Abstract
In the engineering of soft tissues, scaffolds with high elastance and strength coupled with controllable biodegradable properties are necessary. To fulfill such design criteria we have previously synthesized two kinds of biodegradable polyurethaneureas, namely poly(ester urethane)urea (PEUU) and poly(ether ester urethane)urea (PEEUU) from polycaprolactone, polycaprolactone-b-polyethylene glycol-b-polycaprolactone, 1,4-diisocyanatobutane and putrescine. PEUU and PEEUU were further fabricated into scaffolds by thermally induced phase separation using dimethyl sulfoxide (DMSO) as a solvent. The effect of polymer solution concentration, quenching temperature and polymer type on pore morphology and porosity was investigated. Scaffolds were obtained with open and interconnected pores having sizes ranging from several μm to more than 150 μm and porosities of 80–97%. By changing the polymer solution concentration or quenching temperature, scaffolds with random or oriented tubular pores could be obtained. The PEUU scaffolds were flexible with breaking strains of 214% and higher, and tensile strengths of approximately 1.0 MPa, whereas the PEEUU scaffolds generally had lower strengths and breaking strains. Scaffold degradation in aqueous buffer was related to the porosity and polymer hydrophilicity. Smooth muscle cells were filtration seeded in the scaffolds and it was shown that both scaffolds supported cell adhesion and growth, with smooth muscle cells growing more extensively in the PEEUU scaffold. These biodegradable and flexible scaffolds demonstrate potential for future application as cell scaffolds in cardiovascular tissue engineering or other soft tissue applications.
Keywords: Biodegradation, Polyurethane, Scaffold, Thermally induced phase separation
1. Introduction
A variety of hydrolytically degradable polymers have been developed for tissue engineering scaffold applications. However, the majority of these polymers have consisted of high molecular weight linear aliphatic polyesters and their copolymers. These materials often possess mechanical properties best suited for hard tissue engineering because of their relatively higher glass transition temperatures and high modulus [1–6]. For engineering of soft tissues, elastic scaffolds are desirable since they are amenable to mechanical conditioning regimens that might be desirable during tissue development [7]. In our previous work, we have developed families of linear, biodegradable polyurethanes which are highly flexible and strong [8,9]. Given the thermoplastic nature of these polymers, it was hypothesized that these polyurethanes could be fabricated into flexible scaffolds using a variety of techniques.
There are many methods to prepare polyurethane into a porous scaffold, such as electrospinning [10,11], solvent casting/salt leaching [12,13], phase inversion [14], laser excimer [15] and thermally induced phase separation [16,17]. The electrospinning method has resulted in small diameter fibers with enhanced mechanical properties. However, it is difficult to make a scaffold possessing pore sizes that are appropriately large [10,11]. The solvent casting/salt leaching method has the advantage of controlling pore sizes by manipulating the size of the salt particulate. However, the resulting scaffold can have limited interconnectivity, which would adversely impact cell seeding and ingrowth [18]. Scaffolds made using the phase inversion method can exhibit low interconnectivity and controlling the pore size can be difficult [14]. The laser excimer method can make scaffolds with straight pores but achieving connectivity remains a challenge [15]. The thermally induced phase separation method offers the ability to control scaffold pore size by varying the preparation conditions and also provides means to control pore structure [16].
The objective of this work was to prepare flexible polyurethane scaffolds that could be employed in soft tissue engineering or as temporary mechanical scaffolds for application without combining with cells. The polymers were synthesized from polycaprolactone (PCL) and 1,4-diisocyanatobutane (BDI) with putrescine used as a chain extender. BDI was selected as the diisocyanate upon which the hard segment was built since it would be expected to yield putrescine, a polyamine that is essential for cell growth and differentiation, following complete degradation [19]. In order to increase the hydrophilicity of the scaffold, a polyethylene glycol (PEG) containing triblock copolymer was synthesized and used as a soft segment. The thermally induced phase separation method was employed to prepare scaffolds and the effect of preparation conditions on scaffold morphology was investigated. Mechanical properties of the scaffolds were measured following preparation, and polymer degradation in phosphate buffered saline at 37 °C was characterized over an 8 week period. Smooth muscle cells were filtration seeded in the scaffold and cell growth was characterized.
2. Materials and methods
2.1. Polymer synthesis
ε-caprolactone, PCL diol (MW=2000) and PEG (MW=1000) were obtained from Aldrich and dried under vacuum for 48 h to remove the residual water before use. 1,4-diisocyanatobutane (Fluka), putrescine (Aldrich) and stannous octoate (Sigma) were used as received. Solvent dimethyl sulfoxide (DMSO) was dried over molecular sieve (3 Å).
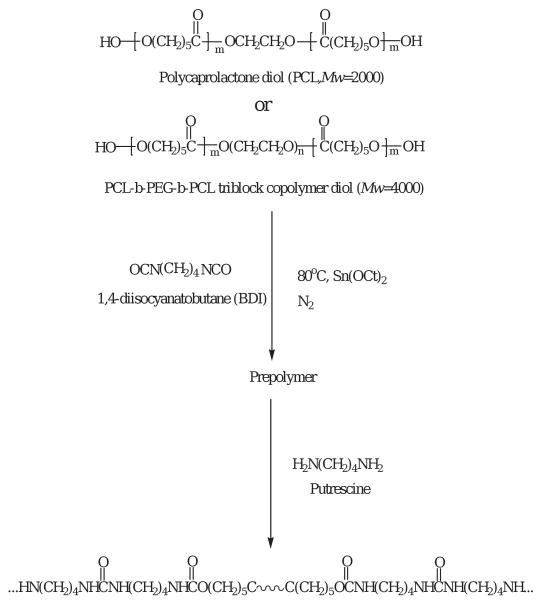
The poly(ester urethane)urea (PEUU) was synthesized using a two-step solution polymerization (Scheme 1) [8]. Synthesis was carried out in a 250 mL three-necked round bottom flask under a dry nitrogen atmosphere. The stoichiometry of the reaction was 2:1:1 of BDI: PCL: putrescine. In a first step of polymerization, an approximate 15 wt% solution of BDI in DMSO was continuously stirred with an approximate 25 wt% solution of diol in DMSO. Two drops of stannous octoate (0.2 final wt%) were then added. This mixture was allowed to react at 80 °C for a period of 3 h. The prepolymer solution was cooled to room temperature, the putrescine solution was added dropwise under stirring and the reaction was continued at room temperature for 18 h. The polymer solution was precipitated in distilled water. The wet polymer was immersed in isopropanol for 1 day to remove the unreacted monomers. Finally, the polymer was dried under vacuum at 50 °C for 24 h.
