Abstract
The affective priming effect has mostly been studied using reaction time measures; however, the neural bases of affective priming are not well established. To understand the neural correlates of cross-domain emotional stimuli presented rapidly, we obtained event-related potential (ERP) measures during an affective priming task using short SOA (stimulus onset asynchrony) conditions. Two sets of 480 picture-word pairs were presented at SOAs of either 150 ms or 250 ms between prime and target stimuli. Participants decided whether the valence of each target word was pleasant or unpleasant. Behavioral results from both SOA conditions were consistent with previous reports of affective priming, with longer RTs for incongruent than congruent pairs at SOAs of 150 ms (771 vs. 738 ms) and 250 ms (765 vs. 720 ms). ERP results revealed that the N400 effect (associated with incongruent pairs in affective processing) occurred at anterior scalp regions at an SOA of 150 ms, and this effect was only observed for negative target words across the scalp at an SOA of 250 ms. In contrast, late positive potentials (associated with attentional resource allocation) occurred across the scalp at an SOA of 250 ms. LPPs were only observed for positive target words at posterior parts of the brain at an SOA of 150 ms. Our finding of ERP signatures at very short SOAs provides the first neural evidence that affective pictures can exert an automatic influence on the evaluation of affective target words.
Keywords: Affective priming, Stimulus onset asynchrony (SOA), Event-related potential (ERP), Evaluative decision task
1. Introduction
Investigation of the neural mechanisms underlying affective processing represents an important step in understanding the relationship between emotional and cognitive processes. Automatic processes play an important role in instantaneous emotional processing, which later influences more reflective processing of affect (Fazio, 2001). Many studies (e.g., Fazio et al., 1986; Hermans et al., 1994; Klauer et al., 1997; Vermeulen et al., 2006; Frings & Wentura, 2008) have found that the time needed to evaluate a target item as either “happy” or “sad” is shorter when prime and target pairs are affectively congruent (e.g. positive-positive: love-win) than when they are affectively incongruent (e.g. positive-negative: love-pain). This phenomenon is referred to as the affective priming effect, which may be useful for investigating how initial affective processing automatically influences the processing of subsequent stimuli. In addition, this affective priming paradigm has been used successfully with a number of populations including schizophrenia patients (e.g., Rossell, 2004), individuals with psychopathy (Blair et al., 2006), Parkinson's patients (Castner et al., 2007), individuals scoring high on neuroticism (e.g., Robinson et al., 2007), and healthy older adults (e.g., Ready et al., 2006; Jiang et al., 2007), providing a means of assessing early emotional processing in a variety of clinical populations.
Previous studies have shown that affective priming is strongly influenced by the interval between the onset of the prime and the onset of the target, i.e. the stimulus onset asynchrony (SOA) (Fazio et al., 1986; Hermans et al., 2001; Hermans et al., 2003; Klauer et al., 1997; Castner et al., 2007). For example, Fazio et al. (1986) observed affective priming using word-word pairs at an SOA of 300 ms, but not at an SOA of 1000 ms. This SOA effect was also reported by Hermans et al. (1994) using picture-picture pairs. Klauer et al. (1997) varied SOA in six steps (-100, 0, 100, 200, 600, and 1200 ms) in a word-word paradigm to investigate the influence of SOA on affective priming. Significant priming effects were found at SOAs of 0 and 100 ms, but not at other SOAs. Similarly, in two experiments, Hermans et al. (2001) found significant priming effects for word-word pairs at short SOAs of 0 and 150 ms but not at 300 and 450 ms SOA levels. In a third experiment, Hermans et al. (2001) found a significant affective priming effect at an SOA of 300 ms when the primes and targets were both presented at the same location in the center of the screen. Affective priming was not found when the prime-target pair was presented one above another, as in previous experiments. Hermans et al. (2001) concluded that the time course of affective priming has a rather quick onset (SOA of 0 ms), with a maximum peak around 150 ms, after which the effect quickly dissipates.
Behavioral performance measures such as RT have provided important information concerning affective priming but do have some limitations. For example, RT can be obtained only after a stimulus has been processed. In contrast to behavioral measures, event-related potentials (ERPs) provide an online measure of mental processes with a temporal resolution in the millisecond range. The N400, a well-known ERP component, is a negative-going component peaking around 400 ms before the positive component. It is often related to semantic processing (e.g., Chwilla et al., 1995; Holcomb & Neville, 1990), and conceptual accessibility by words and pictures (Nigam et al., 1992). Using ERP measurements (especially the N400) allows us to further examine the temporal characteristics and possible mechanisms of affective priming effect during the evaluative decision task.
Previous ERP studies of affective priming have reported an N400 priming effect. For example, Schirmer et al. (2002) investigated the influence of emotional prosody context on written word processing in a lexical decision task. The written emotional words were either congruent or incongruent with the emotional prosody of a preceding spoken sentence. They found that incongruent words evoked a larger N400 than congruent words. The authors suggested that emotional prosody modulates word processing in a similar way to semantic conflicts. In other words, emotional primes influence semantic processing for target words in the lexical decision task. Similar results have also been reported in an auditory affective priming study (Bostanov & Kotchoubey, 2004). Zhang et al. (2006) was the first to use ERPs to examine neural mechanisms underlying visual affective priming, and found that affectively incongruent word-word pairs elicited a larger N400 than affectively congruent trials. However, Zhang et al. did not find cross-domain ERP affective priming effects for picture-word pairs. That is, when using pictures as primes, there was no significant difference in ERPs evoked by affectively congruent trials and affectively incongruent trials. Zhang et al. speculated that the lack of significant findings may have been due to the use of a 300 ms SOA in the picture-word, because an SOA of 300 ms is located at the edge of the automatic activation curve (Hermans et al., 2001). Thus, it is necessary to examine affective priming of picture-word pairs at shorter SOAs using ERP measurements. For this purpose, the current study used two short SOAs of 150 ms and 250 ms to investigate the ERP affective priming effect in a picture-word paradigm, and to examine how neural correlates of affective priming vary with SOA. Although behavioral data has shown that a strong affective priming effect occurs at an SOA of 150 ms, this effect weakens as longer SOAs (Hermans et al., 2001). However, the neural basis of these SOA effects on affective priming remains unclear (see Fazio, 2001; Klauer & Musch, 2003 for reviews; Wentura & Frings, 2008). The current study is the first to examine whether there are ERP response differences in affective priming between SOA conditions.
