Summary
Objectives:
Members of the glycoprotein 130 (gp130) receptor–gp130 ligand family play a role in angiogenesis in different tissues. We tested the effect of this cytokine family on the angiopoietin (Ang)–Tie system, which is involved in blood vessel maturation, stabilization, and regression.
Results:
Oncostatin M (OSM) increased Ang2 expression in human umbilical vein endothelial cells via Janus kinase/signal transducer and activator of transcription (JAK/STAT) and mitogen-activated protein (MAP) kinase activation. Furthermore, OSM induced Ang2 expression in macrovascular endothelial cells isolated from the human aorta and in microvascular endothelial cells isolated from human heart. Our in vivo experiments revealed that mRNA expression of Ang2 in hearts of mice injected with OSM increased significantly, and levels of OSM mRNA significantly correlated with mRNA levels of Ang2 in human hearts. In addition, OSM increased the expression of its own receptors, gp130 and OSM receptor, in endothelial cells in vitro and in mice in vivo, and levels of OSM mRNA significantly correlated with mRNA levels of gp130 and OSM receptor in human hearts.
Conclusion:
Our data, showing the effects of OSM on the Ang–Tie system in endothelial cells, in hearts of mice, and in human heart tissue, provide yet another link between inflammation and angiogenesis.
Keywords: angiogenesis, angiopoietin, cytokine, oncostatin M
Introduction
Angiogenesis, the formation of blood vessels from pre-existing vessels, is a highly regulated process that is essential in embryogenesis, and normal physiologic growth and repair. Pathologic angiogenesis causes or contributes to diseases such as cancer, atherosclerosis, obesity, arthritis, diabetic retinopathy, and ischemic heart disease [1].
There is evidence that angiogenesis and inflammation are associated processes in pathologic situations, including rheumatoid arthritis, diabetes, and cancer [2], owing to the facts that inflammatory mediators such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1 and IL-6 act directly and indirectly on the vasculature, and that angiogenesis sustains inflammation [3].
Members of the glycoprotein 130 (gp130) receptor–gp130 ligand family have been shown to have, in addition to their inflammatory properties, direct and indirect roles in angiogenesis in different tissues [4-6]. For example, IL-6 increases the expression of vascular endothelial growth factor (VEGF) in cervical cancer and gastric carcinoma [7,8]. Oncostatin M (OSM), another gp130 ligand, produced mainly by activated T lymphocytes, monocytes, and macrophages, and which is involved in the regulation of inflammation, tissue remodeling, and cell growth, upregulates VEGF in cardiac myocytes, astroglioma cells, preadipocytes, adipocytes, and smooth muscle cells, as shown by us and others [9-11].
Besides VEGF, the angiopoietin (Ang)–Tie system plays an important role in the regression, maturation and stabilization of blood vessels [12,13]. Ang1 and Ang2 have been identified as ligands for the endothelial receptor tyrosine kinase Tie2 [14-16]. Ang1 activates Tie2; Ang2, predominantly expressed by endothelial cells, primarily binds the receptor without inducing signal transduction, therefore acting mainly as a functional antagonist of Ang1 by an autocrine mechanism [1,17]. Ang2, stored in Weibel–Palade bodies [18], destabilizes the vasculature. This results in the absence of VEGF in vessel regression, whereas in concert with VEGF Ang2 induces endothelial cell migration and proliferation [19]. Ang2 also plays a role in the induction of inflammation by sensitizing endothelial cells to TNF-α [20].
In this study, we wanted to investigate the link between the Ang–Tie system and the gp130 receptor–gp130 ligand system. Therefore, we studied the effect of gp130 ligands, namely OSM, cardiotrophin 1 (CT1), IL-6, leukemia inhibitory factor (LIF), IL-11, ciliary neurotrophic factor (CNTF), and cardiotrophin-like cytokine (CLC), on Ang2 expression in endothelial cells isolated from human umbilical vein, human aorta, and human heart tissue. In addition, we studied the effects of OSM on Ang2 expression in umbilical vein explants ex vivo and in hearts of mice in vivo, and the correlation between OSM and Ang2 levels in human hearts.
Material and methods
Isolation and cultivation of human cells
Human umbilical vein endothelial cells (HUVECs) were isolated from fresh umbilical cords by mild collagenase treatment, and characterized as recently described [21,22]. Human aortic endothelial cells (HAECs) were isolated from fresh aortas obtained from patients undergoing heart transplantation by collagenase treatment, and cultivated and characterized as previously described [23], or obtained from PromoCell (Heidelberg, Germany). Human heart microvascular endothelial cells (HHMECs) were isolated from human hearts from different donors undergoing heart transplantation, and characterized and cultivated as described recently [24]. Human aortic smooth muscle cells were isolated, characterized and cultivated as described recently [25].
All human material was obtained and processed according to the recommendations of the hospital's Ethics Committee, including informed consent.
