Summary
Costimulatory signals from dendritic cells (DCs) are required for naive T cells to respond to antigenic stimulation. To what extent DCs reactivate memory T cells during recall responses is not known. Here, an in vivo depletion system has been used to analyze the role of DCs in reactivating CD8 memory T cells during recall responses to three different microbial infections. We show a profound decrease in the numbers of responding memory CD8 T cells in both lymphoid and nonlymphoid tissues during the recall responses to infection with vesicular stomatitis virus, Listeria monocytogenes (Lm), or influenza virus. These data show that interaction with DCs is a major mechanism driving T cell reactivation in vivo, even during a tissue-specific infection of the respiratory tract.
Introduction
CD8 T cells provide protection against a number of viral and bacterial pathogens (Ahmed and Gray, 1996; Lin et al., 2000). After an infection, naive CD8 T cells are primed and undergo a rapid expansion phase followed by a contraction period in which most effector cells are eliminated, leaving a small, long-lived memory cell pool (Ahmed and Gray, 1996). When rechallenged with an infectious pathogen, antigen-specific memory T cells, unlike naive T cells, can respond swiftly by robust proliferation and upregulation of effector function (Bachmann et al., 1999a; Veiga-Fernandes et al., 2000; Zimmermann et al., 1999). Although the events required for the initiation of a primary CD8 T cell response have been extensively studied, the cellular and molecular mechanisms that initiate and perpetuate an effective memory CD8 T cell recall response are less well defined. Analysis of the cellular requirements for generating a memory CD8 T cell recall response have focused predominantly on the role of CD4 T cell help (Bourgeois et al., 2002; Dirosa and Matzinger, 1996; Janssen et al., 2003; Shedlock and Shen, 2003; Shedlock et al., 2003; Sun and Bevan, 2003). The dependence of the antimicrobial CD8 T cell recall response on CD4 T cells has been controversial. In some cases, CD4 T cells assist the proliferative CD8 T cell recall response (Bourgeois et al., 2002; Shedlock et al., 2003), whereas in other situations, CD4 T cells appear to be dispensable for the secondary response (Shedlock and Shen, 2003; Sun and Bevan, 2003). These differences may be explained in part by the characteristics of the pathogen being studied as well as perhaps by the infectious dose and the mouse strain employed. Recent studies have also suggested that CD8 memory T cell development is dependent on the presence of CD4 T cells in the primary response, but again, this has not been a universal finding (Khanolkar et al., 2004; Marzo et al., 2004; Shedlock and Shen, 2003; Sun et al., 2004; Sun and Bevan, 2003; Shen et al., 2003).
Memory T cells are phenotypically and functionally heterogenous, and the precursor-product relationships among the different subsets is a topic of intense interest. Two generally defined populations of memory cells can be identified based primarily on tissue localization and, in part, by phenotype and function (Masopust et al., 2001; Sallusto et al., 1999; Seder and Ahmed, 2003). Thus, effector memory cells are found in nonlymphoid tissues such as the parenchyma of the lung and the lamina propria (LP) of the intestine and, for CD8 T cells, exhibit direct ex vivo lytic activity, whereas effector memory CD4 T cells rapidly produce effector cytokines (Masopust et al., 2001; Sallusto et al., 1999). In contrast, central memory T cells are present in lymphoid tissues and require reactivation to express effector function (Sallusto et al., 1999), although at least in the mouse, all CD8 memory T cells produce cytokines when stimulated (Masopust et al., 2001; Unsoeld et al., 2002). Although the expression patterns of cell surface homing molecules such as CCR7 and CD62L were originally suggested to define these subsets in human memory T cells, a faithful phenotypic identification has yet to be described, because memory cells with “effector” phenotypes can be found in lymphoid tissues. In addition, blood-borne CD8 and CD4 memory T cells raised by systemic infection rapidly equilibrate into lymphoid and nonlymphoid tissues, suggesting that a common pool of migrating memory T cells may exist (Klonowski et al., 2004). Nevertheless, a recent report suggested that CD8 memory T cell development follows a pathway from naive to effector cell to effector memory cell followed by reversion of effector memory cells to cells with a central memory phenotype (Wherry et al., 2003). In this scheme, the central memory CD8 T cell is primarily responsible for maintaining long-ter m memory and for mounting the proliferative recall response. However, effector memory CD8 T cells isolated from multiple nonlymphoid tissues are able to respond vigorously to infection after adoptive transfer (Masopust et al., 2004), so it remains unclear whether memory cell subsets develop along a continuum or represent distinct lineages.
A rapid response by tissue-based memory T cells to secondary infection makes teliological sense, because such a response would presumably most effectively remove localized infection. Indeed, in cases where memory cells may be apposed directly with parenchymal cells, such as in the intestinal or lung epithelium, it might be expected that tissue-based memory cells would respond directly to antigen presented by parenchymal cells. However, in the case of reactivation of CD4 memory T cells, expression of MHC class II by nonhematopoietic tissue would be required, and in some cases, such as in the intestinal epithelium, this requirement is met (Hershberg et al., 1997; Vidal et al., 1993). In addition, inflammation and infection can induce MHC class II expression as well as upregulate MHC class I levels (Hershberg et al., 1997). The concept of memory cell reactivation by antigen expressed by parenchymal tissue is supported by findings showing that memory T cells are more readily activated than naive T cells and that their reactivation may have altered costimulation requirements (Bachmann et al., 1999b; Bertram et al., 2004; Dawicki and Watts, 2004). Given these findings, it has been postulated that memory T cell recall responses are not DC dependent and that other nonprofessional antigen-presenting cells (APCs) may be sufficient for initiation of a secondary response (Bachmann et al., 1999b; Bertram et al., 2004; Crowe et al., 2003). Furthermore, during influenza infection of the lungs, it has been suggested that the ability of cell types other than DCs to present antigen skews the epitope dominance of the response (Crowe et al., 2003). Nevertheless, the role of DCs in mounting a secondary response to infection has not been directly examined. Our results now show that activation of memory T cells in response to systemic or localized infection is predominantly, though not exclusively, dependent on DCs.
