Abstract
Wnt5a is a representative ligand that activates the Wnt/β-catenin-independent pathway, resulting in the regulation of cell adhesion, migration, and polarity, but its molecular mechanism is poorly understood. This report shows that Dishevelled (Dvl) binds to adenomatous polyposis coli (APC) gene product, and this binding is enhanced by Wnt5a. Dvl was involved in the stabilization of the plus end dynamics of microtubules as well as APC. Frizzled2 (Fz2) was present with Wnt5a at the leading edge of migrating cells and formed a complex with APC through Dvl. Fz2 also interacted with integrins at the leading edge, and Dvl and APC associated with and activated focal adhesion kinase and paxillin. The binding of APC to Dvl enhanced the localization of paxillin to the leading edge and was involved in Wnt5a-dependent focal adhesion turnover. Furthermore, this new Wnt5a signalling pathway was important for the epithelial morphogenesis in the three-dimensional culture. These results suggest that the functional and physical interaction of Dvl and APC is involved in Wnt5a/Fz2-dependent focal adhesion dynamics during cell migration and epithelial morphogenesis.
Keywords: APC, dishevelled, focal adhesion, migration, Wnt5a
Introduction
Wnts are secretory proteins that are essential for animal development (Logan and Nusse, 2004). A subset of Wnts activates the β-catenin-independent pathways (also referred to as non-canonical Wnt pathways) that primarily modulate cell movement, as initially observed during embryogenesis (Veeman et al, 2003; Kikuchi and Yamamoto, 2008). This activation occurs through multiple mechanisms, which overlap with other signalling pathways, including Rac, Rho, Jun N-terminal kinase (JNK), Rho-associated kinase, and protein kinase C (PKC). Wnt5a, which is a representative ligand that activates the β-catenin-independent pathway, is known to regulate cell adhesion, migration, and polarity (Weeraratna et al, 2002; Veeman et al, 2003; Kikuchi and Yamamoto, 2008). Although its molecular mechanism is not well understood, possible mechanisms have been demonstrated. For instance, Wnt5a activates JNK in convergent extension of Xenopus embryos (Yamanaka et al, 2002) or recruits actin, myosin IIB, and melanoma cell adhesion molecule into a polarized structure in migrating melanoma cells (Witze et al, 2008). It has also been shown that Wnt5a activates Rac and focal adhesion kinase (FAK) and that it is required for the turnover of focal adhesions in migrating cells (Kurayoshi et al, 2006, 2007).
Dishevelled (Dvl) is a critical component of Wnt signalling and mediates both the β-catenin-dependent and -independent pathways (Wharton, 2003). It has been shown that Dvl is observed as punctate cytoplasmic structures (Kishida et al, 1999; Capelluto et al, 2002), which correlates to the protein assembling, and translocated to the cell-surface membrane in a Wnt-dependent manner (Schwarz-Romond et al, 2007). It has also been reported that Dvl localizes to focal adhesions in mammalian cultured cells (Torres and Nelson, 2000) and that its translocation to the cell-surface membrane is dependent on fibronectin in Xenopus embryos (Marsden and DeSimone, 2001). In addition, Dvl is known to stabilize microtubules by binding directly to them (Krylova et al, 2000; Ciani et al, 2004). These results suggest that Dvl localizes to specific sites where it regulates the cytoskeleton and cell-substrate adhesion, but the involvement of Dvl in Wnt5a-dependent cell-substrate adhesion and cell migration has not yet been clarified.
The adhesion of a cell to a substrate (cell-substrate adhesion) is necessary for the cell to spread and migrate (Small and Kaverina, 2003). Small focal adhesions, referred to as focal complexes, at the cell periphery of spreading and migrating cells are regulated by small GTP-binding proteins (G proteins) Rac and Cdc42 (Etienne-Manneville and Hall, 2002). On the generation of tension, these small adhesions can mature into large, more organized adhesions, such as focal adhesions, which are found both at the cell periphery and more centrally. Spreading and migrating cells continuously form and disassemble their adhesions at the leading edge by the activation of small G proteins and FAK (Mitra et al, 2005). Recent imaging studies have implicated microtubule targeting to focal adhesions in focal adhesion disassembly, although the molecular mechanism is not fully understood (Small and Kaverina, 2003).
Adenomatous polyposis coli (APC) protein was originally identified as a tumour suppressor of human colon cancer and subsequently found to be involved in the stability of β-catenin in Wnt signalling (Polakis, 2000; Aoki and Taketo, 2007). Evidence has shown that APC regulates cell–cell adhesion, cell polarization, and migration through the stabilization of microtubules by binding to their plus ends (Mimori-Kiyosue et al, 2000; Dikovskaya et al, 2001). APC is transported along microtubules by kinesin motor protein complexes containing KAP3 and accumulates at microtubule plus ends specifically at the protrusion of the migrating cells (Akiyama and Kawasaki, 2006; Kroboth et al, 2007). As Wnt5a and Dvl are involved in the accumulation of APC to the plus end of microtubules (Schlessinger et al, 2007), it has been suggested that Wnt5a, Dvl, and APC interact functionally to regulate cell-substrate adhesions and migration. However, how Wnt5a signalling links to the regulation of focal adhesions remains to be clarified.
Here, it is reported that Dvl bound to APC directly and that the complex played a role in the Wnt5a-dependent formation of cell-substrate adhesions and polarized cell migration. It was also shown that Frizzled2 (Fz2), a Wnt5a receptor, is concentrated to the leading edge of migrating cells where it forms a complex with APC through Dvl. Furthermore, Fz2 interacted with integrins at the leading edge, and Dvl and APC associated and colocalized with FAK and paxillin. These results show that Dvl and APC mediate a Wnt5a-Fz2 signal to focal adhesions to regulate cell-substrate adhesions and migration.
Results
Roles of Wnt5a, Dvl, and APC in cell-substrate adhesion and migration
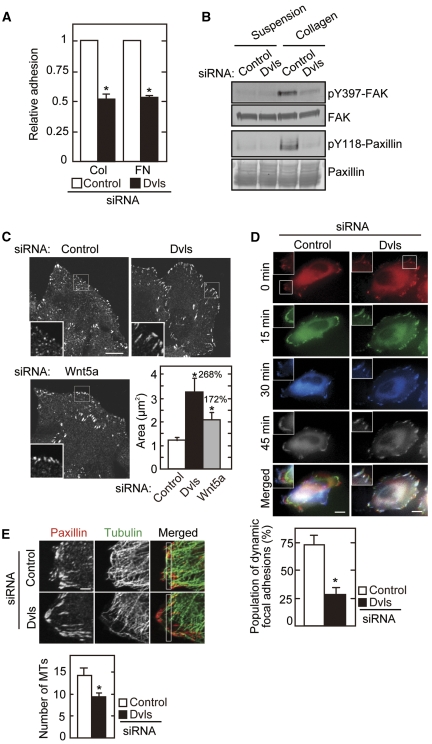
To investigate whether Dvl mediates the ability of Wnt5a to regulate cell-substrate adhesion and migration, the phenotypes by Dvl knockdown cell lines were analysed. The cells in which all Dvls (Dvl1, Dvl2, and Dvl3) were knocked down were used (Supplementary Figure S1A). Adhesion of HeLaS3 (human cervical cancer) cells to collagen or fibronectin was suppressed by the knockdown of Dvls (Figure 1A). In Dvl knockdown cells, the levels of α2, α3, α5, αv, β1, and β3 integrins were unchanged (Supplementary Figure S1B), suggesting that Dvl is necessary for cell-substrate signalling after integrin engagement with extracellular matrix proteins. When cells were seeded into a collagen-coated dish from a suspension culture, FAK was activated as assessed by the phosphorylation of Tyr397 (pY397), and paxillin was phosphorylated at Tyr118 (pY118) (Figure 1B). The cell adhesion-dependent activation of FAK and phosphorylation of paxillin were dramatically reduced in Dvl knockdown cells (Figure 1B). Immunocytochemical analyses also showed that depletion of Dvl inhibits the activation (FAK pY397 staining) and formation (FAK and cortical actin staining) of cell-substrate adhesion at the cell periphery in the initiation of cell spreading (Supplementary Figure S2A). Knockdown of Dvl in Vero (monkey kidney epithelium) cells also suppressed cell-substrate adhesion and FAK activation (Supplementary Figure S3), suggesting that these functions of Dvl are conserved in at least HeLaS3 and Vero cells.
