Abstract
Epidemiological studies suggest that early life infections may contribute to the development of neuropsychiatric disorders later in life. Experimental studies employing infections during neonatal life support this notion by reporting persistent changes in the behaviour of adult animals, including deficits in sensorimotor gating. We have previously described an induction of the kynurenine pathway in neonatal wild-type (WT) mice following a systemic infection with neurotropic influenza A/WSN/33 virus. Here, we use the same model of infection in both WT and Tap1−/− mice (expressing reduced levels of MHC class I) and study long-term effects of the infection on sensorimotor gating, as determined by measuring prepulse inhibition (PPI). Moreover, transcription of genes encoding enzymes in the kynurenine pathway and levels of kynurenic acid (KYNA), in the brain of Tap1−/− mice were investigated. In mice infected on postnatal day (P)3 or P4, the levels of several transcripts in the kynurenine pathway were altered at P7, P13 and P24. Transcripts encoding indoleamine-pyrrole 2,3-dioxygenase (IDO), degrading tryptophan in the first step of the kynurenine pathway were consistently up-regulated at all time-points investigated. The changes in transcript levels were accompanied by a transient elevation of KYNA in the brain of infected mice at P13. At age 5–6 months, neonatally infected Tap1−/−, but not WT, mice exhibited a reduction in PPI. The present data show that a neonatal infection targeting the brain can induce the kynurenine pathway and that such an infection can disrupt sensorimotor gating in adulthood in genetically vulnerable mice.
Keywords: Innate immunity, kynurenic acid, MHC, neuropsychiatric disorders, prepulse inhibition
Introduction
Early life exposure to herpes simplex virus type 2, cytomegalovirus, rubella and influenza viruses, as well as to Toxoplasma gondii, has been associated with an increased risk of developing neuropsychiatric disorders, e.g. schizophrenia or autism (Karlsson, 2003; Libbey et al. 2005; Yolken & Torrey, 2008). Moreover, family studies suggest that these disorders share genetic risk factors (Daniels et al. 2008; Larsson et al. 2005; Lichtenstein et al. 2009). Although several susceptibility genes have been proposed, including genes involved in the immune response (Costa e Silva, 2008; Harrison & Weinberger, 2005; Schwab et al. 2003; Shirts et al. 2007), major risk alleles remain to be identified.
Experimental models support the plausibility that early life infections can give rise to persistent effects on behaviour. Neonatal infections in rodents are used to model human infections during the late 2nd/early 3rd trimester of gestation. Such models employing infections with herpes virus (Crnic & Pizer, 1988), Borna disease virus (Lancaster et al. 2007; Pletnikov et al. 1999; Rubin et al. 1999) or lymphocytic choriomeningitis virus (de la Torre et al. 1996; Gold et al. 1994; Hotchin & Seegal, 1977) have reported behavioural disturbances related to both cognitive and emotional domains, as well as disrupted sensorimotor gating (Engel et al. 2000; Pletnikov et al. 2002) in adult animals. Sensorimotor gating disruptions are considered to reflect deficits in the ability to filter out extraneous stimuli that would interfere with attention and information processing (McGhie & Chapman, 1961). Prepulse inhibition (PPI) of a startle response is a cross-species operational measure of sensorimotor gating that refers to the ability of a non-startling ‘prestimulus’ to inhibit the response to a startling stimulus (Hoffman & Ison, 1980; Swerdlow et al. 2001). There are numerous studies reporting PPI deficits in patients with schizophrenia and other neuropsychiatric disorders (Geyer, 2006; Perry et al. 2007). Although the mechanism underlying the behavioural changes following the different experimental neonatal infections remains elusive, convergence on common inflammatory pathways has been proposed (Meyer & Feldon, 2008). This notion is supported by reports of altered behaviour related to anxiety, cognition and sensorimotor gating in rodents following experimental immune challenges, e.g. by interleukin (IL)-6, IL-1α, polyI:C or lipopolysaccharide, during fetal or neonatal life (Borrell et al. 2002; Ozawa et al. 2006; Shi et al. 2003; Smith et al. 2007; Spencer et al. 2005; Tohmi et al. 2004; Wolff & Bilkey, 2008).
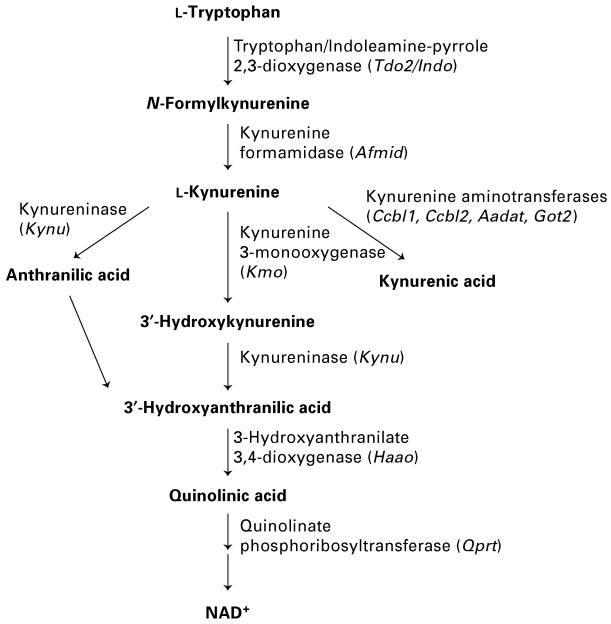
Tryptophan degradation along the kynurenine pathway (Fig. 1) is induced by a number of infections (Atlas et al. 2007; Eastman et al. 1994; Fujigaki et al. 2002; Heyes et al. 1992a, b; Reinhard, 1998; Schwarcz & Hunter, 2007; Silva et al. 2002) and can be considered part of the host's innate immune response (King & Thomas, 2007). Indoleamine-pyrrole 2,3-dioxygenase (IDO) is the first and rate-limiting enzyme of the kynurenine pathway induced by proinflammatory cytokines such as interferon (IFN)-γ and IFN-β (Guillemin et al. 2001a; Takikawa et al. 1988). Such induction appears to inhibit viral replication in vitro (Adams et al. 2004a, b; Bodaghi et al. 1999; Suh et al. 2007). We previously reported a transient induction of the kynurenine pathway in the brain of wild-type (WT) (C57BL/6) mice during the acute stages of a neonatal infection with influenza A/WSN/33 virus (Holtze et al. 2008). Using this model of neonatal infection in both WT and Tap1−/− mice, we recently reported deficits in working memory and increased anxiety in adult Tap1−/−, but not in adult WT mice (Asp et al. 2009). Due to a targeted disruption of the gene encoding ‘transporter associated with antigen processing 1’, Tap1−/− mice express reduced levels of MHC class I and therefore lack functional CD8+ T cells (Van Kaer et al. 1992). Compared to WT mice, the absence of invading CD8+ T cells did not affect virus replication, distribution or clearance in Tap1−/− mice. However, a more pronounced innate immune response was observed in Tap1−/− mice than in WT mice, as indicated by increased expression of glial markers in the brain parenchyma 21 d after infection only in Tap1−/− mice (Asp et al. 2009). Based on these latter findings we here hypothesize a more persistent induction of the kynurenine pathway in neonatally influenza A/WSN/33 virus-infected Tap1−/− mice, than previously reported in WT mice (Holtze et al. 2008). Furthermore, we investigate the potential effects of the neonatal infection on sensorimotor gating in Tap1−/− and WT mice at age 5–6 months.