Results
Systemic Depletion of DCs in DTR Transgenic Mice
In order to selectively ablate DCs, we employed a previously described system in which the diptheria toxin receptor (DTR) and green fluorescent protein (GFP) are expressed under control of the CD11c promoter (Jung et al., 2002). Treatment of these mice with DT results in transient depletion of DCs. Unexpectedly, we found that a single treatment of CD11c-DTR mice with DT (4 ng/g body weight i.p.) resulted in the death of the transgenic mice in 6–7 days. However, when normal C57BL/6 mice were reconstituted with bone marrow from CD11c-DTR mice and were treated with the same dose of DT, no deleterious effects were observed even with prolonged DT treatment (data not shown). This result suggested that essential nonhematopoietic cells expressed the transgene and were effected by DT treatment. Thus, chimeric mice generated with CD11c-DTR bone marrow were used for all of our studies. Because our goal was to examine recall responses in lymphoid and nonlymphoid tissues, we examined the efficacy of DC depletion in these sites. CD11c-DTR bone marrow chimeric mice or normal bone marrow chimeras were injected twice with DT (4 ng/g body weight i.p.) 3 days apart, and 24 hr later, tissues were analyzed for the presence of DCs (Figure 1). As determined by flow cytometric analysis of the expression of CD11c and GFP by gated MHC class II+ cells, DCs in the spleen were reduced by ~90% as previously reported (Jung et al., 2002; Crowe et al., 2003). DCs were also effectively depleted in lymph node (LN) (pooled axillary, brachial, inguinal, and cervical nodes), lung, liver, and LP, although small populations of residual DCs were detected in some cases. In all organs except the LP, a population of MHC class II+ GFP+ CD11c intermediate/low cells were detected that were ablated by DT treatment. Further analysis found this population to contain some B cells and CD11c-low DCs, but not plasmacytoid DCs or macrophages (data not shown). Whether expression of GFP by these cells is a result of bona-fide CD11c expression or due to aberrant expression in the transgenic mice remains to be determined.
Figure 1. DCs Are Ablated in Lymphoid and Nonlymphoid Organs after DT Treatment.
DTR BM chimeras were injected with two doses of either 4 ng/g body weight DT or PBS 3 days apart and 24 hr later analyzed to determine the efficacy of DC ablation. Pooled samples of liver, peripheral LN, and LP from the two groups of mice were used to gain enough numbers of DCs for analysis. DCs were identified as MHC II+ CD11c+ GFP+ by flow cytometry, and the plots shown are gated on MHC class II+ cells. DC numbers in all organs tested were generally depleted by ~90% after treatment. The total number of DCs is shown in each plot. Four mice were used per group and the experiment repeated twice. Data shown is representative of one experiment.
DCs Drive the Secondary Lymphoid and Nonlymphoid Response to Lm Infection
Because CD11c may be expressed by activated CD8 T cells (Huleatt and Lefrançois, 1995; Jung et al., 2002; Lin et al., 2003) and memory T cells (Kim et al., 1999), it is not possible to study endogenous memory cells generated in the CD11c-DTR bone marrow chimeras. To circumvent this problem, we transferred normal C57BL/6-Ly5.2 DC-depleted splenocytes containing memory T cells to the C57BL/6-Ly5.1 DTR chimeras. Our previous results show that transferred splenic memory T cells migrate effectively to the spleen, lung, and liver but poorly to the intestinal LP (Masopust et al., 2004). To address the role of DCs in the recall response to systemic bacterial infection, we employed a recombinant L. monocytogenes-expressing ovalbumin (Lm-ova) (Pope et al., 2001) to allow us to monitor ova-specific CD8 T cells. DC-depleted splenocytes containing Lm-ova-specific CD8+ splenic memory T cells were transferred into DTR or wild-type (wt) chimeras, and the mice were infected i.v. with Lm-ova the next day. DT was administered every 3 days starting 4 days before infection, and the recall response was analyzed 6 days postinfection. It should be noted that DC depletion did not alter memory cell migration to lymphoid and nonlymphoid tissues (data not shown). In infected control chimeras, a robust response by the transferred cells was detected in all lymphoid and nonlymphoid tissues tested (Figure 2A). Surprisingly, the recall response was dramatically reduced in both lymphoid and nonlymphoid organs in DT-treated CD11c-DTR chimeric mice (Figure 2A). The total number of antigen-specific cells in the spleen and LN was decreased by 98% and 95%, respectively (Figure 2B). This decrease was a reflection of both a decreased frequency of antigen-specific cells (Figure 2A) and in total antigen-specific cell number per organ (Figure 2B). Similarly, the total numbers of anti gen-specific cells in the lung, liver, and LP were also greatly reduced (93%, 97%, and 91%, respectively) (Figure 2B). The massive reduction in the recall response to Lm-ova in the absence of CD11c+ cells strongly indicated that DCs are the primary APC required for a maximal recall response. We also examined bacterial burdens in these mice, and although bacterial titers were increased in the livers of DC-depleted mice 3 days after infection, by 6 days postinfection, Lm was cleared from both control and CD11c-DTR chimeras (Figure S1 available with this article online).
Figure 2. DCs Are Required for the Initiation of an Anti-Lm-Ova Recall Response.
DTR or wild-type (wt) bone marrow-reconstituted mice were treated 4 and 1 days before infection with DT to ablate DCs in the DTR mice. Ly5.2+ ova-specific CD8+ memory cells were transferred into both groups 1 day before the mice were infected i.v. with 103 cfu Lm-ova. DT was administered every 3 days, and the mice were analyzed 6 days after infection for Ly5.2+ ova-specific CD8+ T cells. (A) The proportion of Ly5.2+ ova+ cells was decreased in all organs analyzed. The numbers in the upper left corner of each panel represent percent of ova tet+ LFA+ cells among the donor CD8+ T cells. (B) The total number of Ly5.2+ ova-specific cells is dramatically less in the absence of DCs in all organs and was calculated from the proportion of tet+ cells and total cellularity of each organ. The data represent a total of six mice per group from two individual experiments. All values are means ± SEM.
