Summary
During tumorigenesis, normal growth mechanisms are deregulated and safeguards that eliminate abnormal cells by apoptosis are disabled. Tumor cells must also increase nutrient uptake and angiogenesis to support the upregulation of metabolism necessary for unrestricted growth. In addition, they have to rely on inefficient energy production by glycolysis. This glycolytic state can result from mutations that promote cell proliferation, the hypoxic tumor microenvironment and perhaps mitochondrial malfunction. Moreover, the very signals that enable unrestricted cell proliferation inhibit autophagy, which normally sustains cells during nutrient limitation. In tumors, inactivation of the autophagy pathway may enhance necrosis and inflammation and promote genomic instability, which can further enhance tumor growth. Thus, tumor cells cannot adapt efficiently to metabolic stress and could be induced to die by metabolic catastrophe, in which high energy demand is contrasted by insufficient energy production. Efforts to exploit this unique metabolic state clinically previously focused mainly on detecting tissue displaying increased glycolytic metabolism. The challenge now is to induce metabolic catastrophe therapeutically as an approach to killing the unkillable cells.
Keywords: Autophagy, Apoptosis, AKT, mTOR, BCL-2, Beclin1, Cancer
Introduction
Cancer is a disease in which inherent genetic flexibility allows cells to progressively evolve functions that promote cell growth, disable cell death mechanisms and evade immune surveillance and therapy (Hanahan and Weinberg, 2000). Regulation of cell growth is inexorably linked to regulation of metabolism, and the unique ways that cancer cells deregulate cell growth and metabolism distinguish them from normal cells. This inherent difference between normal and tumor cells can be exploited therapeutically. Furthermore, cells possess multiple death mechanisms that differentially impact tumor progression and the response to treatment. Apoptosis is an efficient programmed cell death process (Fig. 1) regulated by cell intrinsic and extrinsic pathways (Adams, 2003; Danial and Korsmeyer, 2004; Gelinas and White, 2005). Activation of apoptosis is controlled by members of the BCL-2 family of proteins and ultimately impinges on the function of the caspase family of proteases. These dismantle the cell through proteolytic cleavage and the activation of nucleases that degrade the genome, which results in rapid cell death (Fig. 1) without inflammation. Metabolic stress is a potent trigger of apoptosis, and activation of apoptosis in tumors is an attractive therapeutic strategy; however, tumor cells evolve defects in apoptosis, which provides a survival advantage that promotes tumorigenesis and confounds treatment (Nelson et al., 2004; Nelson and White, 2004; White, 2006).
Fig. 1.
Distinct morphological features of apoptosis, necrosis and autophagy. Immortal baby mouse kidney epithelial (iBMK) cell lines competent for apoptosis (wild-type), necrosis (apoptosis-defective and autophagy disabled by AKT activation) and autophagy (apoptosis defective) (Degenhardt et al., 2002; Degenhardt et al., 2006; Degenhardt and White, 2006) were subjected to metabolic stress (ischemia) for up to five days. Morphological changes were observed by time-lapse microscopy (100×). Representative individual cells from each cell line were followed for the indicated times, beginning immediately prior to any signs of altered morphology in metabolic stress (13.5 hours for wild-type, 16.6 hours for apoptosis- and autophagy-defective and 52.6 hours for apoptosis-defective cells). Cells undergoing apoptosis or necrosis are not viable at the end of each specified time, whereas cells capable of autophagy by virtue of an apoptotic defect retained viability for more than five days (Degenhardt et al., 2006). Following 112 hours of metabolic stress, apoptosis-defective cells that underwent autophagy were returned to normal culture conditions and photographed to document recovery (Degenhardt et al., 2006). The majority of these cells were able to recover and proliferate upon restoration of nutrients, and a representative cell is shown. Reproduced in part from Degenhardt et al. (Degenhardt et al., 2006) with permission from Elsevier.
Recent evidence suggests that tumor cells that acquire defects in apoptosis can be diverted to another cell death pathway, necrosis, where cells die by a loss of physical integrity (Fig. 1) (Zong and Thompson, 2006), and this provides an alternate therapeutic approach (Degenhardt et al., 2006; Zong et al., 2004). Necrosis in apoptosis-defective tumor cells appears to occur when the rate of energy consumption exceeds that of energy production. This again links cell death to regulation of cell metabolism, supporting the idea that metabolism is an attractive alternative therapeutic target. Even though necrosis is a less efficient mechanism of cell death compared with apoptosis, it remains a tractable means to kill tumor cells with apoptosis defects, despite the possible consequences of promoting inflammation (Degenhardt et al., 2006).
