Abstract
Influenza virus, a negative stranded RNA virus causing severe illness in humans and animals, stimulates the inflammasome through the NOD-like receptor (NLR), NLRP3. However, the mechanism by which influenza virus activates the NLRP3 inflammasome is unknown. Here, we show that the influenza virus M2 protein, a proton-selective ion channel important in viral pathogenesis, stimulates the NLRP3 inflammasome pathway. M2 channel activity was required for influenza activation of inflammasomes, and was sufficient to activate inflammasomes in primed macrophages and dendritic cells. M2-induced inflammasome activation required its localization to Golgi and was dependent on pH gradient. Our results reveal a mechanism by which influenza virus infection activates inflammasomes, and identifies the sensing of disturbances in intracellular ionic concentrations as a novel pathogen recognition pathway.
Influenza virus is responsible for annual epidemics that cause severe illness in ~5 million people worldwide. Recent evidence has unveiled that influenza infection engages the NLRP3 inflammasome complex in dendritic cells and macrophages1–4. NLRP3 forms a multi-protein complex with ASC (also known as Pycard) and caspase-1, leading to the catalytic cleavage of the pro-forms of interleukin 1β (IL-1β), IL-18, and IL-33. Inflammasome activation requires two signals5: signal 1 is induced by Toll-like receptor (TLR) stimulation, leading to the synthesis of pro-forms of IL-1β, IL-18 and IL-33; signal 2, triggered by agents that can cause ionic perturbations, specifically potassium efflux, induces activation of caspase-1 and cleavage of pro-forms of IL-1β, IL-18, and IL-33. Well known examples of signal 2 include pore-forming microbial toxins, maitotoxin, aerolysin, and nigericin, which activate NLRP3 inflammasomes by allowing efflux of potassium from the cytosol5–7. In addition, lysosomal membrane damage caused by phagocytosis of crystals such as asbestos, silica, and aluminum salt (alum) triggers NLRP3 activation8, 9.
The mechanism by which virus infection results in NLRP3 inflammasome activation is unclear. Infection with both DNA and RNA viruses results in NLRP3-dependent inflammasome activation1–4, 10, 11, and recent studies have identified AIM2 as a sensor for dsDNA that is capable of stimulating inflammasomes12–15. AIM2 consists of a HIN200 domain that binds to DNA, and the pyrin domain, which associates with the adaptor molecule, ASC to activate both NF-κB and caspase-1. However, AIM2-induced activation is NLRP3 independent12, 13. In contrast to dsDNA, the mechanism by which influenza virus, a negative stranded ssRNA virus, triggers NLRP3 inflammasome activation is unknown. Here, we examine the cellular mechanism by which influenza virus infection elicits NLRP3 inflammasome.
RESULTS
Influenza virus subtypes induce potent inflammasome activation
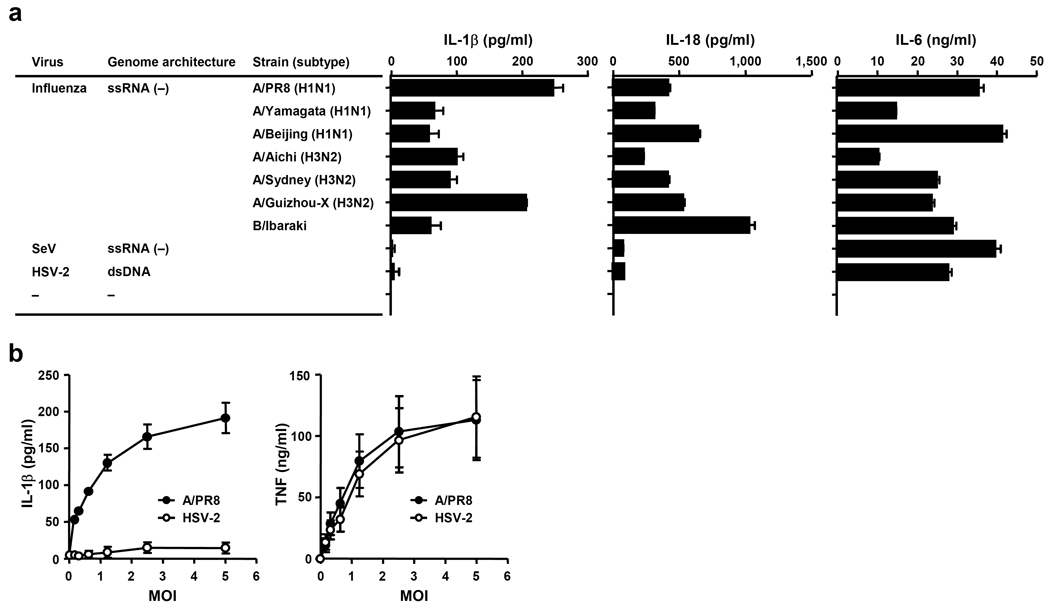
To determine whether inflammasome activation is generally induced by virus infection, we compared the ability of several viruses to trigger inflammasome activation by measuring IL-1β secretion from infected bone marrow-derived macrophages (BMM) (Fig. 1a). All influenza virus strains tested, including influenza A and B types, induced robust IL-1β release from BMM (Fig. 1a). Influenza-induced IL-1β was also NLRP3-, ASC-and caspase-1-dependent (Supplementary Figure 1a & b), as demonstrated previously1–4. In addition, IL-1β secretion by influenza infection (Supplementary Figure 1a & b), but not dsDNA (Supplementary Figure 1c), was NLRP3 dependent. In contrast, at the same MOI, two other ssRNA viruses, Sendai virus (SeV; paramyxovirus), and vesicular stomatitis virus (rhabdovirus, data not shown) or the dsDNA virus, herpes simplex virus type 2 (HSV-2), activated inflammasomes at much lower amounts, despite robust stimulation of inflammasome-independent cytokines, such as IL-6 (Fig. 1a) and TNF (Fig. 1b). These data indicated that influenza virus infection alone is capable of activating both signals 1 and 2 in unprimed BMM or dendritic cells (DCs). In addition, such findings suggest that inflammasome activation is mediated by a specific feature associated with influenza virus infection that is not common to other ssRNA viruses.
