Abstract
In eutherian mammals, the first functional organ is the placenta, a transient structure that is rapidly assembled in the extraembryonic compartment. By necessity the placenta develops in advance of the embryo, which it supports in utero by performing many of the same functions that the lungs, gastrointestinal tract and urinary system carry out after birth. Specialized epithelial cells that arise from the placenta, termed cytotrophoblasts (CTBs), are responsible for redirecting maternal blood to the developing conceptus, which occurs as a result of the cells’ aggressive invasion through the maternal endometrial stroma (interstitial invasion) and resident blood vessels (endovascular invasion). The latter process involves displacement of maternal endothelium and induction of apoptosis in the surrounding smooth muscle. Together, these events result in a reduction of blood vessel elasticity and increased blood flow (Burton et al., 1999). In the past, investigations of human CTB endovascular invasion have been limited to immunohistochemical examination of tissue sections. In this chapter, we will discuss the use of in vitro and in vivo techniques that have been recently adapted for the study of the complex events that occur during CTB endovascular invasion. As an introduction, we provide background on placental anatomy and the molecular basis of CTB behaviors. To follow, we present techniques used in the isolation and culture of primary CTBs and chorionic villous explants. Approaches for identifying trophoblast-modified blood vessels in placental tissue sections are also described. Next, we review methods used by other groups to study CTB/endothelial interactions in culture focusing on techniques that employ isolated cells and chorionic explants. Finally, we conclude with methods devised by our group and others to explore the complex heterotypic cell-cell interactions that occur as CTBs invade blood vessels in vivo in the nude mouse.
1. Introduction
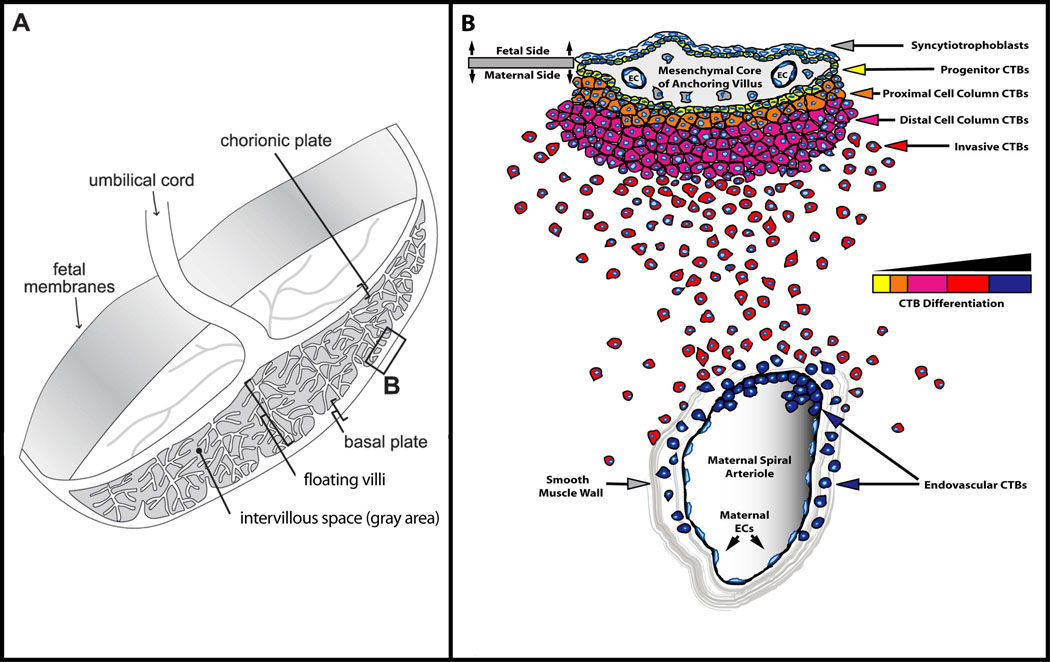
The placenta is commonly depicted as a simple, disc-shaped structure connecting the embryo/fetus to the uterus (Figure 1A). Within the developed placenta is a cavity (intervillous space) that possesses a vast network of chorionic villi that float in circulating maternal blood. Here, nutrients, gases, and wastes are exchanged across the surface of these fetal villi between the maternal blood and the fetal capillary beds underlying the villi. These structures are lined by two layers of trophoblast. The outer layer is composed of a multinucleated syncytium (syncytiotrophoblast) while the inner layer is a polarized CTB epithelial layer. Uterine attachment requires CTBs to differentiate and acquire the ability to aggressively invade maternal tissues (Fisher et al., 1989; Librach et al., 1991). This process also initiates the remarkable cell-cell interactions that occur as CTBs remodel the uterine vasculature into chimeric vessels, comprised of fetal and maternal cells, that supply blood to the placenta. Key morphological aspects of the invasion process are diagrammed in figure 1B. CTB progenitors form a polarized epithelium that is attached to the basement membrane surrounding the mesenchymal cores of chorionic villi. During differentiation along the invasive pathway, CTBs leave this basement membrane to form columns of unpolarized cells that attach to and then penetrate the uterine wall. The ends of the columns terminate within the superficial endometrium, where they give rise to invasive (extravillous) CTBs. During interstitial invasion, a subset of these cells, either individually or in small clusters, commingles with resident decidual, myometrial and immune cells. During endovascular invasion, masses of CTBs breach and plug the vessels (Ramsey et al., 1976). Subsequently, these fetal cells replace the resident maternal endothelium and portions of the smooth muscle wall. Normally, this process encompasses the portions of uterine arterioles that span the decidua and the inner third of the myometrium. In contrast, only the termini of uterine veins are breached (Zhou et al., 1997). Together these two components of CTB invasion anchor the placenta to the uterus and permit a steady increase in the supply of maternal blood that is delivered to the growing embryo/fetus. Endovascular invasion, which is a major determinant of pregnancy outcome, incorporates the placenta into the maternal circulation.
Figure 1. Anatomy of the maternal-fetal interface.
Anchoring chorionic villi (B) attach the placenta to the uterus and give rise to invasive CTBs. (A) Placental chorionic villi stem from the chorionic plate and lie within the intervillous space. The point of attachment between anchoring villi and the underlying tissue is referred to as the basal plate (box B). (B) Enlargement of the area in box B. Undifferentiated CTB progenitors in the anchoring villi give rise to invasive CTBs that invade the uterine interstitium (interstitial invasion) and the maternal endothelium (endovascular invasion).
Some of the key molecular aspects of human CTB differentiation and invasion are known. The cells’ expression of several classes of functionally relevant molecules is precisely modulated as they invade either in situ (the uterine wall) or in vitro (extracellular matrix [ECM]). For example, work from our group and other investigators has produced a detailed picture of how invading CTBs modulate the expression of molecules that facilitate both interstitial and endovascular invasion. With regard to adhesion molecules, the onset of CTB differentiation/invasion is characterized by reduced staining for receptors characteristic of polarized CTB epithelial stem cells—e.g., integrin α6β4 and E-cadherin—and the onset of expression of adhesion receptors characteristic of endothelium—e.g., VE-cadherin, Ig family members VCAM-1 and PECAM-1, and integrins α Vβ3 and α 1β1 (Damsky et al., 1992; Vicovac et al., 1995; Blankenship and Enders, 1997; Zhou et al., 1997a). A portion of this switching program is regulated by the wide array of VEGF family members that CTBs produce (Zhou et al., 2002; 2003). Recently we discovered that CTBs also express L-selectin, a leukocyte cell surface receptor that promotes adhesion under shear stress (Genbacev et al., 2003). Similarly, cadherin-11 is upregulated on extravillous CTBs and on decidualizing endometrial stroma (MacCalman et al., 1996, 1998). Whether this interaction promotes or restrains interstitial CTB invasion is not yet clear. All CTBs in this pathway, regardless of location, stain for Mel-CAM, a melanoma-associated Ig family receptor also expressed on endothelium (Shih and Kurman, 1996). Finally, CTBs within the maternal blood vessels turn on neural cell adhesion molecule (NCAM, CD56) (Blankenship and King, 1996; Y. Zhou et al., unpublished observations), an adhesion receptor that is also expressed by maternal natural killer cells that home to the pregnant uterus (Starkey et al., 1988).