Besides the N400, a late positive potential (LPP) component has been shown to differ in response to affectively incongruent pairs compared to affectively congruent pairs. Werheid et al. (2005) used emotional face pairs to examine the ERP correlates of affective priming in an evaluative decision task. In their study, participants were asked to classify portraits of unfamiliar persons according to their emotional expression (happy or angry). The portraits were either preceded by the face of a different person with the same emotional expression (congruent prime) or a different emotional expression (incongruent prime). At an SOA of 250 ms, ERP results revealed both early and late priming effects, independent of stimulus valence. The early priming effect was characterized by attenuated ERP amplitudes of frontal electrodes between 100 and 200 ms in response to congruently primed targets, possibly reflecting the priming of a very early stage of face processing. The late priming effect consisted of an enhancement of the LPP following incongruently primed targets at parietal electrodes between 500 and 600 ms. The LPP effect may indicate elevated attention due to inconsistency of valence. Other studies of affective processing have also reported that LPP components are evoked by emotional pictures and associated LPP with attentional capture, evaluation or memory encoding (e.g. Keil et al., 2002; Schupp et al., 2003; Kissler et al., 2009). However, the LPP priming effect found in the Werheid et al (2005) study did not occur in previous affective priming studies that used verbal stimuli (Schirmer et al., 2002; Zhang et al., 2006).
In summary, a number of studies have demonstrated affective priming effects for picture-word pairs using standard reaction time measures (e.g., De Houwer & Hermans, 1994; Jiang et al., 2007). In contrast, the ERP correlates of picture-word (cross-domain) affective priming remain unknown. The current study was designed to establish these ERP correlates and, in doing so, to inform the debate about the cognitive processes involved in cross-domain affective priming. Specifically, the present study was designed to determine whether priming effects indexed by N400 (demonstrated by the word-word priming experiment) and/or LPP (demonstrated by the picture-picture priming experiment) occur when evaluating picture-word pairs. Secondly, by employing multiple short SOA conditions, the current experiment examined how affective priming varied with SOA.
2. Results
2.1. Behavioral results
The current experiment utilized both reaction times (RTs) and ERP measures (details are described in the method section) to examine cross-domain affective priming. We first examined whether the current behavioral results matched previous reports on affective priming. Table 1 presents the mean response times to target stimuli (RTs) and response error rates (ERs – consistency to standard responses) for each SOA and affective congruency condition. Consistent with many previous reports mentioned above, our participants were faster and more accurate in determining the valence of target word for affectively congruent trials as compared to affectively incongruent trials.
Table 1. Mean response times (ms) and error rates (%) for each condition.
| affective congruency | SOA of 150 ms | SOA of 250 ms | ||
|---|---|---|---|---|
| Positive target | Negative target | Positive target | Negative target | |
| congruent | 720 (2.5%) | 757 (2.5%) | 706 (3.0%) | 736 (4.2%) |
| incongruent | 765 (6.3%) | 778 (5.8%) | 750 (9.4%) | 780 (6.6%) |
Footnote: Any trial having an error response or having a RT value outside of a predetermined 200-1500 ms range was excluded from mean RT calculations (3.1% to all trials).
A 2 SOA (150 ms, 250 ms) × 2 affective congruency (congruent, incongruent) × 2 target valence (positive, negative) repeated measures ANOVA was performed for RTs. Parallel ANOVAs were performed for ERs. The RT analysis revealed a significant main effect of affective congruency, F(1,17) = 16.337, p < 0.005. When primed by pictures, participants were significantly faster in determining the valence of target words for affectively congruent trials compared to affectively incongruent trials. A main effect of target valence also reached significance, F(1,17) = 10.549, p < 0.01. Participants' responses to positive words were faster than those to negative words. The interaction of SOA and affective congruency was marginally significant, F(1,17) = 3.047, p = 0.099. Tests for simple effects revealed significant effects of affective congruency at the 150 ms SOA condition, F(1,17) = 13.91, p < 0.005, and 250 ms SOA condition, F(1,17) = 15.62, p = 0.005. In addition, when primes and targets were affectively congruent, participants' responses were faster at the 250 ms SOA condition than at the 150 ms SOA condition, F(1,17) = 5.82, p < 0.05.
Analysis of ERs also revealed a significant main effect for affective congruency, F(1,17) = 6.597, p < 0.05, indicating that participants were more correct in evaluation decisions for affectively congruent trials than for affectively incongruent trials. The interaction of SOA, affective congruency, and target valence was marginally significant, F(1,17) = 3.915, p = 0.064. Tests for simple effects indicated significant effects of affective congruency when positive words were used as targets both at the 150 ms SOA condition, F(1,17) = 7.64, p < 0.05, and 250 ms SOA condition, F(1,17) = 8.77, p < 0.01. When negative words were used as targets, the effect of affective congruency was marginally significant at the 150 ms SOA condition, F(1,17) = 4.03, p = 0.061. In addition, when primes and targets were affectively congruent, participants achieved greater accuracy for positive as compared to negative words at the SOA of 250 ms, F(1,17) = 4.48, p < 0.05.
2.2. ERP results
The grand mean ERPs elicited by affectively congruent and affectively incongruent trials with SOA of 150 and 250 ms are shown in Figure 1. Please refer to section 4.4 for details of overall ANOVAs conducted at each time intervals. Only results for the N400 and the LPP are reported here.
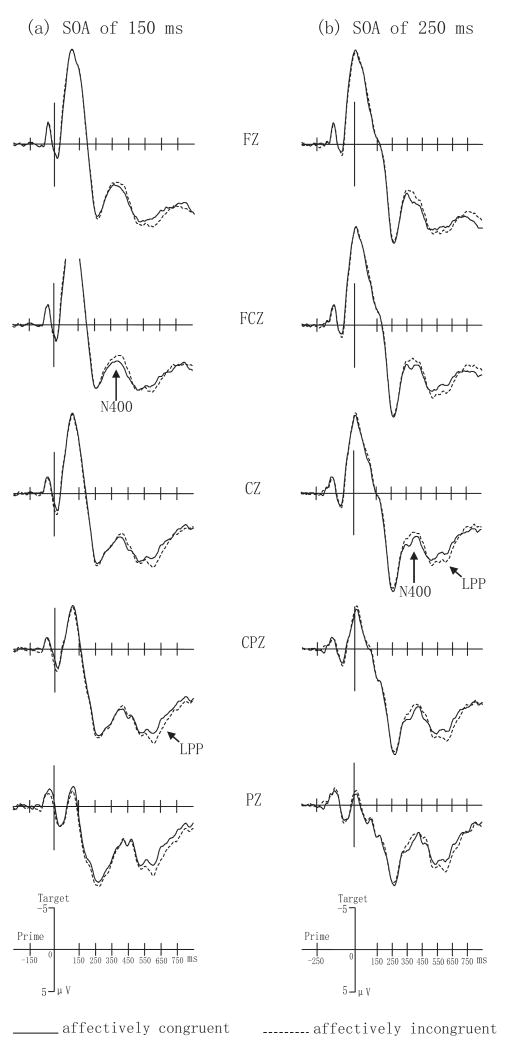
Fig.1.
