Abstract
Background
Relationships of thyroid and iron measures in large cohorts are unreported. We evaluated thyroid-stimulating hormone (TSH) and free thyroxine (T4) in white participants of the primary care–based Hemochromatosis and Iron Overload Screening (HEIRS) Study.
Methods
We measured serum TSH and free T4 in 176 HFE C282Y homozygotes without previous hemochromatosis diagnoses and in 312 controls without HFE C282Y or H63D who had normal serum iron measures and were matched to C282Y homozygotes for Field Center, age group, and initial screening date. We defined hypothyroidism as having TSH >5.00 mIU/L and free T4 <0.70 ng/dL, and hyperthyroidism as having TSH <0.400 mIU/L and free T4 >1.85 ng/dL. Multivariate analyses were performed using age, sex, Field Center, log10 serum ferritin (SF), HFE genotype, log10 TSH, and log10 free T4.
Results
Prevalences of hypothyroidism in C282Y homozygotes and controls were 1.7% and 1.3%, respectively, and of hyperthyroidism 0% and 1.0%, respectively. Corresponding prevalences did not differ significantly. Correlations of log10 SF with log10 free T4 were positive (p = 0.2368, C282Y homozygotes; p = 0.0492, controls). Independent predictors of log10 free T4 were log10 TSH (negative association) and age (positive association); positive predictors of log10 SF were age, male sex, and C282Y homozygosity. Proportions of C282Y homozygotes and controls who took medications to supplement or suppress thyroid function did not differ significantly.
Conclusions
Prevalences of hypothyroidism and hyperthyroidism are similar in C282Y homozygotes without previous hemochromatosis diagnoses and controls. In controls, there is a significant positive association of SF with free T4. We conclude that there is no rationale for routine measurement of TSH or free T4 levels in hemochromatosis or iron overload screening programs.
Introduction
Hemochromatosis in Western European whites is typically associated with homozygosity for C282Y, a mutation in the HFE gene on chromosome 6p (1). Approximately 0.44–0.48% of non-Hispanic whites in North America are C282Y homozygotes (2,3). Some C282Y homozygotes develop iron overload and complications such as liver disease, diabetes mellitus, arthropathy, and hypogonadotrophic hypogonadism (1–4). Among 391 patients with hemochromatosis diagnosed in medical care combined from five studies, 4.1% had primary hypothyroidism and 0.3% had hyperthyroidism (5–9). To date, there is no report of thyroid-related laboratory measures in participants of large population or workplace hemochromatosis screening programs (2,10–12).
The Hemochromatosis and Iron Overload Screening (HEIRS) Study is a cross-sectional, multi-center, multi-ethnic study of primary care clinic attendees (13). The HEIRS Study performed initial screening for hemochromatosis and iron overload using transferrin saturation (TS) and serum ferritin (SF) measurements and HFE genotyping (C282Y and H63D alleles) in 101,168 participants (13). In this HEIRS substudy, we measured and compared serum concentrations of thyroid-stimulating hormone (TSH), free thyroxine (T4), and SF at a post-initial screening examination in 176 white participants with C282Y homozygosity and in 312 white participants without HFE C282Y or H63D designated as control subjects. The implications of the present results for understanding the prevalence and pathogenesis of thyroid abnormalities in whites with hemochromatosis and C282Y homozygosity are discussed.
Materials and Methods
Study approval
The local Institutional Review Boards of the HEIRS Study Coordinating Center, Central Laboratory, and each Field Center approved the study protocol that is described in detail elsewhere (13). The Field Centers recruited participants ≥25 years of age who gave informed consent. It is the privacy policy of the HEIRS Study to display the age of individual subjects by decade.
Initial screening of HEIRS Study subjects
Participants were recruited during the interval February 2001–February 2003 from HEIRS Study Field Centers (13). These clinics serve ethnically and socioeconomically diverse primary care patients. At initial screening, blood samples were obtained from participants for measurement of TS and SF without regard for state of fasting, and for HFE mutation analysis (13). The dataset excluded observations on participants for whom TS, SF, or HFE genotype data on initial screening were not available. All adult volunteers were eligible to participate in the HEIRS Study, but those who participated only because a family member participated or those who reported on their screening questionnaire that they had been previously diagnosed to have hemochromatosis or iron overload were excluded from the present analysis (3).
Post-initial screening clinical examination and subject selection
All C282Y homozygotes diagnosed in the HEIRS Study were invited to participate in a clinical examination that included listing of participants' medications and collection of blood samples for additional testing (13). Control subjects invited to attend a clinical examination were selected from initial screening participants who had (i) HFE genotype wt/wt (defined as absence of alleles C282Y and H63D) and (ii) both TS and SF in the eligible range. Eligible ranges for men were TS 20–34% and SF 87–247 ng/dL. Eligible ranges for women were TS 16–28% and SF 19–121 ng/dL. Among participants eligible to be control subjects, a subset was selected that was frequency matched in a 1:1 ratio to the group of C282Y homozygotes and provisional iron overload cases (defined as participants with confirmed elevations of both SF and TS without evidence of elevated C-reactive protein or elevated serum levels of hepatic transaminases). Variables used for the frequency matching were Field Center (UAB, UCI, Howard, KP-Portland, LHSC, and Toronto), age group (24–44, 45–64, and 65+ years), and date of initial screening visit. Potential control participants who could not be contacted or who declined to participate were replaced. New potential control subjects were selected using frequency matching in a 1:1 ratio to the group of the potential control subjects who declined. Variables used for this frequency matching were Field Center (as above), age group (as above), date of initial screening, gender, and race/ethnicity. Most C282Y homozygotes in the HEIRS Study were white (3). Therefore, we included only those participants and control subjects who reported that they were only “white or Caucasian” (13).