Changes in the CTB adhesion molecule repertoire take place in the context of the cells’ equally dramatic modulation of their proteinase and proteinase inhibitor expression. Some aspects of this phenotypic transformation are undoubtedly linked to CTB acquisition of an invasive phenotype. For example, we found that expression and activation of matrix metalloproteinase (MMP)-9 is required for invasion in vitro (Librach et al., 1991). We speculate that CTBs upregulate the expression of other proteinases/inhibitors to present a nonthrombogenic surface to maternal blood. The urokinase plasminogen activators uPA (Queenan et al., 1987) and PAI-1 (Feinberg et al., 1989), as well as the thrombin receptor (Even-Ram et al., 1998), might function in this manner. Invading CTBs upregulate the expression of immune molecules that likely enable them to escape maternal rejection. Mechanisms used for this purpose include their expression of HLA-G, a unique major histocompatibility class Ib antigen with limited polymorphism (Ellis et al., 1990; Kovats et al., 1990), and interleukin-10, a potent immunosuppressive cytokine (Roth et al., 1996). Finally, CTBs modulate expression of Eph/ephrins that direct their migration through the uterus. Very recently, we found that, as CTBs begin to differentiate toward an invasive phenotype, they downregulate expression of EphB4 and upregulate expression of ephrinB2 (Red-Horse et al., 2005). Our work suggests that, due to repulsive interactions induced by ephrinB2/EphB4 ligation, ephrinB2-expressing CTBs are directed away from their EphB4-expressing progenitors and toward ephrinB2-expressing arteries, where permissive interactions take place (Red-Horse et al., 2005).
In the sections that follow, we discuss methods applicable to the study of human endovascular invasion. Initially we provide basic techniques required for the isolation and culture of primary CTBs (section 2) and explants (section 3), as well as means for identifying CTB-modified blood vessels in tissues sections (section 4). Next, we review methods used by other groups to study CTB/endothelial interactions in both cell culture (section 5) and explant models (section 6). We conclude with in vivo methods devised by our group and others to explore the complex heterotypic cell-cell interactions that occur as human CTBs invade blood vessels in the immunodeficient mouse (section 7).
2. Isolation and culture of human cytotrophoblasts
To study the cells’ behavior in culture, we isolate CTBs from whole placental tissue collected after elective pregnancy termination or at term. CTBs reside within the villous tissue of the placenta, which is also comprised of syncytiotrophoblasts, red blood cells, fibroblasts, endothelial cells, leukocytes, and other mesenchymal progenitors. The major steps in the cell isolation procedure include removal the outer layer of syncytium, release of the underlying CTBs by sequential enzymatic digestion, and purification of the cells by Percoll density gradient centrifugation. Any remaining leukocytes are removed by virtue of their interactions with CD45-coated beads, which are then separated with a magnetic device. Using these methods for CTB isolation, we typically achieve greater than 95% purity. The viability and purity of the cells are subject to a number of factors including tissue quality, gestational age, and tissue handling during the procedure. Our methods have never been published in detail, and as such, we will describe the procedure that we have evolved over the past 20 years.
Cytotrophoblast Isolation Procedure
Working with human tissue requires approval by an institutional human subjects review board.
Obtain the placental tissue as soon as possible after elective termination/delivery and place immediately in cytowash medium (see table 1). If the tissue is exposed to water, the CTBs initiate apotosis.
Keep the tissue on ice prior to dissection.
Perform all work in a biosafety hood using techniques appropriate for working with potentially biohazardous tissues.
Place the tissue in a petri dish and add fresh cytowash. Frequent exchange of the wash medium helps remove contaminating pathogens and maternal blood.
Remove fibrinoid, blood clots, amnion and basement membrane from the placental villi using vannas scissors and forceps. Dissect villi into approximately 2–4 mm pieces.
Filter the tissue through a mesh strainer (1 mm gaps) to remove small pieces of villi that would otherwise undergo digestion too quickly, which results in DNA breakdown, a process that entraps the cells in a viscous gel.
Wash the remaining tissue with 1 liter or more of cytowash to remove any remaining maternal blood.
Pour the dissected tissue into a 50 ml conical tube and fill with cytowash. Centrifuge the tube at 1300 rpm for 5 minutes at 4°C. This centrifugation speed corresponds to 365 rcf in a Sorvall Legend RT centrifuge.
Aspirate the supernatant and weigh the tissue pellet within the tube.
Prewarm the first enzyme dissociation solution by adding a volume of collagenase (see table 1) that is equal to six times the weight of the tissue to a sterile flask and place in a 37°C water bath shaker for approximately 10 minutes or until the desired temperature is reached.
Add the tissue to the warmed collagenase solution and shake in the water bath at 175 rpm for approximately 7 minutes. The amount of time necessary for digestion will depend heavily on the gestational age and health of the tissue. This step removes the syncytiotrophoblast layer (Figure 2A).
Transport the flask from the water bath to the biosafety hood and place it on ice at an angle to allow the tissue to settle at the bottom.
Aspirate and discard the supernatant.
During step 11, pre-warm the trypsin solution (see table 1) to 37°C.
Add a volume of trypsin solution equal to six times the weight of the tissue, return flask to the water bath, and shake at 175 rpm for approximately 10 minutes at 37°C. The exact length of this incubation will again depend greatly on tissue health and gestational age. This step releases the CTBs from the villous tissue (Figure 2B.).
Transport the flask from the water bath to the biosafety hood and place on ice at an angle that allows the tissue to settle at the bottom. Immediately after the remaining tissue settles, collect the supernatant and add an equal volume of cytowash.
Place 2 layers of sterile gauze over the mouth of a 50 ml conical tube that contains 5 ml of fetal bovine serum (Hyclone, SH30071.03). To halt enzymatic digestion, transfer 45 ml through the gauze. Note: this step will require multiple 50 ml tubes, with the exact number depending on the volume of dissociation buffer used.
Centrifuge the tubes at 1,300 rpm for 8 minutes at 4°C
Meanwhile, prepare the Percoll gradient (see table 1) that will be used later. Slowly layer 6 ml of each concentration in a 50 ml conical tube in the following order: 70%, 60%, 50%, 40%, 30% and 20%. Store at 4°C. Make one tube for each 7 grams of starting tissue.
Aspirate and discard the supernatant. Re-suspend and combine the cell pellets in one 50 ml conical tube with 50 ml of cytowash. Centrifuge again at 1,300 rpm for 8 minutes at 4°C.