DCs Are Required for Optimal Secondary Lymphoid and Nonlymphoid Recall Response to Vesicular Stomatitis Virus Infection
Donor mice were infected with vesicular stomatitis virus (VSV) in order to generate nucleoprotein (N)-specific memory T cells. DC-depleted splenocytes containing VSV-specific CD8 memory T cells were then transferred into DTR and wt chimeras, the mice were challenged with VSV, and the recall response was analyzed 6 days later. The mice were treated with DT every 3 days starting 4 days before infection. Similar to what we observed in the recall response to Lm infection, the secondary response to VSV infection was dramatically reduced in the absence of DCs (Figure 3). The number of antigen-specific T cells in the spleen and LN were reduced by 87% and 88%, respectively (Figure 3B). However, unlike in the secondary response to Lm-ova infection, this decrease was predominantly due to a decrease in total cellularity, as the percentage of antigen-specific cells was only decreased by 55% (spleen) and 30% (LN) (Figure 3A). This trend was reflected in the lung, liver, and LP where the total number of antigen-specific cells was decreased by 81%, 86%, and 90%, respectively, yet the percentage of antigen-specific cells was reduced by 21%, 41%, and 47%, respectively (Figures 3B and 3A, respectively). Although the reduction in the percentage of antigen-specific cells was not as dramatic as that seen after Lm-ova challenge, these data strongly supported an important requirement for DCs in generating an optimal recall response to VSV infection. Nevertheless, the response observed in DC-depleted mice represented a significant expansion over unimmunized mice, suggesting that either residual DCs were present or that other cells types could drive some level of CD8 memory T cell reactivation.
Figure 3. DCs Are Required for the Initiation of an Anti-VSV Recall Response.
DTR or wt bone marrow-reconstituted mice were treated after the protocol in Figure 2 except the mice were infected i.v. with 105 pfu VSV. DT was administered every 3 days, and the mice were analyzed 6 days after infection for 5.2+ N tet+ CD8+ T cells.
(A) The proportion of Ly5.2+ N tet+ cells is decreased in all organs analyzed to varying degrees. The numbers in the upper left corner of each panel represent percent of N tet+ LFA+ cells among the donor CD8+ T cells.
(B) The total number of Ly5.2+ N tet+ cells was calculated from the proportion of tet+ cells and total cellularity of each organ. The data is from one experiment of three mice per group, and the values are means ± SEM. The experiment was repeated twice with similar results.
Peptide-Loaded DCs Restore the VSV Recall Response in DC-Depleted CD11c-DTR Chimeras
To determine if the decreased proliferative recall response after DT treatment was due to the loss of DCs in this system, VSV N peptide-loaded DCs were adoptively transferred into DT-treated wt or DTR chimeras containing VSV N peptide-specific CD8 memory T cells. The introduction of peptide-loaded DCs led to a robust recall response in the DTR chimeras of an equivalent magnitude to the wt control mice in both the lung (Figure 4) and the spleen (data not shown). These data demonstrated that the decreased CD8 memory T cell recall response observed in DC-ablated mice could be restored by introducing peptide-loaded DCs and further indicated that the effects observed in CD11c-DTR chimeras were not due to any untoward direct or indirect adverse effects of DT treatment.
Figure 4. CD8 Memory T Cells Respond to Peptide-Loaded DCs in DC-Depleted Mice.
VSV-specific CD8 memory T cells (50,000) were transferred to the indicated chimeras, and all were treated with DT on the day of transfer. One day later, 5 × 105 purified splenic DCs (either coated with the VSV N peptide or not) were transferred i.v. 6 days later, the response in the lung (shown here) and the spleen (data not shown) was measured by flow cytometry using an N peptide H-2Kb tetramer. Data shown is gated on CD8+ T cells. Responses in the spleen, though less robust, were similar between groups.
The Recall Response to Tissue-Specific Infection Exhibits Partial DC Dependence
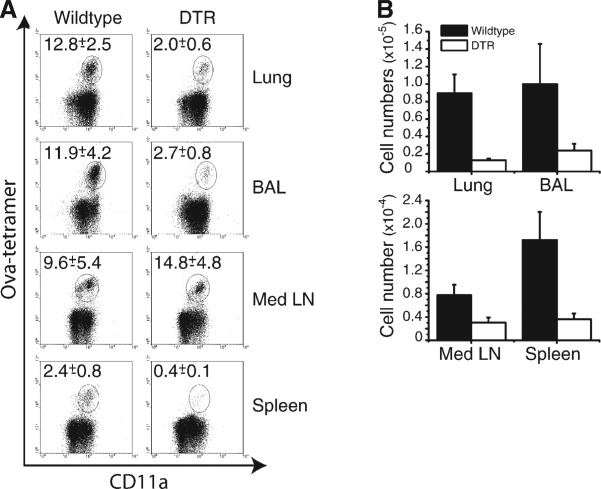
Influenza virus infection is known to be primarily restricted to the epithelium of the lungs, and a previous report shows that in LTα−/− mice, which lack encapsulated LN, an influenza virus-specific CD8 T cell response could be generated albeit with delayed kinetics (Crowe et al., 2003; Lund et al., 2002). To address whether lung infection could initiate a recall response in the absence of DCs, Lm-ova-specific CD8 memory cells were adoptively transferred into DT-treated DTR and wt mice, which were then infected intranasally with the influenza strain WSN-ova (Topham et al., 2001). Initial experiments indicated that the recall response in this system occurred slightly later than in either the Lm-ova or VSV models (data not shown), hence, the mice were sacrificed at day 8 and the magnitude of the response analyzed. In DT-treated mice, the total number of influenza virus-specific CD8 T cells was reduced by 86% in the lung parenchyma, 76% in the lung airways (BAL), 61% in the mediastinal LN (MedLN), and 79% in the spleen (Figure 5B). Interestingly, whereas in the lung parenchyma, BAL and spleen proportionate decreases (~80%) in the percentage of antigen-specific cells were noted, in the MedLN, a ~50% increase in the frequency of antigen-specific cells was noted as compared to controls (Figure 5A). However, as the total cellularity of the MedLN was significantly smaller in the DTR chimeras, the actual number of antigen-specific CD8 T cells was comparatively smaller than in wt animals (Figure 5B).
Figure 5. Partial DC Requirement for the Initiation of an Anti-Influenza Recall Response.
DTR or wt bone marrow-reconstituted mice were treated following the protocol in Figure 2 except the mice were infected i.n. with 103 pfu WSN-ova. DT was administered every 3 days, and the mice were analyzed 8 days after infection for Ly5.2+ ova-specific CD8+ T cells.
(A) The proportion of Ly5.2+ ova+ cells is decreased in all organs analyzed with the exception of the MedLN. The numbers in the upper left corner of each panel represent percent of ova tet+ LFA+ cells among the donor CD8+ T cells.
