Abstract
Little is known regarding the cerebral autoregulation in pediatric traumatic brain injury (TBI). We examined the relationship between cerebral hemodynamic predictors, including cerebral autoregulation, and long-term outcome after severe pediatric TBI. After Institutional Review Board (IRB) approval, a retrospective analysis of prospectively collected data (May 2002 to October 2007) for children age ≤16 years with severe TBI (admission Glasgow Coma Scale [GCS] score <9) was performed. Cerebral autoregulation was assessed within 72 h after TBI. Cerebral hemodynamic predictors (intracranial pressure [ICP], systolic blood pressure [SBP], and cerebral perfusion pressure [CPP]) through the first 72 h after TBI were abstracted. Univariate and multivariate analyses examined the relationship between impaired cerebral autoregulation (autoregulatory index <0.4), intracranial hypertension (ICP >20 mm Hg), and hypotension (SBP <5th percentile and CPP <40 mm Hg). Six-month Glasgow Outcome Scale (GOS) score of <4 defined poor outcome. Ten (28%) of the 36 children examined (9.1 ± 5.3 [0.8–16] years; 74% male) had poor outcome. Univariate factors associated with poor outcome were impaired cerebral autoregulation (p = 0.005), SBP <5th percentile for age and gender (p = 0.02), and low middle cerebral artery flow velocity (<2 SD for age and gender; p = 0.04). Independent risk factors for poor 6-month GOS were impaired cerebral autoregulation (adjusted odds ratio [aOR] 12.0; 95% confidence interval [CI] 1.4–99.4) and hypotension (SBP <5th percentile; aOR 8.8; 95% CI 1.1–70.5), respectively. Previous studies of TBI describing poor outcome with hemodynamics did not consider the status of cerebral autoregulation. In this study, both impaired cerebral autoregulation and SBP <5th percentile were independent risk factors for poor 6-month GOS.
Key words: : cerebral autoregulation, outcome, pediatric, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is the leading cause of morbidity and mortality in children over 1 year of age (Langlois, 2001). Studies show that low admission Glasgow Coma Scale (GCS) score, coagulopathy, hyperglycemia, and early hypotension are associated with poor outcome after pediatric TBI (Luerrsen et. al., 1988; Nakayama et al., 1991; Vavilala et al., 2001, 2003; Jennett et al., 1979; Kokoska et al., 1998; Pigula et al., 1993; Samant et al., 2008). However, there is a paucity of information regarding which of the cerebral hemodynamic factors, such as cerebral autoregulation, cerebral blood flow (CBF), intracranial pressure (ICP), and cerebral perfusion pressure (CPP), most influences outcome.
Two previous studies described impaired cerebral autoregulation after severe pediatric TBI but reported conflicting relationships between cerebral autoregulation status and outcome (Muizelaar et al., 1989; Sharples et al., 1995). Muizelaar et al. (1989) reported no relationship between early (12 h) impaired cerebral autoregulation and outcome, whereas Sharples et al. (1995) described a correlation between loss of CPP and cerebral vascular resistance (indirectly examining cerebral autoregulation) in patients with the worst neurological outcomes. More recently, we (Vavilala et al., 2006) reported that impaired cerebral autoregulation, within the first 72 h after severe pediatric TBI, was associated with poor 6-month outcome, but this was a univariate association and no other cerebral hemodynamic factors were considered.
Other potential cerebral hemodynamic predictors have also been examined. Adelson et al. (1997) described that children under the age of 1 year and children with cerebral hypoperfusion (CBF ≤20 ml/100 g/min) at any time after severe TBI had poor 6-month Glasgow Outcome Scale (GOS) score, and other studies (Catala-Temprano et al., 2007; Downward et al., 2000) have demonstrated that initial CPP <40 mm Hg is associated with unfavorable outcome, and decreased survival in children presented with normal initial ICP (<20 mm Hg). However, none of these studies contemporaneously included the major relevant cerebral hemodynamic risk factors when examining outcome. Therefore, the objective of this study was to simultaneously examine the relationship between select cerebral hemodynamic predictors and poor long-term outcome after pediatric TBI. We hypothesized that impaired cerebral autoregulation in children with severe TBI is an independent risk factor for poor long-term outcome.
Methods
After Institutional Review Board (IRB) approval from the University of Washington (Seattle, WA), a retrospective analysis of prospectively collected data from an ongoing observational cohort study examining cerebral autoregulation after pediatric TBI was performed at Harborview Medical Center (level 1 pediatric trauma center). Data from patients with available 6-month GOS on June 1, 2008 were analyzed. Consent was obtained from parents or legal guardians. Some data from 10 of the subjects in the present study were presented in our previous work, where the univariate association between impaired cerebral autoregulation and poor outcome is described (Vavilala et al., 2006).