It is now apparent that tumor cells with apoptosis defects require the catabolic process of autophagy to provide an alternate energy source in periods of metabolic deprivation to prevent death by necrosis (Degenhardt et al., 2006; Lum et al., 2005). Autophagy is a process in which cellular organelles and bulk cytoplasm are targeted for degradation in lysosomes (Klionsky, 2005). Normal mammalian cells require basal autophagy for normal organelle and protein turnover and autophagy induction is required to sustain metabolism and viability particularly during periods of starvation (Hara et al., 2006; Komatsu et al., 2006; Komatsu et al., 2005; Kuma et al., 2004). In tumor cells with defects in apoptosis, autophagy is needed to maintain cell metabolism and viability during starvation until the nutrient supply is re-established (Fig. 1). If nutrient deprivation persists, progressive autophagy can ultimately lead to autophagic cell death. Thus, in contrast to apoptosis, which is a death process that is rapid and irreversible, autophagy can be thought of as an interruptible pathway to cell death (Fig. 1). In tumor cells that have defects in apoptosis, autophagy enables them to survive metabolic stress. As such, autophagy may also therefore be an appropriate therapeutic target in cancer.
There is thus an emerging appreciation of the interdependence between cell growth, metabolism and death pathways. Below we discuss recent work that has shed light on the interplay between these processes and the ongoing challenge to translate this knowledge for therapeutic benefit.
The PI 3-kinase pathway and nutrient availability
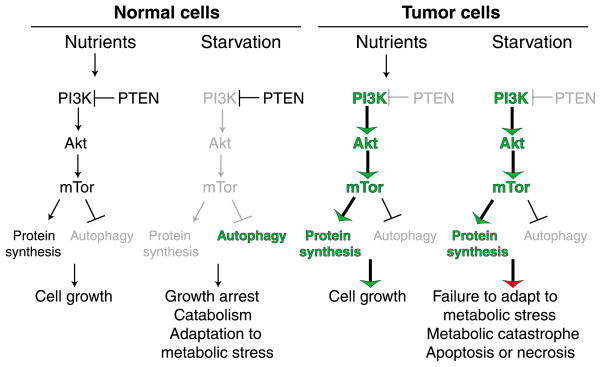
The phosphatidylinositol (PI) 3-kinase pathway functions in growth factor receptor signal transduction to activate the cell growth and proliferative responses to nutrients and growth factors. This is accomplished by activation of the protein kinase AKT, one of whose targets is the tuberous sclerosis complex (TSC), an inhibitor of the protein kinase mammalian target of rapamycin (mTOR) (Hemmings, 1997). AKT inhibits TSC and thereby activates mTOR. mTOR in turn activates pathways that promote translation, cell cycle progression, nutrient uptake and glycolysis, while inhibiting apoptosis and autophagy. The result is cell division, protection from apoptosis and rapid but inefficient ATP production by glycolysis (see below). PI 3-kinase signaling through mTOR shuts down catabolic function that cells do not require when sufficient nutrients are available (Arico et al., 2001). When the supply of nutrients and growth factors is interrupted, PI 3-kinase signaling is inhibited, which leads to suppression of cell growth and protein synthesis (Fig. 2). This also activates autophagy to provide an alternative energy supply (Fig. 2).
Fig. 2.
Differential response of normal and tumor cells to metabolic stress. Normal cells regulate cell growth in response to nutrient availability by modulating the activity of the PI 3-kinase pathway, which through mTOR promotes cell growth and downregulates the catabolic process of autophagy. In periods of starvation, normal cells downregulate mTOR, which slows cell growth, while upregulating autophagy to allow adaptation to metabolic stress. In contrast, tumor cells frequently acquire mutations that constitutively activate the PI 3-kinase pathway that efficiently promotes cell growth in the presence of nutrients. In starvation conditions, however, tumor cells inefficiently adapt to metabolic stress through the failure to downregulate cell growth and upregulate autophagy, which can result in apoptotic or necrotic cell death though metabolic catastrophe.