Scheme 1.
Synthesis of PEUU or PEEUU, the latter utilizing the triblock copolymer diol.
In the synthesis of poly(ether ester urethane) urea (PEEUU), triblock copolymer PCL-b-PEG-b-PCL with molecular weight 4000 was used as the soft segment. PCL-b-PEG-b-PCL was synthesized according to Bogdanov et al. and An et al. [20,21]. Polymers were prepared by using PEG to initiate the polymerization of ε-caprolactone with added stannous octoate (0.2 final wt%). Polymerization was carried out at 120 °C for 24 h under a nitrogen atmosphere. The products obtained were washed with ethyl ether and hexane, then dried in a vacuum oven. PEEUU was synthesized by the same method as for PEUU [9].
The polymers were processed into a 60 μm thick film by solution casting. The properties of PEUU and PEEUU are shown in Table 1.
Table 1.
Chemical and physical properties of synthesized polymers
| Polymer | Mw | Mn | Mw/Mn | Tg (°C) | Tm (°C) | Breaking strain (%) | Breaking stress (MPa) |
|---|---|---|---|---|---|---|---|
| PEUU | 352100 | 92 900 | 3.79 | −53.2 | 40.2 | 660±96 | 29±5 |
| PEEUU | 78500 | 55 600 | 1.41 | −56.5 | 52.8 | 325±156 | 18±5 |
2.2. Scaffold fabrication
PEUU and PEEUU scaffolds were fabricated by thermally induced phase separation and subsequent solvent extraction. The method was similar to that described by Liu and Kodama [16]. Briefly, polymer was dissolved in DMSO to form solutions of a defined concentration. The solution at 80 °C was injected into a glass cylinder mould equipped with two rubber stoppers. The mould comprised the space between two glass tubes, the outer diameter of the inner tube was 5 mm, and the inner diameter of the outer tube was 10 mm. The mould was placed in a freezer at −20 or −80 °C, or liquid nitrogen for 3 h. The mould was then removed and placed in absolute alcohol at −20 °C for 7 days to extract the DMSO. The resulting tubular scaffold was air dried and vacuum dried for 24 h respectively.
2.3. Scaffold characterization
Scaffold morphology was examined with scanning electron microscopy (SEM). Cross-sections were obtained by breaking the specimens after freezing in liquid nitrogen. Pore sizes were calculated from SEM images by IPLab3.5.2 software. Scaffold porosity was determined using a liquid displacement method similar to that reported by Zhang and Ma [22] and Hsuet al. [23]. Ethanol was used as the displacement liquid because it penetrated easily into the pores and did not induce shrinkage or swelling as a non-solvent of the polymers. A scaffold sample was immersed in a cylinder containing a known volume of ethanol (V1). The sample was kept in ethanol for 5 min, and then pressed to force air from the scaffold and allow the ethanol to penetrate and fill the pores. The total volume of ethanol and the ethanol-impregnated scaffold was recorded as V2. The ethanol-impregnated scaffold was removed from the cylinder and the residual ethanol volume was recorded as V3. The porosity of the scaffold was expressed as
p = (V1 − V3)/(V2 − V3).
The tensile properties of the scaffolds were measured according to ASTM D638-98. Testing was conducted in an Instron testing machine equipped with a 5 lb load cell. A cross-head speed of 10 mm/min was used. The longitudinal direction of the scaffold was cut and tested. Four samples were evaluated for each scaffold.
2.4. Scaffold degradation
To measure scaffold hydrolytic degradation, dry scaffolds (1.5 × 0.5 cm) were weighed and immersed in 10 mL phosphate buffered saline (PBS, pH = 7.4) at 37 °C in a water bath. Samples were taken at intervals and weighed after drying in a vacuum for 2 days. The weight remaining was calculated as
Weight remaining (%) = 100 × w2/w1,
where w1 and w2 are the weights of films before and after degradation, respectively.
To investigate the change in mechanical properties during the early stages of degradation, scaffolds made from 10 wt% PEUU and PEEUU that had been quenched at −80 °C (PEUU1080 and PEEUU1080) were cut and immersed in 10 mL PBS (pH = 7.4) at 37 °C for 1 and 2 weeks (n = 3 or 4 per scaffold type per time point). The samples were then freeze-dried and tensile mechanical testing was conducted using the methods described above.
2.5. Smooth muscle cell culture
The ability of scaffolds to support smooth muscle cell adhesion and growth was evaluated for PEUU and PEEUU scaffolds made from 10% polymer solution concentration and quenched at −80 °C. Rat vascular smooth muscle cells were isolated according to the method of Ray et al. [24]. Cells at the fifth passage were used for scaffold seeding. Scaffolds were cut in the transverse direction of the tube with a thickness of 1 mm. The scaffolds were sterilized by immersion in 70% ethanol for 6 h, followed by immersion in PBS and exposure to the ultraviolet light source in a laminar flow hood (Class II A/B3 Biological Safety Cabinet) for 2 h. The scaffolds were pressed to force the ethanol out. After rinsing thoroughly with sterile PBS, scaffolds were then immersed in cell culture medium (DMEM supplemented with 10% FBS) for 24 h. Cells at a density of 7.8 × 106 cells/mL were seeded into scaffolds using the filtration cell seeding method [25]. The seeded samples were then transferred into a 24-well polystyrene tissue culture plate. Culture medium was replaced every second day. Randomly selected wells were destructively evaluated with the MTT mitochondrial cell viability assay [8,9] at 1 and 7 days respectively (n = 4 per polymer type per time point).