Aspirate and discard the supernatant. Then, re-suspend the cell pellet in pre-warmed collagenase solution using 1 ml per gram of starting tissue.
Place the cell suspension in a sterile flask and transfer to the water bath. Shake the flask at 150 rpm for 3 minutes at 37°C. This second collagenase digestion dissociates clumps of CTBs into single cells (Figure 2C).
Add cytowash to the collagenase solution up to 50 ml. Pass the cell suspension through a 100 micron cell strainer into a 50 ml conical tube. Centrifuge at 1,300 rpm for 8 minutes at 4°C.
Aspirate and discard the supernatant. For every 7 grams of starting tissue, re-suspend the cell pellet in 4ml serum-free medium (see table 1). Very slowly add 4 ml of the cell suspension to the top of the Percoll gradient. Note: multiple Percoll tubes are usually required.
Centrifuge the tubes at 2,700 rpm for 25 minutes at 4°C. This centrifugation speed corresponds to 1573 rcf in a Sorvall Legend RT centrifuge. This step separates cells according to their individual densities.
Aspirate the top two bands of the Percoll gradient. Collect the CTB-containing layers between 20–28 ml. Combine the 8 ml harvested from each tube into a fresh 50 ml conical tube and fill with cytowash. Centrifuge at 1300 rpm for 8 minutes. The resulting semi-purified preparation will contain CTBs along with other cells of similar size (Figure 2D).
Aspirate and discard the supernatant, re-suspend the pellet in 50 ml cytowash, and centrifuge at 1300 rpm for 8 minutes at 4°C.
Aspirate and discard the supernatant, re-suspend the pellet in 10 ml serum-free medium.
Count cells using a hemocytometer.
Add cytowash to a final volume of 50 ml and centrifuge at 1300 rpm for 8 minutes at 4°C.
Aspirate the supernatant and re-suspend the pellet in serum-free medium at a concentration of 106 cells per ml. Ideally, the cells are approximately 80–90% pure at this stage. Greater than 95% purity can be achieved by negative selection using CD-45 coated Miltenyi beads (Miltenyi Biotec, according to the manufacturer’s directions).
Prior to plating cells, coat tissue culture wells with a 1:1 mixture of Matrigel (BD Biosciences, 354234) and serum-free medium. For each well of a six-well plate, use 50 microliters of the Matrigel:serum-free medium mixture. Allow the Matrigel to polymerize for 15 minutes at 37°C. Note: We use Matrigel lots prescreened for their ability to support CTB adhesion.
Plate 3–4 million cells per well.
| Serum-Free Medium | 500ml | Cytowash | 500ml |
|---|---|---|---|
| DME/H-21 Medium (Gibco, 11965-092) | 469.5ml | DME/H-21 Medium (Gibco, 11965-092) | 477ml |
| Nutridoma (Roche, 11011375001) | 10ml | Fetal Bovine Serum (Hyclone, SH30071.03) | 12.5ml |
| Sodium Pyruvate (Sigma, S8636) | 5ml | Glutamine Plus (Atlanta Biologicals, B92010) | 5ml |
| HEPES Buffer (Invitrogen, 15630-080) | 5ml | Penicillin/Streptomycin (Invitrogen, 15140-122) | 5ml |
| Glutamine Plus (Atlanta Biologicals, B92010) | 5ml | Gentamycin (Invitrogen, 15750-060) | 0.5ml |
| Penicillin/Streptomycin (Invitrogen, 15140-122) | 5ml | ||
| Gentamycin (Invitrogen, 15750-060) | 0.5ml | ||
| Collagenase Solution | 100ml | Trypsin Solution | 100ml |
| 1× PBS Mg2+ Ca2+ free (Gibco, 14190-144) | 100ml | 1× PBS Mg2+ Ca2+ free (Gibco, 14190-144) | 100ml |
| Collagense (Sigma, C-2674) | 0.062g | Trypsin (Sigma, T-8003) | 0.0069g |
| DNase (Sigma, DN25) | 0.040g | DNase (Sigma, DN25) | 0.0400g |
| Hyaluronidase (Sigma, H-3506) | 0.069g | EDTA (Sigma, ED2SC) | 0.0200g |
| BSA (Sigma, A7906) | 0.100g | ||
| Percoll Gradient Reagents | |||
| Percoll (Amersham, 17-0891-01) | |||
| 10× Hank’s BSS without Phenol Red (Invitrogen, 14185) | |||
| 1× Hank’s BSS without Phenol Red (Invitrogen, 14175) | |||
| 1× Hank’s BSS with Phenol Red (Invitrogen, 14170) | |||
| Percoll Stock Solutions | 90% Percoll* | Hank’s 1X | |
| 70% | 35.0 ml | 10.0 ml ( w/o Phenol Red) | |
| 60% | 30.0 ml | 15.0 ml ( with Phenol Red) | |
| 50% | 25.0 ml | 20.0 ml ( w/o Phenol Red) | |
| 40% | 20.0 ml | 25.0 ml ( with Phenol Red) | |
| 30% | 15.0 ml | 30.0 ml ( w/o Phenol Red) | |
| 20% | 10.0 ml | 35.0 ml ( with Phenol Red) | |
90% Percoll (270 ml Percoll + 30 ml 10× Hanks BSS without Phenol Red)
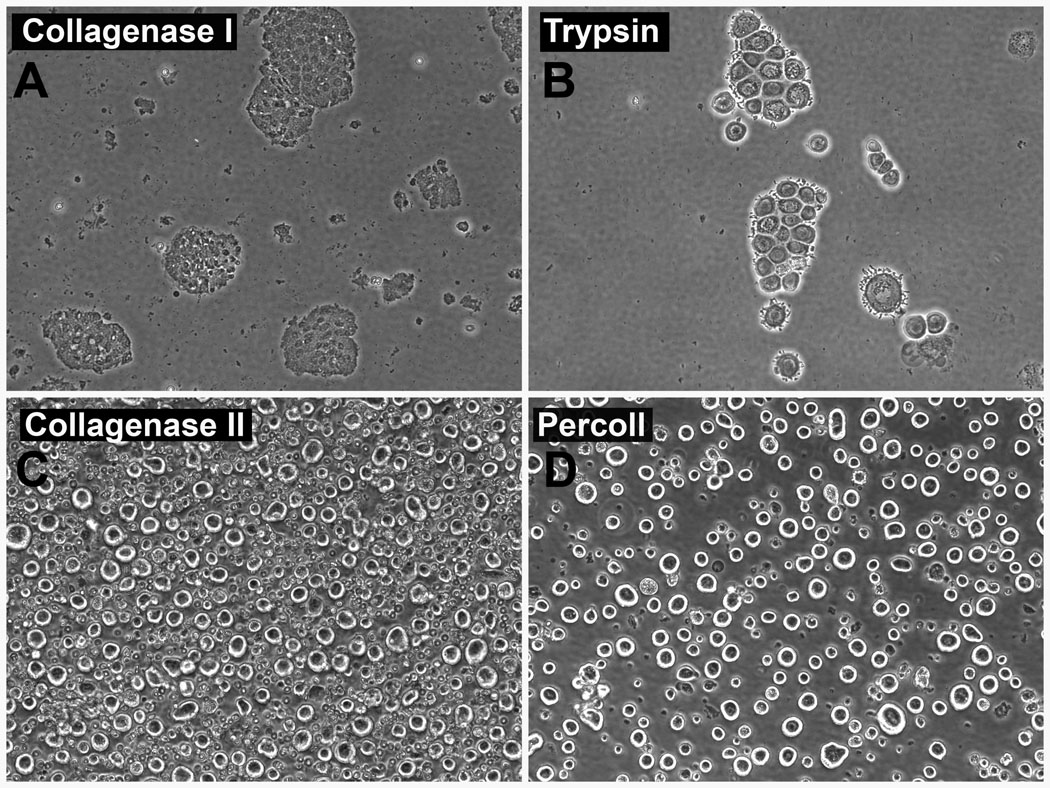
Figure 2. Monitoring enzymatic digestions and cell purification during CTB isolation.