Study participants
Eligibility criteria for prospective study included age 16 years or younger admitted to the Harborview Medical Center pediatric intensive care unit (PICU) with an admission diagnosis of severe TBI (admission GCS score <9), TBI on computed tomography (CT) scan, and tracheal intubation. Children with extracranial injuries were also included. Patients without available parent/guardian were excluded. Study participants underwent cerebral autoregulation testing when they were considered hemodynamically stable by the treating pediatric intensivist. Hemodynamic instability (significant hypotension or hypertension per treating intensivist) was corrected in all cases prior to autoregulation testing for two reasons: (1) cerebral autoregulation testing should not interfere with resuscitation care, and (2) cerebral autoregulation should not be falsely impaired. Some patients initially hemodynamically unstable underwent cerebral autoregulation testing later after stabilization, while some did not due to logistic issues such as being outside the 72 h window or clinical care priorities. Cerebral autoregulation was examined once in the first 72 h (in bilateral cerebral hemispheres) after injury in every patient at bedside in the PICU. The research nurse, who obtained 6-month GOS, was not involved in determining middle cerebral artery (MCA) flow velocity (Vmca) or autoregulatory index (ARI).
Measuring middle cerebral artery blood flow velocity
All patients were receiving medical care in a 30-degree head-up position prior to cerebral autoregulation testing according to clinical practice for patients with TBI. Transcranial Doppler (TCD) ultrasonography (Multidop X; DWL Corp., Sipplingen, Germany) was used to measure flow velocities in the bilateral MCA using 2-MHz ultrasound probe. Previously described age-appropriate depths were referenced and used to insonate the MCA (Bode, 1988). One registered vascular technologist, with more than 10 years of experience with TCD ultrasonography, insonated the MCAs during cerebral autoregulation testing. Investigators who performed the test were blinded to TBI severity. Cerebral autoregulation was then calculated off-line and entered into our database by another investigator.
Testing and quantifying cerebral autoregulation
Study participants underwent static cerebral autoregulation testing after TBI as previously described (Strebel et al., 1995). Briefly, during steady state (technically satisfactory conditions where change or lack of change in Vmca is attributed to increase in CPP; usually within 10 sec), intravenous phenylephrine was titrated using a slow infusion (0.05–0.1 μg · kg−1 · min−1) over a 3–5-min period. CPP or mean arterial blood pressure (MAP), when ICP was not monitored, was increased according to whichever following variable was greater: (1) 20% above baseline; or (2) a set value of 80 mm Hg for the group younger than 9 years and 90 mm Hg for the group aged 9–16 years, respectively. CPP and Vmca were simultaneously and continuously measured, and were recorded in the computer for subsequent off-line analysis. Autoregulatory capacity was quantified with the ARI, which was calculated according to a previously published formula (Strebel et al., 1995). Mathematically, the ARI is the percent change in estimated cerebrovascular resistance per percent change in CPP:
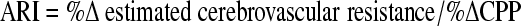 |
The estimated cerebrovascular resistance is the ratio of CPP to Vmca. Therefore, an ARI of 0 represents absent autoregulation (pressure-dependent Vmca), whereas an ARI of 1.0 represents perfect autoregulation. To dichotomize the results for statistical analysis and in accordance with the previous definition of intact cerebral autoregulation, autoregulatory capacity was considered intact if the ARI was 0.4 or greater (Strebel et al., 1995).
Blood pressure and Vmca
Recorded blood pressures were non-invasive (cuff ) measurements in the field and Emergency Department (ED), and arterial catheter measurements in PICU. When both non-invasive and invasive blood pressure readings were available, we used the invasive measurements. Each available lowest systolic blood pressure (SBP), lowest CPP, and highest ICP (irrespective of duration of the value), starting with the first Emergency Medical Services (EMS) responder and continuing through the first 72 h after TBI, including the time of cerebral autoregulatory testing, were abstracted and entered into an Excel spreadsheet and SPSS (SPSS Inc., Chicago, IL) datasheet. Quantifying the duration of hypotension was not feasible since such data, especially in the pre-hospital and ED settings, were lacking. Age- and gender-appropriate SBP percentiles (Kokoska et al., 1998; Adelson et al., 2003) were assigned to each corresponding blood pressure, and Vmca values were compared to available age and gender Vmca data (Udomphorn et al., 2008).
Patient outcome
GOS was determined at 6 months after TBI using either telephone or written questionnaires or in-person evaluations by a research nurse blinded to clinical data determined outcome. The Jennett five-point GOS classification was used (Jennett et al., 1979): GOS 1 = dead; GOS 2 = vegetative state; GOS 3 = alive but functionally impaired; GOS 4 = minimal handicap; and GOS 5 = pre-morbid level of functioning. A GOS of <4 reflected poor outcome, whereas good outcome was defined as a GOS of 4 or 5 (Jennett et al., 1979).