The cultured scaffold surface was examined by SEM. Samples were fixed in 2.5% glutaradehyde, dehydrated in a graded series of ethanol/water solutions, dried, and then sputter coated with gold. The electron microscope was operated at 3 kV to image the samples. For histological analysis, samples were fixed in 10% formaldehyde, frozen in 2-methylbutane and sectioned (15 μm thickness), and stained with hematoxylin and eosin (H&E) for cell visualization.
2.6. Statistical methods
Data are expressed as mean ± standard deviation. Single-factor analysis of variance (ANOVA) was employed together with Neuman-Keuls testing for post hoc evaluations of differences between specific groups.
3. Results
3.1. Preparation of scaffolds
As seen from Table 1 the synthesized polymers, PEUU and PEEUU had high molecular weights and formed films that were highly distensible and strong. Scaffolds prepared by thermally induced phase separation had porosities of 80–96% (Table 2). The porosity varied with the polymer solution concentration, quenching temperature and polymer type. The porosity decreased with increasing the polymer solution concentration. At the same polymer solution concentration and quenching temperature, the scaffolds made from PEEUU had higher porosity than PEUU.
Table 2.
Preparation of porous polyurethane scaffolds
| Scaffold | Polymer concentration (w/v%) | Quenching temperature (°C) | Pore structure | Porosity (%) | Pore size (μm) | Pore size range (μm) |
|---|---|---|---|---|---|---|
| PEUU580 | 5 | −80 | Random | 93.7 | 89±40 | 25–126 |
| PEUU880 | 8 | −80 | Tubular | 92.7 | 62±30 | 14–137 |
| PEUU1080 | 10 | −80 | Random | 85.9 | 63±30 | 23–154 |
| PEUU1020 | 10 | −20 | Random | 80.0 | 132±65 | 36–203 |
| PEUU10LN | 10 | liquid nitrogen | Random | 89.3 | 12±9 | 3–19 |
| PEEUU580 | 5 | −80 | Tubular | 96.6 | 121±36 | 53–182 |
| PEEUU520 | 5 | −20 | Random | 95.2 | 232±130 | 32–343 |
| PEEUU1080 | 10 | −80 | Tubular | 91.5 | 83±44 | 24–146 |
| PEEUU1020 | 10 | −20 | Tubular | 93.8 | 196±68 | 76–387 |
Fig. 1 shows the cross-sectional morphologies of scaffolds prepared under different conditions. The scaffolds were seen to have open and interconnected pores. The polymer type and processing parameters, such as the quenching temperature and polymer concentration were studied for their effects on the architectural and bulk properties of the scaffolds. The polymer type affected scaffold morphology. When comparing the morphologies of scaffolds made from the same polymer solution concentration and quenching temperature but different polymer, the pores of scaffolds made from PEEUU were quantitatively larger than those derived from PEUU (Table 2).
Fig. 1.
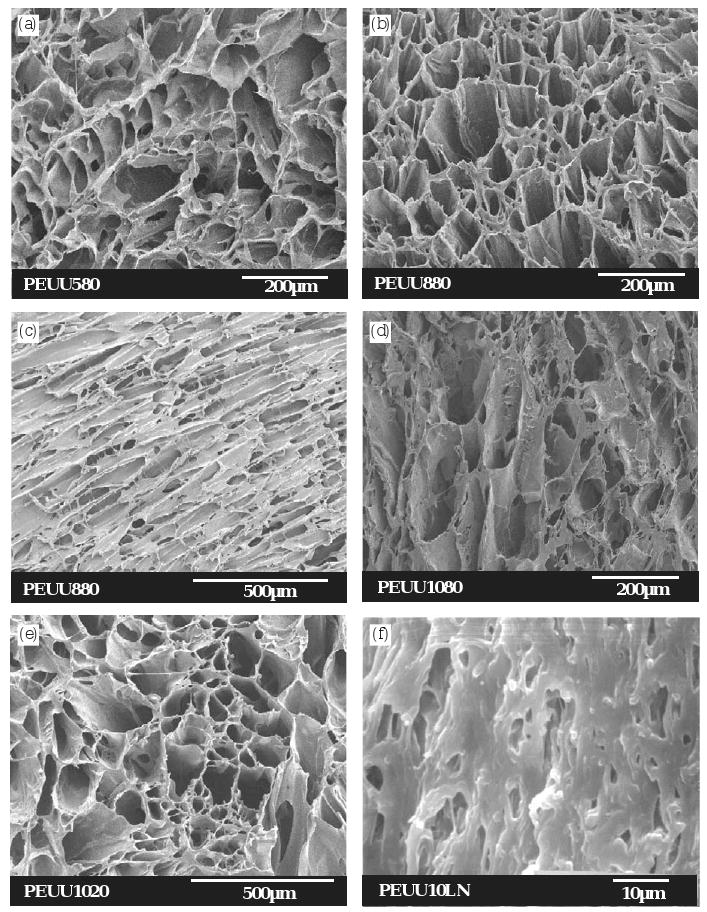
Electron micrographs of PEUU scaffolds prepared from different PEUU solution concentrations and quenching temperatures. (a) PEUU580 (5%, −80 °C), longitudinal cross-section; (b) PEUU880 (8%, −80 °C), longitudinal cross-section; (c) PEUU880 (8%, −80 °C), transverse cross-section (d) PEUU1080 (10%, −80 °C), longitudinal cross-section; (e) PEUU1020 (10%, −20 °C), longitudinal cross-section; (f) PEUU10LN (10%, liquid nitrogen), longitudinal cross-section. The scale bars are shown in the micrographs.