Fig. 1.
Schematic diagram of the kynurenine pathway. Mouse gene symbols are given in parentheses.
Material and methods
Experimental design
In accordance with our previous studies (Asp et al. 2009; Holtze et al. 2008), virus was intraperitoneally administered, mimicking a haematogenous spread to a human fetus following maternal infection. Mice were injected with neurotropic mouse-adapted influenza A/WSN/33 virus or phosphate-buffered saline (PBS) on postnatal day (P)3 or P4, i.e. when pups measured 3.5 cm in head–rump length. With regard to brain development, this time-point corresponds to ∼25 wk of gestation in humans (i.e. late 2nd/early 3rd trimester; Rice & Barone, 2000). In this study, both WT and Tap1−/− mice were included and whole brains, including cerebellum, of the latter group were sampled 4 d (P7), 10 d (P13), and 21 d (P24) after injection in order to determine levels of transcripts encoding enzymes in the kynurenine pathway and kynurenic acid (KYNA). These time-points were selected based on our previous observations on the course of kynurenine pathway induction in WT mice (Holtze et al. 2008). At age 5–6 months, sensorimotor gating was determined in male WT and Tap1−/− mice by measuring PPI of the startle reflex. Mice were subsequently sacrificed and levels of KYNA were determined in their brains. All comparisons were made within each genotype group.
Animals
WT mice (C57BL/6) were obtained from Scanbur AB, Sweden, and mice with targeted disruption of the gene-encoding transporter associated with antigen processing 1 (Tap1−/−; background strain C57BL/6), were obtained from the Jackson Laboratory (USA). Mice of both genotypes were bred at the Department of Neuroscience, Karolinska Institutet, Stockholm, Sweden. Mice were kept under standard laboratory conditions with free access to food pellets and tap water. They were housed (2–5 per cage) in standard transparent type III Macrolon® cages (42 × 26 × 20 cm) in a light-controlled room (12-h light/dark cycle, lights on 06:00 hours) at 21 °C and 60% relative humidity. Experiments were conducted in accordance with permissions from the regional ethical committee for animal experimentation (N264/05, and supplements: N166/06, N101/07 and N225/07).
Influenza A/WSN/33 virus infection of mice
Mice were injected intraperitoneally with 2400 plaque-forming units of influenza A/WSN/33 virus obtained from Dr S. Nakajima (The Institute of Public Health, Tokyo, Japan) suspended in 30 μl PBS (Gibco, UK) or PBS alone, at P3 or P4 as previously described (Asp et al. 2009; Holtze et al. 2008).
Extraction of RNA and reverse transcription
Total RNA was extracted from whole brains using the RNeasy kit (Qiagen GmbH, Germany). One μg of RNA was subsequently DNaseI treated and reverse-transcribed into random hexamer-primed cDNA using reagents from Invitrogen (Carlsbad, USA), as previously described (Asp et al. 2007).
Real-time PCR and data analysis
Real-time PCR assays were performed as previously described (Asp et al. 2007) using reagents from Invitrogen and Applied Biosystems (Palo Alto, USA). Primers (Invitrogen) and probe (Applied Biosystems) sequences are provided in Table 1. Transcript levels were normalized to those encoding glyceraldehyde-3-phosphate dehydrogenase (GAPDH). From these values, relative levels of transcripts in the two groups were calculated according to the formula 2−ΔΔCt (Livak & Schmittgen, 2001).
Table 1.
Transcripts analysed by real-time PCR
| Gene symbol | Gene product | Accession no. (positive strand) | Primer sequence, 5′ to 3′ |
|---|---|---|---|
| Indo | Indoleamine-pyrrole 2,3-dioxygenase (IDO) | NM_008324 | CGGACTGAGAGGACACAGGTTAC ACACATACGCCATGGTGATGTAC |
| Tdo2 | Tryptophan 2,3-dioxygenase (TDO2) | NM_019911 | CAGCATCAGGCTTCCAGAGTCT AGGGACTCTCAAGCTCTGAAGAAC |
| Ccbl1 | Kynurenine aminotransferase 1 (KAT1) | NM_172404 | GGAGATGGACCCACTCAAGAAT GCCTGAAAGGCTGTGAACAAG |
| Aadat | Kynurenine aminotransferase 2 (KAT2) | NM_011834 | CCAGGAACCCTTTATGCTATGAA TGGAATAATCCCATGCTCATCA |
| Ccbl2 | Kynurenine aminotransferase 3 (KAT3) | NM_173763 | AACCCCGGCGACACCTA TGATCGTTCTCCTGGTTCCAA |
| Got2 | Mitochondrial aspartate aminotransferase (mitAAT) | NM_010325 | GATGGCCGAATCTCCGTG CCTGGTGAATGGCATGGG |
| Kmo | Kynurenine 3-monooxygenase (KMO) | NM_133809 | CCTGTAGAGGACAATATAGGATCAACAA GCAAGCCCCATCTACTGCAT |
| Kynu | Kynureninase (KYNU) | NM_027552 | GAGACTCGATCGCCGTGATC TGTTATGGCAGGAATGTTGAACA |
| Haao | 3-hydroxyanthranilate 3,4-dioxygenase (HAAO) |
NM_025325 | GGATGTCCTCTTCGAGAAATGG AACTCTTGGATGATGGGTGCTAA |
| Qprt | Quinolinate phosphoribosyl-transferase (QPRT) | NM_133686 | CACGCTCGCCGGTTCTA GCCCAACAAGCCGCAGTA |
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | NM_008084 | TGCACCACCAACTGCTTAGC CAGTCTTCTGAGTGGCAGTGATG Probe: TGGAAGGGCTCATGACCACAGTCCA |
Analyses of KYNA
Whole brain tissues were homogenized in 0.4 m perchloric acid, 0.1% sodium hydrogen sulfite, and 0.05% EDTA. KYNA levels were measured, using an isocratic reversed-phase high-performance liquid chromatography system connected to a fluorescence detector with an excitation wavelength of 344 nm and an emission wavelength of 398 nm, as previously described (Holtze et al. 2008).