Figure 1.
Dvl is required for cell-substrate adhesion. (A) HeLaS3 cells transfected with Dvl siRNA were plated on collagen (Col) or fibronectin (FN) for 15 min and subjected to the adhesion assay. The results are expressed as the ratio of adhesion activity of cells treated with control siRNA and indicate means±s.e. from three independent experiments. (B) After HeLaS3 cells transfected with Dvl siRNA were suspended in serum-free medium, they were kept in suspension or plated onto collagen-coated dishes for 1 h. Lysates were probed with anti-pY397-FAK and anti-pY118-paxillin antibodies. FAK and paxillin were used as loading controls. (C) HeLaS3 cells transfected with Dvl or Wnt5a siRNA were stained with anti-paxillin antibody. The individual areas of paxillin staining were quantified in five different focal adhesions per cell (counted cells: 20 per siRNA), and the results were shown in the lower right panel. The region in the white box is shown enlarged. (D) The dynamics of GFP-paxillin in HeLaS3 cells transfected with Dvl siRNA were visualized. The percentages of adhesions turning over within 45 min were calculated for 10 different focal adhesions per cell (counted cells: 20 per siRNA), and the results were shown in the bottom panel. The region in the white box is shown enlarged. (E) HeLaS3 cells transfected with Dvl siRNA were stained with anti-paxillin and anti-tubulin antibodies. The numbers of microtubules in a 1 μm × 10 μm area (white box) from the cell edge were counted (counted cells: 30 per siRNA). Scale bars in (C) and (D), 10 μm; in (E), 2 μm. *P<0.01.
Knockdown of Dvl induced enlargement of the sizes of focal adhesions, which indicated the inhibition of the focal adhesion turnover, and expression of FLAG-Dvl2 rescued this phenotype (Figure 1C; Supplementary Figures S1C and S2B). When the processes of focal adhesion formation were visualized in a time-lapse imaging study, dynamic remodelling of focal adhesions was less dynamic in Dvl knockdown cells (Figure 1D). These results suggested that Dvl regulates the dynamics of focal adhesions.
It has been shown that microtubule growth promotes the disassembly of focal adhesions (Small and Kaverina, 2003). Although microtubules extended towards the cell edges and got over focal adhesions in control cells, knockdown of Dvl created a microtubule-free border behind the adhesion sites (Figure 1E). The numbers of microtubule filaments were also decreased more at the periphery of Dvl knockdown cells when cells were treated with a low dose of nocodazole, which affects the dynamics of the plus ends of microtubules (Supplementary Figure S2C). In addition, cell adhesion-dependent extension of microtubules to the cell periphery was reduced in Dvl knockdown cells (Supplementary Figure S2D). Consistent with these observations, knockdown of Dvl in HeLaS3 and NIH3T3 cells reduced cell migration in transwell migration and wound-healing assays (Supplementary Figure S4). Taken together, these results showed that Dvl is involved in the regulation of the dynamics of cell-substrate adhesion and migration probably through the stabilization of microtubules at the cell periphery.
It has already been shown that overexpression of Wnt5a enhances cell-substrate adhesion and adhesion-dependent FAK activation (Kurayoshi et al, 2006, 2007). Consistent with the results, knockdown of Wnt5a decreased these cellular responses (Supplementary Figure S5A–C). In addition, knockdown of Wnt5a induced enlargement of the sizes of focal adhesions and enhanced nocodazole sensitivity of microtubules predominantly at the cell periphery as well as knockdown of Dvl (Figure 1C; Supplementary Figure S5D).
Although it is known that APC regulates cell–cell adhesion, cell migration, and microtubule dynamics, the involvement of APC in cell-substrate adhesion is not clear. Knockdown of APC decreased cell-substrate adhesion activity and suppressed adhesion-dependent FAK activation and paxillin phosphorylation as well as knockdown of Dvl (Supplementary Figure S6A–C). Furthermore, extension of microtubules to the cell periphery in initial cell spreading was reduced in APC knockdown cells (Supplementary Figure S6D). Thus, the knockdown of Wnt5a, Dvl, and APC showed similar phenotypes, suggesting that there are functional relationships between Wnt5a, Dvl, and APC in cell-substrate adhesion and cell migration.
Interaction of Dvl with APC
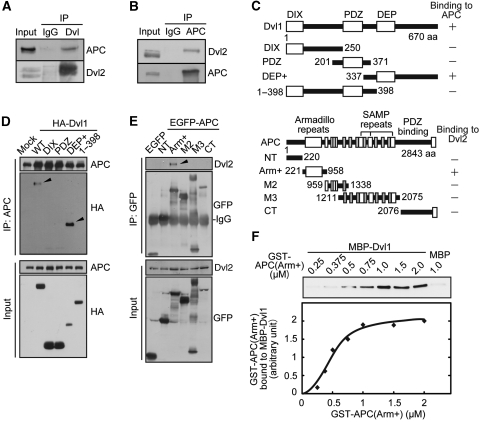
To address this possibility, the physiological interaction between Dvl and APC was examined. Dvl2 and APC formed a complex at endogenous levels (Figure 2A and B). Deletion mutant analyses showed that the C-terminal region of Dvl1 (Dvl1(DEP+)) interacts with APC in COS7 cells and that the armadillo domain of APC (APC(Arm+)) formed a complex with Dvl2 (Figure 2C–E). In vitro binding studies using recombinant proteins revealed that GST-APC(Arm+) binds to MBP-Dvl1 directly in a dose-dependent manner (Figure 2F). Furthermore, MBP-APC(Arm+) bound to GST-Dvl1(395–670) directly (Supplementary Figure S7).
Figure 2.
Dvl interacts with APC. (A) Lysates of HeLaS3 cells were immunoprecipitated with anti-Dvl (DIX) antibody and the immunoprecipitates were probed with the indicated antibodies. (B) Lysates of MDCK cells were immunoprecipitated with anti-APC antibody and the immunoprecipitates were probed with the indicated antibodies. (C) Regions of Dvl and APC required for the interaction are shown. Amino acid numbers are indicated. (D) Lysates of COS7 cells expressing HA-Dvl1 deletion mutants were immunoprecipitated with anti-APC antibody. The immunoprecipitates were probed with anti-HA and anti-APC antibodies. Arrowheads indicate HA-Dvl1(WT) and HA-Dvl1(DEP+) coimmunoprecipitated with endogenous APC. (E) Lysates of COS7 cells expressing EGFP-APC deletion mutants were immunoprecipitated with anti-GFP antibody. The immunoprecipitates were probed with anti-Dvl2 and anti-GFP antibodies. An arrowhead indicates endogenous Dvl2 coimmunoprecipitated with EGFP-APC(Arm+). (F) The indicated concentrations of GST-APC(Arm+) were incubated with MBP-Dvl1. Band intensities of GST-APC(Arm+) precipitated with MBP-Dvl1 were quantified in the bottom panel.