Cell treatment
Human endothelial cells were incubated in M199 containing 1.25%, 2.5% or 5% fetal bovine serum (FBS), depending on the incubation time. For stimulation experiments, recombinant human (rh) OSM, rhIL-11, rhCNTF, rhCLC (R&D Systems, Minneapolis, MN, USA), rhIL-6,rhLIF (Biotrend, Cologne, Germany) or rhCT1 (Calbiochem/Merck, Darmstadt, Germany) was added at a concentration of 100 ng mL−1, if not indicated otherwise, for time periods between 4 h and 72 h. Additionally, we used cycloheximide (1 μg mL−1; Sigma, St. Louis, MO, USA) and the phosphorylation blockers U0126 (10 μm), piceatannol (200 μm), WHI-P131 (30 μg mL−1), SB-202190 (10 μm) and LY-294002 (10 μm; Merck, Darmstadt, Germany). For blocker experiments, cells were preincubated for 1 h with the respective blocker before stimulation with OSM (100 ng mL−1). For RNA stability experiments, cells were stimulated for 12 h with rhOSM (100 ng mL−1), and incubated with actinomycin D (5 μg mL−1; Sigma) for time periods between 30 and 240 min.
Small interfering RNA (siRNA)-mediated gene knockdown
siRNA targeting signal transducer and activator of transcription (STAT) 1 and STAT3, and control siRNA, were obtained from Dharmacon-Thermo Fisher Scientific (Waltham, MA, USA). HUVECs were transfected with 100 nmol L−1 siRNA, using polyethylenimine (Sigma) according to the manufacturer's instructions. Seventy-two hours after transfection, the cells were treated with OSM. The efficiency of STAT1 and STAT3 knockdown was confirmed by western blot analysis.
Tissue samples
Fragments of fresh umbilical vein were incubated for 48 h in M199containing2.5%FBSwithorwithout100 ng mL−1 OSM. Pieces were embedded in Tissue-Tek OCT Compound (Sakura Finetek, Zoeterwoude, The Netherlands) and stored at − 20 °C.
Human heart tissue was obtained from the left ventricles of explanted hearts from donors undergoing heart transplantation, and stored at − 80 °C.
Animals
Male C57/BL6 mice were injected intraperitoneally with recombinant murine OSM or with 0.9% NaCl. Detailed information on animal experiments is given in Doc. S1.
Immunohistochemistry
OCT-embedded frozen fragments of umbilical cord were cut into 10-μm cryosections, fixed in chilled acetone, and air-dried for 10 min. The sections were rinsed with phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA; Sigma), and incubated with goat anti-human Ang2 polyclonal antibody (15 μg mL−1; R&D Systems) and rabbit anti-human von Willebrand factor (VWF) antibody (1 : 500; DakoCytomation Dako, Glostrup, Denmark) for 4 h at room temperature. After being washed with PBS/BSA, the sections were incubated with Northern Lights NL-493 anti-rabbit IgG and Northern Lights Nl-557 anti-goat IgG (both 1 : 1000; R&D Systems) for 1 h, washed three times with PBS/BSA, and coverslipped. Images were taken by a blinded investigator.
Protein determination and western blotting
Cell culture supernatants were collected or cells were lysed in PBS containing 0.1% Triton. Ang1 and Ang2 protein concentrations were determined using a specific enzyme-linked immunosorbent assay according to the manufacturer's instructions (R&D Systems). For detailed information about western blotting, see Doc. S1.
Real-time polymerase chain reaction (PCR)
Frozen human or mouse heart tissue was homogenized using a ball mill (Retsch, Haan, Germany), and mRNA was isolated using a High Pure RNA Tissue Kit (Roche, Basel, Switzerland). mRNA of cell culture was isolated using a High Pure RNA Isolation Kit (Roche). Real-time PCR was performed using a LightCycler system (Roche), as described in detail in Doc. S1.
Statistical analysis
Data were compared statistically by t-test (independent variables). P-values < 0.05 were considered to be significant; a Pearson correlation was calculated for mRNA correlation using spss 16.0 (SPSS, Chicago, IL, USA).
Results
OSM increases Ang2 expression in HUVECs in vitro and in umbilical vein explants ex vivo
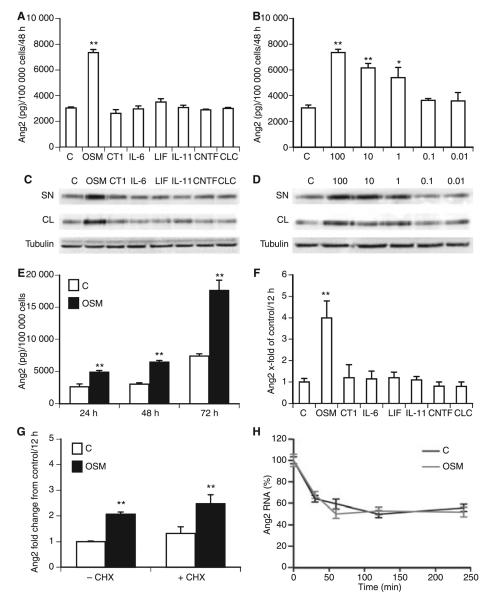
To test the effect of the gp130 ligands on Ang2 expression, we incubated HUVECs for 48 h with or without the gp130 ligands OSM, CT1, IL-6, LIF, IL-11, CNTF, and CLC. In the supernatant of cells treated with OSM, we detected up to three-fold increased levels of Ang2, whereas the other gp130 ligands did not alter Ang2 production (Fig. 1A). The effect of OSM was concentration-dependent, as a significant inducing effect of OSM on Ang2 production was already seen at concentrations of 1 ng mL−1 (Fig. 1B). This stimulating effect of OSM was confirmed by western blot analysis (Fig. 1C,D). Furthermore, we observed increased intracellular expression of Ang2 in OSM-treated cells (Fig. 1C). This effect was concentration-dependent (Fig.1D). Figure 1E shows significant induction of Ang2 secretion after 24, 48 and 72 h, respectively.
