis to an equal number of thyroid cancer cases who had not died. The hazard ratio estimate for the association of mortality with no radiation exposure was 1.0 (95% CI, 0.5–2.3) by univariate and 0.4 (95% CI, 0.5–1.2) by multivariate analyses. A smaller study of thyroid cancer in 33 patients treated for a variety of childhood malignancies reached the same conclusion (5), but another in 32 patients treated for Hodgkin's disease, suggested otherwise (24). In neither study were matched controls included and no statistical tests were performed. Previously, we reviewed six studies published before 2005 that used recurrence as the end-point to measure the behavior of external radiation-related thyroid cancer. In the largest, a case–control study carried out in France (91 radiation-related cases vs. 273 cases with no radiation exposure), the risks of recurrence and thyroid cancer–related death were not related to radiation exposure (3). Five of the six found no difference between cases in exposed and nonexposed patients. In the sixth, controls were not matched to cases and no statistical tests were reported.
The situation may be different for internal radiation-related (Chernobyl) thyroid cancers. Pacini et al. (9) compared 472 pediatric cases from Belarus with 369 in Italy and France. The Chernobyl-related cases were younger, had fewer follicular cancers, were comparatively more frequent in males, and had more frequent extrathyroidal extension and lymph node metastases. In Ukrainian children with thyroid cancer who were up to 18 years at the time of the Chernobyl accident, radioactive iodine ablation of all disease proved difficult. Of 249 children, only 63 were ablated with one treatment (10). The other children received 406 courses of radioactive iodine treatment and only 86 of them were eventually successfully ablated. A similar observation about the difficulty of ablation was made in 740 cases in Belarus with the age at exposure less than 15 years and an average of about 10 years of follow-up (11). The recurrence rate was 27.6% with 131 cases of distant metastases, including those present at diagnosis and developing later. Multivariate analysis showed that younger age at diagnosis, lymph node involvement at diagnosis, extent of lymph node dissection, and multifocality were significant risk factors.
While Chernobyl-related thyroid cancers appear to be aggressive, the significance of this observation remains uncertain and other factors, in addition to radiation, seem to be as important or more so. Iodine deficiency has been well-documented in the affected areas and has only been partially corrected over time (25,26). The resulting TSH stimulation, even by TSH levels within the reference range, may promote cancer development and progression (27,28). The Chernobyl-related cases identified so far have been in pediatric and young adult patients where aggressive characteristics are more commonly seen than in older patients. In accord with the possibility that age and iodine deficiency, but not radiation, account for the aggressive features are the observations of Williams et al. (29). They compared the morphological characteristics of three groups of childhood cases: Chernobyl-exposed; Chernobyl-unexposed from the same countries; and cases from Japan, England, and Wales. The first two groups shared aggressive characteristics, exceeding those in the third group. Additional support is that pediatric thyroid cancer in patients born in Belarus after the accident have the same predominance of RET/PTC rearrangement as cases related to the accident (30). Ethnicity may also play a role, although no population-specific genetic factors related to cancer behavior have been identified. Finally, some reports, especially those describing persistent disease, may be subject to referral bias.
In summary, the clinical behavior of thyroid cancer in patients with external radiation-induced and non–radiation-induced thyroid cancer, as measured by the risk of recurrence, is similar. Not all of the factors seen in non–radiation-induced cases are statistically significant in this cohort, but taking age into account, no factor points to a more aggressive course (1–3). Although the number of cases is too small to independently evaluate the effectiveness of treatment, we recommend that radiation-induced thyroid cancers be treated and followed the same way as non–radiation-induced thyroid cancers.
Acknowledgments
We thank Dr. David Sarne for his valuable input. This work was supported, in part, by a grant to ABS from the National Cancer Institute (CA21518).
Disclosure Statement
No competing financial interests exist.