Statistical analysis
Patients who underwent cerebral autoregulatory testing during the first 72 h after TBI were considered for this analysis. SPSS version 11.5 was used for data entry and analysis. Descriptive statistics were used to describe patient characteristics, clinical data, cerebral hemodynamic parameters, and factors associated with long-term outcome.
Impaired cerebral autoregulation was defined by the lowest ARI (lARI) of either cerebral hemisphere (Vavilala et al., 2008), and lARI of <0.4 defined impaired cerebral autoregulation. SBP of <5th percentile adjusted by age and gender and CPP of <40 mm Hg were both used to define hypotension (Samant et al., 2008). Vmca of <2 SD for age- and gender-defined low Vmca (Udomphorn et al., 2008).
Patients were then divided into two groups: (1) poor outcome (6-month GOS <4) and good outcome (6-month GOS ≥ 4). The Student t-test, chi-square test, or Fisher exact test was used to analyze differences in good (GOS ≥ 4) and poor (GOS <4) outcome by baseline characteristics. Relationships between poor outcome and the following were first determined using Fisher's exact test: (1) impaired cerebral autoregulation, (2) hypotension (SBP <5th percentile and CPP <40 mm Hg), (3) ICP > 20 mm Hg, and (4) low Vmca. These data are presented as mean ± SD or n (%); p < 0.05 reflects significance. Significant (p < 0.05) univariate factors or select cerebral hemodynamic variables with trends toward significance (0.05 ≤ p ≤ 0.25) for poor outcome were entered into multivariate regression models. To determine potential independent risk factors for poor outcome, three models of binary logistic regression were performed. Two using each definition of hypotension (SBP <5th percentile and CPP <40 mm Hg), and both including lARI and Vmca <2 SD were created. The last model included both SBP <5th percentile and CPP <40 mm Hg, lARI and Vmca <2 SD. The covariates tested were not collinear with each other, and the variance inflation factors (VIFs) were less than 1.2. Adjusted odds ratio (aOR) and 95% confidence interval (CI) for the relation between cerebral hemodynamic predictors (variables of interest) and poor outcome (6-month GOS <4) were determined.
Results
Study participants
During the study period, 38 children were eligible for participation (Table 1). We failed to capture 6-month GOS in two participants, leaving 36 patients available for analyses (Table 2).
Table 1.
Clinical Data of 38 Children with Severe Traumatic Brain Injury
| Age (years) | 9.1 ± 5.3 (0.8–16.0) |
| Male | 28 (74) |
| Admission Glasgow Coma Scale | 4 ± 2.0 (3–8) |
| Mechanism of injury | |
| Motor vehicle crash | 11 (29) |
| Fall | 10 (26) |
| Auto-pedestrian | 5 (13) |
| Inflicted trauma | 2 (5) |
| Bike | 4 (11) |
| Gun shot wound | 3 (8) |
| Other | 3 (8) |
| Associated injuriesa | |
| Orthopedic | 21 (55) |
| Abdominal/pulmonary | 12 (32) |
| None | 15 (40) |
| Traumatic brain injury on computed tomography in emergency departmenta | |
| Diffuse axonal injury | 3 (8) |
| Subdural hematoma | 17 (45) |
| Epidural hematoma | 7 (18) |
| Subarachnoid hemorrhage | 14 (37) |
| Intracerebral hemorrhage | 4 (11) |
| Cerebral edema | 6 (16) |
| Skull fracture | 22 (58) |
| Cerebral infarction | 2 (5) |
| In-hospital mortality | 2 (5.3) |
Data are presented as mean ± SD (range) or n (%).
Percentages exceed 100% because some patients have multiple injuries.
Table 2.
Relationship between Lowest Autoregulatory Index (ARI), and Lowest Systolic Blood Pressure (SBP) and Lowest Cerebral Perfusion Pressure (CPP) in the First 72 h after Severe Pediatric Traumatic Brain Injury and with 6-Month Glasgow Outcome Scale (GOS Score)
| Table 2a: SBP (n = 36) | ARI | GOS < 4 (n = 10) | GOS ≥ 4 (n = 26) |
|---|---|---|---|
| Normal | ≥0.4 | 0 (0) | 16 (62) |
| Normal | <0.4 | 3 (30) | 4 (15) |
| <5th percentile | ≥0.4 | 2 (20) | 4 (15) |
| <5th percentile | <0.4 | 5 (50) | 2 (8) |
| Table 2b: CPP (n = 34) | ARI | GOS < 4 (n = 9) | GOS ≥ 4 (n = 25) |
|---|---|---|---|
| ≥40 mm Hg | ≥0.4 | 0 (0) | 13 (52) |
| ≥40 mm Hg | <0.4 | 4 (44) | 4 (16) |
| <40 mm Hg | ≥0.4 | 1 (12) | 6 (24) |
| <40 mm Hg | <0.4 | 4 (44) | 2 (8) |
Hypotension is defined as SBP of <5th percentile adjusted by age and gender and as CPP of <40 mm Hg. Impaired cerebral autoregulation is defined as ARI of <0.4. GOS of <4 defines poor outcome. Data are presented as n (%). Note that n = 36 in Table 2a and n = 34 in Table 2b since two patients did not have intracranial pressure (ICP) monitoring.