Fig. 1.
Oncostatin M (OSM) induces angiopoietin 2 (Ang2) expression in human umbilical vein endothelial cells (HUVECs). Confluent monolayers of HUVECs were incubated in the absence (C) or presence of OSM, cardiotrophin 1 (CT1), interleukin (IL)-6, leukemia inhibitory factor (LIF), IL-11, ciliary neurotrophic factor (CNTF), or cardiotrophin-like cytokine (CLC) (all 100 ng mL−1) (A), or in the absence (C) or presence of different OSM concentrations (0.01-100 ng mL−1), for 48 h (B). HUVECs were incubated for 24, 48 or 72 h in the absence (C, white bars) or presence of 100 ng mL−1 OSM (black bars) (E). Ang2 protein was determined in the supernatant by specific enzyme-linked immunosorbent assay, and is given in pg/100 000 cells. Values represent mean values ± standard deviations (SDs) of three independent determinations. Each experiment was repeated at least three times. A representative experiment is shown. Ang2 expression was determined in the supernatant (SN) and cell lysate (CL) of HUVECs incubated in the absence (C) or presence of OSM, CT1, IL-6, LIF, IL-11, CNTF, or CLC (all 100 ng mL−1) (C), or in the absence (C) or presence of different OSM concentrations (0.01–100 ng mL−1) (D), by western blot analysis. β-Tubulin was used as a loading control. HUVECs were incubated in the absence (C) or presence of OSM, CT1, IL-6, LIF, IL-11, CNTF or CLC (all 100 ng mL−1) for 12 h (F). HUVECs were preincubated for 1 h with or without cycloheximide (CHX), and incubated in the absence (white bars, C) or presence of OSM (black bars, 100 ng mL−1) for 12 h (G). Real-time polymerase chain reaction (PCR) for Ang2 was performed, employing specific primers. Values, given as fold change from control, represent mean values ± SDs of three independent determinations, and were normalized according to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels. Each experiment was repeated at least three times. A representative experiment is shown. HUVECs were incubated in the absence (C, black line) or presence (gray line) of OSM for 12 h before actinomycin D was added for 30, 60, 120 or 240 min (H). Real-time PCR for Ang2 was performed, employing specific primers. Results are expressed as percentage of Ang2 mRNA remaining after being normalized to GAPDH. Values represent mean values ± SDs of three independent determinations. **P < 0.005, *P < 0.05.
As shown in Fig. 1F, specific mRNA for Ang2 was induced significantly in HUVECs treated with OSM for 12 h. Treatment of cells with cycloheximide did not have any effect on OSM-induced Ang2 expression (Fig. 1G), suggesting a direct effect of OSM. Furthermore, we tested the effect of OSM on Ang2-specific mRNA stability by treating HUVECs with actinomycin D. Our data (Fig. 1H) revealed that OSM did not alter mRNA stability.
OSM increases Ang2 expression in umbilical vein explants ex vivo
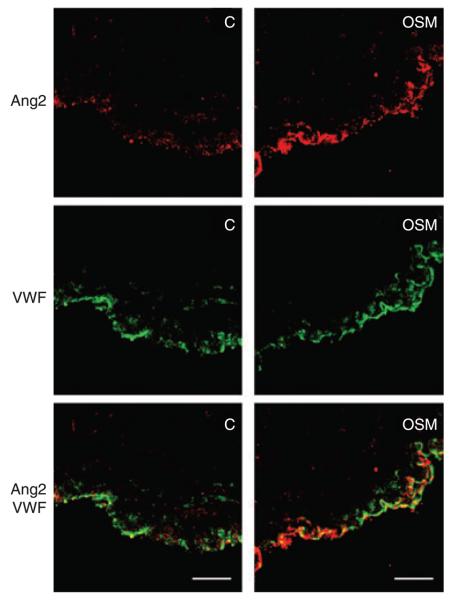
Performing immunocytochemical staining for Ang2 and VWF, we observed stronger staining for Ang2 of the endothelial layer in umbilical vein explants treated for 48 h with OSM than in control explants (Fig. 2).
Fig. 2.
Oncostatin M (OSM) induced angiopoietin 2 (Ang2) expression in umbilical vein explants. Fragments of umbilical vein explants were incubated without (C) or with OSM (100 ng mL−1) for 48 h. Immunhistochemical staining was performed for Ang2 (red) and von Willebrand factor (VWF) (green). This experiment was repeated three times with similar results. One representative experiment is shown. Scale bars: 10 μm.
