Abstract
Because changes in subendothelial matrix composition are associated with alterations of the endothelial immune phenotype, we sought to understand if cytokine-induced NF-κB activity and downstream effects depend on substrate adherence of endothelial cells (EC). We compared the upstream phosphorylation cascade, activation of NF-κB, and expression/secretion of downstream effects of EC grown on tissue culture polystyrene plates (TCPS) with EC embedded within collagen-based matrices (MEEC). Adhesion of natural killer (NK) cells was quantified in vitro and in vivo. NF-κB subunit p65 nuclear levels were significantly lower and p50 significantly higher in cytokine-stimulated MEEC than in EC-TCPS. Despite similar surface expression of TNF-α receptors, MEEC had significantly decreased secretion and expression of IL-6, IL-8, MCP-1, VCAM-1, and ICAM-1. Attenuated fractalkine expression and secretion in MEEC (two to threefold lower than in EC-TCPS; p < 0.0002) correlated with 3.7-fold lower NK cell adhesion to EC (6,335 ± 420 vs. 1,735 ± 135 cpm; p < 0.0002). Furthermore, NK cell infiltration into sites of EC implantation in vivo was significantly reduced when EC were embedded within matrix. Matrix embedding enables control of EC substratum interaction. This in turn regulates chemokine and surface molecule expression and secretion, in particular of those compounds within NF-κB pathways, chemoattraction of NK cells, local inflammation, and tissue repair.
Keywords: Endothelial cell, Extracellular matrix, NF-κB, Natural killer cell
INTRODUCTION
Endothelial integrity regulates vascular tone, luminal patency, and the immune reactivity to tissue and organ transplants. Endothelial dysfunction, characterized by impaired biochemical function and heightened immunogenicity, presages vascular illness, serves to initiate and propagate vascular disease initiation, and is a marker of disease severity (6,10). There is, however, a profound paradox in any attempt to restore diseased endothelium. Injections of isolated endothelial cells (EC) onto the surface or in the general vicinity of injured vessels elicit a heightened immune response without restoration of the biochemical milieu they are designed to replace. It is understood that allo- or xenogeneic EC implants induce a significant immune response that is amplified by host and donor cells. But it must also be appreciated that autologous cells can be immunogenic as many of the diseases that are accompanied by endothelial dysfunction are also associated with anti-EC antibodies. The interactions of activated and diseased EC with leukocytes are critical to recruiting circulating immune cells into the vessel wall destroying implanted cells and even exacerbating the initial injury. Recently, natural killer (NK) cells have been identified as pivotal host immune cells linking innate and adaptive immune responses in a variety of organ rejection reactions (5,11,26).
Beside their role in vascularized organ rejection, EC play a pivotal role in inflammatory diseases as well as chronic diseases such as atherosclerosis and autoimmune diseases. In all these circumstances crosstalk with immune cells depends on the activation of EC via danger signals or proinflammatory mediators (3,29). One signaling cascade pivotally involved in proinflammatory activation of EC is tumor necrosis factor-α (TNF-α)-mediated activation of NF-κB (12).
Interestingly, we discovered that the embedding of EC within a matrix allows EC to assume a natural interaction with a substratum. EC in this more energy-favorable configuration are under less strain, and even matrix-embedded xenogeneic EC do not induce a significant host immune response (18,21). Such implants are not only immunosilent, they are immunomodulatory, downregulating immune reactions in concert with profoundly heightened regulation of local biochemical reactivity.
These findings add to the emerging interdependence of EC–substratum interaction, immune recognition, and subsequent control of immune state and biochemical control. We seek to understand the nature of this relationship and can now cast this in terms of modulation of NF-κB-based signaling and, in particular, control of EC implant interactions with NK cells.
MATERIALS AND METHODS
Endothelial Cells
Primary human aortic EC (HAE) were obtained from Cambrex and grown in optimized endothelial growth medium-2 (EGM-2, Cambrex, MD) supplemented with 5% fetal bovine serum (FBS, Life Technologies, NY). Confluent monolayers of second to fifth passage were used for all experiments. For substrate adherence cells were cultured on Gelfoam sheets (Pfizer, NY) as previously described (21). TCPS experiments were conducted on gelatin-coated plates. EC were stimulated with TNF-α (Sigma, St. Louis, MO) for indicated time periods and concentrations at 37°C in a humidified air atmosphere containing 5% CO2. In some experiments EC were pretreated with 5 µmol/L sulfasalazine (Sigma) or a selective Iκκ-2 inhibitor (100 µM SC-514; EMD Bioscience) (13) before addition of cytokines.
NF-κB Binding Activity Assay
p50 and p65 DNA binding was detected by ELISA using a Mercury TransFactor kit following the manufacturer’s instructions (Clontech Laboratories, Palo Alto, CA). Briefly, nuclear extracts were obtained using the TransFactor extraction kit; protein levels were determined with the BCA method (Pierce). A total of 10 µg of nuclear extract was bound to wells coated with NF-κB consensus oligonucleotide, then incubated with anti-p50 and anti-p65 followed by anti-rabbit HRP-conjugated IgG. Resulting binding was then detected by measuring color development of tetramethylbenzidine at 450 nm using a Multiskan plate reader (Labsystems, Chicago, IL).
RT-PCR
Total RNA was extracted (RNeasy Mini Kit, Qiagen, Valencia, CA) from EC grown on polystyrene tissue culture plates (TCPS), or substrate adherent within Gelfoam sheets, after stimulation with TNF-α and IFN-γ. Complementary DNA was synthesized using the TaqMan reverse transcription reagents from Applied Biosystems (Foster City, CA). Real-time PCR analysis was performed with an Opticon Real Time PCR Machine (MJ Research) using SYBR Green PCR Master Mix Reagent Kit (Applied Biosystems). Primers used were: CX3CL1 sense: 5-CCTGTAGCTTTGCTCATCCACTATC-3 and antisense: 5-TCCAAGATGATTGCGGGTT-3 (sequence accession number NM_002996), GAPDH sense: 5-GGCCTCCAAGGAGTAAGACC-3 and antisense: 5-AGGGGTCTACATGGCAACTG-3 (M33197). Data from the reaction were collected and analyzed by the complementary Opticon computer software (MJ Research).
In addition, the RT2 Profiler Custom PCR Array was used to simultaneously examine the mRNA levels of 89 genes, including five “housekeeping gene,s” in 96-well plates according to the protocol of the manufacturer (SuperArray Bioscience). Each reaction included 1 µg of total RNA and appropriate control samples (without addition of reverse transcription or without template). Relative quantification of gene expression was determined from standard curves and normalized to GAPDH.
Flow Cytometry
EC monolayers (incubated in 1 mmol PBS/EDTA for 5 min) or surface-adherent EC (digested with collagenase type I; Worthington Biochemical, NJ) were harvested, cell suspensions washed, and 3 × 105 EC fixed in 4% paraformaldehyde for 10 min. EC were treated with saponin (0.1% saponin, 0.05% NaN3) for intracellular CX3CL1 staining (clone 51637, R&D Systems, Minneapolis, MN) or left untreated for VCAM-1 (clone 1.G11B1; Research Diagnostics, Flanders, NJ) and ICAM-1 surface staining (clone 15.2; Pharmingen, San Diego, CA) for 45 min at 4°C. After two washing steps 104 cells were analyzed by flow cytometry using a FACScalibur instrument and CellQuest software (Becton Dickinson, San Diego, CA).
Western Blot Analysis
Western blot analysis was conducted as previously described (19) using anti-human fractalkine polyclonal antibody (R&D Systems), phospho-IKKα/β and phospho-IκBα (cell signaling Inc., Danvers, MA) and secondary IgG antibody conjugated to horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA) as the secondary antibody.