References
- 1.Schneider AB. Recant W. Pinsky S. Ryo UY. Bekerman C. Shore-Freedman E. Radiation-induced thyroid carcinoma: clinical course and results of therapy in 296 patients. Ann Intern Med. 1986;105:405–412. doi: 10.7326/0003-4819-105-3-405. [DOI] [PubMed] [Google Scholar]
- 2.Samaan N. Schultz P. Ordonez N. Hickey R. Johnston D. A comparison of thyroid carcinoma in those who have and have not had head and neck irradiation in childhood. J Clin Endocrinol Metab. 1987;64:219–223. doi: 10.1210/jcem-64-2-219. [DOI] [PubMed] [Google Scholar]
- 3.Rubino C. Cailleux AF. Abbas M. Diallo I. Shamsaldin A. Caillou B. De Vathaire F. Schlumberger M. Characteristics of follicular cell-derived thyroid carcinomas occurring after external radiation exposure: results of a case control study nested in a cohort. Thyroid. 2002;12:299–304. doi: 10.1089/10507250252949423. [DOI] [PubMed] [Google Scholar]
- 4.Gow KW. Lensing S. Hill DA. Krasin MJ. McCarville MB. Rai SN. Zacher M. Spunt SL. Strickland DK. Hudson MM. Thyroid carcinoma presenting in childhood or after treatment of childhood malignancies: an institutional experience, review of the literature. J Pediat Surg. 2003;38:1574–1580. doi: 10.1016/s0022-3468(03)00563-3. [DOI] [PubMed] [Google Scholar]
- 5.Acharya S. Sarafoglou K. LaQuaglia M. Lindsley S. Gerald W. Wollner N. Tan C. Sklar C. Thyroid neoplasms after therapeutic radiation for malignancies during childhood or adolescence. Cancer. 2003;97:2397–2403. doi: 10.1002/cncr.11362. [DOI] [PubMed] [Google Scholar]
- 6.Furlan JC. Rosen IB. Prognostic relevance of previous exposure to ionizing radiation in well-differentiated thyroid cancer. Langenbecks Arch Surg. 2004;389:198–203. doi: 10.1007/s00423-003-0424-0. [DOI] [PubMed] [Google Scholar]
- 7.Kikuchi S. Perrier ND. Cheah K. Siperstein AE. Duh QY. Clark OH. Complication of thyroidectomy in patients with radiation-induced thyroid neoplasms. Arch Surg. 2004;139:1185–1188. doi: 10.1001/archsurg.139.11.1185. [DOI] [PubMed] [Google Scholar]
- 8.Schneider AB. Sarne DH. Long-term risks for thyroid cancer and other neoplasms after exposure to radiation. Nat Clin Pract Endocrinol Metab. 2005;1:82–91. doi: 10.1038/ncpendmet0022. [DOI] [PubMed] [Google Scholar]
- 9.Pacini F. Vorontsova T. Demidchik EP. Molinaro E. Agate L. Romei C. Shavrova E. Cherstvoy ED. Ivashkevitch Y. Kuchinskaya E. Schlumberger M. Ronga G. Filesi M. Pinchera A. Post-Chernobyl thyroid carcinoma in Belarus children and adolescents: comparison with naturally occurring thyroid carcinoma in Italy and France. J Clin Endocrinol Metab. 1997;82:3563–3569. doi: 10.1210/jcem.82.11.4367. [DOI] [PubMed] [Google Scholar]
- 10.Oliynyk V. Epshtein O. Sovenko T. Tronko M. Elisei R. Pacini F. Pinchera A. Post-surgical ablation of thyroid residues with radioiodine in Ukrainian children and adolescents affected by post-Chernobyl differentiated thyroid cancer. J Endocrinol Invest. 2001;24:445–447. doi: 10.1007/BF03351045. [DOI] [PubMed] [Google Scholar]
- 11.Demidchik YE. Demidchik EP. Reiners C. Biko J. Mine M. Saenko VA. Yamashita S. Comprehensive clinical assessment of 740 cases of surgically treated thyroid cancer in children of Belarus. Ann Surg. 2006;243:525–532. doi: 10.1097/01.sla.0000205977.74806.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell MJ. Statistics at Square Two: Understanding Modern Statistical Applications in Medicine. BMJ Publication Group; London: 2001. [Google Scholar]
- 13.Ron E. Lubin JH. Shore RE. Mabuchi K. Modan B. Pottern LM. Schneider AB. Tucker MA. Boice JD., Jr Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res. 1995;141:259–277. [PubMed] [Google Scholar]
- 14.Bucci A. Shore-Freedman E. Gierlowski T. Mihailescu D. Ron E. Schneider AB. Behavior of small thyroid cancers found by screening radiation-exposed individuals. J Clin Endocrinol Metab. 2001;86:3711–3716. doi: 10.1210/jcem.86.8.7742. [DOI] [PubMed] [Google Scholar]
- 15.Mazzaferri EL. Young RL. Papillary thyroid carcinoma: a 10 year follow-up report of the impact of therapy in 576 patients. Am J Med. 1981;70:511–518. doi: 10.1016/0002-9343(81)90573-8. [DOI] [PubMed] [Google Scholar]