Grand mean ERPs from FZ, FCZ, CZ, CPZ, and PZ to affectively congruent and incongruent trials (a) when SOA is 150 ms and (b) when SOA is 250 ms.
N400 Effects (350—450 ms)
This time interval corresponded to a negative-going wave (N400) that peaked around 350-400 ms. The analyses of this epoch showed a significant main effect of affective congruency, F(1,17) = 5.201, p < 0.05. The interaction between SOA and anterior-posterior location reached significance, F(1,17) = 25.194, p < 0.001. Tests for simple effects indicated significant effects of SOA at both the anterior location, F(1,17) = 10.53, p < 0.01, and posterior location, F(1,17) = 14.9, p < 0.005. Because the interaction between SOA, anterior-posterior location, and affective congruency reached marginal significance, F(1,17) = 3.525, p = 0.078, mean amplitude data for the SOAs of 150 ms and 250 ms were then each subjected to a 2 affective congruency (congruent, incongruent) × 2 target valence (positive, negative) × 2 hemisphere (left, right) × 2 location (anterior, posterior) four-way ANOVA test, respectively. The test for the 150 ms SOA condition showed a significant interaction of affective congruency and anterior-posterior location, F(1,17) = 9.905, p < 0.01. Tests of simple effects indicated significant affective priming effects at anterior parts of the scalp when the SOA was 150 ms.
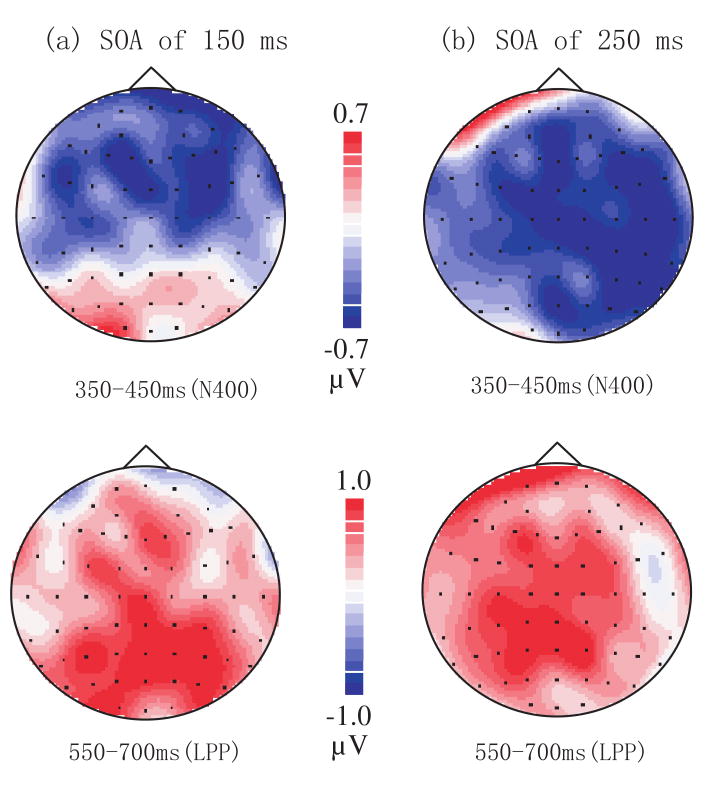
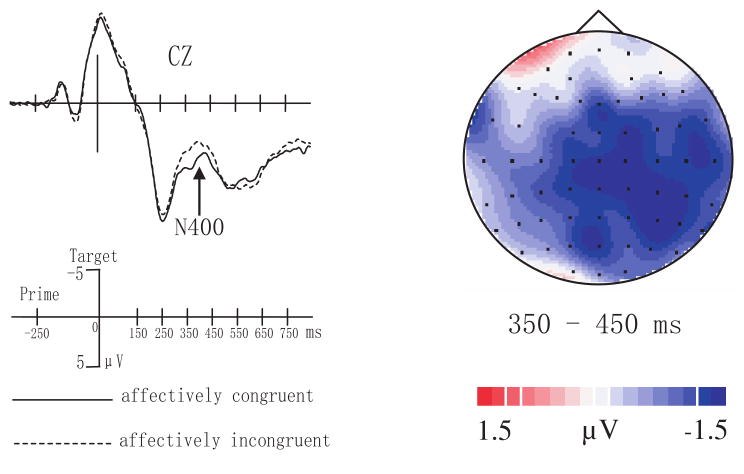
Figure 2 shows the topography of difference waves (subtracting congruent trials from incongruent trials) with SOAs of 150 ms and 250 ms. In the 350-450 ms time window, the affective priming effect was primarily at anterior locations when the SOA was 150 ms. When the SOA was 250 ms, our analysis showed that the main effect of affective congruency reached significance, F(1,17) = 5.181, p < 0.05. The interaction of hemisphere, target valence, and affective congruency was marginally significant, F(1,17) = 3.86, p = 0.066. Further tests showed a significant effect of affective congruency for negative word targets in the right hemisphere, F(1,17) = 20.68, p < 0.001, and a marginally significant effect of affective congruency for negative word targets in the left hemisphere, F(1,17) = 4.44, p = 0.05 (see Figure 3).
Fig.2.
Topographic maps of N400 and LPP difference waves (subtracting congruent trials from incongruent trials) for the interval 350-450 ms and 550-700 ms after target onset (a) when SOA is 150 ms and (b) when SOA is 250 ms.
Fig.3.
Grand mean ERP from CZ to affectively congruent and incongruent trials (left figure) and topographic map of difference waves (subtracting congruent trials from incongruent trials) during 350-450 ms after target onset (right figure) when SOA is 250 ms and targets were negative words.