(B) The total number of Ly5.2+ ova+ cells was calculated from the proportion of tet+ cells and total cellularity of each organ,. The data represents five mice (wt) or six mice (DTR) from two individual experiments. All values are means ± SEM.
It is known that during the primary T cell response to influenza virus infection, the increase in antigen-specific T cells is first observed in the MedLN before a noticeable increase is detected in the lung parenchyma or the BAL (Lawrence and Braciale, 2004). Hence, as the MedLN showed the smallest reduction in the proliferative recall response, the possibility existed that the response in the DTR chimeras was delayed compared to control mice, comparable to what has been observed in the primary anti-influenza virus CD8 T cell response in LTα−/− mice (Lund et al., 2002). To test this, cohorts of influenza virus-infected DTR and wt chimeras were sacrificed 11 days after infection. In all cases, the infected DTR chimeras exhibited signs of respiratory distress by day 11, whereas wt animals appeared normal, suggesting that DCs are required for protection against infection in this setting. Indeed, on days 5 and 8 after infection, the lungs of DC-depleted mice contained ~100- to 1000-fold greater viral loads than did control mice (Figure S1). The number of antigen-specific T cells in the spleen and lung airways remained substantially decreased compared to wt controls (84% and 80%, respectively) despite an equivalent total cellularity in these organs (Figures 6B and 6A, respectively). Furthermore, the total number of antigen-specific cells in the spleen and airways of both wt and DTR mice had increased from day 8 to day 11, whereas in the lung parenchyma, the number had decreased during this time. Interestingly, unlike at day 8, the response in the lung parenchyma was not reduced by the ablation of DCs (Figure 6B). This effect was due in part to an increase in the overall cell numbers in DT-treated mice, because the proportion of antigen-specific cells in these mice was half that present in wt chimeras (Figure 6A and data not shown). In DT-treated mice at day 11 postinfection, the total cellularity of the MedLN was dramatically lower than that observed on day 8 (Figure 5A). Although the total number of antigen-specific cells could not be accurately determined, the approximate values obtained were also significantly lower than those observed on day 8 (data not shown). Combined, these data demonstrated that without DCs to initiate a recall response to influenza virus infection, neither other APCs nor infected lung epithelium could generate a recall response of similar magnitude to that seen in DC-sufficient wt mice.
Figure 6. The Reduced Recall Response to Influenza Is Delayed in the Absence of DCs.
(A) The total cellularity of the lungs, BAL fluid, and spleen is equal in the DTR and wt mice, 11 days after infection. The cellularity of the MedLN is still dramatically reduced at this time point.
(B) Despite the equal total cellularity in the BAL fluid and spleen, there is still a reduced number of ova+ CD8+ cells in these organs. By day 11, the number of ova+ CD8+ cells in the lungs of wt and DTR mice are comparable. The data represent six mice (wt) or four mice (DTR) from two individual experiments. All values are means ± SEM.
Kinetics of DC Requirements during the Recall Response
The first 24 hr after infection has been identified as a critical period for the initiation of a naive CD8+ T cell response to infectious agents (Van Stipdonk et al., 2003; Mercado et al., 2000; Kaech and Ahmed, 2001). In order to determine when DCs are required during initiation of a secondary response, ova-specific memory CD8+ T cells were transferred into either wt or DTR bone marrow chimeras, and the DCs were ablated at 0, 1, 2, 3, or 5 days after infection with VSV-ova. Depletion of DCs at the time of infection resulted in a greatly reduced (88%) recall response in the spleen (Figure 7A). A similar decrease in the response was observed when the DCs were ablated 24 hr after infection. Surprisingly, even when DCs were removed 48 hr after the secondary challenge, the response was reduced to 47% of the equivalent wt response (Figure 7A). From this initial data, the time before DT treatment was extended. As before, depletion of DCs at the time of infection resulted in a greatly reduced (93%) recall response in the spleen (Figure 7B). When DCs were ablated 24 hr after infection, the response was inhibited by 73% in the experiment shown. When DCs were depleted on day 3 or 5, the inhibition observed was not significant. These data indicated a continuing requirement for DCs over at least the first 2 days after infection in order for the generation of a maximum recall response.
Figure 7. DCs Are Required for More than 48 hr to Generate a Full Recall Response.
(A) Ly5.2+ ova -specific CD8+ memory cells were transferred into both groups of mice 1 day before the mice were infected i.v. with 105 pfu VSV-ova. A single dose of DT was administered at either day 0, 1, or 2 of infection and CD8+ Ly5.2+ ova-tet+ cells in the spleens were quantitated six days after infection. The percent decrease was calculated based on total tet+ cell numbers in each group. The data represent four mice in each group from two individual experiments.
(B) CFSE-labeled Ly5.2+ VSV N protein-specific CD8+ memory cells were transferred into the indicated chimeras 1 day before the mice were infected i.v. with 105 pfu VSV. A single dose of DT was administered at either day 0, 1, 3, or 5, and CD8+ Ly5.2+ N tet+ cells in the spleens were quantitated six days after infection. Values represent total tet+ cells from three mice per group.
(C) CFSE analysis of the mice in (B) treated at day 0 with DT. Analysis is of gated Ly5.2+ CD8+ tet+ cells. The total number of CFSE-high cells is indicated.
The inhibition of the memory response could be the result of decreased recruitment of memory cells into the response and/or due to reduced expansion. To test these possibilities, memory cells were carboxyfluorescein diacetate succinimidyl ester (CFSE) labeled prior to transfer, and DT was administered at the time of infection (the treatment resulting in the greatest inhibition). Interestingly, 6 days after infection, nearly all memory cells in control and CD11c-DTR chimeras had lost CFSE (Figure 7C). In addition, in terms of total cell numbers, ~2-fold more CFSE bright-tetramer+ cells remained in the spleens of CD11c-DTR chimeras as com pared to control mice (Figure 7C and data not shown). Because this difference is unlikely to account for the level of inhibition observed, decreased overall proliferation and/or decreased survival of responding memory cells may have also contributed to the reduced response.