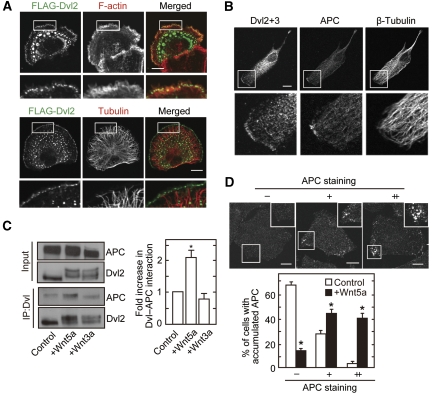
FLAG-Dvl2 was localized to the leading edge where F-actin was accumulated and microtubules were extended in polarized Vero cells (Figure 3A). Endogenous Dvl2, Dvl3, and APC were detected at the leading edge to where the ends of microtubules were extended, and localization of APC was more restrictive than that of Dvl (Figure 3B). Knockdown of Dvl or APC reduced their staining at the leading edge (Supplementary Figure S8A and B), indicating that these findings are not simply because of non-specific signals by antibodies. It was also observed that ectopically expressed FLAG-Dvl2 is colocalized with APC-GFP to the cell edge in liver progenitor HPPL cells (Supplementary Figure S8C). Consistent with the previous observations using embryonic fibroblasts (Schlessinger et al, 2007), knockdown of Dvl in Vero and HPPL cells decreased the localization of APC to the leading edge (Supplementary Figure S9A and B), whereas knockdown of APC did not affect the localization of Dvl (Supplementary Figure S9C). Localization of Dvl and APC at the leading edge was decreased significantly by a high dose of nocodazole to disrupt microtubules (Supplementary Figure S9D). These results suggested that Dvl is localized to the leading edge independently of APC, whereas Dvl is required for the localization of APC to the leading edge of polarized cells. In addition, their membrane localization would depend on microtubules.
Figure 3.
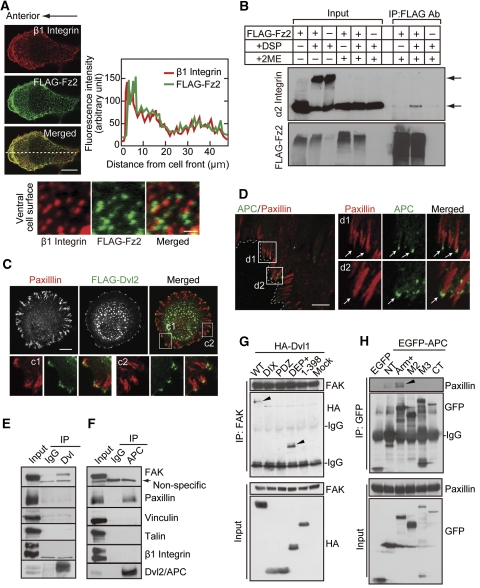
APC localizes to the cell periphery with Dvl in response to Wnt5a. (A) Vero cells expressing FLAG-Dvl2 were plated onto collagen-coated dishes for 1 h, followed by the staining with anti-FLAG antibody and phalloidin or anti-tubulin antibody. Cortical localization of FLAG-Dvl2 was observed in 42% of 30 cells expressing FLAG-Dvl2. (B) Vero cells were fixed with methanol and stained with anti-β-tubulin, anti-Dvl2+3 (a mixture of anti-Dvl2 and anti-Dvl3 antibodies), and anti-APC antibodies. Polarized Dvl at the cell cortex was observed in 42% of 96 cells and colocalization of Dvl with APC was observed in 27% of 41 cells with cortical Dvl staining. (C) After NIH3T3 cells were treated with Wnt5a or Wnt3a for 30 min, lysates were immunoprecipitated with anti-Dvl (DIX) antibody. The immunoprecipitates were probed with anti-APC and anti-Dvl2 antibodies, and band intensities of precipitated APC were quantified in the right-hand panel. (D) After HeLaS3 cells were serum-starved for 36 h and stimulated with or without 400 ng/ml purified Wnt5a for 60 min, the cells were stained with anti-APC antibody. Cells with a high intensity of APC at the cell periphery that was 1.5–2 times stronger than at the cell centre were defined as (+), and 2 times or more stronger were defined as (++). Cells with accumulated APC (−, +, ++) at the cell periphery was counted in at least 50 cells per each treatment. Scale bars in (A), (B), and (D), 10 μm. *P<0.01.
To clarify the role of interaction of Dvl and APC at the cell periphery, the effect of Wnts on the complex formation was examined. Wnt5a enhanced the formation of a complex between Dvl and APC in intact cells but Wnt3a did not (Figure 3C). Consistent with these results, Wnt5a induced the accumulation of APC to the cell periphery (Figure 3D). Furthermore, Wnt5a-dependent localization of APC to the cell periphery was reduced in Dvl knockdown cells (Supplementary Figure S10). Taken together, these results suggest that the binding of Dvl and APC is involved in Wnt5a-dependent recruitment of APC to the cell periphery.
Wnt5a and Fz2 are colocalized with Dvl and APC to the leading edge
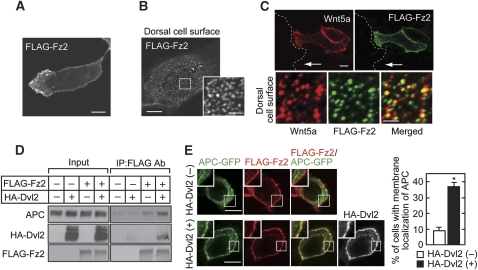
FLAG-Fz2, a Wnt5a receptor, was concentrated to the leading edge (Figure 4A) and was present as punctate structures at the dorsal cell surface (Figure 4B) in polarized Vero cells. The findings were also observed in the front cells of directionally migrating cells (Supplementary Figure S11A). When Wnt5a was expressed alone, it could not be detected at the cell surface (data not shown). When Wnt5a and FLAG-Fz2 were expressed simultaneously, both proteins were colocalized to the leading edge and also to the boundary of cell-to-cell adhesions (Figure 4C). To show the colocalization of Wnt5a and Fz2 more clearly, their expression at the dorsal cell surface was examined. FLAG-Fz2 was indeed colocalized with Wnt5a as punctate structures (Figure 4C). FLAG-Fz2 expressed on the cell surface formed a weakly visible complex with endogenous APC, and expression of HA-Dvl2 enhanced the complex formation in HEK293T cells (Figure 4D). When FLAG-Fz2, HA-Dvl2, or APC-GFP was expressed alone, they revealed different subcellular localizations (Supplementary Figure S11B–D). It has been shown that Dvl binds to the Wnt receptor Fz (Schulte and Bryja, 2007). Consistent with these observations, when FLAG-Fz2 and HA-Dvl2 were coexpressed, HA-Dvl2 was localized to the cell-surface membrane with FLAG-Fz2 (Supplementary Figure S11E). Coexpression of HA-Dvl2 and APC-GFP induced their colocalization as cytoplasmic punctate structures (Supplementary Figure S11F). APC-GFP was distributed in the cytoplasm along microtubules without the expression of HA-Dvl2 even though FLAG-Fz2 was expressed (Figure 4E, top panels). However, APC-GFP was colocalized with FLAG-Fz2 at the cell-surface membrane when HA-Dvl2 was expressed (Figure 4E, bottom panels). Taken together, these results suggest that Dvl and APC are localized to the leading edge of polarized cells with the Wnt5a and Fz2 complex.
Figure 4.