OSM regulates Ang2 expression via Janus kinase (JAK)/signal transducer and activator of transcription (STAT) and mitogen-activated protein (MAP) kinase activation
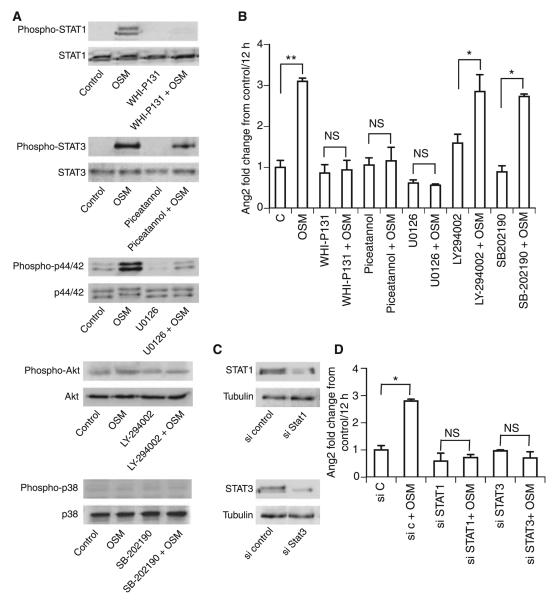
OSM downstream signaling events include activation of the JAK/STAT, p44/42 MAP kinase (extracellular signal-regulated kinase 1/2), p38 and phosphoinositol-3-kinase (PI3K)/Akt pathways [6]. Stimulation of HUVECs with OSM for 30 min resulted in the phosphorylation of STAT1, STAT3, and p44/42, whereas Akt and p38 phosphorylation could not be detected (Fig. 3A). To elucidate the pathway involved in the regulation of Ang2 in more detail, we performed blocking experiments. Figure 3A shows the blocking effect of WHIP131 (30 μg mL−1) on STAT1 phosphorylation, of piceatannol (200 μm) on STAT3 phosphorylation, and of U0126 (1 μm) on p44/42 phosphorylation. Blocking the phosphorylation of STAT1, STAT3 or p44/42 eliminated the effect of OSM on Ang2 expression (Fig. 3B), whereas blockers of the p38 (10 μm SB-202190) and PI3K/Akt (10 μm LY-294002) pathways did not inhibit OSM-induced Ang2 expression, suggesting the involvement of the JAK/STAT and MAP kinase pathways.
Fig. 3.
Oncostatin M (OSM) regulates angiopoietin 2 (Ang2) expression via activation of the Janus kinase/signal transducer and activator of transcription (STAT) and mitogen-activated protein kinase pathway. Human umbilical vein endothelial cells (HUVECs) were incubated with or without the respective blockers WHI-P131 (30 μg mL−1), piceatannol (200 μm), U0126 (1 μm), LY-294002 (10 μm), or SB-202190 (10 μm). Blockers were administered 1 h before OSM addition (100 ng mL−1). Cells were incubated for 30 min with OSM, and STAT1, STAT3, p44/42, Akt and p38 phosphorylation was determined by western blotting (A). Real-time polymerase chain reaction (PCR) for Ang2 (B) was performed, employing specific primers. Values, given as fold change from control, represent mean values ± standard deviations (SDs) of three independent determinations, and were normalized according to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels. HUVECs were transfected with small interfering (si)RNA against STAT1 or STAT3, or control siRNA. Gene knockdown after 72 h was analyzed by western blot (C). Transfected cells were stimulated for 12 h with OSM (100 ng mL−1), and real-time PCR for Ang2 (D) was performed, employing specific primers. Values, given as fold change from control, represent mean values ± SDs of three independent determinations, and were normalized according to the GAPDH levels. **P < 0.005, *P < 0.05. C, control; NS, not significant.
siRNA-mediated gene knockdown of STAT1 and STAT3 resulted in reduced expression of STAT1 and STAT3 72 h after transfection in HUVECs (Fig. 3C). No effect of OSM on Ang2 expression was observed in cells transfected with siRNA against STAT1 and STAT3 (Fig. 3D).
OSM induces Ang2 expression in HAECs and HHMECs
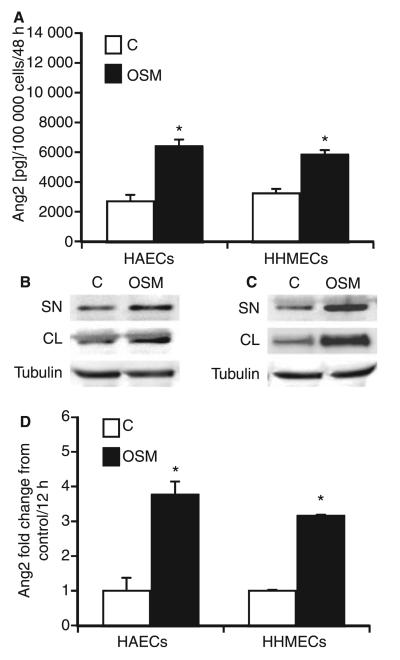
As there is evidence for heterogeneity of endothelial cells isolated from different vascular beds [26], we tested the effect of gp130 ligands on HAECs and HHMECs. We detected gp130, OSM receptor (OSMR), LIF receptor (LIFR), IL-6 receptor, IL-11 receptor and CNTF receptor mRNA expression in HAECs and HHMECs by real-time PCR (data not shown). Similar to what was found in HUVECs, OSM induced Ang2 production in HAECs and HHMECs up to 2.5-fold (Fig. 4A). None of the other gp130 ligands tested showed any effect under these conditions (data not shown). These data were confirmed by western blot analysis (Fig. 4B,C). Furthermore, we detected increased intracellular Ang2 expression in HAECs and HHMECs incubated with OSM (Fig. 4B,C). It is of note that OSM also induced Ang2 at the level of specific mRNA expression (Fig. 4D).