LPP effects (550—700 ms)
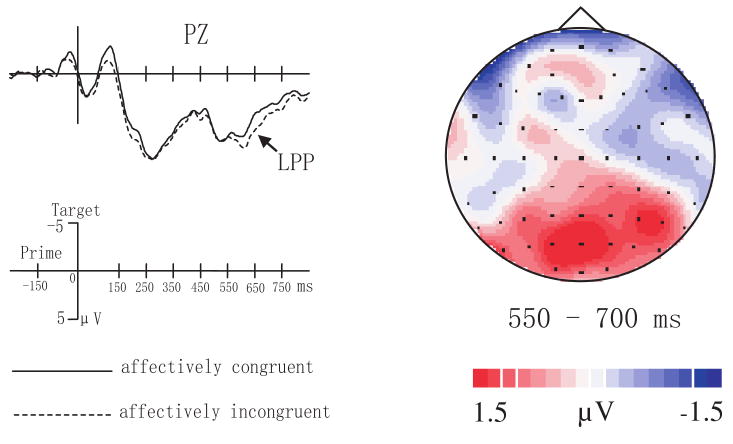
This time interval encompassed a late positive-going ERP potential (LPP) peaking around 600 ms. A significant main effect of affective congruency, F(1,17) = 17.103, p < 0.005, indicated that affectively incongruent trials had larger overall positive amplitudes than affectively congruent trials. The main effect of target valence also was significant, F(1,17) = 21.497, p < 0.001. The interaction between SOA and hemisphere reached significance, F(1,17) = 17.947, p < 0.005. Tests of simple effects indicated a marginally significant effect of SOA in the right hemisphere, F(1,17) = 3.97, p = 0.063. The interaction between SOA and anterior-posterior location was significant, F(1,17) = 6.144, p < 0.05. The interaction between SOA, anterior-posterior location, affective congruency, and target valence was also significant, F(1,17) = 7.621, p < 0.05. Further tests for the 250 ms SOA condition indicated significant main effects for affective congruency, F(1,17) = 6.009, p < 0.05, and for target valence, F(1,17) = 11.625, p < 0.005., Our tests for the 150 ms SOA condition also found significant main effects for affective congruency, F(1,17) = 7.472, p < 0.05, and for target valence, F(1,17) = 18.617, p < 0.001. In addition, the interaction of affective congruency and anterior-posterior location reached significance, F(1,17) = 5.09, p < 0.05. The interaction of affective congruency, anterior-posterior location, and target valence was marginally significant, F(1,17) = 4.133, p = 0.058. Tests of simple effects showed a significant effect of affective congruency for positive word targets at posterior regions of the scalp, F(1,17) = 6.49, p < 0.05 (see Figure 4).
Fig.4.
Grand mean ERP from PZ to affectively congruent and incongruent trials (left figure) and topographic map of difference waves (subtracting congruent trials from incongruent trials) during 550-700 ms after target onset (right figure) when SOA is 150 ms and targets were positive words.
Tests for shift in topography
To test the neural activity responsible for the anterior to posterior shift of the N400 component, we conducted a four-way ANOVA, i.e. 2 affective congruency (congruent, incongruent) × 2 target valence (positive, negative) × 2 hemisphere (left, right) × 2 location (anterior, posterior), for the N400 data at the 150 ms SOA. The electrodes were grouped into anterior-left, anterior-right, posterior-left and posterior-right regions. The ANOVA indicated a significant interaction of affective congruency and anterior-posterior location, F(1,17) = 9.905, p < 0.01. Tests of simple effects indicated significant affective priming effects at anterior parts of the brain when the SOA was 150 ms. Similarly, in LPP component for the 150 ms SOA, the interaction of affective congruency and anterior-posterior location reached significance, F(1,17) = 5.09, p < 0.05. Tests of simple effects indicated a significant effect of affective congruency at posterior parts of the brain, F(1,17) = 16.54, p < 0.005.
3. Discussion
In order to investigate the neural correlates of cross-domain affective priming and the role of SOA in an affective evaluation task, the current study used ERP to investigate evaluation of affective words primed by affective pictures using two SOAs known to recruit automatic processes. Consistent with previous studies using RTs and ERs as measures (e.g., Fazio et al., 1986; Hermans et al., 1994; Klauer et al., 1997; Vermeulen et al., 2006; Zhang et al., 2006), our behavioral data showed that responses to affectively congruent stimulus pairs were significantly faster and more accurate than those to affectively incongruent pairs in both short SOA conditions. Our behavioral data also showed that participants' responses to positive words were faster than those to negative words. It should be noted that because the word familiarity and arousal level between negative and positive words were not perfectly balanced in the stimuli sets, we are unable to explain the source of the target valence effect found in the current study. The purpose of including the factor of target valence in the current study, however, was to investigate whether affective priming effects were influenced by target valence, and not to study the main effect of target valence.
In addition, our behavioral data indicated that participants' responses to targets were faster at the SOA of 250 ms than at the SOA of 150 ms when primes and targets were affectively congruent. This suggests that perhaps there is increased interference with evaluative decision processes when the SOA is 150 ms than when the SOA is 250 ms for affectively congruent prime-target pairs. When primes and targets were affectively incongruent, response conflict induced by affective incongruency between primes and targets possibly concealed this interference effect, so no significant effect of SOA was found in affectively incongruent trials.
To date, there have been few ERP studies of the neurobiological correlates of affective priming. Our ERP results revealed an N400 component that distinguished affectively incongruent picture-word pairs from affectively congruent pairs, consistent with previous research on affective priming (Bostanov & Kotchoubey, 2004; Schirmer et al., 2002). Many previous studies (e.g., Chwilla et al., 1995; Holcomb & Neville, 1990; Matsumoto et al., 2005; Nobre & McCarthy, 1994) have demonstrated that the N400 is related to semantic processing. The underlying mechanisms for the N400 priming effect have been interpreted in terms of spreading of activation within a semantic network (e.g., Deacon et al., 1999; Deacon et al., 2000; Franklin et al., 2007) or the integration of semantic information (e.g., Brown & Hagoort, 1993; Brown et al., 2000; Chwilla et al., 1995). The present study revealed that affectively incongruent trials elicited a larger N400 compared to affectively congruent trials. Combined with previous literature on the N400 priming effect, our N400 effect in the affective priming paradigm possibly reflected monitoring of affective conflict and difficulty of semantic integration.
Furthermore, at an SOA of 150 ms, the N400 priming effect in the current study was more frontal than the typical central-posterior N400. Although these ERP results are novel, they are consistent with those from previous functional MRI studies of affective and semantic priming. For example, Schirmer et al. (2004) used fMRI to investigate the brain regions that mediate the processing of emotional speech by presenting positive and negative words that were spoken with happy or angry prosody. Emotional prosody and word valence were either congruent or incongruent. Schirmer and colleagues found that the left inferior frontal gyrus was more strongly activated by emotionally incongruent than by congruent trials. Given an established link between the left inferior frontal gyrus and semantic processing (e.g., Kang et al., 1999; Kiehl et al., 2002; Newman et al., 2001; Gold & Buckner, 2002; Gold et al., 2006), Schirmer et al. suggested that their finding demonstrated the influence of emotional prosody on semantic processing.
