Abstract
A better understanding of high-cost kidney transplant patients would be useful for informing value-based purchasing strategies by payers. This retrospective cohort study was based on the Medicare Provider Analysis and Review (MEDPAR) files from 2003 to 2006. The focus of this analysis was high-cost kidney transplant patients (patients that qualified for Medicare outlier payments and 30-day readmission payments). Using regression techniques, we explored relationships between high-cost kidney transplant patients, center-specific case mix, and center quality. Among 43 393 kidney transplants in Medicare recipients, 35.2% were categorized as high-cost patients. These payments represented 20% of total Medicare payments for kidney transplantation and exceeded $200 million over the study period. Case mix was associated with these payments and was an important factor underlying variation in hospital payments high-cost patients. Hospital quality was also a strong determinant of future Medicare payments for high-cost patients. Compared to high-quality centers, low-quality centers cost Medicare an additional $1185 per kidney transplant. Payments for high-cost patients represent a significant proportion of the total costs of kidney transplant surgical care. Quality improvement may be an important strategy for reducing the costs of kidney transplantation.
Keywords: Economics, financial analysis, quality of care, transplantation outcomes
Introduction
Kidney transplantation is expensive. The costs of an uncomplicated renal transplant event are approximately $100 000 for the first in 90 days of care (1,2). CMS is the primary payer for most of kidney transplant patients and though kidney transplantation is cost effective compared to long-term dialysis, kidney transplant recipients consume significant healthcare resources (3). CMS spends $27 billion/year on the care of renal failure patients, and approximately 13% of this amount is for kidney transplantation (4).
Reducing adverse outcomes following kidney transplantation may be one strategy for reducing these high costs. Clearly, payments associated with adverse surgical outcomes represent a significant expense for CMS (5,6). For example, postkidney transplant sepsis the costs insurers $48 000, posttransplant pneumonia costs insurers $38 000 and postkidney transplant urinary complications are associated with $28 000 of additional insurer costs (1,2,7). Significant variation among hospitals in outcomes suggests opportunities to avoid excess costs associated with adverse outcomes and high-cost patients (8,9).
In this context, we studied Medicare payments associated with high-cost patients following the kidney transplant event. We focused on two specific sources of Medicare payments, 30-day readmission payments to hospitals and outlier payments. The link between adverse outcomes and 30-day readmissions is clear. Although less well known, outlier payments may be equally as important. Medicare outlier payment and 30-day readmission policies are designed to promote access to care for extremely costly patients. It remains unclear the degree to which these policies are supporting high-risk patients compared to poor-quality care.
Within this context, the goals of this article include:
Describe the epidemiology of Medicare payments for high-cost patients in kidney transplantation.
Describe the relationship between these all payments and both measures of high-risk patients and poor quality.
Methods
Subjects and data bases
This study was based on the Medicare Provider Analysis and Review (MEDPAR) files from 2003 to 2006. Because services provided to Medicare managed care patients are not consistently captured in the MEDPAR files, such patients were excluded from our study (<1% of kidney transplant recipients). Patients undergoing isolated kidney transplant (living and deceased donor) were identified using the procedure codes from the International Classification of Diseases, version 9. Medicare was identified as the primary payer for all patients with claims of $15 000 or greater for the kidney transplant event (10).
Determining payments for adverse events
A high proportion of the costs of kidney transplantation are from payments to hospitals for the single kidney transplant diagnosis-related group (DRG). DRG payments were devised under the prospective payment system to bundle payments for hospitals for the care of a patient with a particular diagnosis. Importantly, there is a single DRG for kidney transplantation that cannot be adjusted for illness severity or case complexity.
For the purposes of this analysis, we identified two additional types of payments in addition to DRG payments. Hospitals may receive payments for readmissions and outlier payments, both of which are associated with adverse outcomes. Readmission payments, outlier payments and payments for adverse events are defined as:
Readmission payments
Payments made to hospitals for any admission within 30 days of the kidney transplant operation.
Outlier payments
How Medicare pays hospitals for unusually expensive cases. Medicare identifies outlier kidney transplant cases by comparing the estimated costs for the case to a fixed loss threshold that is specific to DRG 302 (approximately $45 000 for kidney transplant). Once a case qualifies as an outlier, the hospital is reimbursed as a fixed percentage of submitted charges. Importantly, DRG 302 is not modifiable for case complexity. The presence of an outlier payments and the associated amount is specifically identified in Medicare claims.