(A) The first collagenase digestion releases the syncytiotrophoblasts from the surface of placental villi, which appear as multinucleated flattened cells at 20× magnification. The presence of any CTBs in the supernatant from the first collagenase digestion indicates that this step is complete and must be stopped immediately (not shown). Large clumps of CTBs are released by the Trypsin digestion (B), which are broken down into single cells during the second collagenase treatment (C). Also present at this stage are blood cells, fibroblasts, leukocytes, and other cell types. (D) CTBs are preferentially isolated by the Percoll density gradient centrifugation step.
Preparation of medium, enzyme solutions, and Percoll density gradient
Enzymatic digestions, time considerations
First Collagenase Digestion
The gestational age and health of the tissue greatly affects the digestion time period. For example, first trimester tissue between 6 and 10 weeks of gestation usually requires about 6–8 minutes of digestion, tissue aged 13 weeks needs about 12 minutes, and villi from a 20 week placenta need 18 minutes or more. A good rule of thumb is to digest one minute for every week in age. With regard to the health of the tissue, fibrinoid deposits and/or clotted blood will add to the digestion time. Collagenase digestion can be monitored in real-time by placing a drop of the reaction under a phase contrast microscope and checking whether the syncytium has lifted from the surface of the villi (Figure 2A). Clumps of syncytium should be visible in the enzymatic supernatant. The first appearance of large, single cells (CTBs) indicates that the collagenase reaction has reached completion and should be halted. Note that the exact times used in this and all the other digestion steps are recalculated for each new batch of enzyme, which we purchase in lots.
Trypsin Digestion
The time required also increases with gestational age. Most first trimester tissues will be adequately digested in about 10 minutes. Second trimester villi require about 14 minutes of incubation. As in the collagenase digestion, the enzymatic reaction progress can be verified under the microscope. Clumps should be visible along with single large CTBs (Figure 2B). The larger clumps of CTBs will be dissociated in the second collagenase digestion (Figure 2C.). The presence of smaller fibroblast cells from the villous core indicates that the tissue has been over digested and the reaction must be stopped immediately.
Second Collagenase Digestion
This step is less subject to factors that change the required time. Digestion for 3 minutes is almost always sufficient and should never exceed 5 minutes.
3. Isolation and culture of first trimester human placental villous explants
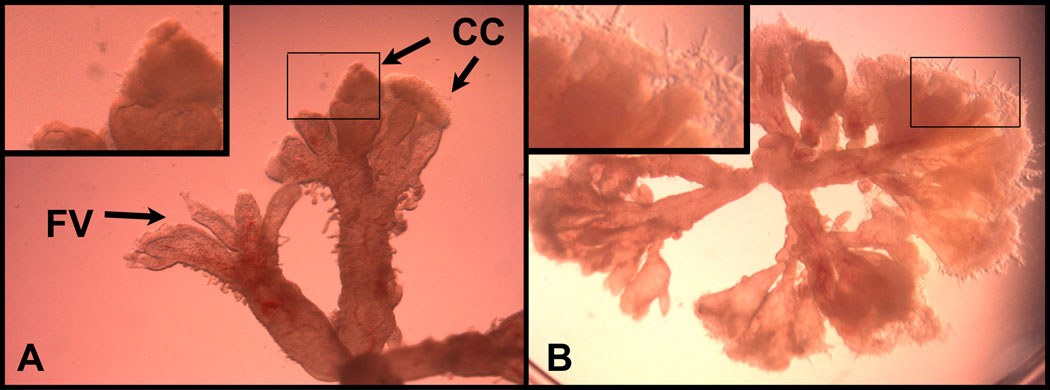
Different culture conditions can be used to study a wide variety of CTB behaviors, including differentiation and invasion (recently reviewed in Miller et al., 2005). We have used the techniques described, herein, to generate explants for analysis in vitro (Prakobphol et al., 2006) and in vivo (Red-Horse et al., 2006) using the nude mouse as a host. In general, explant culture requires careful selection of anchoring villous remnants with attached cell columns. Floating villi are not adequate in studies of invasion because the component CTBs are programmed to differentiate into syncytium rather than invasive cells. Under a phase microscope, cell columns extend well beyond the translucent villous core, appearing more opaque with an irregularly shaped border (see Figure 3A). Cell columns are most frequently observed in association with placentas up to 7 weeks of gestation. Placentas are collected and transported as described in section 2 and dissected under stereomicroscopic visualization into explants between 2 and 5 millimeters in diameter. Depending on the application, the villous explants may be cultured in any number of conditions.
Figure 3. Chorionic villous explants.
(A) Remnants of anchoring villi containing CTB cell columns can be distinguished from floating villi by their irregular outlines and opaque caps that extend from their termini (inset). (B) Explant cultured on Matrigel showing CTB outgrowths (inset). Abbreviations used: CCs; cell columns, FV; floating villi.
For illustrative purposes, the following is a description of one method for culturing anchoring villous explants that we have used in many different applications. Briefly, villi are transferred to Matrigel-coated 12-mm inserts that are placed in 24-well plates and completely submerged in several drops of medium (Ham's F-12/Dulbecco's modified Eagle's medium [1:1, vol/vol]) supplemented with an antibiotic/antimycotic mixture (100 U/ml penicillin, 100 µg/ml streptomycin, 50 µg/ml gentamycin) and 10% fetal calf serum. 8–24 hours later, when the cell columns have adhered to the matrix, additional medium is added. The cultures are incubated at 37°C under standard conditions (5% CO2/95% air) for 72 to 96 hours. Medium is changed daily. The morphology of the villi and their CTB outgrowths should be monitored at regular intervals using an inverted-phase microscope (Figure 3B). Explants with similar growth patterns should be paired as experimental and control samples.
4. Identification of cytotrophoblast-modified blood vessels in tissue sections
Microscopic analysis of histological sections of the pregnant uterus that contain spiral arterioles is often the starting point for gathering information about molecules that are relevant to CTB endovascular invasion. While this approach offers only a snapshot of the invasion process, it is a necessary prerequisite in forming hypotheses about the molecules that are involved. To play a role, they must be expressed in a relevant location at the right time. By way of background, CTB invasion of maternal blood vessels occurs in several stages. The fetal CTBs breach the termini of both arteries and veins. Within veins, invasion rarely progresses beyond the point of entry. In contrast, invasion within the spiral arterioles ultimately involves almost the entire intra-uterine course of these vessels, and normally goes as deep as the first third of the myometrium (Robertson AND Brosen, 1972–73). During much of the first trimester, these fetal cells plug uterine arterioles, an arrangement that is thought to maintain an hypoxic environment that favors CTB proliferation (Burton et al., 1999). Starting at about 10 weeks of gestation, the cells begin to migrate in a retrograde fashion up the vessel lumina, which rapidly recanulate (Enders and Blankenship, 1997). Once the vessels are fully modified, which includes a 10–50 fold increase in diameter, they can deliver liters of blood per minute to the intervillous space.
