Abstract
We recently found that moderate consumption of two unrelated red wines generate from different grape species, a Cabernet Sauvignon and a muscadine wine that are characterized by distinct component composition of polyphenolic compounds, significantly attenuated the development of Alzheimer's disease (AD)-type brain pathology and memory deterioration in a transgenic AD mouse model. Interestingly, our evidence suggests that the two red wines attenuated AD phenotypes through independent mechanisms. In particular, we previously found that treatment with Cabernet Sauvignon reduced the generation of AD-type amyloid-β (Aβ) peptides. In contrast, evidence from our present study suggests that muscadine treatment attenuates Aβ neuropathology and Aβ-related cognitive deterioration in Tg2576 mice by interfering with the oligomerization of Aβ molecules to soluble high-molecular-weight Aβ oligomer species that are responsible for initiating a cascade of cellular events resulting in cognitive decline. Collectively, our observations suggest that distinct polyphenolic compounds from red wines may be bioavailable at the organism level and beneficially modulate AD phenotypes through multiple Aβ-related mechanisms. Results from these studies suggest the possibility of developing a “combination” of dietary polyphenolic compounds for AD prevention and/or therapy by modulating multiple Aβ-related mechanisms.
Keywords: Alzheimer's disease dementia, amyloid-β protein precursor (AβPP), high-molecular-weight amyloid-β oligomer species, polyphenols
INTRODUCTION
Alzheimer's disease (AD) is a growing public health concern with potentially devastating effects. There is presently no cure for or means of preventing AD. There is increasing consensus that the production and accumulation of amyloid-β (Aβ) peptides is central to the pathogenesis of AD. Deposition of Aβ peptides into insoluble fibrous aggregates known as amyloid plaques in the brain is a major hallmark of AD neuropathology. Moreover, increasing evidence suggests that cognitive decline in AD may be directly caused by accumulation of soluble high molecular weight (HMW) oligomeric Aβ species in the brain that are generated by aggregation of Aβ peptides [1–3]. Genetic factors are important risk factors for AD, especially for early-onset AD cases. Despite a relatively lower penetrance of genetic factors among late-onset sporadic AD cases, genetic factors remained to be highly relevant for the vast majority of late-onset sporadic AD cases, which is the most common form of AD [4]. Non-genetic factors, including modifiable lifestyle dietary regimens such as moderate consumption of certain alcoholic beverages, are receiving increasing attention in AD research, especially in light of recent epidemiological studies indicating that moderate wine consumption may reduce the relative risk for AD clinical dementia [5].
Consistent with the hypothesis that red wines might provide beneficial disease-modifying activities in AD, we recently demonstrated that moderate consumption of a red Cabernet Sauvignon wine generated from Vitis Vinifera L. grapes attenuated the onset of cognitive deterioration in the Tg2576 transgenic AD mouse model by reducing the accumulation of amyloid pathology in the brain [6]. Moreover, our evidence suggests that the polyphenolic components from Cabernet Sauvignon may attenuate the development of AD-phenotypes, in part, by promoting α-secretase activity in the brain that is responsible for “non-amyloidogenic” processing of the amyloid-β protein precursor (AβPP) that precludes the generation of amyloidogenic Aβ peptides [6].
To gather insights on the specific polyphenolic component(s) in red wines that might exert beneficial AD-modifying activities in vivo, we recently assessed for potential AD-disease modifying activity in a red muscadine wine, which is generated from Vitis rotundifolia grapes and is characterized by a distinctly different polyphenol composition compared to the Cabernet Sauvignon used in our previous study.
We report that moderate consumption of the muscadine wine significantly reduced AD-type Aβ neuropathology and attenuated spatial memory decline in the Tg2576 AD mouse model. However, in contrast to our previous observation with Cabernet Sauvignon [6], we found that muscadine treatment did not modulate α-secretase activity in the brain. Instead, we demonstrated that the beneficial impact of muscadine treatment is associated with reducing the aggregation of Aβ peptides into soluble HMW oligomeric Aβ species in the brain. Results from these studies suggest the possibility of developing a “combination” of dietary polyphenolic compounds for AD prevention and/or therapy by modulating multiple Aβ-related mechanisms.
MATERIALS AND METHODS
Muscadine wine
Muscadine was generated from Vitis rotundifolia at the University of Florida as previously described [7]. Red muscadine grapes (cv. Noble) were obtained from a local vineyard. Grapes were crushed, de-stemmed and allowed to ferment on the skins for 7 days at 13 °C. The soluble solids of the Noble grapes were adjusted to 21% before fermentation. The must was pressed and allowed to finish fermenting to dryness (<0.05% reducing sugar) at 13°C. The muscadine wine was then treated with 100 mg/L of potassium metabisulfite, cold-stabilized at 3°C for 2 months, filtered and stored at 13°C for approximately 3.5 years. The muscadine wine contains approximately 12% alcohol – as determined by ebulliometry [8], a titratable acidity (as tartaric acid) of 6.9 g/L and a pH of 3.00. The phenolic composition (as gallic acid and measured by the Folin-Coicalteau method [8] was 1.731 mg/L.
Chemical analysis of wine polyphenolic component composition
Chemical compositions of muscadine (and Cabernet Sauvignon) were assessed by reverse phase chromatography using HPLC and an Octadecyl silane column (4.6 × 250 mm) and the solvent conditions of Talcott and Lee [9]. Briefly, 2 ml of each wine was freeze dried. Dried extracts were re-dissolved in 0.1 M citric acid buffer at pH 3.0 and injected into a Waters Alliance 2695 HPLC system that linked to a Waters 996 Photodioarray detector. Compounds were separated on a Dionex Acclaim 120 C18 column (4.6 × 250 mm) using a gradient mobile phase that consisted of Phase A (100% water adjusted to pH 2.4 with o-phosphoric acid) and Phase B (60:40 methanol:water, also at pH 2.4 with o-phosphoric acid). Phase A changed from 100% to 50% in 30 min, to 20% in 10 min, and to 0% in 10 min and held isocratic for 10 min. Compounds were detected at 280 nm (phenolic acids), 370 nm (flavonoids), and 520 nm (anthocyanins), with select compounds identified based on spectroscopic interpretations from 200–600 nm [9,10].