Behavioural testing
Startle response and PPI testing were performed on WT and Tap1−/− mice at age 5–6 months in commercial startle chambers (SR-LAB System, San Diego Instruments, USA) at the Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden. Within each chamber there was a Plexiglas cylinder (3.7 cm diameter) into which the mouse was placed. Sudden movements by the mouse were detected by a piezoelectric accelerometer attached below the cylinder. A loudspeaker provided the broadband background noise and acoustic stimuli, and the whole apparatus was housed within the ventilated, sound-attenuating chamber (35 × 33 × 46 cm). A standard computer controlled presentations of acoustic stimuli.
The experimental session consisted of a 5-min acclimatization period to a 65-dB background noise (continuous throughout the session), followed by a variable stimulus intensity block (i.e. to measure startle threshold), a variable prepulse intensity block, and a variable interstimulus interval (ISI) block. This session was designed to fully characterize the startle and PPI phenotype associated with neonatal infection similar to what we have reported previously in isolation-reared mice (Varty et al. 2006). Throughout the session, hidden nostim trials (i.e. no acoustic stimulus) were presented in between each trial. During the variable stimulus intensity block, five trial types were presented: 40-ms startle pulses of 80, 90, 100, 110 and 120 dB. Each trial was presented four times. The varied prepulse intensity block consisted of four trial types: a 40-ms, 120-dB startle pulse (pulse alone); and three 20-ms prepulse + pulse combinations (69, 73 or 81 dB prepulses followed 100 ms later by a 120 dB stimulus; prepulse + pulse). There were 12 presentations of the pulse alone trial and 10 presentations of each prepulse + pulse combination. During the varied ISI block, six trial types were presented: a 40-ms, 120-dB startle pulse (pulse alone); and five 20-ms prepulse + pulse combinations (73-dB prepulses followed 25, 50, 100, 200, 500 ms later by a P120 stimulus; prepulse + pulse). There were eight presentations of the pulse alone trial, and four presentations of each prepulse + pulse trial. Throughout the session, trial types were presented in a pseudo-random order with an average inter-trial interval (ITI) of 15 s, not including the hidden nostim trials. In addition, five pulse alone trials were presented at the beginning (Block 1: to assess startle reactivity before appreciable habituation) and the end of the acoustic test session (Block 5). Mean startle magnitude for each trial type presentation, the dependent measure, was determined by averaging 65 1-ms readings taken from the onset of the startle pulse stimulus.
Statistical analysis
Relative transcript levels (i.e. ΔCt values) and levels of KYNA in Tap1−/− mice were compared between virus-infected and uninfected mice using the Mann–Whitney U test in GraphPad Prism (GraphPad Software Inc., USA). Startle and PPI data from Tap1−/− and WT mice were analysed separately. Startle reactivity data and mean startle magnitudes within the test session were analysed using one-factor ANOVAs with infection status as a between-subjects factor. Percent acoustic PPI from the ISI and prepulse intensity blocks of the test was calculated using the following formula:
Percent acoustic PPI data were analysed using two-factor ANOVAs with prepulse intensity, or ISI, as a within-subject factor, and infection status as the between-subject factor. Post-hoc comparisons of means were performed with Tukey's test. Significance was set at p < 0.05.
Results
Following systemic influenza A/WSN/33 virus infection in WT and Tap1−/− mice, the virus targeted neurons in several different brain regions. Virus replication, distribution or rates of clearance did not differ between the two genotypes. At 6–12 d post-infection a number of mice developed signs of disease, such as reduced weight gain, and were therefore sacrificed and excluded from the study according to our institutional guidelines. The survival rates of virus-infected WT and Tap1−/− mice did not differ (∼54%), as previously described (Asp et al. 2009; Holtze et al. 2008).