Figure 1.
Influenza viruses are specifically capable of inducing inflammasome activation. (a) Wild-type BMM were infected with A/PR8, A/Yamagata, A/Beijing, A/Aichi, A/Sydney, A/Guizhou-X, B/Ibaraki, SeV, or HSV-2 at MOI of 2.5. Supernatants were collected 24 h after infection and analyzed for IL-1β, IL-18, and IL-6 by ELISA. (b) Wild-type BMM were infected with A/PR8 (closed circle) or HSV-2 (open circle) at the indicated MOIs. Supernatants were collected 24 h after infection and analyzed for IL-1β and TNF-α by ELISA. Data represent the mean ± S.D. Similar results were obtained from three separate experiments.
Influenza virus activates pro-IL-1β synthesis via TLR7
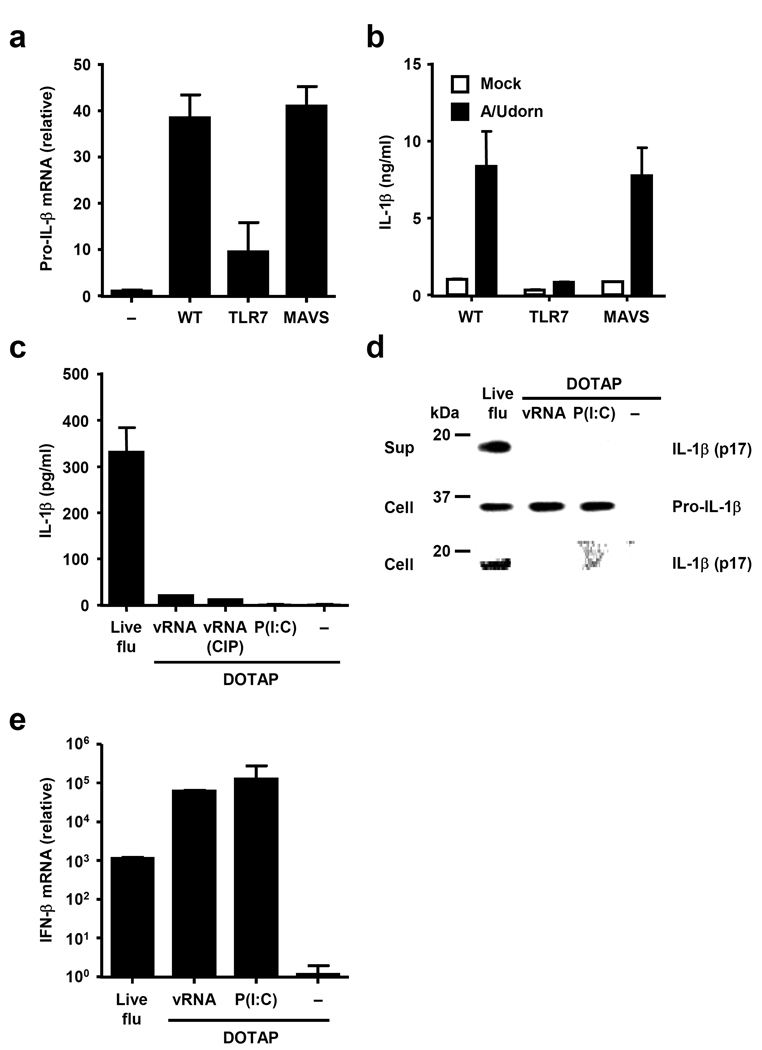
To determine the nature of signal 1 induced by influenza infection, we examined the two known innate recognition pathways for influenza virus. Influenza genomic RNA is recognized in the endosome by TLR7 (refs. 16, 17), whereas RIG-I recognizes the 5’ triphosophate end of viral RNA in the cytosol18, 19. Analysis of IL-1β release from BM DCs lacking TLR7 or MAVS (adaptor of RIG-I signaling) revealed that TLR7, but not RIG-I, signaling is required for the transcription of pro-IL-1β (Fig. 2a) and release of mature IL-1β following influenza infection (Fig. 2b). Next, we tested whether influenza virus RNA alone might be uniquely capable of stimulating both signals 1 and 2 upon infection. We compared the ability of influenza genomic RNA and, as a control, synthetic dsRNA (Poly I:C) to stimulate IL-1β release. Unlike live infection, influenza virus RNA complexed with liposomes, with or without the 5- triphosphate (CIP, calf intestinal phosphatase-treated viral RNA), while inducing robust type I IFN expression (Fig. 2e), failed to stimulate IL-1β secretion from BMM (Fig. 2c). In addition, Poly I:C also failed to elicit inflammasome activation in the absence of ATP, as previously reported11. The failure of the transfected RNA to induce IL-1β secretion (Fig. 2c) was not due to their inability to induce pro-IL-1β protein synthesis, but due to the lack of IL-1β processing by caspase-1 in the cytosol (Fig. 2d). These data indicate that viral RNA alone is insufficient to trigger robust NLRP3 inflammasome activation, and that signal 2 is likely derived from a virally-encoded gene(s).
Figure 2.
Influenza virus activates signal 1 through TLR7. (a, b) BMM prepared from WT, TLR7−/−, or MAVS−/− mice were infected with A/Udorn virus. Expression levels of pro-IL-1β were assessed by RT quantitative PCR 24 h after infection (a). Supernatants were collected 24 h after stimulation and analyzed for IL-1β by ELISA (b). (c–e) BMM prepared from WT mice were infected with A/PR8 virus or transfected with CIP-treated, or non-treated A/PR8 genomic RNA or poly (I:C) with DOTAP. Supernatants were collected 24 h after stimulation and analyzed for IL-1β by ELISA (c). Supernatants were analyzed for the presence of mature IL-1β and cell extracts, for the presence of mature IL-β and pro-IL-1β by Western blotting (d). (e) Six hours after infection, total RNA was extracted from virus-infected and RNA-transfected BMM. IFN-β mRNA levels were assessed by RT quantitative PCR. GAPDH was used as an internal control. Data represent the mean ± S.D. Similar results were obtained from three separate experiments.