Fz2 forms a complex with APC and Dvl. (A, B) Vero cells expressing FLAG-Fz2 were fixed and incubated with polyclonal anti-FLAG antibody for 1 h without permeabilization. After the removal of free antibody, cells were probed with Alexa 546-conjugated anti-rabbit Ig antibodies. Polarized localization of FLAG-Fz2 was observed in 32% of 76 cells expressing FLAG-Fz2 (A). Punctate structures of FLAG-Fz2 were observed at the dorsal cell surface (B). (C) Vero cells expressing FLAG-Fz2 and Wnt5a were wounded. After 4 h, the cells were incubated with anti-FLAG and anti-Wnt5a antibodies for 1 h before fixation. White arrows indicate migration direction. Dashed lines indicate the front line of scratched cells. Bottom panels, colocalization of FLAG-Fz2 with Wnt5a was observed in 65% of 150 FLAG-Fz2 puncta at the dorsal cell surface. (D) HEK293T cells expressing FLAG-Fz2 and HA-Dvl2 as indicated were incubated with anti-FLAG antibody for 1 h before lysis. Lysates were incubated with protein sepharose-A beads, and the immunoprecipitates were probed with the indicated antibodies. (E) HEK293T cells expressing FLAG-Fz2 and APC-GFP with (bottom panels) or without (top panels) HA-Dvl2 were fixed and stained with polyclonal anti-FLAG antibody before permeabilization. Thereafter, Dvl and APC were stained with anti-HA and anti-GFP antibodies. Cells with membrane localization of APC were counted in at least 50 cells per condition, and the results are shown in the right-hand panel. *P<0.01. Scale bars in (A), (B) (left panel), (C) (top panels), and (E), 10 μm; in (B) (right panel) and (C) (bottom panels), 2 μm.
Axin has an essential role in the regulation of β-catenin stability by associating with GSK-3β, APC, and Dvl (Kikuchi et al, 2009). HA-Axin was observed as cytoplasmic punctate structures in Vero cells (Supplementary Figure S12A). When HA-Axin and FLAG-Dvl2 were coexpressed, HA-Axin was colocalized with cytoplasmic FLAG-Dvl2 but not with cell-surface FLAG-Dvl2 (Supplementary Figure S12B). Overexpression of FLAG-Fz2 did not affect the distribution of HA-Axin, either (Supplementary Figure S12C). Therefore, it is conceivable that Axin is not recruited with APC to the Fz2-associated Dvl.
Dvl and APC bind to components of the focal adhesion complex
Integrins are essential molecules to regulate cell-substrate adhesion and migration (Legate et al, 2009). Endogenous cell-surface β1 integrin was accumulated at the leading edge where FLAG-Fz2 was also accumulated in polarized Vero cells (Figure 5A, top three panels). When localization of β1 integrin and FLAG-Fz2 at the ventral (substrate facing) cell surface was examined, β1 integrin puncta localized adjacent to puncta of FLAG-Fz2 (Figure 5A, bottom panels). Furthermore, complex formation between FLAG-Fz2 and α2 integrin in HEK293T cells was observed using cross-linker methods (Figure 5B). These findings raised the possibility that there is a connection between Wnt/Fz and integrin signalling.
Figure 5.
Fz2, Dvl, and APC associate with focal adhesions. (A) Vero cells expressing FLAG-Fz2 were fixed and stained with anti-β1 integrin and anti-FLAG polyclonal antibodies without permeabilization (top three panels). Right panel, the distribution of fluorescence intensity was measured along the dotted line. Bottom panels, localization of FLAG-Fz2 adjacent to β1 integrin was observed in 45% of 118 FLAG-Fz2 puncta at the ventral (substrate facing) cell surface. (B) HEK293T cells expressing FLAG-Fz2 were treated with or without 2 mM Dithiobis (succinimidyl propionate) (DSP) for 1 h at 4°C. The lysates were immunoprecipitated with polyclonal anti-FLAG antibody, and the immunoprecipitates were probed with anti-α2 integrin and anti-FLAG antibodies. The samples for immunoblotting were treated with or without 2-mercaptoethanol (2ME). Upper and lower arrows indicate cross-linked and monomeric α2 integrin, respectively. (C) Vero cells expressing FLAG-Dvl2 were plated on collagen for 1 h and stained with anti-FLAG and anti-paxillin antibodies. The regions in the white boxes (c1 and c2) are shown enlarged in bottom panels. Localization of FLAG-Dvl2 at the tip of paxillin staining was observed in 83% of 60 cortical Dvl staining. (D) Wounded HPPL cells were stained with anti-APC and anti-paxillin antibodies. The regions in the white boxes (d1 and d2) are shown enlarged in the right-hand panels. Localization of APC at the tip of paxillin was observed in 61% of 96 cortical APC staining. (E, F) Lysates of HeLaS3 cells were immunoprecipitated with anti-Dvl(DIX) (E) or anti-APC (F) antibody, and the immunoprecipitates were probed with indicated antibodies. (G) Lysates of COS7 cells expressing HA-Dvl1 deletion mutants were immunoprecipitated with anti-FAK antibody and the immunoprecipitates were probed with anti-HA and anti-FAK antibodies. Arrowheads indicate HA-Dvl1(WT) and HA-Dvl1(DEP+) coimmunoprecipitated with endogenous FAK. (H) Lysates of COS7 cells expressing EGFP-APC deletion mutants were immunoprecipitated with anti-GFP antibody and the immunoprecipitates were probed with anti-paxillin and anti-GFP antibodies. An arrowhead indicates endogenous paxillin coimmunoprecipitated with EGFP-APC(Arm+). Scale bars in (A) (top three panels), (C), and (D), 10 μm; in (A) (bottom panels), 1 μm.
In the initiation of spreading of Vero cells, paxillin was observed throughout the cell periphery and FLAG-Dvl2 was found to be localized at the tip of the paxillin-positive region (Figure 5C). APC was also localized at the tip of the paxillin-positive region in the focal complex of migrating and spreading HPPL cells (Figure 5D; Supplementary Figure S13). These results suggested that Dvl and APC are colocalized to focal adhesions.
To clarify the roles of Dvl and APC in focal complex formation, the interaction of Dvl and APC with the components of cell-substrate adhesion was examined. Dvl formed a complex with FAK at endogenous levels in HeLaS3 cells, but it did not interact with paxillin (Figure 5E). In contrast, APC associated with paxillin at endogenous levels but not with FAK (Figure 5F). Dvl and APC did not form a complex with talin, vinculin, or β1 integrin (Figure 5E and F). Deletion mutant analyses showed that Dvl1(DEP+) forms a complex with FAK and that APC(Arm+) associates with paxillin (Figure 5G and H).
The binding activity of GFP-Dvl2(ΔDEP) or GFP-Dvl2(1-509) to APC was similar to that of GFP-Dvl2, but GFP-Dvl2(506–736) did not form a complex with APC (Supplementary Figure S14). Therefore, either the DEP domain or the C-terminal region after the DEP domain is necessary for the binding of Dvl to APC, but the C-terminal region alone is not sufficient for it. GFP-Dvl2(ΔDEP) and GFP-Dvl2(506-736) associated with FAK as well as GFP-Dvl2, but GFP-Dvl2(1-509) did not (Supplementary Figure S14). Therefore, the DEP domain is not necessary for the binding of Dvl to FAK. The C-terminal region after the DEP domain of Dvl is necessary and sufficient for the binding of Dvl to FAK. These results suggest that Dvl forms a complex with APC and FAK at different sites although they might be overlapped partially.
Paxillin formed a complex with EGFP-APC(Arm) with the similar efficiency with EGFP-APC(Arm+), but the binding of Dvl to EGFP-APC(Arm) was weaker than that of Dvl to EGFP-APC(Arm+) (Supplementary Figure S15). In addition, EGFP-APC(Arm1) or EGFP-APC(Arm4) was sufficient for the binding to paxillin, but neither of them bound to Dvl (Supplementary Figure S15). These results suggest that APC forms a complex with Dvl and paxillin at different sites.