Tg2576 mice and wine treatment
In this study, 4 month old female Tg2576 mice (Taconic, Germantown Inc.) were randomly assigned to muscadine-treatment or control, non-treatment groups. Muscadine was delivered to mice by diluting the wine into the drinking water to a final ethanol concentration of 6%. Animals had free access to the liquid and standard rodent chow. Drinking solutions were changed every three days. Liquid consumption, food intake and animal body weight were monitored weekly throughout the study. Mice were treated chronically with the muscadine wine for 10 months. In parallel control studies, wild-type (WT) mice matching with Tg2576 mice in respect to strain, gender and age were similarly treated with the muscadine wine for 10 months; control, non-treated WT mice received unadulterated drinking water. At 14 months of age, spatial memory functions of muscadine-treated and non-treated Tg2576 and WT mice were assessed by the Morris water maze (MWM) test (see below). Thereafter, all mice were anesthetized with the general inhalation anesthetic 1-chloro-2,2,2-trifluoroethyl difluoromethyl ether (Baxter Healthcare, Deerfield, IL) and sacrificed by decapitation. Brains were harvested and hemidissected. One hemisphere was fixed in 4% paraformalde-hyde for 24 hours for morphological studies; hippocampus, and cingulate and parietal neocortex were dissected from the opposite hemisphere, rapidly frozen, pulverized in liquid nitrogen and stored at –80°C for biochemical studies. All studies involving animal subjects are conducted in accord with the guide of the Mount Sinai School of Medicine Institutional Animal Care and Use Committee.
Assessment of wine toxicity
Blood was collected after sacrifice by cardio puncture. Serum was collected by clotting blood specimens for 10 minutes at room temperature followed by centrifugation at 2,500 g for 20 min at 4 °C. Serum was immediately subjected to total bilirubin, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) analysis using commercial enzyme assays according to the manufacturer's instructions (Stanbio laboratory, Texas). Serum alcohol content was measured using a commercially available kit (BioAssay Systems, CA).
Behavioral assessment of cognitive functions by the Morris water maze (MWM) test
Spatial learning memory was assessed at 14 months of age by the MWM behavioral test, as previously described [2]. Following 10 months of treatment with the muscadine wine, approximately 14 month old mice were tested in a 1.25 m circular pool filled with water mixed with non-toxic white paint (Dick Blick Art Materials, Galesburg, IL). The temperature of the water was maintained at 27°C. Mice were trained in absence of visual cue to mount a hidden/submerged (1.5 cm below-water surface) escape platform (14 × 14 cm) in a restricted region of the pool. Thereafter, learning trials (four 60 second trials per day with a 10-min inter-trial, for 7 consecutive days) were conduct to test spatial memory by evaluating the latency to locate the hidden escape platform in an environment enriched in spatial cues as a function of learning trials. At the end of the learning trials, animals were rested for 24 hours before they were subjected to a 45 second probe trial in the water maze in the presence of the identical spatial cues used in the learning trials, but in the absence of the hidden escape platform. The pool was further divided into four hypothetical quadrants of identical surface area. The percentage of time spent in the target quadrant where the former escape platform was located was recorded to reflect the capability of the animals to the use spatial cues to identify the appropriate location in the pool where the escape platform ought to be found.
In control studies, a visible platform test was used to determine whether differences in visual and/or swimming capability might confound interpretations of the behavioral performance data gathered from the MWM tests. Visual capability was assessed by the latency of the animals to escape onto the visible escape platform as a function of trial days. Motor functions were reflected by swimming speed (cm/second) as a function of trial days during the visible platform test.
All water maze activities were monitored with the San Diego Instrument Poly-Track video tracking system (San Diego, CA). All behavior analyses were conducted during the last 4 hours of the day portion of the light cycle in an environment with minimal stimuli (e.g., noise, movement, or changes in light or temperature).
Assessment of AD-type amyloid neuropathology in Tg2576 mice
AD-type amyloid plaque density in the brain at 14 months of age was assessed using stereological procedures. Freshly harvested mouse brain hemispheres were immersion fixed overnight in 4% paraformalde-hyde. They were then sectioned in the coronal plane on a Vibratome at a nominal thickness of 50 μm. Amyloid plaques were visualized by thioflavin-S staining. Every 12th section was selected from a random start position and processed for thioflavin-S staining, as previously described [13,14]. All stereological analysis was performed using a Zeiss Axiophoto photomicroscope equipped with a Zeiss motorized stage and MSP65 stage controller, a high-resolution MicroFire digital camera, and a Dell computer running the custom-designed software Stereo Investigate (MBF Bioscience). Amyloid plaque burden was estimated using the Cavalieri principle with a small-size grid (25 × 25 μm) for point counting, as previously described [6, 13].
Assessments of α-, β-, γ-secretase activity in the brain
α-, β-, and γ- secretase activities in brain specimen from 14 month old Tg2576 mice were assessed using commercially available kits (R&D Systems) [6,12,13]. Brain samples were homogenized in supplied buffers. Homogenate was then added to secretase-specific AβPP peptide conjugated to the reporter molecules EDANS and DABCYL. In the un-cleaved form, fluorescent emissions from EDANS were quenched by the physical proximity of the DABCYL moiety. Cleavage of AβPP peptide by secretase physically separates the EDANS and DABCYL reporter molecules, allowing for the release of a fluorescent signal. The level of secretase enzymatic activity is proportional to the fluorometric reaction in the homogenate (R&D Systems). In parallel control studies, holo-AβPP expression was examined by Western blot analysis with the C8 antibody (raised against aa 676–695 of human AβPP cytoplasmic domain; gift of Dennis Selkoe, Brigham and Women's Hospital, Boston, Massachusetts, USA).
Assessment of HMW soluble oligomeric Aβ oligomerization in the brain by dot blot and western blot assays
Soluble proteins were extracted from brain samples (cerebral cortex) of 14 month old Tg2576 mice with phosphate-buffered saline in the presence of protease inhibitors followed by centrifugation at 78,500 g for 1 hour at 4°C [15]. In dot-blot assays, 4 μg of extra-cellular soluble protein isolated from the cerebral cortex or from the hippocampal formation of muscadine-treated or control, non-treated Tg2576 mice was directly applied to a nitrocellulose membrane, air dried, and blocked with 5% nonfat milk, as previously described [16]. The membrane was probed with either the anti-oligomer A11 antibody (1:1,000; Biosource) or the 6E10 antibody (1:1,000; Signet) [16]. In western blot assays, 75 μg of soluble proteins were resolved by electrophoresis through a 10–20% Tris-Tricine gel, transferred to a nitrocellulose membrane and blocked with 10% non-fat milk for 1 hour. The membrane was then incubated with the 6E10 antibody (Signet). For both dot-blot and western blot analysis, immunoreactive signals were visualized by using enhanced chemiluminescence detection and quantified densitometrically (Quantity One; Bio-Rad).
Polyphenolic extraction for in vitro studies
For in vitro Aβ peptide oligomerization studies, a muscadine polyphenol extract was prepared by solid phase extraction using C18 cartridges (Waters). Muscadine was diluted 1:4 in acidified water (water buffered to pH 2.4 with o-phosphoric acid) to optimize preservation of the polyphenol compounds in the wine. Diluted wine was loaded onto C18 cartridges followed by washing the columns with 3-volumns of acidified water. Bound organic compounds were eluted in 12% methanol diluted in acidified water. The eluants were dispensed into small aliquots and dried in a speedivac. For anti-oligomerization assays, dried polyphenol extracts were dissolved into phosphate-buffered saline immediately before use.