Reactivation of Central versus Effector Memory Cells
Although we observed a DC requirement in the CD8 recall response in all tissues, it remained possible that memory cell subsets were differentially DC dependent. In the studies above, transferred memory cells were comprised of ~85% CD62Llow and 15% CD62Lhigh memory cells, so the responses we measured were perhaps predominantly mediated by CD62Llow cells. However, it has been reported that CD62Llow CD8 memory cells do not respond as robustly as CD62Lhigh memory cells. To test this in our system, we transferred similar numbers of splenic memory cells that had been sorted based on CD62L expression into B6 control or CD11c-DTR bone marrow chimeras. After DT treatment and i.v. VSV infection, the expansion of the transferred memory cells was measured (Figure S2). The expansion of CD62Llow and CD62Lhigh memory cells was similar in the spleen, lung, and liver. This result is in contrast to a previous report using vaccinia virus challenge of transferred LCMV-specific memory cells (Wherry et al., 2003) in which expansion of CD62Lhigh cells substantially outpaced that of CD62Llow memory cells. When DCs were depleted, the response of CD62Lhigh memory cells was inhibited ~90% in all tissues. Interestingly, the response by CD62Llow memory cells was less DC dependent, with inhibition of ~60%–80%. Although further studies will be needed to learn the basis of this difference, the results support the intriguing possibility that nonlymphoid memory cells are more readily able to interact with nonprofessional APCs.
Discussion
The data presented in this study identified an important role for DCs in generating efficient memory recall responses to multiple pathogens. Thus far, a potential role for DCs in mounting a secondary CD8 T cell response to infection has not been described. Indeed, only limited data are available with regard to the role of endogenous DCs in initiating immune responses in vivo. Although the transfer of antigen-loaded, in vitro-generated DCs has been used to drive responses in vivo, only recently has a system become available to study DC involvement in immune response initiation in situ (Jung et al., 2002). This system was employed to demonstrate a DC requirement for driving a primary CD8 T cell response in the spleen to Lm infection. We have now utilized this model to analyze the secondary CD8 T cell response to various infectious agents. Considering that many memory cells are located in nonlymphoid tissues and are closely apposed to potentially infected MHC class I-bearing parenchymal cells (Masopust et al., 2001), we reasoned that DCs might be dispensable for secondary immune response initiation. Moreover, the lower activation threshold of memory CD8 T cells suggests that costimulation may not be required, and thus, nonprofessional APCs could mediate reactivation as has been previously suggested (Bachmann et al., 1999b). However, our results indicated an important role for DCs in activation of CD8 memory T cells after secondary infection.
Our findings demonstrated that the recall response to Lm infection was decreased >90% in all organs analyzed when DCs were depleted at the time of infection. Other effects of the absence of DCs in our system have potential to influence the CD8 T cell recall response. For example, DC removal could affect lymphoid architecture and thus the location of memory T cells. Distinguishing such effects from the role of DCs themselves in memory cell reactivation, however, will be difficult if both occur simultaneously. In addition, DC ablation may also prevent the DCs from acting as a conduit between the helper CD4 T cell and the CD8 memory cell. Nevertheless, the inhibition of the Lm-specific CD8 T cell response could not be solely attributed to a loss of CD4 T cell help, because depletion of CD4 T cells generally reduces the CD8 T cell recall response by ~50% (Marzo et al., 2004). Thus, our data indicated that other cell types such as macrophages, which can be directly infected by Lm (Unanue, 1997), were unable to replace the role provided by DCs after Lm rechallenge. Unlike the recall response to Lm, the secondary CD8 T cell response to VSV is not CD4 T cell dependent (Marzo et al., 2004; Sun et al., 2004), and VSV is thought to infect a wide range of cell types. Hence, many cell types are potentially capable of presenting viral antigens via MHC class I. Nevertheless, in the absence of DCs, the recall response to VSV infection was reduced by ~81%–90% in all organs analyzed. Although this is a dramatic reduction compared to control mice, the response obtained is nevertheless the result of a significant increase in the numbers of antigen-specific cells that were originally transferred, and this was the case for Lm and influenza virus recall responses as well. This finding suggested that either not all DCs were depleted by DT treatment or that other APCs or parenchymal cells were involved in antigen presentation and activation of memory cells. Importantly, the reduced recall responses were not due to any unintended effects of DT treatment, as the VSV N peptide recall response could be restored in our model by the transfer of N peptide-loaded DCs. Likewise, it seems unlikely that the small population of GFP+ CD11clow B cells deleted by DT treatment contributed substantially to the decreased memory recall responses observed, although this has not been formally excluded.
In the case of influenza virus, infection is known to occur primarily in the epithelial lining of the lungs (Collins et al., 2004). Hence, the initiation of a primary immune response is thought to occur when antigen-loaded DCs migrate to the draining mediastinal LN (Banchereau and Steinman, 1998). However, a recent study demonstrates that in mice lacking the spleen and LN, the primary CD8 T cell response to influenza virus infection can be initiated, with delayed kinetics, in bronchus associated lymphoid tissue (BALT), whose formation is induced by the infection (Lund et al., 2002; Moyron-Quiroz et al., 2004). In our experiments, although many influenza virus-specific memory cells are located in the lung parenchyma, the recall response to infection was reduced by ~60%–90% in the various organs analyzed. Like in the studies by Lund et al. (2002), a delayed increase in total antigen-specific cells in the lung parenchyma, BAL, and spleen was observed. Thus, the number of antigen-specific cells in the parenchyma doubled in the DT-treated mice between days 8 and 11 after infection, whereas increases of 54% and 85% were observed in the BAL and spleen, respectively. In contrast, the response in control mice decreased during this time in the lung parenchyma, presumably due to clearance of the infection. Both the BAL and spleens of the control mice exhibited slight increases in antigen-specific cells though not of the same magnitudes as that seen in the DTR mice. Despite an increase in antigen-specific cells in the BAL, the response was still considerably lower when DCs were removed, although there was an equivalent total cellularity compared to control BAL fluid. The large cell number in the DT-treated animals was a reflection of the nonspecific recruitment of cells into the lung airways due to inflammation (Ely et al., 2003). In any case, the results suggested that as compared to secondary responses to VSV and Lm infection, the recall response to influenza virus infection was less dependent on DCs. In accordance with this finding, a previous report examining epitope dominance in the CD8 T cell response to influenza virus infection suggested that non-DCs are involved in initiation of at least a portion of the secondary response (Crowe et al., 2003). Despite this result, protection against infection was clearly DC dependent (Figure S1).