Gene expression in brains of Tap1−/− mice
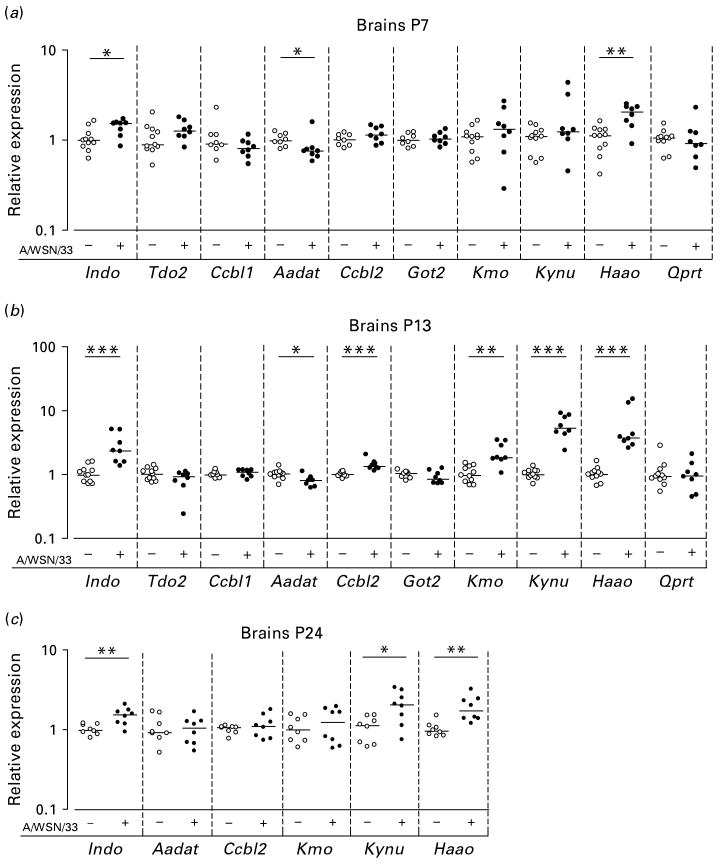
Levels of transcripts encoding enzymes in the kynurenine pathway were investigated during 3 wk after the infection at P3 or P4. At P7, brains from virus-infected mice expressed elevated levels of transcripts encoding IDO (p < 0.05) as well as those encoding 3-hydroxyanthranilate 3,4-dioxygenase (HAAO) (p < 0.01) compared to brains from uninfected mice. Transcripts encoding kynurenine aminotransferase (KAT)2 were detected at lower levels in the virus-infected brains compared to uninfected brains (p < 0.05). All other transcripts investigated were detected at similar levels in the two groups (Fig. 2a).
Fig. 2.
Levels of transcripts in brains of Tap1−/− mice. Symbols (○, ●) represent individual mice. The levels of transcripts from the genes Indo (encoding IDO), Tdo2 (encoding TDO), Ccbl1 (encoding KAT1), Aadat (encoding KAT2), Ccbl2 (encoding KAT3), Got2 (encoding mitAAT), Kmo (encoding KMO), Kynu (encoding KYNU), Haao (encoding HAAO), and Qprt (encoding QPRT) in brains at (a) postnatal day (P)7, (b) P13, and (c) P24 following intraperitoneal injection with 2400 plaque-forming units of influenza A/WSN/33 virus (+) or phosphate-buffered saline (−) on P3 or P4. The levels of transcripts in virus-infected brains are relative to those observed in uninfected brains. The horizontal lines indicate median values (n = 8–11). * p < 0.05, ** p < 0.01, *** p < 0.001. Mann–Whitney U test.
At P13, levels of transcripts encoding IDO (p < 0.001) and those encoding HAAO (p < 0.001) remained elevated in virus-infected brains. While levels of transcripts encoding KAT2 remained reduced in virus-infected brains (p < 0.05), those encoding KAT3 (p < 0.001), kynurenine 3-monooxygenase (KMO) (p < 0.01) or kynureninase (KYNU) (p < 0.001) were now detected at increased levels in virus-infected brains compared to uninfected brains. All other transcripts investigated were detected at similar levels in the two groups (Fig. 2b).
Transcripts found to be differentially expressed at P13 were subsequently analysed at P24. At this time-point, the levels of transcript encoding IDO (p < 0.01), KYNU (p < 0.05) or HAAO (p < 0.01) all remained elevated in the virus-infected brains. Other transcripts analysed were detected at similar levels in the two groups of mice at this time-point (Fig. 2c).
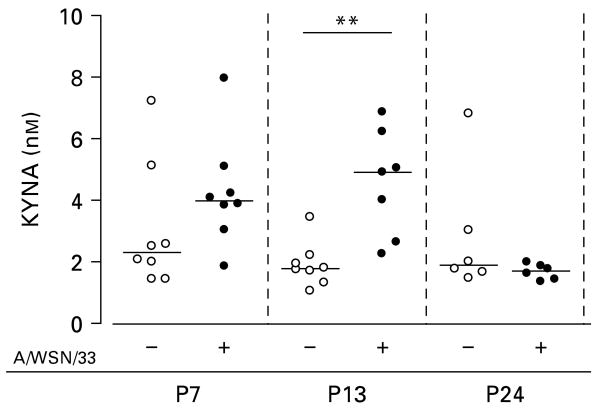
KYNA in brains of Tap1−/− mice at P7, P13 and P24
In parallel to the gene expression analyses, we determined levels of KYNA; an end product of the kynurenine pathway (Stone, 1993) in the brains of Tap1−/− mice. No significant differences in the levels of KYNA were observed between virus-infected and uninfected mice at P7 or P24. However, at P13 levels of KYNA in virus-infected mice (4.6, range 2.3–6.9 nm) exceeded the levels observed in uninfected mice (1.9, range 1.1–3.5 nm, p < 0.01; Fig. 3).
Fig. 3.
Levels of kynurenic acid (KYNA) in brains of Tap1−/− mice at postnatal days (P)7, P13, P24 following intraperitoneal injection with 2400 plaque-forming units of influenza A/WSN/33 virus (+) or phosphate-buffered saline (−) on P3 or P4. Symbols (○, ●) represent individual mice. The horizontal lines indicate median values (n = 6–8). ** p < 0.01. Mann–Whitney U test.
Effects of neonatal influenza virus infection on PPI
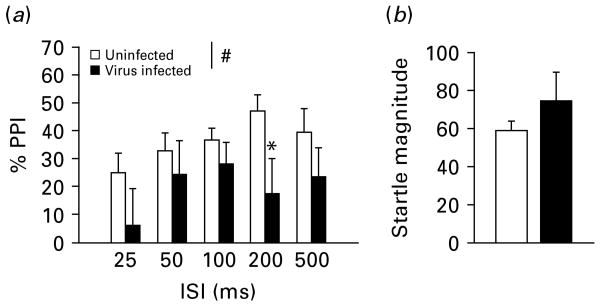
At age 5–6 months, measurements of PPI were performed on WT and Tap1−/− mice following injection with PBS or influenza A/WSN/33 virus at P3 or P4. In the varied ISI block of the session, virus-infected Tap1−/− mice displayed impaired PPI as evidenced by a main effect of virus infection [F(1, 26) = 5.67, p < 0.05; Fig. 4a] and no interaction between virus infection and ISI. The post-hoc test revealed a decrease in PPI in virus-infected mice at the 200-ms ISI (p < 0.05). Startle magnitude in the P120 trials in the varied ISI blocks did not differ between the groups [F(1, 26) < 1, n.s.; Fig. 4b]. There was no effect of virus infection [F(1, 26) < 1, n.s.] or any interaction between prepulse intensity and virus infection in the varied prepulse intensity block. In the startle threshold block, designed to measure any differences in startle threshold between uninfected and virus-infected Tap1−/− mice, there was a main effect of stimulus intensity [F(4, 104) = 38.1, p < 0.001] and a trend for higher startle in virus-infected compared to uninfected mice [values are mean (s.e.m.)] [uninfected: 42.3 (3.0); virus-infected: 58.8 (10.4); F(1, 26) = 3.81, p = 0.062]. However, there was no interaction between acoustic stimulus and virus infection suggesting that the groups did not differ in their threshold for startle response.