High-cost patients
The sum of the total readmission payments and outlier payments following kidney transplantation.
We first quantified payments for high-cost patients in kidney transplantation, as a proportion of total Medicare payments. We calculated the percentage of cases associated with such payments, the mean amount of each payment, and the ratio of these payments to total Medicare payments.
We then examined the extent to which payments for high-cost patients are associated with specific patient and hospital characteristics. Additional patient level clinical data was obtained from MEDPAR, including age at transplant, sex, race, donor source (living or deceased) and comorbidities (11). Provider level data were collected, including hospital teaching status and kidney transplant operative volume (ranked in terciles). A patient level analysis was done using a multivariable logistic regression model with the outlier payment status as the dependant variable (measured as a binary variable) and the covariates mentioned above as the independent variables. Another model was created with 30-day readmission payment status as the dependent variable.
Variation in payments across hospitals
We then assessed variation in rates of payments for high-cost patients among hospitals. For this analysis we calculated the proportion of total hospital payments that were attributable to high-cost patients (2003–2006). Though data in this analysis were not explicitly adjusted for differences in pricing across regions and hospitals, our reliance on the ratio of adverse outcome payments to total payments implicitly deals with price adjustment.
We then investigated the relationship between payments for high-cost patients and hospital case mix (illness severity of kidney transplant recipients and donor source). For this analysis, we first ranked hospitals by predicted mortality. Predicted mortality was derived by creating a multiple logistic regression equation using the following dependant variables: patient characteristics (age, race, sex, comorbidities and donor source) and hospital characteristics (transplant volume and teaching status) (12,13). Comorbidities were identified by ICD-9 codes and we used the methods of Elixhauser to adjust for these comorbidities at the patient level (11). We then related hospital predicted risk of mortality (our measure of case mix) to two center-specific outcome measures: the proportion of cases qualifying for payments for high-cost patients and the proportion of hospital reimbursement from payments for high-cost patients. Hospitals were then ranked and sorted into terciles according to case mix. We then assessed relationships between hospital case mix and to two center-specific outcome measures: the proportion of kidney transplant cases qualifying for payments for high-cost patients and the proportion of hospital reimbursement from payments for high-cost patients following kidney transplantation.
Using similar methods, we assessed associations between hospital quality and Medicare payments for high-cost patients. To characterize hospital quality, we used a previously validated composite measure (14,15). In brief, this measure was created by using an empirical Bayes approach to combine two important domains of quality: risk-adjusted 30-day mortality and center-specific kidney transplant volume. The weight placed on each input varied across hospitals, with more weight placed on 30-day mortality when it was measured reliably (i.e. hospitals have higher caseloads). The remaining weight was then placed on hospital volume. The risk adjusted mortality was calculated as above. We then calculated the ‘volume predicted mortality’ by shrinkage of the observed mortality rate back toward the mortality rate expected given the volume at that hospital. The combined measure was calculated as follows: composite mortality prediction = (weight) × (observed mortality) + (1 – weight) × (volume – predicted mortality). This approach ensures an optimal combination of these two measures, as the direct measure (adjusted mortality) is weighted to the extent it is reliable, and the proxy measure (volume) is weighted only to the extent necessary. Hospitals were then ranked and sorted into terciles according to this composite measure of quality. Using multiple linear regression techniques, we then related hospital predicted risk of mortality (our measure of case mix) to two center-specific outcome measures: the proportion of cases qualifying for payments for adverse events and the proportion of hospital reimbursement from payments for high-cost patients.
Since mortality is a significant component of our composite measure for quality, and these patients may be more likely to qualify as high-cost patients, assessing payments and quality on contemporaneous data samples would result in spuriously strong correlation between the two measures. As a result, hospital quality was characterized based on data from 2003 to 2004, and Medicare payments for high-cost patients were reported using data from 2005 to 2006.
All statistical analyses were conducted using STATA 10.0 (College Station, TX). This study was judged exempt by the Institutional Review Board at the University of Michigan.
