Abstract
Driven by remarkable advances in the understanding of structure and reaction mechanisms, organic synthesis will be increasingly directed to producing bioinspired and newly designed molecules.
Molecular evolution on Earth over the past 3.8 billion years has produced an extraordinary library of chemical structures, unsurpassed in number, diversity and function. Each structure is a treasure-trove of information and inspiration, a molecular textbook encoded in the language of chemistry. Collectively, these molecules comprise the chemical genome of our planet, and represent a universe ripe for exploration. With modern analytical tools, each of these structural tomes can now be read, enabling an understanding of how structure relates to function. More significantly, we can now use organic synthesis not only to make copies of these molecules, but also to prepare bioinspired or designed compounds, some with functions unheard of in the natural world — compounds that will influence, if not shape, every facet of our existence.
Our emerging molecular literacy is creating a revolution that will transform our world. The ability to design, create and control molecules has opened a vast frontier of research and an age of unprecedented opportunity. Scientists from every background are being drawn to this molecular frontier, creating a melting pot of disciplinary fusions and the resultant ability to address problems that transcend the boundaries of individual fields. From molecular biology to molecular computing, molecular medicine, molecular (nano) technology and even molecular gastronomy, science is becoming increasingly integrated and ‘molecularized’.
Chemists have been laying the foundations for this molecular revolution for the past two centuries. Before that, nature’s archive was the sole or primary source of chemicals used by humans. This has now changed. Through extraordinarily innovative advances in tools, theories and methods, synthesis has provided a reliable supply of many natural compounds as well as others created by design. Indeed the question of whether a molecule from nature could be made is increasingly giving way to whether it could be made in a way that impacts on supply and science. Of increasing importance now is the question of what molecules to make. Naturally occurring molecules are produced in their ecosystems for uses other than what we seek or need. Their activities in humans are thus serendipitous and unoptimized but provide a rich source of information and inspiration. We are now on the cusp of a period in which we can use this inspiration to design molecules with superior or new functions and make them in increasingly efficient, practical and environmentally friendly ways1–11.
Interpreting the chemical genome
In 1861, the best that Alexander Butlerow could do to characterize the reaction product of aqueous formaldehyde and calcium hydroxide was to describe it as a golden liquid that tastes of liquorice. Today, molecular spectroscopy and microscopy allow us to detect the structural features of molecules, and even to observe their dynamic behaviour. To those schooled in the static representations of molecules in books, the movie of a single molecule moving in the barrel of a nanotube12 inspires awe, along with a plethora of previously unimaginable ideas that span disciplinary boundaries.
Nuclear magnetic resonance (NMR) spectroscopy and single-crystal X-ray analysis have proven to be of particular importance to organic chemists historically, and are keys to the increasing ability to make known as well as designed functional molecules. In bygone times when spectroscopic methods for structural characterization were limited, natural products were targeted for synthesis — at least in part — because structural validation of the product could be made by comparing it to a natural sample. Making a non-natural, designed molecule 50 years ago would thus have carried the additional burden of establishing its structure. Analytical tools and techniques have now reduced or eliminated this problem for most molecules, thereby opening the door to molecular design.
Lessons from natural products
Nature’s molecular archive encodes the evolutionary record of millions of species and of innumerable chemical entities: compounds that are part of complex biochemical systems involved in all aspects of life, reproduction and death. Some ants, for example, communicate through the use of hydrocarbons. The exchange of these chemical messages through antennal contact determines whether a recipient will harvest, forage or ‘work at home’ on a given day. In other organisms, chemical systems provide a sophisticated defence against predation. Bugula neritina, a marine bryozoan found along the Californian and other coasts, is proposed to be in a symbiotic partnership with a ‘guest’ bacterium in its gut. Together, they produce an agent known as bryostatin that is thought to serve as an antifeedant, protecting the bryozoan’s larvae from predators (Fig. 1a)13.
Figure 1. Structural and functional inspirations from natural products.
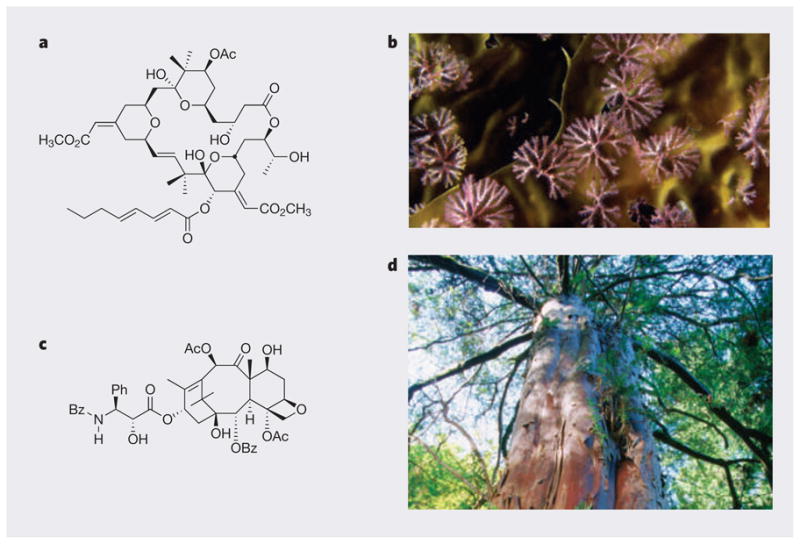
Bryostatin 1 (a) is putatively an antifeedant compound produced in the marine bryozoan Bugula neritina (b). This ornate natural product also has useful anticancer activity. Taxol (c) was isolated from the bark of the Pacific yew (d). It interferes with cytoskeletal dynamics to arrest the cell cycle and is used for the treatment of breast and ovarian cancers. Ac, acetyl; Bz, benzoyl; Ph, phenyl.