- 16.Mazzaferri EL. Young RL. Oertel JE. Kemmerer WT. Page CP. Papillary thyroid carcinoma: the impact of therapy in 576 patients. Medicine (Baltimore) 1977;56:171–196. [PubMed] [Google Scholar]
- 17.Samaan NA. Schultz PN. Hickey RC. Goepfert H. Haynie TP. Johnston DA. Ordonez NG. The results of various modalities of treatment of well differentiated thyroid carcinoma—a retrospective review of 1599 patients. J Clin Endocrinol Metab. 1992;75:714–720. doi: 10.1210/jcem.75.3.1517360. [DOI] [PubMed] [Google Scholar]
- 18.Mazzaferri EL. Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 19.Morris DM. Boyle PJ. Stidley CA. Altobelli KK. Parnell T. Key C. Localized well-differentiated thyroid carcinoma: survival analysis of prognostic factors and I-131 therapy. Ann Surg Oncol. 1998;5:329–337. doi: 10.1007/BF02303496. [DOI] [PubMed] [Google Scholar]
- 20.Sanders LE. Cady B. Differentiated thyroid cancer—reexamination of risk groups and outcome of treatment. Arch Surg. 1998;133:419–424. doi: 10.1001/archsurg.133.4.419. [DOI] [PubMed] [Google Scholar]
- 21.Hay ID. McConahey WM. Goellner JR. Managing patients with papillary thyroid carcinoma: insights gained from the Mayo Clinic's experience of treating 2,512 consecutive patients during 1940 through 2000. Trans Am Clin Climatol Assoc. 2002;113:241–260. [PMC free article] [PubMed] [Google Scholar]
- 22.Bilimoria KY. Bentrem DJ. Ko CY. Stewart AK. Winchester DP. Talamonti MS. Sturgeon C. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246:375–384. doi: 10.1097/SLA.0b013e31814697d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundgren CI. Are possible risk factors for differentiated thyroid cancer of prognostic importance? Thyroid. 2006;16:659–666. doi: 10.1089/thy.2006.16.659. [DOI] [PubMed] [Google Scholar]
- 24.Robinson E. Neugut AI. The clinical behavior of radiation-induced thyroid cancer in patients with prior Hodgkins disease. Radiother Oncol. 1990;17:109–113. doi: 10.1016/0167-8140(90)90098-h. [DOI] [PubMed] [Google Scholar]
- 25.Gembicki M. Stozharov AN. Arinchin AN. Moschik KV. Petrenko S. Khmara IM. Baverstock KF. Iodine deficiency in Belarusian children as a possible factor stimulating the irradiation of the thyroid gland during the Chernobyl catastrophe. Environ Health Perspect. 1997;105:1487–1490. doi: 10.1289/ehp.97105s61487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tronko M. Kravchenko V. Fink D. Hatch M. Turchin V. McConnell R. Shpak V. Brenner A. Robbins J. Lusanchuk I. Howe G. Iodine excretion in regions of Ukraine affected by the Chornobyl accident: experience of the Ukrainian-American cohort study of thyroid cancer and other thyroid diseases. Thyroid. 2005;15:1291–1297. doi: 10.1089/thy.2005.15.1291. [DOI] [PubMed] [Google Scholar]
- 27.Cardis E. Kesminiene A. Ivanov V. Malakhova I. Shibata Y. Khrouch V. Drozdovitch V. Maceika E. Zvonova I. Vlassov O. Bouville A. Goulko G. Hoshi M. Abrosimov A. Anoshko J. Astakhova L. Chekin S. Demidchik E. Galanti R. Ito M. Korobova E. Lushnikov E. Maksioutov M. Masyakin V. Nerovnia A. Parshin V. Parshkov E. Piliptsevich N. Pinchera A. Polyakov S. Shabeka N. Suonio E. Tenet V. Tsyb A. Yamashita S. Williams D. Risk of thyroid cancer after exposure to 131I in childhood. J Natl Cancer Inst. 2005;97:724–732. doi: 10.1093/jnci/dji129. [DOI] [PubMed] [Google Scholar]
- 28.Haymart MR. Repplinger DJ. Leverson GE. Elson DF. Sippel RS. Jaume JC. Chen H. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab. 2008;93:809–814. doi: 10.1210/jc.2007-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams ED. Abrosimov A. Bogdanova T. Demidchik EP. Ito M. Livolsi V. Lushnikov E. Rosai J. Tronko MD. Tsyb AF. Vowler SL. Thomas GA. Morphologic characteristics of Chernobyl-related childhood papillary thyroid carcinomas are independent of radiation exposure but vary with iodine intake. Thyroid. 2008;18:847–852. doi: 10.1089/thy.2008.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogounovitch T. Saenko V. Mankovskaya S. Demidchik Y. Matsuse M. Mitsutake N. Yamashita S. Molecular, clinico-pathological analysis of pediatric thyroid cancers in Belarus; The Endocrine Society Annual Meeting; San Francisco. 2008. Abstract P2–261. [Google Scholar]