Results
Determining payments for adverse events
A total of 43 393 kidney transplants were completed on Medicare recipients during the study period (2003–2006). As described in Table 1, 6.7% of kidney transplant patients qualified for Medicare outlier payments with a mean outlier payment amount of $29 579. Outlier payments represented 8.2% of total Medicare payments for the kidney transplant event. In addition, 28.5% of kidney transplant recipients had payments for a readmission within 30 days and the mean payment for this readmission from Medicare was $9962. Over the study period, Medicare paid kidney hospitals over $200 million in payments for high-cost patients, representing 20.0% of Medicare payments for the kidney transplant event.
Table 1.
Medicare payments for high-cost kidney transplant patients (N = 43 393)
| Outlier payments |
30-day readmission payments |
Total | |
|---|---|---|---|
| Total # patients | 2894 | 12 384 | 15 278 |
| Percentage of patients | 6.7 | 28.5 | 35.2 |
| Mean payment | $29 579 | $9962 | $13 678 |
| Proportion of total Medicare payments | 8.2 | 11.8 | 20.0 |
| Total payments | $85 602 000 | $123 363 000 | $208 965 000 |
Patient characteristics had a significant effect on the likelihood of outlier payments (Table 2). Specifically, black patients and patients with more comorbidities were more likely to qualify for outlier payments. In addition, recipients of living donor kidney transplants were less likely to receive outlier payments. Similarly, patient-specific characteristics had a significant effect on the rates of 30-day readmission payments. Black patients and patients with more comorbidities were more likely to be readmitted within 30 days. In addition, recipients of living donor kidney transplants were less likely to require a readmission.
Table 2.
Patient and hospital demographics by outlier payment status or readmission status for kidney transplantation (MEDPAR 2005–2006)
| Outlier payments status | Readmission status | |||||||
|---|---|---|---|---|---|---|---|---|
| Demographics | Patients with outlier payment |
Patients without outlier payment |
OR (95% CI) | Patients with 30-day readmission |
Patients without 30-day readmission |
OR (95% CI) | ||
| Number of patients | 1663 | 20 889 | NA | NA | 6420 | 16 132 | NA | NA |
| Patient characteristics | ||||||||
| Age (% 65+ years) | 18.1 | 17.9 | 1.03 | (0.89, 1.18) | 18.6 | 17.7 | 1.07 | (0.99, 1.16) |
| Sex (% female) | 40.5 | 38.8 | 1.06 | (0.95, 1.18) | 39.5 | 38.8 | 1.01 | (0.95, 1.07) |
| Race (% black) | 33.1 | 26.6 | 1.32 | (1.17, 1.48) | 30.8 | 25.7 | 1.22 | (1.14, 1.31) |
| Comorbidity (% >2) | 50.1 | 46.3 | 1.12 | (1.01, 1.24) | 51.7 | 44.5 | 1.32 | (1.25, 1.41) |
| Living donor (%) | 23.0 | 30.3 | 0.71 | (0.63, 0.81) | 24.3 | 31.9 | 0.72 | (0.67, 0.77) |
| Provider characteristics | ||||||||
| Teaching (%) | 77.1 | 76.7 | 0.99 | (0.87, 1.13) | 79.2 | 75.7 | 1.15 | (1.07, 1.24) |
| Hospital volume tercile 1 | 35.7 | 33.6 | Reference | 32.1 | 34.4 | Reference | ||
| 2 | 34.7 | 33.5 | 0.92 | (0.81, 1.05) | 35.1 | 33.0 | 1.13 | (1.05, 1.22) |
| 3 | 29.7 | 32.9 | 0.90 | (0.79, 1.03) | 32.8 | 32.7 | 1.10 | (1.01, 1.19) |
Hospital teaching status did not significantly affect rates of outlier payments. Conversely, patients who received their kidney transplant at a teaching institution were more likely to be readmitted within 30 days of the transplant. There were nonsignificant trends suggesting lower rates of outlier payments among higher-volume kidney hospitals (>50 Medicare cases per year). Patients that received a kidney transplant at a medium (between 25 and 50 Medicare cases per year) and high-volume hospitals were more likely to require 30-day readmission compared to low-volume hospitals (<25 Medicare cases per year).