Remarkable as is bryostatin’s natural role, the molecule also has other lessons for us, serving as a new lead for treating cancer14 and, more recently, as an agent that facilitates learning and extends memory in animals15. Similarly, Taxol (paclitaxel; a compound found in the bark of the Pacific yew tree) arrests the cell cycle by interfering with cytoskeletal dynamics, thus teaching us much about cell proliferation and providing the collateral benefit of a highly effective anticancer drug (Fig. 1c)16. Indeed, many of the most important therapeutic agents introduced in the past 50 years are, or were inspired by, natural products17. More are surely to follow. Prostratin, for example, another plant-derived compound, is known to flush latent HIV from cells. Used in combination with antiretroviral therapy, this agent offers a promising strategy to eradicate HIV/AIDS18,19.
In addition to therapeutic uses, many naturally occurring compounds have been the source of inspiration for the design of materials, diagnostics and imaging agents. Green fluorescent protein, for example, a molecule found in jellyfish, is now widely used for cellular imaging (see http://tinyurl.com/npe3r2) and the proteins and processes involved in photosynthesis have provided inspiration for solar collection devices20, optical storage systems21 and retinal prosthetics22. Clearly, we have much to learn from nature’s molecular library.
Much like the exploration of our cosmic frontier, exploration of the molecular frontier has created vast new bodies of information. But a major difference between the two frontiers exists: not only can we observe the molecules of nature, we can also make them and, more significantly, even design and create new ones. Molecules that once were available only in trace amounts from natural sources can now be prepared and sometimes manufactured in quantity through total synthesis, semi-synthesis (Box 1), biosynthesis (using enzymes or even whole organisms) and combinations thereof. Synthesis in this way plays the metaphorical role of the printing press, creating copies of compounds that are otherwise scarce or found only in unreliable, inaccessible, or ecologically fragile natural sources. Complementing this capability, synthesis can also produce new molecules with improved or new functions, thereby expanding our frontier.
Box 1. Concepts in synthesis.
Total synthesis
The production of a molecule from commercially or readily available chemicals through a series of chemical reactions.
Semi-synthesis
The production of a molecule from a naturally derived starting material through a series of chemical reactions.
Convergent synthesis
A total synthesis in which two (or more) molecular fragments are synthesized independently, then joined together.
Linear synthesis
A total synthesis proceeding stepwise from commercially available starting materials, without the introduction of independently synthesized molecular fragments.
Ideal synthesis
The production of a target molecule in a single synthetic operation from readily available starting materials, in 100% yield and without side-product formation. The synthesis should be simple, safe, economically acceptable and environmentally friendly.
Target-relevant complexity
Bonds and stereochemical configurations that are retained in the final synthetic target.
Diversity-oriented synthesis
The production of structurally diverse libraries of molecules.
P.A.W & B.L.M.
The ability to replicate nature’s molecules has profound consequences. Not only can it be used to supplement supply, but the step-by-step synthesis of natural compounds also allows us to better understand how the parts of a molecule influence the properties and function of the whole — a form of reverse molecular engineering and thus inspiration on how it might be modified to create new medicines, materials, devices or probes. Throughout, the mantra of chemistry is repeated: structure, function, design and synthesis. Knowledge of structure leads to an understanding of function that informs design, leading to the synthesis of new structures with improved or totally new functions.