Gel electrophoresis Aβ peptide oligomerization assay in vitro
Lyophilized Aβ1–42 peptide was dissolved in 1,1,1,3,3,3,-hexafluoro-2-propanol (HFIP; Sigma-Aldrich), incubated at room temperature for 60 min, aliquoted, vacuum dried, and stored at –80°C. Aβ peptide was dissolved in DMSO and diluted into ddH2O to a final concentration of 100 μg/ml. 5 μl of the peptide was then mixed with 1–2 μl of muscadine wine, the final volume adjusted to 10 μl and the reaction was incubated at 37°C for 3 hours [17]. Following incubation, samples were mixed with 2× SDS sample buffer and separated on a 10%–20% Tris-Tricine gradient SDS gel (Invitrogen). The separated peptides were subjected to Western blotting using 6E10 antibody (1:1,000; Signet). Immunoreactive signals were visualized using ECL detection (Amersham) and quantified densitometrically (Quantity One; Bio-Rad).
Photo-induced cross-linking of unmodified proteins (PICUP) assay
Freshly isolated low molecular weight Aβ1–40 (30–40 μM) peptide was mixed with 1 μl of 1 mM tris(2,2’-bipyridyl)dichlororuthenium (II) (Ru(bpy)) and 1 μl of 20 mM ammonium persulfate in 10 mM phosphate, pH 7.4, either in the presence or absence of muscadine wine or a muscadine polyphenolic extract. The mixture was irradiated for 1 second, and quenched immediately with 10 μl of Tricine sample buffer (Invitrogen) containing 5% β-mercaptoethanol [1]. The reaction was subjected to SDS-PAGE and visualized by silver staining (SilverXpress, Invitrogen).
Statistical analysis
All values are expressed as mean and standard error of the mean (SEM). Differences between means were analyzed using either 2-way repeated measures ANOVA or 2-tailed Student t test. In all analyses, the null hypothesis was rejected at the 0.05 level. All statistical analyses were performed using the Prism Stat program (GraphPad Software, Inc., San Diego CA).
RESULTS
Chemical analysis of muscadine and Cabernet Sauvignon wines
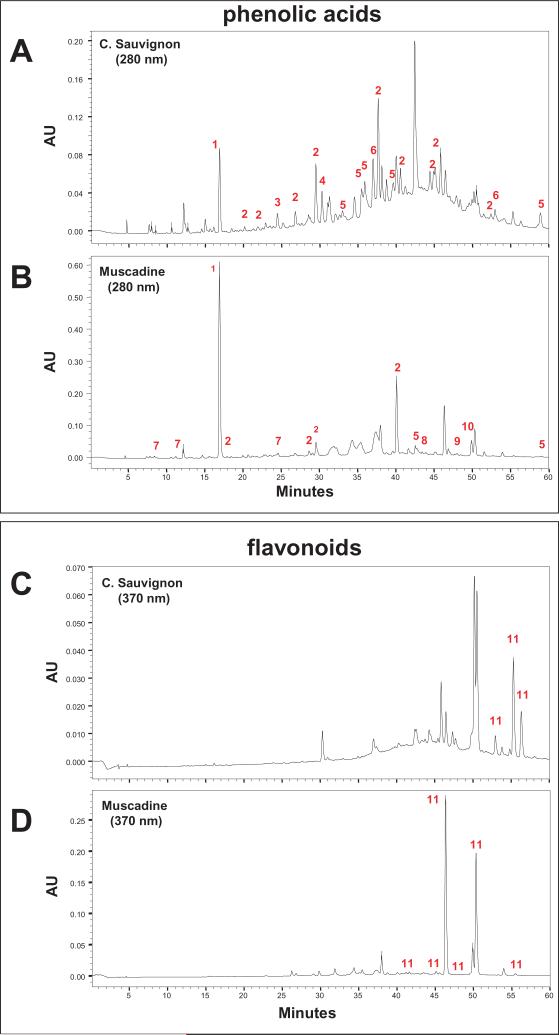
Chemical compositions of the muscadine wine used in this study and the red Cabernet Sauvignon wine we used in our previous study [6] were analyzed using HPLC C18 reverse phase chromatography. We confirmed that the two wines are characterized by distinct component compositions of phenolic acid, flavonoid and anthocyanin polyphenolic compounds (Fig. 1A–F).
Fig. 1a.
Chemical analysis of Cabernet Sauvignon and muscadine wines. Constituent polyphenolic components in Cabernet Sauvignon (A,C,E) and muscadine (B,D,F) wines were analyzed by reverse phase HPLC using a C18 column. (A,B) Detection of phenolic acid compounds at 280 nm. (C,D) Detection of flavonoids at 370 nm. (E,F) Detection of anthocyanins at 520 nm. (G) Identification of polyphenols corresponding to peaks detected in panel (A-F) based on spectroscopic interpretations
Fig. 1b.
Chemical analysis of Cabernet Sauvignon and muscadine wines. Constituent polyphenolic components in Cabernet Sauvignon (A,C,E) and muscadine (B,D,F) wines were analyzed by reverse phase HPLC using a C18 column. (A,B) Detection of phenolic acid compounds at 280 nm. (C,D) Detection of flavonoids at 370 nm. (E,F) Detection of anthocyanins at 520 nm. (G) Identification of polyphenols corresponding to peaks detected in panel (A-F) based on spectroscopic interpretations
Moderate consumption of muscadine is well-tolerated in Tg2576 mice
Based on our observation that each mouse consumed an approximately 4 ml of wine-adulterated water per day (Online Supplemental Information, Fig. 1A), we calculated that daily wine consumption by muscadine-treated Tg2576 mice in this study is equivalent to moderate wine consumption in the human as defined by the United States Department of Agriculture and Health and Human Services [19]. Moreover, we found that moderate consumption of muscadine for 10 months is compatible with general good health in Tg2576 mice, as reflected by no detectable changes in food/water intakes, body weights, and indexes of liver functions in 14-month old muscadine-treated, compared to age- gender-matched control, non-treated Tg2576 mice (Online Supplemental Information). This observation is consistent with our recent report that moderate red wine consumption is well-tolerated by Tg2576 mice [6]. Details on translating daily wine intake in muscadine-treated Tg2576 mice to equivalent wine consumption in the human, and assessments of food/liquid consumptions, body weights, and liver functions are provided in Online Supplemental Information.
Muscadine treatment attenuates AD-type cognitive deterioration in Tg2576 mice
In this study, we explored the potential impact of muscadine treatment on cognitive behavioral functions and AD-type amyloid neuropathology in 14 month old Tg2576 mice, when these animals typically exhibit moderate-to-severe amyloid neuropathology and cognitive impairment [20].