M2 channel activity induces inflammasome activation
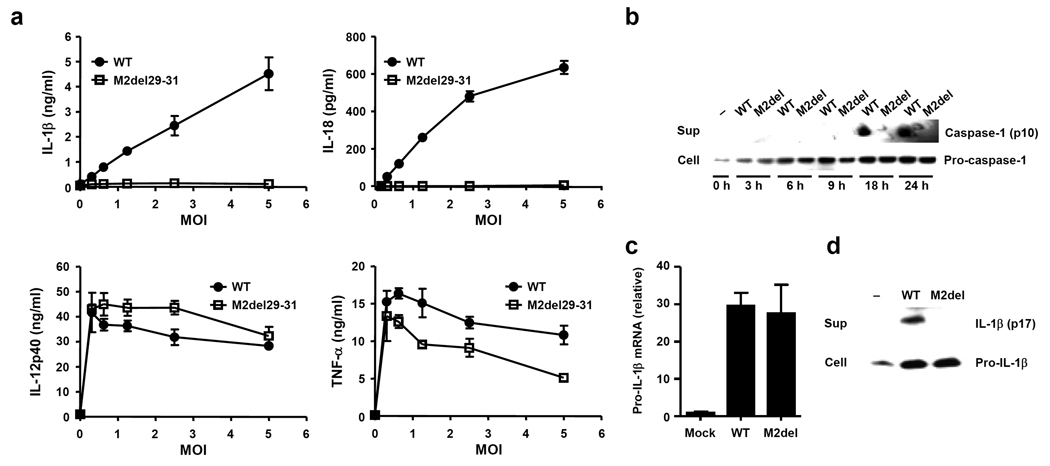
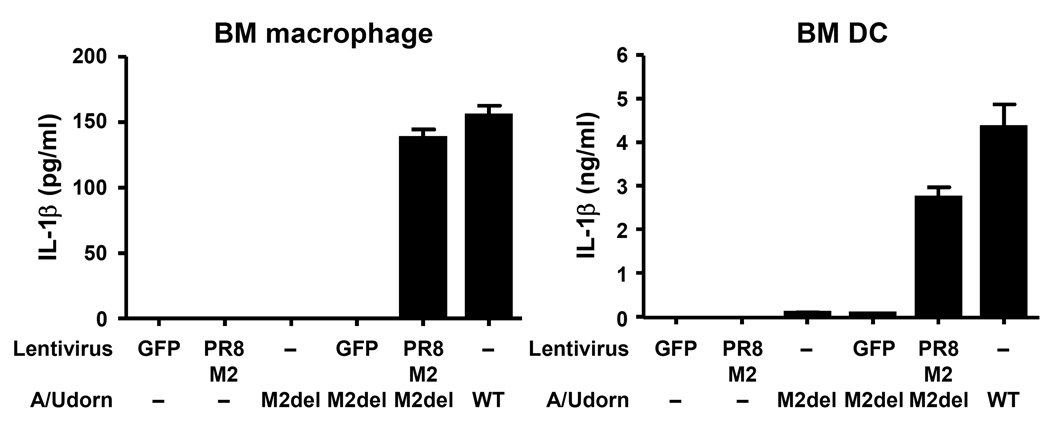
To investigate how influenza virus infection stimulates signal 2 for inflammasome activation, we focused on a feature shared by other known NLRP3 agonists – the ability to perturb cellular membranes. Influenza virus encodes a proton-specific ion channel, M2, which plays a key role in both fusion during viral entry and synthesis of new virions20. M2 acts in at least two subcellular locations. Within the acidifying endosomes containing the invading virions, M2 serves to import H+ ions into the virions and help release viral ribonucleocapsid into the cytosol21. Within the acidic trans-Golgi network (TGN), M2 plays a key role in transporting H+ ions out of the lumen, leading to neutralization of TGN pH and the prevention of hemagglutinin from becoming fusogenic22. Because M2 can alter ionic concentrations within intracellular compartments, we hypothesized that it may serve as signal 2 during inflammasome triggering. To test whether M2 ion channel activity is required to elicit influenza-induced inflammasome stimulation, BMM and BM DCs were infected with influenza virus lacking amino acids 29–31 from the transmembrane region of the M2 protein21. These amino acids are required for M2 to transport H+ (ref. 23). However, such deletion still allows the mutant M2 to be expressed in the cell23. Strikingly, the M2del29-31 mutant influenza virus completely failed to stimulate inflammasome activation and release of IL-1β or IL-18 from BMM (Supplementary Figure 2) and BM DCs (Fig. 3a). In addition, mature caspase-1 and IL-1β were only detected in the supernatants of cells infected with wild-type influenza, but not M2del29-31 mutant virus (Fig. 3b,d). The M2del29-31 mutant has either similar24 or reduced21 replicative capacity in vitro. A trivial explanation is that M2del29-31 failed to infect the target cells. However, three sets of evidence ruled out this possibility. First, the extent of infection by the M2del29-31 mutant virus was comparable to wild-type virus (Supplementary Figure 3a). Second, M2del29-31 mutant virus-infected cells expressed comparable amounts of pro-IL-1β mRNA (Fig. 3c) and protein (Fig. 3d). Third, the M2del29-31 mutant induced non-inflammasome-dependent cytokines such as TNF and IL-12 from infected BM DCs at amounts comparable to wild-type virus (Fig. 3a). To provide definitive evidence that the loss of the M2 ion channel activity is responsible for the failure of M2del29-31 virus to activate inflammasomes, we performed a complementation experiment. Intact M2 channel expressed from a lentivirus was able to completely rescue the ability of M2del29-31 virus to trigger inflammasomes (Fig. 4). In addition, the influenza A M2 channel blocker Amantadine inhibited influenza A-, but not influenza B-, induced IL-1β release from BMM (Supplementary Figure 3 b – e). Finally, UV-irradiated virus, which is capable of attachment, fusion and entry with intact M2 channel, but lack the ability to produce M2 de novo, failed to trigger IL-1β secretion1. These data collectively indicated that channel activity of de novo synthesized M2 of influenza virus is required for stimulation of NLRP3 inflammasomes.