In these types of studies, several criteria should be considered, including the gestational age of the tissue and the site of placental insertion. Regarding gestational age, it should be noted that elective pregnancy termination in the first trimester is most commonly achieved by vacuum aspiration, a process that rarely, if ever, yields intact CTB-modified maternal blood vessels. In our experience, such vessels are best obtained following hysterectomies performed during the early gestation period. However, tissue acquired from terminations that are performed during the second trimester period by curettage, often contains intact maternal blood vessels, which are grossly visible on the surface of the basal plate (Figure 1A). These vessels are also found in association with placental tissue acquired after caesarian section. In addition, there are regional differences within the placenta. At the periphery, invasion is usually superficial as compared to the central region.
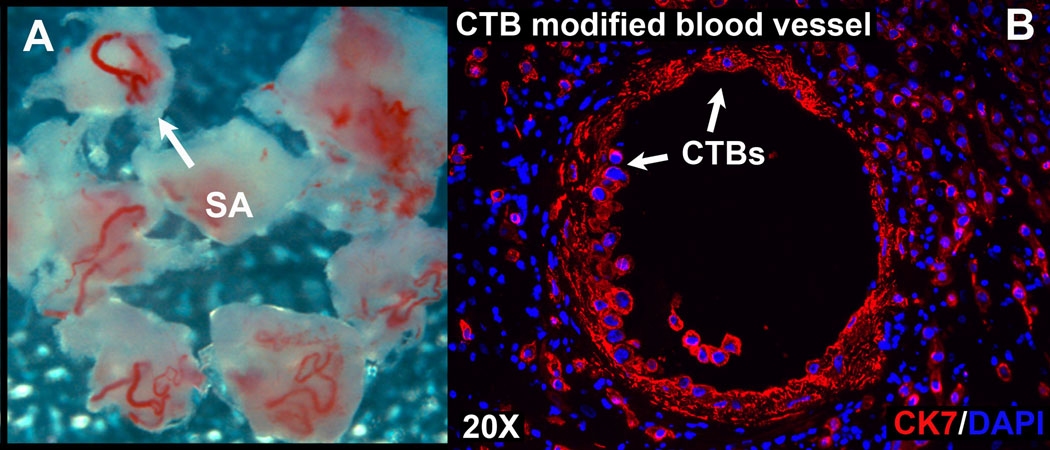
Although morphological analyses are very informative, the value of these studies can be vastly increased when they are performed in the context of immunolocalization experiments. In this case, samples of the basal plate that contain spiral arterioles are collected and quickly processed following elective pregnancy termination to prevent degradation of the tissue. The vessels and the immediately adjacent decidua, 2–3 mm in diameter (Figure 4A), are dissected free of the remaining tissue and fixed in 3% paraformaldehyde for 30 minutes. Then, the tissue is washed three times in PBS (4°C), infiltrated with gradually increasing concentrations of sucrose (5–15%) followed by OCT compound and freezing in liquid nitrogen. Placental tissues are processed using immunolocalization protocols that we published previously (Zdravkovic et al., 2006). Nonspecific antibody reactivity is blocked by incubation in 3% bovine serum albumin in PBS for 1 hour. Primary antibodies for identification of CTBs in the basal plate include a rat anti-human cytokeratin 8 and 18 produced in our laboratory and a mouse anti-human cytokeratin 7 (Dako). The antibodies used for the identification of endothelial cells in incompletely remodeled blood vessels include a rabbit anti-human Von Willebrand Factor (Dako). CTB remodeling of large bore vessels often leaves no endothelial cells behind, and therefore, antibodies that recognize smooth muscle actin may help to visualize blood vessel remnants, although it should be noted that the muscular walls of the arterioles are also disrupted by endovascular invasion. Cytokeratin-positive glandular epithelial cells are often present in earlier samples from the 1st and 2nd trimester. In such cases, endovascular CTBs can be distinguished from glands by virtue of their HLA-G expression (McMaster et al., 1995). Glands may also be distinguished morphologically by their columnar epithelial organization relative to the more stellate, disorganized distribution of endovascular CTBs. Tissue sections are incubated with primary antibodies overnight at 4°C, washed three times in PBS and incubated in the appropriate species-specific FITC- or rhodamine-conjugated secondary antibodies. Negative control sections are incubated in the absence of primary antibodies. After staining, antibody reactivity is photographed using a Leica DM 500B fluorescence microscope (Leica Microsystems, Wetzlar, GDR). An example of a CTB-remodeled uterine spiral arteriole is shown in figure 4B. In this case, the tissue section was stained with antibodies that specifically reacted with cytokeratin 7 and nuclei were visualized by staining with DAPI.
Figure 4. Spiral arterioles and a histological section of the uterus that contains a CTB-modified blood vessel.
(A) Spiral arterioles dissected from the decidua at 12 weeks of gestation. (B) Frozen section of an 18 week basal plate biopsy containing a CTB-modified blood vessel. CTBs were distinguished from other cell types by staining for cytokeratin 7 (CK7; red); nuclei were visualized by staining with DAPI (blue).
5. Cytotrophoblast migration and induction of endothelial cell apoptosis during co-culture
Given the limited number of approaches available to study interactions between CTBs and host endothelial cells in vivo, several groups have attempted to model such behavior with co-culture systems. While the unique experimental conditions differed, for example, in the use of freshly isolated cells versus choriocarcinoma cell lines and 2-dimensional versus 3-dimensional culture conditions, several commonalities have emerged in the results. First, CTBs migrate toward and adhere to the endothelial cells. Second, the state of the endothelium, e.g., activation or differentiation, influences CTB behavior. Third, CTBs can induce apoptosis of the cultured endothelial cells. The methods used in these studies will be described with results highlighting the CTB behaviors that were observed.
Coculture of Jar choriocarcinoma cells with endothelial monolayers (Chen et al., 2005; Chen et al., 2007)
Rather than using freshly isolated cells, which are costly to prepare and have a limited lifespan in culture, the authors utilized choriocarcinoma cells. As background, choriocarcinoma arises during or after pregnancy with the common feature that the abnormal cells are derived from CTB progenitors (Reviewed in Benirschke and Kaufmann, 2000; Shih and Kurman, 2002). This neoplasm is characterized by solid sheets of CTBs and syncytial trophoblasts with the distinctive features of aggressive vascular invasion (Benirschke and Kaufmann, 2000), frequent metastases (Berkowitz and Goldstein, 1996), and human chorionic gonadotropin (hCG) production. While it is certain that malignant choriocarcinoma cells do not replicate all the behaviors exhibited by primary CTBs in culture, they do share many of their properties, and therefore, are widely used as models in the study of CTB biology. As to the methods of this study, human umbilical vein endothelial cells (HUVECs) and human microvascular endothelial cells (HMECs) were stained with green cell tracker dye (CMFDA) and seeded (5×104cells/mL) onto plastic cover slips in 6-well plates. Once confluent, Jar cells (2.5×104 cells/mL) that had been labeled with a red cell tracker dye (SNARF) were added to the endothelial cultures. Interactions among the cells were characterized at multiple time points up to 48 hours. The Jar cells displaced the endothelial cells from the surface of the dish, a phenomenon that occurred progressively over time. Further investigation revealed that the choriocarcinoma cells induced apoptosis in the endothelial cells, which they phagocytosed. Interestingly, activation of the endothelial cells by addition of TNF alpha, interferon gamma, or necrotic cells prevented their displacement by Jar cells as well as their apoptotic cell death. These results suggest that the state of the endothelium regulates CTB-endothelial interactions, a novel observation.