ELISA
Conditioned medium from nonadherent and surface-adherent EC was harvested after cytokine stimulation for indicated time periods. Secreted fractalkine, IL-6, IL-8, and MCP-1 were detected with commercially available ELISA detection kits (R&D Systems, BD Pharmingen). Samples were stored at −80°C and measured at the same time by the same ELISA to avoid variations of assay conditions.
Confocal and Electron Microscopy
For immunofluorescence analysis, EC were washed in PBS and fixed with 3.7% paraformaldehyde for 15 min at room temperature. After blocking for 60 min in blocking buffer (PBS/10% FCS/0.1% sodium azide) cells were twice washed in washing buffer (PBS/0.05% glycine/0.75% BSA). Some of the cells were incubated with brefeldin A (Sigma; 10 µg/ml for 4 h) before treatment with 0.2% Triton X-100 for 5 min at room temperature. Immunostainings were performed by incubating cells with primary antibodies overnight at 4°C diluted in blocking buffer (10 µg/ml goat-anti human TNF-α receptor I, 55 kDa; 25 µg/ml mouse-anti human TNF-α receptor II, 75 kDa; both R&D). After three washes with washing buffer detection of bound primary antibodies was visualized by the addition of specific Alexa Fluor 488-conjugated secondary antibodies (7.5 µg/ml, Invitrogen) for 1 h at room temperature. Images were collected with a Zeiss LSM510 confocal laser-scanning microscope (Thornwood, NY).
SEM images of uranyl acetate-stained EC were obtained on a Hitachi S4300 SE-N (Hitachi High Technology, Pleasanton, CA) using Quantomix TM QX-102 capsules (Quantomix Ltd, Nes-Ziona, Israel).
Natural Killer Cell Endothelial Cell Binding Assays
Fresh heparinized whole blood was diluted 1:2 in PBS (Gibco, Grand Island, NY), then overlaid on Lymphoprep (density 1.077 g/L, Nycomed, Oslo, Norway) and centrifuged at 900 × g for 30 min. Mononuclear cells were collected and further purified by incubation on prewashed sterile nylon wool columns (Polysciences, Warrington, PA) in RPMI-1640 medium supplemented with glutamine, 25 mmol HEPES buffer, and 10% heat-inactivated FBS for 1 h at 37°C/5% CO2. After gently eluting, remaining mononuclear cells were enriched for NK cells by negative isolation with a Dynal kit following the manufacturer’s instructions (Dynal Biotech, Oslo, Norway). Two-color flow cytometry revealed purity of the negatively selected NK cells with >90% of all cells being CD56+ (clone B159) and very few CD3+ T lymphocytes (<5%; clone HIT3a, both Pharmingen; data not shown). NK cells (106) were incubated with 10 µCi 51Cr, washed in PBS, and then resuspended (5 × 105/well) in 400 µ1 of medium alone or medium containing anti-CX3CR1 antibody at 20 µg/ml for 20 min.
EC were grown to confluence in six-well plates (6 × 105 cell/well) or surface adherent to 3D Gelfoam sheets and activated with 100 U/ml IFN-γ and TNF-α for 20 h at 37°C. Gelfoam matrices were digested with collagenase type I; cells were counted and plated at a concentration of 6 × 105 cells/well in six-well plates for 1 h to allow adherence. Then NK cell suspensions were added to the endothelial monolayer under gentle rocking conditions (10 cycles/min). After 30 min medium was decanted and wells were gently washed before lysis with 1% Triton in PBS. Total binding was determined by measuring individual well-associated counts per minutes using a gamma counter.
In another set of experiments, 6 × 105 EC were cytokine stimulated, plated on cover slips, and fixed with 3% paraformaldehyde for 20 min. Primary staining with mouse anti-human PECAM (clone WM59, Serotec, Raleigh, NC) was followed after three washing steps with washing buffer by secondary (Alexa Fluor 488-conjugated goat anti-mouse, Invitrogen) and nuclear staining (Hoechst 33258, Sigma) for 30 min was followed by a 20-min incubation with PKH26-labeled NK cells (Sigma). Attachment of NK cells to EC was visualized with a Leica DMRA2 fluorescence microscope (Bannockburn, IL).
In Vivo Adhesion of Natural Killer Cells
A previously published animal model (21) was employed to examine NK cell chemoattraction to sites of EC implantation. In brief, 5 × 105 porcine aortic EC (PAE), isolated from naive Large White swine, were implanted in the subcutaneous dorsal space of Sprague-Dawley rats (Taconic, Germantown, NY) as either matrix-embedded or saline-suspended cell pellets. Control animals received saline injections alone. NK cell infiltration was evaluated immunohistochemically 28 days after implantation. Paraffin sections (5 µm) were cut and antigen retrieval performed by microwave heating for 10 min in a 0.01 mol/L citrate buffer. Sections were labeled for NK cells (mouse anti-rat CD161a, clone 10/78, dilution 1:100, Pharmingen) and identified by an avidinbiotin peroxidase complex method (biotinylated horse anti-mouse IgG, Vector Laboratories, Burlingame, CA). Rat spleen was used as a positive control and mouse IgG as negative control. Primary antibodies were applied for 1 h at room temperature, and all sections were counterstained with Mayer’s hematoxylin solution (Sigma).
Statistics
Statistical analyses were performed with JMP software (SAS Institute Inc., USA). Data were normally distributed and expressed as mean ± SD. Comparisons between two groups were analyzed by Student t-test, and comparisons between more than two groups were analyzed by ANOVA followed by Bonferroni post hoc test. A Spearman correlation determined relations between soluble fractalkine levels and intracellular fractalkine expression. A value of p < 0.05 was considered statistically significant.
RESULTS
Substrate Adherence of EC Alters NF-κB Activation and Regulation
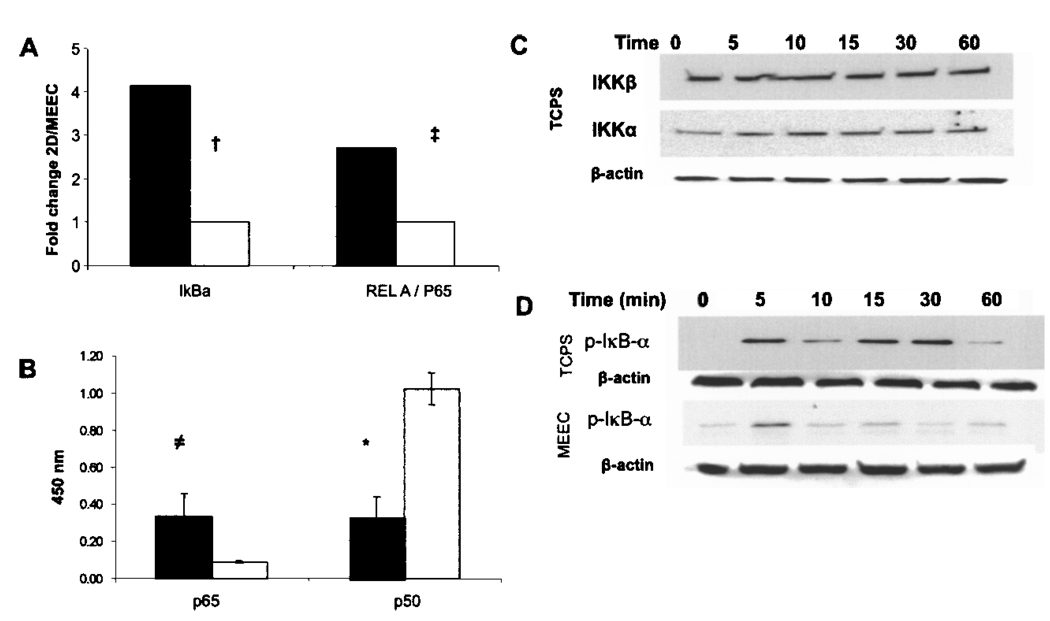
The TNF-α-induced expression of mRNA of the transcriptional active NF-κB subunit p65 in TCPS-grown EC was significantly muted when these same cells were embedded within collagen matrices (p < 0.02) (Fig. 1A). The 2.75-fold reduction in expression of the subunit p65 in MEEC when compared to TCPS-grown EC was accompanied by a 3.0-fold lower protein concentration of p65 in the nuclear extracts upon TNF-α stimulation (p < 0.001) (Fig. 1B). Whereas in MEEC nuclear translocation of the subunit p50 exceeded translocation of p65 12-fold (p < 0.0001), we did not find a significant difference in nuclear protein levels of p65 and p50 subunits in TNF-α-stimulated EC-TCPS (Fig. 1B). Nuclear translocation of the transcriptional inhibitory NF-κB subunit p50 was 3.8-fold higher in MEEC upon TNF-α stimulation than in EC-TCPS (p < 0.0001) (Fig. 1B).