Figure 3.
M2 channel activity of influenza virus is required for inflammasome activation. Cells were infected with WT (A/Udorn) or M2del29-31 (A/Udorn) (a–d) virus. (a) Supernatants from BM DCs infected at indicated MOIs were collected 24 h after infection and analyzed for IL-1β, IL-18, IL-12p40, and TNF-α by ELISA. (b) Supernatants and cell extracts from infected BMM were collected at the indicated time points and analyzed for the presence of pro- and mature-caspase-1 by Western blotting. (c) Pro-IL-1β mRNA levels from infected BMM were assessed by RT quantitative PCR. (d) Supernatants and cell extracts from infected BMM were collected 24 h after infection and the presence of pro- and mature IL-1β were analyzed by Western blotting. Data represent the mean ± S.D, and are representative of at least three independent experiments.
Figure 4.
Ectopic expression of M2 channel rescues IL-1β production from M2del29-31 virus infected cells. BMM and BM DC transduced with A/PR8 M2- or GFP-expressing lentivirus were infected with WT or M2del29-31 A/Udorn virus. Supernatants were collected 24 h after infection and analyzed for IL-1β by ELISA. Data represent the mean ± S.D, and are representative of at least three independent experiments.
M2 is sufficient to trigger inflammasome activation
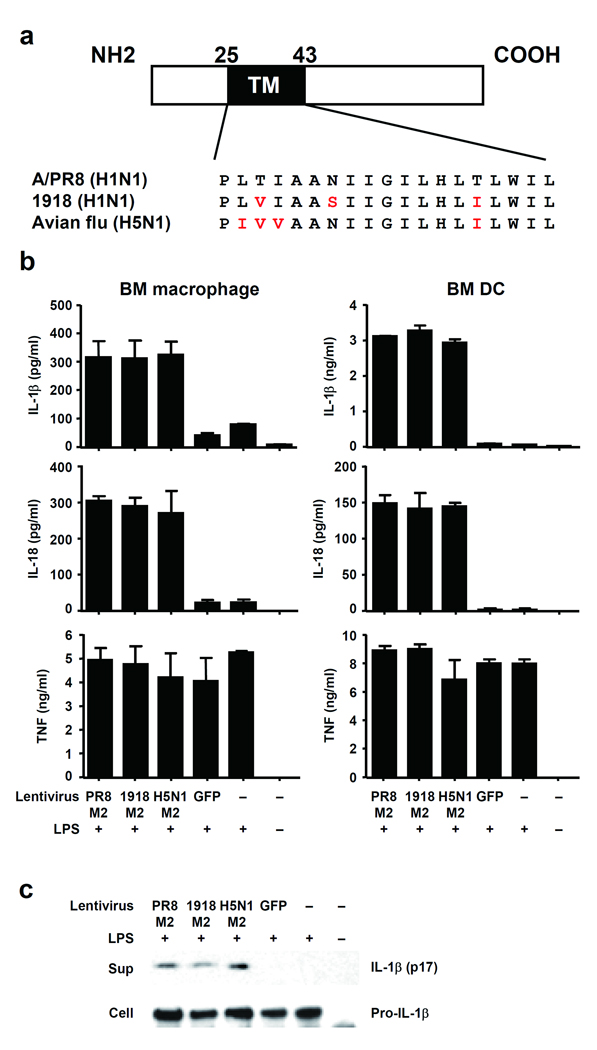
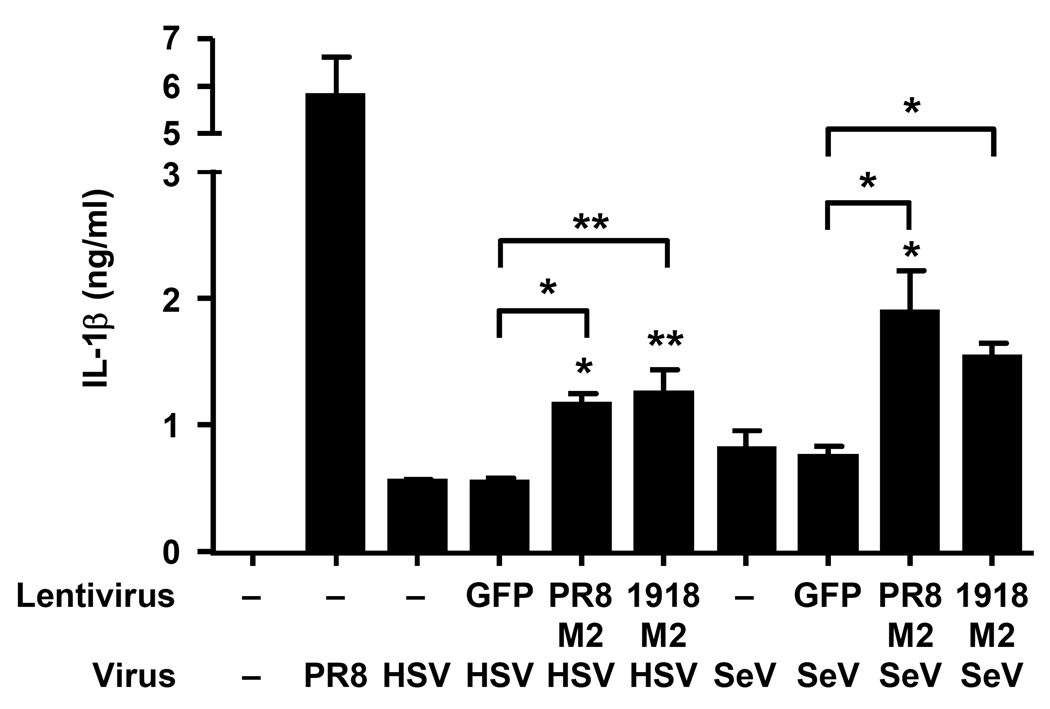
Next, we assessed whether M2 is sufficient to trigger the NLRP3 inflammasome. BMM and BM DCs stimulated with LPS (signal 1) were transduced with a lentivirus expressing the M2 protein derived from A/PR/8/34 (H1N1). Both IL-1β and IL-18 were specifically released from LPS-primed cells transduced with M2-expressing but not control (GFP) lentivirus constructs (Fig. 5b–c). In unprimed BMM, M2 expression alone was not sufficient to trigger IL-1β release (Supplementary Figure 4a–c). In addition, we compared inflammasome stimulation following ectopic expression of lentiviral-driven M2 from highly pathogenic influenza virus strains, A/Viet Nam/1194/2004 (H5N1), and 1918 Spanish flu A/Brevig Mission/1/1918 (H1N1) (Fig. 5a), in order to decipher whether the inflammation induced by these subtypes25 could be explained in part by the ability of the respective M2 to trigger inflammasome activation. M2 from all three viral strains elicited comparable inflammasome activation (Fig. 5b–c). In contrast, TNF and pro-IL-1β were produced in cells under all conditions in which LPS was present, regardless of M2 expression (Fig. 5b–c), indicating a specific effect of M2 on inflammasome activation. Next, we asked if M2 could stimulate inflammasomes in the presence of other viral triggers of signal 1. Similar to BMM ( Fig. 1a), SeV and HSV-2 induced marginal amounts of inflammasome activation in BM DCs (Fig. 6). However, M2 expression significantly potentiated NLRP3-dependent inflammasome activation in SeV-, or HSV-2-infected cells (Fig. 6 and Supplementary Figure 4d,e). These data provide clear evidence that M2 is sufficient to trigger signal 2 for NLRP3 inflammasome activation, and indicate that it is the expression of M2 that is responsible for inflammasome activation by influenza but not by other viruses tested (Fig. 1).