Bioinspired reaction science
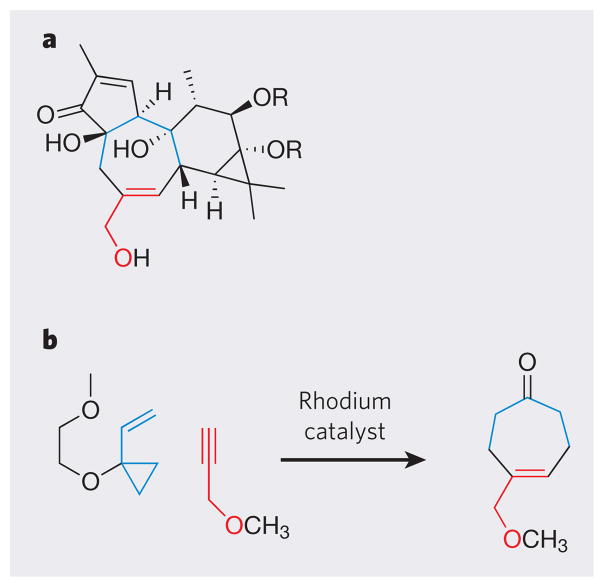
Nature’s cornucopia of diverse molecular structures has also served another profoundly important purpose: providing synthetic targets that inspire and often require the development of new reactions, methods, catalysts and strategies. Synthetic methods have evolved impressively over the past half century in response to this challenge. For example, a consideration of syntheses of the phorbol esters — remarkably active tumour promoters used in models of carcinogenesis — led to the design of a new metal-catalysed reaction for the synthesis of seven-membered rings (Fig. 2)23. A consideration of how nature introduces chemical groups into molecules has led to the design of dazzling ‘C–H activation’ strategies, in which ordinarily inert carbon–hydrogen bonds take part in reactions24–26.
Figure 2. The development of new reactions for step-economical total synthesis.
Examination of the structures of phorbol esters (a) — remarkably active tumour promoters used in studies of carcinogenesis — inspired the development of a new rhodium-catalysed reaction for the synthesis of seven-membered rings (b).
Whereas 50 years ago, literature reports of total syntheses of natural products were rare, such reports now appear daily, as a result of the many advances in reaction science. Numerous compounds, once considered to be beyond the reach of synthesis, are now produced synthetically in research or commercial quantities — even genomes are within reach of synthesis, in its broadest sense. This is both a testimonial to the impressive progress of the field, and a signal that it is time to take on the next level of challenges.
Not unlike the evolution of X-ray crystallography from studies on simple salts (such as sodium chloride) to studies on biomacromolecular complexes and even their dynamics, synthesis is poised to address a new range of problems, societal needs and scientific opportunities. Many — some would argue most — natural products can now be synthesized if suitable resources are provided. The challenge in synthesis is therefore increasingly not whether a molecule can be made, but whether it can be made in a practical fashion, in sufficient quantities for the needs of research and/or society, and in a way that is environmentally friendly if not ‘ideal’ (Box 1). Such syntheses require reactions that are more selective (minimizing the number of side-products) and efficient (providing good yields) than many of those currently available. But there are many more considerations.
Although selectivity and efficiency are important, their beneficial effects can be significantly compromised, or even offset, in syntheses that are too long to be commercially viable. For example, a synthesis of 70 steps, even if perfectly efficient (100% overall yield) and selective (no side-products), would be too expensive for most applications. This is because each step requires its own starting materials, reagents, solvents and energy costs, and produces a waste stream that requires disposal. More significantly, the biggest costs are in manpower: each step must be developed and executed by individuals skilled in synthesis. In contrast, shorter syntheses generally offer better economies, lower environmental impact, easier access to valuable synthetic targets and conservation of our most precious resource — those few skilled in the science and practice of synthesis. To appreciate the importance of ‘step-economical’ synthesis one need only reflect on how the one-step synthesis of carbon fullerenes (some of which are familiarly known as ‘buckyballs’) and nanotubes has enabled research around the globe in many different fields and has contributed to the emergence of nanotechnology.
The idea of ‘step economy’ is not new (although the term itself was coined recently)27, but it is a singularly important issue in considering the current state and future of synthesis. A classic — and still spectacular — example of step economy is provided by a pair of syntheses of the alkaloid tropinone, a key intermediate in the production of atropine (a drug with many therapeutic applications). Tropinone was first synthesized in 1901 by Richard Wilstätter, in what was regarded at the time as a tour de force of organic chemistry. Even so, the synthesis required about 20 steps and had an overall yield of 0.75%. The compound was subsequently synthesized in one step in much better yield (17%) by Robert Robinson28, who devised a multi-reactant, multi-bond-forming process inspired by biosynthetic reactions. Such dramatic improvements in syntheses are uncommon, but they call attention to the unique impact of new reactions, and the strategies they enable, on step economy. New reactions change the very way chemists think about a synthesis, and their views of what is possible. They create transformative rather than incremental change. The olefin metathesis reaction, in which two carbon–carbon bonds are broken and two new ones formed to create new products, is a recent example (see http://tinyurl.com/mkxmo6).
Towards the ideal synthesis
The step count of a synthesis is a function of which reactions are used and the sequence in which they are performed. Syntheses based on reactions that provide only small increases in target-relevant complexity (TRC; Box 1) generally require more steps than those based on reactions providing greater increases in TRC. The literature is replete with examples of this, as several different research groups succeed in synthesizing a given natural product, but with greatly differing step counts29.