Figure 1.
Matrix embedding influences TNF-α-induced NF-κB activation in endothelial cells. (A) Real-time PCR fold change (ΔΔCt) values of IκBα, and REL-A (p65) for MEEC (opened histogram) and EC-TCPS (closed histogram) stimulated for 6 h with 5 ng/ml TNF-α. (B) Protein levels in the EC nucleus of p65 and p50, after 6-h stimulation with 5 ng/ml TNF-α in MEEC (opened histogram) and EC-TCPS (closed histogram). (C) Western blots of phosphorylated IKKα/β in EC-TCPS (levels were not detected in MEEC). (D) Western blots of phosphorylated IκBα over time in EC grown on TCPS. Western blots are representative for three independent experiments; equal loading was verified with β-actin.†p < 0.002, ‡p < 0.02, ≠p < 0.008, *p < 0.0001.
Embedding of EC Alters Protein Phosphorylation Upstream of NF-κB
TNF-α stimulation (5 ng/ml) resulted in rapid phosphorylation of IKKα and IKKβ in EC-TCPS but not in MEEC where neither phosphorylated IKKα nor phosphorylated IKKβ could be detected (Fig. 1C, data for MEEC not shown). Attenuated phosphorylation of IKK in MEEC was followed by attenuated and curtailed phosphorylation of IκBα in MEEC upon TNF-α stimulation (p < 0.04) (Fig. 1D). This correlated with significant reduced mRNA expression the inhibitory signaling molecule IκBA in MEEC when compared to EC-TCPS (4.15-fold reduction, p<0.002) (Fig. 1A). Phosphorylation and subsequent separation of IκBα from the p50/p65 complex is a prerequisite for the nuclear translocation of the NF-κB subunits.
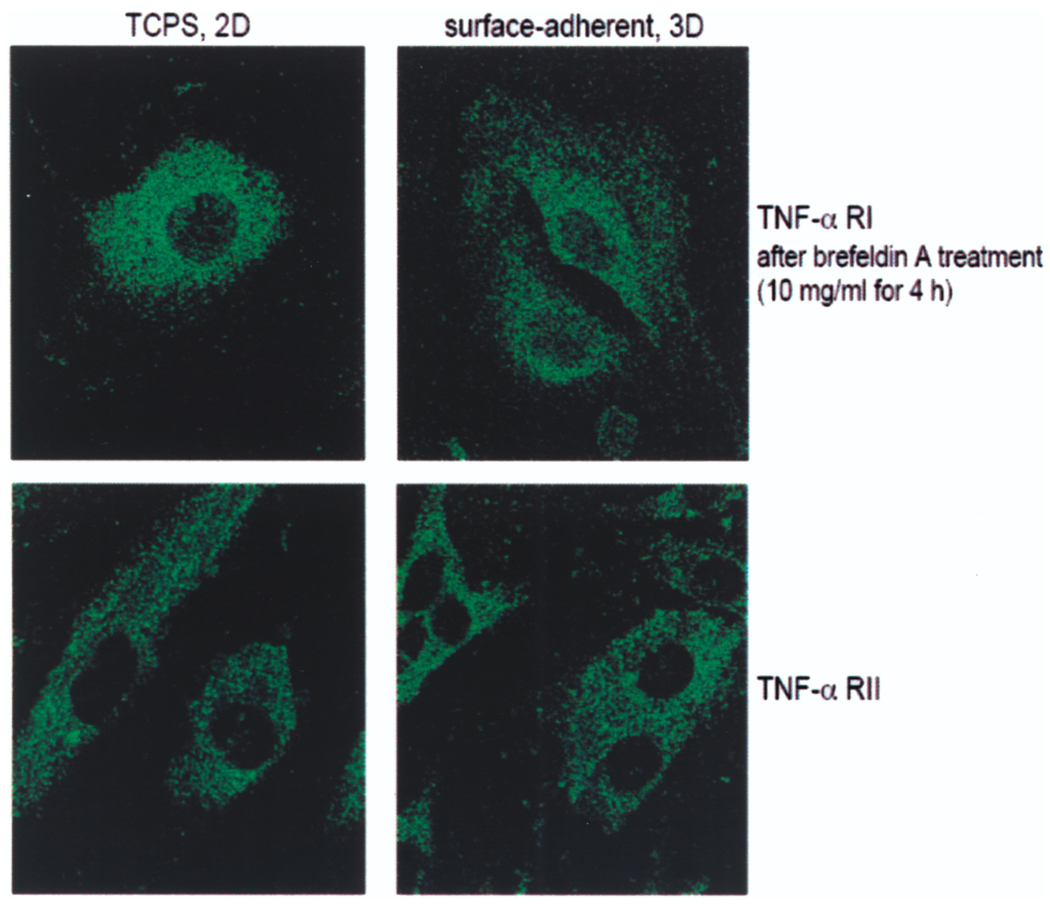
Interestingly, we could not find significant differences in surface and cytoplasmatic staining intensities of TNF-α receptor subunits I and II in EC for the two cell vehicles under confocal immunofluorescence microscopy (Fig. 2).
Figure 2.
Expression patterns of TNF-α receptors subunits I and II are similar between EC-TCPS and MEEC. EC were stained with antibodies against both subunits of TNF-α receptors. For intracellular staining cells were pretreated with brefeldin A and Triton X-100. Bound primary antibodies were visualized by the addition of specific Alexa Fluor 488-conjugated secondary antibodies and analyzed by confocal laser-scanning microscope (100× magnification).