Fig. 4.
Oncostatin M (OSM) increases angiopoietin 2 (Ang2) expression in human aortic and human microvascular endothelial cells. Confluent monolayers of human aortic endothelial cells (HAECs) and human heart microvascular endothelial cells (HHMECs) were incubated in the absence (white bars, C) or presence (black bars, 100 ng mL−1) of OSM for 48 h. Ang2 (A) protein was determined in the supernatant by specific enzyme-linked immunosorbent assay. Values, given in pg/100 000 cells, represent mean values ± standard deviations (SDs) of three independent determinations. Each experiment was repeated at least three times. One representative experiment is shown. Ang2 expression was determined in the supernatant (SN) and cell lysate (CL) of HAECs (B) and HHMECs (C) incubated in the absence (C) or presence of OSM by western blot analysis. b-Tubulin was used as a loading control. HAECs and HHMECs were incubated in the absence (white bars, C) or presence (black bars, 100 ng mL−1) of OSM for 12 h. Real-time polymerase chain reaction for Ang2 (D) was performed, employing specific primers. Values, given as fold change from control, represent mean values ± SDs of three independent determinations, and were normalized according to the glyceraldehyde-3-phosphate dehydrogenase levels. Each experiment was repeated at least three times. One representative experiment is shown. *P < 0.005.
Effect of OSM on the expression of gp130 and OSMR
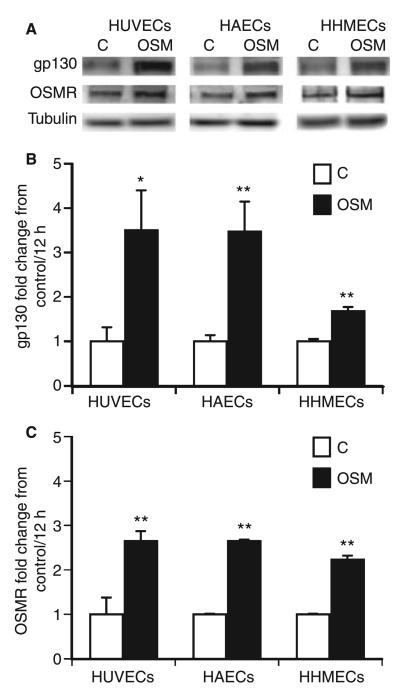
In humans, OSM signal transduction occurs via receptor complexes composed of LIFR or OSMR and gp130 [27]. We tested the effects of OSM on the receptors gp130, OSMR and LIFR in HUVECs, HAECs, and HHMECs. OSM induced gp130 and OSMR at the protein level (Fig. 5A) and mRNA level (Fig. 5B,C) in all cell types, whereas LIFR expression was not altered (data not shown).
Fig. 5.
Oncostatin M (OSM) induces the expression of glycoprotein 130 (gp130) and OSM receptor (OSMR). Human umbilical vein endothelial cells (HUVECs), human aortic endothelial cells (HAECs) and human heart microvascular endothelial cells (HHMECs) were incubated in the absence (C) or presence of OSM (100 ng mL−1) for 24 h, cells were lysed, and gp130 and OSMR expression were determined by western blot. β-Tubulin was used as loading control (A). Each experiment was repeated at least three times. One representative experiment is shown. HUVECs, HAECs and HHMECs were stimulated in the absence (C) or presence of OSM (100 ng mL−1) for 12 h, and real-time PCR for gp130 (B) and OSMR (C) was performed, employing specific primers. Values, given as fold change from control, represent mean values ± standard deviations of three independent determinations, and were normalized according to the glyceraldehyde-3-phosphate dehydrogenase levels. **P < 0.005, *P < 0.05.
OSM increases the expression of Ang2, gp130 and OSMR in heart tissue in mice in vivo
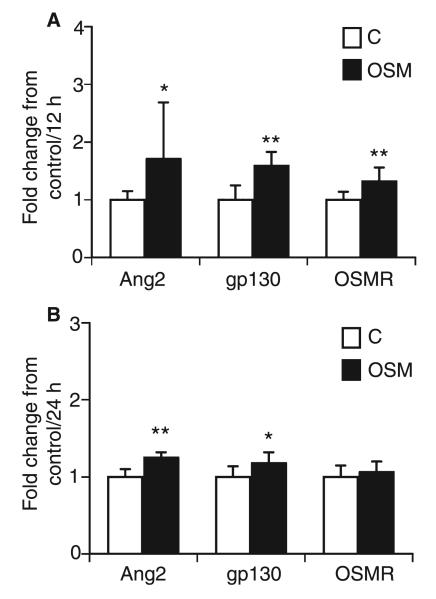
To test the effects of OSM on Ang2 and the OSM receptors in vivo, we injected OSM intraperitoneally into C57/Bl6 mice. The animals were killed 12 or 24 h after injection. Real-time PCR analysis revealed significant increases in the levels of mRNA specific for Ang2, gp130, and OSMR, 12 h after injection, in heart tissue of the left ventricles of OSM-injected mice as compared with control animals (Fig. 6A), and increased Ang2 and gp130 mRNA levels 24 h after injection (Fig. 6B).
Fig. 6.