To study the effect of polymer solution concentration on the scaffold structure, scaffolds made from PEUU with solution concentrations 5, 8, and 10 wt% and a quenching temperature of −80 °C were compared. The scaffold made from PEUU at 5% was composed of bonded thin PEUU leaflets (Fig. 1a). The scaffolds made from polymer solution concentration 5% and 10% showed an irregular pore structure in both longitudinal and transverse cross-section (transverse not shown) (Fig. 1a, d, e). However, when the polymer solution concentration was 8%, the longitudinal and transverse morphology of the scaffold showed the pores oriented in a parallel tubular structure (Fig. 1b, c). When PEUU was cooled in liquid nitrogen during processing, a unique structure with irregular, small pores were observed in the cross-section (Fig. 1f). Most of the pores were less than 10 μm in size.
For PEEUU scaffolds, when the polymer solution concentration was 5% and the quenching temperature was −20 °C, the pores were random (Fig. 2a). However, scaffolds made from 10% PEEUU and the same quenching temperature had pores that were tubular (Fig. 2c). PEEUU scaffolds made from 5% (Fig. 2b) and 10% (Fig. 2d) polymer and quenched at −80 °C also had pores that were tubular. The pore sizes of scaffolds made from a polymer solution of 5% were larger than those from a 10% polymer scaffold when the quenching temperature was the same.
Fig. 2.
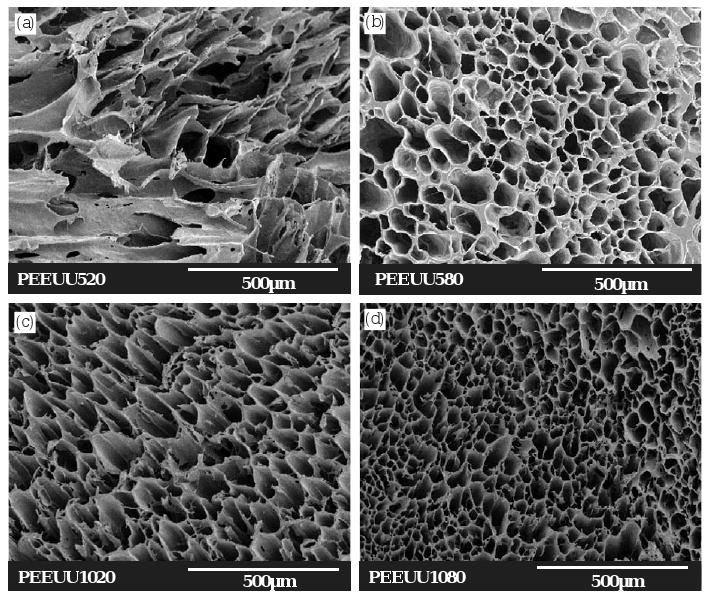
Electron micrographs of PEEUU scaffolds prepared from different PEEUU solution concentrations and quenching temperatures. (a) PEEUU520 (5%, −20 °C), longitudinal cross-section; (b) PEEUU580 (5%, −80 °C), longitudinal cross-section; (c) PEEUU1020 (10%, −20 °C), longitudinal cross-section; (d) PEEUU1080 (10%, −80 °C), longitudinal cross-section. Scale bar = 500 μm.
3.2. Mechanical properties
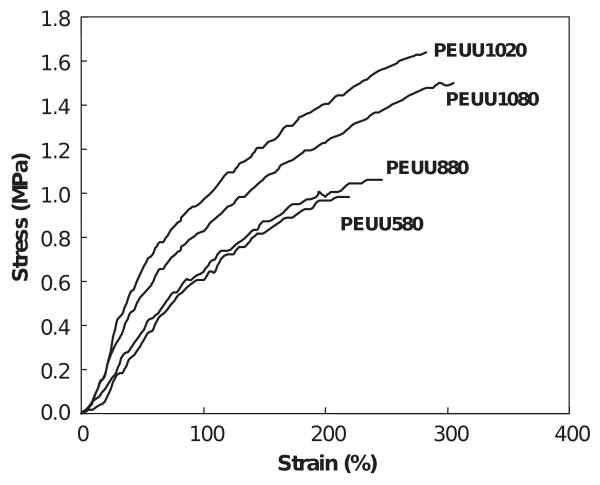
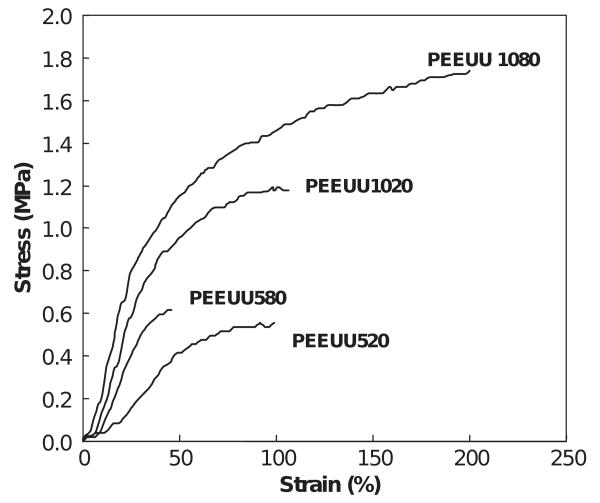
Figs. 3 and 4 show typical tensile stress-strain curves for four types of scaffolds made from each polymer. Scaffolds made from a polymer solution concentration of 10% PEUU and quenched by liquid nitrogen were not measured because the formed scaffold was fragile and fragmented with manipulation. Measured mechanical parameters are summarized in Table 3. The PEUU scaffolds were flexible with tensile strengths ranging from 0.97 to 1.64 MPa, and breaking strains from 214% to 294%. PEEUU scaffolds had tensile strengths from 0.59 to 1.68 MPa and breaking strains from 47% to 197%. It can be seen that the polymer solution concentration affected the mechanical properties of the scaffolds made at the same quenching temperature. The PEUU scaffolds made from a polymer solution concentration of 10% showed significantly higher tensile strength (p < 0.03) and breaking strain (p < 0.03) than scaffolds made from polymer concentrations of 5% and 8%. The tensile strength and breaking strain of scaffolds made from a polymer solution concentration of 8% appeared slightly higher than scaffolds made from a polymer solution concentration of 5%, though this was not statistically significant (p > 0.1). Comparing the scaffolds made from a polymer solution concentration of 10% but quenched at different temperatures, there was no significant difference.