Surface Molecule Expression and Cytokine Receptor Expression and Secretion Downstream of NF-κB
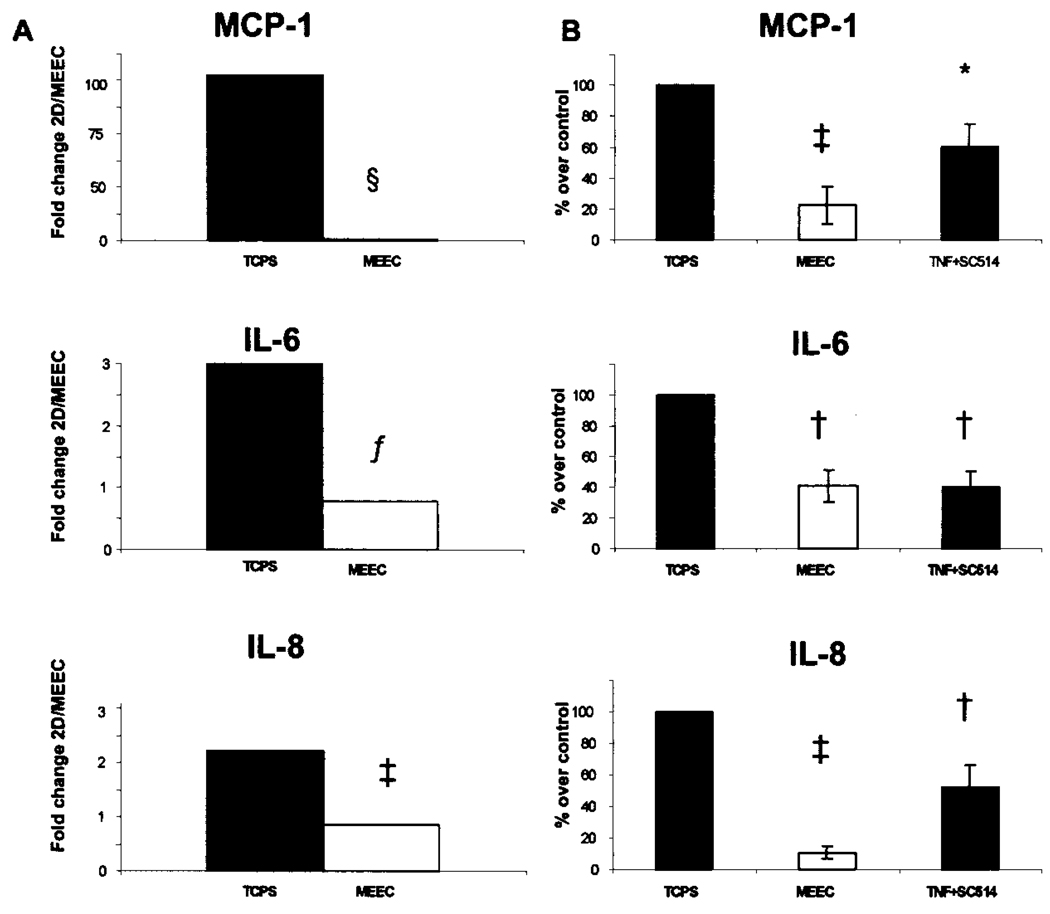
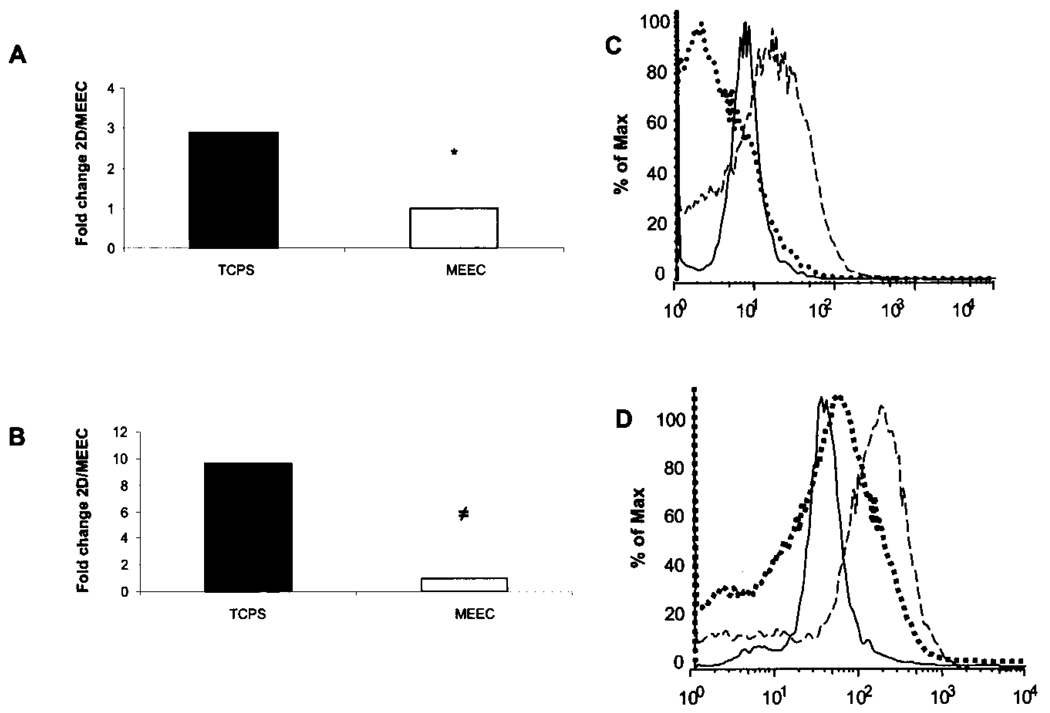
Attenuated nuclear translocation of the transcriptional active NF-κB subunit p65 upon TNF-α stimulation in matrix-embedded EC associated with lower mRNA levels of pivotal proinflammatory cytokines (IL-6: 3.1-fold lower than in EC-TCPS, p < 0.02; IL-8: 2.1-fold, p < 0.01) (Fig. 3), and adhesion molecules ICAM-1 (1.6-fold, p < 0.01), VCAM-1 (1.3-fold, p < 0.02) (Fig. 4A and B). Protein levels followed suit (Figs. 3B, 4C and D). Preincubation of EC-TCPS with a selective IKK-2 inhibitor (100 µM SC-514) (13) resulted in a marked downregulation of the cytokine-induced expression of the studied molecules (Figs. 3B, 4C and D).
Figure 3.
EC–substratum interaction influences NF-κB-dependent expression and secretion of proinflammtory mediators. (A) Real-time PCR ΔΔCt values for MCP-1, IL-6, and IL-8 following 6-h stimulation with 5 ng/ml TNF-α. (B) Protein levels of MCP-1, IL-6, and IL-8 following 6-h stimulation with 5 ng/ml TNF-α with and without SC514 100 µM, a IKK-2 inhibitor applied to EC-TCPS. §p < 0.0001, fp < 0.02, ‡p < 0.001, *p < 0.05, †p < 0.01.
Figure 4.
EC–substratum interaction influences NF-κB-dependent expression of adhesion molecules. Real-time PCR ΔΔCt of (A) VCAM-1 and (B) ICAM in MEEC (opened histogram) and EC-TCPS (closed histogram) stimulated for 6 h with 5 ng/ml TNF-α. Flow cytometry analysis of (C) VCAM and (D) ICAM expression on embedded EC (solid line), TCPS (dashed line), and TCPS with SC514 100 µM (dotted line) following 24-h stimulation with TNF-α. ≠p < 0.008, *p < 0.05.