Laboratory methods for serum thyroid-related and SF measures
Serum concentrations of TSH and free T4 were measured at the HEIRS Study Central Laboratory at the University of Minnesota Medical Center-Fairview (13) using a Siemens/Bayer ADVIA Centaur® immunoassay analyzer (Siemens, New York, NY). The coefficients of variation for the TSH (TSH-3) and free T4 (FrT4) assays in the normal range were 5.0% and 4.0%, respectively. The TSH assay has a functional sensitivity of approximately 0.019 mIU/L. Reference ranges were TSH 0.400–5.00 mIU/L and free T4 0.70–1.85 ng/dL. These ranges represent the central 95% confidence intervals (CIs) of serum TSH and free T4 measurements in ostensibly healthy young adults. We defined hypothyroidism as having TSH >5.00 mIU/L and free T4 <0.70 ng/dL, and hyperthyroidism as having TSH <0.400 mIU/L and free T4 >1.85 ng/dL, regardless of cause. SF levels were measured using a turbidometric immunoassay (Roche Diagnostics/Hitachi 911, Indianapolis, IN) (3,14). The SF reference ranges defined by the HEIRS Study were 15–200 ng/mL (women) and 15–300 ng/mL (men) (3,14). We defined severe hyperferritinemia as SF >1000 ng/mL.
Statistical considerations
We evaluated observations on 176 C282Y homozygotes and 312 wt/wt control subjects. Statistical analyses were performed using SAS (15), Excel 2000® (Microsoft, Redmond, WA), and GB-Stat® (v. 10.0, 2003; Dynamic Microsystems, Silver Spring, MD). Initial evaluations revealed that TSH and free T4 data were not normally distributed. This is characteristic of thyroid screening programs, largely due to the relatively high prevalence of hypothyroidism (16–20). Similarly, SF values are not normally distributed (3,14). Therefore, TSH, free T4, and SF data were normalized using log10 transformation for analysis; some results were reported as antilog values, as appropriate. For display of TSH values, we used three significant figures. Descriptive data are displayed as enumerations, percentages, or mean ± 1 SD (or 95% CIs, as appropriate). Frequency values were compared using chi-square analysis or Fisher exact test, as indicated. Mean values were compared using Student's t-test. We determined the correlations of log10 SF as a dependent variable with log10 free T4 and with log10 TSH as independent variables. Multivariate analyses were performed using age, sex (male or female), Field Center, HFE genotype (either C282Y/C282Y or wt/wt), log10 SF, and log10 TSH as independent variables, and log10 free T4 as the dependent variable. In other multivariate analyses, we evaluated the determinants of log10 SF as the dependent variable. For correlation and multivariate analyses, we included only those observations from the 437 study participants who reported taking neither thyroid supplements nor medications to suppress thyroid function. Values of p < 0.05 were defined as significant.
Results
General characteristics of study subjects
There was a predominance of women among the C282Y homozygotes and wt/wt control subjects, consistent with the overall greater participation of women than men in the HEIRS Study (Table 1) (3). C282Y homozygotes were significantly younger and had a significantly higher mean free T4 than control subjects in univariate analyses, although the magnitudes of these respective differences were small (Table 1). The prevalence of abnormal values of TSH, free T4, or both did not differ significantly between HFE C282Y homozygotes and control subjects (Table 1). Mean SF was significantly greater in C282Y homozygotes than in control subjects (Table 1).
Table 1.
Characteristics of 488 White HEIRS Study Participants
| Characteristica |
HFE C282Y homozygotes (n = 176; 64.2% F) |
HFE wt/wt controls (n = 312; 60.6% F) |
p-valueb |
|---|---|---|---|
| Mean age ± 1 SD (range), years | 50 ± 13 | 55 ± 14 | <0.0001 |
| Mean TSH, mIU/L (95% CI) | 1.67 (1.42, 1.96) | 2.00 (1.80, 2.22) | 0.0652 |
| TSH <0.400 mIU/L, % (n) | 5.1 (9) | 2.9 (9) | 0.2097 |
| TSH >5.00 mIU/L, % (n) | 8.0 (14) | 8.3 (26) | 0.8835 |
| Mean free T4, ng/dLc (95% CI) | 1.12 (1.09, 1.15) | 1.01 (1.05, 1.10) | 0.0492 |
| Free T4 <0.70 ng/dL, % (n) | 1.7 (3) | 1.0 (3) | 0.3741 |
| Free T4 >1.85 ng/dL, % (n) | 1.7 (3) | 1.3 (4) | 0.4925 |
| TSH <0.400 mIU/L and free T4 >1.85 ng/dL, % (n) | 0 | 1.0 (3) | 0.2604 |
| TSH >5.00 mIU/L and free T4 <0.70 ng/dL, % (n) | 1.7 (3) | 1.3 (4) | 0.4925 |
| Mean SF, ng/mL (95% CI) | 308 (252, 376) | 77 (71, 84) | <0.0001 |
TSH, thyroid-stimulating hormone; T4, thyroxine; SF, serum ferritin. For display of TSH values, we used three significant figures.
Comparisons were made between HFE C282Y homozygotes and HFE wt/wt controls using Student's t-tests, Fisher exact tests, or chi-square tests, as appropriate.
When mean log10 free T4 was computed in C282Y homozygotes and in controls after all participants with free T4 values outside the reference range of 0.70–1.85 ng/dL were removed, a slightly higher mean was still observed in C282Y homozygotes (p = 0.0423).
Prevalence of hypothyroidism and hyperthyroidism
Three participants had TSH and free T4 levels defined as hypothyroidism; each was a control subject (Table 2). Seven participants had TSH and free T4 levels defined as hyperthyroidism: three were C282Y homozygotes and four were control subjects (Table 2). The mean SF in the three participants with hypothyroidism was significantly lower than the mean SF in the seven participants with hyperthyroidism (Table 2). The respective prevalence estimates for hypothyroidism and hyperthyroidism did not differ significantly between C282Y homozygotes and control subjects (Table 1), or between male and female C282Y homozygotes and control subjects of the corresponding sex (data not shown).
Table 2.