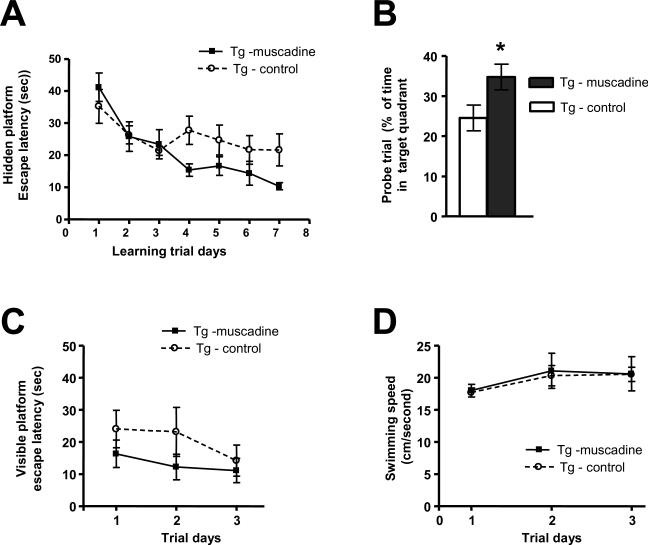
Using the MWM, we found that muscadine treatment for 10 months significantly attenuated spatial memory activity decline in approximately 14 month old Tg2576 mice relative to age- and gender-matched control, non-treated Tg2576 mice, as reflected by a shorter escape latency as a function of learning trials during the learning phase [2-way ANOVA analysis of Tg-muscadine vs. Tg-control groups for muscadine treatment (< 0.05, F = 4.24, DFn = 1, DFd = 84) and for training days (p < 0.05, F = 6.43, DFn = 6, DFd = 84)] (Fig. 2A). That muscadine-treatment significantly attenuated cognitive impairment in Tg2576 mice was confirmed by analysis of spatial memory retention in a probe trial conducted following removal of the escape platform, which showed that muscadine-treated mice spent significantly more time in the target quadrant area relative to non-treated Tg2576 mice (p < 0.05, 2-tailed Student t test) (Fig. 2B). Moreover, we confirmed that improved MWM behavioral performance in muscadine-treated mice is not due to non-specific promotion in either visual or motor functions as both muscadine-treated and non-treated Tg2576 mice exhibited comparable capability to locate a visible escape platform (Fig. 2C) and demonstrated similar swim speeds (Fig. 2D).
Fig. 2.
Muscadine treatment improves spatial memory function in Tg2576 mice. Assessments of spatial memory behavioral functions of 14 month old muscadine-treated (Tg-muscadine) and control, gender- and age-matched non-treated (Tg-control) Tg2576 mice using the Morris water maze (MWM) protocol. (A) Learning trial hidden-platform acquisition curves. Tg-muscadine group performed significantly better than the control, non-treated group (Tg-control) [2-way ANOVA analysis of Tg-muscadine vs. Tg-control groups for muscadine treatment (p < 0.05, F = 4.24, DFn = 1, DFd = 84) and for training days (p < 0.05, F = 6.43, DFn = 6, Dfd = 84)]. (B) Probe trial conducted 24 hours after completion of hidden-platform training. Muscadine-treated Tg2576 mice exhibited a significantly higher preference for the target platform compared to control, non-treated Tg2576 mice (p < 0.05, 2-tailed Student t test). (C) Visible-platform learning curves. There is no significant difference in visible platform performance among Tg-muscadine compared to Tg-control mice. (D) Average swimming speed. There is no significant difference in swimming ability of muscadine-treated animals compared to control, non-treated animals. In (A-D) Values represent group mean (+SEM); n = 7–9 mice per group.
In parallel control studies, we assess for MWM performances of 14 month old WT mice that are strain-, gender-, and age-matched with Tg2576 mice used in behavioral testing. We found that 10 months of muscadine treatment did not lead to any detectable change in MWM performances in muscadine-treated compared to non-treated WT mice (data not shown). This suggests that muscadine treatment attenuated cognitive impairments in Tg2576 mice through mechanisms associated with expression of the AβPP transgene in this AD mouse model.
Muscadine treatment attenuates the development of AD-type Aβ neuropathology in Tg2576 mice
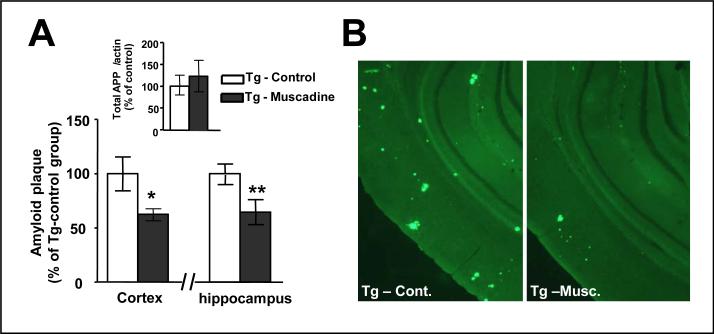
The initiation and progression of cognitive decline with increasing age in Tg2576 mice is due to age-dependent progressive accumulations of AD-type Aβ neuropathology in the brain [20]. We used stereological histological methodologies to quantitatively explore the accumulation of AD-type amyloid neuritic plaques in the brain of 14 month old Tg2576 mice in response to 10 months of muscadine treatment. We found significantly lower amyloid neuritic plaque density in both the cortical and the hippocampal formation regions of the brain of 14 month old muscadine-treated compared to age- and gender-matched control, non-treated Tg2576 mice (Fig. 3A,B). Thus, coincidental to attenuating the development of AD-type spatial memory functions, muscadine treatment also reduced the accumulation of AD-type Aβ neuropathology in the Tg2576 AD mouse model. Ongoing studies exploring the impact of muscadine treatment on amyloid neuropathology as a function of age in the Tg2576 mouse AD model will clarify whether the red wine might benefit AD by delaying the onset of amyloid plaque deposition, and/or by attenuating the continue progression of existing amyloid neuropathology.
Fig. 3.
Muscadine treatment significantly reduced Aβ neuropathology in Tg2576 mice. Tg2576 mice were assessed for indexes of AD-type amyloid burden in the brain in response to muscadine treatment using stereological technologies; the same 14 month old Tg2576 mice used for behavioral assessments in Fig. 2 were used in this study. (A) Assessments of amyloid neuritic plaque density in cerebral cortex and in the hippocampal formation of brain specimens from muscadine-treated and control, non-treated Tg2576 mice. (A, inset) Control studies confirming muscadine treatment did not modulate expression of the holo-AβPP in the brain (cerebral cortex). Bar graphs represent means ± SEM., n = 6–8 per group; * P < 0.05 vs. non-treated control Tg2576 group (2-tailed Student's t test). (B) Representative micrograph of brain specimen stained for amyloid neuritic plaques in muscadine-treated (Tg-musc.) or in control, non-treated (Tg-cont.) Tg2576 mice.