Whether the Dvl and APC complex binds to FAK or paxillin was also examined. The complex formation between Dvl and APC at endogenous levels was inhibited by the overexpression of EGFP-APC(Arm+) or HA-Dvl1(DEP+) (Supplementary Figure S16A and B). However, under the same conditions the interaction of Dvl with FAK was not inhibited by EGFP-APC(Arm+) (Supplementary Figure S16C). The complex formation of APC with paxillin was not suppressed by HA-Dvl1(DEP+), either (Supplementary Figure S16D). Taken together, these results suggest that Dvl binds to APC and FAK simultaneously and that APC also binds to Dvl and paxillin simultaneously.
Wnt5a stimulates focal adhesion dynamics through Dvl and APC
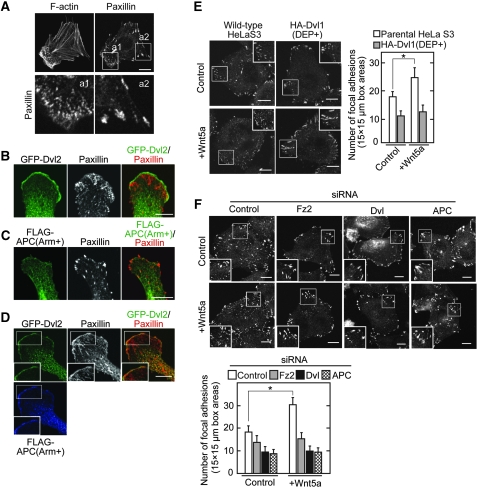
The roles of the binding of Dvl and APC in Wnt5a-dependent focal adhesion dynamics were examined. The strong staining of F-actin and the small size of the paxillin-positive region were observed at the cell front of polarized Vero cells, whereas a large area of paxillin staining was detected in other regions (Figure 6A). This reflects the rapid turnover of focal complexes containing paxillin at the front of motile cells and the slow turnover of focal adhesions in other regions (Ridley et al, 2003). When GFP-Dvl2 was expressed in Vero cells, it localized along the leading edge where a number of small paxillin-positive regions were observed (Figure 6B). In contrast, when FLAG-APC(Arm+), which binds to Dvl, was expressed, it was distributed throughout the cytosol, and the numbers of small paxillin-positive regions were reduced, instead large areas of paxillin staining were observed (Figure 6C). These findings are consistent with the previous report that APC(Arm+) acts as a dominant negative form to suppress cell migration activity (Watanabe et al, 2004). When both GFP-Dvl2 and FLAG-APC(Arm+) were expressed, they were present along the leading edge where paxillin was also colocalized (Figure 6D). These results suggested that the armadillo repeats of APC links Dvl and paxillin at the leading edge for cells to migrate.
Figure 6.
The Dvl and APC complex stimulates the focal adhesion dynamics in response to Wnt5a. (A) Vero cells were stained with anti-paxillin antibody and phalloidin. The region in white boxes (a1 and a2) are shown enlarged in bottom panels. (B, C) Vero cells expressing GFP-Dvl2 (B) or FLAG-APC(Arm+) (C) were stained with the indicated antibodies. (D) Vero cells coexpressing FLAG-APC(Arm+) with GFP-Dvl2 were stained with anti-paxillin, anti-FLAG, and anti-GFP antibodies. The region in the white box is shown enlarged. (E, F) HeLaS3 cells expressing HA-Dvl1(DEP+) (E) or transfected with siRNA for Fz2, Dvl, or APC (F) were stimulated with Wnt5a conditioned medium for 60 min and then the cells were stained with anti-paxillin antibody. The region in the white box is shown enlarged. The numbers of focal adhesions in three different areas (15 μm × 15 μm) at the cell periphery (white box) were counted in 20 cells per treatment. Scale bars, 10 μm. *P<0.01.
Wnt5a increased focal adhesion numbers, reflecting that it increases rates in focal adhesion turnover (Figure 6E and F). Overexpression of HA-Dvl1(DEP+), which inhibits complex formation between Dvl and APC at endogenous levels, suppressed the Wnt5a-dependent increase in focal adhesion numbers (Figure 6E). In addition, knockdown of Fz2, Dvl, and APC inhibited it (Figure 6F). Therefore, Wnt5a might regulate focal adhesion turnover through Fz2, and the binding of Dvl to APC is important for this process.
The Wnt5a signalling is required for 3D cell morphogenesis
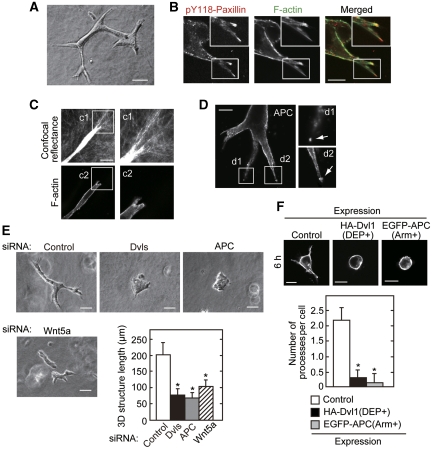
It has been shown that both β-catenin-dependent and -independent Wnt signalling pathways are important in the epithelial morphogenesis of tubular organs such as the lungs, kidneys, guts, and mammary glands (Logan and Nusse, 2004; Grigoryan et al, 2008). Indeed, Wnt5a mutant mouse exhibits severe morphological defect including shortening of the small intestine and truncated trachea (Li et al, 2002; Cervantes et al, 2009). As epithelial morphogenesis is a complex process involving cell polarization, migration, and cell-substrate adhesion (O'Brien et al, 2002; Schock and Perrimon, 2002), the involvement of the new Wnt5a signalling through Dvl and APC in epithelial morphogenesis was examined. As an in vitro morphogenesis model, HPPL cells proliferate and differentiate to form multicellular epithelial tube-like structures when cultured in type I collagen gel (Figure 7A; Supplementary Figure S17) (Tanimizu et al, 2004, 2007). Paxillin phosphorylated at Tyr118 and F-actin localized at the tips of extended structures (Figure 7B). Collagen fibrils at the front of leading cells oriented towards the tips of extended structures, suggesting that the leading edge of tube-like structures generate a force to contact with the extracellular matrix (Figure 7C). Consistent with these results, it has been reported that cell-substrate adhesion through FAK and paxillin is required for 3D epithelial morphogenesis (Ishibe et al, 2004). As observed in monolayer culture experiments, APC was accumulated at the tips of branching processes as well as tyrosine-phosphorylated paxillin (Figure 7D). Short-length branching structures were formed in Wnt5a knockdown cells, and branching morphogenesis was dramatically inhibited and cells were aggregated by knockdown of Dvl or APC (Figure 7E). To exclude the effects of cell-to-cell adhesion and cell proliferation on branching formation, the branching processes extended from a single cell for a short time (6 h) were examined. Overexpression of Dvl1(DEP+) or APC(Arm+) suppressed the formation of initial branching processes (Figure 7F). These results suggested that Wnt5a signalling through the binding of Dvl and APC regulates 3D epithelial morphogenesis.
Figure 7.
A Wnt5a signalling through Dvl and APC is involved in 3D epithelial morphogenesis. (A) HPPL cells were cultured in collagen gel for 3 days. A representative structure was photographed using phase contrast microscopy on day 3. (B) HPPL cells were cultured in collagen gel for 2 days and stained with anti-pY118-paxillin antibody and phalloidin. Localization of pY118-paxillin at the leading cell of extended structure was observed. The region in the white box is shown enlarged. (C) HPPL cells were cultured in collagen gel for 2 days and stained with phalloidin. Top panels, confocal reflectance image around the leading cell, which reveals the morphology of the collagen network. Bottom panels, staining with phalloidin for F-actin. The regions in the white boxes (c1 and c2) are shown enlarged. (D) HPPL cells were cultured in collagen gel for 2 days and stained with anti-APC antibody. Localization of APC in the leading cell of the extended structure was observed. The regions in the white boxes (d1 and d2) are shown enlarged. (E) HPPL cells transfected with siRNA for Wnt5a, Dvl, or APC were cultured in collagen gel for 2 days. The length of the major axis of the extended structure was measured (counted structures: 30 per siRNA). (F) HPPL cells expressing GFP (control), HA-Dvl1(DEP+), or GFP-APC(Arm+) were cultured in collagen gel for 6 h and stained with phalloidin. The numbers of processes were counted (counted cells: 30 per siRNA). Scale bars in (A) and (E), 50 μm; in (C) and (E), 20 μm; in (B) and (D), 10 μm. *P<0.01.