Characteristics of 10 White HEIRS Study Participants with Abnormal Thyroid-Related Measures
| Conditiona | Age, sexb | HFE genotype | Thyroid-stimulating hormone (TSH), mIU/L | Free thyroxine (T4), ng/dL | Serum ferritin, ng/mLc |
|---|---|---|---|---|---|
| Hypothyroidism | 20s F | wt/wt | 291 | 0.16 | 23 |
| Hypothyroidism | 50s F | wt/wt | 88.9 | 0.36 | 90 |
| Hypothyroidism | 50s F | wt/wt | 19.4 | 0.69 | 27 |
| Hyperthyroidism | 50s M | C282Y/C282Y | 0.014 | 2.04 | 24 |
| Hyperthyroidism | 20s F | C282Y/C282Y | 0.009 | 1.98 | 124 |
| Hyperthyroidism | 70s M | C282Y/C282Y | 0.007 | 4.59 | 1,462 |
| Hyperthyroidism | 40s M | wt/wt | 0.064 | 2.11 | 144 |
| Hyperthyroidismd | 50s M | wt/wt | 0.054 | 2.07 | 230 |
| Hyperthyroidism | 80s M | wt/wt | 0.015 | 2.35 | 199 |
| Hyperthyroidismd | 40s F | wt/wt | 0.011 | 2.73 | 124 |
We defined hypothyroidism as the concurrence of TSH >5.00 mIU/L and free T4 <0.70 ng/dL, and hyperthyroidism as the concurrence of TSH <0.400 mIU/L and free T4 >1.85 ng/dL. These measurements were performed at the time of post-initial screening examination. For display of TSH values, we used three significant figures.
It is the privacy policy of the HEIRS Study to display the age of individual subjects by decade.
The mean serum ferritin level was lower in participants with hypothyroidism than in those with hyperthyroidism (38 ng/mL vs. 167 ng/mL, respectively; p = 0.0446).
These participants reported that they took thyroid supplements.
Participants with severe hyperferritinemia
Twenty-two HFE C282Y homozygotes (12.5%; 18 men and 4 women) had SF >1000 ng/mL. None had hypothyroidism as defined herein, although one man reported taking thyroid supplements. Another man had hyperthyroidism. By study design, no HFE wt/wt control subjects had hyperferritinemia.
Participants who reported taking thyroid supplements
Forty-nine participants (15 HFE C282Y homozygotes, 34 HFE wt/wt control subjects) reported that they took thyroid supplements (Table 3). These respective proportions of these participants were 8.5% and 10.9% (p = 0.4019). Mean values of age, TSH, free T4, proportions of participants with elevated or subnormal TSH or free T4, or those who had hypothyroidism or hyperthyroidism as defined herein did not differ significantly between C282Y homozygotes and control subjects (Table 3). Mean SF was significantly higher in C282Y homozygotes than in control subjects (Table 3). Two control participants who reported taking thyroid supplements met the present definition for having hyperthyroidism, suggesting that their elevated T4 and subnormal TSH values were due to thyroid supplements (Table 2).
Table 3.
Characteristics of 49 White HEIRS Study Participants Who Reported Taking Thyroid Supplementsa
| Characteristicb |
HFE C282Y homozygotes (n = 15; 86.7% F) |
HFE wt/wt controls (n = 34; 85.3% F) |
p-valuec |
|---|---|---|---|
| Mean age ± 1 SD (range), years | 53 ± 11 | 53 ± 15 | 0.9351 |
| Mean TSH, mIU/L (95% CI) | 1.51 (0.651, 3.51) | 1.79 (0.875, 3.64) | 0.7604 |
| TSH <0.400 mIU/L, % (n) | 20.0 (3) | 14.7 (5) | 0.4677 |
| TSH >5.00 mIU/L, % (n) | 20.0 (3) | 17.6 (6) | 0.5670 |
| Mean free T4, ng/dL (95% CI) | 1.28 (1.14, 1.43) | 1.10 (0.93, 1.30) | 0.1294 |
| Free T4 <0.70 ng/dL, % (n) | 0 | 5.9 (2) | 0.4770 |
| Free T4 >1.85 ng/dL, % (n) | 0 | 5.9 (2) | 0.4770 |
| TSH <0.400 mIU/L and free T 4 >1.85 ng/dL, % (n) | 0 | 5.9 (2) | 0.4770 |
| TSH >5.00 mIU/L and free T4 <0.70 ng/dL, % (n) | 0 | 5.9 (2) | 0.4770 |
| Mean SF, ng/mL (95% CI) | 301 (129, 705) | 68 (54, 85) | 0.0036 |
Thyroid supplements reported in medication lists provided by participants, by descending numbers of reports, were Synthroid®, levothyroxine, dessicated thyroid, Levoxyl®, Thyrolar®, Liotrix®, liothyronine, Levothroid®, and Cytomel®.
TSH, thyroid-stimulating hormone; T4, thyroxine; SF, serum ferritin. For display of TSH values, we used three significant figures.
Comparisons were made between HFE C282Y homozygotes and HFE wt/wt controls using Student's t-tests, Fisher exact tests, or chi-square tests, as appropriate.
Participants who reported taking medications used to suppress thyroid function
A man in his 70s with HFE C282Y homozygosity and SF 1462 ng/mL reported taking propylthiouracil; he met the present definition for having hyperthyroidism. A woman in her 30s with HFE wt/wt and SF 22 ng/mL reported that she took methimazole; her values of TSH and free T4 were within the respective reference limits. The proportions of C282Y homozygotes and control subjects who reported taking medications to suppress thyroid function did not differ significantly (p = 0.5883).