As most reactions involve the formation of only one or two bonds, one might conclude that syntheses of very complex compounds would invariably require many reactions and therefore many steps. There are, however, two ways around this apparent problem. The first entails performing a series of reactions in one vessel so that, as the products of each reaction form, they immediately go on to take part in the next reaction of the sequence, ultimately ending up in a final isolatable product. Because the products of each individual reaction are not isolated, the sequence constitutes a single synthetic operation in which many more than two bonds are formed and much time is saved, as only a single set-up, reaction and work-up is required. The second approach to step economy is to invent new reactions that generate greater increases in TRC per step. Both strategies shorten the journey from simple commercial materials to complex targets, with the ultimate goal being to arrive at an ideal synthesis (Box 1). Both save time, while minimizing cost and especially solvent use — solvents are usually removed and discarded at each step of a synthesis, and so are the greatest source of waste in most syntheses.
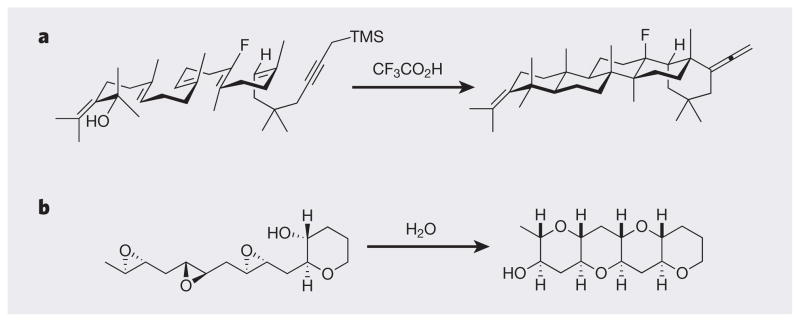
Such single-operation, multiple-step processes — described variously as serial, cascade, chain, tandem, regenerative or domino reactions — are an increasingly important focus of many research groups30, and are certainly having an impact on the ability of synthesis to produce complex targets in a step-economical fashion. This strategy draws inspiration, if not validation, from biosynthetic pathways that often proceed through such serial processes. For example, the bio-synthetic route to terpenes and steroids has been mimicked with great success through the efforts of Bill Johnson and others (Fig. 3a)31. Johnson’s synthesis features a biomimetic complexity-building step in which a carbocation intermediate is generated; this is used to make a bond in a way that generates a second carbocation, and the cycle is repeated until the process is terminated. This is a wonderful example of intermediate recycling (regeneration). A related process (Fig. 3b)32 that builds on a long-standing biosynthetic hypothesis allows access to complex polyether molecules (found in many marine natural products). Similar cascades have been reported that involve other reactive intermediates, including anions, carbenes3 and radicals33. Even cross-over processes, in which one reactive intermediate produces a second, different kind of intermediate34, are emerging as innovative features of step-economical syntheses.
Figure 3. The use of cascade reactions to rapidly generate complexity and value.
a, A biomimetic complexity-building process involving carbocation intermediates (not shown) provides a powerful strategy for terpene and steroid synthesis. b, A related process that can be used to access complex polyethers via an epoxide-opening cascade initiated by water.
The chain reactions used to make polymers and oligomers from monomers are also obvious examples of successful cascade processes that involve the regeneration of reactive intermediates. An initiator I reacts with a monomer A to produce an intermediate I–A, which reacts with another monomer to produce I–A–A and on to the final I–[A–A]n product. Although promising35, this approach is not yet applicable to a broad range of targets, because of the still unsolved challenges of sequencing the reaction of different monomers (A, B, C) to make, say, I–A–B–C rather than I–A–C–B. Nature choreographs such feats through compartmentalization, proximity and templating processes. Mimicking these processes in the lab represents a priority research opportunity for chemists.