Discussion
The molecular mechanism by which Wnt5a regulates focal adhesion dynamics was examined in this study. It was shown that the Dvl and APC (Dvl/APC) complex mediates a Wnt5a-Fz2 signal to focal adhesions by associating with FAK and paxillin, thereby regulating cell-substrate adhesion, polarized migration, and epithelial morphogenesis.
Functional interaction between Wnt5a, Fz2, Dvl, and APC
The present results together with previous reports showed that the loss of function mutants of Wnt5a, Dvl, and APC exhibit similar phenotypes in cell-substrate adhesion and cell migration, suggesting that these molecules are functionally interacted. Consistent with these results, it was shown that Dvl directly interacts with APC and that the interaction was enhanced by Wnt5a. Although Dvl and APC were already localized at the leading edge without the addition of Wnt5a in Vero cells, endogenously secreted Wnt5a may be involved in their localization. Wnt5a indeed induced the accumulation of APC at the cell periphery of HeLaS3 cells, and Dvl was necessary for this process. Therefore, it seems that Dvl and APC bind each other without Wnt5a stimulation, but Wnt5a might determine the localization of APC at the cell periphery through Dvl.
It has been reported that Dvl is required for polarized cell orientation and migration in vertebrate gastrulation and Wnt-induced cell migration in vitro (Wallingford et al, 2000; Endo et al, 2005). Dvl has also been shown to bind to the C-terminus or intracellular loops of Fz (Schulte and Bryja, 2007). In this study, it was found that Dvl is localized to the F-actin-rich leading edge in migrating cells, which is consistent with the observation that Dvl is recruited from the cytoplasm to the distal cell cortex and determines planar cell polarity in Drosophila (Veeman et al, 2003). In addition, it was shown that Fz2, a possible receptor for Wnt5a, is concentrated to the leading edge of polarized cells, where Wnt5a and Dvl are also present. Although the determinants of the restricted localization of Fz2 are not known, the interaction with integrins may allow Fz2 to localize to the leading edge.
It became clear that membrane trafficking pathways that recycle adhesion molecules such as integrins contribute to cell migration (Polo and Di Fiore, 2006; Caswell and Norman, 2008). Integrin heterodimers are internalized from the plasma membrane through the clathrin-dependent or -independent routes and then recycled back to the cell surface through Rab4- or Rab11-dependent manners. The recycling processes that move towards the leading edge are likely to be dispensable for directionality of cell migration. It has been shown recently that Wnt5a-dependent activation of Rac requires clathrin-dependent internalization of Fz2 (Sato et al, 2010). Therefore, taken together with the observation that Fz2 associates with intergrins, it is intriguing to speculate that the recycling of Fz2 with integrins is involved in the accumulation of Fz2 at the leading edge of migrating cells. Alternatively, polarized secretion of Wnt5a to this specialized region might trigger the concentration of Fz2. Thus, the complex of Wnt5a and Fz2 at the leading edge, where integrins are also present, could be important for the localization of Dvl and APC.
Impact of the interaction of Dvl with APC in cell-substrate adhesion
How do Dvl and APC regulate cell-substrate adhesion? This study found that Dvl and APC preferentially interact with FAK and paxillin, respectively, among focal adhesion proteins. The C-terminal region of Dvl interacted with FAK, and the armadillo repeat of APC bound to paxillin. Furthermore, in Vero cells overexpressing Dvl and APC(Arm+), both proteins were colocalized to the leading edge and paxillin was recruited to the same locations. Therefore, it is possible that the binding of Dvl and APC enhances the formation of a complex between FAK and paxillin, leading to the formation of focal complexes or focal adhesions. IQ-motif-containing GTPase activation protein 1 (IQGAP1) was also shown to be necessary for the localization of APC at the leading edge (Watanabe et al, 2004). However, IQGAP1 did not recruit paxillin to the leading edge although overexpression of IQGAP1 allowed FLAG-APC(Arm+) to localize at the leading edge (Supplementary Figure S18). Therefore, it is conceivable that Dvl and IQGAP1 have different functions as APC-binding proteins.
Another possible role of Dvl and APC in cell-substrate adhesion and migration might be to stabilize microtubules at the cell periphery, because APC is present at the end of microtubules and can stabilize them (Dikovskaya et al, 2001; Aoki and Taketo, 2007; Barth et al, 2008). It has been reported that the Cdc42, Par6, and atypical PKC (aPKC) signalling pathway are required for the accumulation of APC at the leading edge (Etienne-Manneville and Hall, 2003). As Dvl interacts with aPKC (Schlessinger et al, 2007), it is possible that the interaction of the Dvl/APC complex with the Cdc42/Par6/aPKC complex promotes the polarization of microtubules at the leading edge. It was shown that microtubules target the substrate adhesion complex specifically, thereby inducing the dissolution of adhesion sites or the release of adhesion molecules from the substrate (Small and Kaverina, 2003). Therefore, the binding of Dvl and APC at the cell periphery may regulate cell migration by promoting the dynamics of cell-substrate adhesion through the stabilization of microtubules.
The importance of this new Wnt5a signalling through the interaction between Dvl and APC was confirmed in 3D epithelial morphogenesis. It was shown that HPPL cells have proliferative activity and differentiate into cholangiocytes in type I collagen gel (Tanimizu et al, 2004). The present results showed that siRNAs for Wnt5a, Dvl, and APC inhibit the formation of tube-like structures. Furthermore, the branching processes extended from a single cell were also suppressed by the overexpression of Dvl1(DEP+) or APC(Arm+), which inhibited the Wnt5a-dependent increase in focal adhesion numbers. Therefore, it is intriguing to speculate that this new Wnt5a signalling pathway through Dvl and APC is important for the formation of tubular organs.
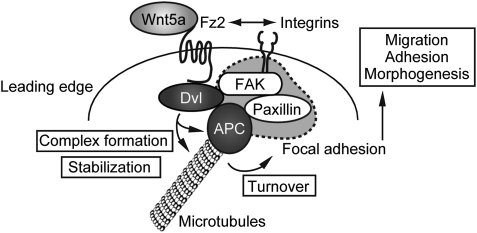
Taken together, the possible model is as follows (Figure 8). When a cell attaches to the substrate or migrates in a certain direction, Fz2 may be accumulated to the leading edge where integrins are also present. Secreted Wnt5a might be accumulated at the leading edge and the binding of Wnt5a and Fz2 could enhance the association of Dvl with APC and induce the recruitment of the Dvl/APC complex to the cell periphery, which lead to the activation of FAK. Then, paxillin is translocated to the vicinity of FAK through the Dvl/APC complex, and the focal complex and focal adhesion are formed. In parallel, the binding of Dvl and APC might stabilize microtubules, which induce the disassembly of the focal adhesion, thereby enhancing cell migration. As some of results in this study were obtained in cell lines such as Vero and HPPL cells, which were easily polarized, further investigations are necessary to clarify the significance of the Dvl and APC interaction in other types of cells. However, the present findings provide new insights into understanding of the regulation of cell-substrate adhesion, cell migration, and epithelial morphogenesis by Wnt5a signalling.
Figure 8.