Fig. 3.
Typical tensile stress–strain curves for PEUU scaffolds.
Fig. 4.
Typical tensile stress–strain curves for PEEUU scaffolds.
Table 3.
Mechanical properties of porous polyurethane scaffolds
| Scaffold | Strain (%) | Tensile stress (MPa) |
|---|---|---|
| PEUU580 | 229±8 | 0.97±0.02 |
| PEUU880 | 214±11 | 1.01±0.19 |
| PEUU1080 | 294±4 | 1.64±0.01 |
| PEUU1020 | 290±9 | 1.53±0.06 |
| PEEUU580 | 47±11 | 0.59±0.04 |
| PEEUU520 | 96±10 | 0.67±0.04 |
| PEEUU1080 | 197±24 | 1.68±0.13 |
| PEEUU1020 | 93±18 | 1.22±0.07 |
For PEEUU scaffolds, tensile strengths of scaffolds made from polymer solution concentration 10% were significantly higher than for those made from 5% (p < 0.004). For the scaffolds made from 10% polymer solution, those quenched at −80 °C had significantly higher tensile strength (p < 0.0004) and strain (p < 0.0002) than for those quenched at temperature −20 °C. The scaffolds made from 5% polymer solution and quenched at −20 °C had significantly higher strain at failure than the scaffolds quenched −80 °C (p < 0.02).
3.3. Degradation properties
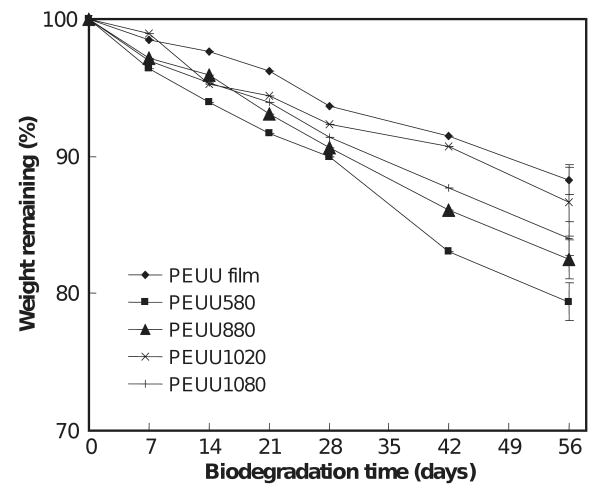
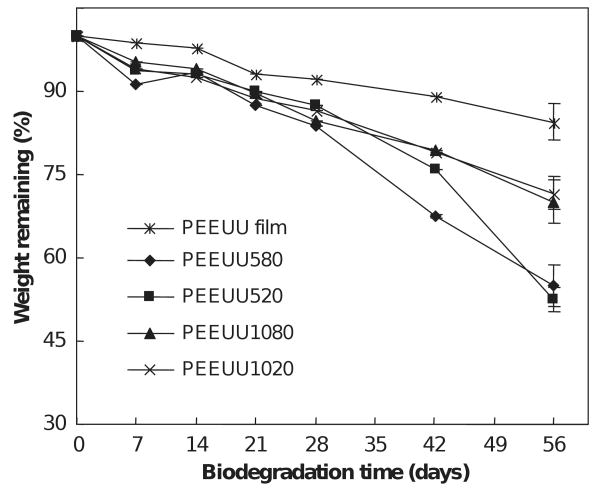
Scaffold degradation curves are shown in Figs. 5 and 6. The scaffolds exhibited progressive mass loss over the 8-week period ranging from 13.3% to 20.7% for PEUU scaffolds and 25.4% to 47.3% for PEEUU scaffolds. The degradation rates depended upon scaffold characteristics. Comparing the weight loss at 56 days of PEUU scaffolds and PEUU film, it was found that the scaffolds had higher degradation rates than the PEUU film (p < 0.05) except for the scaffold made from 10% polymer and quenched at −20 °C. The scaffolds made from different polymer solution concentrations but the same quenching temperatures had different levels of degradation at 8 weeks. The PEUU scaffold made from 5% polymer had greater degradation than those made from higher polymer solution concentrations of 8% and 10% (p < 0.04). For PEEUU, scaffolds made from 5% polymer degraded more at 56 days than those made from 10% polymer (p < 0.002). There was no significant difference between scaffolds made from the same concentration but different quenching temperatures.
Fig. 5.
Effect of biodegradation time on the weight remaining for PEUU scaffolds.
Fig. 6.
Effect of biodegradation time on the weight remaining for PEEUU scaffolds.