Figure 5.
M2 is sufficient to trigger signal 2 for inflammasome activation. (a) Schematic diagram of influenza virus M2 protein. The amino acid sequence of the transmembrane domain (residues 25 to 43) is shown in the expanded section of the diagram. (b) BMM or BM DC were infected with M2- or GFP-expressing lentivirus in the presence or absence of LPS (50 ng/ml). Supernatants were collected at 24 h post infection and analyzed for IL-1β, IL-18, and TNF. (c) BMM were infected with M2- or GFP-expressing lentiviruses in the presence or absence of LPS (50 ng/ml), and analyzed for the presence of pro- or mature IL-1β by immunoblotting. Data represents the mean ± S.D., and are representative of at least three independent experiments.
Figure 6.
Influenza virus M2 protein stimulates IL-1β production from HSV-2 or SeV infected cells. BM DCs were infected with influenza, HSV-2 or SeV in combination with M2- or GFP-lentiviruses. A/PR8 virus was used as a positive control. Supernatants were collected at 24 h post infection and analyzed for IL-1β. Data represents the mean ± S.D., and are representative of at least three independent experiments. *, p < 0.05; **, p < 0.01 compared to non-transduced cells or as indicated, as determined by ANOVA.
M2-mediated perturbation of ionic concentrations
We next investigated the mechanism by which M2 triggers NLRP3 inflammasomes. We first tested the role of previously identified factors that potentiate NLRP3 inflammasome activation26. We found that treatment of cells with high extracellular concentration of KCl, despite having little effect on M2 expression (Supplementary Figure 5c) or on viral replication (Supplementary Figure 5j), prevented M2-dependent IL-1β production (Supplementary Figure 5a & b). In addition, inhibition of IL-1β production by KCl was not due to general toxicity, as demonstrated by normal secretion of IL-1β following dsDNA-dependent inflammasome activation (Supplementary Figure 5d). We also found partial requirements for P2X7 receptor for influenza- (Supplementary Figure 5e) but not dsDNA- (Supplementary Figure 5f) induced inflammasome. ATP (Supplementary Figure 5g & h) and reactive oxygen species (Supplementary Figure 5i) also appeared to play a role in influenza and M2-induced inflammasome activation. However, only picomolar concentrations of ATP were secreted from BM DCs infected with wild-type or M2del29-31 influenza (Supplementary Figure 5h), which alone is not sufficient to trigger K+ efflux27 and NLRP3 inflammasomes.
Next, we examined whether the proton specificity of the M2 channel is required for NLRP3 activation. To this end, cells were transduced with a lentivirus encoding M2 proteins that carry mutation at position 37 (His37Gly). The proton selectivity of M2 is lost when transmembrane domain His37 is mutated, enabling other cations (Na+, K+) to be transported28. The His37Gly mutant M2 induced almost a two-fold increase in secretion of IL-1β from LPS- or poly (I:C)-activated BMM and BM DCs compared to wild-type M2 (Supplementary Figure 4, 6), indicating that, in addition to H+, disturbance in the concentrations of other cationic species act as triggers for inflammasome activation. Similarly, lentiviral complementation of M2His37Gly in BMM or BM DCs infected with the Mdel29–31 influenza virus resulted in a two-fold increase in inflammasome activation compared to wild-type M2 (Supplementary Figure 6). Therefore, these data indicated that many of the pathways common to other NLRP3 stimuli are required for M2 to elicit inflammasomes. Although proton selectivity of the channel is not required, the direct mechanism by which the M2 ion channel induces NRLP3 inflammasome activation still remained unclear.