The efficacy of muscadine treatment to attenuate AD-type Aβ neuropathology in Tg2576 mice is not due to ethanol exposure from the wine. In control studies, we found that long-term exposure of Tg2576 mice to a drinking water solution containing 6% ethanol (the same ethanol content in the muscadine-adulterated drinking water used in this study) did not lead to any detectable changes Aβ neuropathology in this AD mouse model [6].
Muscadine treatment is not associated with detectable changes in AβPP processing in the brain of Tg2576 mice
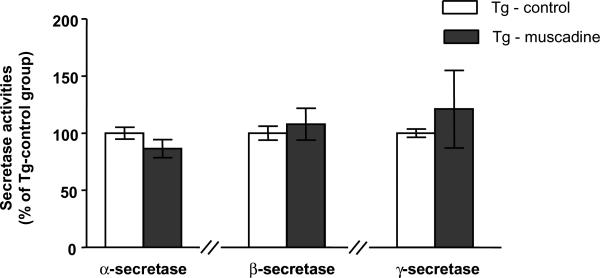
Multiple enzyme activities are known to regulate the generation of Aβ peptides from the ubiquitous AβPP [21]. Aβ species with different carboxy termini are generated from AβPP through sequential proteolysis by β- and γ-secretase. AβPP can also be processed by a non-amyloidogenic pathway in which α-secretase cleaves AβPP within the Aβ domain, thereby precluding the generation (and deposition) of intact amyloidogenic Aβ peptides in the brain [22]. In an initial step to explore the mechanism by which the muscadine treatment might have attenuate AD-type Aβ neuropathology, we examined the potential impact of muscadine treatment on the regulation of α-, β- and γ-secretase activities in the brain of the same 14 month old muscadine-treated and control Tg2576 animals we have used for behavioral and Aβ neuropathology studies. We found no detectable change in α-, β-, or γ-secretase activity in the brain of the muscadine-treated in comparison to control, non-treated Tg2576 mice (Fig. 4). Thus, it is unlikely that muscadine treatment might have attenuated the development of AD-type Aβ neuropathology and cognitive deterioration in the Tg2576 AD mouse model by modulating secretase activities in the brain that play key roles in the generation of Aβ peptides from AβPP.
Fig. 4.
No detectable changes in α-, β- and γ-secretase activities in the brain of Tg2576 mice in response to muscadine treatment. α–, β-, and γ-secretase enzymatic activities were assessed in cerebral cortex brain specimens from the same 14 month old Tg2576 animals used in the behavioral studies in Fig. 2 and neuropathology assessments in Fig. 3. (A) α-secretase activity, (B) β-secretase activity, and (C) γ-secretase activity in Tg-muscadine and Tg-control mice. (A-C). Bar graphs represent group mean (± SEM), n = 6–8 per group.
Attenuation of Aβ neuropathology in response to muscadine treatment is associated with reduced accumulation of soluble HMW oligomeric Aβ species in the brain
Recent evidence indicates that accumulations of soluble HMW oligomeric Aβ species in the brain, rather than deposition of amyloid plaques per se, may be specifically related to spatial memory reference deficits in mouse models of AD [1,2]. Based on this, we continue to explore whether muscadine treatment in this study might have attenuated the development of AD phenotypes in Tg2576 mice at 14 months of age by interfering with the accumulation of soluble HMW oligomeric Aβ species in the brain.
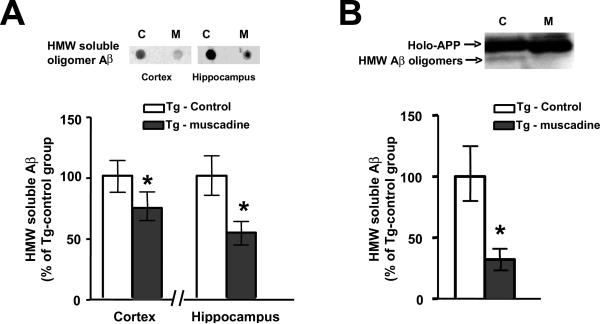
In a dot blot assay using the A11 antibody that detects oligomeric species [16], we found significantly reduced contents of A11-immunoreactive oligomeric Aβ species in the cerebral cortex (Fig. 5A, left panel) and in the hippocampal formation (Fig. 5A, right panel) of brain specimens from muscadine-treated relative to control, non-treated Tg2576 mice. We also assess the content of Aβ oligomers in the brain of Tg2576 mice using an independent western blot analysis that identifies Aβ oligomers based on their characteristic molecular size. Consistent with our observation from the dot blot assay, we found significantly lower contents of HMW oligomeric Aβ peptides in the cerebral cortex of muscadine-treated compared to control Tg2576 mice (Fig. 5B). Thus based on two independent assays, we found that muscadine treatment might have attenuated AD pathologic and cognitive phenotypes in Tg2576 mice, in part, by reducing the accumulation of HMW oligomeric Aβ peptides in the brain.
Fig. 5.
Muscadine treatment significantly attenuated the accumulation soluble HMW Aβ species in the brain of Tg2576 mice. The contents of HMW Aβ oligomeric species in cerebral cortex brain specimens from muscadine-treated or control, non-treated 14 month old Tg2576 mice were assessed by independent dot-blot (A) and western blot studies (B). (A) Immunodetection of HMW Aβ oligomeric species in the cerebral cortex and in the hippocampal formation using the oligomer-specific antibody A11 antibody in an dot blot assay. (A, inset) Representative A11-immunoreactive dot-blot analysis of cortical and hippocampal formation brain specimens. Abbreviations: C: control, non-treated Tg2576 mice; M: muscadine-treated Tg2576 mice. (B) Western blot analysis in which HMW Aβ oligomeric species are resolved by gel electrophoresis followed by immunodetection using a pan-Aβ 6E10 antibody. (B, Inset) Representative western blot analysis of muscadine-treated and control non-treated Tg2576 mice. Immunoreactive holo-AβPP and relatively less abundant HMW Aβ species are identified. In (A, B), bar graphs represent means ± SEM., n = 6–8 per group; * P < 0.05 vs. non-treated control Tg2576 group (2-tailed Student's t test).