Effect of oncostatin M (OSM) on angiopoietin 2 (Ang2), glycoprotein 130 (gp130) and OSM receptor (OSMR) expression in mice in vivo. Mice (n = 9 in each group) were injected with vehicle (200 μL of 0.9% NaCl; white bars, C) or recombinant murine OSM (100 ng per 200 μL of 0.9% NaCl; black bars, OSM) intraperitoneally, and killed after 12 h (A) and 24 h (B), respectively. mRNA from heart tissue of the left ventricle was extracted, and real-time polymerase chain reaction was performed for Ang2, gp130, and OSMR. Values, given as fold change from control, represent mean values ± standard deviations, and were normalized according to the glyceraldehyde-3-phosphate dehydrogenase levels. *P < 0.05, **P < 0.005.
Correlation of OSM, Ang2, gp130 and OSMR expression in human heart tissue
To determine whether OSM expression is linked to Ang2, gp130 and OSMR expression in human cardiac tissue in vivo, we analyzed the levels of mRNA specific for OSM, Ang2, gp130 and OSMR in the left ventricles of explanted failing human hearts from 13 donors undergoing heart transplantation. OSM mRNA levels correlated significantly with Ang2 (Pearson correlation coefficient, R2 = 0.768; P = 0.002), gp130 (R2 = 0.700; P = 0.008) and OSMR (R2 = 0.760; P = 0.003) expression.
Discussion
The role of the gp130 ligand system in angiogenesis is emphasized by different studies demonstrating effects of gp130 ligands on angiogenesis in vitro and in vivo [28,29]. In particular, OSM has been shown to stimulate expression of the major angiogenic factor VEGF in different cell types and tissues [9-11]. Here, we provide evidence for a link between the gp130 ligand system and yet another system of biomolecules involved in the regulation of angiogenesis, namely the Ang–Tie system. We have shown that, of all the gp130 ligands tested, only OSM increases Ang2 expression in HUVECs in a concentration-dependent and time-dependent way, via the JAK/STAT and MAP kinase pathway. Blocking of either STAT1 or STAT3 alone completely abolished the effect of OSM on Ang2 expression, in line with previously reported findings [30,31]. It is thought that the formation of STAT1–STAT3 heterodimers could be responsible for these effects. Furthermore, our experiments with cycloheximide and actinomycin D suggest that OSM directly increased the levels of mRNA specific for Ang2 without affecting its stability.
It should be noted that, in a recent study, OSM stimulated angiogenesis but did not induce Ang2 expression in rheumatoid synovial tissue cultured alone or in combination with synovial fibroblasts [32]. This difference from our findings could result from cell-specific patterns of response to OSM and/or from the particular microenvironment of the synovial tissue.
Furthermore, we also observed an increase in Ang2 expression induced by OSM in macrovascular human endothelial cells isolated from the aorta and microvascular endothelial cells isolated from human heart tissue. Our in vitro data are supported by the findings that Ang2 expression is increased in endothelial cells in tissue cultures of pieces of human umbilical veins treated ex vivo with OSM. Our in vivo data, showing an increase in Ang2 mRNA in hearts of mice injected with OSM and a strong correlation between OSM and Ang2 mRNA levels in explanted human hearts, emphasize a possible impact of OSM on Ang2 expression in the heart in vivo. With respect to our experiments in mice, it should be noted, however, that the increase in Ang2 mRNA in the hearts of mice 12 and 24 h after OSM injection was smaller than the increase seen in the in vitro experiments. This could be because 100 ng of OSM was injected into each mouse and this amount was diluted in, and probably also partially cleared, from the body fluids before it reached the heart. Thus, the actual concentration of OSM in the hearts of the mice might have been much lower than the concentrations of OSM that were shown to give maximal induction of Ang2 in the in vitro experiments. In agreement with our data, the expression of OSM in the human heart has recently been shown in explanted hearts from patient with end-stage heart failure and in samples of inflamed aorta [28,29]. Involvement of the gp130 ligand system in maintaining cardiac function has been suggested by studies showing that cardiac deletion of gp130 results in severe cardiac defects [33].
Increased Ang2 expression was seen in the infarct region of the myocardium in a rat model of myocardial infarction [34] and in the plasma of patients suffering from myocardial infarction or congestive heart failure [35,36]. Cardiovascular diseases such as heart failure or myocardial infarction are strongly associated with inflammation. Given our results showing an OSM-induced increase in Ang2 expression in microvascular endothelial cells of the human heart and in heart tissue of mice, and a significant correlation between Ang2 and OSM levels in human failing hearts, one could speculate that OSM might also contribute to increased Ang2 levels in response to such cardiovascular pathologies as described above [35,36].
As shown in Doc. S1, in contrast to its effects on Ang2, OSM decreased Ang1 expression not only in macrovascular endothelial cells, but also in perivascular cells such as smooth muscle cells, which are thought to be the main sources of Ang1 [14,37]. Thus, we provide evidence for antagonistic regulation of Ang1 and Ang2 in vascular cells.
Furthermore, we have described a positive autocrine feedback loop in which OSM increases the expression of its own receptors, OSMR and gp130, in human endothelial cells. It should be noted that such an effect of OSM has also been described for fibroblasts and epithelial cells [38,39]. In addition, we show that OSM increases gp130 and OSMR expression in mice in vivo, and that OSM levels correlate with gp130 and OSMR expression in human heart tissue. Such increased receptor expression could, in turn, lead to enhanced responsiveness of the affected cells to the receptor ligand OSM.