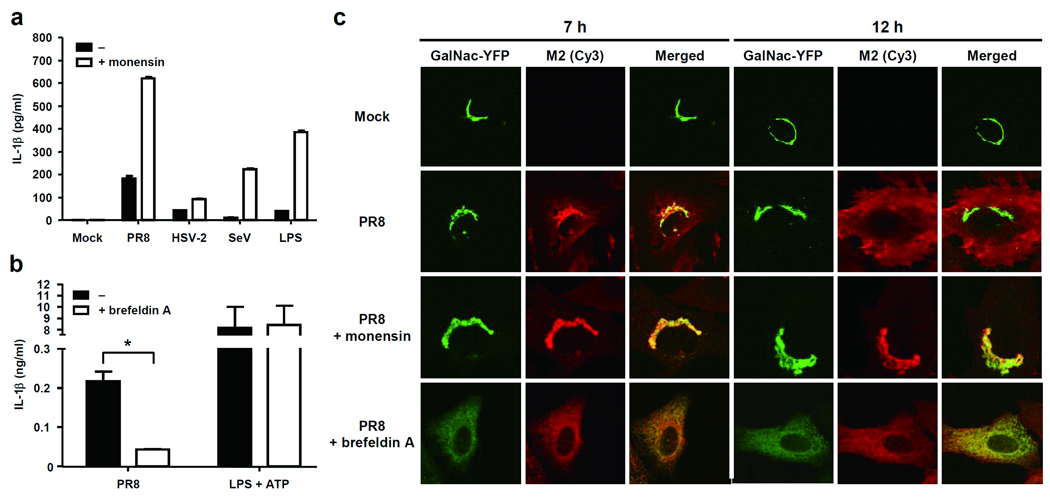
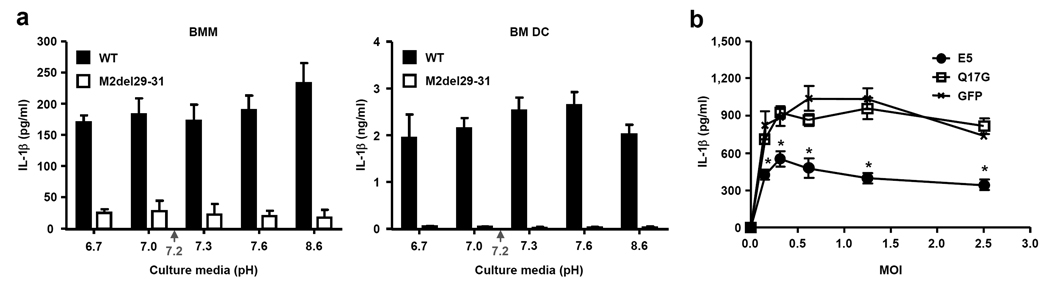
To address the intracellular mechanism by which M2 triggers inflammasome activation, we undertook three separate approaches. First, since M2 is a pH-gated H+ channel that neutralizes the pH of TGN22, we tested whether we can mimic the activity of M2 using monensin - a Na+/H+ antiporter that exports H+ from the TGN29. Monensin-treatment enabled inflammasome activation in LPS-treated, SeV-, or HSV-2-infected BMM (Fig. 7a). In addition, influenza-induced IL-1β release was dramatically enhanced by monensin treatment. Mature IL-1β undergoes a non-canonical protein export independent of ER and Golgi6. Because monensin effectively blocks transport of proteins through the classical secretory pathway, its ability to enhance IL-1β secretion could be in some way related to its ability to alter trafficking and secretion of proteins. Thus, we tested the ability of Brefeldin A, another inhibitor of classical secretory pathway, in IL-1β secretion upon influenza infection. In stark contrast to monensin, treatment of cells with Brefeldin A, which causes the collapse of the Golgi into the ER and the accumulation of proteins in the ER30, blocked inflammasome activation by influenza virus, but not LPS + ATP (Fig. 7b). Intracellular localization analysis of M2 revealed that Monensin treatment indeed restricted M2 to almost exclusively within the Golgi compartment, while Brefeldin A treatment resulted in the collapse of Golgi to the ER (Fig. 7c)30 and distribution of M2 in the ER (Supplementary Fig. 7). In untreated cells, M2 trafficked from mostly Golgi (7 h post infection) to the plasma membrane within 12 h of infection (Fig. 7c and Supplementary Figure 8). Cells treated with or without Monensin or Brefeldin A expressed similar amounts of M2 protein intracellularly (Supplementary Figure 8). Thus, these data indicated that M2 localization to the Golgi, but not ER, correlates with inflammasome activation, and indicated that a general blockade of protein transport in the classical secretory pathway does not account for the ability of monensin to elicit inflammasome activation. Second, since M2 is localized to both Golgi and the plasma membrane, it remained unclear which of these locations are important to activate the inflammasomes. Thus, to examine whether plasma membrane-localized M2 is capable of activating NLRP3 inflammasome, we took advantage of the fact that M2 localizes to the plasma membrane in influenza-infected cells after 12 h of infection (Fig. 7c and Supplementary Figure 8). The M2 channel conduct protons only when the pH of the medium bathing the N-terminal ectodomain, pHout is lowered below pH 7 (ref. 20). We utilized this feature of the M2 channel to probe the site of M2 activity required for inflammasome activation. Thus, we reasoned that if M2 at the plasma membrane activates inflammasomes, incubating cells in pH below 7 would elicit robust inflammasome activity by facilitating H+ transport into the cytosol. Conversely, extracellular pH above 7 should block the ion channel activity and thus prevent inflammasome activation. However, in both BMM and in DCs, alteration of extracellular pH to either lower or higher amounts had absolutely no effect on the inflammasome activation (Fig. 8a). These data indicated that plasma membrane M2 does not mediate inflammasome activation. Finally, to examine whether M2-induced activation of inflammasome requires acidified pH in Golgi, we utilized the E5 molecule from bovine papillomavirus (BPV), which specifically neutralizes TGN without affecting other intracellular compartments by binding to and inhibiting vacuolar H+ ATPase31. Cells were first transduced with retrovirus encoding BPV E5 protein or its inactive mutant (E5 Q17G) incapable of alkalinizing Golgi, and subsequently infected with different doses of influenza virus. These analyses revealed that only the WT, but not mutant, E5 blocked the activity of influenza-induced inflammasomes (Fig. 8b) without affecting the secretion of IL-6 and TNF, or inflammasome activation by LPS + ATP (Supplementary Fig. 9). Thus, these data indicated that acidification of Golgi is a prerequisite for influenza-induced inflammasome activation. Taken together, our data revealed that M2 channel-induced inflammasome activation correlates with its Golgi but not ER localization, is independent of its plasma membrane localization, and requires acidified Golgi compartment (Supplementary Figure 10).
Figure 7.