Variation in payments across hospitals
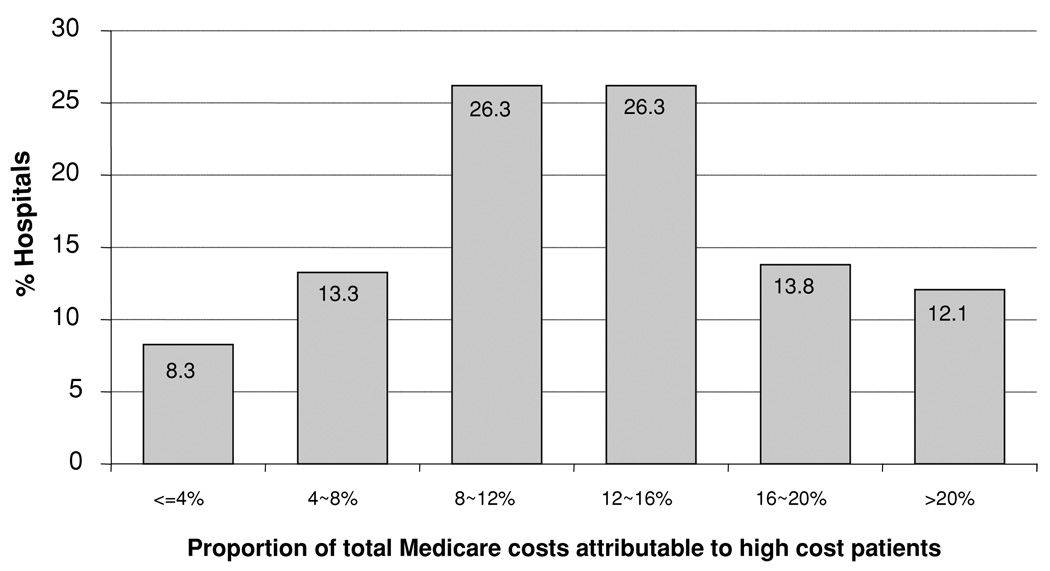
Among kidney hospitals, there was significant variation in the average proportion of total Medicare payments attributable to high-cost patients (Figure 1). The proportion of patients qualifying for payments for high-cost patients ranged across hospitals, from 5% to 50%.
Figure 1. The proportion of total costs attributable to high-cost patients; the distribution across US hospitals.
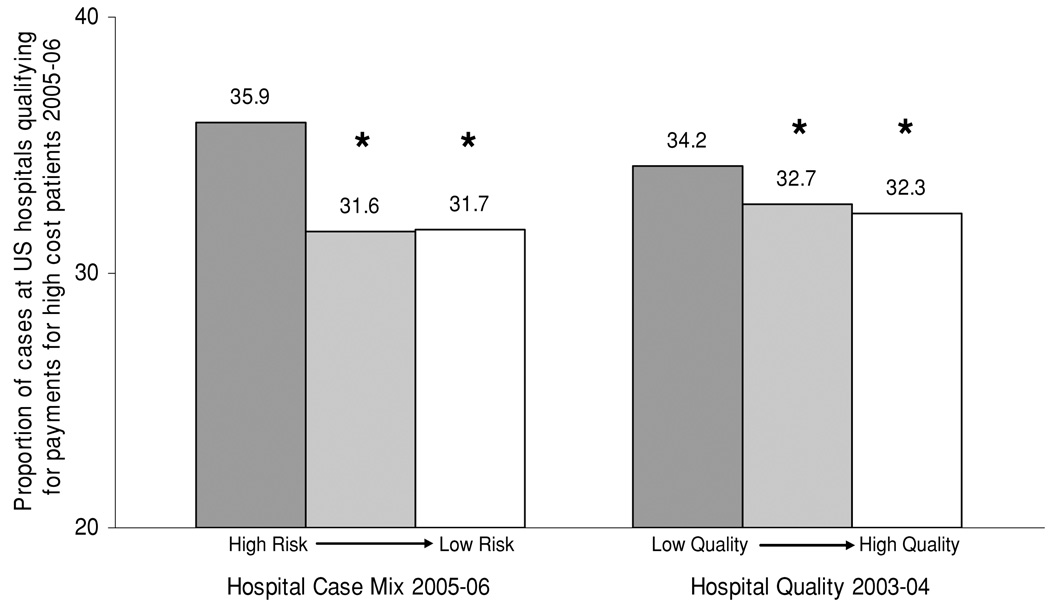
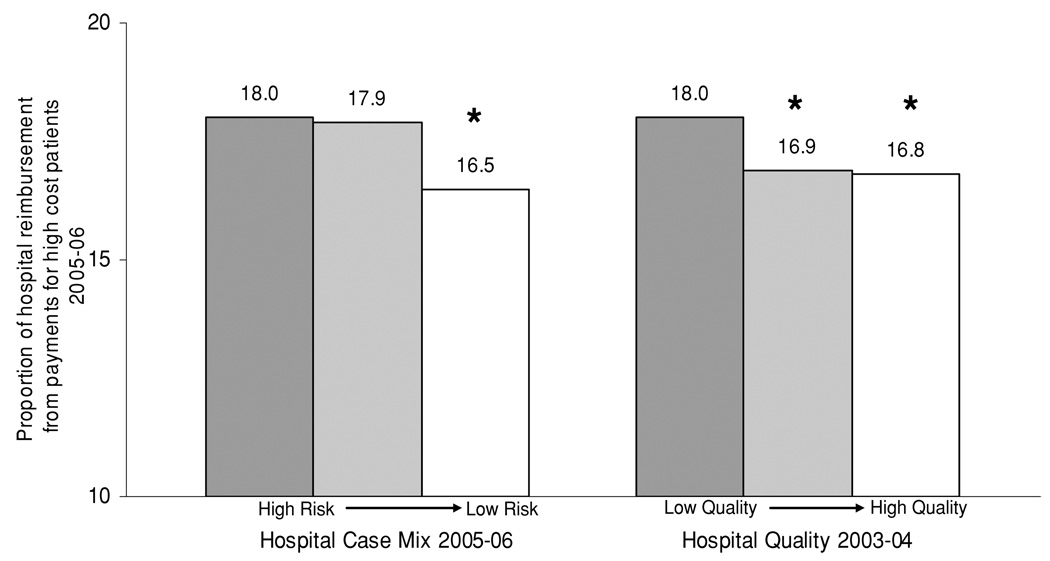
We then assessed the relationship between hospital case mix and payments for high-cost patients. Hospital case mix was stratified by terciles (ranging from high risk to low risk) and then related to the proportion of cases that qualified for payments for high-cost patients (Figure 2). Hospitals with the highest risk case mix had the highest proportion of cases that qualified as high-cost patients. Similarly, hospital case mix was stratified by terciles and then related to the proportion of hospital reimbursement from payments for high-cost patients (Figure 3). Hospitals with the highest risk case mix had the highest proportion of hospital reimbursement from payments for high-cost patients.
Figure 2. The relationship between kidney hospital-specific case mix, quality and the proportion of cases which qualify for payments for high-cost patients across US hospitals.
For comparisons of case mix, *indicates p < 0.001 compared to hospitals with a high case mix risk profile. For comparisons of hospital quality, *indicates p < 0.001 compared to hospitals with low measures of quality.
Figure 3. The relationship between kidney hospital-specific case mix, quality and the proportion of hospital reimbursement from payments for high-cost patients across US hospitals.
For comparisons of case mix, * indicates p < 0.001 compared to hospitals with high-risk case mix risk profile. For comparisons of hospital quality, * indicates p < 0.001 compared to hospitals with low measures of quality
We then assessed the relationship between historical hospital performance (hospital quality 2003–2004) and future payments for high-cost patients (2005–2006). Hospital performance was stratified by terciles (ranging from low quality to high quality) and then related to the proportion of cases that qualified for payments for high-cost patients (Figure 2). Similar to the hospitals with the highest risk case mix, hospitals of the lowest historical performance had the highest future proportion of cases that qualified for payments for high-cost patients. Then, hospital quality was stratified by terciles and related to the proportion of hospital reimbursement from payments for high-cost patients (Figure 3). Similar to hospitals with the highest risk case mix, hospitals with the lowest historical performance had the highest future proportion of hospital reimbursement from payments for high-cost patients.