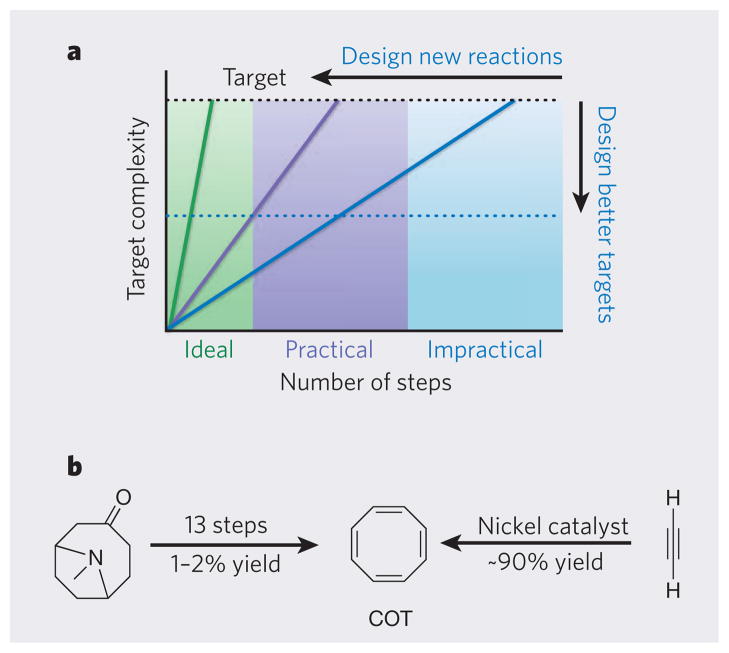
So what of the second approach for achieving greater step economy: the design or discovery of new reactions that greatly increase TRC? As we have seen, such reactions offer tremendous opportunities to move syntheses towards the ideal (Fig. 4a). A historical example is the synthesis of cyclooctatetraene, a relatively simple-looking compound of significant theoretical interest, which originally required more than 10 steps to produce in a 1–2 % overall yield (Fig. 4b). Although the efficiency and selectivity of this synthesis could no doubt be optimized further, the introduction of a new reaction — a nickel-catalysed process in which four acetylene (H–C≡C–H) molecules react to form four bonds in one step — allowed cyclooctatetraene to be made in one step, in more than 90% yield36. One of the more striking examples yet of a complexity-generating reaction is the arene–alkene metaphotocycloaddition, in which three new bonds and up to six chiral carbon centres are formed, an exceptional increase in complexity. This reaction has greatly shortened the syntheses of numerous natural products, underscoring the profound impact that new complexity-generating reactions can have on step economy37.
Figure 4. Developing ideal syntheses.
a, In an ideal synthesis (green line), a single step converts simple reactants into a structurally complex product. The syntheses of complex natural products often require too many steps (blue line), rendering them impractical for making large quantities of product. Reactions that generate a large increase in structural complexity per step allow shorter, more practical syntheses to be developed (purple line). For natural products that have valuable functional properties, another way to achieve step economy is to target structurally simpler compounds (blue dotted line) that have the same (or better) biological properties than the natural product. b, The first published synthesis of cyclooctatetraene (COT) required 13 steps, and the overall yield was very low. The discovery of a nickel-catalysed reaction allowed COT to be made in 90% yield in one step.
Despite impressive progress in the field of synthesis, we are currently awash with complex, potentially useful natural products that cannot be made in the quantities required for scientific research or therapeutic use. In some cases, enzymes, cells, or even whole organisms can be used to produce such compounds, simple analogues of them, or precursor materials easily converted to the compound of interest38. This approach benefits from nature’s refined pathways, but the specific nature of biosynthetic machinery means that there are limits to the range of structures it can prepare. For fields such as drug discovery, this limitation suggests that, in addition to natural products and natural-product-like compounds available through biosynthesis, one must embrace a broader range of structural (and synthetic) possibilities. New molecular scaffolds are needed if we are to truly exploit the potential of organic synthesis.
Some parts are greater than the whole
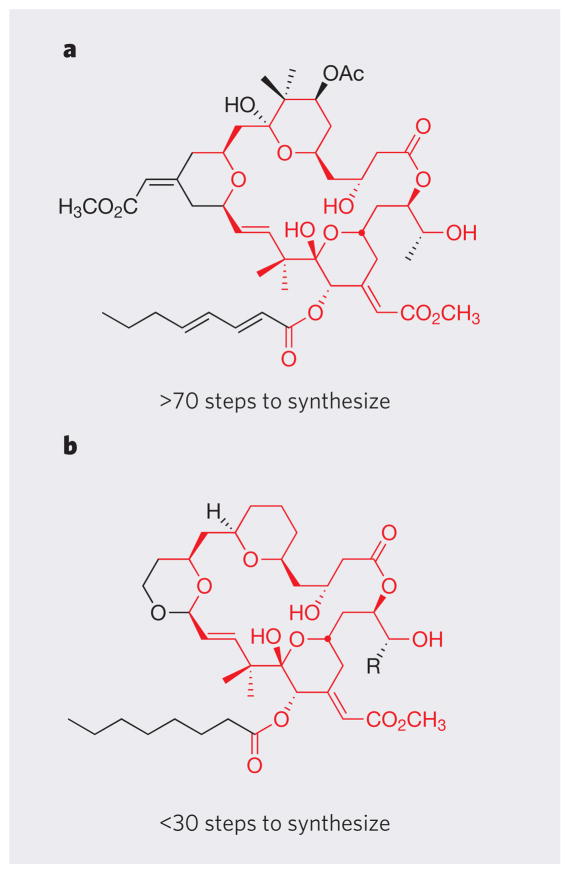
It is now recognized that, in many cases, only certain parts of naturally occurring molecules are needed for functional activity27. This expands the focus and challenge of synthesis from making a known, complex structure, to designing a simplified but functionally superior target that might be more readily prepared. Bryostatin, for example, has been tested in clinical trials as an anticancer agent and is a promising candidate for drug-discovery programmes targeting cognitive dysfunction and Alzheimer’s disease. But realization of the full clinical potential of bryostatin has been hampered by its limited supply: natural sources have very low levels of bryostatin, and total syntheses of the clinically relevant, potent bryostatins are at present too long to be a source of supply (exceeding 70 steps)39.