Demographic and baseline clinical characteristics
Children were 9.1 ± 5.3 (0.8–16) years old (Table 1). Most (74%) were male. Motor vehicle crash (29%) and fall (26%) accounted for the majority of injuries. Two children had inflicted trauma. All patients received a head CT scan in the ED. All children had severe TBI (GCS ≤ 8, mean GCS 4 ± 2) at the time of admission to the PICU. ICP data were available in 34 patients (94%) during the first 72 h after TBI. The mortality rate was 5.3%, and both deaths occurred during the first week of PICU stay. Cerebral autoregulation was impaired (ARI <0.4) in 14 patients, with both sides being involved in seven patients, and only one side being involved in the remaining seven patients.
Univariate factors associated with poor outcome
Ten (28%) patients had poor outcome. Five of 10 children had both hypotension (SBP <5th percentile) and impaired autoregulation (ARI <0.4). Three (30%) who had normal SBP but impaired autoregulation had poor outcome, and two (20%) patients who had intact autoregulation but SBP of <5th percentile had poor outcome (Table 2a). When CPP of <40 mm Hg was defined as hypotension, nine (25%) children had poor outcome. Of these poor outcome patients, 44% had CPP of ≥40 mm Hg and impaired ARI. Four patients had both CPP of <40 mm Hg and impaired ARI, and one patient had CPP of <40 mm Hg and intact ARI (Table 2b). Univariate factors associated with poor outcome (GOS <4) included higher hematocrit at the time of autoregulation testing (p = 0.005), impaired cerebral autoregulation (ARI <0.4; p = 0.005), SBP of <5th percentile by age and gender during the first 72 h after TBI (p = 0.02), and low Vmca at time of testing (Vmca less than 2 SD by age and gender; p = 0.04). ICP of >20 mm Hg and CPP of <40 mm Hg during the first 72 h after TBI had no association with poor outcome (Table 3).
Table 3.
Univariate Factors Associated with Poor 6-Month GOS after Severe Pediatric Traumatic Brain Injury (n = 36)
| GOS < 4 (n = 10) | GOS ≥ 4 (n = 26) | p | |
|---|---|---|---|
| Clinical characteristics | |||
| Age (years) | 8.1 ± 6.0 | 9.6 ± 4.8 | 0.46 |
| Male (n = 26) | 7 (70) | 19 (73) | 1.0 |
| GCS at PICU admission | 4 ± 1.0 | 5 ± 2.0 | 0.07 |
| PaCO2 at the time of testing (mm Hg) | 34 ± 2.8 | 35 ± 3.4 | 0.2 |
| Hematocrit at the time of testing (%) | 33.6 ± 4.5 | 28.5 ± 4.6 | 0.005 |
| Number of patients with | |||
| Impaired cerebral autoregulation (n = 14) | 8 (80) | 6 (23) | 0.005 |
| Low Vmca at time of testing (n = 6) | 4 (40) | 2 (8) | 0.04 |
| SBP <5th percentile in 72 h after injury (n = 13) | 7 (70) | 6 (23) | 0.02 |
| ICP >20 mm Hg in first 72 h after injury (n = 27) | 8 (80) | 19 (73) | 0.64 |
| CPP <40 mm Hg in first 72 h after injury (n = 13) | 5 (50) | 8 (31) | 0.25 |
| Subdural hematoma on the first CT (n = 16) | 5 (50) | 11 (42) | 0.7 |
| Edema or diffuse axonal injury on the first CT (n = 8) | 3 (30) | 5 (19) | 0.7 |
| Sedation (propofol or benzodiazepine) at the time of testing (n = 26) | 7 (70) | 19 (73) | 1.0 |
| Fever (temperature >38.5°C ) at the time of testing (n = 6) | 2 (20) | 4 (15) | 1.0 |
Of 36 patients, 34 had intracranial pressure (ICP) monitoring. Glasgow Outcome Scale (GOS) score of <4 = poor outcome. Autoregulatory Index (ARI) of <0.4 = impaired cerebral autoregulation. CPP, cerebral perfusion pressure; low Vmca, middle cerebral artery flow velocity of <2 SD for age and gender. Data are presented as mean ± SD, or n (column %).
GCS, Glasgow Coma Scale; PICU, pediatric intensive care unit; CT, computed tomography.