Correlations of log10 SF with log10 free T4
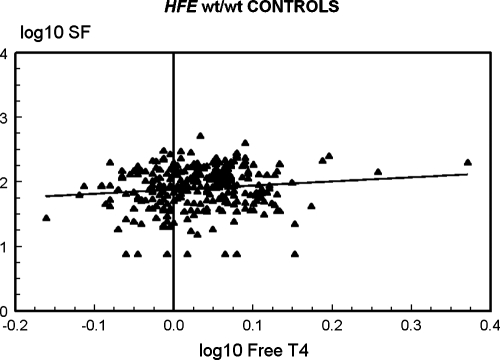
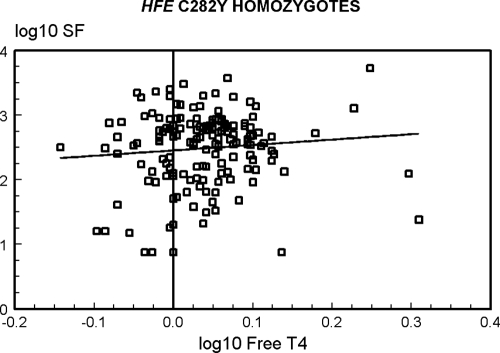
The correlation of log10 SF with log10 free T4 in all 437 study participants who reported taking neither thyroid supplements nor medications to suppress thyroid function was significant (Pearson correlation coefficient 0.1148; p = 0.0166) (data not shown). To determine if the relationship of log10 SF with log10 free T4 were significant in both C282Y homozygotes and in control subjects, we computed correlations in each group of subjects. In 160 C282Y homozygotes, the correlation was positive but not significant (Pearson correlation coefficient 0.0947; p = 0.2368) (Fig. 1). In 277 control subjects, the correlation was positive and significant (Pearson correlation coefficient 0.1183; p = 0.0492) (Fig. 1).
FIG. 1.
Correlations of log10 serum ferritin (SF) and log10 free thyroxine (T4) in Hemochromatosis and Iron Overload Screening (HEIRS) Study participants who reported taking neither thyroid supplements nor medications to suppress thyroid function. In 160 C282Y homozygotes, the correlation was positive but not significant (p = 0.2368) (above). In 277 control subjects, the correlation was positive and significant (p = 0.0492) (below).
Predictors of log10 free T4 in multivariate analyses
We used age, sex, Field Center, HFE genotype, log10 SF, and log10 TSH as independent variables, and log10 free T4 as the dependent variable. Values of p for these analyses were as follows: Field Center, 0.4687; age, 0.0142; gender, 0.3238; HFE genotype, 0.9689; log10 SF, 0.2102; and log10 TSH, <0.0001. These analyses reveal significant direct (positive) relationships of age and log10 TSH with log10 free T4.
Predictors of log10 SF in multivariate analyses
We used age, sex, Field Center, HFE genotype, log10 TSH, and log10 free T4 as independent variables, and log10 SF as the dependent variable. Values of p for these analyses were as follows: Field Center, 0.8192; age, 0.0006; gender, <0.0001; HFE genotype, <0.0001; log10 TSH, 0.1571; and log10 free T4, 0.2102. These analyses reveal significant direct (positive) relationships of age, male gender, and HFE C282Y homozygosity with SF.
Discussion
The prevalence of hypothyroidism or hyperthyroidism as defined in the present HEIRS substudy did not differ significantly between white HFE C282Y homozygotes and white HFE wt/wt control subjects, and is similar to that reported in other large population studies in which screening of whites for thyroid disorders was performed (16–20). Multivariate analyses demonstrated that there is a significant inverse relationship of age with log10 free T4 levels, consistent with other population studies of thyroid-related measures (16–20).
In a large population hemochromatosis screening program in Norway, 12.5% of women aged 20–49 years with C282Y homozygosity reported having hypothyroidism, whereas only 3.0% of the control participants reported having hypothyroidism (11). Thyroid-related laboratory measures were not quantified in participants in this study (11). By design, only study participants with elevated TS at initial screening underwent HFE genotyping, and therefore C282Y homozygotes without elevated TS would have been excluded from the Norway screening study (11). This difference of hypothyroidism reports between C282Y homozygotes and control subjects in the Norway screening study could be due to misunderstanding of the screening questionnaire by study participants, the higher proportion of thyroid peroxidase antibody positivity in younger than older women observed in other studies, or a consequence of multiple comparisons in any large study (11,13,16,20,21). The HEIRS Study identified all C282Y homozygotes at initial screening, and afterward obtained reports of thyroid supplement use and measurements of TSH and free T4 in selected participants. Therefore, it is not possible to compare the results of the present HEIRS substudy directly with the hypothyroidism reports in the Norway screening study.
There is evidence that iron deposits in the anterior pituitary gland or thyroid gland do not contribute to the pathogenesis of hypothyroidism or hyperthyroidism in most persons with hemochromatosis. In persons with severe iron overload due to hemochromatosis, iron deposits were visualized in a minority of thyrotrophs; the deposits were much less prominent than those in gonadotrophs (22,23). There are few well-documented hemochromatosis patients who had hypothyroidism due to impaired thyrotroph function; most of them also had hypogonadotrophic hypogonadism (24–29). In rare cases, secondary hypothyroidism resolves after therapeutic phlebotomy to achieve iron depletion (29). Iron deposits were detected in the thyroid gland in a majority of persons diagnosed to have hemochromatosis in medical care or at autopsy (23,30,31). Nonetheless, only 4.1% of 391 patients with hemochromatosis diagnosed in medical care had primary hypothyroidism and only 0.3% had hyperthyroidism (5–9). It has been suggested but remains unproven that iron deposits in the thyroid glands of persons with hemochromatosis cause Hashimoto thyroiditis or Graves disease to develop (6,23).
In persons with hemochromatosis, there is a significant positive correlation of SF with hepatic iron content and iron removed by phlebotomy to achieve iron depletion (32,33). Mean SF in the present HFE C282Y homozygotes was significantly greater than that of HFE wt/wt control subjects, but our prevalence estimates for hypothyroidism and hyperthyroidism did not differ significantly between these groups. The proportion of C282Y homozygotes who reported taking thyroid supplements did not differ significantly from that of control subjects, although two control subjects who reported that they took thyroid supplements met present criteria for having hyperthyroidism. Mean SF in participants with hyperthyroidism was significantly greater than mean SF in participants with hypothyroidism, but both means were within the reference range for SF. None of 22 C282Y homozygotes with severe hyperferritinemia had hypothyroidism; the only C282Y homozygote with hyperthyroidism did not report taking thyroid supplements. Although direct measures of hepatic iron content or quantitative phlebotomy in these substudy participants were not available, our SF observations agree with previous evidence that hypothyroidism or hyperthyroidism in persons with hemochromatosis is usually unrelated to the severity of iron overload (23).