Overall, hospitals determined to be low quality in 2003–2004 had an average payment for high-cost patients that was $1185 larger than the average payments made to high-quality centers in 2005–2006.
Discussion
Medicare payments for high-cost patients (outlier and 30-day readmission payments) represent a significant proportion of a total cost of kidney transplant surgical care in the United States. Among kidney hospitals, there is significant variation in the rates of these payments. High-risk patients were more likely to qualify for these payments and hospitals with more high-risk patients received larger payments. Upon adjusting for these differences in case mix, historically high-performing hospitals had lower rates of payments for adverse outcomes in the future. This finding suggests that hospital quality has significant impact on Medicare reimbursements in kidney transplantation.
There is little previous work in transplantation focusing on the relationship between quality and costs. Clearly, post-operative complications have been linked to increased hospital costs (16–19). Similarly, variations in case mix such as donor quality or recipient burden of illness have been associated with hospital costs (20–22). Unfortunately, previous work investigating the financial implications of adverse outcomes and high-cost patients have been limited to single institutions, thus center quality could not be assessed.
Outlier payments and payments for 30-day readmission can, in part be considered ‘excess’ costs to Medicare, and would potentially be significantly impacted by quality improvement initiatives. Our observation that high-quality hospitals have low payments for high-cost patients is perhaps not surprising. High-quality hospitals may have fewer patients requiring reoperation, dialysis, prolonged length of stay, intensive care or interventions that might results in outlier payments and readmissions. Nonetheless, our work is among the first to demonstrate a direct relationship between hospital quality and payments in kidney transplantation.
Our study has several important limitations. First, this analysis was based on Medicare claims data. Certainly, this administrative data has limitations in capturing clinical variables, and as a result we may underestimate the impact of illness severity on payments for high-cost patients. For example, we adjust only for living donor and deceased donor, and do not adjust the significant variation in the quality of deceased donor grafts (20). Previous work has suggested that there is little variation in hospital case mix for other surgical procedures, but this has not been shown in transplantation (23). Similarly, this analysis is limited to a single payer (Medicare) and can not be generalized to private payers. That having been said, considering Medicare’s role as the primary payer for the majority of kidney transplants in the United States, our findings are likely to inform policy makers. Presumably, most hospitals bill for services rendered, but variation in billing practices may affect hospital outlier payment rates. We are unable to control for these variations. Second, we used the word quality in a very narrow sense, not considering the multidimensional nature of this measure within the context of transplantation. More specifically, we have focused on only one domain of hospital quality, operative (30 day) mortality. Other measures of quality, such as complication rates, graft function rates, or waitlist mortality, or quality of life measures, may be even more predictive of center performance. Future analyses will focus on developing a more robust composite measure of hospital quality. Our composite measure is better in capturing systematic variation and forecasting future hospital performance than individual quality indicators (e.g. volume, risk-adjusted mortality alone). To the extent that this composite remains an imperfect proxy of hospital quality, however, our study may underestimate the relationship between quality and payments for high-cost patients. Nonetheless, these data do suggest that better measures of quality may not only discriminate hospitals based on quality, but they may also be useful in selecting lower-cost hospitals. Third, perioperative payments are only portion of the costs associated with kidney transplantation, and assessment of total costs, over longer periods of time and including the costs of long-term dialysis, would be essential. Similarly, it is important to note that poor quality will affect more than just payments for adverse outcomes. These patients will presumably also require additional resources to pay for outpatient care, home health care and extended care facilities after discharge. None of these payments by Medicare have been quantified in this analysis.
As Medicare pursues value-based purchasing strategies in kidney transplantation, understanding the relationship between quality and costs will be paramount. On one hand, it is plausible that higher quality could, in fact, lead to higher costs. For example, hospitals may have better outcomes related to closer surveillance or more testing, all of which have real costs. If so, and costs were directly associated with quality, this would imply the need for payers to make explicit trade-offs, balancing both quality and costs. On the other hand, our analysis suggests that the alternative hypothesis is valid and that value-based purchasing strategies built around optimizing quality may also reduce costs. Moreover, the findings of our work suggest a viable business case for quality improvement at all kidney hospitals in the United States. Within this context, selective contracting for kidney transplant care at ‘centers of excellence’ could lead to cost savings, assuming that accurate measures of ‘excellence’ are employed. Unfortunately, center of excellent models may affect access to kidney transplant care for some candidates. Another potential model for quality improvement involves a systematic identification of best practices. More specifically, a comparison of the practices at the highest performing centers compared to lowest performing centers may elucidate specific practice patterns associated with high quality and efficient kidney transplant care. Another approach to quality improvement in kidney transplantation involves higher payments by Medicare to centers with the best performance. Unfortunately, Medicare demonstration projects using ‘pay for performance’ strategies have been questionably effective.