Fig. 4.
(a) Prepulse inhibition (PPI) and (b) startle magnitude in uninfected (n = 16) and influenza A/WSN/33 virus-infected (n = 12) Tap1−/− mice at age 5–6 months, during the varied interstimulus interval (ISI) block of the startle session. # p < 0.05, main effect of virus infection on PPI during the varied ISI block (details of statistics in Results section). * p < 0.05, statistically different from respective uninfected mice, Tukey's post-hoc comparison. Data are presented as mean + s.e.m.
In contrast to Tap1−/− mice, neonatally infected WT mice did not differ from uninfected mice in PPI in the varied prepulse intensity block (F < 1, n.s.) or in the varied ISI block (F < 1, n.s.) when tested in adulthood. WT uninfected and virus-infected mice also did not differ in startle magnitude in the startle threshold block (F < 1, n.s.).
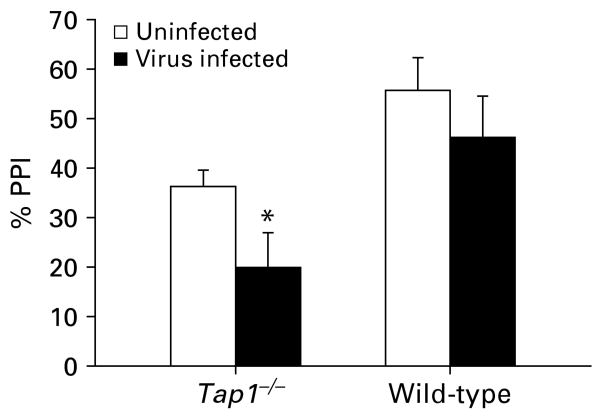
In order to evaluate the effect of the influenza virus infection on PPI in the two genotypes, we examined the average PPI during the ISI block in the two experiments. In the ISI block, virus-infected WT mice had slightly less average PPI than uninfected WT mice [values are mean (s.e.m.)] [uninfected: 55.6 (6.6); virus-infected: 46.3 (8.1); 19% reduction], but this decrease was much less than that observed in the Tap1−/− mice [uninfected: 36.2 (3.3); virus-infected: 19.9 (7.0); 45% reduction]. A comparison of the effect of the neonatal virus infection on PPI across the two experiments yielded a greater effect size (Cohen, 1988) in Tap1−/− mice (d = 1.23) than in WT mice (d = 0.61; Fig. 5).
Fig. 5.
Comparison of the effects of neonatal influenza in Tap1−/− mice (left) and wild-type (WT) mice (right) on average PPI during the ISI block. Data represent the mean (+s.e.m.). * p < 0.05, significantly different from respective control. Data were compared across experiments by computing the effect size (Cohen's d): Tap1−/− mice, d = 1.23; WT mice, d = 0.61. For details of statistics see Results section.
KYNA in brains of adult mice
After completion of PPI, mice were sacrificed and the brains sampled. In these brains, KYNA was detected at similar levels in virus-infected and uninfected mice of both genotypes (data not shown).
Discussion
In the present study, we report that a neonatal infection with a neurotropic strain of influenza A virus reduced PPI in adult Tap1−/− mice. Such reduction in PPI was not observed in adult WT mice following neonatal infection. With regard to changes in gene expression and KYNA levels in brains of virus-infected Tap1−/− mice during the early stages of the infection, the present findings are in general agreement with our previously reported findings in WT mice (Holtze et al. 2008). However, a notable finding was the consistent elevation of transcripts encoding IDO throughout the time-points investigated.
The changes in gene expression were accompanied by a transient increase in the levels of KYNA in the brain parenchyma at P13. In agreement with a previous study, reporting a close relationship between transcripts encoding IDO and enzyme activity (Fujigaki et al. 2002), our findings support functionality of the induction of the kynurenine pathway observed at the gene expression level. In accordance, levels of transcript encoding IDO have been correlated with the production of quinolinic acid (QUIN; Smith et al. 2001), another metabolite of tryptophan degradation (Stone, 1993). Thus, levels of QUIN and other neuroactive (Stone, 1993) and/or immunomodulatory (Moffett & Namboodiri, 2003) metabolites of the kynurenine pathway can also be altered in the brain parenchyma of infected Tap1−/− mice, although measurements of these metabolites were not performed in the present study.
In humans and rats, KAT2 is considered to play a major role in KYNA formation in the brain (Guidetti et al. 2007). However, mice lacking KAT2, exhibit reduced cerebral KYNA levels only during the first weeks of life (Yu et al. 2004). It has therefore been proposed that mitAAT accounts for most of the KYNA formation in the adult mouse brain (Guidetti et al. 2007). During P7–P24, levels of transcripts encoding mitAAT were not altered in virus-infected mice. In contrast, decreased levels of transcripts encoding KAT2 (P7 and P13), and increased levels of transcripts encoding KAT3 (P13) were observed. Thus, the present and previous findings (Holtze et al. 2008) suggest that, in addition to the availability of l-kynurenine, transcriptional regulation of genes encoding KAT activity might modulate KYNA formation during an infection.