We observed a significant positive correlation of log10 SF with log10 free T4 in control subjects, and a nonsignificant positive correlation of these variables in C282Y homozygotes. The mean SF in the three participants with hypothyroidism was significantly lower than the mean SF in the seven participants with hyperthyroidism. Nonetheless, multivariate analysis of observations from all subjects who reported taking neither thyroid supplements nor medications to suppress thyroid function did not reveal that log10 free T4 was a significant, independent determinant of log10 SF. Hashimoto reported that SF was significantly lower in patients with primary hypothyroidism than in those who were euthyroid, and that SF increased significantly in patients with primary hypothyroidism after normalization of thyroid function (34). In patients with hyperthyroidism due to Graves disease, SF was significantly higher than in patients with primary hypothyroidism (34). In rats made hypothyroid with propylthiouracil or hyperthyroid with L-T4, the liver ferritin synthesis rate was reduced by 36% in hypothyroid rats, and elevated by 38% in hyperthyroid rats; a similar trend was observed in liver ferritin concentration (35). Thus, the present results confirm and extend previous reports that SF is positively correlated with free T4 in control subjects without HFE C282Y homozygosity. The present observations also suggest that increased absorption of iron characteristic of many persons with C282Y homozygosity has a greater influence on SF than free T4, and that increasing quantities of storage iron in C282Y homozygotes are not typically associated with decreasing levels of free T4.
It is unlikely that a non-HFE determinant of either TSH or free T4 on chromosome 6 could explain the occurrence of hypothyroidism or hyperthyroidism in most patients with hemochromatosis. HFE C282Y occurs in linkage disequilibrium with class I alleles at human leukocyte antigen (HLA)-A and -B loci and with the hemochromatosis ancestral haplotype HLA-A*03,B*07 on chromosome 6p (1,36). In Western European whites, Hashimoto thyroiditis and Graves' disease have been associated with HLA class II loci (37–39), but there are few reports of these disorders in whites with hemochromatosis phenotypes or C282Y homozygosity (6,40,41). This suggests that linkage disequilibrium of HFE C282Y with HLA class II loci is not great, that the ancestral hemochromatosis haplotype does not include susceptibility alleles for common autoimmune thyroid disorders, or that there are other susceptibility factors for Hashimoto thyroiditis and Graves' disease. Thyroid-related abnormalities are not typical of chromosome 6p deletion syndromes that involve the region 6p24-pter (42). The CGA gene that encodes the alpha chain subunit common to TSH, gonadotrophin, luteinizing hormone, and follicle-stimulating hormone occurs on the long arm of chromosome 6 (6q12–q21) (43–45).
The HEIRS Study was not designed to determine the clinical severity of or need for treatment of thyroid dysfunction. Our review of medications reported by participants suggests that some were previously diagnosed and treated for thyroid dysfunction, causing their TSH and free T4 values to return to normal before they enrolled in this post-initial screening evaluation. Obtaining self-reports or family history of thyroid gland disease, examining the thyroid gland and related attributes, measuring thyroid peroxidase and thyroglobulin antibodies, and performing thyroid ultrasonography and biopsy were beyond the scope of the HEIRS Study. Long-term follow-up of participants that would have permitted estimation of incidence rates of thyroid conditions was not possible.
In routine medical care settings, some patients who report having fatigue, weakness, or malaise need evaluation for thyroid-related disease, iron overload, or both (8,23,46). In hemochromatosis and iron overload screening programs, however, the prevalence of these symptoms in participants with hemochromatosis phenotypes and C282Y homozygosity was similar to that in age- and sex-matched control subjects (11,47). In this HEIRS substudy, the prevalence of abnormal values of free T4, TSH, or both is similar in white persons with or without C282Y homozygosity. Based on these observations, we conclude that there is no rationale for routine measurement of TSH or free T4 levels in hemochromatosis or iron overload screening programs.
Acknowledgments
The HEIRS Study was initiated and funded by the National Heart, Lung, and Blood Institute, in conjunction with the National Human Genome Research Institute. The study is supported by contracts N01-HC05185 (University of Minnesota); N01-HC05186, N01-CM-07003–74, and Minority CCOP (Howard University); N01-HC05188 (University of Alabama at Birmingham); N01-C05189 (Kaiser Permanente Center for Health Research); N01-HC05190 (University of California, Irvine); N01-HC05191 (London Health Sciences Centre); and N01-C05192 (Wake Forest University). Additional support was provided by the University of Alabama at Birmingham General Clinical Research Center (GCRC) grant M01-RR00032, Howard University GCRC grant M01-RR10284, and the University of California, Irvine UCSD/UCI Satellite GCRC grant M01-RR00827, sponsored by the National Center for Research Resources, National Institutes of Health; Howard University Research Scientist Award UH1-HL03679-05 from the National Heart, Lung and Blood Institute and the Office of Research on Minority Health (VRG); and Southern Iron Disorders Center (JCB, RTA).
Participating HEIRS Study investigators and institutions
FIELD CENTERS
Birmingham, AL—University of Alabama at Birmingham:
Dr. Ronald T. Acton (Principal Investigator), Dr. James C. Barton (Co-Principal Investigator), Ms. Deborah Dixon, Dr. Susan Ferguson, Dr. Richard Jones, Dr. Jerry McKnight, Dr. Charles A. Rivers, Dr. Diane Tucker, and Ms. Janice C. Ware.
Irvine, CA—University of California, Irvine:
Dr. Christine E. McLaren (Principal Investigator), Dr. Gordon D. McLaren (Co-Principal Investigator), Dr. Hoda Anton-Culver, Ms. Jo Ann A. Baca, Dr. Thomas C. Bent, Dr. Lance C. Brunner, Dr. Michael M. Dao, Dr. Korey S. Jorgensen, Dr. Julie Kuniyoshi, Dr. Huan D. Le, Dr. Miles K. Masatsugu, Dr. Frank L. Meyskens, Dr. David Morohashi, Dr. Huan P. Nguyen, Dr. Sophocles N. Panagon, Dr. Chi Phung, Dr. Virgil Raymundo, Dr. Thomas Ton, Prof. Ann P. Walker, Dr. Lari B. Wenzel, and Dr. Argyrios Ziogas.