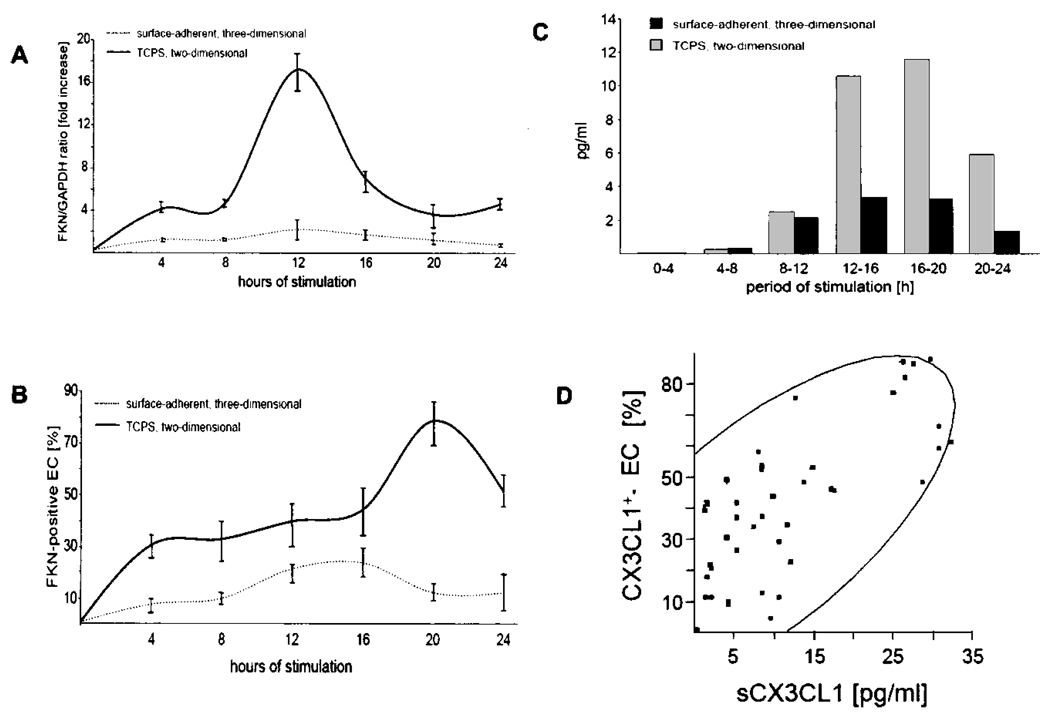
Furthermore, substrate adherence of EC within the matrix attenuated the cytokine-induced expression and secretion of the endothelial chemokines MCP-1 and fractalkine (Figs. 3 and Fig. 5). Resting EC-TCPS or MEEC did not express fractalkine (Fig. 5). TNF-α induced fractalkine mRNA expression in a time-dependent manner in EC-TCPS with a maximum induction after 12 h and a sustained effect over the entire 24-h study period. In contrast, induction of fractalkine mRNA expression was significantly reduced throughout the study period in MEEC (Fig. 5A). Maximum was here too attained 12 h after cytokine stimulation, but at only ~10% of the expression levels in EC-TCPS [0.031 ± 0.01 relative units (RU) vs. 0.3 ± 0.04 RU; p < 0.0001] (Fig. 5A). Increase of cytokine concentrations (up to 400 U/ml IFN-γ and TNF-α) as well as prolongation of the duration was without additional effect on fractalkine mRNA expression in MEEC (data not shown). Following suit, maximal intracellular fractalkine expression upon cytokine stimulation was 3.3-fold higher in EC-TCPS than in MEEC as detected by flow cytometry analysis (76.5 ± 8.6% vs. 22.8 ± 5.7%; p < 0.0001) (Fig. 5B). In addition Western blot analysis revealed lower total fractalkine expression in MEEC compared to EC-TCPS (data not shown). Interestingly, preincubation of EC-TCPS with the unspecific NF-κB inhibitor sulfasalazine (5 mol/L, Sigma) resulted in an attenuated expression profile of fractalkine upon TNF-α stimulation similar to the one observed in MEEC (real-time PCR: 0.038 ± 0.02, flow cytometry: 25.4 ± 10.2%; p < 0.0001 vs. TNF-α-stimulated EC-TCPS without sulfasalazine; data not shown). Soluble fractalkine concentrations were similarly significantly lower in the supernatant of cytokine-stimulated MEEC when compared to EC-TCPS in each 4-h time interval studied and as a cumulative result over the 24-h study period (32.2 ± 2.4 vs. 13.8 ± 1.7 pg/ml; p < 0.0002) (Fig. 5C). A strong correlation was noted between soluble fractalkine levels and intracellular protein expression in both EC groups (r = 0.70, p < 0.01) (Fig. 5D).
Figure 5.
Matrix embedding attenuates induction of fractalkine mRNA expression in EC. Fractalkine mRNA expression was normalized to GAPDH mRNA expression. (A) The fractalkine/GAPDH ratio for all treatment conditions was expressed as fold induction or repression of the fractalkine/GAPDH ratio determined for stimulation of MEEC with 100 U/ml TNF-α and IFN-γ for 4 h. Data are presented as mean ± SD from eight different experiments. (B) Cytokine stimulation induces significantly lower endothelial fractalkine expression when EC are matrix embedded. Intracellular expression of fractalkine on EC was analyzed by flow cytometry. Ten thousand cells were analyzed. Data are expressed as mean percentage ± SD of EC from eight different experiments at each time point; MEEC secrete lower amounts of soluble fractalkine. (C) Detection of secreted fractalkine levels in supernatants of cytokine-stimulated EC was performed with ELISA. Each value represents mean ± SD from eight different experiments for the denoted sampling period of 4 h. (D) Spearman correlation of soluble fractalkine levels (sCX3CL1) and percentage of EC expressing fractalkine intracellular (CX3CL1+-EC). Area of the density ellipse represents the 95% confidence interval.
Reduced In Vitro Adhesion of Natural Killer Cells to Matrix-Embedded EC
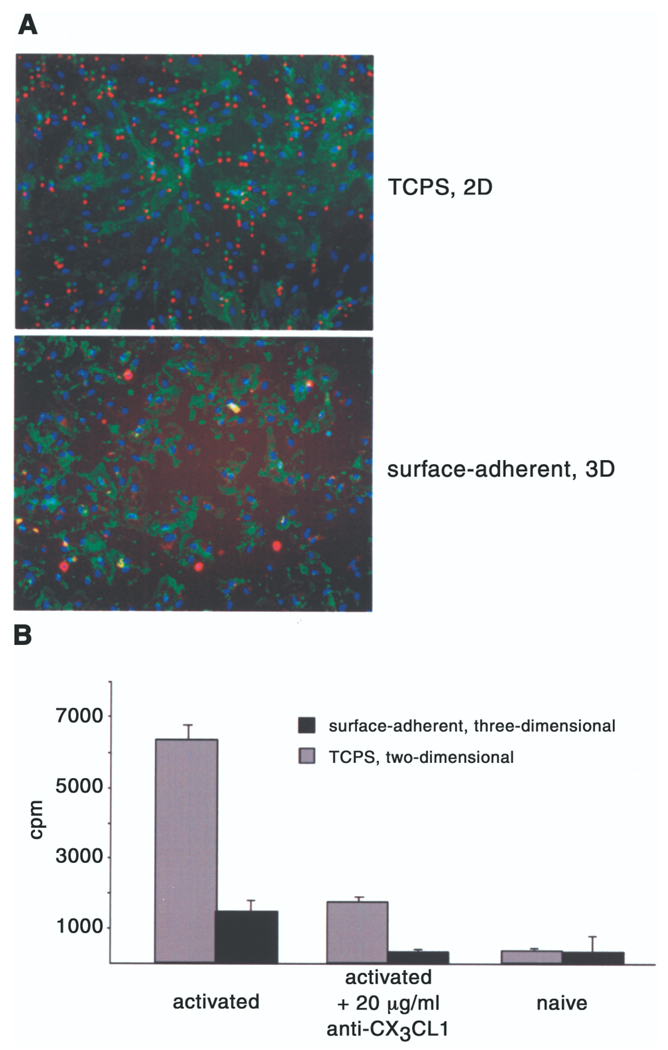
Fluorescence microscopy revealed substantial NK cell attachment to EC-TCPS and minimal attachment to MEEC (Fig. 6A). This effect was quantified with 51Cr-labeled NK cell adhesion assay: 3.7-fold more NK-cells adhered to cytokine-stimulated allogeneic EC-TCPS than to MEEC (6335 ± 420 vs. 1735 ± 135 cpm; p < 0.0002) (Fig. 6B). Fractalkine expression was critical for NK cell adhesion to activated EC, as the addition of 20 µg/ml anti-CX3CL1 decreased adhesion of NK cells to cytokine-stimulated EC by ~74% (p < 0.005 vs. without anti-CX3CL1) (Fig. 6B). NK cells did not adhere to TCPS or 3D matrix alone (data not shown).