A model for Wnt5a-dependent focal adhesion dynamics through Fz2, Dvl, and APC. When a cell attaches to the substrate or migrates in a certain direction, Fz2 is accumulated to the leading edge where integrins are also present. Secreted Wnt5a is accumulated at the leading edge and the binding of Wnt5a and Fz2 enhances the association of Dvl with APC and induces the recruitment of the Dvl/APC complex to the cell periphery, which leads to the activation of FAK. Then, paxillin is translocated to the vicinity of FAK through the Dvl/APC complex, and the focal complex and focal adhesion are formed. The binding of Dvl and APC stabilizes microtubules, which induce the disassembly of the focal adhesion, thereby enhancing cell migration.
Materials and methods
Immunocytochemistry
Cells grown on glass coverslips were fixed for 10 min at room temperature in PBS containing 4% (w/v) paraformaldehyde and then permeabilized in PBS containing 0.2% (w/v) Triton X-100 and 2 mg/ml BSA for 10 min. When necessary, the cells were fixed in 100% methanol for 5 min at −20°C. The cells were incubated with antibodies against FAK, pTyr397-FAK, vinculin, β-tubulin, tubulin, FLAG, GFP, Dvl2, Dvl3, APC, Wnt5a, β1 integrin, phalloidin, paxillin, pTyr118-paxillin, and HA, and viewed using a confocal microscope (LSM510, Carl-Zeiss, Jana, Germany).
To examine Wnt5a-dependent APC accumulation at the cell periphery (Figure 3D), the mean intensity of APC staining in a 5 μm × 5 μm area at the cell edge and centre was measured using Image J soft ware (National Institute of Health, Bethesda, MD, USA).
Complex formation and immunoprecipitation
HeLaS3, COS7, or MDCK cells (100-mm diameter dish) were lysed in 300 μl of lysis buffer (10 mM Tris–HCl [pH 7.4], 140 mM NaCl, 5 mM EDTA, 1% NP40, 25 mM NaF, 20 μg/ml leupeptin, 20 μg/ml aprotinin, and 10 mM PMSF). The lysates were immunoprecipitated with anti-Dvl (DIX) or anti-APC (rabbit) antibody, and the immunoprecipitates were probed with the indicated antibodies.
To determine which regions of Dvl and APC interact with each other, FAK, paxillin, COS7 cells (100-mm diameter dish) were transfected with various mutants of HA-Dvl1, GFP-Dvl2, or EGFP-APC and the cells were lysed in 300 μl of lysis buffer. The lysates were immunoprecipitated with anti-GFP, anti-APC, or anti-FAK antibodies, and the immunoprecipitates were probed with the indicated antibodies.
To examine the complex states of Dvl, APC, FAK, and paxillin, HEK293T cells (100-mm diameter dish) expressing HA-Dvl1(DEP+), EGFP-APC(Arm+), or GFP-Dvl2 were lysed in 300 μl of lysis buffer. The lysates were immunoprecipitated with anti-APC or anti-Dvl(DIX) antibodies, and the immunoprecipitates were probed with the indicated antibodies.
Interaction of APC with Dvl in vitro
For direct binding of Dvl1 (full length) to APC (Arm+), various concentrations of GST-APC(Arm+) were incubated with 0.1 μM MBP-Dvl1 bound to amylose resin in 100 μl of reaction buffer (20 mM Tris–HCl [pH7.5], 1 mM dithiothreitol, and 0.05% CHAPS) for 1 h. The resin was then washed with reaction buffer three times and precipitated by the centrifugation. The precipitates were probed with anti-GST antibody.
For direct binding of Dvl1(395–670) to APC(Arm+), 1 μM of MBP-APC(Arm+) was incubated with 0.3 μM GST-Dvl1(395–670) or GST bound to glutathione–sepharose in 100 μl of reaction buffer (20 mM Tris–HCl [pH7.5], 1 mM dithiothreitol, and 0.05% CHAPS) for 1 h. The beads were then washed with reaction buffer three times and precipitated by the centrifugation. The precipitates were probed with anti-MBP antibody.
Cell adhesion assays
To measure the cell adhesion activity, HeLaS3 cells (5 × 104 cells in 100 μl) were added to a 96-well dish that was precoated with 10 μg/ml fibronectin or 40 μg/ml collagen type 1 (INAMED) in PBS for 2 h. After 15 min incubation, the cells were washed with PBS three times. Adherent cells were fixed, stained with 0.1% crystal violet in 20% methanol for 5 min at room temperature, and then washed with PBS extensively. The stain was eluted with 100 μl of 50% methanol, and the absorbance at 590 nm was measured using a spectrofluorometer (Fusion, Packard, Perkin Elmer Life Sciences, Boston, MA, USA) as described earlier (Oshiro et al, 2002). The results are expressed as the ratio of adhesion activity of cells treated with control siRNA and indicate means±s.e. from three independent experiments.
Focal adhesion turnover assays
The dynamics of GFP-paxillin in HeLaS3 cells transfected with control or Dvl siRNA were visualized by time-lapse fluorescence microscopy (Figure 1D). The images from time points at 0, 15, 30, and 45 min are represented in red, green, blue, and white, respectively, and these are superimposed at the bottom. White spots were counted as a non-dynamic adhesion, and spots containing different colours were counted as a dynamic adhesion.
Cell migration assays
To measure the cell migration activity, Transwell and wound-healing assays were performed. The Transwell cell migration assay was performed using a modified Boyden chamber (tissue culture treated, 6.5 mm diameter, 10 μm thickness, 8 μm pore; Transwell, Costar, Cambridge, MA, USA) as described earlier (Kurayoshi et al, 2006, 2007). The lower surface of filters was coated with 40 μg/ml collagen or 10 μg/ml fibronectin. Cells (2.5 × 104 cells in 100 μl) suspended in serum-free DMEM containing 0.1% bovine serum albumin were applied to the upper chamber. The same medium was added to the lower chamber. After the cells were incubated with 37°C for 8 h, the number of cells that migrated to the lower side of the upper chamber was counted.
For the wound-healing assay, cells were plated onto fibronectin-coated cover slips. The monolayer cells were then scratched manually with a plastic pipette tip, and after being washed with PBS, the wound monolayers of the cells were allowed to heal for 10 h in DMEM containing 10% FBS.
Branching morphogenesis in 3D collagen gel
HPPL cells were suspended in type I collagen gel at a density of 1 × 105 cells/ml, and 80 μl of the cell suspension was mounted on a round coverslip. After incubation at 37°C for 30 min with solid the gel, the coverslip was transferred to 24-well culture plate and 1 ml of growth medium containing 5 ng/ml hepatocyte growth factor and 5 ng/ml epidermal growth factor was added on collagen gel (Tanimizu et al, 2004, 2007).
Statistical analysis
The experiments were performed at least four times and the results were expressed as means±s.e. Statistical analysis was performed using StatView software (SAS Institute Inc.). Differences between the data were tested for statistical significance using t-test. P-values <0.01 were considered statistically significant.
Others
Western blotting data were representative of at least four independent experiments.
Supplementary Material
Acknowledgments
We are grateful to Drs A Miyajima, R Habas, and Y Mimori-Kiyosue for donating cells, plasmids, and antibodies. We thank all of our laboratory members for helpful discussions. This work was supported by Grants-in-Aid for Scientific Research and for Scientific Research on Priority Areas from the Ministry of Education, Science, and Culture, Japan (2007, 2008, 2009), and by grants from The Uehara Memorial Foundation (2008) and Takeda Science Foundation (2009).
Footnotes
The authors declare that they have no conflict of interest.