At an SOA of 250 ms, our results showed significant N400 priming effects only for negative target words. This may suggest that affective congruency for negative words contributes more to overall N400 priming effects than that for positive words, especially when the SOA is longer (250 ms SOA is longer as compared to 150 ms SOA). We hypothesize that integration of semantic information is more important during the processing of negative words, as larger effects of congruent or incongruent emotional context were found for negative words than for positive words. However, in order to confirm this explanation, more evidence will be needed.
The present study also revealed that affectively incongruent words elicited larger late positive amplitudes (i.e., LPP) than affectively congruent words in the interval 550-700 ms after the target onset. Interestingly, Werheid et al. (2005) found an enhancement of the LPP at parietal electrodes in response to face-face pairs with different affective expressions (e.g., happy-angry). They argued that the LPP effect may represent greater relevance that is attributed to a change of emotional expressions. The LPP effect in the current study is consistent with that in the Werheid et al. (2005) study. An LPP after the N400 might be a P300 component. Previous studies have indicated that the P300 is linked to working memory updating, encoding, or retrieval (Donchin & Coles, 1988; Kutas et al., 1977). In the present study, the LPP effect might reflect the increased attentional engagement evoked by affectively incongruent trials.
In addition, our results showed the interaction of affective congruency, SOA, anterior-posterior location, and target valence in the LPP component. When the SOA was 250 ms, there was significant main effect of affective priming, but no interaction of affective congruency and other factors. When the SOA was 150 ms, affective priming effects occurred mainly for positive words at posterior parts of the brain. The affective priming LPP effects found in the 150 ms SOA condition may be due to the influence of different affective priming pictures. When positive words were targets, negative pictures were used as primes in affectively incongruent trials. However, positive pictures were used as primes in affectively incongruent trials when negative words were targets. Because negative stimuli can generally produce stronger emotional effects than positive stimuli (Cacioppo, Gardner, & Berntson, 1999; Crawford & Cacioppo, 2002; Öhman & Mineka, 2001; see Olofsson et al., 2008) and affective pictures have generally higher arousal levels than affective words, participants could have stronger feeling of incongruence for affectively incongruent trials including negative picture primes than for those including positive picture primes, especially at shorter SOAs of 150 ms. Consequently, the affective congruence effect on the LPP mainly occurred during evaluation of positive words at an SOA of 150 ms.
Because Zhang et al. (2006) did not find the LPP effect in word-word paradigm, the present LPP effect might only relate to picture processing during affective priming. Additionally, Li et al. (2008) studied subliminal affective priming by recording brain potentials to surprised faces preceded by 30 ms happy or fearful prime faces. Their results suggested that unconsciously perceived affective information influences social judgments by altering early perceptual processing. Because the LPP effect was not observed in the subliminal face-face paradigm, the present LPP effect may indicate a conscious processing of prime pictures during affective priming.
Recent neuroimaging studies have implicated cortical and subcortical structures, such as the insula, amgydala and orbitalfrontal cortex (OFC) in affective processing and emotional decision-making and regulation (e.g. Damasio et al., 2000). Functional MRI offers the best spatial resolution for the localization of brain responses. Using co-registration of both ERP and fMRI, O'Hare et al. (2007) reported that word or picture-related N300, P2 and frontal word negativity (which has been associated with reflective cognitive processes rather than automatic spreading activation) are likely generated from the dorsal posterior cingulate cortex (dPCC). Other regions involved in automatic cognitive processes should also be examined. The dorsal anterior cingulate cortex (ACC), for example, has long been implicated in perceptual and response conflict monitoring, and its role in ERP priming effects, such as the N400 and LPP effects seen here, should be investigated using fMRI. The mechanisms underlying the anterior to posterior shift for the N400 affective priming effect from an SOA of 150 to 250 ms also demand further investigation using fMRI. Finally, prior research (e.g., Schirmer et al., 2002; Schirmer et al., 2005) has revealed sex differences in cross-modal affective priming and has demonstrated that the presence of sex differences depends on the task used. Sex differences and individual differences in response to affective primes should also be examined in future studies.
Taken together, the present results concerning picture-word affective priming demonstrate that ERP effects are observed at short SOAs of 150 ms and 250 ms. At both short SOAs, affectively incongruent pairs evoked stronger N400 responses than congruent pairs in the evaluative decision task, when an affective picture was used to prime an affective word. However, the N400 effect (likely indexing detection of conflict in affective processing) mainly occurred at anterior parts of the brain at an SOA of 150 ms, and only for negative words at an SOA of 250 ms. The N400 priming effect seen in the current experiment suggests that the difficulty of semantic integration contributes to the affective priming effect in the evaluative decision task. Additionally, the affectively incongruent trials were associated with a larger LPP than affectively congruent trials, which might reflect the increased attentional engagement evoked by affectively incongruent trials. The current ERP results provide first neurobiological evidence that affective pictures used as priming stimuli exert an automatic influence on the processing of target affective words as early as 150ms.
4. Experimental procedures
4.1. Participants
Eighteen right-handed undergraduate students (mean age = 21; 9 males and 9 females) from the Capital Normal University in Beijing, China participated in the study. All participants who consented to participation were native Chinese speakers and had normal or corrected-to-normal vision. Each subject received 30 RMB (about $5) compensation for participating in the study.
4.2. Stimuli
The stimuli consisted of 960 picture prime - word target pairs. There were 480 affectively congruent prime-target pairs (240 positive-positive, 240 negative-negative) and 480 affectively incongruent prime-target pairs (240 negative-positive, 240 positive-negative). The same stimuli were presented in affectively congruent and incongruent pairs, counterbalanced across subjects. The pictures used as primes consisted of 120 positive and 120 negative pictures from the International Affective Picture System (IAPS) (Lang, Bradley, & Cuthbert, 1999). The mean valence on a 1-9 point scale (with 9 being the most positive in valence dimension) was 7.08 (SD=0.66) for positive pictures and 2.60 (SD=0.77) for negative pictures, respectively. The mean arousal value (on a 1-9 point scale with 9 being the highest arousal) was 4.47 (SD=0.95) for positive pictures and 5.86 (SD=0.88) for negative pictures, respectively. The valence difference between positive and negative pictures was significant (p<.05). The arousal difference between positive and negative pictures was also significant (p<.05). In order to make 960 prime-target pairs, each picture was used in four separate pairs.