Figure 6.
NK cell binding to EC is reduced when EC are matrix embedded. EC were grown to confluence on polystyrene plates or in Gelfoam matrices, activated with 100 U TNF-α and IFN-γ for 20 h. (A) Attachment of NK cells to cytokine-stimulated EC was visualized via immunofluorescence microscopy (10×) and quantified with attachment of 51Cr-labeled NK cells (0.5 × 106/well). Labeled cells were resuspended in medium alone or medium containing 20 µg/ml anti-CX3CR1 antibody and added to the EC under gentle rocking conditions (10 cycles/min). After 30 min the medium was decanted and the wells were gently washed. Adherent cells were lysed by treating with 1% Triton in PBS. (B) Total binding was determined by measuring individual well-associated cpm using a gamma counter. Each value represents mean ± SD from eight different experiments.
Natural Killer Cell Infiltration of EC Implantation Sites Is Reduced When EC Are Matrix Embedded
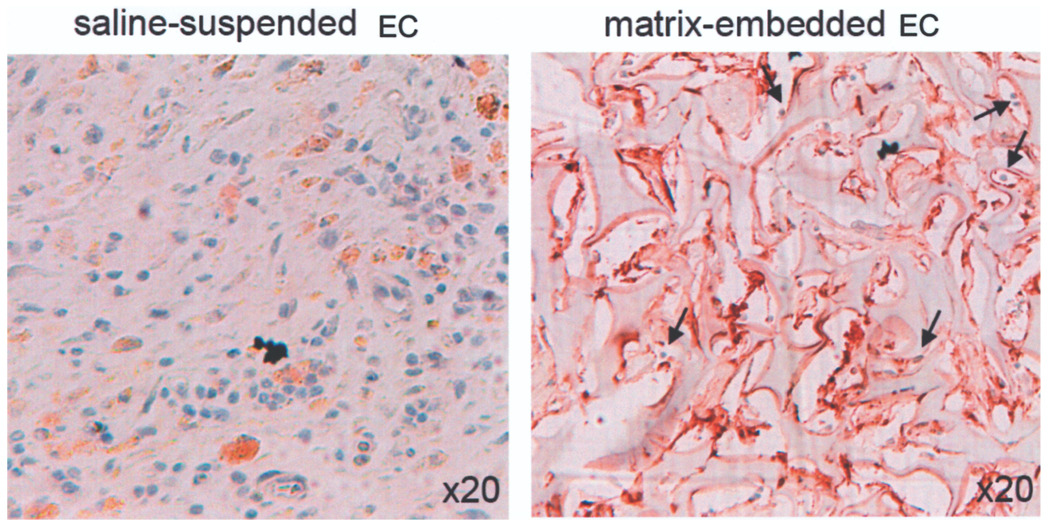
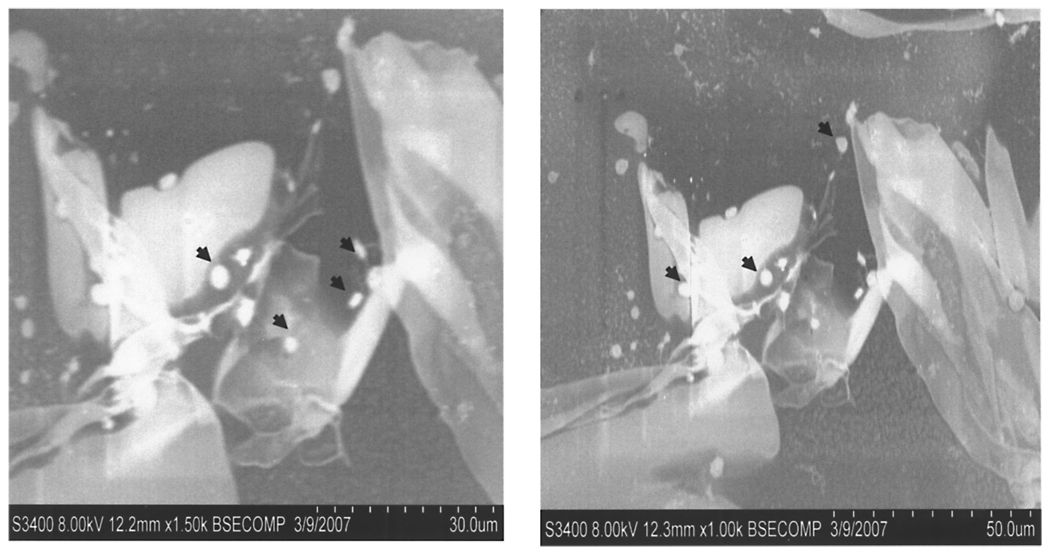
Matrix embedding has previously been shown to inhibit macrophage and lymphocyte infiltration of implantation sites of xenogeneic EC (21,24). Specific immunostaining now reveals a marked reduction of NK cell infiltration into subcutaneous implants of PAE surface adherent within a 3D matrix when compared with EC saline suspended pellets (Fig. 7). Saline injection alone (control animals) did not result in NK cell infiltration (data not shown). To further rule out the possibility that the used matrix serves as a physical barrier to immune cells, we incubated EGM-2-hydrated 3D matrices with fresh isolated monocytes for 3 h at 37°C in vitro, and noted ample monocyte penetration into the matrix under wet electron microscopy (Fig. 8).
Figure 7.
MEEC chemoattract fewer NK cells to implantation sites of EC. EC were implanted in the subcutaneous dorsal space of Sprague-Dawley rats as either matrix-embedded or saline-suspended cell pellets. On the 28th postoperative day, marked rat NK cell infiltration was noted in and around the injection site of saline-suspended EC pellets. Only few NK cells (blue, black arrows) were found in and around the implantation site of MEEC.
Figure 8.
Wet SEM image of monocytes (indicated with black arrows) incubated on a collagen-based matrix for 2 h at 37°C, indicating the lack of a physical barrier for monocyte penetration.
DISCUSSION
The importance of EC polarity and interaction with extracellular matrix components as key regulators of EC phenotype and function continue to be elucidated. EC are naturally basement membrane surface adherent, and disruption of extracellular matrix structure, EC–matrix contact, and 3D architecture are early features of inflammatory diseases and metabolic derangements. Substratum interactions dictate EC control of vascular homeostasis. MEEC are far more potent inhibitors of local and systemic immune reactivity than identical cells injected as pellets or adherent to material surfaces (12,17–21). The data presented here are in agreement with previous reports that EC–substratum interactions pivotally influence the proinflammatory intracellular signaling cascade (28), in particular the critical regulation of NF-κB activity in EC. These findings extend our understanding of the relationship between extracellular matrix signaling and the spatial organization of cells within tissues as determining factors in cell and tissue repair (4,16). Furthermore, given the fact that cells grown on TCPS were actually gelatin coated, the same protein the matrix is composed of, it is intriguing that integrin expression as well as extracelluar proteins (e.g., αVβ3, α5β1, collagen IV, fibronectin) are differentially expressed between MEEC and TCPS (data not shown), indicating a differential type of adhesion that is spatial driven.