Independent cerebral hemodynamic risk factors for poor outcome
When SBP was used to define hypotension, independent risk factors for poor 6-month outcome were impaired cerebral autoregulation (aOR 12.0; 95% CI 1.4–99.4) and SBP of <5th percentile for age and gender during the first 72 h after TBI (aOR 8.8; 95% CI 1.1–70.5; Table 4). When CPP was used to define hypotension, only impaired cerebral autoregulation remained as an independent predictor of poor outcome (aOR 23.1; 95% CI 1.9–279.0). Only impaired autoregulation was an independent risk factor when we entered CPP of <40 mm Hg, SBP of <5th percentile for age and gender during the first 72 h after TBI, low Vmca by age and gender, and impaired autoregulation (aOR 29.8; 95% CI 1.7–521.4) into the regression model.
Table 4.
Model 1 Describes Independent Cerebral Hemodynamic Risk Factors for Poor 6-Month GOS (GOS <4) after Severe Pediatric TBI Using SBP of <5th Percentile to Define Hypotension (n = 36) and CPP of <40 mm Hg (n = 32/36) Using CPP of <40 mm Hg to Define Hypotension
| Table 4a: n = 36 | Poor 6-month GOS, aOR (95% CI) |
|---|---|
| Impaired cerebral autoregulation (ARI <0.4) | 12.0 (1.4–99.4) |
| Hypotension (SBP <5th percentile at first 72 h after TBI) | 8.8 (1.1–70.5) |
| Low Vmca at time of testing | 7.1 (0.6–78.0) |
| Table 4b: n = 32 | Poor 6-month GOS, aOR (95% CI) |
|---|---|
| Impaired cerebral autoregulation (ARI <0.4) | 23.1 (1.9–279.0) |
| CPP <40 mm Hg (at first 72 h after injury) | 2.0 (0.2–17.7) |
| Low Vmca at time of testing | 6.4 (0.4–103.0) |
Of the 36 patients enrolled in the study, four patients were excluded because of cerebral perfusion pressure (CPP) of <40 mm Hg at the time of cerebral autoregulation testing and no intracranial pressure (ICP) monitoring during the first 72 h after traumatic brain injury (TBI) (n = 32/36). Autoregulatory Index (ARI) < 0.4 =impaired cerebral autoregulation. Glasgow Outcome Scale (GOS) score of <4 = poor outcome. Low Vmca = middle cerebral artery flow velocity of <2 SD for age and gender.
aOR, adjusted odds ratio; CI, confidence interval.
Discussion
The main finding of this study is that, in our small sample of children with severe TBI, impaired cerebral autoregulation was an independent risk factor for poor 6-month GOS, regardless of whether SBP or CPP was used to define hypotension. Low Vmca (Vmca <2 SD for age and gender) did not predict poor outcome. These results suggest that, like hypotension, early impaired cerebral autoregulation, is an important secondary insult after severe pediatric TBI.
Poor 6-month outcome was predicted by SBP of <5th percentile and impaired autoregulation, but not by CPP of <40 mm Hg or low Vmca
In the present study, 6-month outcomes were good if children with severe TBI had neither SBP of <5th percentile nor ARI of <0.4 during the first 72 h after injury (Table 2a). However, the occurrence of either of these insults was associated with poor outcome (GOS <4), and when both these risk factors were present, poor outcome was more common (Table 2a). While it is possible that cerebral autoregulation may have been impaired because the lower limit of autoregulation was higher than SBP of <5th percentile, not all patients with SBP of <5th percentile had impaired cerebral autoregulation, and SBP of ≥5th percentile did not result in intact cerebral autoregulation. These facts suggest that cerebral autoregulation status is a risk factor for poor outcome, independent of blood pressure.
We examined hypotension using two different definitions based on the 2003 pediatric guidelines' definitions of hypotension (Adelson et al., 2003). We first defined systemic hypotension as SBP of <5th percentile for age during the first 72 h after injury (Coates et al., 2005) and CPP of <40 mm Hg (Adelson et al., 2003; Downard et al., 2000; Elias-Jones et al., 1992; Català-Temprano et al., 2007). Since ICP monitoring is not performed in the pre-hospital setting and it is not consistently used in TBI patients, and early hypotension may impact outcome more so than hypotension occurring later after hospital admission (Samant et al., 2008), it was important to consider hypotension using a definition that many clinicians use. Therefore, in this study, both CPP <40 mm Hg and SBP <5th percentile defined hypotension. It is possible that poor outcome was predicted by SBP of <5th percentile but not by CPP of <40 mm Hg because ICP monitoring was instituted only after arrival in the hospital. We, therefore, might have missed early (pre-hospital) cerebral hypotension, which would have been captured with earlier ICP monitoring. Other possible explanations might include that we could not evaluate duration of hypotension. Therefore, it is possible that the duration of low SBP may have been longer than duration of low CPP (due to increases in ICP). It is also possible that SBP of <5th percentile represents more severe intracranial hypotension than CPP of <40 mm Hg. Finally, SBP values are corrected for age and gender, whereas CPP is not because such referenced norms are lacking. Regardless of the definition of hypotension used and after considering the impact of blood pressure, 6-month outcome was poor when cerebral autoregulation was impaired during the first 72 h after TBI.