The PI 3-kinase pathway is frequently deregulated in cancer
Activation of the PI 3-kinase pathway is an exquisite formula for driving proliferation of tumor cells, which is why constitutive activation of this pathway is so common in tumors (Downward, 2004). Constitutive activation of the PI 3-kinase pathway is achieved in multiple ways, such as activation of PI 3-kinase itself, inactivation of its inhibitor, the tumor suppressor PTEN, or activation of AKT downstream. This may also be the Achilles heel of tumor cells because it renders them unable to adapt to nutrient and growth factor limitation because the pathway promotes cell growth and inhibits autophagy (Figs 2 and 3). These findings should be considered in the development of anti-cancer agents that target mTOR as opposed to targeting the downstream autophagy pathway.
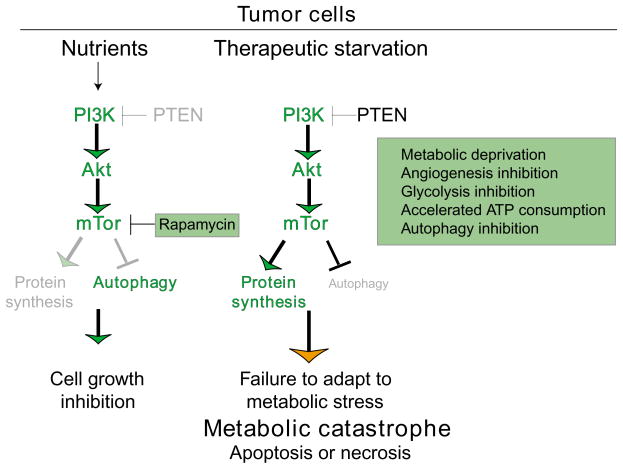
Fig. 3.
How manipulation of tumor cell metabolism can be used to induce cell death by metabolic catastrophe. The difference between normal and tumor cell metabolism can be exploited for cancer therapy by promoting metabolic catastrophe. mTOR inhibitors, such as rapamycin and its analogues in this case, are an effective means to limit tumor cell growth (Faivre et al., 2006). Alternatively, metabolic catastrophe can be achieved by therapeutic starvation, by restricting nutrient availability, uptake and utilization, by enhancing consumption or by preventing catabolism through autophagy.
Efforts to target mTOR in the clinic have yielded promising initial results, and indeed further drug development (both single agents and combinations) is warranted. Agents such as rapamycin and analogues including CCI-779 and RAD001, for example, inhibit downstream signaling and are currently in clinical trials (Chan, 2004; Rowinsky, 2004). Inhibition of mTOR in this way should block tumor growth, which is obviously desirable, but it should also induce autophagy (Fig. 3). The potential impact of such induction on overall tumor cell death therefore needs to be considered and studied further.
Metabolic stress in tumors
Constitutive activation of the PI 3-kinase pathway in tumor cells prevents the downregulation of cell metabolism, protein synthesis and cell growth when nutrients and growth factors become limiting. The resulting metabolic stress is compounded by the tumor’s frequent reliance on glycolysis. A common feature of human solid tumors is their reliance on glycolysis even under aerobic conditions – the Warburg effect (Warburg, 1956). Glycolysis is triggered by oncogene activation, including activation of RAS, AKT and MYC, and by hypoxia in the tumor microenvironment through HIF-1α induction. It might also be triggered by the accumulation of damaged mitochondria that have an impaired capacity for ATP generation through oxidative phosphorylation (Dang et al., 1997; Elstrom et al., 2004; Gatenby and Gillies, 2004; Semenza, 2003). All of these events commonly occur in human tumors. Whether or not the dependence on glycolysis directly contributes to tumor growth is unclear, but glycolysis is clearly a substantially less efficient means for ATP generation compared with oxidative phosphorylation. Thus, the more dependent a tumor cell is on glycolysis, the more crucial is its requirement for nutrients such as glucose. To compensate for inefficient energy production, many of the mechanisms that promote glycolytic metabolism also facilitate uptake of nutrients from extracellular sources (Thompson et al., 2005).
Another source of nutrients is catabolism achieved by activation of the autophagy pathway (Jin and White, 2007). In turn, the high energy demand and glycolytic state in tumor cells can potentially feedback to activate autophagy. Note, however, that the ability of the tumor to use an alternate internal nutrient supply through autophagy is restricted (see above). What this therefore creates is a situation in which tumor cells are forced to grow whether or not they have access to nutrients or the capacity to efficiently produce energy. This can result in cell death by apoptosis or necrosis (Fig. 2).