In our previous study of infected WT mice, no evidence of an activated kynurenine pathway was observed at P24 (Holtze et al. 2008). The increased levels of transcripts encoding IDO, KYNU, and HAAO observed in the present study at this time-point, suggest a more persistent induction of the kynurenine pathway in the brains of Tap1−/− mice. Although the cellular origins of these transcripts were not further investigated, IDO, KYNU, and HAAO can all be expressed by resident cells in the brain parenchyma, as well as by invading cells (Alberati-Giani et al. 1996; Guillemin et al. 2001b, 2003, 2007; Holtze et al. 2008). These observations thus provide further support for a more pronounced innate immune response in the brains of Tap1−/− mice than in WT mice following a neonatal virus infection (Asp et al. 2009). This difference in innate immune response between the two genotypes may be an effect of the absence of functional CD8+ T cells in Tap1−/− mice. These and CD4+ T cells were recently reported to be critically important in down-regulating the systemic innate immune response in young mice (Zhao et al. 2008).
The kynurenine pathway has previously been implicated in PPI. For example, acute pharmacological elevation of the N-methyl d-aspartate (NMDA) receptor antagonist KYNA in the brain disrupts PPI, and is suggested to be an endogenous modulator of sensorimotor gating (Erhardt et al. 2004). However, it should be noted that levels of KYNA were not elevated in the brain of infected Tap1−/− mice at the time of PPI testing in the present study. The persistent effects of other neonatally administered NMDA receptor antagonists, e.g. phencyclidine or MK-801, on sensorimotor gating in rats (Harris et al. 2003; Wang et al. 2001) indicate that the transient elevation of KYNA contributed to the deficits in PPI observed in adulthood. The transient increase in KYNA concentration in Tap1−/− mice was similar to that reported previously in WT mice (Holtze et al. 2008). However, it is possible that Tap1−/− mice are more vulnerable than WT mice to perturbed synaptic functions due to their reduced expression of MHC class I molecules, which are suggested to be of importance for synaptic plasticity and regeneration (Goddard et al. 2007; Oliveira et al. 2004).
In conclusion, the present results show that a neonatal CNS infection can cause long-term deficits in sensorimotor gating in genetically vulnerable individuals. Although innate immunity appears to be an important mediator of these effects, a specific role for the kynurenine pathway in the establishment of PPI deficits remains to be determined.
Acknowledgments
The present study was supported by the Stanley Medical Research Institute, the Swedish Research Council, Hållstens Forskningsstiftelse, the Swedish Brain Foundation, Torsten och Ragnar Söderbergs Stiftelse, Karolinska Institutets stiftelse för virusforskning, Stiftelsen Sigurd och Elsa Goljes Minne, National Institute of Health grant MH52885, and Jeansons stiftelse. We thank Victoria Risbrough for helpful discussions on the manuscript.
Footnotes
Statement of Interest: None.
References
- Adams O, Besken K, Oberdorfer C, MacKenzie CR, et al. Inhibition of human herpes simplex virus type 2 by interferon gamma and tumor necrosis factor alpha is mediated by indoleamine 2,3-dioxygenase. Microbes and Infection. 2004a;6:806–812. doi: 10.1016/j.micinf.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Adams O, Besken K, Oberdorfer C, MacKenzie CR, et al. Role of indoleamine-2,3-dioxygenase in alpha/beta and gamma interferon-mediated antiviral effects against herpes simplex virus infections. Journal of Virology. 2004b;78:2632–2636. doi: 10.1128/JVI.78.5.2632-2636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberati-Giani D, Ricciardi-Castagnoli P, Kohler C, Cesura AM. Regulation of the kynurenine metabolic pathway by interferon-gamma in murine cloned macrophages and microglial cells. Journal of Neurochemistry. 1996;66:996–1004. doi: 10.1046/j.1471-4159.1996.66030996.x. [DOI] [PubMed] [Google Scholar]
- Asp L, Beraki S, Kristensson K, Ove Ogren S, et al. Neonatal infection with neurotropic influenza A virus affects working memory and expression of type III Nrg1 in adult mice. Brain, Behavior, and Immunity. 2009 doi: 10.1016/j.bbi.2009.04.004. Published online : 10 April 2009. [DOI] [PubMed] [Google Scholar]
- Asp L, Nellaker C, Karlsson H. Influenza A virus transactivates the mouse envelope gene encoding syncytin B and its regulator, glial cells missing 1. Journal of Neurovirology. 2007;13:29–37. doi: 10.1080/13550280601103125. [DOI] [PubMed] [Google Scholar]
- Atlas A, Gisslen M, Nordin C, Lindstrom L, et al. Acute psychotic symptoms in HIV-1 infected patients are associated with increased levels of kynurenic acid in cerebrospinal fluid. Brain, Behavior, and Immunity. 2007;21:86–91. doi: 10.1016/j.bbi.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Bodaghi B, Goureau O, Zipeto D, Laurent L, et al. Role of IFN-gamma-induced indoleamine 2,3 dioxygenase and inducible nitric oxide synthase in the replication of human cytomegalovirus in retinal pigment epithelial cells. Journal of Immunology. 1999;162:957–964. [PubMed] [Google Scholar]
- Borrell J, Vela JM, Arevalo-Martin A, Molina-Holgado E, et al. Prenatal immune challenge disrupts sensorimotor gating in adult rats. Implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology. 2002;26:204–215. doi: 10.1016/S0893-133X(01)00360-8. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. New Jersey: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Costa e Silva JA. Autism, a brain developmental disorder: some new pathopysiologic and genetics findings. Metabolism Clinical and Experimental. 2008;57(Suppl. 2):40–43. doi: 10.1016/j.metabol.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Crnic LS, Pizer LI. Behavioral effects of neonatal herpes simplex type 1 infection of mice. Neurotoxicology and Teratology. 1988;10:381–386. doi: 10.1016/0892-0362(88)90042-6. [DOI] [PubMed] [Google Scholar]
- Daniels JL, Forssen U, Hultman CM, Cnattingius S, et al. Parental psychiatric disorders associated with autism spectrum disorders in the offspring. Pediatrics. 2008;121:1357–1362. doi: 10.1542/peds.2007-2296. [DOI] [PubMed] [Google Scholar]
