Abstract
The molecular mechanism by which dual-specificity RasGAPs of the Gap1 subfamily activate the GTP hydrolysis of both Rap and Ras is an unresolved phenomenon. RasGAPs and RapGAPs use different strategies to stimulate the GTPase reaction of their cognate G-proteins. RasGAPs contribute an arginine finger to orient through the Gln61 of Ras the nucleophilic water molecule. RapGAP contributes an asparagine (Asn thumb) into the active site to substitute for the missing Gln61. Here, by using steady-state kinetic assays and time-resolved Fourier-transform infrared spectroscopy (FTIR) experiments with wild type and mutant proteins, we unravel the remarkable mechanism for the specificity switch. The plasticity of GAP1IP4BP and RASAL is mediated by the extra GTPase-activating protein (GAP) domains, which promote a different orientation of Ras and Rap's switch-II and catalytic residues in the active site. Thereby, Gln63 in Rap adopts the catalytic role normally taken by Gln61 of Ras. This re-orientation requires specific interactions between switch-II of Rap and helix-α6 of GAPs. This supports the notion that the specificities of fl proteins versus GAP domains are potentially different.
Keywords: GAP1IP4BP, GAP-mechanism, Rap, Ras, RASAL
Introduction
Ras and Rap are two types of GTP-binding proteins (G-proteins in short) of the Ras family. They control a wide range of cellular processes by switching between an inactive (GDP-bound) and an active (GTP-bound) conformation. The latter is able to bind to downstream effectors. This switch is regulated by guanine nucleotide-exchange factors (GEFs), which act as activators and promote the release of GDP and the binding of GTP. GTPase-activating proteins (GAPs) are responsible for acceleration of the intrinsically slow GTPase activity. Oncogenic Ras mutants incapable of hydrolysing GTP are found in 20–30% of human tumours, underscoring the importance of the GTPase activity (Downward, 2003). These mutations are single point mutations in codon-12, 13 or 61 (Gln61) that render Ras insensitive to GAP catalysis. Deregulation or mutations of RasGAPs also contributes to tumour formation. Thus, RASAL is epigenetically silenced in multiple tumours (Jin et al, 2007; Ohta et al, 2009) and neurofibromin is the product of the gene responsible for neurofibromatosis-1 (NF1), an inherited tumour predisposition syndrome (Dasgupta et al, 2003). Furthermore, knockout of the Rap-specific GAP Spa-1 leads to a CML-like phenotype (Ishida et al, 2003).
The molecular mechanism for the RasGAP-mediated GTPase hydrolysis (commonly referred to as GAP activity) involves two major residues: Gln61 from Ras (for nomenclature see Supplementary Table I), situated in a highly mobile motif called switch-II; and an arginine (arginine finger) provided by GAP. The latter moves into the binding site and orients Gln61. This positions a water molecule for nucleophilic attack (Scheffzek et al, 1997) and already induces a GDP-like charge distribution in GTP. Thereby the free activation energy is reduced (Kötting and Gerwert, 2004). Rap-subfamily proteins (Rap1 A, B; Rap2 A, B, C) belong to the few small G-proteins that do not possess a residue corresponding to Gln61, but rather a non-essential threonine. Rap-specific RapGAPs, which are structurally unrelated to RasGAPs, do not supply an arginine finger, but rather an asparagine (the asparagine thumb), which recapitulates the function of the intrinsic Gln (belonging to the G-protein) (Daumke et al, 2004; Scrima et al, 2008). Surprisingly, in spite of these very different mechanisms by which finally the attacking water molecule is oriented and charge shift is induced in GTP, there are several RasGAPs with dual specificity for Ras and Rap (Rap in short; Supplementary Table I); these are: SynGAP, a synaptic protein involved in neuronal development (Kim et al, 2003); and three members of the Gap1 family, GAP1IP4BP, RASAL and CAPRI (Kupzig et al, 2006). A mechanistic explanation of this intriguing dual activity is still elusive. To unravel the mechanism might also provide important clues for approaches to stimulate the GTPase activity of oncogenic Gln61 Ras mutants.
GAP1IP4BP was the first dual GAP to be studied biochemically (Kupzig et al, 2006). As other Gap1-family proteins, it contains two N-terminal C2 domains (C2A and C2B), a GAP domain and a pleckstrin-homology domain (PH) connected to a Bruton's tyrosine kinase (Btk) motif (Figure 1A). The C-terminal part, Ct from here on, has no homology with any known domain. Full-length (fl) GAP1IP4BP and its GAP domain were previously characterized. While the fl protein is an effective GAP for Ras and Rap, the GAP domain is only active with Ras. Using pull-down assays with cell extracts, Pro489 in α6 of the GAP domain was found to be important for RapGAP activity (Kupzig et al, 2009), and the arginine-finger residue of the GAP domain was considered crucial for both activities (Kupzig et al, 2006). So far however, no catalytic asparagine or glutamine or any other crucial residue in the dual-specificity RasGAP could be detected, which might mediate GTP hydrolysis on Rap (Kupzig et al, 2009).
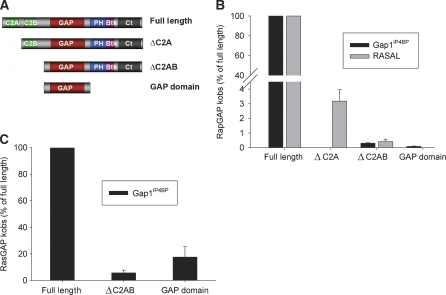
Figure 1.
Effect of C2 and PH-Ct domains on GAP activity. (A) A schematic representation of GAP1IP4BP/RASAL fl and the deletion mutants used for biochemical experiments. (B, C) GAP activity of deletion mutants with Rap (B) and Ras (C), represented as percentage of the fl activity, measured by charcoal method using standard buffer (see Materials and methods) with 200 μM Rap or Ras bound to GTP and 50 nM fls, 1 μM Gap1IP4BPΔC2AB, Gap1IP4BPGap domain for RasGAP activity, and 1 μM RASALΔC2A, RASALΔC2AB; 10 μM Gap1IP4BPΔC2AB and 20 μM Gap1IP4BPGap domain for RapGAP activity. Data represent the means±s.d.
SynGAP, a dual-specificity GAP from another family, which is more active on Rap than on Ras, possesses a PH domain, followed by a C2 domain and a GAP domain (Bernards, 2003). Although the PH domain is dispensable, the C2 domain is necessary for RapGAP activity. On the basis of a crystal structure of a C2-GAP domain fragment of SynGAP, a possible contact between the C2 domain and the nucleotide-binding site of Rap has been suggested. In addition, it has been shown there that not only the arginine finger, but also a conserved asparagine located two residues downstream of the catalytic arginine, is important for RapGAP activity (Pena et al, 2008).
RASAL and CAPRI are members of the Gap1 family also described as dual-specificity GAPs using GST-RBD pull-down assays of cell extracts. Surprisingly, RASAL responds to Ca2+ oscillations and activates the GTPase activity of Ras only when bound to membranes through its C2 domains. Therefore, purified recombinant RASAL is not active with Ras (Walker et al, 2004). By contrast, its RapGAP activity is independent of membrane binding (Kupzig et al, 2006).
The mechanism used by dual GAPs to activate the GTPase activity of Rap is not known. In this paper we present an extensive mutational study to describe the residues and domains in Rap and GAP1IP4BP important for the dual activity. By combining multiple-turnover solution kinetics with single-turnover GTPase experiments through time-resolved FTIR at higher protein concentrations, we obtained a mechanistic understanding of the remarkable specificity switch between the Ras- and a Rap-specific GTPase catalysis mode by dual-specificity GAPs.