Taken together, our findings provide evidence that the inflammatory cytokine OSM is involved in modulation of the Ang–Tie system by increasing Ang2 expression in human endothelial cells in vitro, and in murine hearts and human hearts in vivo. As OSM has been shown to induce VEGF expression in various cells and tissues, and as Ang2 only acts in concert with VEGF to induce endothelial cell migration and proliferation, two key events in the formation of new blood vessels, one could speculate that these effects might contribute considerably to the angiogenic properties of OSM [19].
Supplementary Material
Acknowledgements
This work was supported by the Austrian Science Fund (FWF JRP ‘Angiogenesis in Disease’ S9409-B11) and by the Association for the Promotion of Scientific Research in Arteriosclerosis, Thrombosis and Vascular Biology.
Footnotes
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Doc. S1. Supporting materials.
Fig. S1. OncostatinMdecreases Ang1 expression inHUVEC.
Table. S1. Primers used for real-time polymerase chain reaction.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 2.Autiero M, Luttun A, Tjwa M, Carmeliet P. Placental growth factor and its receptor, vascular endothelial growth factor receptor-1: novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J Thromb Haemost. 2003;1:1356–70. doi: 10.1046/j.1538-7836.2003.00263.x. [DOI] [PubMed] [Google Scholar]
- 3.Costa C, Incio J, Soares R. Angiogenesis and chronic inflammation: cause or consequence? Angiogenesis. 2007;10:149–66. doi: 10.1007/s10456-007-9074-0. [DOI] [PubMed] [Google Scholar]
- 4.Vasse M, Pourtau J, Trochon V, Muraine M, Vannier JP, Lu H, Soria J, Soria C. Oncostatin M induces angiogenesis in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1999;19:1835–42. doi: 10.1161/01.atv.19.8.1835. [DOI] [PubMed] [Google Scholar]
- 5.Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271:736–41. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- 6.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang SP, Wu MS, Shun CT, Wang HP, Lin MT, Kuo ML, Lin JT. Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. J Biomed Sci. 2004;11:517–27. doi: 10.1007/BF02256101. [DOI] [PubMed] [Google Scholar]
- 8.Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB, Lee CN, Hsieh CY. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22:1517–27. doi: 10.1038/sj.onc.1206226. [DOI] [PubMed] [Google Scholar]
- 9.Weiss TW, Speidl WS, Kaun C, Rega G, Springer C, Macfelda K, Losert UM, Grant SL, Marro ML, Rhodes AD, Fuernkranz A, Bialy J, Ullrich R, Holzmann P, Pacher R, Maurer G, Huber K, Wojta J. Glycoprotein 130 ligand oncostatin-M induces expression of vascular endothelial growth factor in human adult cardiac myocytes. Cardiovasc Res. 2003;59:628–38. doi: 10.1016/s0008-6363(03)00463-2. [DOI] [PubMed] [Google Scholar]
- 10.Repovic P, Fears CY, Gladson CL, Benveniste EN. Oncostatin-M induction of vascular endothelial growth factor expression in astroglioma cells. Oncogene. 2003;22:8117–24. doi: 10.1038/sj.onc.1206922. [DOI] [PubMed] [Google Scholar]
- 11.Faffe DS, Flynt L, Mellema M, Whitehead TR, Bourgeois K, Panettieri RA, Jr, Silverman ES, Shore SA. Oncostatin M causes VEGF release from human airway smooth muscle: synergy with IL-1beta. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1040–8. doi: 10.1152/ajplung.00333.2004. [DOI] [PubMed] [Google Scholar]
- 12.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 13.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–8. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 14.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–9. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 15.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 16.Brindle NP, Saharinen P, Alitalo K. Signaling and functions of angiopoietin-1 in vascular protection. Circ Res. 2006;98:1014–23. doi: 10.1161/01.RES.0000218275.54089.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eklund L, Olsen BR. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp Cell Res. 2006;312:630–41. doi: 10.1016/j.yexcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103:4150–6. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- 19.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci USA. 2002;99:11205–10. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006;27:552–8. doi: 10.1016/j.it.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Tal Cohen E, Hohensinner PJ, Kaun C, Maurer G, Huber K, Wojta J. Statins decrease TNF-alpha-induced osteoprotegerin production by endothelial cells and smooth muscle cells in vitro. Biochem Pharmacol. 2007;73:77–83. doi: 10.1016/j.bcp.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Wojta J, Zoellner H, Gallicchio M, Hamilton JA, McGrath K. Gamma-interferon counteracts interleukin-1 alpha stimulated expression of urokinase-type plasminogen activator in human endothelial cells in vitro. Biochem Biophys Res Commun. 1992;188:463–9. doi: 10.1016/0006-291x(92)92407-o. [DOI] [PubMed] [Google Scholar]