Mechanical properties of PEUU1080 and PEEUU1080 scaffolds were measured after 1 and 2 weeks of degradation (Table 4). The tensile strength of PEUU1080 decreased from 1.64 to 1.44 MPa at 1 week (p < 0.04) and to 1.13 MPa at 2 weeks (p < 0.01 vs. 1 week). PEEUU1080 tensile strength decreased from 1.68 to 1.03 MPa at 1 week (p < 0.001) and to 0.81 MPa at 2 weeks (p < 0.02 vs. 1 week). The breaking strain also decreased during the early degradation period for both scaffolds (p < 0.05 at 1 week vs. initial measurement; no difference between 1 and 2 weeks), however the elongation at break remained above 150% after 2 weeks degradation.
Table 4.
Mechanical properties of porous polyurethane scaffolds with degradation
| Scaffold | Degradation time (days) | Strain (%) | Tensile stress (MPa) |
|---|---|---|---|
| PEUU1080 | 0 | 294±4 | 1.64±0.01 |
| PEUU1080 | 7 | 193±34 | 1.44±0.02 |
| PEUU1080 | 14 | 186±8 | 1.13±0.15 |
| PEEUU1080 | 0 | 197±24 | 1.68±0.13 |
| PEEUU1080 | 7 | 164±33 | 1.03±0.07 |
| PEEUU1080 | 14 | 159±21 | 0.81±0.04 |
3.4. Smooth muscle cell growth
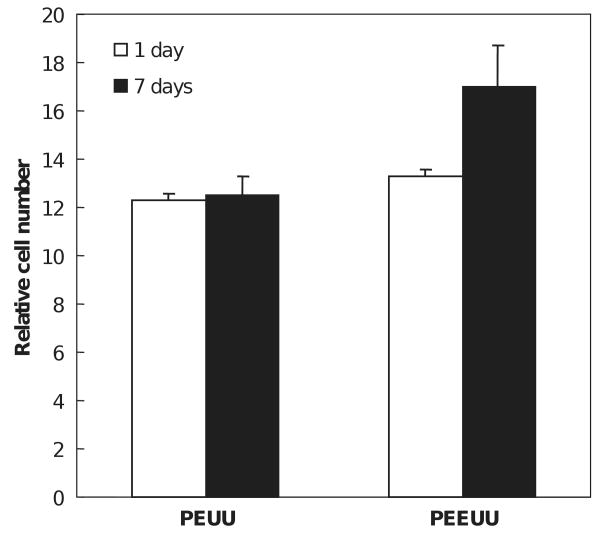
Vascular smooth muscle cells cultured on PEUU and PEEUU scaffolds were quantified by measuring the MTT absorbance of the seeded scaffolds (Fig. 7). The relative cell number in the PEUU scaffold did not change from 1 to 7 days (p = 0.42), whereas the cell number in PEEUU scaffolds at 7 days was significantly higher than at 1 day (p = 0.03). Comparing the cell number in PEUU and PEEUU scaffolds, there was no significant difference at day 1 (p = 0.11). However, by day 7, the PEEUU scaffolds had significantly higher cell numbers (p = 0.01). Electron micrographs (Fig. 8) showed that both scaffold surfaces covered were populated with cells that had started to spread after 1 day. By 7 days the cells had formed dense confluent layers on the surface. The cellular ingrowth was examined by H&E staining (Fig. 9). Smooth muscle cells were uniformly distributed in the scaffolds (Fig. 9a and b) and generally retained a rounded morphology after 1 day of culture. Cells at 7 days were spread and had a slightly higher density in PEEUU scaffolds (Fig. 9c and d).
Fig. 7.
Growth of vascular smooth muscle cells seeded in PEUU and PEEUU scaffolds. Relative cell number refers to relative mitochondrial activity as determined by the MTT assay.
Fig. 8.
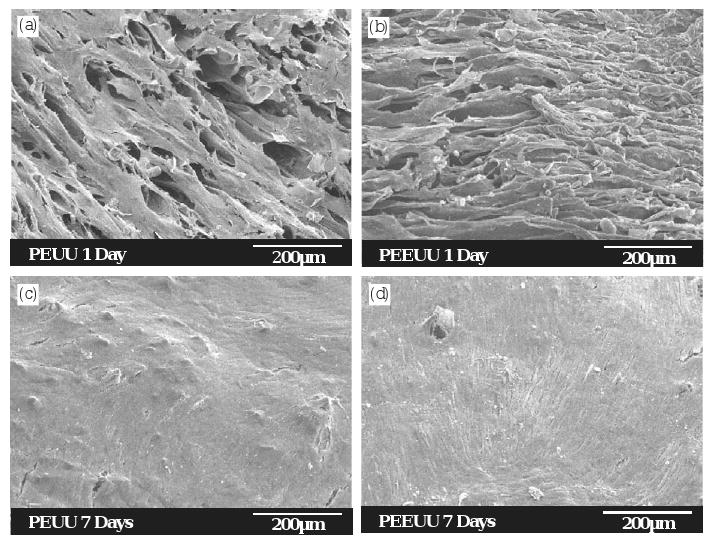
Electron micrographs of the scaffold surfaces showing the morphology of vascular smooth muscle cells seeded in PEUU (a, c) and PEEUU (b, d) scaffolds after 1 day (a, b) and 7 days (c, d) of culture.
Fig. 9.