Results
Contribution of C2 and PH-Ct to GAP activity
We previously showed that the isolated GAP domain of GAP1IP4BP has no effective RapGAP activity, whereas it still has substantial RasGAP activity, which is however lower than that of fl and not saturable under the conditions used (Kupzig et al, 2006). Moreover, recent work using pull-down assays of extracts of cells transiently expressing the G-protein and GAP1IP4BP (Kupzig et al, 2009) showed that deletion mutants lacking the N- or the C-terminal part of GAP1IP4BP retain RasGAP but not RapGAP activity. To analyse their mechanistic contribution, here we measure the effect of the C2 and PH-Ct domains on the GAP activity of GAP1IP4BP more quantitatively. To establish possible general features of dual-specificity GAPs, we similarly analyse the GAP activity of RASAL and the corresponding deletion mutants (Figure 1A) (for a list of proteins and their simplified nomenclature, see Supplementary Table I). The assays were performed under multiple-turnover conditions by following Pi release from radioactive [γ-32P]-labelled GTP (Brinkmann et al, 2002; Supplementary Table II). The kinetic constants (kobs) obtained were represented as percentage of the fl constant (Figure 1B and C).
The experiments show that deletion of the C2 domains of GAP1IP4BP strongly decreases RapGAP activity by more than 1000-fold to 0.03% (kobs: 0.015 s−1) and is further decreased by deletion of the C-terminus. However, the RasGAP activity of GAP1IP4BPΔC2AB and GAP domain is reduced only 17- and 6-fold as compared with that in the wild type (wt). Thus, deletion of the C2 and PH-Ct domains has an effect on RasGAP activity, although it is much smaller than with RapGAP activity. Deletion of only the first C2 domain (C2A) of GAP1IP4BP would show if this domain is also implicated in RapGAP activity. As it was not possible to isolate this GAP1IP4BP mutant as a soluble protein, the effect of C2A deletion was measured in RASAL instead. RASAL, as previously described, has no RasGAP activity in solution. Its RapGAP activity is successively reduced by deletion of one or two C2 domains. Deletion of one C2 domain reduces activity 30-fold (to 3%), whereas deletion of both reduces activity 250-fold, a similar effect as for GAP1IP4BP. We could not isolate the GAP domain of RASAL as a soluble protein, but on the basis of GAP1IP4BP results we would expect it to have even lower RapGAP activity.
FTIR measurements of GAP1IP4BP-catalysed GTPase reactions
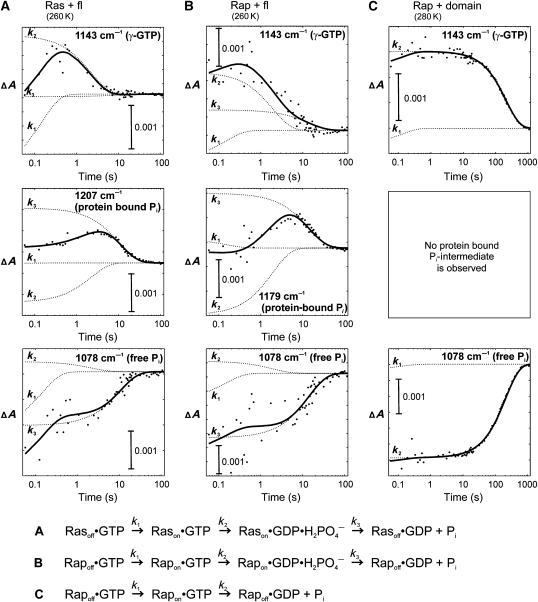
RasGAP or RapGAP activities using radioactive GTP could not be easily saturated under the experimental conditions due to low affinities. As we have shown earlier that FTIR measurements of the GTPase reaction can easily be performed under higher protein concentrations and can be used to study partial reaction steps under single-turnover conditions (Cepus et al, 1998; Kötting et al, 2006; Chakrabarti et al, 2007), we turned to FTIR to get more mechanistic insight. The results for GAP1IP4BP (fl) and its GAP domain are presented in Figure 2 and Table 1.
Figure 2.
Single-frequency kinetics of the GAP1IP4BP-catalysed GTPase reaction using the fl protein with Ras (A) and Rap (B), and the GAP1IP4BP-GAP-domain-catalysed reaction of Rap (C). The IR band amplitude changes with time of GTP (1143 cm−1) and free Pi (1078 cm−1) are shown for all three systems. In the GAP1IP4BP fl, an intermediate is found for the GTPase reactions of Ras (at 1207 cm−1) and Rap (at 1179 cm−1). The experimental data are represented as dots, which were fitted (continuous curve) to a multi-exponential equation that describes the global reaction as shown at the bottom of the Figure. The contributions from the apparent rate constant (ki) of each partial reaction to the global fit are presented as dotted lines. The contribution of k1 to the 1078 cm−1 kinetic is due to an artefact from the buffer.
Table 1. FTIR with wild-type and mutants Ras, Rap and Gap1IP4BP.
| G-protein | GAP1IP4BP | T (K) | k2 (s−1) | k3 (s−1) |
|---|---|---|---|---|
| Ras | Full length | 260 | 4.5 × 10−1 | 9.7 × 10−2 |
| Ras | ΔC2AB | 260 | 4.7 × 10−1 | 8.4 × 10−2 |
| Ras | GAP domain | 260 | 1.8 × 10−1 | 1.3 × 10−2 |
| Ras | N373T | 260 | 1.2 × 10−1 | |
| Ras | P489V | 260 | 7.8 × 10−1 | 4.6 × 10−2 |
| Rap | Full length | 260 | 6.6 × 10−1 | 7.8 × 10−2 |
| Rap | ΔC2AB | 260 | 4.9 × 10−3 | |
| Rap | GAP domain | 280 | 4.8 × 10−3 (5.3 × 10−4) | |
| Rap | N373T | 260 | 9.4 × 10−3 | |
| Rap | P489V | 260 | 1.2 × 10−3 | |
| Rap-T61Q | Full length | 260 | 4.0 × 10−1 | 7.9 × 10−2 |
| Rap-T61Q | GAP domain | 280 | 1.8 × 10−1 (2.0 × 10−2) | |
| Rap-T61A | Full length | 260 | 9.1 × 10−1 | 5.8 × 10−2 |
| Rap-T61A | GAP domain | 280 | 1.1 × 10−2 (1.2 × 10−3) | |
| Rap-Q63E | Full length | 285 | 5.9 × 10−4 (4.3 × 10−5) | |
| Rap-Q63A | Full length | 285 | 2.3 × 10−4 (1.7 × 10−5) | |
| G-protein | Rap1GAP | T (K) | k2 (s−1) | k3 (s−1) |
| Rap | Rap1GAP | 260 | 1.7 × 10−1 | 5.0 × 10−2 a |
| Rap-Q63E | Rap1GAP | 260 | 7.9 × 10−2 | |
| Rap-Q63A | Rap1GAP | 260 | 3.6 × 10−1 | |
| For the sake of comparison, we have enclosed in parentheses all rates extrapolated to 260 K, using a factor of three for each 10K step. | ||||
| aData obtained from Chakrabarti et al (2007). | ||||
The GAP-mediated GTPase reaction of Ras and Rap with GAP1IP4BP (fl) can be described by three rate constants, as shown previously for Ras with the GAP domain of NF1 and for Rap with RapGAP (Chakrabarti et al, 2004; Kötting et al, 2006). After triggering reaction by photolysis of G-protein-bound caged-GTP to GTP, the first step with rate k1 represents the off-to-on conformational change towards the GTPase-competent ‘ON' state (Kötting et al, 2008). In the next step described by k2, the γ-phosphate is cleaved and an intermediate is formed. This intermediate represents doubly protonated inorganic phosphate (previously identified as H2PO4−) non-covalently bound within the binding pocket (Kötting et al, 2006). In the final process described by the rate-determining k3, Pi is released into the bulk and the conformation of the GTPase returns into the ‘OFF' state. This series of events can be followed in real-time by time-resolved infrared spectroscopy. The time-resolved absorbance changes of marker bands for GAP1IP4BP fl reaction are shown in Figure 2A and B. Assignment of marker bands is clear-cut by site-specific isotopic labelling (for details see Materials and methods and Supplementary Figure 3).