Muscadine treatment might reduce the accumulation of HMW oligomeric Aβ species by interfering with aggregation of Aβ peptides
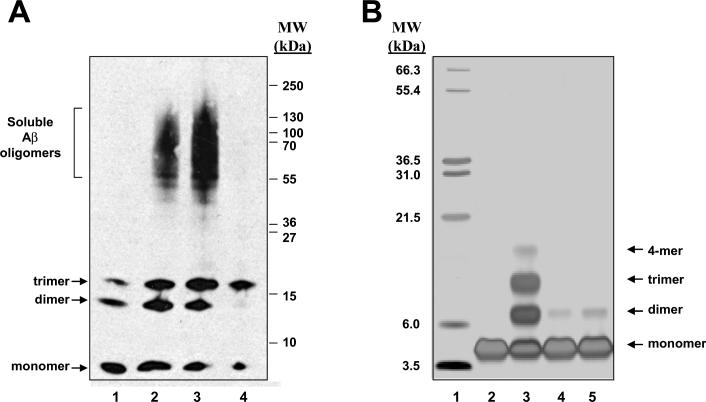
We explored whether muscadine wine or bioactive component from the wine may mechanistically reduce the accumulation of HMW Aβ oligomers in the brain by interfering with aggregations of Aβ peptides that is necessary for the generation of HMW Aβ oligomers. In initial in vitro studies using a gel-electrophoresis protocol, we found that muscadine wine prevented aggregations of synthetic Aβ1–42 peptides into SDS-stable HMW oligomers (Fig. 6A). In parallel control studies, we found that the application of ethanol does not have any detectable influence on aggregations of synthetic Aβ1–42 peptides into HMW oligomeric species (Fig. 6A), suggesting that the alcohol contents in the muscadine is not responsible for the anti-Aβ oligomerization bioactivity of the wine. Interestingly, evidence from our gel-electrophoresis study suggests that the generation of Aβ1–42 trimers might be relatively resistant to interference by muscadine wine. Ongoing studies are exploring whether bioactive components from the wine might preferentially interfere with specific Aβ aggregation activities.
Fig. 6.
Muscadine polyphenols exert anti-Aβ oligomerization bioactivity in vitro. (A) Muscadine wine interferes with aggregation of synthetic Aβ1–42 peptides into HMW oligomer Aβ species, in vitro. Synthetic Aβ1–42 peptides were aggregated in the absence or in the presence of muscadine wine. Aβ species were then resolved by molecular size, transblotted onto a nitrocellulose membrane, followed by immunodetection of Aβ peptides using and the 6E10 antibody. Lane 1 represents non-aggregated Aβ1–42 peptides; Lane 2, aggregated Aβ1–42 peptides; Lane 3, Aβ1–42 peptides aggregated in the presence of 1.2% ethanol (the same amount of ethanol presented in the aggregation assay in the presence of muscadine in Lane 4); Lane 4, Aβ1–42 peptides aggregated in the presence of 1 μl muscadine wine. (B) Application of an independent PICUP assay to explore the role of muscadine wine and its polyphenolic components to interfere with aggregations of synthetic Aβ1–40 peptides. The PICUP assay is designed to explore the initial protein-protein interactions that are necessary for the formation of HMW Aβ oligomeric species. Aβ aggregates are stabilized by photo-cross-linking. Monomeric and multimeric Aβ species are separated by molecular size and visualized by silver staining. Lane 1 represents molecular weight marker; Lane 2, non-aggregated Aβ1–40 peptide; Lane 3, aggregated Aβ1–40 peptide; Lane 4, Aβ1–40 peptide aggregated in the presence of muscadine wine (2 μl of the wine); Lane 5, Aβ1–40 peptide aggregated in the presence of a polyphenolic extract from 2 μl of the muscadine wine. The position of Aβ1–40 monomer, dimmer, trimer and 4-mer are as indicated.
Using an independent in vitro PICUP assay [23,24], we continue to explore whether muscadine treatment might interferes with initial protein-protein interactions that are necessary for aggregations of Aβpeptides into HMW oligomeric species. Consistent with our observation from gel-electrophoresis studies, we found that the muscadine wine (but not ethanol) interferes with initial aggregation of synthetic Aβ1–40 peptides into small multimeric species in the PICUP assay (Fig. 6B).
We continue to explore whether polyphenol contents in muscadine might be responsible for the anti-Aβ oligomerization activity of the red wine. We prepared an alcohol-free polyphenolic extract from the muscadine wine by solid phase extraction and tested for in vitro anti-Aβ oligomerization activity using the PICUP assay. We found that similar to the intact muscadine wine, the polyphenolic isolate from the wine almost completely prevented aggregations of Aβ1–40 peptides in the PICUP assay (Fig. 6B).
Thus, based on observations from independent in vitro assays, we demonstrated that muscadine treatment might, in part, attenuated accumulations of HMW Aβ oligomers in the brain of Tg2576 mice by mechanistically inhibiting aggregations of Aβ peptides into higher-ordered oligomers. More importantly, our evidence implicated polyphenol compounds in the muscadine wine as the bioactive components that exert beneficial AD-disease modifying activity by inhibiting Aβ aggregations.
DISCUSSION
Genetic factors are important risk factors for AD, especially for early-onset AD cases. Despite a relatively lower penetrance of genetic factors among late-onset sporadic AD cases, genetic factors remained to be highly relevant for the vast majority of late-onset sporadic AD cases, which is the most common form of AD [4]. Non-genetic factors, including modifiable lifestyle dietary regimens such as moderate consumption of alcoholic beverages, are receiving increasing attention in AD research, especially in light of the recent epidemiological studies indicating that moderate wine consumption may influence the relative risk for AD clinical dementia [5,25]. Our evidence that moderate consumption of a red Cabernet Sauvignon wine [6] and a red muscadine wine (in this study) significantly attenuated the accumulation of AD-type Aβ neuropathology and the development of cognitive deterioration in the Tg2576 AD mouse model supports the hypothesis that red wine consumption might provide preventive and/or therapeutic value in AD. However, despite our encouraging evidence, a major concern for translating this observation into clinical application is the health risks associated with chronic consumption of alcoholic beverages [26]. Based on this, ongoing studies are focused on identifying the bioactive component(s) in red wines and clarifying the mechanisms of action to provide the necessary basis for developing red wine mimetic(s) for applications in AD.