Although practical access to bryostatin could eventually be addressed by optimizing and innovating around the existing synthetic approaches, it is important to remember that bryostatin, like most of nature’s compounds, is neither produced nor optimized for human use. The design of structurally simpler but functionally superior analogues — an approach dubbed ‘function-oriented synthesis’27 — could be faster than optimizing syntheses of intricate natural products. Significantly, function-oriented synthesis also offers a way to tune, if not totally redesign, promising natural materials for optimal function.
In 1986, we analysed the structural features that contribute to bryostatin’s unique biological activity, and used this analysis to design simplified bryostatin analogues that are easier to make than the natural product (Fig. 5). In cell and animal assays, many of these analogues performed as well as (and often better than) bryostatin in binding to cellular protein targets and in arresting the growth of cancer cells14; a second generation of compounds performed even better40. Crucially, these analogues can be prepared in a convergent synthesis (Box 1) that requires fewer than 30 steps, more than 45 steps fewer than required to make the clinically relevant natural product. This step-economical and tunable route to highly potent bryostatin analogues now drives research and preclinical studies on these compounds in drug-discovery programmes.
Figure 5. Function-oriented synthesis as applied to bryostatin.
Currently available syntheses of bryostatin 1 (a) involve more than 70 steps, meaning that they will have no impact on supply and are as yet impractical for industrial-scale production of the compound. An analysis of the complex structural features of bryostatin suggested which parts of the molecule are essential for the compound’s biological activity and which parts are not. This allowed a structurally simpler analogue of bryostatin 1 to be designed (b), which was made in fewer than 30 steps. In cell and animal assays, this compound (and related designed analogues) performed as well as bryostatin in binding to cellular protein targets and in arresting the growth of cancer cells. Bonds and atom labels shown in red have been used to highlight parts of the two molecules that are identical.
Similarly, the anticancer activity of the dimeric alkaloid stephacidin B can be mimicked by a monomeric alkaloid, avrainvillamide, which in turn can be mimicked by much simpler analogues41. Synthetic studies on the highly complex molecule halichondrin B led to the discovery that its antitumour activity was contained in one part of the structure42. A highly simplified analogue was then made that has even better activity than the natural product. The supply problem for halichondrin B has thus been solved by developing an efficient synthesis of a simpler analogue, which has entered human trials. Function-oriented synthesis can also be used to reprogram the properties and biological activities of compounds, as shown with amphotericin43, Taxol44, DNA binders and many others.
Function-oriented design and synthesis offers several advantages when compared with target-oriented synthesis. First, the natural product is not necessarily seen as the synthetic target, but as a source of structural and functional information that informs design. Traditionally, natural products were often selected for synthesis because their structures were previously established when isolated, frequently through lengthy research. Chemists would devise a route to make the proposed structure of a natural product, and if the compound thus obtained was identical to that isolated from a natural source, then the structure of the synthetic material and its precursors were validated. With some exceptions, modern analytical techniques have virtually eliminated the need to establish structure by comparison with a structurally characterized natural product. This allows one to make designed molecules with confidence in their structures and the precursor structures leading to their syntheses.
So in addition to targets served up by nature, chemists can now design their own targets — molecules that have improved or new functions compared with their natural counterparts. This change of emphasis — from producing known structures to translating them into functionally superior but simplified structures — accounts in part for the recent explosion of non-natural targets and opportunities in synthesis. The rapidly growing interest in function provides a new range of exciting targets that have immediate relevance and application to many other areas of science.
Form follows function
Focusing on designed targets allows one to address a wider range of questions beyond those posed by the natural product, and often yields faster and superior results. Of course, natural products that incorporate unusual structural features are also justifiably recognized as inspiration for synthetic innovation. Function-oriented synthesis can also be used for this purpose, and often to better advantage, as one can design the target to fit both functional and synthetic goals. For example, designing a molecular muscle — a molecule that shortens or elongates electrochemically — poses a rich set of new synthetic challenges not unlike those encountered with natural products. The same is true of many other functionally desirable targets, such as smart molecules that detect (or prevent) disease, report on molecular events or interfere with biological processes. All of these could transform therapy and profoundly reduce the burgeoning cost of health care. Designed targets also often allow one to rapidly test hypotheses about the structural foundations of chemical reactivity, and to develop or improve synthetic methods, and the translation of a natural-product structure into a simplified, but functionally superior, designed compound is itself a great challenge to creativity.