Apoptosis resistance and metabolic stress
In cells capable of apoptosis, starvation-induced metabolic stress is a potent activator of this form of cell death. It requires pro-apoptotic BAX or BAK and is inhibited by anti-apoptotic BCL-2, although exactly how apoptosis is activated remains to be determined (Degenhardt et al., 2002; Nelson et al., 2004; Tan et al., 2005; White, 2006). Many anti-cancer drugs work by indirectly inducing apoptosis, and newer therapies designed to activate the apoptotic pathway directly are in development (Fesik, 2005; Johnstone et al., 2002; Tan et al., 2005). Most tumor cells, however, acquire defects in apoptosis that provide a distinct survival advantage in the hostile microenvironment and in response to cancer therapies that activate apoptosis. If these cells retain the normal function of the PI 3-kinase pathway, they can adapt to starvation by downregulating cell growth and metabolism as normal cells do. Indeed, autophagy is evident in metabolically stressed regions of apoptosis-defective tumors and sustains the viability of tumor cells that can still regulate the PI 3-kinase pathway (Fig. 2) (Degenhardt et al., 2006).
When tumor cells have inactivated apoptosis and constitutively activated the PI 3-kinase pathway, which is a common occurrence, there is a significant difference in the response to metabolic stress. These cells cannot downregulate cell growth, activate autophagy or die by apoptosis (Degenhardt et al., 2006; Jin and White, 2007). The unrelenting demand for energy, exacerbated by inefficient ATP production by glycolysis and the absence of an alternative energy source generated by autophagy, leads to a necrotic pathway to cell death, which may be the ultimate default mechanism (Degenhardt et al., 2006; Jin and White, 2007; Zong et al., 2004; Zong and Thompson, 2006). We have designated this ‘metabolic catastrophe’ (Figs 2 and 3). Because this represents a means to induce death in tumor cells resistant to apoptosis, it is a potential therapeutic approach worth exploiting that is applicable to many tumors that are not responsive to treatment (Fig. 3).
Therapeutic induction of necrosis through metabolic catastrophe
Modulation of tumor cell metabolism is a therapeutic approach that takes advantage of inherent metabolic differences between normal and cancer cells. As we begin to understand how metabolism is linked to death and survival signaling and how this is influenced by tumor genotype, we can start to predict the types of treatment that will drive tumor cells toward cell death – particularly necrosis. Tumor cells displaying constitutive activation of the PI 3-kinase pathway can be driven into metabolic catastrophe therapeutically by various methods. Angiogenesis inhibitors such as Avastin, which targets vascular endothelial growth factor (Carmeliet, 2005; Ferrara and Kerbel, 2005), can reduce nutrient availability. Similar approaches that block nutrient uptake, transport or utilization are worth consideration (Fig. 3). Additional therapeutic opportunities include approaches that target other steps in metabolism and those that increase energy requirements. Metabolic stress can be amplified by accelerating ATP depletion with DNA alkylating agents that stimulate poly(ADP-ribose) polymerase (PARP), which consumes ATP (Zong et al., 2004). Alternatively, one can increase ATP demand by upregulating protein synthesis – for example, by blocking eEF2-α kinase, which also inhibits autophagy (Wu et al., 2006). These and other means for acute induction of necrotic cell death in tumor cells should be explored further for cancer therapy.
Ironically, necrotic tumors in fact have a poorer prognosis, and chronic necrotic cell death in tumors can be associated with inflammation; one consequence of diversion from apoptotic to necrotic cell death may therefore be altered tumor-host interaction (Degenhardt et al., 2006; Nelson and White, 2004). Chronically necrotic tumors provoke chronic inflammation analogous to wounds in which the wound healing cytokine response is usurped to favor tumor growth (Balkwill et al., 2005; Nelson and White, 2004; Zeh and Lotze, 2005). Apoptosis and autophagy could therefore serve to prevent necrosis, and thus limit inflammation and tumor progression in some situations (Degenhardt et al., 2006; Jin and White, 2007), although activation of an effective immune response can ultimately favor tumor regression. Thus, controlling necrosis to modulate the appropriate inflammatory response to favor tumor regression remains the challenge.