The potential health benefits of wine consumption are generally ascribed to the polyphenol compounds that are present in high abundance, particularly in red wines [27,28]. Since many of the wine-derived polyphenols are strong antioxidants, it is thought that red wine polyphenols may benefit AD (and other neurodegenerative disorders) by reducing the contents of reactive oxygen species in the brain [29]. Aside from potential antioxidant activities, our accumulating pre-clinical evidence suggests that red wine polyphenols may also benefit AD by directly modulating Aβ-related mechanisms in the brain. In particular, we recently demonstrated that polyphenolic components from a red Cabernet Sauvignon wine may protects against the onset of AD-type Aβ-neuropathology and cognitive deterioration in the Tg2576 AD mouse model by promoting non-amyloidogenic α-secretase activity in the brain that reduces Aβ neuropathology [6]. Similar to our observation with Cabernet Sauvignon, results from our present studies showed that polyphenolic compounds from a red muscadine wine may also exert beneficial AD-disease modifying activity in vivo and inhibit the onset of Aβ neuropathology and cognitive dysfunction in Tg2576 mice. However, in contrast to Cabernet Sauvignon, our new evidence suggests that bioactive polyphenols from the muscadine wine may modulate AD phenotypes by interfering with aggregation of Aβ peptides into HMW oligomeric Aβ species in the brain that play a major role in AD-type cognitive dysfunction [1–3]. Recent evidence suggests that multiple polyphenols found in red wines may block oligomerization of synthetic Aβ peptides in vitro [30–32]. Thus, it is possible that more than one polyphenolic components in the muscadine wine might have contribute to the in vivo efficacy of the wine to attenuated Aβ-related neuropathology and cognitive dysfunction in Tg2576 mice. Interestingly, our recent evidence demonstrated that polyphenolic components from Cabernet Sauvignon also blocked oligomerization of synthetic Aβ peptides in vitro (data not shown). Thus, it is possible that in our previous study [6] Cabernet Sauvignon treatment might have attenuated AD phenotypes by promoting α-secretase activity as well as inhibiting aggregation of Aβ peptides in the brain. Ongoing studies will clarify whether similar (or different) polyphenolic compounds in Cabernet Sauvignon and muscadine are responsible for anti-Aβ oligomerization activities in the brain.
In addition to providing antioxidant activities and/or modulating Aβ neuropathology,select dietary polyphenols may benefit AD dementia through non-Aβ related mechanisms by directly promoting cellular processes relevant to cognitive functions. In particular, Joseph et al. [33] reported that dietary supplement with blueberries, which contains high contents of polyphenols, prevented the development of cognitive impairment in a 12 month old double transgenic AβPP/PS1 AD mouse model by promoting cellular signaling process associated with learning and memory without detectable effect on amyloid deposition. In our studies with two red wines, we found that dietary supplement with Cabernet Sauvignon [6] or muscadine (in the present study) did not promote cognitive behavior performance in WT mice. Thus in contrast to blueberries, polyphenolic from the two red wines may benefit AD phenotypes by attenuating Aβ neuropathology instead of promoting Aβ-unrelated mechanisms.
While our evidence to date with the Cabernet Sauvignon and the muscadine wine suggests that red wine polyphenolic compounds may benefit AD by modulating multiple Aβ-related mechanisms, there is no information on the specific bioactive polyphenolic compounds that are involved. Based on our observation that the two red wines are characterized by different polyphenol component compositions, we posit that distinct polyphenols from the two red wine are responsible for anti-Aβ aggregation and the promotion of α-secretase in the brain.
The health benefits of dietary polyphenols depend not only on the type and amount consumed, and also on their bioavailability [34,35]. Accumulating in vitro evidence suggests that certain polyphenolic compound, include a number of polyphenolic compounds that are found in red wines and grape byproducts, are capable of inhibiting the extension, and eventually destabilize the polymerization of amyloidogenic Aβ peptides [30,31]. However, with the exceptions of curcumin from turmeric spice that have been shown to attenuate AD phenotypes in an AD mouse model [36,37], it is not know whether other polyphenols could exert anti-Aβ oligomerization activity in vivo and modulate AD-type Aβ neuropathology and cognitive deterioration at the organism level. Based on our observation from the muscadine wine and the fact that curcumin is not found in red wines, we hypothesized there are additional dietary polyphenols from red wines (or other grape-derived products) that are capable of exerting anti-Aβ oligomerization activity in vivo and modulate AD phenotype. Consistent with this hypothesis, we recently found that a certain grape-seed/skin polyphenolic extract exerts anti-Aβ oligomerization activity in vivo and attenuated the development of AD-type Aβ-related neuropathology and cognitive deterioration in Tg2576 mice [38].
In summary, in conjunction with our previously reported pre-clinical study with a red Cabernet Sauvignon wine [6], results from our present pre-clinical study with a red muscadine wine suggest that distinct polyphenolic compounds from red wines may be bioavailable at the organism level and beneficially modulate AD phenotypes through multiple Aβ-related mechanisms. Thus, it might be possible to develop a combination of grape-derived bioactive polyphenolic compounds that modulate multiple Aβ-related mechanisms for AD prevention and/or therapy. Identification and mechanistic characterization of individual polyphenols capable of exerting bioactivity in vivo will promote the development of selective bioactive dietary polyphenol(s) as lead compounds for clinical testing in AD. Moreover, the information gathered will also provide the necessary basis for identifying food sources enriched in targeted bioactive polyphenols that ultimately could be incorporated as key components in the development of potential dietary guidelines for AD prevention and/or management.
Supplementary Material
ACKNOWLEDGMENTS
These studies were supported by 1P01 AT004511-01 Project 1 to LH and 1P01 AT004511-01 Project 3 to GMP, MERIT Review grant from Dept. of Veterans Affairs, and James J. Peters VA GRECC Program to GMP.