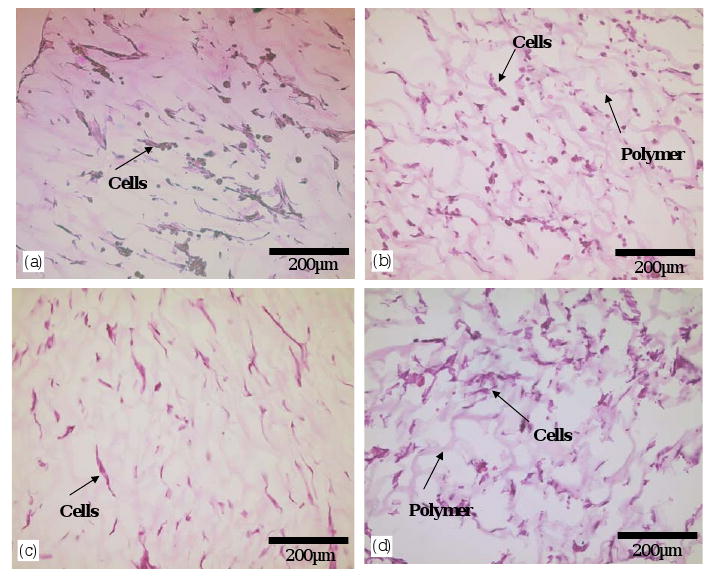
Hematoxylin and eosin (H&E) staining of vascular smooth muscle cells in the cross-section of PEUU (a, c) and PEEUU (b, d) scaffolds after 1 day (a, b) and 7 days (c, d) of culture.
4. Discussion
In an effort to develop an elastomeric, biodegradable scaffold that would be applicable in the engineering of soft tissues, PEUU and PEEUU were synthesized based upon a PCL or PCL-b-PEG-b-PCL soft segment, a BDI hard segment and chain extension with putrescine. Stannous octoate, an US Food and Drug Administration approved food stabilizer was used as a catalyst [26]. The molecular weights of the polymers were high enough, and the glass transition temperatures low enough, to impart elastomeric behavior to the solid films [27]. Thermally induced phase separation was chosen as the method to create scaffolds with open pores, which together with pore interconnectivity, are essential for cellular ingrowth.
With the thermally induced phase separation technique, solvent selection can affect the structure of the resulting scaffolds. Spaans et al. [28] used dioxane as a solvent for PEUU and found that it was difficult to effectively obtain a scaffold and only very thin porous material could be obtained. This might have been due to limited solubility of their polyurethaneurea in dioxane. In this work, DMSO was used as the solvent since it could dissolve both PEUU and PEEUU and had a relative high melting point (18 °C). When quenching the polymer solution, it was able to form a polymer rich phase and a DMSO rich phase, the latter being removable by ethanol extraction.
The quenching temperature (specifically the cooling rate) can affect the crystallization process of DMSO during phase separation and thus affect the morphologies of the resulting scaffolds. At a lower temperature, DMSO has a higher nucleation rate and a lower crystal growth rate, which leads to the formation of a large number of small crystals, resulting in small pore sizes. In contrast, at a higher temperature, DMSO has a lower nucleation rate and a higher crystal growth rate, which leads to the formation of larger crystals, and thus larger pore sizes. The pore sizes of the PEUU scaffold processed in liquid nitrogen were found to be much smaller than for the scaffolds cooled to −80 and −20 °C. Also, the pore sizes of PEUU and PEEUU scaffolds made at −80 °C were found to be smaller than for those made at −20 °C.
The polymer solution concentration used in processing would be expected to affect pore morphology and porosity. As the polymer solution concentration increases, the content of DMSO in the system decreases, therefore, porosity should decrease. This trend was observed. The effect of polymer solution concentration on pore size could be attributed to the effect of polymer solution viscosity and the crystallization process of DMSO. When the polymer solution concentration is increased, the viscosity of the solution increases, upon quenching, the growth rate of DMSO crystals decreases and forms a large number of smaller crystals resulting in smaller pores.
Pore morphologies in the produced PEUU and PEEUU scaffolds were found to exhibit either random or oriented structures, depending on the processing conditions. The mould employed has an open outer and inner surface, thus heat transfer occurs from these surfaces to the middle of the scaffold and DMSO crystals might grow following these directions and form oriented pores. Ma and Zhang [29] described the creation of tubular pores by employing a heat transfer gradient and crystal growth in the heat transfer direction when preparing poly(lactic acid) scaffolds. The variability observed in morphologies in the current system might be attributed to the effect of viscosity on crystal growth kinetics. When the polymer solution concentration and viscosity are low, during quenching, the crystals may grow rapidly and not exhibit sensitivity to the heat transfer direction. Alternatively, if the polymer solution concentration is high, the viscosity of the polymer/DMSO system may lead to slow crystal growth during quenching, minimizing the impact of the transient temperature gradient and resulting in random pore structures.
The PEUU and PEEUU scaffolds were found to be flexible with tensile strengths from 0.97 to 1.64 MPa and breaking strains from 214% to 294% for PEUU scaffolds, and tensile strengths from 0.59 to 1.68 MPa and breaking strains from 47% to 197% for PEEUU scaffolds. The tensile strengths of the scaffolds were comparable to the canine thoracic aorta (0.9±0.1 MPa) [30] while the breaking strains were generally greater than that of human femoral and popliteal arteries (about 90%) [31]. The mechanical properties of the scaffold depended on the pore structure and the mass of polymer in the scaffolds. In general, as the concentration of polymer solution utilized increased, the volume of the pores in scaffold decreased and the tensile strength increased.
The in vitro degradation studies demonstrated that the scaffolds degraded at rates that might be attractive for tissue engineering applications. The scaffolds degraded faster than non-porous films of the same material. This is attributed to the increase in surface area with porosity and thus the availability of more surface contact area for polymer hydrolysis. The scaffolds made from lower polymer solution concentrations had higher degradation rates than those made from higher polymer solution concentrations presumably due to their higher porosities.