Low Vmca did not independently predict poor outcome. This may be because, in this sample, low Vmca may not have resulted in cerebral ischemia. However, we did not have information on cerebral metabolic rate. Alternatively, low Vmca was associated with poor outcome in the univariate analysis, suggesting that while it may be associated with poor outcome, cerebral hypoperfusion per se may not be as important a predictor of outcome when considered alongside the other cerebral hemodynamic predictors examined. Although high hematocrit at time of testing was associated with poor outcome, we did not enter it into multivariate analysis because (1) our aim was to examine the relationship between cerebral hemodynamic predictors and poor long-term outcome after pediatric TBI and (2) hematocrit directly affects Vmca, which we wanted to examine as a predictor of outcome.
Impaired cerebral autoregulation is an important secondary insult
Although poor outcome is associated with impaired autoregulation in severe pediatric TBI (Vavilala et al., 2004, 2006, 2008; Sharples et al., 1995), it has been debated as to whether impairment of cerebral autoregulation is a marker of severity of TBI or the causal pathway for poor outcome. Results of this study suggest that impaired autoregulation within 72 h after TBI could be involved in the causal pathway for outcome after TBI in children. Since the incidence of impaired cerebral autoregulation is related to TBI severity and approximates 40% after severe TBI (Vavilala et al., 2004), empirically augmenting or lowering blood pressure after TBI may seem appealing as a therapeutic strategy, particularly since cerebral autoregulation testing is not routinely performed in patients with TBI. However, cerebral autoregulation is a dynamic process and can change during the first week to 10 days after TBI, coincident with worsening TBI. Alterations in this homeostatic process occur early after TBI, when the brain is particularly vulnerable (Tontisirin et al., 2007; Muizelaar et al., 1989), and although cerebral autoregulation is often dichotomized as intact or absent, it represents a continuous spectrum of adaptive responsiveness of cerebral vasculature to changes in CPP and is not an “all or none” phenomenon. Additionally, despite efforts to maintain tight control of blood pressure, the likelihood of blood pressure fluctuations is high early after TBI, increasing the risk of secondary cerebral injury if cerebral autoregulation changes (Kokoska et al., 1998). Therefore, even minor fluctuations in blood pressure when cerebral autoregulation changes may lead to cerebral ischemia or hyperemia. Physiological strategies that may improve autoregulatory status include moderate hyperventilation (Newell et al., 1996) and indomethacin (Puppo et al., 2007) due to cerebral vasoconstriction and improved cerebrovascular tone. But whether improvement in autoregulation can translate into improved outcome is not yet known.
Limitations of the study
Some important limitations of this study deserve discussion. First, while the SBP data for 72-h period post-injury included the pre-hospital period, CPP data were available only after hospital arrival. Therefore, we might not have captured all patients with CPP of <40 mm Hg. However, we included blood pressure data from the pre-hospital period because SBP of <5th percentile for age, occurring in the field and/or ED, is a better predictor of poor outcome than hypotension occurring later. Blood pressure cuff data were used for out-of-hospital and ED measurements, whereas blood pressures from arterial catheters were recorded for PICU measurements. While the two methods of blood pressure measurements are different, this represents clinical practice at our institution. We used TCD ultrasonogrpahy, which measures CBF velocity and not CBF; however, TCD ultrasonography is an appropriate surrogate for studying CBF since changes in flow velocity are proportional to changes in flow, and its relatively noninvasive nature and feasibility for bedside measurement makes it a clinically suitable method in critically ill children. We tested cerebral autoregulation only once in each patient, and hence we do not know whether impairment of autoregulation was temporary or permanent, or whether autoregulatory status changed over time. Although we would have liked to quantify the duration of hypotension, such data, especially from the pre-hospital and ED settings, were lacking. For this reason, we could not estimate lengths of time for which hypotension occurred and cannot comment if hypotension coincided with impaired ARI. Additionally, we did not have CMRO2 data or biomarkers for TBI. Finally, our sample size was too small to formally examine for interactions between hypotension and impaired cerebral autoregulation. Despite these limitations, our data are new and shed light on the potentially important role of cerebral autoregulation in children with severe TBI.
In summary, this study builds on previous work and is the first study to contemporaneously examine the importance of cerebral autoregulation alongside other cerebral secondary insults in pediatric TBI. We have provided new information showing that impaired cerebral autoregulation is an independent risk factor for poor 6-month outcome after severe pediatric TBI. These data may support the need for early data collection on cerebral autoregulation in patients with TBI.