Whether DC-dependent recall responses are being initiated in the BALT, LN, or nonlymphoid tissue is unknown, but our results suggested that at least some measure of memory cell activation may occur in the parenchymal tissues, and further, that effector memory cells in these tissues were responding to secondary challenge. A recent study suggests that although effector and central memory T cells both exhibit rapid effector function, central memory T cells are more proficient at mounting a proliferative recall response (Wherry et al., 2003). In contrast, our results showed that splenic CD62Lhigh and CD62Llow memory cells both mounted robust recall responses in response to VSV infection (Figure S2). Moreover, effector memory cells in the lung can mount a vigorous proliferative recall response to respiratory virus infection, perhaps more effectively than central memory cells (Roberts and Woodland, 2004). Interestingly, CD62Llow memory cells were less dependent on DCs for reactivation than were CD62Lhigh cells. This may be due to a greater DC requirement for activation of lymphoid versus nonlymphoid memory cells, though additional analysis is required to address this possibility. Whether DCs, non-DC APCs, or parenchymal tissues are capable of inducing effector functions in either memory T cell population remains to be seen and is a subject of further investigation.
Our findings also shed light on the duration of memory cell-DC interaction required for optimal reactivation after VSV infection. Not surprisingly, DCs were essential in the first 24 hr of the recall response. However, substantial DC dependence was noted when DCs were removed 24 and 48 hr after infection. Whether this effect was the result of disruption of preexisting T cell-DC conjugates or due to inhibition of formation of new interactions will require analysis with in situ visualization techniques. These results were perhaps unexpected given the current data for naive CD8 T cell activation, which suggests that a short (<24 hr) encounter with antigen is sufficient to drive an optimal response (Badovinac et al., 2002; Kaech and Ahmed, 2001; Mercado et al., 2000; Van Stipdonk et al., 2001). Because memory cells respond more rapidly than do naive T cells, one would not expect memory cells to require a longer period of antigen stimulation to reach a full response. The differences observed may be due to the use of in vitro systems in some cases and the incomplete removal of antigen/APCs in the in vivo studies. For example, in a study of naive CD8 T cell activation, removal of infectious Lm by antibiotic treatment 24 hr after infection did not affect the overall CD8 T cell response (Mercado et al., 2000). In our model, the infectious agent was not removed, but the means to present antigen via DCs was eliminated at various time points after infection. Thus, as previously noted (Mercado et al., 2000), removal of the pathogen in vivo does not remove DCs that are primed and loaded with antigen, and our data indicated that, at least for memory T cells, antigen presentation by DCs is required for more than 24 hr in order to drive the maximal proliferative response of memory CD8 T cells.
By relying on DCs to initiate a recall response, the immune system adds a layer of protection against auto-reactivity in the same manner as it does for naive T cells. The rapidity and strength of the recall response, which are the advantages gained in having memory T cells, occur primarily by increased sensitivity at the level of the T cell without sacrificing the need for antigen presentation by DCs. The relative contribution of other potential APCs, professional or otherwise, in mounting and sustaining the recall response remains the focus of further investigation.
Experimental Procedures
Mice
C57Bl/6 (CD45.2) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and C57Bl/6 (CD45.1) mice were purchased from Charles River through the NCI program (Bethesda, MD). DTR transgenic mice were a gift from Drs. Steffen Jung and D. Littman (Skirball Institute, New York, NY). The mice were backcrossed ten times to C57Bl/6 at the UCONN Health Center facilities. DTR Tg+ mice were screened by PCR from tail DNA as previously described (Jung et al., 2002). Mice were maintained in specific pathogen-free conditions and handled under protocols approved by the UCHC Animal Care Committee according to federal guidelines.
Bone Marrow Chimeras
Femurs and tibias were taken from DTR Tg+ mice or non-Tg+ litter-mates. The bone marrow was flushed out with a syringe and passed through a 70 μm nylon mesh to generate a single-cell suspension. Red blood cells (RBCs) were lysed, and the cells resuspended in HBSS supplemented with HEPES, L-glutamine, penicillin, streptomycin, and gentamycin sulfate (HBSS-HGPG). To remove mature T cells from the bone marrow, cells were incubated with anti-Thy1 ascites fluid (T24), washed once in HBSS-HGPG, then incubated with Low-Tox-M rabbit complement (Cedarlane Laboratories, Ontario, Canada) for 45 min at 37°C. Cells were washed twice and resuspended between 10 × 106 cells/ml and 25 × 106 cells/ml. CD45.1 recipient B6 mice were irradiated (1000 rad) before 2 × 106 to 5 × 106 bone marrow cells were transferred i.v. The mice were allowed to rest for 8 weeks before use.
DT Treatment of Mice
DT (Sigma, St Louis, MO) was administered to mice at 4 ng/g body-weight, in saline, i.p. To determine the efficacy of DC ablation, two doses of DT were administered 3 days apart to DTR bone marrow chimeras, and 24 hr later, various organs were analyzed by flow cytometry for the presence of CD11c+, MHC class II+ GFP+ cells. DT treatment of DTR or wt BM chimeras for memory cell recall experiments consisted of two doses of DT prior to pathogen rechallenge and was administered every 3 days for the duration of the experiments.
Generation of Memory Cells and Adoptive Cell Transfer
B6 mice were infected with either Lm-ova (103 cfu i.v.) or VSV (Indiana strain, 105 pfu i.v.). The mice were rested for 35–60 days to allow for the formation of memory cells. Spleens were then harvested and crushed between glass slides, the RBC lysed, and the remaining lymphocytes washed in HBSS-HGPG. Cells were then incubated with an anti-MHC class II mAb (M5), incubated on ice for 20 min, and washed before being mixed with beads coupled to anti-rat IgG and anti-mouse IgG (Dynal, Oslo, Norway). Unwanted MHC class II+ cells and B cells were then removed by magnetic separation. The cells were then analyzed for the number of CD8+ memory cells, CD4 cells, B cells, NK cells, and DCs by flow cytometry. Cells for adoptive transfer had typically less than 0.2% DCs after purification. For analysis of division history, cells were resuspended in HBSS/HEPES, L-Glutamine, Pen/Strep, and gentamycin sulfate (HGPG) at a concentration of 10 × 106 cells/ml and warmed to 37°C. 5- (and 6-) CFSE (Molecular Probes, Eugene, OR) was added to the cell suspension at a final concentration of 5 μM and incubated for 10 min at 37°C (Lyons and Parish, 1994). Cells were washed with HBSS/10% FCS then resupended in PBS prior to i.v. injection.