An attractive model for quality improvement in kidney transplantation involves a Medicare funded ‘pay for participation’ program. In short, Medicare would offer a premium to the DRG 302 for hospitals who participates in a regional or national quality improvement collaborative. This premium would in part fund additional data reporting on specific process and outcome measures. Regional quality improvement collaboratives have been successful in cardiac surgery in Northern New England, and general surgery in Michigan (24,25). Quality collaboratives in transplantation would not require comprehensive reform of current fee structures and would be unlikely to reduce access to kidney transplantation for high-risk populations (25,26). Overall, a collaborative focusing on improving kidney transplant may offer significant opportunities to improve quality while reducing costs.
In summary, Medicare payments for high-cost patients made to hospitals following kidney transplantation represent a significant portion of the costs of kidney transplant surgery. As expected, payments for adverse event or more likely, in cases of high-risk patients. Just as much if not even to a larger degree, payments for high-cost patients are linked to variations in hospital quality. As a result, current Medicare payment policies for adverse events are not only supporting the care of high-risk kidney transplant recipients, as they should, but they are also supporting poor-quality care. Thus, efforts aimed at quality improvement could reduce Medicare costs by reducing payments for adverse perioperative outcomes to hospitals. Further research should focus on broader and longer-term measures of kidney transplant costs and quality and utilize more robust data sets.
Acknowledgment
Dr. Englesbe is supported by a grant from the American Surgical Association Foundation.
References
- 1.Englesbe MJ, Dubay DA, Gillespie BW, et al. Risk factors for urinary complications after renal transplantation. Am J Transplant. 2007;7:1536–1541. doi: 10.1111/j.1600-6143.2007.01790.x. [DOI] [PubMed] [Google Scholar]
- 2.DuBay DA, Lynch R, Cohn J, et al. Is routine ureteral stenting cost-effective in renal transplantation? J Urol. 2007;178:2509–2513. doi: 10.1016/j.juro.2007.08.037. discussion 13. [DOI] [PubMed] [Google Scholar]
- 3. [Accessed March 26, 2009];The USRDS 2006 Annual Report. Available from: www.usrds.org.
- 4.World Health Organization (WHO) [Accessed March 26, 2009];World Health Statistics. 2005 Available from: http://www.who.int/healthinfo/statistics/en/.
- 5.Dimick JB, Chen SL, Taheri PA, Henderson WG, Khuri SF, Camp-bell DA., Jr Hospital costs associated with surgical complications: A report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531–537. doi: 10.1016/j.jamcollsurg.2004.05.276. [DOI] [PubMed] [Google Scholar]
- 6.Dimick JB, Weeks WB, Karia RJ, Das S, Campbell DA., Jr Who pays for poor surgical quality? Building a business case for quality improvement. J Am Coll Surg. 2006;202:933–937. doi: 10.1016/j.jamcollsurg.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Kutinova A, Woodward RS, Ricci JF, Brennan DC. The incidence and costs of sepsis and pneumonia before and after renal transplantation in the United States. Am J Transplant. 2006;6:129–139. doi: 10.1111/j.1600-6143.2005.01156.x. [DOI] [PubMed] [Google Scholar]
- 8. [Accessed March 26, 2009];The 2006 OPTN/SRTR Annual Report – US Department of Health and Human Services. Available from: www.ustransplant.org.