480 word targets were selected from the Chinese Affective Words System (CAWS) (Luo & Wang, 2004) and were equally divided in valence (240 positive words and 240 negative words). The mean valence values were 6.89 (SD=0.27) for positive words and 2.92 (SD=0.30) for negative words. The mean arousal value was 5.16 (SD=0.69) for positive words and 5.51 (SD=0.79) for negative words. The mean familiarity value was 5.94 (SD=0.58) for positive words and 4.75 (SD=0.60) for negative words. The valence difference between positive and negative words was significant (p<. 05). The arousal and familiarity differences between positive and negative words were also significant (both p<.05). Each target word was used in two different pairs.
To minimize repetition effects of primes and targets, each of the 960 prime-target pairs was assigned to one of two lists. Within each list, each prime picture was presented two times and each target word appeared only once. Half of the participants were presented with one list, whereas the remaining participants were presented with the other list. Each list contained 240 prime-target pairs (120 affectively congruent, 120 affectively incongruent) used with the 150 ms SOA condition and 240 pairs (120 affectively congruent, 120 affectively incongruent) used with the 250 ms SOA condition.
4.3. Procedure
Figure 5 illustrates the trial structure. Each trial began with a fixation point presented for 300 ms. Following the fixation, a prime picture was displayed on the screen for 100 ms, and was replaced by a fixation point for either 50 ms (SOA=150 ms) or 150 ms (SOA = 250 ms). A target word was then presented for 300 ms, followed by a 2500∼2700 ms presentation of another fixation point. The participants' task was to judge the pleasantness of the target word. All words and pictures were presented against a black background on a computer screen. Participants pressed a keypad with buttons showing either a happy face or a sad face to indicate their choices. The assignment of key to response hand was counterbalanced across subjects. Participants were also instructed to respond as quickly and accurately as possible, and to refrain from head movements and eye blinking. A two-minutes break after each 60-trial block was provided to help offset excessive movement. Prior to testing, each participant performed 20 practice trials to ensure that the testing procedure was well understood. To avoid possible order effects, presentation order of the two SOA conditions was counterbalanced across subjects.
Fig.5.
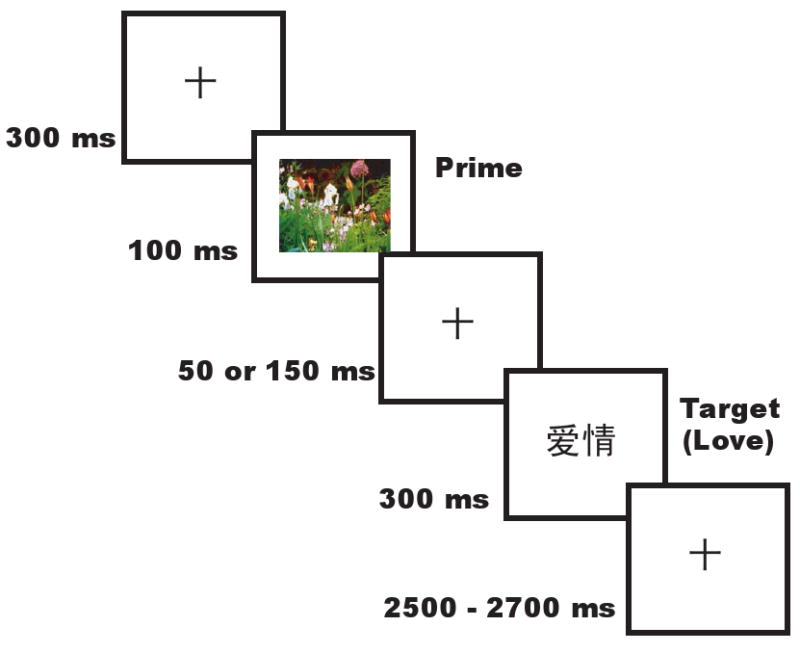
The affective priming task (picture prime – word target) and timing of stimuli presentation (The target is Chinese word “love”).
4.4. ERP recordings and analysis
Electroencephalogram (EEG) was recorded from 64 scalp electrodes using an electrode cap with Ag/AgCl inserts. All scalp electrodes were referenced to the average of left and right mastoid recording. Vertical electrooculogram (VEOG) and horizontal electrooculogram (HEOG) were recorded with two pairs of electrodes, one pair placed above and below the left eye, and another pair placed beside the two eyes. EEG signals were filtered with a bandpass of 0.05∼ 40 Hz and sampled at a rate of 500 Hz. Impedance was less than 5KΩ. Average ERPs were formed offline from correct-response trials free of ocular and movement artifacts (>±75μV). Each averaging epoch lasted 1100 ms (for SOA of 150 ms) or 1200 ms (for SOA of 250 ms), including 100 ms prior to prime onset. Each scalp site resulted in eight separate ERPs. The mean number of individual trials per waveform was 48.
Visual inspection of the waveforms and topographies was initially conducted to detect obvious differences between affectively congruent and affectively incongruent trials. Such differences were found mainly at 350 ∼ 450 and 550 ∼ 700 ms time windows post target onset. ERP responses should reflect the difference in the relationship between prime-target pairs (congruent vs. incongruent).
Mean amplitudes were computed at time windows 350 ∼ 450 and 550 ∼ 700 ms for each subject and condition type. The amplitude measurements were referred to the pre-prime baseline. Regional ANOVA analysis was conducted by selecting 24 electrodes from left vs. right hemisphere, at anterior vs. posterior locations (Dien & Santuzzi, 2005). The left anterior region included electrodes F5, F3, F1, FC5, FC3 and FC1. The right anterior region included electrodes F2, F4, F6, FC2, FC4, and FC6. The left posterior region included electrodes P5, P3, P1, PO7, PO5, and PO3. The right posterior region included electrodes P2, P4, P6, PO4, PO6, and PO8. The mean amplitude for each of the four regions under each condition was calculated. Mean amplitude data for the SOAs of 150 ms and 250 ms were subjected to a five-way repeated measures analysis of variance (ANOVA) test, with the following factors and levels: 2 affective congruency (congruent, incongruent) × 2 target valence (positive, negative) × 2 SOA (150 ms, 250 ms) × 2 hemisphere (left, right) × 2 location (anterior, posterior). Significance levels were set at 0.05. All significant or marginally significant interaction effects were decomposed with simple main effects comparisons and post hoc analyses. When significant or marginally significant interaction of SOA and affective congruency were found, the following ANOVAs on amplitude values for 150 ms and 250 ms SOAs were conducted respectively: 2 affective congruency (congruent, incongruent) × 2 target valence (positive, negative) × 2 hemisphere (left, right) × 2 location (anterior, posterior).