M2 triggers inflammasomes through perturbation of ionic homeostasis of the Golgi. (a) BMM were stimulated with A/PR8, HSV-2, SeV, or LPS and cultured in the presence or absence of Monensin (10 µM). (b) BMM were stimulated with A/PR8 virus or LPS + ATP in the presence or absence of Brefeldin A (10µg/ml). Supernatants were collected at 24 h (A/PR8) or 6 h (LPS + ATP) post stimulation and analyzed for IL-1β by ELISA. (c) BSC-1 cells that stably produce the resident Golgi enzyme N-acetylgalactosaminyltransferase II fused to yellow fluorescent protein (GalNAc-T2-YFP) (green) were infected with A/PR8 virus in the presence or absence of Monensin or Brefeldin A. Cells were stained with M2-specific antibody (red) and analyzed by confocal microscopy.
Figure 8.
M2 channel-induced inflammasome activation requires acidified Golgi compartment. (a) BMM and BM DC were infected with wild-type or M2del29-31 A/Udorn virus. Six hours later, culture media were replaced with media with the indicated pH levels. Green arrows indicate cytosolic pH level of 7.2. (b) RAW264.7 cells transduced with E5 WT, E5 Q17G, or GFP-expressing retroviruses were infected with A/PR8 at the indicated MOIs. Supernatants were analyzed for IL-1β by ELISA 24 h after infection. Data represents the mean ± S.D. Similar results were obtained from at least three separate experiments.
DISCUSSION
Our findings reveal a novel mechanism by which inflammasomes are triggered through detection of activity of a virally encoded ion channel. Specifically, influenza infection activates signal 1 through stimulation of macrophages and DCs via TLR7, leading to synthesis of pro-forms of IL-1β and IL-18. Upon infection, virally encoded M2 is expressed in the secretory compartment including TGN. Ion channel activity of M2 enables H+ export from acidified Golgi, and such activity is a trigger for signal 2 required for the formation of NLRP3 inflammasome complex. We also showed that M2-His37Gly mutant, which is capable of transporting Na+ and K+ (ref. 28), can induce elevated amounts of inflammasome activation. These data indicated that in addition to H+, imbalances in the concentrations of other cations can signal the activation of inflammasomes. The enhanced capacity of the M2-His37Gly channel to elicit inflammasome activation may be related to its potential capacity to export K+ in exchange for Na+ at the plasma membrane, as K+ efflux is a well-known activator of NLRP3 inflammasomes7, 32. Based on the requirement for K+ efflux and partial requirements for ATP, P2X7 and ROS in influenza-induced inflammasome activation, we speculate that the dysregulated ionic concentrations in the Golgi in some way lead to the activation of plasma membrane channels, resulting in K+ efflux and activation of the NLRP3 inflammasome complex. In addition, it is also possible that ATP released from influenza-infected dying cells33 amplify the inflammasome activation.
Our data demonstrated that the viral RNA is insufficient to trigger robust NLRP3 inflammasome activation. Thus, neither transfection of viral RNA or Poly (I:C) into BM DCs, nor treatment of BM DCs with UV-irradiated influenza virus1 elicited significant IL-1β secretion. Instead, viral infection and activity of the M2 channel was required to trigger full NLRP3 inflammasome activation. While some IL-1β is released by RNA agonists alone3, 4, our direct comparison revealed that the amounts secreted by cells infected with influenza virus are considerably higher. These data are consistent with a previous report showing that poly (I:C) or infection with the RNA viruses (reovirus and vesicular stomatitis virus) failed to elicit inflammasome activation11. Our data also demonstrated that TLR7, but not MAVS, is required for transcriptional activation of IL-1β. However, a recent report indicates that cytosolic RNA recognition by RIG-I can stimulate both signal 1 (via MAVS, CARD9) and signal 2 (via ASC independent of MAVS, CARD9 and NLRP3) (Poeck et al Nat. Immunol. 11, 63 – 69 (2009)). Therefore, cells infected with RNA viruses could utilize distinct molecular complexes, NLRP3-ASC and RIG-I-ASC, to trigger inflammasome activation.
In the infected cells, M2 is expressed in the ER, Golgi and then at the plasma membrane in a sequential manner. We provide several lines of evidence that suggest that the relevant location from which M2 triggers inflammasome activation is likely not the plasma membrane or the ER, as acidification of extracellular space or retention of M2 in ER by Brefeldin A was not associated with inflammasome activation. Instead, acidification of Golgi was a prerequisite, as evidenced by the ability of BHV E5 protein to block the activity of influenza-induced inflammasomes. Limited by lack of technology, we were unable to directly address whether ionic imbalance within the Golgi compartment was sufficient to trigger M2-mediated inflammasome activation. However, these data collectively suggested that sensing of cellular stress imposed by imbalances in ionic concentrations in intracellular vesicles could serve as a pathogen recognition pathway.
Our data also unveil that pathogen-encoded ion channels, in addition to more drastic disruption of membranes by pore-forming toxins or membrane rupture, signals inflammasome activation. Since ion channels are utilized by other viruses, notably, by HIV, which encodes the protein Vpu whose transmembrane domain acts as potassium channel34 to enhance viral particle release35, it is tempting to speculate that ion channel activity of multiple viral and bacterial pathogens might be sensed by the eukaryotic cells to trigger the NLR inflammasomes. Such a model represents a previously unappreciated mechanism by which the innate immune system senses critical pathogen-catalyzed cellular stress pathways to facilitate viral clearance, with important implications for both viral evolution and host-pathogen interactions. Future studies to understand the relevance of other microbial ion channels in the activation of innate receptors will aid in our ability to design effective interventions and treatments for the prevention of infectious diseases.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/natureimmunology/.
Supplementary Material
ACKNOWLEDGMENTS
We thank Michael Caplan, Robert Lamb, Craig Roy, Jonathan Kagan, Igor Brodsky, Wai Kee Eddie Ip, Heung Kyu Lee, Yusuke Yanagi, Satoshi Ikegame, and Daniel DiMaio for expert advice and/or reagents, and Ruslan Medzhitov and Joseph. M. Thompson for critical reading of the manuscript. This work is supported by NIH grants to A. I. (AI062428, AI064705, AI083242). T. I. is a Japan Society for the Promotion of Science for Postdoctoral Fellow for Research Abroad. A. I. is a recipient of the Burroughs Wellcome Investigators in Pathogenesis of Infectious Disease.