Glycolysis as a target
Interfering with the glycolytic pathway is now also attracting interest as a possible therapeutic means to limit energy production in a tumor cell-specific fashion. The fact that tumors need glycolysis to convert glucose to ATP formed the basis of tumor imaging by fluorine-18 fluorodeoxyglucose ([18F]FDG) positron emission tomography (PET), which demonstrates the increase in glucose uptake in tumors compared with normal tissue (Zhang et al., 2004). Although prior efforts focused on detecting tissue with increased glycolytic metabolism, recent strategies use agents capable of inducing tumor cell death by confounding metabolic demand. Such approaches include inhibition of glucose utilization with 2-deoxyglucose, an agent currently in clinical trials that is phosphorylated by hexokinase but which cannot be metabolized further and thus blocks glucose metabolism (Mohanti et al., 1996). Lonidamine, also in clinical trials, is a derivative of indazole-3-carboxylic acid that inhibits hexokinase (Brawer, 2005). Such agents exploit increased activity of the glucose transporter system in tumor cells to enter cells (Pelicano et al., 2006). Glufosfamide, a conjugate of D-glucose with a toxic mustard, is an alkylating agent that damages DNA and activates PARP, and is currently in clinical trials (Giaccone et al., 2004). Furthermore, an inhibitor SB-204990 that targets ATP citrate lyase, which links glucose metabolism to lipid synthesis, has shown efficacy in preclinical models (Hatzivassiliou et al., 2005). Many such agents may have additional activities, and ongoing efforts are likely to support the development of a panoply of more specific agents for clinical use.
Autophagy as a target
Now that it is becoming clear that tumor cells can use autophagy to survive metabolic stress, the development of autophagy inhibitors will be another approach to render cells susceptible to cell death (Fig. 3). Putative targets for development of inhibitors in the mammalian autophagy pathway include the protein kinase ATG1, which relays signals that activate autophagy, and the class III PI 3-kinase VPS34, which associates with ATG6/Beclin1 and is required for autophagosome formation (Klionsky, 2005). The protease ATG4, the E1-like enzyme ATG7, and the E2-like enzymes ATG3 and ATG10 are also possible targets for anti-autophagy drug development. Note that agents such as mTOR inhibitors that stimulate autophagy could promote autophagic cell death as well as inhibit growth. Note, however, that progressive autophagy may eventually lead to cell death but this may be more difficult to achieve therapeutically, and it is not yet clear whether specificity for tumor cells can be achieved. Inhibition of autophagy may thus be a more effective strategy.
Concluding remarks
Common mutations in human tumors render tumor cells unable to adapt to metabolic stress by constitutively activating cell growth while compromising the capacity to survive through autophagy. This phenotypic difference between normal and tumor cells can be exploited by use of therapies that inflict metabolic stress, to which many tumor cells are preferentially sensitive. Moreover, autophagy is a survival pathway that enables tumor cells to tolerate metabolic stress. Thus, therapeutic strategies that involve induction of metabolic catastrophe through metabolic stress while compromising the autophagy survival mechanism may be a unique, rational combinatorial approach for treating solid tumors. Importantly, metabolic catastrophe can promote death of tumor cells that have disabled apoptosis. Since it causes apoptosis-defective tumor cells to undergo necrotic cell death, which can lead to inflammation, we should now seek to further understand the process of necrosis and how to use it to favor tumor regression.
References
- Adams JM. Ways of dying: multiple pathways to apoptosis. Genes Dev. 2003;17:2481–2495. doi: 10.1101/gad.1126903. [DOI] [PubMed] [Google Scholar]
- Arico S, Petiot A, Bauvy C, Dubbelhuis PF, Meijer AJ, Codogno P, Ogier-Denis E. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2001;276:35243–35246. doi: 10.1074/jbc.C100319200. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Brawer MK. Lonidamine: basic science and rationale for treatment of prostatic proliferative disorders. Rev Urol. 2005;7:S21–S26. [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Chan S. Targeting the mammalian target of rapamycin (mTOR): a new approach to treating cancer. Br J Cancer. 2004;91:1420–1424. doi: 10.1038/sj.bjc.6602162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV, Lewis BC, Dolde C, Dang G, Shim H. Oncogenes in tumor metabolism, tumorigenesis, and apoptosis. J Bioenerg Biomembr. 1997;29:345–354. doi: 10.1023/a:1022446730452. [DOI] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Degenhardt K, White E. A mouse model system to genetically dissect the molecular mechanisms regulating tumorigenesis. Clin Cancer Res. 2006;12:5298–5304. doi: 10.1158/1078-0432.CCR-06-0439. [DOI] [PubMed] [Google Scholar]