Acknowledgments
This work was presented at the 115th Annual Convention of APA, 2007 and was supported by NIH AG00986 to YJ, and Beijing grant (KM200810028020) to QZ. We thank E Walsh, C Black for helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bargh JA, Chaiken S, Govender R, Pratto F. The generality of the attitude activation effect. J Pers Soc Psychol. 1992;62:893–912. doi: 10.1037//0022-3514.62.6.893. [DOI] [PubMed] [Google Scholar]
- Blair KS, Richell RA, Mitchell DGV, Leonard A, Morton J, Blair RJR. They know the words, but not the music: Affective and semantic priming in individuals with psychopathy. Biol Psychol. 2006;73:114–123. doi: 10.1016/j.biopsycho.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Bostanov V, Kotchoubey B. Recognition of affective prosody: Continuous wavelet measures of event-related brain potentials to emotional exclamations. Psychophysiology. 2004;41:259–268. doi: 10.1111/j.1469-8986.2003.00142.x. [DOI] [PubMed] [Google Scholar]
- Brown C, Hagoort P. The processing nature of the N400: evidence from masked priming. J Cogn Neurosci. 1993;5:34–44. doi: 10.1162/jocn.1993.5.1.34. [DOI] [PubMed] [Google Scholar]
- Brown C, Hagoort P, Chwilla D. An event-related brain potential analysis of visual word-priming effects. Brain Lang. 2000;72:158–190. doi: 10.1006/brln.1999.2284. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Gardner WL, Berntson GG. The affect system has parallel and integrative processing components: form follows function. J Pers Soc Psychol. 1999;76:839–855. [Google Scholar]
- Castner JE, Chenery HJ, Copland DA, Coyne TJ, Sindair F, Silburn PA. Semantic and affective priming as a function of stimulation of the subthalamic nucleus in Parkinson's disease. Brain. 2007:1–13. doi: 10.1093/brain/awm059. [DOI] [PubMed] [Google Scholar]
- Chwilla DJ, Brown CM, Hagoort P. The N400 as a function of the level of processing. Psychophysiology. 1995;32:274–285. doi: 10.1111/j.1469-8986.1995.tb02956.x. [DOI] [PubMed] [Google Scholar]
- Crawford LE, Cacioppo JT. Learning where to look for danger: integrating affective and spatial information. Psychol Sci. 2002;13:449–453. doi: 10.1111/1467-9280.00479. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski T, Bechara A, Damasio H, Ponto LB, Parvizi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3(10):1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Deacon D, Hewitt S, Yang CH, Nagata M. Event-related potential indices of semantic priming using masked and unmasked words: evidence that the N400 does not reflect a post-lexical process. Cogn Brain Res. 2000;9:137–146. doi: 10.1016/s0926-6410(99)00050-6. [DOI] [PubMed] [Google Scholar]
- Deacon D, Uhm TJ, Ritter W, Hewitt S, Dynowska A. The lifetime of automatic semantic priming effects may exceed two seconds. Cogn Brain Res. 1999;7:465–472. doi: 10.1016/s0926-6410(98)00034-2. [DOI] [PubMed] [Google Scholar]
- De Houwer J, Hermans D. Differences in the affective processing of words and pictures. Cogn Emot. 1994;8:1–20. [Google Scholar]
- De Houwer J, Hermans D, Rothermund K, Wentura D. Affective priming of semantic categorization responses. Cogn Emot. 2002;16:643–666. [Google Scholar]
- Dien J, Santuzzi AM. Application of repeated measures ANOVA to high-density ERP datasets: A review and tutorial. In: Handy TC, editor. Event-Related Potentials: A Methods Handbook. Cambridge, Massachusetts: MIT Press; 2005. pp. 57–82. [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behavi Brain Sci. 1988;11:355–372. [Google Scholar]
- Fazio RH. On the automatic activation of associated evaluations: an overview. Cogn Emot. 2001;15:115–141. [Google Scholar]
- Fazio RH, Sanbonmatsu DM, Powell MC, Kardes FR. On the automatic activation of attitudes. J Pers Soc Psychol. 1986;50:229–238. doi: 10.1037//0022-3514.50.2.229. [DOI] [PubMed] [Google Scholar]
- Franklin MS, Dien J, Neely JH, Huber E, Waterson LD. Semantic priming modulates the N400, N300, and N400RP. Clin Neurophysiol. 2007;118:1053–1068. doi: 10.1016/j.clinph.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Frings C, Wentura D. Trial-by-trial effects in the affective priming paradigm. Acta Psychologica. 2008;128:318–323. doi: 10.1016/j.actpsy.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Jones SJ, Powell DK, Smith CD, Andersen AH. Dissociation of automatic and strategic lexical-semantics: Functional magnetic resonance imaging evidence for differing roles of multiple frontotemporal regions. J Neurosci. 2006;26:6523–6532. doi: 10.1523/JNEUROSCI.0808-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Buckner RL. Common prefrontal regions co-activate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35:803–812. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Hermans D, De Houwer J, Eelen P. The affective priming effect: Automatic activation of evaluative information in memory. Cogn Emot. 1994;8:515–533. [Google Scholar]
- Hermans D, De Houwer J, Eelen P. A time course analysis of the affective priming effect. Cogn Emot. 2001;15:143–165. [Google Scholar]
- Hermans D, Spruyt A, Eelen P. Automatic affective priming of recently acquired stimulus valence: Priming at SOA 300 but not at SOA 1000. Cogn Emot. 2003;17:83–99. doi: 10.1080/02699930302276. [DOI] [PubMed] [Google Scholar]
- Holcomb PJ, Neville HJ. Auditory and visual semantic priming in lexical decision: a comparison using event-related potentials. Lang Cogn Process. 1990;5:281–312. [Google Scholar]
- Jiang Y, Vagnini V, Clark J, Zhang Q. Reduced sensitivity of affective mismatches in older adults. TheScientificWorldJOURNAL. 2007;7:641–648. doi: 10.1100/tsw.2007.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang AM, Constable RT, Gore JC, Avrutin S. An eventrelated fMRI study of implicit phrase-level syntactic and semantic processing. NeuroImage. 1999;10:55–61. doi: 10.1006/nimg.1999.0493. [DOI] [PubMed] [Google Scholar]
- Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, Lang PJ. Large-scale neural correlates of affective picture processing. Psychophysiology. 2002;39:641–649. doi: 10.1017.S0048577202394162. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, Liddle PF. Reading anomalous sentences: an event-related fMRI study of semantic processing. NeuroImage. 2002;17:842–850. [PubMed] [Google Scholar]