- de la Torre JC, Mallory M, Brot M, Gold L, et al. Viral persistence in neurons alters synaptic plasticity and cognitive functions without destruction of brain cells. Virology. 1996;220:508–515. doi: 10.1006/viro.1996.0340. [DOI] [PubMed] [Google Scholar]
- Eastman CL, Urbanska E, Love A, Kristensson K, et al. Increased brain quinolinic acid production in mice infected with a hamster neurotropic measles virus. Experimental Neurology. 1994;125:119–124. doi: 10.1006/exnr.1994.1015. [DOI] [PubMed] [Google Scholar]
- Engel JA, Zhang J, Bergstrom T, Conradi N, et al. Neonatal herpes simplex virus type 1 brain infection affects the development of sensorimotor gating in rats. Brain Research. 2000;863:233–240. doi: 10.1016/s0006-8993(00)02149-1. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Schwieler L, Emanuelsson C, Geyer M. Endogenous kynurenic acid disrupts prepulse inhibition. Biological Psychiatry. 2004;56:255–260. doi: 10.1016/j.biopsych.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Fujigaki S, Saito K, Takemura M, Maekawa N, et al. L-tryptophan-L-kynurenine pathway metabolism accelerated by Toxoplasma gondii infection is abolished in gamma interferon-gene-deficient mice: cross-regulation between inducible nitric oxide synthase and indoleamine-2,3-dioxygenase. Infection and Immunity. 2002;70:779–786. doi: 10.1128/iai.70.2.779-786.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA. The family of sensorimotor gating disorders: comorbidities or diagnostic overlaps? Neurotoxicity Research. 2006;10:211–220. doi: 10.1007/BF03033358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard CA, Butts DA, Shatz CJ. Regulation of CNS synapses by neuronal MHC class I. Proceedings of the National Academy of Sciences USA. 2007;104:6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LH, Brot MD, Polis I, Schroeder R, et al. Behavioral effects of persistent lymphocytic choriomeningitis virus infection in mice. Behavioral and Neural Biology. 1994;62:100–109. doi: 10.1016/s0163-1047(05)80031-7. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Amori L, Sapko MT, Okuno E, et al. Mitochondrial aspartate aminotransferase: a third kynurenate-producing enzyme in the mammalian brain. Journal of Neurochemistry. 2007;102:103–111. doi: 10.1111/j.1471-4159.2007.04556.x. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Cullen KM, Lim CK, Smythe GA, et al. Characterization of the kynurenine pathway in human neurons. Journal of Neuroscience. 2007;27:12884–12892. doi: 10.1523/JNEUROSCI.4101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin GJ, Kerr SJ, Pemberton LA, Smith DG, et al. IFN-beta1b induces kynurenine pathway metabolism in human macrophages: potential implications for multiple sclerosis treatment. Journal of Interferon and Cytokine Research. 2001a;21:1097–1101. doi: 10.1089/107999001317205231. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Kerr SJ, Smythe GA, Smith DG, et al. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. Journal of Neurochemistry. 2001b;78:842–853. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Smith DG, Smythe GA, Armati PJ, et al. Expression of the kynurenine pathway enzymes in human microglia and macrophages. Advances in Experimental Medicine and Biology. 2003;527:105–112. doi: 10.1007/978-1-4615-0135-0_12. [DOI] [PubMed] [Google Scholar]
- Harris LW, Sharp T, Gartlon J, Jones DN, et al. Long-term behavioural, molecular and morphological effects of neonatal NMDA receptor antagonism. European Journal of Neuroscience. 2003;18:1706–1710. doi: 10.1046/j.1460-9568.2003.02902.x. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Molecular Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Saito K, Crowley JS, Davis LE, et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. 1992a;115:1249–1273. doi: 10.1093/brain/115.5.1249. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Jordan EK, Lee K, Saito K, et al. Relationship of neurologic status in macaques infected with the simian immunodeficiency virus to cerebrospinal fluid quinolinic acid and kynurenic acid. Brain Research. 1992b;570:237–250. doi: 10.1016/0006-8993(92)90587-y. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychological Review. 1980;87:175–189. [PubMed] [Google Scholar]
- Holtze M, Asp L, Schwieler L, Engberg G, et al. Induction of the kynurenine pathway by neurotropic influenza A virus infection. Journal of Neuroscience Research. 2008;86:3674–3683. doi: 10.1002/jnr.21799. [DOI] [PubMed] [Google Scholar]
- Hotchin J, Seegal R. Virus-induced behavioral alteration of mice. Science. 1977;196:671–674. doi: 10.1126/science.854742. [DOI] [PubMed] [Google Scholar]
- Karlsson H. Viruses and schizophrenia, connection or coincidence. Neuroreport. 2003;14:535–542. doi: 10.1097/00001756-200303240-00001. [DOI] [PubMed] [Google Scholar]
- King NJ, Thomas SR. Molecules in focus: indoleamine 2,3-dioxygenase. International Journal of Biochemistry and Cell Biology. 2007;39:2167–2172. doi: 10.1016/j.biocel.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Lancaster K, Dietz DM, Moran TH, Pletnikov MV. Abnormal social behaviors in young and adult rats neonatally infected with Borna disease virus. Behavioural Brain Research. 2007;176:141–148. doi: 10.1016/j.bbr.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Larsson HJ, Eaton WW, Madsen KM, Vestergaard M, et al. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. American Journal of Epidemiology. 2005;161:916–925. doi: 10.1093/aje/kwi123. discussion 926–918. [DOI] [PubMed] [Google Scholar]
- Libbey JE, Sweeten TL, McMahon WM, Fujinami RS. Autistic disorder and viral infections. Journal of Neurovirology. 2005;11:1–10. doi: 10.1080/13550280590900553. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. British Journal of Medical Psychology. 1961;34:103–116. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J. Neural basis of psychosis-related behaviour in the infection model of schizophrenia. Behavioural Brain Research. 2008 doi: 10.1016/j.bbr.2008.12.022. Published online : 30 December 2008. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunology and Cell Biology. 2003;81:247–265. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x. [DOI] [PubMed] [Google Scholar]