Author Disclosure Statement
No competing financial interests exist.
References
- Adelson P.D. Bratton S.L. Carney N.A. Chesnut R.M. du Coudray H.E. Goldstein B. Kochanek P.M. Miller H.C. Partington M.D. Selden N.R. Warden C.R. Wright D.W. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 4. Resuscitation of blood pressure and oxygenation and prehospital brain-specific therapies for the severe pediatric traumatic brain injury patient. Pediatr. Crit. Care Med. 2003;4:S12–S18. [PubMed] [Google Scholar]
- Adelson P.D. Clyde B. Kochanek P.M. Wisniewski S.R. Marion D.W. Yonas H. Cerebrovascular response in infants and young children following severe traumatic brain injury: a preliminary report. Pediatr. Neurosurg. 1997;26:200–207. doi: 10.1159/000121192. [DOI] [PubMed] [Google Scholar]
- Adelson P.D. Bratton S.L. Carney N.A. Chesnut R.M. du Coudray H.E. Goldstein B. Kochanek P.M. Miller H.C. Partington M.D. Selden N.R. Warden C.R. Wright D.W. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 8. Cerebral perfusion pressure. Pediatr. Crit. Care Med. 2003;4, Suppl:S31–S33. [PubMed] [Google Scholar]
- Bode H. Application of Pediatric Transcranial Doppler Ultrasonography. Springer-Verlag; New York: 1988. [Google Scholar]
- Catala-Temprano A. Claret-Teruel G. Cambra-Lasaosa F.J. Pons-Odena M. Noguera-Julian A. Palomeque-Rico A. Intracranial pressure and cerebral perfusion pressure as risk factors in children with traumatic brain injuries. J. Neurosurg. 2007;106:463–466. doi: 10.3171/ped.2007.106.6.463. [DOI] [PubMed] [Google Scholar]
- Coates B.M. Vavilala M.S. Mack C.D. Muangman S. Suz P. Sharar S.R. Bulger E. Lam A.M. Influence of definition and location of hypotension on outcome following severe pediatric traumatic brain injury. Crit. Care Med. 2005;33:2645–2650. doi: 10.1097/01.ccm.0000186417.19199.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downard C. Hulka F. Mullins R.J. Piatt J. Chesnut R. Quint P. Mann N.C. Relationship of cerebral perfusion pressure and survival in pediatric brain-injured patients. J. Trauma. 2000;49:654–658. doi: 10.1097/00005373-200010000-00012. [DOI] [PubMed] [Google Scholar]
- Elias-Jones A.C. Punt J.A. Turnbull A.E. Jaspan T. Management and outcome of severe head injuries in the Trent region 1985–90. Arch. Dis. Child. 1992;67:1430–1435. doi: 10.1136/adc.67.12.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennett B. Teasdale G. Braakman R. Minderhoud J. Heiden J. Kurze T. Prognosis of patients with severe head injury. Neurosurgery. 1979;4:283–289. doi: 10.1227/00006123-197904000-00001. [DOI] [PubMed] [Google Scholar]
- Kokoska E.R. Smith G.S. Pittman T. Weber T.R. Early hypotension worsens neurological outcome in pediatric patients with moderately severe head trauma. J. Pediatr. Surg. 1998;33:333–338. doi: 10.1016/s0022-3468(98)90457-2. [DOI] [PubMed] [Google Scholar]
- Langlois J.A. Traumatic Brain Injury in the United States: Assessing Outcomes in Children. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention (CDC); Atlanta: 2001. [Google Scholar]
- Luerrsen T.G. Klauber M.R. Marshall L.F. Outcome from head injury related to patient's age: a longitudinal prospective study of adult and pediatric study of adult and pediatric head injury. J. Neurosurg. 1988;68:409–416. doi: 10.3171/jns.1988.68.3.0409. [DOI] [PubMed] [Google Scholar]
- Marion D.W. Darby J. Yonas H. Acute regional cerebral blood flow changes caused by severe head injuries. J. Neurosurg. 1991;74:407–414. doi: 10.3171/jns.1991.74.3.0407. [DOI] [PubMed] [Google Scholar]
- Muizelaar J.P. Ward J.D. Marmarou A. Newlon P.G. Wachi A. Cerebral blood flow and metabolism in severely head-injured children. Part 2: Autoregulation. J. Neurosurg. 1989;71:72–76. doi: 10.3171/jns.1989.71.1.0072. [DOI] [PubMed] [Google Scholar]