- 9.Transplant Services 2003 Benchmarking Project Findings and Conclusions; University Health Systems Consortium kidney transplant web conference; 2004. Apr 5, [Google Scholar]
- 10.Buchanan PM, Lentine KL, Burroughs TE, Schnitzler MA, Salvalaggio PR. Association of lower costs of pulsatile machine perfusion in renal transplantation from expanded criteria donors. Am J Transplant. 2008;8:2391–2401. doi: 10.1111/j.1600-6143.2008.02412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Birkmeyer JD, Sun Y, Goldfaden A, Birkmeyer NJ, Stukel TA. Volume and process of care in high-risk cancer surgery. Cancer. 2006;106:2476–2481. doi: 10.1002/cncr.21888. [DOI] [PubMed] [Google Scholar]
- 13.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 14.Staiger DO, Dimick JB, Baser O, Birkmeyer JD. Empirically derived composite measures of surgical performance. Medical Care. 2009;47:226–233. doi: 10.1097/MLR.0b013e3181847574. [DOI] [PubMed] [Google Scholar]
- 15.Dimick JB, Birkmeyer JB. Composite measures for predicting hospital mortality with surgery: The 2008 Leapfrog standards. Washington, DC: The Leapfrog Group; 2008. [Google Scholar]
- 16.Ammori JB, Pelletier SJ, Mathur A, et al. Financial implications of surgical complications in pediatric liver transplantation. Pediatr Transplant. 2008;12:174–179. doi: 10.1111/j.1399-3046.2007.00783.x. [DOI] [PubMed] [Google Scholar]
- 17.Ammori JB, Pelletier SJ, Lynch R, et al. Incremental costs of post-liver transplantation complications. J Am Coll Surg. 2008;206:89–95. doi: 10.1016/j.jamcollsurg.2007.06.292. [DOI] [PubMed] [Google Scholar]
- 18.Cohn JA, Englesbe MJ, Ads YM, et al. Financial implications of pancreas transplant complications: A business case for quality improvement. Am J Transplant. 2007;7:1656–1660. doi: 10.1111/j.1600-6143.2007.01791.x. [DOI] [PubMed] [Google Scholar]
- 19.Englesbe MJ, Dimick J, Mathur A, et al. Who pays for biliary complications following liver transplant? A business case for quality improvement. Am J Transplant. 2006;6:2978–2982. doi: 10.1111/j.1600-6143.2006.01575.x. [DOI] [PubMed] [Google Scholar]
- 20.Englesbe MJ, Ads Y, Cohn JA, et al. The effects of donor and recipient practices on transplant center finances. Am J Transplant. 2008;8:586–592. doi: 10.1111/j.1600-6143.2007.02098.x. [DOI] [PubMed] [Google Scholar]
- 21.Axelrod DA, Koffron AJ, Baker T, et al. The economic impact of MELD on liver transplant centers. Am J Transplant. 2005;5:2297–2301. doi: 10.1111/j.1600-6143.2005.01025.x. [DOI] [PubMed] [Google Scholar]
- 22.Axelrod DA, Schnitzler M, Salvalaggio PR, Swindle J, Abecassis MM. The economic impact of the utilization of liver allografts with high donor risk index. Am J Transplant. 2007;7:990–997. doi: 10.1111/j.1600-6143.2006.01724.x. [DOI] [PubMed] [Google Scholar]
- 23.Dimick JB, Welch HG, Birkmeyer JD. Surgical mortality as an indicator of hospital quality: The problem with small sample size. JAMA. 2004;292:847–851. doi: 10.1001/jama.292.7.847. [DOI] [PubMed] [Google Scholar]
- 24.O’Connor GT, Plume SK, Olmstead EM, et al. The Northern New England Cardiovascular Disease Study Group. A regional intervention to improve the hospital mortality associated with coronary artery bypass graft surgery. JAMA. 1996;275:841–846. [PubMed] [Google Scholar]
- 25.Englesbe MJ, Dimick JB, Sonnenday CJ, Share DA, Campbell DA., Jr The Michigan surgical quality collaborative: Will a statewide quality improvement initiative pay for itself? Ann Surg. 2007;246:1100–1103. doi: 10.1097/SLA.0b013e31815c3fe5. [DOI] [PubMed] [Google Scholar]
- 26.Englesbe MJ, Pelletier SJ, Kheterpal S, O’Reilly M, Campbell DA., Jr A call for a national transplant surgical quality improvement program. Am J Transplant. 2006;6:666–670. doi: 10.1111/j.1600-6143.2006.01267.x. [DOI] [PubMed] [Google Scholar]