London, Ontario, Canada—London Health Sciences Center:
Dr. Paul C. Adams (Principal Investigator), Ms. Erin Bloch, Dr. Subrata Chakrabarti, Ms. Arlene Fleischhauer, Ms. Helen Harrison, Ms. Kelly Jia, Ms. Sheila Larson, Dr. Edward Lin, Ms. Melissa Lopez, Ms. Lien Nguyen, Ms. Corry Pepper, Dr. Tara Power, Dr. Mark Speechley, Dr. Donald Sun, and Ms. Diane Woelfle.
Portland, OR, and Honolulu, HI—Kaiser Permanente Center for Health Research, Northwest and Hawaii, and Oregon Health and Science University:
Dr. Emily L. Harris (Principal Investigator), Dr. Mikel Aickin, Dr. Elaine Baker, Ms. Marjorie Erwin, Ms. Joan Holup, Ms. Carol Lloyd, Dr. Nancy Press, Dr. Richard D. Press, Dr. Jacob Reiss, Dr. Cheryl Ritenbaugh, Ms. Aileen Uchida, Dr. Thomas Vogt, and Dr. Dwight Yim.
Washington, D.C.—Howard University:
Dr. Victor R. Gordeuk (Principal Investigator), Dr. Fitzroy W. Dawkins (Co-Principal Investigator), Ms. Margaret Fadojutimi-Akinsiku, Dr. Oswaldo Castro, Dr. Debra White-Coleman, Dr. Melvin Gerald, Ms. Barbara W Harrison, Dr. Ometha Lewis-Jack, Dr. Robert F. Murray, Dr. Shelley McDonald-Pinkett, Ms. Angela Rock, Dr. Juan Romagoza, and Dr. Robert Williams.
CENTRAL LABORATORY
Minneapolis, MN—University of Minnesota and University of Minnesota Medical Center Fairview:
Dr. John H. Eckfeldt (Principal Investigator and Steering Committee Chair), Ms. Susie DelRio-LaFreniere, Ms. Catherine Leiendecker-Foster, Dr. Ronald C. McGlennen, Mr. Greg Rynders, Dr. Michael Y. Tsai, and Dr. Xinjing Wang.
COORDINATING CENTER
Winston-Salem, NC—Wake Forest University:
Dr. David M. Reboussin (Principal Investigator), Dr. Beverly M. Snively (Co-Principal Investigator), Dr. Roger Anderson, Ms. Elease Bostic, Ms. Brenda L. Craven, Ms. Shellie Ellis, Dr. Curt Furberg, Mr. Jason Griffin, Dr. Mark Hall, Mr. Darrin Harris, Ms. Leora Henkin, Dr. Sharon Jackson, Dr. Tamison Jewett, Mr. Mark D. King, Mr. Kurt Lohman, Ms. Laura Lovato, Dr. Joe Michaleckyj, Ms. Shana Palla, Ms. Tina Parks, Ms. Leah Passmore, Dr. Pradyumna D. Phatak, Dr. Stephen Rich, Ms. Andrea Ruggiero, Dr. Mara Vitolins, Mr. Gary Wolgast, and Mr. Daniel Zaccaro.
NHLBI PROJECT OFFICE
Bethesda, MD—Ms. Phyliss Sholinsky (Project Officer), Dr. Ebony Bookman, Dr. Henry Chang, Dr. Richard Fabsitz, Dr. Cashell Jaquish, Dr. Teri Manolio, and Ms. Lisa O'Neill.
NHGRI PROJECT OFFICE
Bethesda, MD—Dr. Elizabeth Thomson.
Dr. Jean MacCluer, Southwest Foundation for Biomedical Research, also contributed to the design of this study.
References
- 1.Feder JN. Gnirke A. Thomas W. Tsuchihashi Z. Ruddy DA. Basava A. Dormishian F. Domingo R., Jr. Ellis MC. Fullan A. Hinton LM. Jones NL. Kimmel BE. Kronmal GS. Lauer P. Lee VK. Loeb DB. Mapa FA. McClelland E. Meyer NC. Mintier GA. Moeller N. Moore T. Morikang E. Prass CE. Quintana L. Starnes SM. Schatzman RC. Brunke KJ. Drayna DT. Risch NJ. Bacon BR. Wolff RK. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 2.Beutler E. Felitti V. Gelbart T. Ho N. The effect of HFE genotypes on measurements of iron overload in patients attending a health appraisal clinic. Ann Intern Med. 2000;133:329–337. doi: 10.7326/0003-4819-133-5-200009050-00008. [DOI] [PubMed] [Google Scholar]
- 3.Adams PC. Reboussin DM. Barton JC. McLaren CE. Eckfeldt JH. McLaren GD. Dawkins FW. Acton RT. Harris EL. Gordeuk VR. Leiendecker-Foster C. Speechley M. Snively BM. Holup JL. Thomson E. Sholinsky P. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med. 2005;352:1769–1778. doi: 10.1056/NEJMoa041534. [DOI] [PubMed] [Google Scholar]
- 4.Barton JC. Wiener HW. Acton RT. Go RC. HLA haplotype A*03-B*07 in hemochromatosis probands with HFE C282Y homozygosity: frequency disparity in men and women and lack of association with severity of iron overload. Blood Cells Mol Dis. 2005;34:38–47. doi: 10.1016/j.bcmd.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Bezwoda WR. Bothwell TH. Van Der Walt LA. Kronheim S. Pimstone BL. An investigation into gonadal dysfunction in patients with idiopathic haemochromatosis. Clin Endocrinol (Oxf ) 1977;6:377–385. doi: 10.1111/j.1365-2265.1977.tb02024.x. [DOI] [PubMed] [Google Scholar]
- 6.Edwards CQ. Kelly TM. Ellwein G. Kushner JP. Thyroid disease in hemochromatosis. Increased incidence in homozygous men. Arch Intern Med. 1983;143:1890–1893. [PubMed] [Google Scholar]
- 7.Varela C. Hurtado A. Boixeda D. Garcia RR. Calle H. Zurita P. Sancho J. [Gonadal and pituitary function in idiopathic hemochromatosis] Med Clin (Barc) 1983;81:606–609. [PubMed] [Google Scholar]
- 8.Barton JC. Barton NH. Alford TJ. Diagnosis of hemochromatosis probands in a community hospital. Am J Med. 1997;103:498–503. doi: 10.1016/s0002-9343(97)00276-3. [DOI] [PubMed] [Google Scholar]
- 9.Murphy MS. Walsh CH. Thyroid function in haemochromatosis. Ir J Med Sci. 2004;173:27–29. doi: 10.1007/BF02914520. [DOI] [PubMed] [Google Scholar]