Coculture of first trimester CTBs with differentiated endometrial endothelial cells in a 3-dimensional model (Aldo et al., 2007)
The authors tracked CTB migration in a 3- dimensional matrix environment that promoted endothelial differentiation and tube formation. Human endometrial endothelial cells (HEECs) were isolated from endometrium by virtue of their affinity for biotinylated Ulex europaeus lectin and grown in EMB-2 medium (Cambrex). The HEECs were immortalized by retroviral transfection of telomerase (Krikun et al., 2004). For endothelial tube formation, 300 microliters of Matrigel were added to 24-well tissue culture plates and polymerized at 37°C for 30 minutes. HEECs were stained with a green fluorescent dye PKH67 (Sigma-Aldrich) and added to the Matrigel-coated wells at a concentration of 100,000 cells per well. Endothelial tube formation occurred over the next 4–8 hours in culture. First trimester CTBs, isolated as described in section 2, were labeled with a red fluorescent dye, PKH26 (Sigma-Aldrich), and seeded onto the endothelial tubes, 80,000 cells per well. Over the next 24–96 hours, cell interactions were observed by fluorescence microscopy and photographed. CTBs migrated towards the endothelial tubes within 2 hours and were either positioned on top or had intercalated within the lumina after 8 hours. Interestingly, intercalation within the tubes was blocked by LPS activation of the endothelial cells prior to CTB addition.
Using similar methods as those described above, other investigators have shown by time lapse microscopy that both primary CTBs and CTB cell lines can induce endothelial cell and smooth muscled apoptosis through Fas/FasL signaling (Ashton et al., 2004; Harris, et al., 2006). A role for Tumor Necrosis Factor α-Related Apoptosis Inducing Ligand (TRAIL) signaling in this process has also been described (Keogh et al., 2007)
6. In vitro models of CTB endovascular invasion using explanted spiral arterioles
While cell culture models of CTB/endothelial interactions are very useful, they fail to provide many elements that are present during endovascular invasion in vivo such as the stromal and extracellular matrix factors present in decidual tissue, the complex vessel architecture, and the variety of cell types that are present in this complex milieu. Additionally, endothelial and CTB cell lines used in the studies described above may not possess the same characteristics as their living equivalents. Therefore, several investigators have attempted to model CTB invasion using explanted tissues. Three unique approaches will be presented. In the first, spiral arterioles were dissected from myometrial biopsies obtained at term and infused with CTBs (Cartwright, et al., 2002; Ashton et al., 2004; Crocker et al., 2005). In the second, explanted spiral arterioles were denuded of endothelium to expose the smooth muscle lining (Harris, et al., 2006; Keogh et al., 2007). In the last, chorionic villous explants were co-cultured with explanted decidual tissue under conditions of hypoxia (Dunk et al., 2004).
CTB invasion of term myometrial spiral artery segments in vitro (Cartwright, et al., 2002; Ashton et al., 2004; Crocker et al., 2005)
Spiral arteries were dissected under sterile conditions from myometrial biopsies obtained following caesarian section. In choosing this approach, it is important to keep in mind that CTB invasion does not normally progress beyond the inner third of the myometrial segments of spiral arterioles. These arterial explants, which included adjacent decidua, allowed study of both the interstitial and endovascular components of CTB invasion. Either a CTB cell line (SGHPL-4) or primary CTBs, isolated from first trimester tissue (see section 2), were fluorescently labeled with CellTracker Orange (Invitrogen, C2927). To assay interstitial invasion, the ends of the spiral arterioles were tied off to prevent CTB infiltration along a luminal path. Ligated arteries were embedded in fibrin gels. To prepare the gels, fibrinogen (Sigma) was suspended in PBS (2.5 mg/ml) with 200 Units/ml aprotinin (Trasylol, Bayer, Germany) in a 6-well plate. Polymerization of the gel was induced by the addition of thrombin (0.625 units/ml). 5×104 fluorescently labeled CTBs were resuspended in large EC growth medium (TCS Biologicals) at a concentration of 105 cells/ml and added to the top of the fibrin gel containing the arterial explant. Cultures were incubated for 5 days and the fresh unfixed explants were embedded in OCT and sections 5 and 10 microns were prepared. To assay endovascular invasion, spiral arterioles placed in a pressurized perfusion chamber (Living Systems), cannulated, and the lumina were maintained by exerting low pressure. Control vessels were perfused with medium alone (10 microliters) and experimental vessels were exposed to medium containing fluorescently labeled CTBs (5×104 in 10 microliters). Then, the vessels ends were tied off and the explants were embedded in fibrin gels, incubated for 5 days, and analyzed as described above.
As to results, in the original publication describing this model (Cartwright et al., 2002) the authors observed CTB endovascular invasion through both interstitial and lumenal routes, with CTBs intercalating within the vessel wall and lining the lumen, respectively. Invasion was accompanied by apoptosis and loss of the resident endothelial cells as revealed by TUNEL staining and the presence of cleaved PARP within the remaining endothelial cells (Ashton et al., 2004). In later work, immunohistochemical analyses and electron microscopy (Crocker et al., 2005) revealed that CTBs disrupted both the endothelial and smooth muscle compartments, although the overall integrity of the vessel was not compromised. Vessels perfused with CTBs often lacked endothelium and showed reduced staining for smooth muscle actin. CTBs adherent to the vessel wall occasionally exhibited cellular processes extending deep into the smooth muscle layer. Invasion, which depended on oxygen concentration, was reduced in hypoxia.
The same group studied interactions between CTBs and spiral arterioles that were denuded of endothelium by passing a column of air through the cannulated vessels (Harris, et al., 2006; Keogh et al., 2007). Perfusion of either CTBs or CTB conditioned medium induced apoptosis in the smooth muscle wall of these vessels. Additional experiments showed that this phenomenon depended of Fas/FasL signaling (Harris, et al., 2006) and TRAIL signaling (Keogh et al., 2007).
Chorionic villous CTBs invade first trimester decidual explants in vitro (Dunk et al., 2004)
The authors devised a novel model to study endovascular invasion. Chorionic villi were cocultured with decidua parietalis expants from the same pregnancy. Experiments were conducted under conditions of physiological hypoxia, which were required for CTB invasion. This is in contrast to the experiments described above in which hypoxia inhibited CTB invasion of spiral arterioles. As to methods, tissue samples were collected following elective termination of pregnancies, 6–9 weeks of gestation. Chorionic villous explants were prepared as described in section 3 and cocultured with decidual tissue that was dissected into cubes of approximately 2–3mm3. Millicell-CM culture inserts with 0.4 micron pores (Millipore) were coated with 200 microliters of Matrigel (BD Biosciences) and incubated at 37°C until the Matrigel polymerized. Decidual explants were transferred to the culture insert, epithelial surface facing upwards, and placental villi added. Culture medium was changed every 48 hours and consisted of a 50/50 mix of DMEM/Ham’s F12 medium supplemented with 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, and 0.25 µg/ml ascorbic acid (pH 7.4). Cultures were incubated under conditions of physiological hypoxia (3% oxygen) with 5% carbon dioxide and were maintained up to 6 days. Decidual cultures without villous explants served as controls. Following termination of the experiments, tissues were fixed in 4% paraformaldehyde and 5 micron sections were prepared.