There are many other opportunities for function-oriented synthesis, both in chemistry and in the growing list of molecularized sciences. As we have seen, synthesis is already enhanced by the use of biosynthetic machinery; synthetic biology represents an emergent field that takes the idea of biosynthesis and inverts it, by using synthesis to create new types of biology45. Diversity-oriented synthesis (Box 1) specifically targets non-natural compounds with natural-product-like complexity46, and has identified small molecules with biological activities not yet displayed by known natural products47. Dynamic combinatorial chemistry is another synthesis-driven discovery concept that incorporates aspects of the ideal synthesis. It is a process in which a structure is selected and amplified from an equilibrating mixture of components on the basis of a desirable molecular property, for example binding to a biological target48. Recent efforts combining structural design (inspired by natural products) with dynamic combinatorial chemistry have led to the discovery of molecules that target RNA sequences critical to HIV replication49, and to the most common form of adult-onset muscular dystrophy50. Molecular self-assembly and directed molecular evolution offer yet more opportunities to those with skills in synthesis and knowledge of function.
Conclusion
Synthesis is increasingly driven by an interest in molecules that not only have intriguing structures, but also have special functions. Added to this mix are exciting opportunities for designing molecular systems that go far beyond any natural function: from barcodes to electronic devices to self-assembled replicating systems.
Over the past two centuries, chemistry and synthesis have evolved from relatively pure disciplinary pursuits to positions of central importance in the physical and life sciences. More generally, they have provided the language and methodology that have unified, integrated and, indeed, molecularized the sciences, shaping our understanding of our molecular world and the direction, development and destiny of scientific research. From new reagents, reactions, strategies and processes to materials, medicinals and machines, chemistry and synthesis have shaped, and been shaped by, science and societal needs. Their continuing importance as central rather than as service sciences is partly a function of critical introspection (that is, can we do better?) but largely a function of sensing new opportunities in science of consequence to society. The human enterprise was once largely limited to what nature produced. We are now limited only by what we can imagine.
Contributor Information
Paul A. Wender, Email: wenderp@stanford.edu, Chemistry Department and, by courtesy, the Department of Chemical and Systems Biology, Stanford University, 333 Campus Drive, Stanford, California 94305-5080, USA
Benjamin L. Miller, Email: Benjamin_miller@urmc.rochester.edu, School of Medicine and Dentistry, University of Rochester, 601 Elmwood Avenue, Box 697, Rochester, New York 14642, USA
References
- 1.Seebach D. Angew Chem Int Edn. 1990;29:1320–1367. [Google Scholar]
- 2.Cornforth JW. Aust J Chem. 1993;46:157–170. [Google Scholar]
- 3.Wender PA, Miller BL. In: Connectivity Analysis and Multibond-forming Processes in Organic Synthesis: Theory and Applications. Hudlicky T, editor. JAI Press; 1993. pp. 27–66. [Google Scholar]
- 4.Tietze LF. Domino Reactions in Organic Synthesis. Wiley-VCH; 2006. [DOI] [PubMed] [Google Scholar]
- 5.Nicolaou KC. Tetrahedron. 2003;59:6683–6738. [Google Scholar]
- 6.Wilson RM, Danishefsky SJ. J Org Chem. 2006;71:8329–8351. doi: 10.1021/jo0610053. [DOI] [PubMed] [Google Scholar]
- 7.Horvath I, Anastas PT. Chem Rev. 2007;107:2169–2173. doi: 10.1021/cr078380v. [DOI] [PubMed] [Google Scholar]
- 8.Corey EJ, Czakó B, Kürti L. Molecules and Medicine. John Wiley; 2007. [Google Scholar]
- 9.Hudlicky T, Reed JW. The Way of Synthesis: Evolution of Design and Methods for Natural Products. Wiley-VCH; 2007. [Google Scholar]
- 10.Trost BM. Science. 1991;254:1471–1477. doi: 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]
- 11.Maimone TJ, Baran PS. Nature Chem Biol. 2007;3:396–407. doi: 10.1038/nchembio.2007.1. [DOI] [PubMed] [Google Scholar]
- 12.Koshino M, Solin N, Tanaka T, Isobe H, Nakamura E. Nature Nanotechnol. 2008;3:595–597. doi: 10.1038/nnano.2008.263. [DOI] [PubMed] [Google Scholar]