The first marker band is the γ-phosphate of GTP bound to the G-protein in its ‘ON' conformation at 1143 cm−1 (Kötting et al, 2007). It appears with k1 and decays with k2. The second marker band at 1207 cm−1 (Ras) and 1179 cm−1 (Rap) represents the protein-bound phosphate intermediate, which is formed with k2 and decays with k3. Finally formation of free phosphate with k3 can be followed at 1078 cm−1 (Klähn et al, 2004). While the absorption bands for free Pi are the same for the Ras and Rap reaction, as expected, and the γ-GTP band is also at the same frequency, the G-protein-bound Pi absorbs at different frequencies (see below). Apart from the intermediate bands, the nucleotide absorption bands for the catalysed Ras reaction are similar to that of the NF1-catalysed reaction, but for the Rap-catalysed reaction they are different from those of the Rap-RapGAP catalysis reaction (Chakrabarti et al, 2004, 2007).
RasGAP activity was also measured with the deletion construct GAP1IP4BPΔC2AB and GAP1IP4BP-GAP domains. The absorbance changes can be described in the same way as above with three rate constants and an intermediate. The results are summarized in Table 1; selected absorbance changes are shown in Supplementary Figure 1. The absorbance bands are the same for fl and deletion constructs, arguing for a very similar microenvironment around the active site in the presence or absence of the extra domains (Supplementary Figure 2). The rates are almost the same for ΔC2AB and somewhat slower for the GAP domain, supporting previous conclusions (Kupzig et al, 2006, 2009). This shows that the lower activity measured under multiple-turnover conditions must be due to a decrease in affinity, suggesting that the C2 domains and/or the C-terminal end are implicated in Ras binding rather than catalysis (Chakrabarti et al, 2007).
The RapGAP activity of the GAP1IP4BPΔC2AB and GAP1IP4BP-GAP domain as defined by the cleavage step k2, is ∼100 and ∼1000 times slower than that of the fl (Figure 2C; Table 1; Supplementary Figure 1). The GTP marker band at 1143 cm−1 is at the same position, whereas other absorptions of the nucleotide are slightly shifted, indicating a similar but not identical environment compared with the intrinsic hydrolysis reaction of Rap (Chakrabarti et al, 2004). The reduction in RapGAP activity is similar to what has been measured with the radioactivity assay, which shows that deletion of N- and C-terminal residues directly affects catalysis rather than affinity. Furthermore, for both deletion mutants, decrease of GTP (1143 cm−1) and formation of free Pi (1078 cm−1) proceed with the same rate k2 (Figure 2C). There is no apparent accumulation of an intermediate due to the much slower bond-cleavage step. This is in contrast to the reaction of Rap with fl GAP1IP4BP and to the GTPase reaction of Ras with any of the constructs where Pi release is described by k3 and is rate-limiting.
The reaction intermediate
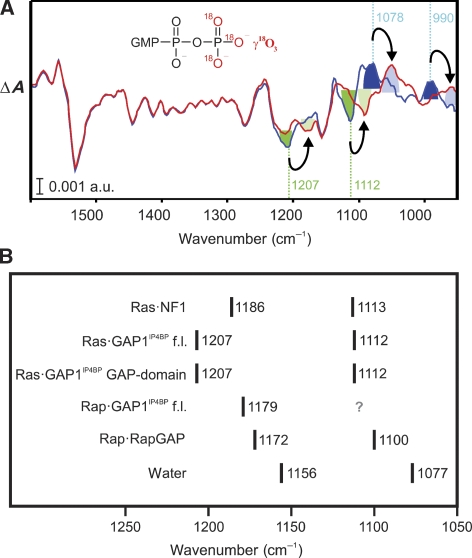
To compare features of the active site during GAP1IP4BP-mediated catalysis on Ras versus Rap, we compared infrared bands of the reaction intermediate. The assignments of the spectral features of the intermediates in different reactions are shown in Figure 3 and Supplementary Figure 3. In Figure 3A the amplitude spectra for k3 describing the Pi release is shown in blue. Bands facing downwards are from the protein-bound Pi state and bands facing upwards are due to the free Pi after its release. Assignment of the peaks is shown here by use of γ-18O-labelled GTP (red line), which leads to downshifts of only those vibrations involving the γ-phosphate, as indicated by the arrows. Due to this downshift the bands at 1207 and 1112 cm−1 (green) can be assigned to the protein-bound phosphate. The bands at 1078 and 990 cm−1 (blue) are the well-known bands of the released phosphate (HPO42−) (Klähn et al, 2004). The remaining part of the spectrum is not affected by the label. The bands of the intermediate with identical shifts upon 18O labelling are also seen in the amplitude spectra of k2 (Supplementary Figure 3).
Figure 3.
Spectral features of the intermediate. (A) An amplitude spectra of k3 (Pi release) obtained by a global fit of the GAP1IP4BP-GAP domain-catalysed GTPase reaction of Ras using unlabelled (blue) or γ-18O3-labelled (red) GTP. The bands facing downwards belong to the phosphate-bound intermediate and bands facing upwards belong to the GDP state, respectively. Absorptions of the phosphate-bound intermediate are labelled in green and the absorptions of the free Pi are labelled in blue. (B) Band positions of the asymmetric and the symmetric PO2 vibrations of Pi (H2PO4−) within the binding pocket of various G-protein–GAP complexes (Chakrabarti et al, 2004; Kötting et al, 2006) and in water (Klähn et al, 2004).
We have previously shown that during the RapGAP- and RasGAP-mediated reaction, G-protein-bound Pi is in a special activated state where it can efficiently perform the reverse reaction and synthesize GTP before release of Pi (Kötting et al, 2008). The FTIR spectra of the protein-bound Pi intermediate are thus an indication of the chemical nature of the active site (Chakrabarti et al, 2004; Kötting et al, 2006). Absorption of the antisymmetric PO2 stretching mode of the protein-bound Pi intermediate in the GAP1IP4BP-catalysed Ras GTPase reaction is shifted as compared with the intermediate in the NF1-catalysed Ras hydrolysis, which absorbs at 1186 cm−1 (Kötting et al, 2006) rather than at 1207 cm−1 (Figure 3B). It is identical to the reaction of the GAP domain. The corresponding intermediate of the GAP1IP4BP fl-catalysed Rap reaction absorbs at 1179 cm−1 (Figure 2B), again deviating from the Rap RapGAP complex (1172 cm−1) (Chakrabarti et al, 2004) and both Ras complexes. The shifts for the symmetric PO2 stretching mode also deviate, but they are smaller compared with the shifts observed for the asymmetric mode. The different absorptions show that the nature of the protein-bound Pi intermediate has a different chemical environment for each active site. The different absorption frequencies might reflect different positions along the reaction pathway with different distances of GDP and the protein-bound phosphate. QM/MM calculations will resolve the origin of these spectral features (Klähn et al, 2005).