- 10.Phatak PD. Sham RL. Raubertas RF. Dunnigan K. O'Leary MT. Braggins C. Cappuccio JD. Prevalence of hereditary hemochromatosis in 16031 primary care patients. Ann Intern Med. 1998;129:954–961. doi: 10.7326/0003-4819-129-11_part_2-199812011-00006. [DOI] [PubMed] [Google Scholar]
- 11.Asberg A. Hveem K. Kruger O. Bjerve KS. Persons with screening-detected haemochromatosis: as healthy as the general population? Scand J Gastroenterol. 2002;37:719–724. doi: 10.1080/00365520212510. [DOI] [PubMed] [Google Scholar]
- 12.Delatycki MB. Allen KJ. Nisselle AE. Collins V. Metcalfe S. du SD. Halliday J. Aitken MA. Macciocca I. Hill V. Wakefield A. Ritchie A. Gason AA. Nicoll AJ. Powell LW. Williamson R. Use of community genetic screening to prevent HFE-associated hereditary haemochromatosis. Lancet. 2005;366:314–316. doi: 10.1016/S0140-6736(05)63012-7. [DOI] [PubMed] [Google Scholar]
- 13.McLaren CE. Barton JC. Adams PC. Harris EL. Acton RT. Press N. Reboussin DM. McLaren GD. Sholinsky P. Walker AP. Gordeuk VR. Leiendecker-Foster C. Dawkins FW. Eckfeldt JH. Mellen BG. Speechley M. Thomson E. Hemochromatosis and Iron Overload Screening (HEIRS) study design for an evaluation of 100,000 primary care-based adults. Am J Med Sci. 2003;325:53–62. doi: 10.1097/00000441-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Barton JC. Acton RT. Dawkins FW. Adams PC. Lovato L. Leiendecker-Foster C. McLaren CE. Reboussin DM. Speechley MR. Gordeuk VR. McLaren GD. Sholinsky P. Harris EL. Initial screening transferrin saturation values, serum ferritin concentrations, and HFE genotypes in whites and blacks in the Hemochromatosis and Iron Overload Screening Study. Genet Test. 2005;9:231–241. doi: 10.1089/gte.2005.9.231. [DOI] [PubMed] [Google Scholar]
- 15.SAS v. 9.02004 SAS Institute; Cary, NC: [Google Scholar]
- 16.Hollowell JG. Staehling NW. Flanders WD. Hannon WH. Gunter EW. Spencer CA. Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 17.Jensen E. Hyltoft PP. Blaabjerg O. Hansen PS. Brix TH. Kyvik KO. Hegedus L. Establishment of a serum thyroid stimulating hormone (TSH) reference interval in healthy adults: the importance of environmental factors, including thyroid antibodies. Clin Chem Lab Med. 2004;42:824–832. doi: 10.1515/CCLM.2004.136. [DOI] [PubMed] [Google Scholar]
- 18.Kratzsch J. Fiedler GM. Leichtle A. Brugel M. Buchbinder S. Otto L. Sabri O. Matthes G. Thiery J. New reference intervals for thyrotropin and thyroid hormones based on National Academy of Clinical Biochemistry criteria and regular ultrasonography of the thyroid. Clin Chem. 2005;51:1480–1486. doi: 10.1373/clinchem.2004.047399. [DOI] [PubMed] [Google Scholar]
- 19.Volzke H. Alte D. Kohlmann T. Ludemann J. Nauck M. John U. Meng W. Reference intervals of serum thyroid function tests in a previously iodine-deficient area. Thyroid. 2005;15:279–285. doi: 10.1089/thy.2005.15.279. [DOI] [PubMed] [Google Scholar]
- 20.Spencer CA. Hollowell JG. Kazarosyan M. Braverman LE. National Health and Nutrition Examination Survey III thyroid-stimulating hormone (TSH)-thyroperoxidase antibody relationships demonstrate that TSH upper reference limits may be skewed by occult thyroid dysfunction. J Clin Endocrinol Metab. 2007;92:4236–4240. doi: 10.1210/jc.2007-0287. [DOI] [PubMed] [Google Scholar]
- 21.Waalen J. Beutler E. Beware of multiple comparisons: a study of symptoms associated with mutations of the HFE hemochromatosis gene. Clin Chim Acta. 2005;361:128–134. doi: 10.1016/j.cccn.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Bergeron C. Kovacs K. Pituitary siderosis: a histologic, immunocytologic, and ultrastructural study. Am J Pathol. 1978;93:295–309. [PMC free article] [PubMed] [Google Scholar]
- 23.Walsh CH. Non-diabetic endocrinopathy in hemochromatosis. In: Barton JC, editor; Edwards CQ, editor. Hemochromatosis: genetics, pathogenesis, diagnosis and treatment. Cambridge University Press; Cambridge: 2000. p. 278. [Google Scholar]
- 24.Stocks AE. Martin FI. Pituitary function in haemochromatosis. Am J Med. 1968;45:839–845. doi: 10.1016/0002-9343(68)90182-4. [DOI] [PubMed] [Google Scholar]
- 25.Stocks AE. Powell LW. Pituitary function in idiopathic haemochromatosis and cirrhosis of the liver. Lancet. 1972;2:298–300. doi: 10.1016/s0140-6736(72)92905-4. [DOI] [PubMed] [Google Scholar]
- 26.Siemons LJ. Mahler CH. Hypogonadotropic hypogonadism in hemochromatosis: recovery of reproductive function after iron depletion. J Clin Endocrinol Metab. 1987;65:585–587. doi: 10.1210/jcem-65-3-585. [DOI] [PubMed] [Google Scholar]