- 13.Sudek S, et al. J Nat Prod. 2007;70:67–74. doi: 10.1021/np060361d. [DOI] [PubMed] [Google Scholar]
- 14.Wender PA, et al. In: Drug Discovery Research. Huang Z, editor. John Wiley; 2007. pp. 127–162. [Google Scholar]
- 15.Sun MK, Alkon DL. Eur J Pharmacol. 2008;584:328–337. doi: 10.1016/j.ejphar.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Kingston DG, Newman DJ. Curr Opin Drug Discov Dev. 2007;10:130–144. [PubMed] [Google Scholar]
- 17.Newman DJ, Cragg GM. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 18.Wender PA, Kee JM, Warrington JM. Science. 2008;320:649–652. doi: 10.1126/science.1154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson HE, Banack SA, Cox PA. J Nat Prod. 2008;71:2041–2044. doi: 10.1021/np800295m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaVan DA, Cha JN. Proc Natl Acad Sci USA. 2006;103:5251–5255. doi: 10.1073/pnas.0506694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hampp N. Chem Rev. 2000;100:1755–1776. doi: 10.1021/cr980072x. [DOI] [PubMed] [Google Scholar]
- 22.Greenbaum E, et al. Tech Dig Int Electron Device Meet. 2002:496–498. [Google Scholar]
- 23.Wender PA, et al. Pure Appl Chem. 2002;74:25–31. [Google Scholar]
- 24.Hinman A, Du Bois J. J Am Chem Soc. 2003;125:11510–11511. doi: 10.1021/ja0368305. [DOI] [PubMed] [Google Scholar]
- 25.Chen MS, White MC. Science. 2007;318:783–787. doi: 10.1126/science.1148597. [DOI] [PubMed] [Google Scholar]
- 26.Chen H, Schlecht S, Semple TC, Hartwig JF. Science. 2000;287:1995–1997. doi: 10.1126/science.287.5460.1995. [DOI] [PubMed] [Google Scholar]
- 27.Wender PA, Verma VA, Paxton TJ, Pillow TH. Acc Chem Res. 2008;41:40–49. doi: 10.1021/ar700155p. [DOI] [PubMed] [Google Scholar]
- 28.Robinson R. J Chem Soc. 1917:762–768. [Google Scholar]
- 29.Chanon M, Barone R, Baralotto C, Julliard M, Hendrikson JB. Synthesis. 1998;1998:1559–1583. [Google Scholar]
- 30.Wender PA, editor. Chem Rev. Vol. 96. 1996. pp. 1–600. [DOI] [PubMed] [Google Scholar]
- 31.Yoder RA, Johnston JN. Chem Rev. 2005;105:4730–4756. doi: 10.1021/cr040623l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vilotijevic I, Jamison TF. Science. 2007;317:1189–1192. doi: 10.1126/science.1146421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curran DP, Kuo S-C. Tetrahedron. 1987;43:5653–5661. [Google Scholar]
- 34.Padwa A, Bur SK. Tetrahedron. 2007;63:5341–5378. doi: 10.1016/j.tet.2007.03.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicewicz DA, Satterfield AD, Schmitt DC, Johnson JS. J Am Chem Soc. 2008;130:17281–17283. doi: 10.1021/ja808347q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reppe W, et al. Liebigs Ann Chem. 1948;560:1–92. [Google Scholar]
- 37.Chappell D, Russell AT. Org Biomol Chem. 2006;4:4409–4430. doi: 10.1039/b614011b. [DOI] [PubMed] [Google Scholar]
- 38.Das A, Khosla C. Acc Chem Res. 2009;42:631–639. doi: 10.1021/ar8002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hale KJ, Hummersone MG, Manaviazar S, Frigerio M. Nat Prod Rep. 2002;19:413–453. doi: 10.1039/b009211h. [DOI] [PubMed] [Google Scholar]
- 40.Wender PA, DeChristopher BA, Schrier AJE. J Am Chem Soc. 2008;130:6658–6659. doi: 10.1021/ja8015632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wulff JE, Herzon SB, Siegrist R, Myers AG. J Am Chem Soc. 2007;129:4898–4899. doi: 10.1021/ja0690971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Towle MJ, et al. Cancer Res. 2001;61:1013–1021. [PubMed] [Google Scholar]
- 43.Szpilman AM, Manthorpe JM, Carreira EM. Angew Chem Int Edn. 2008;47:4339–4342. doi: 10.1002/anie.200800590. [DOI] [PubMed] [Google Scholar]
- 44.Dubikovskaya EA, et al. Proc Natl Acad Sci USA. 2008;105:12128–12133. doi: 10.1073/pnas.0805374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krueger AT, Kool ET. Chem Biol. 2009;16:242–248. doi: 10.1016/j.chembiol.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schreiber SL. Science. 2000;287:1964–1969. doi: 10.1126/science.287.5460.1964. [DOI] [PubMed] [Google Scholar]
- 47.Thomas GL, et al. Angew Chem Int Edn. 2008;47:2808–2812. doi: 10.1002/anie.200705415. [DOI] [PubMed] [Google Scholar]
- 48.Corbett PT, et al. Chem Rev. 2006;106:3652–3711. doi: 10.1021/cr020452p. [DOI] [PubMed] [Google Scholar]
- 49.McNaughton BR, Gareiss PC, Miller BL. J Am Chem Soc. 2007;129:11306–11307. doi: 10.1021/ja072114h. [DOI] [PubMed] [Google Scholar]
- 50.Gareiss PC, et al. J Am Chem Soc. 2008;130:16254–16261. doi: 10.1021/ja804398y. [DOI] [PMC free article] [PubMed] [Google Scholar]