Mutation of GAP domain residues
Previous results showed that the arginine finger is required for efficient RasGAP and RapGAP activity of dual-specificity GAPs (Kupzig et al, 2006; Pena et al, 2008), whereas Rap-specific GAP RapGAP operates without it (Scrima et al, 2008). Pena et al showed that an asparagine (Asn472, corresponding to Asn373 in GAP1IP4BP) is important for efficient RapGAP catalysis of SynGAP in contrast to Kupzig et al (2009) where mutation of conserved asparagine residues of the GAP domain of GAP1IP4BP did not affect RapGAP activity. Pro489 of GAP1IP4BP is situated close to the FLR-motif of helix-α6 in the GAP domain, which in the Ras–RasGAP complex interacts with the switch-II motif of Ras containing Gln61 (Scheffzek et al, 1997). This proline residue is conserved in RasGAPs except GAP1m, the only GAP1-family member without dual activity, and has been considered important for GTPase activation of the Rap of dual-specificity GAPs (Kupzig et al, 2009). To more specifically investigate the contribution of these residues, they were investigated by the methods described above.
The GAP1IP4BP mutants N373T and P489V were tested for Ras and RapGAP activities by HPLC (Brinkmann et al, 2002; Supplementary Figure 4) and FTIR (Table 1). Multi-turnover analysis shows that both residues seem to be important for RapGAP activity, whereas RasGAP activity is somewhat dependent on Asn373 but not Pro489. FTIR data support the importance of both Asn373 and Pro489 for RapGAP activity, which is reduced to 1.42 and 0.18% (compared by k2 value) of wt activity for N373T and P489V, respectively. The much lower activity has the consequence that there is no accumulation of intermediate and the reaction can be described by a single rate. The large loss of activity seen in the multiple-turnover assay of N373T with Ras is likely due to lower affinity, while the chemical step itself is only mildly slowed down. This is in line with studies of NF1, where this Asn was implicated in Ras binding (Ahmadian et al, 2003).
The relatively modest 70-fold decrease in activity observed with GAP1IP4BP(N373T) in the RapGAP mode of catalysis shows that this residue is unlikely responsible for positioning the attacking water molecule. Mutations of the Asn thumb of Rap-specific GAP RapGAP (Chakrabarti et al, 2007) or the intrinsic Gln61 of Ras lead to an almost complete loss of activity (Bollag and McCormick, 1991). Pro489 has an important role in RapGAP catalysis, as the P489V mutation has a 500-fold reduction in activity but due to the nature of the residue is unlikely to directly contribute to the chemistry of the reaction. Mutations of the same residues in RASAL (P476V and N344T) have a similar effect on catalysis as measured by HPLC under the multi-turnover reaction conditions (data not shown), suggesting that these residues are generally important for dual-specificity GAPs.
A structural model of the Rap-GAP1IP4BP complex
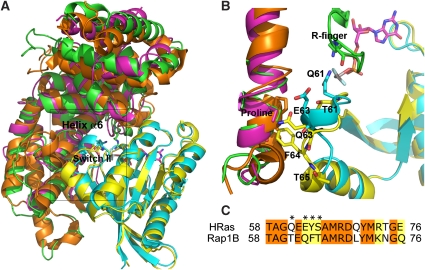
So far, none of the residues considered above seem to have an essential role in RapGAP catalysis, similar to what would be expected for a Gln/Asn residue after previous studies of the RasGAP- and RapGAP-mediated GTPase reactions (Gideon et al, 1992; Daumke et al, 2004; Scrima et al, 2008). We prepared a model of binding of Rap to GAP1IP4BP-GAP domain on the basis of the structure of the Ras–RasGAP complex (Scheffzek et al, 1997) and the structure of Rap from the Rap–RapGAP complex (Scrima et al, 2008; Figure 4A and B). To try to have an approximate idea about how the Rap–GAP1IP4BP GAP domain complex could look like, we superimposed the Rap structure from the Rap–RapGAP complex (Scrima et al, 2008) onto the Ras–p120GAP complex (Scheffzek et al, 1997; Figure 4A and B). As the G-proteins are highly homologous and the GAP1IP4BP′s arginine finger is important for RapGAP activity (Kupzig et al, 2006), the structural model should allow us to identify possible catalytic residues of the active site. Thus, Rap-Thr61 in switch-II is superimposing with the crucial Gln61 of Ras. The residues in switch-II different between Ras and Rap (Figure 4C) would be located close to helix-α6 of the GAP domains. Pro489, which is required for RapGAP activity, is a central residue of that helix. We also aligned all known RasGAP domain structures (SynGAP and NF1) onto the Ras–p120GAP complex structure, to check that helix-α6 is always in the same orientation.
Figure 4.
A comparison between the switch-II of Ras and Rap. (A) Superimposition of Rap1B (yellow) (PDB entry 3BRW) extracted from Rap1B-Rap1GAP onto the structure (PDB entry 1WQ1) of the complex between Ras (cyan) and the GAP domain of p120GAP (green) domain. SynGAP (orange) (PDB entry 3BXJ) and NF1 (magenta) (PDB entry 1NF1) GAP domain structures are aligned to p120GAP to show the similarity between different GAP domains. (B) A detailed view of the active centre. Important residues in Ras and Rap switch-II are highlighted, as well as the proline in helix-α6 in the three GAP domains. (C) Sequence alignment of the switch-II regions of Ras and Rap. Residues differing around position 61 and mutated in this work are indicated by an asterisk.
The role of the Rap switch-II region
Ras(Q61T) is almost completely insensitive to GTPase activation by GAP1IP4BP (Figure 5B) (or any other RasGAP), whereas in the reverse situation, introduction of Gln61 into Rap partially rescues the inability of the GAP domain of GAP1IP4BP to activate Rap (Kupzig et al, 2006; Table 1). It seems to suggest that introduction of Gln61 into Rap partially switches the catalysis mode to that of Ras, whereas the reverse is not true. Both charcoal assay and FTIR show that the Rap(T61Q) or (T61A) mutations do not appreciably affect the reaction with both fl RASAL and GAP1IP4BP (Figure 5A; Table 1), supporting the notion that residue 61 is not important for Rap catalysis. However, activity of the GAP1IP4BP GAP domain towards Rap is increased 37-fold with Rap(T61Q) and is only 22-fold lower than that of the fl protein (Table 1). In summary we can conclude that Rap-Thr61 has no role in the Rap catalysis mode of dual-specificity fl GAPs, whereas Gln61 is essential for the Ras mode.
Figure 5.