- 27.Gama R. Smith MJ. Wright J. Marks V. Hypopituitarism in primary haemochromatosis: recovery after iron depletion. Postgrad Med J. 1995;71:297–298. doi: 10.1136/pgmj.71.835.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilkinson H. Haemochromatosis associated with arthritis and hypopituitarism. Ann Clin Biochem. 1996;33(Pt 2):171–173. doi: 10.1177/000456329603300217. [DOI] [PubMed] [Google Scholar]
- 29.Hudec M. Grigerova M. Walsh CH. Secondary hypothyroidism in hereditary hemochromatosis: recovery after iron depletion. Thyroid. 2008;18:255–257. doi: 10.1089/thy.2007.0140. [DOI] [PubMed] [Google Scholar]
- 30.Sheldon J. Haemochromatosis. Oxford University Press; London: 1935. pp. 128–152. [Google Scholar]
- 31.MacDonald RA. Mallory GK. Hemochromatosis and hemosiderosis. Study of 211 autopsied cases. Arch Intern Med. 1960;105:686–700. [PubMed] [Google Scholar]
- 32.Olynyk JK. Luxon BA. Britton RS. Bacon BR. Hepatic iron concentration in hereditary hemochromatosis does not saturate or accurately predict phlebotomy requirements. Am J Gastroenterol. 1998;93:346–350. doi: 10.1111/j.1572-0241.1998.00346.x. [DOI] [PubMed] [Google Scholar]
- 33.Beutler E. Felitti V. Ho NJ. Gelbart T. Relationship of body iron stores to levels of serum ferritin, serum iron, unsaturated iron binding capacity and transferrin saturation in patients with iron storage disease. Acta Haematol. 2002;107:145–149. doi: 10.1159/000057632. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto T. Matsubara F. Changes in the tumor marker concentration in female patients with hyper-, eu-, and hypothyroidism. Endocrinol Jpn. 1989;36:873–879. doi: 10.1507/endocrj1954.36.873. [DOI] [PubMed] [Google Scholar]
- 35.Deshpande UR. Nadkarni GD. Relation between thyroid status and ferritin metabolism in rats. Thyroidology. 1992;4:93–97. [PubMed] [Google Scholar]
- 36.Barton JC. Acton RT. HLA-A and -B alleles and haplotypes in hemochromatosis probands with HFE C282Y homozygosity in central Alabama. BMC Med Genet. 2002;3:9. doi: 10.1186/1471-2350-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tandon N. Zhang L. Weetman AP. HLA associations with Hashimoto's thyroiditis. Clin Endocrinol (Oxf ) 1991;34:383–386. doi: 10.1111/j.1365-2265.1991.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 38.Levin L. Ban Y. Concepcion E. Davies TF. Greenberg DA. Tomer Y. Analysis of HLA genes in families with autoimmune diabetes and thyroiditis. Hum Immunol. 2004;65:640–647. doi: 10.1016/j.humimm.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 39.Ratanachaiyavong S. McGregor AM. HLA-DPB1 polymorphisms on the MHC-extended haplotypes of families of patients with Graves' disease: two distinct HLA-DR17 haplotypes. Eur J Clin Invest. 1994;24:309–315. doi: 10.1111/j.1365-2362.1994.tb01090.x. [DOI] [PubMed] [Google Scholar]
- 40.Williams RS. Increased thyroid function in haemochromatosis. Br Med J. 1959;1:1078–1080. doi: 10.1136/bmj.1.5129.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barton JC. Bertoli LF. Acton RT. Common variable immunodeficiency and IgG subclass deficiency in central Alabama hemochromatosis probands homozygous for HFE C282Y. Blood Cells Mol Dis. 2003;31:102–111. doi: 10.1016/s1079-9796(03)00116-5. [DOI] [PubMed] [Google Scholar]
- 42.Mirza G. Williams RR. Mohammed S. Clark R. Newbury-Ecob R. Baldinger S. Flinter F. Ragoussis J. Refined genotype-phenotype correlations in cases of chromosome 6p deletion syndromes. Eur J Hum Genet. 2004;12:718–728. doi: 10.1038/sj.ejhg.5201194. [DOI] [PubMed] [Google Scholar]
- 43.Naylor SL. Chin WW. Goodman HM. Lalley PA. Grzeschik KH. Sakaguchi AY. Chromosome assignment of genes encoding the alpha and beta subunits of glycoprotein hormones in man and mouse. Somatic Cell Genet. 1983;9:757–770. doi: 10.1007/BF01539478. [DOI] [PubMed] [Google Scholar]
- 44.Dracopoli NC. Rettig WJ. Whitfield GK. Darlington GJ. Spengler BA. Biedler JL. Old LJ. Kourides IA. Assignment of the gene for the beta subunit of thyroid-stimulating hormone to the short arm of human chromosome 1. Proc Natl Acad Sci USA. 1986;83:1822–1826. doi: 10.1073/pnas.83.6.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johns Hopkins University. *118850. Chorionic gonadotrophin, alpha chain; CGA. Online Mendelian Inheritance in Man (OMIM) 2007. http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=118850 http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=118850
- 46.Witte DL. Crosby WH. Edwards CQ. Fairbanks VF. Mitros FA. Practice guideline development task force of the College of American Pathologists. Hereditary hemochromatosis. Clin Chim Acta. 1996;245:139–200. doi: 10.1016/0009-8981(95)06212-2. [DOI] [PubMed] [Google Scholar]
- 47.Waalen J. Felitti V. Gelbart T. Ho NJ. Beutler E. Prevalence of hemochromatosis-related symptoms among individuals with mutations in the HFE gene. Mayo Clin Proc. 2002;77:522–530. doi: 10.4065/77.6.522. [DOI] [PubMed] [Google Scholar]