- Degenhardt K, Chen G, Lindsten T, White E. BAX and BAK mediate p53-independent suppression of tumorigenesis. Cancer Cell. 2002;2:193–203. doi: 10.1016/s1535-6108(02)00126-5. [DOI] [PubMed] [Google Scholar]
- Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gelinas C, Fan Y, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004;15:177–182. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- Gelinas C, White E. BH3-only proteins in control: specificity regulates MCL-1 and BAK-mediated apoptosis. Genes Dev. 2005;19:1263–1268. doi: 10.1101/gad.1326205. [DOI] [PubMed] [Google Scholar]
- Giaccone G, Smit EF, de Jonge M, Dansin E, Briasoulis E, Ardizzoni A, Douillard JY, Spaeth D, Lacombe D, Baron B, et al. Glufosfamide administered by 1-hour infusion as a second-line treatment for advanced non-small cell lung cancer; a phase II trial of the EORTC-New Drug Development Group. Eur J Cancer. 2004;40:667–672. doi: 10.1016/j.ejca.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thompson CB. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Hemmings BA. Akt signaling: linking membrane events to life and death decisions. Science. 1997;275:628–630. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- Jin S, White E. Role of autophagy in cancer: management of metabolic stress. Autophagy. 2007;3:28–31. doi: 10.4161/auto.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone RW, Ruefli A, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ. The molecular machinery of autophagy: unanswered questions. J Cell Sci. 2005;118:7–18. doi: 10.1242/jcs.01620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Mohanti BK, Rath GK, Anantha N, Kannan V, Das BS, Chandramouli BA, Banerjee AK, Das S, Jena A, Ravichandran R, et al. Improving cancer radiotherapy with 2-deoxy-D-glucose: phase I/II clinical trials on human cerebral gliomas. Int J Radiat Oncol Biol Phys. 1996;35:103–111. doi: 10.1016/s0360-3016(96)85017-6. [DOI] [PubMed] [Google Scholar]
- Nelson DA, White E. Exploiting different ways to die. Genes Dev. 2004;18:1223–1226. doi: 10.1101/gad.1212404. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Tan TT, Rabson AB, Anderson D, Degenhardt K, White E. Hypoxia and defective apoptosis drive genomic instability and tumorigenesis. Genes Dev. 2004;18:2095–2107. doi: 10.1101/gad.1204904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- Rowinsky EK. Targeting the molecular target of rapamycin (mTOR) Curr Opin Oncol. 2004;16:564–575. doi: 10.1097/01.cco.0000143964.74936.d1. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Tan TT, Degenhardt K, Nelson DA, Beaudoin B, Nieves-Neira W, Bouillet P, Villunger A, Adams JM, White E. Key roles of BIM-driven apoptosis in epithelial tumors and rational chemotherapy. Cancer Cell. 2005;7:227–238. doi: 10.1016/j.ccr.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Thompson CB, Bauer DE, Lum JJ, Hatzivassiliou G, Zong WX, Zhao F, Ditsworth D, Buzzai M, Lindsten T. How do cancer cells acquire the fuel needed to support cell growth? Cold Spring Harb Symp Quant Biol. 2005;70:357–362. doi: 10.1101/sqb.2005.70.011. [DOI] [PubMed] [Google Scholar]
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- White E. Mechanisms of apoptosis regulation by viral oncogenes in infection and tumorigenesis. Cell Death Differ. 2006;13:1371–1377. doi: 10.1038/sj.cdd.4401941. [DOI] [PubMed] [Google Scholar]
- Wu H, Yang JM, Jin S, Zhang H, Hait WN. Elongation factor-2 kinase regulates autophagy in human glioblastoma cells. Cancer Res. 2006;66:3015–3023. doi: 10.1158/0008-5472.CAN-05-1554. [DOI] [PubMed] [Google Scholar]
- Zeh HJ, 3rd, Lotze MT. Addicted to death: invasive cancer and the immune response to unscheduled cell death. J Immunother. 2005;28:1–9. doi: 10.1097/00002371-200501000-00001. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Li H, Liu Q, Zhou L, Zhang M, Luo Q, Glickson J, Chance B, Zheng G. Metabolic imaging of tumors using intrinsic and extrinsic fluorescent markers. Biosens Bioelectron. 2004;20:643–650. doi: 10.1016/j.bios.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]
- Zong WX, Ditsworth D, Bauer DE, Wang ZQ, Thompson CB. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 2004;18:1272–1282. doi: 10.1101/gad.1199904. [DOI] [PMC free article] [PubMed] [Google Scholar]