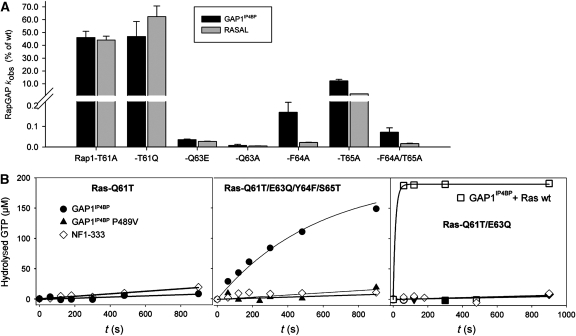
Gln63 as the main catalytic residue for Rap catalysis. (A) A bar diagram of the GAP activity of GAP1IP4BP and RASAL with Rap mutants, measured by reverse-phase HPLC, except the Rap(T61Q) mutant that was measured by charcoal assay, presented as percentage of the kinetic constant (kobs) of wt. wt (200 μM) or mutant Rap was mixed with 50 nM (for Rap(T61) mutants), 1 μM (for Rap(T64A)) or 50 μM (for Rap-Q63 mutants, Rap(F64A) and Rap(F64A/T65A)) GAP1IP4BP/RASAL in standard buffer. (B) The GTPase reaction kinetics of 200 μM GTP-loaded Ras(Q61T) (first panel), Ras(Q61T/E63Q/Y64F/S65T) (second panel) and Ras(Q61T/E63Q) (third panel) in the presence of 10 μM GAP1IP4BP wt, P489V mutant and NF1-333. The GTPase reaction of 200 μM Ras wt in the presence of 10 μM GAP1IP4BP is shown in the third panel. Hydrolysis of GTP was monitored by reverse-phase HPLC and plotted against reaction time.
This leaves still open the question of the Rap catalysis mode. From the superimposition of Rap and Ras, and by comparing their sequences (Figure 4), we reasoned that, as the only residue close to γ-phosphate in our Rap–RasGAP complex model, Rap-Thr61, is not implicated in catalysis, the difference in activity is very likely due to other differences in switch-II. Thus, Rap-Gln63, which is close to GAP1IP4BP-Pro489 and pointing away from the γ-phosphate (Figure 4B), might become localized into the active site by a structural rearrangement. The proximity of Rap-Gln63 and GAP1IP4BP-Pro489 in the model would suggest an implication of this residue in this rearrangement, what would explain its importance in the RapGAP activity. In Ras, the corresponding Glu63 contacts Arg903 (in the FLR motif) of p120GAP to stabilize Ras-Gln61 and switch-II. Its contribution to catalysis is however marginal as activity of GAP1IP4BP towards Ras(E63A) is half that of the wt (data not shown), and a threefold lower stimulation was previously observed for Ras(E63H) (Gideon et al, 1992). Although residue 63 is not close to the γ-phosphate in the Ras complex, it could potentially adopt the role of Gln61 by assuming a different binding mode of switch-II in case of Rap. We thus measured the GTPase activity of the Rap mutants Q63E and Q63A in the presence of RASAL and Gap1IP4BP by HPLC (Figure 5A) and FTIR (Table 1). The mutations strongly impair GTPase activation in both types of assays. In the more quantitative FTIR experiments, a single rate for cleavage is observed which is 15 000 and an estimated 40 000 times lower than that for wt for Q63E and Q63A, respectively. These results suggest that in the fl GAP1IP4BP, Rap-Gln63 is the main catalytic residue for Rap catalysis. As similar results are obtained for RASAL, this feature seems conserved along the dual-specificity GAPs of the Gap1 family. Interestingly, Gln63 has no catalytic role in the Rap-specific GAP (RapGAP)-catalysed reaction of Rap. As measured by FTIR, RapGAP is able to hydrolyse GTP with Rap(Q63A) as fast as with the wt (Table 1).
Switching the binding and catalysis mode of Ras towards Rap
The results presented so far suggest that Ras and Rap use different binding modes to position either Gln61 or Gln63 for catalysis. As switch-II in Ras/Rap is an important element of binding and catalysis, we compared the sequences in switch-II (Figure 4C). Having established the role of the position 61 and 63 substitutions, we turned our attention to Phe64 and Thr65 of Rap, which are replaced by Tyr and Ser in Ras. If Gln63 is the main catalytic residue of the GAP1IP4B-stimulated Rap GTPase activity, the neighbouring residues Phe64/Thr65 might have a role in achieving its correct position and orientation. The GTPase reaction with GAP1IP4BP was measured for the Rap mutants F65A, T65A and F64A/T65A (Figure 5A). The F64A mutation reduces GTPase activity by 600-fold (to 0.17%), whereas the T65A mutation is less drastic and reduces activity to 12%. The double mutation decreases the activity to 0.07%. Similar results were obtained with RASAL. The mutations decrease its GAP activity to 0.02, 2.1 and 0.015%, respectively.
Having established the importance of switch-II for Rap·GTP hydrolysis, we wondered whether we can induce inert Ras(Q61T) to hydrolyse GTP. The GTPase of Ras(Q61T) is not stimulated by GAP1IP4BP and not by the GAP domain of NF1 even at high (10 μM) concentrations (Figure 5B), which is not unexpected considering that any mutation of Gln61 is oncogenic and not responsive to RasGAPs. FTIR measurements show that the catalytic rate constant of the GAP reaction with Ras(Q61A) is 4–5 orders of magnitude lower than with Ras wt (Table 2). To convert Ras into Rap, we made the Ras Q61T/E63Q double and the Q61T/E63Q/Y64F/S65T quadruple switch-II mutants and measured their activity with GAP1IP4BP by HPLC and FTIR and compared that with the NF1-catalysed RasGAP activity measured by HPLC. GAP1IP4BP is not very active on the double mutant, but becomes a rather effective GAP for the quadruple mutant. Although it is still about 100-fold less active than with wt Rap, it adopts the Rap catalysis mode and stimulates Ras GTPase by three orders of magnitude (Table 2). This is corroborated by the finding that, as observed for Rap catalysis, the activity depends on GAP1IP4BP-Pro489 in helix-α6 (Figure 5B and Table 2) and the C2 and PH-Ct domains (Table 2), because GAP1IP4BP GAP domain and GAP1IP4BP(P489V) and are one and two orders of magnitude less active, respectively.
Table 2. FTIR with Ras switch-II mutants.
| G-protein | GAP1IP4BP | T (K) | k2 (s−1) | k3 (s−1) |
|---|---|---|---|---|
| Ras | Full length | 260 | 4.6 × 10−1 | 9.7 × 10−2 |
| Ras-Q61A | Full length | 285 | 8.4 × 10−5 (6.2 × 10−6) | |
| Ras-Q61A | GAP domain | 285 | 2.5 × 10−4 (1.9 × 10−5) | |
| Ras-Q61T/E63Q | Full length | 285 | 3.8 × 10−4 (2.8 × 10−5) | |
| Ras-Q61T/E63Q | GAP domain | 285 | 5.5 × 10−4 (4.1 × 10−5) | |
| Ras-Q61T/E63Q/Y64F/S65T | Full length | 260 | 4.0 × 10−3 | |
| Ras-Q61T/E63Q/Y64F/S65T | GAP domain | 260 | 2.9 × 10−4 | |
| Ras-Q61T/E63Q/Y64F/S65T | P489V | 260 | 3.0 × 10−5 | |
| For the sake of comparison, we have enclosed in parentheses all rates extrapolated to 260 K, using a factor of three for each 10-K step. | ||||
Discussion
Activities of multiple specificity in GAPs have been measured in vivo and in vitro, including RasGAPs (Bernards, 2003), RhoGAPs (Tcherkezian and Lamarche-Vane, 2007) and ArfGAPs (Kahn et al, 2008). However, in most of the cases, and unlike dual Ras/RapGAP specificity, these GAPs activate G-proteins from the same subfamily using the same catalytic strategy. For example, p120GAP, GAP1IP4BP and GAP1m are active with Ras and R-Ras (Li et al, 1997). Even when multiple specificities between GAPs and the G-proteins of different subfamilies have been reported, it does not mean a challenge for the catalytic mechanism. In this way, p120GAP and SynGAP are supposed to also stimulate Rab5 GTPase activity (Liu and Li, 1998; Oh et al, 2004), but here the Rab5 switch-II is highly homologous to that of Ras and includes a catalytic Gln. Furthermore, the dual-specificity switch described here is likely to be evolutionarily conserved, as Bud2p, a RasGAP-related protein in Saccharomyces cerevisiae, regulates Bud1p, the only Rap homologue (Park et al, 1993), in that organism. This would indicate that Rap-specific RapGAP with an extrinsic (not belonging to G-protein) asparagine appeared later in evolution, replacing and complementing the function of dual-specificity GAPs.