- Kissler J, Herbert C, Winkler I, Junghofer M. Emotion and attention in visual word processing- An ERP study. Biol Psychol. 2009;80:75–83. doi: 10.1016/j.biopsycho.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Klauer KC, Musch J. Does sunshine prime loyal? Affective priming in the naming task. Q J Exp Psychol. 2001;54A:727–751. doi: 10.1080/713755986. [DOI] [PubMed] [Google Scholar]
- Klauer KC, Rossnagel C, Musch J. List-context effects in evaluative priming. J Exp Psychol Learn Mem Cogn. 1997;23:246–255. doi: 10.1037//0278-7393.23.1.246. [DOI] [PubMed] [Google Scholar]
- Klauer KC, Musch J. Affective priming: Findings and theories. In: Musch J, Klauer KC, editors. The psychology of evaluation: Affective processes in cognition and emotion. Mahwah, NJ: Erlbaum; 2003. pp. 7–49. [Google Scholar]
- Kounios J, Holcomb PJ. Structure and process in semantic memory: Evidence from event-related brain potentials and reaction times. J Exp Psychol Gen. 1992;121:459–479. doi: 10.1037//0096-3445.121.4.459. [DOI] [PubMed] [Google Scholar]
- Kutas M, McCarthy G, Donchin E. Augmenting mental chronometry. The P300 as a measure of stimulus evaluation time. Science. 1977;197:792–795. doi: 10.1126/science.887923. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Instruction Manual and Affective Ratings, Technical Report A-4. The Center for Research in Psychophysiology; Gainesville, Florida: 1999. [Google Scholar]
- Li W, Zinbarg RE, Boehm SG, Paller KA. Neural and behavioral evidence for affective priming from unconsciously perceived emotional facial expressions and the influence of trait anxiety. J Cogn Neurosci. 2008;20:95–107. doi: 10.1162/jocn.2008.20006. [DOI] [PubMed] [Google Scholar]
- Luo YJ, Wang YN. Chinese affective words system (CAWS) Institute of Psychology CAS; Beijing, China: 2004. [Google Scholar]
- Matsumoto A, Iidaka T, Haneda K, Okada T, Sadato N. Linking semantic priming effect in functional MRI and event-related potentials. NeuroImage. 2005;24:624–634. doi: 10.1016/j.neuroimage.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Neely JH. Semantic priming effects in visual word recognition: a selective review of current findings and theories. In: Besner D, Humphreys GW, editors. Basic processes in reading: visual word recognition. Hillsdale, NJ: Lawrence Erlbaum; 1991. pp. 264–336. [Google Scholar]
- Newman AJ, Pancheva R, Ozawa K, Neville HJ, Ullman MT. An event-related fMRI study of syntactic and semantic violations. J Psycholing Res. 2001;30:339–364. doi: 10.1023/a:1010499119393. [DOI] [PubMed] [Google Scholar]
- Nigam A, Hoffman JE, Simons RF. N400 to semantically anomalous pictures and words. J Cogn Neurosci. 1992;4:15–22. doi: 10.1162/jocn.1992.4.1.15. [DOI] [PubMed] [Google Scholar]
- Nobre AC, McCarthy G. Language-related ERPs: scalp distributions and modulation by word type and semantic priming. J Cogn Neurosci. 1994;6:233–255. doi: 10.1162/jocn.1994.6.3.233. [DOI] [PubMed] [Google Scholar]
- O'Hare AJ, Dien J, Waterson LD, Savage CR. Activation of the posterior cingulate by semantic priming: A co-registered ERP/fMRI study. Brain Res. 2007;1189(2):97–114. doi: 10.1016/j.brainres.2007.10.095. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychol Rev. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: An integrative review of ERP findings. Biol Psychol. 2008;77:247–265. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ready RE, Robinson MD, Weinberger M. Age differences in the organization of emotion knowledge: effects involving valence and time frame. Psychol Aging. 2006;21:726–736. doi: 10.1037/0882-7974.21.4.726. [DOI] [PubMed] [Google Scholar]
- Robinson MD, Ode S, Moeller SK, Goetz PW. Neuroticism and affective priming: Evidence for a neuroticism-linked negative schema. Pers Individ Differ. 2007;42:1221–1231. doi: 10.1016/j.paid.2006.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossell SL. Affective semantic priming in patients with schizophrenia. Psychiatry Res. 2004;129:221–228. doi: 10.1016/j.psychres.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Schirmer A, Kotz SA, Friederici AD. Sex differentiates the role of emotional prosody during word processing. Cogn Brain Res. 2002;14:228–233. doi: 10.1016/s0926-6410(02)00108-8. [DOI] [PubMed] [Google Scholar]
- Schirmer A, Kotz SA, Friederici AD. On the role of attention for the processing of emotions in speech: Sex differences revisited. Cogn Brain Res. 2005;24:442–452. doi: 10.1016/j.cogbrainres.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Schirmer A, Zysset S, Kotz SA, von Cramon DY. Gender differences in the activation of inferior frontal cortex during emotional speech perception. NeuroImage. 2004;21:1114–1123. doi: 10.1016/j.neuroimage.2003.10.048. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghofer M, Weike AI, Hamm AO. Emotional facilitation of sensory processing in the visual cortex. Psychol Sci. 2003;14:7–13. doi: 10.1111/1467-9280.01411. [DOI] [PubMed] [Google Scholar]
- Vermeulen N, Luminet O, Corneille O. Alexithymia and the automatic processing of affective information: Evidence from the affective priming paradigm. Cogn Emot. 2006;20:64–91. [Google Scholar]
- Wentura D. Activation and inhibition of affective information: Evidence for negative priming in the evaluation task. Cogn Emot. 1999;13:65–91. [Google Scholar]
- Wentura D. Dissociative affective and associative priming effects in the lexical decision task: Responding with “yes” vs. “no” to word targets reveals evaluative judgment tendencies. J Exp Psychol Learn Mem Cogn. 2000;26:456–469. doi: 10.1037//0278-7393.26.2.456. [DOI] [PubMed] [Google Scholar]
- Wentura D, Frings C. Response-bound primes diminish affective priming in the naming task. Cogn Emot. 2008;22:374–384. [Google Scholar]
- Werheid K, Alpay G, Jentzsch I, Sommer W. Priming emotional facial expressions as evidenced by event-related brain potentials. Inter J Psychophysi. 2005;55:209–219. doi: 10.1016/j.ijpsycho.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Lawson A, Guo CY, Jiang Y. Electrophysiological correlates of visual affective priming. Brain Res Bull. 2006;71:316–323. doi: 10.1016/j.brainresbull.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]