References
- 1.Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-[beta] protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 2.Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 3.Jacobsen JS, Wu CC, Redwine JM, Comery TA, Arias R, Bowlby M, Martone R, Morrison JH, Pangalos MN, Reinhart PH, Bloom FE. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings BJ, Cotman CW. Image-analysis of beta-amyloid load in Alzheimer's disease and relation to dementia severity. Lancet. 1995;346:1524–1528. doi: 10.1016/s0140-6736(95)92053-6. [DOI] [PubMed] [Google Scholar]
- 5.Luchsinger JA, Tang MX, Siddiqui M, Shea S, Mayeux R. Alcohol intake and risk of dementia. J Am Geriatr Soc. 2004;52:540–546. doi: 10.1111/j.1532-5415.2004.52159.x. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Ho L, Zhao Z, Seror I, Humala N, Dickstein DD, Thiyagarajan M, Percival SS, Talcott ST, Pasinetti GM. Moderate consumption of Cabernet Sauvignon attenuates Aβ neuropathology in a mouse model of Alzheimer's disease. FASEB J. 2006;20:2313–2320. doi: 10.1096/fj.06-6281com. [DOI] [PubMed] [Google Scholar]
- 7.Percival SS, Sims CA. Wine modifies the effects of alcohol on immune cells of mice. J Nutr. 2000;130:1091–1094. doi: 10.1093/jn/130.5.1091. [DOI] [PubMed] [Google Scholar]
- 8.Zoecklin B, Fugelsang K, Gump B, Nury F, Abraham S. Production Wine Analysis. Van Nostrand Reinhold; New York: 1990. [Google Scholar]
- 9.Talcott ST, Lee JH. Ellagic acid and flavonoid antioxidant content of muscadine wine and juice. J Agric Food Chem. 2002;50:3186–3192. doi: 10.1021/jf011500u. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Johnson JV, Talcott ST. Identification of ellagic acid congjugates and other polyphenolics in muscadine grapes by HPLC-ESI-MS. J Agric Food Chem. 2005;53:6003–6010. doi: 10.1021/jf050468r. [DOI] [PubMed] [Google Scholar]
- 11.Morris R. Developments of a water-maze procedure for studying spatial-learning in the rat. J Neurosci Meth. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 12.Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, Peng Y, Cambareri G, Rocher A, Mobbs CV, Hof PR, Pasinetti GM. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer's disease. FASEB J. 2004;18:902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Ho H, Qin W, Rocher AB, Seror I, Humala N, Maniar K, Dolios G, Wang R, Hof PR, Pasinetti GM. Caloric restriction attenuates β-amyloid neuropathology in a mouse model of Alzheimer's disease. FASEB J. 2005;19:659–661. doi: 10.1096/fj.04-3182fje. [DOI] [PubMed] [Google Scholar]
- 14.Vallet PG, Guntern R, Hof PR, Golaz J, Delacourte A, Robakis NK, Bouras C. A comparative study of histological and immunohistochemical methods for neurofibrillary tangles and senile plaques in Alzheimer's disease. Acta Neuropathol. 1992;83:170–178. doi: 10.1007/BF00308476. [DOI] [PubMed] [Google Scholar]
- 15.McLaurin J, Kierstead ME, Brown ME, Hawkes CA, Lambermon MH, Phinney AL, Darabie AA, Cousins JE, French JE, Lan MF, Chen F, Wing SS, Mount HT, Fraser PE, Westaway D, St George-Hyslop P. Cyclohexanehexol inhibitors of Aβ aggregation prevent and reverse Alzheimer phenotype in a mouse model. Nat Med. 2006;12:801–808. doi: 10.1038/nm1423. [DOI] [PubMed] [Google Scholar]
- 16.Kin HJ, Chae SC, Lee DK, Chromy B, Lee SC, Park YC, Klein WL, Krafft GA, Hong ST. Selective neuronal degeneration induced by soluble oligomeric amyloid beta protein. FASEB J. 2003;17:118–120. doi: 10.1096/fj.01-0987fje. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Ho L, Chen L, Zhao Z, Zhao W, Qian X, Humala N, Seror I, Bartholomew S, Rosendorff C. Pasinetti Valsartan lowers brain beta-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J Clin Invest. 2007;117:3393–3402. doi: 10.1172/JCI31547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bitan G, Lomakin A, Teplow DB. Amyloid beta-protein oligomerization: prenucleation interactions revealed by photo-induced cross-linking of unmodified proteins. J Biol Chem. 2001;276:35176–35184. doi: 10.1074/jbc.M102223200. [DOI] [PubMed] [Google Scholar]
- 19.US Department of Health and Human Services. US Department of Agriculture Dietary guidelines for Americans 2005. 2005 http://www.health.gov/DIETARYGUIDELINES/dga2005/document/pdf/DGA2005.pdf.
- 20.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 21.Zhang YW, Xu H. Molecular and cellular mechanisms for Alzheimer's disease: understanding APP metabolism. Curr Mol Med. 2007;7:687–696. doi: 10.2174/156652407782564462. [DOI] [PubMed] [Google Scholar]
- 22.Vassar R, Citron M. Abeta-generating enzymes: recent advances in beta- and gamma-secretase research. Neuron. 2000;27:419–422. doi: 10.1016/s0896-6273(00)00051-9. [DOI] [PubMed] [Google Scholar]
- 23.Vallers SS, Teplow DB, Bitan G. Determination of peptide oligomerization state using rapid photochemical crosslinking. Methods Mol Biol. 2005;299:11–18. doi: 10.1385/1-59259-874-9:011. [DOI] [PubMed] [Google Scholar]
- 24.Bitan G. Structural study of metastable amyloidogenic protein oligomers by photo-induced cross-linking of unmodified proteins. Methods Enzymol. 2006;413:217–236. doi: 10.1016/S0076-6879(06)13012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luchsinger JA, Mayeux R. Dietary factors and Alzheimer's disease. Lancet Neurol. 2004;3:579–587. doi: 10.1016/S1474-4422(04)00878-6. [DOI] [PubMed] [Google Scholar]
- 26.Reid MC, Boutros NN, O'Connor PG, Cadariu A, Concato J. The health-related effects of alcohol use in older persons: a systematic review. Subst Abus. 2002;23:149–164. doi: 10.1080/08897070209511485. [DOI] [PubMed] [Google Scholar]
- 27.Urquiaga I, Leighton F. Plant polyphenol antioxidants and oxidative stress. Biol Res. 2000;33:55–64. doi: 10.4067/s0716-97602000000200004. [DOI] [PubMed] [Google Scholar]
- 28.Scalbert A, Manach C, Morand C, Remesy C, Jimenez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 29.Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol. 2006;545:51–64. doi: 10.1016/j.ejphar.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 30.Porat Y, Abramowitz A, Gazit E. Inhibition of amyloid fibril formation by polyphenols: structural similarity and aromatic interactions as a common inhibition mechanism. Chem Biol Drug Des. 2006;67:27–37. doi: 10.1111/j.1747-0285.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 31.Ono K, Hasegawa K, Naiki H, Yamada M. Anti-amyloidogenic activity of tannic acid and its activity to destabilize Alzheimer's beta-amyloid fibrils in vitro. Biochim Biophys Acta. 2004;1690:193–202. doi: 10.1016/j.bbadis.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M. Potent anti-amyloidogenic and fibrildestabilizing effects of polyphenols in vitro: implications for the prevention and therapeutics of Alzheimer's disease. J Neurochem. 2003;87:172–181. doi: 10.1046/j.1471-4159.2003.01976.x. [DOI] [PubMed] [Google Scholar]
- 33.Joseph JA, Denisova NA, Arendash G, Gorden M, Diamond D, Shukitt-Hale B, Morgan D. Blueberry supplemntation enhances signaling and prevents behaviroal deficits in an Alzheimer disease model. Nutr Neurosci. 2003;6:153–162. doi: 10.1080/1028415031000111282. [DOI] [PubMed] [Google Scholar]
- 34.Cheynier V. Polyphenols in foods are more complex than often thought. Am J Clin Nutr. 2005;81(Suppl):223S–229S. doi: 10.1093/ajcn/81.1.223S. [DOI] [PubMed] [Google Scholar]
- 35.Manach C, Scalbert A, Morand C, Remesy C, Jimenez I. Polyphenols: food sources and bioavailablity. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 36.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 37.Lim GP, Chu T, Yang F, Beech W, Frautsch SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Ho L, Zhao W, Ono K, Rosensweig C, Chen L, Humala N, Teplow DB, Pasinetti GG. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer's disease. J Neurosci. 2008;28:6388–6392. doi: 10.1523/JNEUROSCI.0364-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.