An interesting factor of GAP1-family dual specificity is the role of the N- and C-terminal domains. Biochemical studies of Ras-specific GAPs have mostly been conducted with isolated GAP domains on the basis of the assumption that the catalytic mechanism and probably the specificity is identical to that of the fl GAPs. Here we show that GAP1-family proteins have a GAP domain that functions as a conventional RasGAP with an arginine finger and supports the intrinsic Gln. The catalytic activity of the GAP domain on Ras is very similar to that of the fl protein, and the extra domains only modify the affinity. The chemistry and kinetics of the individual steps are similar to that of the NF1-catalysed reaction as observed by FTIR (Kötting et al, 2008). The most remarkable feature is the switch to a different binding and catalysis mode in the presence of Rap, where the extra domains are required for this switch. The additional domains are not just increasing the affinity but are in fact essential for catalysis. Although the ΔPH-Ct deletion mutants of GAP1IP4BP or RASAL cannot be purified, the PH-Ct region is implicated in catalysis independently, as the GAP-domain catalytic constant for RapGTP hydrolysis is 10-fold lower than the ΔC2AB-deletion mutant, supporting previous conclusions (Kupzig et al, 2009).
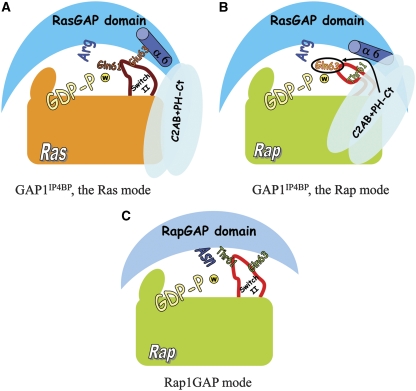
The requirement of an N-terminal C2 domain for the RapGAP activity has also been described for SynGAP. On the basis of a partial SynGAP structure (Pena et al, 2008), the N-terminal C2 domain would be in proximity to switch-II in a Rap-SynGAP model. As the N- and the C-termini of the RasGAP domain are very close to each other, one could speculate the C2 and PH domains of GAP1IP4BP to be close to each other also. For SynGAP, it has been suggested that the possible contact of the mobile C2 domain with Rap and/or the GAP domain would promote catalysis, for example by making accessible a catalytic residue of GAP or Rap. The results presented here lead us to propose a model for the specificity switch of dual-specificity RasGAPs (Figure 6A and B). They act as normal RasGAPs where the arginine finger interacts and positions a catalytic glutamine. For the Rap mode of catalysis (Figure 6B), the interaction of switch-II and helix-α6 of the GAP domain are modulated by the C- and N-terminal domains, to allow Rap-Gln63 to move into the Ras-Gln61 position and act as catalytic residue fixing the water for nucleophilic attack. The structural rearrangement in the active site is supported by the model of the Rap-GAP domain complex (Figure 4A and B), where Rap-Gln63 is close to the proline in helix-α6. It shows that Rap-Gln63, although not oriented towards the γ-phosphate, is not far away, and could be positioned closer to the GTP by conformational rearrangements in switch-II. Furthermore, the proximity of Rap-Gln63 to helix-α6 and the important proline would suggest its implication, together with C2AB and PH-Ct domains, in switch-II conformational change. This mode of catalysis is completely different from that of the RapGAP-mediated catalysis (Figure 6C), where neither Thr61 nor Gln63 are required. In support of differences in catalysis between Ras and Rap, we have shown that the G-protein- bound Pi-intermediate observed in the course of the reaction is different between the Ras and Rap mode, and is again different from Ras-specific catalysis of NF1 and of Rap-specific catalysis of RapGAP.
Figure 6.
A schematic model of the different catalysis modes of GAP1IP4BP (blue) with Ras (orange) or Rap (green). In the Ras mode (A) the C2AB and PH-Ct domains are not implicated in catalysis, and switch-II (dark red) is in the conventional RasGAP mode, with Gln61 pointing towards the nucleophilic water molecule (w, yellow sphere) and the γ-phosphate. In the Rap mode (B), the extra domains of GAP and helix-α6 with Pro489 induce a conformational change of the switch-II of Rap (red), allowing Gln63 to act as the catalytic residue. (C) In RapGAP-mediated catalysis on Rap, the Asn thumb inserts into the active site to adopt the role of the intrinsic Gln.
This model is confirmed by the Q63A mutation, which has a rate very similar to that of the unstimulated reaction, just like Q61A in Ras completely eliminates catalysis (Table 2; Der et al, 1986). As the Rap(T61Q) mutant can be partially activated by the GAP domain, we would assume that in this case hydrolysis can take place in the Ras mode, while with fl GAP the presence of Gln63 is required. The mechanism we propose also explains the inability of Gap1IP4BP to stimulate the GTPase reaction of Ras(Q61T), which misses a catalytic Gln. In fact, for Ras to adopt the catalysis mode of Rap, residues divergent between Ras and Rap have to be introduced to achieve efficient Rap mode catalysis, in addition to the ones in switch-II mutated here.
Materials and methods
Protein purifications
Truncated Ras and Rap1B (residues 1–167) were prepared as described previously (Tucker et al, 1986). The GAP1IP4BP cDNA was synthesized for optimized expression in prokaryotes (GeneArt, Regensburg, Germany). This and ΔC2AB constructs (residues 291–834) (Figure 1) were cloned into a pProEx plasmid (Invitrogen). N-terminally His6-tagged proteins were expressed in Escherichia coli strain BL21DE3 (RIL). Cells were lysed in a microfluidizer using a lysis buffer (50 mM Tris (pH 8.5), 600 mM NaCl, 3 mM β-mercaptoethanol, 20% glycerol); the soluble extract was applied to a Ni-NTA column equilibrated in buffer-A (25 mM Tris (pH 8.5), 300 mM NaCl, 3 mM β-mercaptoethanol, 10% glycerol, 10 mM imidazol), washed with buffer-A and with buffer-A containing 100 mM KCl, 10 mM MgCl2 and 1 mM ATP. Proteins were eluted on a gradient of 0–300 mM imidazol in buffer-A, and further purified by gel filtration using Superdex S200 26/60 in buffer-C (25 mM Tris (pH 8.5), 250 mM NaCl, 5 mM dithioerythritol (DTE)). The Gap1IP4BP-GAP domain (291–576) was expressed and purified as described by Kupzig et al (2006).