Immunostaining for trophoblast, endothelial and smooth muscle markers revealed that CTBs invaded through the decidua (interstitial invasion) and colonized blood vessels (endovascular invasion), which lost most of their resident endothelial cells. Those remaining often appeared to be partially detached from the underlying basement membrane. In addition, CTB-modified vessels showed reduced staining for smooth muscle actin. In contrast, decidual explants cultured without placental villi contained vessels with intact endothelial and smooth muscle layers. Interestingly, when experiments were carried out in a hyperoxic environment (17% oxygen) CTB invasion of the decidual explants was not observed.
7. In vivo models of human cytotrophoblast vascular remodeling
During human pregnancy, placental CTBs of fetal origin invade the uterine wall. This process has two components. In the first, CTBs invade the uterine parenchyma where they interact with the stromal compartment and a resident maternal immune population. In the second, a subpopulation of CTBs invades uterine blood vessels with subsequent colonization of the arterial side of the circulation. Although some information is known about the molecular bases of these unique processes, a great deal remains to be learned. One reason is the lack of in vivo model for studying these unique heterotypic cell-cell interactions. For example, the mechanisms whereby CTBs replace the maternal endothelial lining of uterine arterioles and intercalate within the surrounding smooth muscle layer are difficult to study in vitro and almost certainly do not recapitulate the complex series of events that occur in vivo. Thus, the mechanisms of CTB vascular remodeling are of considerable interest.
Placental xenograft of the murine mammary fat pad and kidney capsule
In a recent study, we devised a novel in vivo model to study CTB invasion in which human placental chorionic villi are implanted into the mammary fat pads or under the kidney capsules of SCID mice (Red-Horse et al., 2006). These in vivo models were very successful as CTBs exhibited robust interstitial and endovascular invasion in both regions. Within the highly vascularized kidney capsule, CTBs invaded deeply, moving along the surfaces of capillaries. Xenograft in the mammary fat pad, which contains large bore vessels, was more suited for the analysis of CTB interactions with arterioles. These experiments yielded a variety of interesting information such as the observation that CTB invasion of arterioles in this region was associated with endothelial apoptosis. The success of these experiments was not surprising given that CTBs exhibit invasive behavior in a variety of extrauterine sites. As examples, ectopic pregnancies can occur in the fallopian tube, abdominal wall, cervix and ovary (Molinaro and Barnhart, 2007). In addition, choriocarcinomas frequently metastasize to the lungs, brain, and liver (Cheung, 2003).
As to the methods, protocols involving animals and human fetal tissue were approved by the UCSF Committee on Animal Research and the UCSF Committee on Human Research, respectively. Homozygous C.B-17 scid/scid mice (Taconic) were the recipients. Anchoring placental villi were dissected into 2–5mm pieces (as described in section 3.) Prior to surgery, mice were anesthetized with isoflurane anesthesia or ketamine/zylazine for mammary or kidney capsule procedures, respectively. For transplantation to the mammary fat pad, placental villi were surgically placed within a small incision made below this region, which was subsequently sutured. For transplantation under the kidney capsule, a small incision was made through the skin covering the lumbar area and the organ was exteriorized. Placental villi were implanted under the capsular membrane using techniques that have been described previously (McCune et al., 1988; Stoddart et al., 2001). Following both mammary fat pad and kidney capsule surgeries, mice were maintained under pathogen-free conditions for one to three weeks, at which time the experiments were terminated and either whole kidneys containing implants or implants dissected from a portion of the fat pads were immediately placed in 3% paraformaldehyde. Tissues were fixed for three hours at 4°C before being passed through a sucrose gradient, snap-frozen in liquid nitrogen, and sectioned. Histological analyses allowed quantitative estimates of invasion that were made by calculating the distance between the implanted villi and the outer perimeter of placental cells that had invaded murine tissues.
Choriocarcinoma xenograft of the murine skin
Another group reported an alternative model for investigating CTB invasive behavior (Grummer et al., 1999). Rather than studying primary CTBs or explants isolated from placental tissue, this group utilized the tumorigenic properties of the Jeg-3 human choriocarcinoma cell line, which was injected subcutaneously in the murine flank. In this location, Jeg-3 cells established tumors that grew rapidly within 2 weeks. Regarding endovascular invasion, two primary observations were made. First, Jeg-3 tumors had lacunar blood spaces that appeared to be the result of tumor cells colonizing host vessels; Von Willebrand factor-positive endothelial cells and hCG-positive Jeg-3 tumor cells lined the blood-filled lacunae. Second, Jeg-3 cells replaced the host endothelium at junctional zones between the tumor and the host tissues. Taken together, these results indicate that Jeg-3 cells remodel the host vasculature.
As to methods, the experiments were carried out in athymic nude mice (Han:NMRI nu/nu) maintained under pathogen-free conditions. Prior to transfer into 10 week-old recipient mice, Jeg-3 cells were grown to 80–90% of confluence and detached with trypsin/EDTA. One million cells in a volume of 200 microliters were injected into the subcutaneous space of the flanks of male mice. To reduce the numbers of animals required, each mouse received injections on both sides of its body. Tumor tissue was collected at the termination of experiments 21 days following injection. For histological studies, tumor and adjacent host tissues were subject to one of the following fixation procedures. Some tissues were immediately embedded in OCT medium and frozen in liquid nitrogen. Others were fixed in 3% paraformaldehyde and processed in paraffin. Alternatively, specimens were fixed in 2.5% glutaraldehyde and embedded in Epon/Araldite for fine structural analysis.
Contributor Information
Nathan M Hunkapiller, nathan.hunkapiller@ucsf.edu, 415-476-1092, Department of Cell and Tissue Biology, University of California San Francisco.