Footnotes
AUTHOR CONTRIBUTIONS
T.I. and A.I. designed the experiments and prepared the manuscript; T.I. and I.P. performed experiments; and T.I., I.P. and A.I. analyzed data.
References
- 1.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. The Journal of experimental medicine. 2009 doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanneganti TD, et al. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 3.Allen IC, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas PG, et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: first line of the immune response to cell stress. Cell. 2006;126:659–662. doi: 10.1016/j.cell.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Yu HB, Finlay BB. The caspase-1 inflammasome: a pilot of innate immune responses. Cell host & microbe. 2008;4:198–208. doi: 10.1016/j.chom.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Dostert C, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science (New York, N.Y.) 2008;320:674. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nature immunology. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston JB, et al. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity. 2005;23:587–598. doi: 10.1016/j.immuni.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Muruve DA, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009 doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009 doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burckstummer T, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nature immunology. 2009 doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 15.Roberts TL, et al. HIN-200 Proteins Regulate Caspase Activation in Response to Foreign Cytoplasmic DNA. Science %R 10.1126/science.1169841. 2009 doi: 10.1126/science.1169841. 1169841. [DOI] [PubMed] [Google Scholar]
- 16.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e, Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 17.Lund JM, et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hornung V, et al. 5'-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 19.Pichlmair A, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5,Ä≤-phosphates. Science. 2006;314:997. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 20.Pinto LH, Lamb RA. The M2 proton channels of influenza A and B viruses. J Biol Chem. 2006;281:8997–9000. doi: 10.1074/jbc.R500020200. [DOI] [PubMed] [Google Scholar]
- 21.Takeda M, Pekosz A, Shuck K, Pinto LH, Lamb RA. Influenza a virus M2 ion channel activity is essential for efficient replication in tissue culture. Journal of virology. 2002;76:1391–1399. doi: 10.1128/JVI.76.3.1391-1399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakaguchi T, Leser GP, Lamb RA. The ion channel activity of the influenza virus M2 protein affects transport through the Golgi apparatus. J. Cell Biol. 1996;133:733–747. doi: 10.1083/jcb.133.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holsinger LJ, Nichani D, Pinto LH, Lamb RA. Influenza A virus M2 ion channel protein: a structure-function analysis. Journal of virology. 1994;68:1551–1563. doi: 10.1128/jvi.68.3.1551-1563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe T, Watanabe S, Ito H, Kida H, Kawaoka Y. Influenza A virus can undergo multiple cycles of replication without M2 ion channel activity. Journal of virology. 2001;75:5656–5662. doi: 10.1128/JVI.75.12.5656-5662.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.La Gruta NL, Kedzierska K, Stambas J, Doherty PC. A question of self-preservation: Immunopathology in influenza virus infection. Immunology and Cell Biology. 2007;85:85. doi: 10.1038/sj.icb.7100026. [DOI] [PubMed] [Google Scholar]
- 26.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annual review of immunology. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 27.Hara N, Ichinose M, Sawada M, Imai K, Maeno T. Activation of single Ca2(+)-dependent K+ channel by external ATP in mouse macrophages. FEBS Lett. 1990;267:281–284. doi: 10.1016/0014-5793(90)80945-f. [DOI] [PubMed] [Google Scholar]
- 28.Venkataraman P, Lamb RA, Pinto LH. Chemical rescue of histidine selectivity filter mutants of the M2 ion channel of influenza A virus. J Biol Chem. 2005;280:21463–21472. doi: 10.1074/jbc.M412406200. [DOI] [PubMed] [Google Scholar]
- 29.Mollenhauer HH, Morre DJ, Rowe LD. Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity. Biochim Biophys Acta. 1990;1031:225–246. doi: 10.1016/0304-4157(90)90008-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lippincott-Schwartz J, Yuan LC, Bonifacino JS, Klausner RD. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schapiro F, et al. Golgi alkalinization by the papillomavirus E5 oncoprotein. J Cell Biol. 2000;148:305–315. doi: 10.1083/jcb.148.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 33.Aymeric L, et al. Tumor Cell Death and ATP Release Prime Dendritic Cells and Efficient Anticancer Immunity. Cancer Res. doi: 10.1158/0008-5472.CAN-09-3566. [DOI] [PubMed] [Google Scholar]
- 34.Fischer WB, Sansom MS. Viral ion channels: structure and function. Biochim Biophys Acta. 2002;1561:27–45. doi: 10.1016/s0304-4157(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 35.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 36.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science (New York, N.Y. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 37.Franchi L, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nature immunology. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 38.Sutterwala FS, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Adachi O, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 40.Jones CA, Taylor TJ, Knipe DM. Biological properties of herpes simplex virus 2 replication-defective mutant strains in a murine nasal infection model. Virology. 2000;278:137–150. doi: 10.1006/viro.2000.0628. [DOI] [PubMed] [Google Scholar]
- 41.Rowe HM, et al. Immunization with a lentiviral vector stimulates both CD4 and CD8 T cell responses to an ovalbumin transgene. Mol Ther. 2006;13:310–319. doi: 10.1016/j.ymthe.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 42.Besnier C, Takeuchi Y, Towers G. Restriction of lentivirus in monkeys. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11920–11925. doi: 10.1073/pnas.172384599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein O, et al. Role of glutamine 17 of the bovine papillomavirus E5 protein in platelet-derived growth factor beta receptor activation and cell transformation. Journal of virology. 1998;72:8921–8932. doi: 10.1128/jvi.72.11.8921-8932.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Axelsson MA, Warren G. Rapid, endoplasmic reticulum-independent diffusion of the mitotic Golgi haze. Molecular biology of the cell. 2004;15:1843–1852. doi: 10.1091/mbc.E03-07-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.