RASAL fl, ΔC2A (111–804) and ΔC2AB (270–804) were cloned into a pGex-T4-TEV plasmid using Gateway technology (Invitrogen, Carlsbad, CA, USA). GST-fusion proteins were expressed in BL21DE3 (RIL), lysed by a microfluidizer in buffer (50 mM Tris (pH 8.0), 600 mM NaCl, 5 mM DTE, 10 mM EDTA, 20% glycerol), applied to and purified from GSH–Sepharose in buffer-A (25 mM Tris (pH 8.0), 200 mM NaCl, 5 mM DTE, 10% glycerol), and eluted in buffer-A with 30 mM glutathione. GST was cleaved overnight using TEV protease and purified using Superdex S200 in buffer-C. Mutants were generated using the QuickChange protocol (Stratagene, La Jolla, CA, USA).
Protein methods
Nucleotide exchange was performed according to the procedure described by Tucker et al (1986). Reverse-phase HPLC was used to monitor the kinetics of GTP hydrolysis by GAP1IP4BP and RASAL as described by Scrima et al (Scrima and Wittinghofer, 2006). H-Ras-GTP or Rap1B-GTP (200 μM) in 25 mM Tris (pH 7.5), 100 mM NaCl, 5 mM MgCl2 and 5 mM DTE (standard buffer) was incubated at 25°C with different concentrations of GAP. Aliquots were removed and analysed by HPLC.
For charcoal assay, Ras and Rap1B preloaded with GTP were partially exchanged with [γ-32P]GTP by incubating 1.5 mM H-Ras-GTP or Rap1B-GTP with 20 μCi [γ-32P]GTP in the presence of 12 mM EDTA for 30 min on ice. The reaction was stopped by 30 mM MgCl2. [γ-32P]GTP-loaded Ras or Rap1 were incubated at 25°C with GAPs, aliquots were added to a charcoal solution and the amount of free inorganic phosphate were determined by scintillation counting as described by Brinkmann et al (2002).
FTIR measurements
The P3-para-hydroxyphenacyl ester of GTP (caged-GTP) was synthesized according to a procedure described by Park and Givens (1997), and exchanges into G-proteins as described (John et al, 1990). The IR sample was prepared between two CaF2 windows as detailed by Cepus et al (1998). The composition was 3 mM of a 1:1 complex of GTPase with caged-GTP, 3.3 mM GAP, 20 mM MgCl2, 20 mM DTT and 100 mM HEPES (pH 7.5–8.0). The experiments performed below 273 K contained 12% ethylene glycol to prevent freezing. Isotopically labelled GTP was prepared as described in the literature (Allin et al, 2001). Note that the band assignment (Figure 3) was performed by using the P3-1-(2-nitrophenyl)ethyl ester (NPE) of GTP (Walker et al, 1988) because the synthesis of γ-18O3-pHP-GTP is too costly.
Photolysis of caged-GTP was performed using an LPX 240 XeCl-excimer laser (308 nm; Lambda Physics, Göttingen, Germany) by applying 12 flashes within 24 ms (or by 40 flashes within 80 ms when using the NPE cage). A modified Bruker IFS 66v/s spectrometer in the fast-scan mode was used for the measurement (Gerwert et al, 1990). The data were analysed between 1800 and 950 cm−1 with a global fit method (Hessling et al, 1993). In this analysis, the absorbance changes ΔA were analysed with sums of nr exponentials using apparent rate constants kl and amplitudes al:
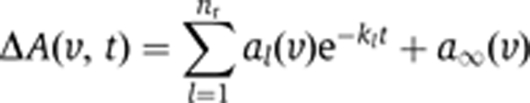 |
To get reliable results, two types of measurements were taken: (1) Temperature was varied between 260 and 285 K. Otherwise some measurements would be either too fast to get a reasonable S/N ratio, or too slow, leading to huge baseline drifts. (2) For the slower measurements, progress of GTPase was monitored as the difference of the two ΔA of the asymmetric GTP- and GDP-stretching vibration of α-PO2− at 1263 and 1236 cm−1. The advantage of taking two reference points close to each other instead of monitoring the trace of a single wavenumber is an annihilation of baseline drifts (Kötting and Gerwert, 2004).
Supplementary Material
Acknowledgments
We thank Dorothee Vogt and Carolin Koerner for technical assistance. BS is supported by an Alexander von Humboldt foundation research fellowship. This work was supported by the DFG within the SFB 642.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ahmadian MR, Kiel C, Stege P, Scheffzek K (2003) Structural fingerprints of the Ras-GTPase activating proteins neurofibromin and p120GAP. J Mol Biol 329: 699–710 [DOI] [PubMed] [Google Scholar]
- Allin C, Ahmadian MR, Wittinghofer A, Gerwert K (2001) Monitoring the GAP catalyzed H-Ras GTPase reaction at atomic resolution in real time. Proc Natl Acad Sci USA 98: 7754–7759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards A (2003) GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim Biophys Acta 1603: 47–82 [DOI] [PubMed] [Google Scholar]
- Bollag G, McCormick F (1991) Differential regulation of rasGAP and neurofibromatosis gene product activities. Nature 351: 576–579 [DOI] [PubMed] [Google Scholar]
- Brinkmann T, Daumke O, Herbrand U, Kuhlmann D, Stege P, Ahmadian MR, Wittinghofer A (2002) Rap-specific GTPase activating protein follows an alternative mechanism. J Biol Chem 277: 12525–12531 [DOI] [PubMed] [Google Scholar]
- Cepus V, Scheidig AJ, Goody RS, Gerwert K (1998) Time-resolved FTIR studies of the GTPase reaction of H-ras p21 reveal a key role for the beta-phosphate. Biochemistry 37: 10263–10271 [DOI] [PubMed] [Google Scholar]
- Chakrabarti PP, Daumke O, Suveyzdis Y, Kötting C, Gerwert K, Wittinghofer A (2007) Insight into catalysis of a unique GTPase reaction by a combined biochemical and FTIR approach. J Mol Biol 367: 983–995 [DOI] [PubMed] [Google Scholar]
- Chakrabarti PP, Suveyzdis Y, Wittinghofer A, Gerwert K (2004) Fourier transform infrared spectroscopy on the Rap.RapGAP reaction, GTPase activation without an arginine finger. J Biol Chem 279: 46226–46233 [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Dugan LL, Gutmann DH (2003) The neurofibromatosis 1 gene product neurofibromin regulates pituitary adenylate cyclase-activating polypeptide-mediated signaling in astrocytes. J Neurosci 23: 8949–8954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumke O, Weyand M, Chakrabarti PP, Vetter IR, Wittinghofer A (2004) The GTPase-activating protein Rap1GAP uses a catalytic asparagine. Nature 429: 197–201 [DOI] [PubMed] [Google Scholar]
- Der CJ, Finkel T, Cooper GM (1986) Biological and biochemical properties of human rasH genes mutated at codon 61. Cell 44: 167–176 [DOI] [PubMed] [Google Scholar]
- Downward J (2003) Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer 3: 11–22 [DOI] [PubMed] [Google Scholar]