- Muizelaar J.P. Marmarou A. DeSalles A.A. Ward J.D. Zimmerman R.S. Li Z. Choi S.C. Young H.F. Cerebral blood flow and metabolism in severely head-injured children. Part 1: Relationship with GCS score, outcome, ICP, and PVI. J. Neurosurg. 1989;71:63–71. doi: 10.3171/jns.1989.71.1.0063. [DOI] [PubMed] [Google Scholar]
- Nakayama D.K. Copes W.S. Sacco W.J. The effect of patient age upon survival in pediatric trauma. J. Trauma. 1991;31:1521–1526. doi: 10.1097/00005373-199111000-00010. [DOI] [PubMed] [Google Scholar]
- Newell D.W. Weber J.P. Watson R. Aaslid R. Winn H.R. Effect of transient moderate hyperventilation on dynamic cerebral autoregulation after severe head injury. Neurosurgery. 1996;39:35–43. doi: 10.1097/00006123-199607000-00008. [DOI] [PubMed] [Google Scholar]
- Pigula F.A. Wald S.L. Shackford S.R. Vane D.W. The effect of hypotension and hypoxia on children with severe head injuries. J. Pediatr. Surg. 1993;28:310–314. doi: 10.1016/0022-3468(93)90223-8. [DOI] [PubMed] [Google Scholar]
- Puppo C. Lopez. L. Farina G. Caragna E. Moraes L. Iturralde A. Biestro A. Indomethacin and cerebral autoregulation in severe head injured patients: a transcranial Doppler study. Acta Neurochir. (Wien) 2007;149:139–149. doi: 10.1007/s00701-006-1074-0. [DOI] [PubMed] [Google Scholar]
- Samant U.B. Mack C.D. Koepsell T. Rivara F.P. Vavilala M.S. Time of hypotension and discharge outcome in children with severe traumatic brain injury. J. Neurotrauma. 2008;25:495–502. doi: 10.1089/neu.2007.0491. [DOI] [PubMed] [Google Scholar]
- Sharples P.M. Matthews D.S. Eyre J.A. Cerebral blood flow and metabolism in children with severe head injuries. Part 2: Cerebrovascular resistance and its determinants. J. Neurol. Neurosurg. Psychiatry. 1995;58:153–159. doi: 10.1136/jnnp.58.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebel S. Lam A.M. Matta B. Mayberg T.S. Aaslid R. Newell D.W. Dynamic and static cerebral autoregulation during isoflurane, desflurane, and propofol anesthesia. Anesthesiology. 1995;83:66–76. doi: 10.1097/00000542-199507000-00008. [DOI] [PubMed] [Google Scholar]
- Tontisirin N. Armstead W. Waitayawinyu P. Moore A. Udomphorn Y. Zimmerman J.J. Chesnut R. Vavilala M.S. Change in cerebral autoregulation as a function of time in children after severe traumatic brain injury: a case series. Childs Nerv. Syst. 2007;23:1163–1169. doi: 10.1007/s00381-007-0339-0. [DOI] [PubMed] [Google Scholar]
- Udomphorn Y. Armstead W.M. Vavilala M.S. Cerebral blood flow and autoregulation after pediatric traumatic brain injury. Pediatr. Neurol. 2008;38:225–234. doi: 10.1016/j.pediatrneurol.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavilala M.S. Bowen A. Lam A.M. Uffman J.C. Powell J. Winn H.R. Rivara F.P. Blood pressure and outcome after severe pediatric traumatic brain injury. J. Trauma. 2003;55:1039–1044. doi: 10.1097/01.TA.0000101759.23607.57. [DOI] [PubMed] [Google Scholar]
- Vavilala M.S. Dunbar P.J. Rivara F.P. Lam A.M. Coagulopathy predicts poor outcome following head injury in children less than 16 years of age. J. Neurosurg. Anesthesiol. 2001;13:13–18. doi: 10.1097/00008506-200101000-00003. [DOI] [PubMed] [Google Scholar]
- Vavilala M.S. Lee L.A. Boddu K. Visco E. Newell D.W. Zimmerman J.J. Lam A.M. Cerebral autoregulation in pediatric traumatic brain injury. Pediatr. Crit. Care Med. 2004;5:257–263. doi: 10.1097/01.pcc.0000123545.69133.c3. [DOI] [PubMed] [Google Scholar]
- Vavilala M.S. Muangman S. Tontisirin N. Fisk D. Roscigno C. Mitchell P. Kirkness C. Zimmerman J.J. Chesnut R. Lam A.M. Impaired cerebral autoregulation and 6-month outcome in children with severe traumatic brain injury: preliminary findings. Dev. Neurosci. 2006;28:348–353. doi: 10.1159/000094161. [DOI] [PubMed] [Google Scholar]
- Vavilala M.S. Tontisirin N. Udomphorn Y. Armstead W. Zimmerman J.J. Chesnut R. Lam A.M. Hemispheric differences in cerebral autoregulation in children with moderate and severe traumatic brain injury. Neurocrit. Care. 2008;9:45–54. doi: 10.1007/s12028-007-9036-9. [DOI] [PubMed] [Google Scholar]