Requirements for DCs in Memory Recall Responses and Protection
DTR or wt BM chimeras received DT at day −4 and day −1 before rechallenge with a pathogen. At day −1, DC-depleted splenocytes containing ~20,000–40,000 memory CD8+ T cells were transferred i.v., and one day later, mice were infected with either Lm-ova (103 cfu i.v.), VSV (Indiana strain, 105 pfu i.v.); or the WSN strain of influenza virus (103 pfu i.n.) containing the SIINFEKL epitope (Topham et al., 2001). Lymphocytes were harvested from various organs at the indicated days and analyzed by flow cytometry. Influenza virus titers were measured from homogenized lung tissue as previously described (Tobita et al., 1975). Colony-forming units (cfus) of Lm in infected tissues were quantitated as previously described (Marzo et al., 2002).
Kinetics of DC Requirements in Generating a Memory Recall Response
DC-depleted splenocytes containing ova- or VSV-specific memory CD8+ T cells were transferred into DTR and wt BM chimeras one day before infection with either VSV-ova or wt VSV (105 pfu i.v.). The mice then received a single dose of DT at either day 0, 1, 2, 3, or 5 after infection. At day 6, the lymphocytes were harvested from the spleen, and the recall response was analyzed by flow cytometry.
Cell Surface Staining and Flow Cytometry Analysis
Lymphocytes were prepared from various organs as previously described (Klonowski et al., 2004). They were then resuspended in PBS with 1% BSA and 0.01% NaN3 at 1 × 106 to 5 × 106 cells/tube and incubated at 4°C in 50 μl of the appropriately diluted cocktail of monoclonal antibodies. MHC class I tetramer staining was carried out for 60 min at room temperature. The following conjugated mAbs were used: anti-CD11c-biotin, anti-MHC class II-PE, anti-CD45.1-PE, anti-CD8-PERCP, anti-CD11a-FITC, anti-CD4-APC, anti-B220-PE, anti-DX5-PE, anti-CD11c-PE, and anti-MHC class II-FITC (Pharmingen, San Diego, CA). Streptavidin-PE-CY7 (Caltag Laboratories, Burlingame, CA) was used to detect biotinylated antibodies. Antigen-specific CD8 T cells were detected with ova-tetramer APCs and N-tetramer APCs. The relative fluorescence intensities of the cells were measured by using a FACSCalibur (Becton Dickinson, San Jose, CA).
Restoration of the Recall Response by Peptide-Loaded DC Adoptive Transfer
Spleens from B6 mice were chopped into small pieces and digested in 5 ml HBSS/HGPG containing 5% FCS, collagenase D (1 mg/ml), and DNase I (0.02 mg/ml) (Roche, Indianapolis, IN) for 30 min at 37°C before being pushed through a cell strainer. DCs were isolated by using CD11c MACS beads and an LS25 column (Miltenyi Biotech, Auburn, CA) per the manufacturer protocol. The purified DCs were coated with 2 μg/ml VSV N peptide for 45 min at 37°C and washed twice before transfer. DC-depleted splenocytes containing ~50,000 VSV N peptide-specific memory CD8+ T cells were transferred into DT-treated CD11c-DTR and wt BM chimeras, and the next day, 5 × 105 purified splenic DCs, either coated with VSV N peptide or not, were adoptively transferred. Lymphocytes were harvested from the spleen and lungs and analyzed by flow cytometry 6 days later.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants DK45260, AI41576, and AI56172 to L.L. and American Lung Association grant RG1119 to L.S.C. We thank L. Puddington and her lab for assistance with purifying DCs.
Footnotes
Supplemental Data
Supplemental data include two figures and are available with this article online at http://www.immunity.com/cgi/content/full/22/5/561/DC1/.
References
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Barner M, Viola A, Kopf M. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. Eur. J. Immunol. 1999a;29:291–299. doi: 10.1002/(SICI)1521-4141(199901)29:01<291::AID-IMMU291>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Gallimore A, Linkert S, Cerundolo V, Lanzavecchia A, Kopf M, Viola A. Developmental regulation of Lck targeting to the CD8 coreceptor controls signaling in naive and memory T cells. J. Exp. Med. 1999b;189:1521–1530. doi: 10.1084/jem.189.10.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat. Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Bertram EM, Dawicki W, Sedgmen B, Bramson JL, Lynch DH, Watts TH. A switch in costimulation from CD28 to 4-1BB during primary versus secondary CD8 T cell response to influenza in vivo. J. Immunol. 2004;172:981–988. doi: 10.4049/jimmunol.172.2.981. [DOI] [PubMed] [Google Scholar]
- Bourgeois C, Veiga-Fernandes H, Joret AM, Rocha B, Tanchot C. CD8 lethargy in the absence of CD4 help. Eur. J. Immunol. 2002;32:2199–2207. doi: 10.1002/1521-4141(200208)32:8<2199::AID-IMMU2199>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Collins P, Chanock R, McIntosh K. Parainfluenza viruses. In: Fields B, editor. Fields Virology. Lippincott-Raven; Philadelphia: 2004. pp. 1205–1241. [Google Scholar]
- Crowe SR, Turner SJ, Miller SC, Roberts AD, Rappolo RA, Doherty PC, Ely KH, Woodland DL. Differential antigen presentation regulates the changing patterns of CD8+ T cell immunodominance in primary and secondary influenza virus infections. J. Exp. Med. 2003;198:399–410. doi: 10.1084/jem.20022151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawicki W, Watts TH. Expression and function of 4-1BB during CD4 versus CD8 T cell responses in vivo. Eur. J. Immunol. 2004;34:743–751. doi: 10.1002/eji.200324278. [DOI] [PubMed] [Google Scholar]
- Dirosa F, Matzinger P. Long-lasting CD8 T cell memory in the absence of CD4 T cells or B cells. J. Exp. Med. 1996;183:2153–2163. doi: 10.1084/jem.183.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely KH, Cauley LS, Roberts AD, Brennan JW, Cookenham T, Woodland DL. Nonspecific recruitment of memory CD8+ T cells to the lung airways during respiratory virus infections. J. Immunol. 2003;170:1423–1429. doi: 10.4049/jimmunol.170.3.1423. [DOI] [PubMed] [Google Scholar]