- Gerwert K, Souvignier G, Hess B (1990) Simultaneous monitoring of light-induced changes in protein side-group protonation, chromophore isomerization, and backbone motion of bacteriorhodopsin by time-resolved Fourier-transform infrared spectroscopy. Proc Natl Acad Sci USA 87: 9774–9778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gideon P, John J, Frech M, Lautwein A, Clark R, Scheffler JE, Wittinghofer A (1992) Mutational and kinetic analyses of the GTPase-activating protein (GAP)-p21 interaction: the C-terminal domain of GAP is not sufficient for full activity. Mol Cell Biol 12: 2050–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessling B, Souvignier G, Gerwert K (1993) A model-independent approach to assigning bacteriorhodopsin's intramolecular reactions to photocycle intermediates. Biophys J 65: 1929–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida D, Kometani K, Yang H, Kakugawa K, Masuda K, Iwai K, Suzuki M, Itohara S, Nakahata T, Hiai H, Kawamoto H, Hattori M, Minato N (2003) Myeloproliferative stem cell disorders by deregulated Rap1 activation in SPA-1-deficient mice. Cancer Cell 4: 55–65 [DOI] [PubMed] [Google Scholar]
- Jin H, Wang X, Ying J, Wong AH, Cui Y, Srivastava G, Shen ZY, Li EM, Zhang Q, Jin J, Kupzig S, Chan AT, Cullen PJ, Tao Q (2007) Epigenetic silencing of a Ca(2+)-regulated Ras GTPase-activating protein RASAL defines a new mechanism of Ras activation in human cancers. Proc Natl Acad Sci USA 104: 12353–12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John J, Sohmen R, Feuerstein J, Linke R, Wittinghofer A, Goody RS (1990) Kinetics of interaction of nucleotides with nucleotide-free H-ras p21. Biochemistry 29: 6058–6065 [DOI] [PubMed] [Google Scholar]
- Kahn RA, Bruford E, Inoue H, Logsdon JM Jr, Nie Z, Premont RT, Randazzo PA, Satake M, Theibert AB, Zapp ML, Cassel D (2008) Consensus nomenclature for the human ArfGAP domain-containing proteins. J Cell Biol 182: 1039–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee HK, Takamiya K, Huganir RL (2003) The role of synaptic GTPase-activating protein in neuronal development and synaptic plasticity. J Neurosci 23: 1119–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klähn M, Mathias G, Kötting C, Nonella M, Schlitter J, Gerwert K, Tavan P (2004) IR spectra of phosphate ions in aqueous solution: predictions of a DFT/MM approach compared with observations. J Phys Chem A 108: 6186–6194 [Google Scholar]
- Klähn M, Schlitter J, Gerwert K (2005) Theoretical IR spectroscopy based on QM/MM calculations provides changes in charge distribution, bond lengths, and bond angles of the GTP ligand induced by the Ras-protein. Biophys J 88: 3829–3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kötting C, Blessenohl M, Suveyzdis Y, Goody RS, Wittinghofer A, Gerwert K (2006) A phosphoryl transfer intermediate in the GTPase reaction of Ras in complex with its GTPase-activating protein. Proc Natl Acad Sci USA 103: 13911–13916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kötting C, Gerwert K (2004) Time-resolved FTIR studies provide activation free energy, activation enthalpy and activation entropy for GTPase reactions. Chem Phys 307: 227–232 [Google Scholar]
- Kötting C, Kallenbach A, Suveyzdis Y, Eichholz C, Gerwert K (2007) Surface change of Ras enabling effector binding monitored in real time at atomic resolution. ChemBioChem 8: 781–787 [DOI] [PubMed] [Google Scholar]
- Kötting C, Kallenbach A, Suveyzdis Y, Wittinghofer A, Gerwert K (2008) The GAP arginine finger movement into the catalytic site of Ras increases the activation entropy. Proc Natl Acad Sci USA 105: 6260–6265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupzig S, Bouyoucef-Cherchalli D, Yarwood S, Sessions R, Cullen PJ (2009) The ability of Gap1ip4bp to function as a Rap1 Gap requires its Ras Gap-related domain and an arginine finger rather than an asparagine thumb. Mol Cell Biol 29: 3929–3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupzig S, Deaconescu D, Bouyoucef D, Walker SA, Liu Q, Polte CL, Daumke O, Ishizaki T, Lockyer PJ, Wittinghofer A, Cullen PJ (2006) GAP1 family members constitute bifunctional Ras and Rap GTPase-activating proteins. J Biol Chem 281: 9891–9900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Nakamura S, Hattori S (1997) Activation of R-Ras GTPase by GTPase-activating proteins for Ras, Gap1(m), and p120GAP. J Biol Chem 272: 19328–19332 [DOI] [PubMed] [Google Scholar]
- Liu K, Li G (1998) Catalytic domain of the p120 Ras GAP binds to RAb5 and stimulates its GTPase activity. J Biol Chem 273: 10087–10090 [DOI] [PubMed] [Google Scholar]
- Oh JS, Manzerra P, Kennedy MB (2004) Regulation of the neuron-specific Ras GTPase-activating protein, synGAP, by Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 279: 17980–17988 [DOI] [PubMed] [Google Scholar]
- Ohta M, Seto M, Ijichi H, Miyabayashi K, Kudo Y, Mohri D, Asaoka Y, Tada M, Tanaka Y, Ikenoue T, Kanai F, Kawabe T, Omata M (2009) Decreased expression of the RAS-GTPase activating protein RASAL1 is associated with colorectal tumor progression. Gastroenterology 136: 206–216 [DOI] [PubMed] [Google Scholar]
- Park C-H, Givens RS (1997) New photoactivated protecting groups. 6. p-Hydroxyphenacyl: a phototrigger for chemical and biochemical probes. J Am Chem Soc 119: 2453–2463 [Google Scholar]
- Park HO, Chant J, Herskowitz I (1993) BUD2 encodes a GTPase-activating protein for Bud1/Rsr1 necessary for proper bud-site selection in yeast. Nature 365: 269–274 [DOI] [PubMed] [Google Scholar]
- Pena V, Hothorn M, Eberth A, Kaschau N, Parret A, Gremer L, Bonneau F, Ahmadian MR, Scheffzek K (2008) The C2 domain of SynGAP is essential for stimulation of the Rap GTPase reaction. EMBO Rep 9: 350–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffzek K, Ahmadian MR, Kabsch W, Wiesmuller L, Lautwein A, Schmitz F, Wittinghofer A (1997) The Ras–RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science 277: 333–338 [DOI] [PubMed] [Google Scholar]
- Scrima A, Thomas C, Deaconescu D, Wittinghofer A (2008) The Rap–RapGAP complex: GTP hydrolysis without catalytic glutamine and arginine residues. EMBO J 27: 1145–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrima A, Wittinghofer A (2006) Dimerisation-dependent GTPase reaction of MnmE: how potassium acts as GTPase-activating element. EMBO J 25: 2940–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkezian J, Lamarche-Vane N (2007) Current knowledge of the large RhoGAP family of proteins. Biol Cell 99: 67–86 [DOI] [PubMed] [Google Scholar]
- Tucker J, Sczakiel G, Feuerstein J, John J, Goody RS, Wittinghofer A (1986) Expression of p21 proteins in Escherichia coli and stereochemistry of the nucleotide-binding site. EMBO J 5: 1351–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J, Reid GP, McCray JA, Trentham DR (1988) Photolabile 1-(2-nitrophenyl)ethyl phosphate esters of adenine nucleotide analogs. Synthesis and mechanism of photolysis. J Am Chem Soc 110: 7170–7177 [Google Scholar]
- Walker SA, Kupzig S, Bouyoucef D, Davies LC, Tsuboi T, Bivona TG, Cozier GE, Lockyer PJ, Buckler A, Rutter GA, Allen MJ, Philips MR, Cullen PJ (2004) Identification of a Ras GTPase-activating protein regulated by receptor-mediated Ca2+ oscillations. EMBO J 23: 1749–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.