Susan J. Fisher, sfisher@cgl.ucsf.edu, 415-476-1092, Professor, Department of Cell and Tissue Biology, University of California San Francisco
References
- Aldo PB, et al. A novel three-dimensional in vitro system to study trophoblast-endothelium cell interactions. Am J Reprod Immunol. 2007;58:98–110. doi: 10.1111/j.1600-0897.2007.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton SV, et al. Uterine spiral artery remodeling involves endothelial apoptosis induced by extravillous trophoblasts through Fas/FasL interactions. Arterioscler Thromb Vasc Biol. 2005;25:102–108. doi: 10.1161/01.ATV.0000148547.70187.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship TN, King BF. Macaque intra-arterial trophoblast and extravillous trophoblast of the cell columns and cytotrophoblastic shell express neural cell adhesion molecule (NCAM) Anat Rec. 1996;245:525–531. doi: 10.1002/(SICI)1097-0185(199607)245:3<525::AID-AR9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Blankenship TN, Enders AC. Expression of platelet-endothelial cell adhesion molecule-1 (PECAM) by macaque trophoblast cells during invasion of the spiral arteries. Anat Rec. 1997;247:413–419. doi: 10.1002/(SICI)1097-0185(199703)247:3<413::AID-AR13>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Burton GJ, et al. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. Am J Obstet Gynecol. 1999;181:718–724. doi: 10.1016/s0002-9378(99)70518-1. [DOI] [PubMed] [Google Scholar]
- Cartwright JE, et al. Trophoblast invasion of spiral arteries: a novel in vitro model. Placenta. 2002;23:232–235. doi: 10.1053/plac.2001.0760. [DOI] [PubMed] [Google Scholar]
- Chen Q, et al. Interaction of Jar choriocarcinoma cells with endothelial cell monolayers. Placenta. 2005;26:617–625. doi: 10.1016/j.placenta.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Chen Q, et al. Activated endothelial cells resist displacement by trophoblast in vitro. Placenta. 2007;28:743–747. doi: 10.1016/j.placenta.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Cheung AN. Pathology of gestational trophoblastic diseases. Best Pract Res Clin Obstet Gynaecol. 2003;17:849–868. doi: 10.1016/s1521-6934(03)00094-4. [DOI] [PubMed] [Google Scholar]
- Crocker IP, et al. The effect of vascular origin, oxygen, and tumour necrosis factor alpha on trophoblast invasion of maternal arteries in vitro. J Pathol. 2005;206:476–485. doi: 10.1002/path.1801. [DOI] [PubMed] [Google Scholar]
- Damsky CH, et al. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J Clin Invest. 1992;89:210–222. doi: 10.1172/JCI115565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunk C, et al. A novel in vitro model of trophoblast-mediated decidual blood vessel remodeling. Lab Invest. 2003;83:1821–1828. doi: 10.1097/01.lab.0000101730.69754.5a. [DOI] [PubMed] [Google Scholar]
- Ellis SA, et al. Human trophoblast and the choriocarcinoma cell line BeWo express a truncated HLA Class I molecule. J Immunol. 1990;144:731–735. [PubMed] [Google Scholar]
- Enders AC, Blankenship TN. Modification of endometrial arteries during invasion by cytotrophoblast cells in the pregnant macaque. Acta Anat (Basel) 1997;159:169–193. doi: 10.1159/000147983. [DOI] [PubMed] [Google Scholar]
- Even-Ram S, et al. Thrombin receptor overexpression in malignant and physiological invasion processes. Nat Med. 1998;4:909–914. doi: 10.1038/nm0898-909. [DOI] [PubMed] [Google Scholar]
- Fisher SJ, et al. Adhesive and degradative properties of human placental cytotrophoblast cells in vitro. J Cell Biol. 1989;109:891–902. doi: 10.1083/jcb.109.2.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genbacev OD, et al. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science. 2003;299:405–408. doi: 10.1126/science.1079546. [DOI] [PubMed] [Google Scholar]
- Grummer R, et al. Characteristic growth of human choriocarcinoma xenografts in nude mice. Placenta. 1999;20:547–553. doi: 10.1053/plac.1999.0406. [DOI] [PubMed] [Google Scholar]
- Harris LK, et al. Invasive trophoblasts stimulate vascular smooth muscle cell apoptosis by a fas ligand-dependent mechanism. Am J Pathol. 2006;169:1863–1874. doi: 10.2353/ajpath.2006.060265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh RJ, et al. Fetal-derived trophoblast use the apoptotic cytokine tumor necrosis factor-alpha-related apoptosis-inducing ligand to induce smooth muscle cell death. Circ Res. 2007;100:834–841. doi: 10.1161/01.RES.0000261352.81736.37. [DOI] [PubMed] [Google Scholar]
- Kovats S, et al. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248:220–223. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- Krikun G, et al. A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology. 2004;145:2291–2296. doi: 10.1210/en.2003-1606. [DOI] [PubMed] [Google Scholar]
- Librach CL, et al. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol. 1991;113:437–449. doi: 10.1083/jcb.113.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCalman CD, et al. Regulated expression of cadherin-11 in human epithelial cells: a role for cadherin-11 in trophoblast-endometrium interactions? Dev Dyn. 1996;206:201–211. doi: 10.1002/(SICI)1097-0177(199606)206:2<201::AID-AJA9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- MacCalman CD, et al. Type 2 cadherins in the human endometrium and placenta: their putative roles in human implantation and placentation. Am J Reprod Immunol. 1998;39:96–107. doi: 10.1111/j.1600-0897.1998.tb00341.x. [DOI] [PubMed] [Google Scholar]
- McCune JM, et al. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- McMaster MT, et al. Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J Immunol. 1995;154:3771–3778. [PubMed] [Google Scholar]
- Miller RK, et al. Human placental explants in culture: approaches and assessments. Placenta. 2005;26:439–448. doi: 10.1016/j.placenta.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Molinaro TA, Barnhart KT. Ectopic pregnancies in unusual locations. Semin Reprod Med. 2007;25:123–130. doi: 10.1055/s-2007-970051. [DOI] [PubMed] [Google Scholar]
- Perry E. The realities of primary nursing care. Needed changes in education. NLN Publ. 1978:23–28. [PubMed] [Google Scholar]
- Prakobphol A, et al. A role for the L-selectin adhesion system in mediating cytotrophoblast emigration from the placenta. Dev Biol. 2006;298:107–117. doi: 10.1016/j.ydbio.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Queenan JT, Jr, et al. Regulation of urokinase-type plasminogen activator production by cultured human cytotrophoblasts. J Biol Chem. 1987;262:10903–10906. [PubMed] [Google Scholar]
- Ramsey EM, et al. Interactions of the trophoblast and maternal tissues in three closely related primate species. Am J Obstet Gynecol. 1976;124:647–652. doi: 10.1016/0002-9378(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Red-Horse K, et al. EPHB4 regulates chemokine-evoked trophoblast responses: a mechanism for incorporating the human placenta into the maternal circulation. Development. 2005;132:4097–4106. doi: 10.1242/dev.01971. [DOI] [PubMed] [Google Scholar]
- Red-Horse K, et al. Cytotrophoblast induction of arterial apoptosis and lymphangiogenesis in an in vivo model of human placentation. J Clin Invest. 2006;116:2643–2652. doi: 10.1172/JCI27306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth I, et al. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J Exp Med. 1996;184:539–548. doi: 10.1084/jem.184.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih IM, Kurman RJ. Expression of melanoma cell adhesion molecule in intermediate trophoblast. Lab Invest. 1996;75:377–388. [PubMed] [Google Scholar]
- Shih Ie M, Kurman RJ. Molecular basis of gestational trophoblastic diseases. Curr Mol Med. 2002;2:1–12. doi: 10.2174/1566524023362960. [DOI] [PubMed] [Google Scholar]
- Starkey PM, et al. Cell populations in human early pregnancy decidua: characterization and isolation of large granular lymphocytes by flow cytometry. Immunology. 1988;65:129–134. [PMC free article] [PubMed] [Google Scholar]
- Stoddart CA, et al. Impaired replication of protease inhibitor-resistant HIV-1 in human thymus. Nat Med. 2001;7:712–718. doi: 10.1038/89090. [DOI] [PubMed] [Google Scholar]
- Vicovac L, et al. Trophoblast differentiation during formation of anchoring villi in a model of the early human placenta in vitro. Placenta. 1995;16:41–56. doi: 10.1016/0143-4004(95)90080-2. [DOI] [PubMed] [Google Scholar]
- Zhou Y, et al. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. 1997 doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]