References
- Akiyama T, Kawasaki Y (2006) Wnt signalling and the actin cytoskeleton. Oncogene 25: 7538–7544 [DOI] [PubMed] [Google Scholar]
- Aoki K, Taketo MM (2007) Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. J Cell Sci 120: 3327–3335 [DOI] [PubMed] [Google Scholar]
- Barth AI, Caro-Gonzalez HY, Nelson WJ (2008) Role of adenomatous polyposis coli (APC) and microtubules in directional cell migration and neuronal polarization. Semin Cell Dev Biol 19: 245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelluto DG, Kutateladze TG, Habas R, Finkielstein CV, He X, Overduin M (2002) The DIX domain targets dishevelled to actin stress fibres and vesicular membranes. Nature 419: 726–729 [DOI] [PubMed] [Google Scholar]
- Caswell P, Norman J (2008) Endocytic transport of integrins during cell migration and invasion. Trends Cell Biol 18: 257–263 [DOI] [PubMed] [Google Scholar]
- Cervantes S, Yamaguchi TP, Hebrok M (2009) Wnt5a is essential for intestinal elongation in mice. Dev Biol 326: 285–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani L, Krylova O, Smalley MJ, Dale TC, Salinas PC (2004) A divergent canonical WNT-signaling pathway regulates microtubule dynamics: dishevelled signals locally to stabilize microtubules. J Cell Biol 164: 243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikovskaya D, Zumbrunn J, Penman GA, Nathke IS (2001) The adenomatous polyposis coli protein: in the limelight out at the edge. Trends Cell Biol 11: 378–384 [DOI] [PubMed] [Google Scholar]
- Endo Y, Wolf V, Muraiso K, Kamijo K, Soon L, Uren A, Barshishat-Kupper M, Rubin JS (2005) Wnt-3a-dependent cell motility involves RhoA activation and is specifically regulated by dishevelled-2. J Biol Chem 280: 777–786 [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A (2002) Rho GTPases in cell biology. Nature 420: 629–635 [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A (2003) Cdc42 regulates GSK-3β and adenomatous polyposis coli to control cell polarity. Nature 421: 753–756 [DOI] [PubMed] [Google Scholar]
- Grigoryan T, Wend P, Klaus A, Birchmeier W (2008) Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev 22: 2308–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibe S, Joly D, Liu ZX, Cantley LG (2004) Paxillin serves as an ERK-regulated scaffold for coordinating FAK and Rac activation in epithelial morphogenesis. Mol Cell 16: 257–267 [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto H (2008) Tumor formation due to abnormalities in the β-catenin-independent pathway of Wnt signaling. Cancer Sci 99: 202–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto H, Sato A (2009) Selective activation mechanisms of Wnt signaling pathways. Trends Cell Biol 19: 119–129 [DOI] [PubMed] [Google Scholar]
- Kishida S, Yamamoto H, Hino S-I, Ikeda S, Kishida M, Kikuchi A (1999) DIX domains of Dvl and Axin are necessary for protein interactions and their ability to regulate β-catenin stability. Mol Cell Biol 19: 4414–4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroboth K, Newton IP, Kita K, Dikovskaya D, Zumbrunn J, Waterman-Storer CM, Nathke IS (2007) Lack of adenomatous polyposis coli protein correlates with a decrease in cell migration and overall changes in microtubule stability. Mol Biol Cell 18: 910–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krylova O, Messenger MJ, Salinas PC (2000) Dishevelled-1 regulates microtubule stability: a new function mediated by glycogen synthase kinase-3β. J Cell Biol 151: 83–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, Yasui W, Kikuchi A (2006) Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res 66: 10439–10448 [DOI] [PubMed] [Google Scholar]
- Kurayoshi M, Yamamoto H, Izumi S, Kikuchi A (2007) Post-translational palmitoylation and glycosylation of Wnt-5a are necessary for its signalling. Biochem J 402: 515–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legate KR, Wickstrom SA, Fassler R (2009) Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev 23: 397–418 [DOI] [PubMed] [Google Scholar]
- Li C, Xiao J, Hormi K, Borok Z, Minoo P (2002) Wnt5a participates in distal lung morphogenesis. Dev Biol 248: 68–81 [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20: 781–810 [DOI] [PubMed] [Google Scholar]
- Marsden M, DeSimone DW (2001) Regulation of cell polarity, radial intercalation and epiboly in Xenopus: novel roles for integrin and fibronectin. Development 128: 3635–3647 [DOI] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Shiina N, Tsukita S (2000) The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr Biol 10: 865–868 [DOI] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD (2005) Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 6: 56–68 [DOI] [PubMed] [Google Scholar]
- O'Brien LE, Zegers MM, Mostov KE (2002) Opinion: Building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol 3: 531–537 [DOI] [PubMed] [Google Scholar]
- Oshiro T, Koyama S, Sugiyama S, Kondo A, Onodera Y, Asahara T, Sabe H, Kikuchi A (2002) Interaction of POB1, a downstream molecule of small G protein Ral, with PAG2, a paxillin-binding protein, is involved in cell migration. J Biol Chem 277: 38618–38626 [DOI] [PubMed] [Google Scholar]
- Polakis P (2000) Wnt signaling and cancer. Genes Dev 14: 1837–1851 [PubMed] [Google Scholar]
- Polo S, Di Fiore PP (2006) Endocytosis conducts the cell signaling orchestra. Cell 124: 897–900 [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR (2003) Cell migration: integrating signals from front to back. Science 302: 1704–1709 [DOI] [PubMed] [Google Scholar]
- Sato A, Yamamoto H, Sakane H, Koyama H, Kikuchi A (2010) Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO J 29: 41–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger K, McManus EJ, Hall A (2007) Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity. J Cell Biol 178: 355–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock F, Perrimon N (2002) Molecular mechanisms of epithelial morphogenesis. Annu Rev Cell Dev Biol 18: 463–493 [DOI] [PubMed] [Google Scholar]
- Schulte G, Bryja V (2007) The Frizzled family of unconventional G-protein-coupled receptors. Trends Pharmacol Sci 28: 518–525 [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Metcalfe C, Bienz M (2007) Dynamic recruitment of axin by Dishevelled protein assemblies. J Cell Sci 120: 2402–2412 [DOI] [PubMed] [Google Scholar]
- Small JV, Kaverina I (2003) Microtubules meet substrate adhesions to arrange cell polarity. Curr Opin Cell Biol 15: 40–47 [DOI] [PubMed] [Google Scholar]
- Tanimizu N, Miyajima A, Mostov KE (2007) Liver progenitor cells develop cholangiocyte-type epithelial polarity in three-dimensional culture. Mol Biol Cell 18: 1472–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimizu N, Saito H, Mostov K, Miyajima A (2004) Long-term culture of hepatic progenitors derived from mouse Dlk+ hepatoblasts. J Cell Sci 117: 6425–6434 [DOI] [PubMed] [Google Scholar]
- Torres MA, Nelson WJ (2000) Colocalization and redistribution of dishevelled and actin during Wnt-induced mesenchymal morphogenesis. J Cell Biol 149: 1433–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT (2003) A second canon. Functions and mechanisms of β-catenin-independent Wnt signaling. Dev Cell 5: 367–377 [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM (2000) Dishevelled controls cell polarity during Xenopus gastrulation. Nature 405: 81–85 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Wang S, Noritake J, Sato K, Fukata M, Takefuji M, Nakagawa M, Izumi N, Akiyama T, Kaibuchi K (2004) Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell 7: 871–883 [DOI] [PubMed] [Google Scholar]
- Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM (2002) Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 1: 279–288 [DOI] [PubMed] [Google Scholar]
- Wharton KAJ (2003) Runnin' with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev Biol 253: 1–17 [DOI] [PubMed] [Google Scholar]
- Witze ES, Litman ES, Argast GM, Moon RT, Ahn NG (2008) Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science 320: 365–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R, Takada S, Nishida E (2002) JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep 3: 69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.