- 23.al-Azhary DB, Wojta J, Binder BR. Fibrinolytic system of cultured rabbit aortic endothelial cells. Thromb Res. 1994;75:559–68. doi: 10.1016/0049-3848(94)90230-5. [DOI] [PubMed] [Google Scholar]
- 24.Wojta J, Zoellner H, Gallicchio M, Filonzi EL, Hamilton JA, McGrath K. Interferon-alpha 2 counteracts interleukin-1 alpha-stimulated expression of urokinase-type plasminogen activator in human foreskin microvascular endothelial cells in vitro. Lymphokine Cytokine Res. 1994;13:133–8. [PubMed] [Google Scholar]
- 25.Demyanets S, Kaun C, Rychli K, Rega G, Pfaffenberger S, Afonyushkin T, Bochkov VN, Maurer G, Huber K, Wojta J. The inflammatory cytokine oncostatin M induces PAI-1 in human vascular smooth muscle cells in vitro via PI 3-kinase and ERK1/2-dependent pathways. Am J Physiol Heart Circ Physiol. 2007;293:H1962–8. doi: 10.1152/ajpheart.01366.2006. [DOI] [PubMed] [Google Scholar]
- 26.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100:174–90. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 27.Gomez-Lechon MJ. Oncostatin M: signal transduction and biological activity. Life Sci. 1999;65:2019–30. doi: 10.1016/s0024-3205(99)00296-9. [DOI] [PubMed] [Google Scholar]
- 28.Eiken HG, Oie E, Damas JK, Yndestad A, Bjerkeli V, Aass H, Simonsen S, Geiran OR, Tonnessen T, Christensen G, Froland SS, Gullestad L, Attramadal H, Aukrust P. Myocardial gene expression of leukaemia inhibitory factor, interleukin-6 and glycoprotein 130 in end-stage human heart failure. Eur J Clin Invest. 2001;31:389–97. doi: 10.1046/j.1365-2362.2001.00795.x. [DOI] [PubMed] [Google Scholar]
- 29.Modur V, Feldhaus MJ, Weyrich AS, Jicha DL, Prescott SM, Zimmerman GA, McIntyre TM. Oncostatin M is a proinflammatory mediator. In vivo effects correlate with endothelial cell expression of inflammatory cytokines and adhesion molecules. J Clin Invest. 1997;100:158–68. doi: 10.1172/JCI119508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Liu W, Wang J, Wang X, Yu Z. STAT1 and STAT3 mediate thrombin-induced expression of TIMP-1 in human glomerular mesangial cells. Kidney Int. 2002;61:1377–82. doi: 10.1046/j.1523-1755.2002.00283.x. [DOI] [PubMed] [Google Scholar]
- 31.Lin SK, Kok SH, Yeh FT, Kuo MY, Lin CC, Wang CC, Goldring SR, Hong CY. MEK/ERK and signal transducer and activator of transcription signaling pathways modulate oncostatin M-stimulated CCL2 expression in human osteoblasts through a common transcription factor. Arthritis Rheum. 2004;50:785–93. doi: 10.1002/art.20058. [DOI] [PubMed] [Google Scholar]
- 32.Fearon U, Mullan R, Markham T, Connolly M, Sullivan S, Poole AR, FitzGerald O, Bresnihan B, Veale DJ. Oncostatin M induces angiogenesis and cartilage degradation in rheumatoid arthritis synovial tissue and human cartilage cocultures. Arthritis Rheum. 2006;54:3152–62. doi: 10.1002/art.22161. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida K, Taga T, Saito M, Suematsu S, Kumanogoh A, Tanaka T, Fujiwara H, Hirata M, Yamagami T, Nakahata T, Hirabayashi T, Yoneda Y, Tanaka K, Wang WZ, Mori C, Shiota K, Yoshida N, Kishimoto T. Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc Natl Acad Sci USA. 1996;93:407–11. doi: 10.1073/pnas.93.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandhu R, Teichert-Kuliszewska K, Nag S, Proteau G, Robb MJ, Campbell AI, Kuliszewski MA, Kutryk MJ, Stewart DJ. Reciprocal regulation of angiopoietin-1 and angiopoietin-2 following myocardial infarction in the rat. Cardiovasc Res. 2004;64:115–24. doi: 10.1016/j.cardiores.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 35.Chong AY, Caine GJ, Freestone B, Blann AD, Lip GY. Plasma angiopoietin-1, angiopoietin-2, and angiopoietin receptor tie-2 levels in congestive heart failure. J Am Coll Cardiol. 2004;43:423–8. doi: 10.1016/j.jacc.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 36.Lee KW, Lip GY, Blann AD. Plasma angiopoietin-1, angiopoietin-2, angiopoietin receptor tie-2, and vascular endothelial growth factor levels in acute coronary syndromes. Circulation. 2004;110:2355–60. doi: 10.1161/01.CIR.0000138112.90641.7F. [DOI] [PubMed] [Google Scholar]
- 37.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–80. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 38.Blanchard F, Wang Y, Kinzie E, Duplomb L, Godard A, Baumann H. Oncostatin M regulates the synthesis and turnover of gp130, leukemia inhibitory factor receptor alpha, and oncostatin M receptor beta by distinct mechanisms. J Biol Chem. 2001;276:47038–45. doi: 10.1074/jbc.M107971200. [DOI] [PubMed] [Google Scholar]
- 39.Mahboubi K, Karras J, Pober JS. Desensitization of signaling by oncostatin M in human vascular cells involves cytoplasmic Tyr residue 759 in gp130 but is not mediated by either Src homology 2 domain-containing tyrosine phosphatase 2 or suppressor of cytokine signaling 3. J Biol Chem. 2003;278:25014–23. doi: 10.1074/jbc.M211867200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.