Immune cell recruitment by activated endothelium is central to tissue repair and host defenses against invading microbes, but it is also a critical determinant for the pathophysiology of atherosclerosis, ischemia-reperfusion injury, and autoimmune diseases with underlying endothelial dysfunction (8,10,34). A central mediator in inflammatory activation of EC is the NF-κB pathway (12), which is under tight control of cytokines and phosphorylation of intracellular kinases (29). Proinflammatory genes under control of NF-κB in EC include a wide variety of cytokines, chemokines, and adhesion molecules (12). Chemokines and adhesion molecules play pivotal roles in the initial encounter between circulating immune and EC. Endothelial chemokine expression has been specifically linked with initiation and progression of atherosclerotic disease (7,14,15,32) and allograft rejection (11,31). Metabolic diseases such as hyperlipidemia and diabetes mellitus are associated with subtle changes in subendothelial matrix composition, promoting changes in EC matrix interconnectivity and spatial formation, thereby advancing development of endothelial dysfunction (1,27,33). The importance of cell–matrix contact and spatial formation for endothelial function and immune regulation is increasingly appreciated (21). Matrix embedding allows EC to attain a physical conformation that maximizes secretion of biochemical regulators of vascular repair (22,24,25) and minimizes implant immunogenicity (17,21,23,24). Even allo- and xenografts of EC can effectively regulate tissue injury and with none of the immune response seen from injections of the identical cells in their free state.
We can now report that nuclear translocation of the transcriptional active NF-κB subunit p65 upon cytokine stimulation was significantly lower in EC within a physiologic milieu when compared with two-dimensional growing patterns (Fig. 1). This anti-inflammatory effect is amplified by increased ratio of nuclear translocated subunit p50 in MEEC, suggested to be of inhibitory nature in certain cases (9). Further analysis revealed reduced phosphorylation of upstream kinases in dependence of the underlying endothelial substratum as one explanation for the observed immunosilencing effect. Downstream-attenuated NF-κB activation is followed by reduced expression and secretion of chemokines and proinflammatory surface molecules on MEEC when compared with EC grown on 2D surfaces. Chemokines (e.g., fractalkine, IL-6, IL-8, MCP-1) and surface molecules (e.g., VCAM-1, ICAM) are all regulated by NF-κB and have been identified as important initial mediators for immune cell attraction and adhesion to activated EC (7). Fractalkine is expressed on activated and/or dysfunctional EC and is an important vascular modulator, attracting fractalkine receptor-expressing NK cells, cytotoxic lymphocytes, and macrophages (18,31).
The importance of the fractalkine system for initiation and progression of different vascular diseases has been recently demonstrated, as fractalkine receptor polymorphisms are associated with a reduced incidence of coronary atherosclerosis (32). Enhanced fractalkine, IL-8, and MCP-1 expression has been demonstrated in patients suffering from atherosclerosis and diabetes mellitus (33,34), in which changes in extracelluar matrix composition and integrin expression are an early feature predisposing to endothelial dysfunction. In addition, fractalkine deficiency protects animals from intimal hyperplasia after vascular injury with decreased smooth muscle proliferation and reduced intimal accumulation of immune cells (14,15). Neutralizing fractalkine antibodies block transplant rejection in animal models (35), and fractalkine receptor knockout mice survive longer after cardiac transplantation (5). As one consequence of attenuated fractalkine expression and secretion by MEEC, we can now demonstrate muted in vitro and in vivo adhesion of NK cells to MEEC compared with adhesion to EC-TCPS. On a functional level, NK-cells react significantly less with ME EC than with EC grown on 2D surfaces, with modulated expression and secretion of chemokines and surface molecules following attenuated NF-κB activation. NK cell adhesion to activated EC on TCPS was reduced almost 4-fold when EC were substrate adherent within a matrix. NK cell infiltration into sites of EC implantation in vivo was restricted when EC were embedded within matrices. Attenuated NK cell adhesion is a direct consequence of reduced expression and secretion of the chemokine fractalkine by MEEC.
The central role of NK cells as interlinkage between innate and adaptive immune responses is increasingly appreciated (5,11,26,31). Unlike T and B lymphocytes, NK cells lyse target cells without prior antigen priming. They recruit and activate other immune cells through the production of proinflammatory cytokines, but only after chemokine-directed adherence and transmigration through endothelium to target tissues. NK cells express chemokine receptors, including CX3CR1 and MCP-1 (CCL-2) (2), and migrate along concentration gradients of these receptors’ ligands (30).
In concert with the overall reduced expression and secretion of cytokines and adhesion molecules downstream of NF-κB in EC cultured within a three-dimensional undisturbed substratum, our findings link our previous in vivo observations of reduced immune cell infiltration to sites of MEEC (21,24) with a general scheme of the effect of cell–substratum contact on the regulation of immune reactivity. This downregulation reflects an altered response rather than limited stimulation. TNF-α receptor expression is not influenced by the EC–substratum interaction but intracellular signaling is. This might further emphasize the pivotal role of endothelial state and conformation in expressing critical chemokines on the one hand, and stimulating adhesion and activation of NK cells on the other hand, in initiation of graft rejection. As fractalkine binding enhances the cytolytic activity of NK cells in a dose- and time-dependent manner (31,35), EC–matrix contact might well govern endothelial expression of fractalkine to injury stimuli, and the net local destruction and pace of tissue repair.
Taken together, our results identify a critical role for surface adherence and spatial formation in modulating endothelial expression and secretion of the chemokines and surface molecules that govern NK cell biology. These findings might enhance our understanding of various endothelial-mediated diseases, and the role of endothelial cell conformation on inflammatory state and tissue repair. Materials formulated with controlled architectures might serve as culture models to further reveal how tissue architecture might influence cellular immune behavior to exogenous stimuli.
ACKNOWLEDGMENTS
S.H. was supported by the NIH Post-Doctoral T32 Ruth L. Kirschstein Research Service Award. H.M. was supported by fellowships from Philip Morris USA Inc. and by Philip Morris International. The research was supported in part by a grant to E.R.E. from the USA National Institutes of Health (HL 49039). We are grateful for the critical advice of Prof. Gail E. Sonenshein, Boston University School of Medicine.