- Hershberg RM, Framson PE, Cho DH, Lee LY, Kovats S, Beitz J, Blum JS, Nepom GT. Intestinal epithelial cells use two distinct pathways for HLA class II antigen processing. J. Clin. Invest. 1997;100:204–215. doi: 10.1172/JCI119514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huleatt JW, Lefrançois L. Antigen-driven induction of CD11c on intestinal intraepithelial lymphocytes and CD8+ T cells in vivo. J. Immunol. 1995;154:5684–5693. [PubMed] [Google Scholar]
- Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- Jung S, Unutmaz D, Wong P, Sano G, De los SK, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanolkar A, Fuller MJ, Zajac AJ. CD4 T cell-dependent CD8 T cell maturation. J. Immunol. 2004;172:2834–2844. doi: 10.4049/jimmunol.172.5.2834. [DOI] [PubMed] [Google Scholar]
- Kim SK, Schluns KS, Lefrançois L. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J. Immunol. 1999;163:4125–4132. [PubMed] [Google Scholar]
- Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrançois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- Lawrence CW, Braciale TJ. Activation, differentiation, and migration of naive virus-specific CD8+ T cells during pulmonary influenza virus infection. J. Immunol. 2004;173:1209–1218. doi: 10.4049/jimmunol.173.2.1209. [DOI] [PubMed] [Google Scholar]
- Lin MY, Selin LK, Welsh RM. Evolution of the CD8 T-cell repertoire during infections. Microbes Infect. 2000;2:1025–1039. doi: 10.1016/s1286-4579(00)01257-0. [DOI] [PubMed] [Google Scholar]
- Lin Y, Roberts TJ, Sriram V, Cho S, Brutkiewicz RR. Myeloid marker expression on antiviral CD8+ T cells following an acute virus infection. Eur. J. Immunol. 2003;33:2736–2743. doi: 10.1002/eji.200324087. [DOI] [PubMed] [Google Scholar]
- Lund FE, Partida-Sanchez S, Lee BO, Kusser KL, Hartson L, Hogan RJ, Woodland DL, Randall TD. Lymphotoxin-alpha-deficient mice make delayed, but effective, T and B cell responses to influenza. J. Immunol. 2002;169:5236–5243. doi: 10.4049/jimmunol.169.9.5236. [DOI] [PubMed] [Google Scholar]
- Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Marzo AL, Vezys V, Williams K, Tough DF, Lefrançois L. Tissue-level regulation of Th1 and Th2 primary and memory CD4 T cells in response to Listeria infection. J. Immunol. 2002;168:4504–4510. doi: 10.4049/jimmunol.168.9.4504. [DOI] [PubMed] [Google Scholar]
- Marzo AL, Vezys V, Klonowski KD, Lee SJ, Muralimohan G, Moore M, Tough DF, Lefrancois L. Fully functional memory CD8 T cells in the absence of CD4 T cells. J. Immunol. 2004;173:969–975. doi: 10.4049/jimmunol.173.2.969. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, Ward RL, Woodland DL, Lefrançois L. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J. Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- Mercado R, Vijh S, Allen SE, Kerksiek K, Pilip IM, Pamer EG. Early programming of T cell populations responding to bacterial infection. J. Immunol. 2000;165:6833–6839. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 2004;10:927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- Pope C, Kim S-K, Marzo A, Masopust D, Williams K, Jiang J, Shen H, Lefrançois L. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 2001;166:3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- Roberts AD, Woodland DL. Cutting edge: effector memory CD8+ T cells play a prominent role in recall responses to secondary viral infection in the lung. J. Immunol. 2004;172:6533–6537. doi: 10.4049/jimmunol.172.11.6533. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- Shedlock DJ, Whitmire JK, Tan J, MacDonald AS, Ahmed R, Shen H. Role of CD4 T cell help and costimulation in CD8 T cell responses during Listeria monocytogenes infection. J. Immunol. 2003;170:2053–2063. doi: 10.4049/jimmunol.170.4.2053. [DOI] [PubMed] [Google Scholar]
- Shen H, Whitmire JK, Fan X, Shedlock DJ, Kaech SM, Ahmed R. A specific role for B cells in the generation of CD8 T cell memory by recombinant Listeria monocytogenes. J. Immunol. 2003;170:1443–1451. doi: 10.4049/jimmunol.170.3.1443. [DOI] [PubMed] [Google Scholar]
- Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobita K, Sugiura A, Enomote C, Furuyama M. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med. Microbiol. Immunol. (Berl.) 1975;162:9–14. doi: 10.1007/BF02123572. [DOI] [PubMed] [Google Scholar]
- Topham DJ, Castrucci MR, Wingo FS, Belz GT, Doherty PC. The role of antigen in the localization of naive, acutely activated, and memory CD8(+) T cells to the lung during influenza pneumonia. J. Immunol. 2001;167:6983–6990. doi: 10.4049/jimmunol.167.12.6983. [DOI] [PubMed] [Google Scholar]
- Unanue ER. Inter-relationship among macrophages, natural killer cells and neutrophils in early stages of Listeria resistance. Curr. Opin. Immunol. 1997;9:35–43. doi: 10.1016/s0952-7915(97)80156-2. [DOI] [PubMed] [Google Scholar]
- Unsoeld H, Krautwald S, Voehringer D, Kunzendorf U, Pircher H. Cutting edge: CCR7+ and CCR7− memory T cells do not differ in immediate effector cell function. J. Immunol. 2002;169:638–641. doi: 10.4049/jimmunol.169.2.638. [DOI] [PubMed] [Google Scholar]
- Van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat. Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- Van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat. Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nat. Immunol. 2000;1:47–53. doi: 10.1038/76907. [DOI] [PubMed] [Google Scholar]
- Vidal K, Samarut C, Magaud JP, Revillard JP, Kaiserlian D. Unexpected lack of reactivity of allogeneic anti-Ia monoclonal antibodies with MHC class-II molecules expressed by mouse intestinal epithelial cells. J. Immunol. 1993;151:4642–4650. [PubMed] [Google Scholar]
- Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- Zimmermann C, Prevost-Blondel A, Blaser C, Pircher H. Kinetics of the response of naive and memory CD8 T cells to antigen: similarities and differences. Eur. J. Immunol. 1999;29:284–290. doi: 10.1002/(SICI)1521-4141(199901)29:01<284::AID-IMMU284>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.