- Oliveira AL, Thams S, Lidman O, Piehl F, et al. A role for MHC class I molecules in synaptic plasticity and regeneration of neurons after axotomy. Proceedings of the National Academy of Sciences USA. 2004;101:17843–17848. doi: 10.1073/pnas.0408154101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, et al. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biological Psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Lopez B, Maron L, et al. Sensorimotor gating deficits in adults with autism. Biological Psychiatry. 2007;61:482–486. doi: 10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pletnikov MV, Rubin SA, Schwartz GJ, Moran TH, et al. Persistent neonatal Borna disease virus (BDV) infection of the brain causes chronic emotional abnormalities in adult rats. Physiology and Behavior. 1999;66:823–831. doi: 10.1016/s0031-9384(99)00021-9. [DOI] [PubMed] [Google Scholar]
- Pletnikov MV, Rubin SA, Vogel MW, Moran TH, et al. Effects of genetic background on neonatal Borna disease virus infection-induced neurodevelopmental damage. I. Brain pathology and behavioral deficits. Brain Research. 2002;944:97–107. doi: 10.1016/s0006-8993(02)02723-3. [DOI] [PubMed] [Google Scholar]
- Reinhard JF., Jr Altered tryptophan metabolism in mice with herpes simplex virus encephalitis: increases in spinal cord quinolinic acid. Neurochemical Research. 1998;23:661–665. doi: 10.1023/a:1022438822023. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environmental Health Perspectives. 2000;108(Suppl. 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin SA, Sylves P, Vogel M, Pletnikov M, et al. Borna disease virus-induced hippocampal dentate gyrus damage is associated with spatial learning and memory deficits. Brain Research Bulletin. 1999;48:23–30. doi: 10.1016/s0361-9230(98)00133-6. [DOI] [PubMed] [Google Scholar]
- Schwab SG, Mondabon S, Knapp M, Albus M, et al. Association of tumor necrosis factor alpha gene -G308A polymorphism with schizophrenia. Schizophrenia Research. 2003;65:19–25. doi: 10.1016/s0920-9964(02)00534-0. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Hunter CA. Toxoplasma gondii and schizophrenia: linkage through astrocyte-derived kynurenic acid? Schizophrenia Bulletin. 2007;33:652–653. doi: 10.1093/schbul/sbm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. Journal of Neuroscience. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirts BH, Wood J, Yolken RH, Nimgaonkar VL. Comprehensive evaluation of positional candidates in the IL-18 pathway reveals suggestive associations with schizophrenia and herpes virus seropositivity. American Journal of Medical Genetics. Part B: Neuropsychiatric Genetics. 2007;147B:343–350. doi: 10.1002/ajmg.b.30603. [DOI] [PubMed] [Google Scholar]
- Silva NM, Rodrigues CV, Santoro MM, Reis LF, et al. Expression of indoleamine 2,3-dioxygenase, tryptophan degradation, and kynurenine formation during in vivo infection with Toxoplasma gondii: induction by endogenous gamma interferon and requirement of interferon regulatory factor 1. Infection and Immunity. 2002;70:859–868. doi: 10.1128/iai.70.2.859-868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DG, Guillemin GJ, Pemberton L, Kerr S, et al. Quinolinic acid is produced by macrophages stimulated by platelet activating factor, Nef and Tat. Journal of Neurovirology. 2001;7:56–60. doi: 10.1080/135502801300069692. [DOI] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, et al. Maternal immune activation alters fetal brain development through interleukin-6. Journal of Neuroscience. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Heida JG, Pittman QJ. Early life immune challenge – effects on behavioural indices of adult rat fear and anxiety. Behavioural Brain Research. 2005;164:231–238. doi: 10.1016/j.bbr.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacological Reviews. 1993;45:309–379. [PubMed] [Google Scholar]
- Suh HS, Zhao ML, Rivieccio M, Choi S, et al. Astrocyte indoleamine 2,3-dioxygenase is induced by the TLR3 ligand poly(I:C): mechanism of induction and role in antiviral response. Journal of Virology. 2007;81:9838–9850. doi: 10.1128/JVI.00792-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berlin) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Takikawa O, Kuroiwa T, Yamazaki F, Kido R. Mechanism of interferon-gamma action. Characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. Journal of Biological Chemistry. 1988;263:2041–2048. [PubMed] [Google Scholar]
- Tohmi M, Tsuda N, Watanabe Y, Kakita A, et al. Perinatal inflammatory cytokine challenge results in distinct neurobehavioral alterations in rats: implication in psychiatric disorders of developmental origin. Neuroscience Research. 2004;50:67–75. doi: 10.1016/j.neures.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Van Kaer L, Ashton-Rickardt PG, Ploegh HL, Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4-8+ T cells. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- Varty GB, Powell SB, Lehmann-Masten V, Buell MR, et al. Isolation rearing of mice induces deficits in prepulse inhibition of the startle response. Behavioural Brain Research. 2006;169:162–167. doi: 10.1016/j.bbr.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Wang C, McInnis J, Ross-Sanchez M, Shinnick-Gallagher P, et al. Long-term behavioral and neurodegenerative effects of perinatal phencyclidine administration: implications for schizophrenia. Neuroscience. 2001;107:535–550. doi: 10.1016/s0306-4522(01)00384-0. [DOI] [PubMed] [Google Scholar]
- Wolff AR, Bilkey DK. Immune activation during mid-gestation disrupts sensorimotor gating in rat offspring. Behavioural Brain Research. 2008;190:156–159. doi: 10.1016/j.bbr.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Torrey EF. Are some cases of psychosis caused by microbial agents? A review of the evidence. Molecular Psychiatry. 2008;13:470–479. doi: 10.1038/mp.2008.5. [DOI] [PubMed] [Google Scholar]
- Yu P, Di Prospero NA, Sapko MT, Cai T, et al. Biochemical and phenotypic abnormalities in kynurenine aminotransferase II-deficient mice. Molecular and Cellular Biology. 2004;24:6919–6930. doi: 10.1128/MCB.24.16.6919-6930.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Kim KD, Yang X, Auh S, et al. Hyper innate responses in neonates lead to increased morbidity and mortality after infection. Proceedings of the National Academy of Sciences USA. 2008;105:7528–7533. doi: 10.1073/pnas.0800152105. [DOI] [PMC free article] [PubMed] [Google Scholar]