REFERENCES
- 1.Aznar-Salatti J, Escolar G, Cases A, Gomez-Ortiz G, Anton P, Castillo R, Revert L, Ordinas A. Uraemic medium causes endothelial cell dysfunction characterized by an alteration of the properties of its subendothelial matrix. Nephrol. Dial. Transplant. 1995;10(12):2199–2204. doi: 10.1093/ndt/10.12.2199. [DOI] [PubMed] [Google Scholar]
- 2.Berahovich RD, Lai NL, Wei Z, Lanier LL, Schall TJ. Evidence for NK cell subsets based on chemokine receptor expression. J. Immunol. 2006;177(11):7833–7840. doi: 10.4049/jimmunol.177.11.7833. [DOI] [PubMed] [Google Scholar]
- 3.Borlongan CV, Thanos CG, Skinner SJ, Geaney M, Emerich DF. Transplants of encapsulated rat choroid plexus cells exert neuroprotection in a rodent model of Huntington’s disease. Cell Transplant. 2007;16(10):987–992. [PubMed] [Google Scholar]
- 4.Boudreau N, Werb Z, Bissell MJ. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc. Natl. Acad. Sci. USA. 1996;93(8):3509–3513. doi: 10.1073/pnas.93.8.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao G, Lu Y, Gao R, Xin Y, Teng D, Wang J, Li Y. Expression of fractalkine, CX3CR1, and vascular endothelial growth factor in human chronic renal allograft rejection. Transplant. Proc. 2006;38(7):1998–2000. doi: 10.1016/j.transproceed.2006.06.081. [DOI] [PubMed] [Google Scholar]
- 6.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91(10):3527–3561. [PubMed] [Google Scholar]
- 7.Cybulsky MI, Hegele RA. The fractalkine receptor CX3CR1 is a key mediator of atherogenesis. J. Clin. Invest. 2003;111(8):1118–1120. doi: 10.1172/JCI18237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson A, Diamond B. Autoimmune diseases. N. Engl. J. Med. 2001;345(5):340–350. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109 Suppl.:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 10.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 11.Haskell CA, Hancock WW, Salant DJ, Gao W, Csizmadia V, Peters W, Faia K, Fituri O, Rottman JB, Charo IF. Targeted deletion of CX(3)CR1 reveals a role for fractalkine in cardiac allograft rejection. J. Clin. Invest. 2001;108(5):679–688. doi: 10.1172/JCI12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kempe S, Kestler H, Lasar A, Wirth T. NF-kappaB controls the global pro-inflammatory response in endothelial cells: Evidence for the regulation of a pro-atherogenic program. Nucleic Acids Res. 2005;33(16):5308–5319. doi: 10.1093/nar/gki836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kishore N, Sommers C, Mathialagan S, Guzova J, Yao M, Hauser S, Huynh K, Bonar S, Mielke C, Albee L, Weier R, Graneto M, Hanau C, Perry T, Tripp CS. A selective IKK-2 inhibitor blocks NF-kappa B-dependent gene expression in interleukin-1 beta-stimulated synovial fibroblasts. J. Biol. Chem. 2003;278(35):32861–32871. doi: 10.1074/jbc.M211439200. [DOI] [PubMed] [Google Scholar]
- 14.Liu P, Patil S, Rojas M, Fong AM, Smyth SS, Patel DD. CX3CR1 deficiency confers protection from intimal hyperplasia after arterial injury. Arterioscler. Thromb. Vasc. Biol. 2006;26(9):2056–2062. doi: 10.1161/01.ATV.0000234947.47788.8c. [DOI] [PubMed] [Google Scholar]
- 15.Liu P, Yu YR, Spencer JA, Johnson AE, Vallanat CT, Fong AM, Patterson C, Patel DD. CX3CR1 deficiency impairs dendritic cell accumulation in arterial intima and reduces atherosclerotic burden. Arterioscler. Thromb. Vasc. Biol. 2008;28(2):243–250. doi: 10.1161/ATVBAHA.107.158675. [DOI] [PubMed] [Google Scholar]
- 16.Lukashev ME, Werb Z. ECM signalling: Orchestrating cell behaviour and misbehaviour. Trends Cell Biol. 1998;8(11):437–441. doi: 10.1016/s0962-8924(98)01362-2. [DOI] [PubMed] [Google Scholar]
- 17.Methe H, Edelman ER. Cell-matrix contact prevents recognition and damage of endothelial cells in states of heightened immunity. Circulation. 2006;114(1 Suppl.):I233–I238. doi: 10.1161/CIRCULATIONAHA.105.000687. [DOI] [PubMed] [Google Scholar]
- 18.Methe H, Groothuis A, Sayegh MH, Edelman ER. Matrix adherence of endothelial cells attenuates immune reactivity: Induction of hyporesponsiveness in allo- and xenogeneic models. FASEB J. 2007;21(7):1515–1526. doi: 10.1096/fj.06-7051com. [DOI] [PubMed] [Google Scholar]
- 19.Methe H, Hess S, Edelman ER. Endothelial cell–matrix interactions determine maturation of dendritic cells. Eur. J. Immunol. 2007;37(7):1773–1784. doi: 10.1002/eji.200636495. [DOI] [PubMed] [Google Scholar]
- 20.Methe H, Hess S, Edelman ER. Endothelial immunogenicity—a matter of matrix microarchitecture. Thromb. Haemost. 2007;98(2):278–282. [PubMed] [Google Scholar]
- 21.Methe H, Nugent HM, Groothuis A, Seifert P, Sayegh MH, Edelman ER. Matrix embedding alters the immune response against endothelial cells in vitro and in vivo. Circulation. 2005;112(9 Suppl.):I89–I95. doi: 10.1161/01.CIRCULATIONAHA.105.524991. [DOI] [PubMed] [Google Scholar]
- 22.Nathan A, Nugent MA, Edelman ER. Tissue engineered perivascular endothelial cell implants regulate vascular injury. Proc. Natl. Acad. Sci. USA. 1995;92(18):8130–8134. doi: 10.1073/pnas.92.18.8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navarro-Alvarez N, Rivas-Carrillo JD, Soto-Gutierrez A, Yuasa T, Okitsu T, Noguchi H, Matsumoto S, Takei J, Tanaka N, Kobayashi N. Reestablishment of microenvironment is necessary to maintain in vitro and in vivo human islet function. Cell Transplant. 2008;17(1–2):111–119. doi: 10.3727/000000008783907125. [DOI] [PubMed] [Google Scholar]
- 24.Nugent HM, Edelman ER. Endothelial implants provide long-term control of vascular repair in a porcine model of arterial injury. J. Surg. Res. 2001;99(2):228–234. doi: 10.1006/jsre.2001.6198. [DOI] [PubMed] [Google Scholar]
- 25.Nugent HM, Rogers C, Edelman ER. Endothelial implants inhibit intimal hyperplasia after porcine angioplasty. Circ. Res. 1999;84(4):384–391. doi: 10.1161/01.res.84.4.384. [DOI] [PubMed] [Google Scholar]
- 26.Obara H, Nagasaki K, Hsieh CL, Ogura Y, Esquivel CO, Martinez OM, Krams SM. IFN-gamma, produced by NK cells that infiltrate liver allografts early after transplantation, links the innate and adaptive immune responses. Am. J. Transplant. 2005;5(9):2094–2103. doi: 10.1111/j.1600-6143.2005.00995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okruhlicova L, Tribulova N, Weismann P, Sotnikova R. Ultrastructure and histochemistry of rat myocardial capillary endothelial cells in response to diabetes and hypertension. Cell Res. 2005;15(7):532–538. doi: 10.1038/sj.cr.7290322. [DOI] [PubMed] [Google Scholar]
- 28.Orr AW, Sanders JM, Bevard M, Coleman E, Sarembock IJ, Schwartz MA. The subendothelial extracellular matrix modulates NF-kappaB activation by flow: A potential role in atherosclerosis. J. Cell Biol. 2005;169(1):191–202. doi: 10.1083/jcb.200410073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007;7(10):803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 30.Robertson MJ. Role of chemokines in the biology of natural killer cells. J. Leukoc. Biol. 2002;71(2):173–183. [PubMed] [Google Scholar]
- 31.Robinson LA, Nataraj C, Thomas DW, Cosby JM, Griffiths R, Bautch VL, Patel DD, Coffman TM. The chemokine CX3CL1 regulates NK cell activity in vivo. Cell Immunol. 2003;225(2):122–130. doi: 10.1016/j.cellimm.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Umehara H, Imai T. Role of fractalkine in leukocyte adhesion and migration and in vascular injury. Drug News Perspect. 2001;14(8):460–464. doi: 10.1358/dnp.2001.14.8.858415. [DOI] [PubMed] [Google Scholar]
- 33.Vogl-Willis CA, Edwards IJ. High-glucose-induced structural changes in the heparan sulfate proteoglycan, perlecan, of cultured human aortic endothelial cells. Biochim. Biophys. Acta. 2004;1672(1):36–45. doi: 10.1016/j.bbagen.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: Molecules, Function and pathophysiological aspects. Lab Invest. 2000;80(5):617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 35.Yoneda O, Imai T, Goda S, Inoue H, Yamauchi A, Okazaki T, Imai H, Yoshie O, Bloom ET, Domae N, Umehara H. Fractalkine-mediated endothelial cell injury by NK cells. J. Immunol. 2000;164(8):4055–4062. doi: 10.4049/jimmunol.164.8.4055. [DOI] [PubMed] [Google Scholar]