In this study, the polymer films and scaffolds did not show evidence of an autocatalytic effect during the monitored degradation process. This degradation behavior is different from that of poly (α-hydroxyester)s such as poly(l-lactide) and poly(lactide-co-glycide) [32,33]. During the biodegradation of poly(l-lactide) and poly(lactide-co-glycide), scaffolds of low porosity have been found to have higher hydrolytic degradation rates than high porosity scaffolds. This could be attributed to the accumulation of carboxylic groups near the pore walls of low porosity scaffold and acceleration of the hydrolytic reaction. Higher porosities presumably allow better acid transport from the surfaces. The degradation behavior of PEUU and PEEUU scaffolds in this study is similar to scaffolds made by Gomes [34] and van Tienen [35] in which higher porosity lead to faster degradation.
In discussing the degradation properties of the scaffolds and their appropriateness for tissue engineering applications it is worth noting that this initial study has examined degradation in the absence of plasma enzymes and cells. Degradation in a cell-culture or in vivo environment would be expected to be faster. Second, a proposed advantage for an elastomeric, biodegradable scaffold is the ability to mechanically condition a tissue engineered cell/matrix construct in vitro prior to placement (e.g. as in the case for a vascular replacement). Subjecting the PEUU scaffolds to cyclic loads might accelerate the degradation rates substantially. Seeded cells would need to reach a state where they were able to bear increasing loads as the polymeric matrix degraded and lost mechanical strength. Many parameters would determine whether such stress transfer occurs appropriately, but this study suggests that manipulation of polymer solution concentration and porosity in the scaffold forming process will allow manipulation of the degradation characteristics in these PEUU and PEEUU scaffolds. This can be done in addition to altering the presence and relative amount of ether in the selected polymer backbone [9]. The mechanical properties were gradually lost during the first 2 weeks of degradation. At the 2 week time point, the scaffolds were still relatively strong and flexible as evidenced by tensile strengths higher than 0.8 MPa and elongations at break higher than 150% for the two scaffolds studied.
A common concern in developing a biodegradable scaffold for implantation is the potential for cytotoxic or carcinogenic degradation products or the release of residual solvent used during processing. It has been noted by earlier studies that commonly used diisocyanates in non-degradable polyurethanes were not appropriate to use due to concerns with carcinogenicity and cytotoxicity in the diamine degradation products [36]. Our use of BDI as a hard segment with chain extension by putrescine would be expected to ultimately yield a hard segment degradation product of putrescine, which is already present in the body and has been implicated as an important mediator of cellular growth and differentiation in response to growth factors [37–39]. Supporting our expectation of non-cytotoxic degradation products, we did not previously find evidence of cytotoxicity in our culture of endothelial cells with PEUU or PEEUU degradation products [8,9]. In preparing scaffolds, DMSO was selected as the solvent due to its lower toxicity relative to other commonly used solvents such as dioxane, and its use in cell storage procedures.
Using the filtration method, smooth muscle cells were found to uniformly distribute in the scaffold and spread over time. The cells in the PEEUU scaffold grew faster than those seeded in the PEUU scaffold. This may be attributed to better metabolite transport in the PEEUU scaffold because of its increased hydrophilicity and its faster degradation rate. Of note, the cell culture occurred in a static system. With mixed culture in, for instance, a spinner flask or a lightly perfused scaffold, cell densities and growth might be expected to be higher due to improved mass transfer, and a difference between the scaffold types might not appear.
5. Conclusions
In summary, we have demonstrated the synthesis and processing of two types of biodegradable polyurethanes into porous scaffolds using a thermally induced phase separation technique. The resulting scaffolds were shown to have mechanical and chemical properties attractive for future application in soft tissue engineering. Future investigations with these materials will necessarily involve further evaluation of cell/material constructs, and biocompatibility evaluation in vivo.
Acknowledgments
We would like to thank Ms. Angela Fu and Mr. John Stankus for the collection of mechanical property data. This work was funded through NIH grant #HL069368.
References
- 1.Heller J, Sparer RV, Zentner GM. Poly(ortho esters) In: Chasin M, Langer R, editors. Biodegradable polymers as drug delivery systems. New York: Marcel Dekker; 1990. pp. 121–61. [Google Scholar]
- 2.Lowry KJ, Hamson KR, Bear L, Peng YB, Calaluce R, Evans ML, Anglen JO, Allen WC. Polycaprolactone/glass bioabsorble implant in a rabbit humerus fracture model. J Biomed Mater Res. 1997;36:536–41. doi: 10.1002/(sici)1097-4636(19970915)36:4<536::aid-jbm12>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal CM, Ray RB. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J Biomed Mater Res. 2001;55:141–50. doi: 10.1002/1097-4636(200105)55:2<141::aid-jbm1000>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Ishaug- Riley S. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J Biomed Mater Res. 1997;36:17–28. doi: 10.1002/(sici)1097-4636(199707)36:1<17::aid-jbm3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 5.Freed LE, Vunjak-Novakovic G, Biron RJ, Eagles DB, Lesnoy DC, Barlow SK, Langer R. Biodegradable polymer scaffolds for tissue engineering. Biotechnology. 1994;12:689–93. doi: 10.1038/nbt0794-689. [DOI] [PubMed] [Google Scholar]
- 6.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–93. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 7.Takahara A, Coury AJ, Hergenrother RW, Cooper SL. Effect of soft segment chemistry on the biostability of segmented polyurethanes. I. In vitro oxidation. J Biomed Mater Res. 1991;25:341–56. doi: 10.1002/jbm.820250306. [DOI] [PubMed] [Google Scholar]
- 8.Guan J, Sacks MS, Beckman EJ, Wagner WR. Synthesis, characterization and cytocompatibility of elastomeric, biodegradable poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. J Biomed Mater Res. 2002;61:493–503. doi: 10.1002/jbm.10204. [DOI] [PubMed] [Google Scholar]
- 9.Guan J, Sacks MS, Beckman EJ, Wagner WR. Biodegradable poly(ether ester urethane)urea elastomers based on poly(ether ester) triblock copolymers and putrescine: synthesis, characterization and cytocompatibility. Biomaterials. 2004;25:85–96. doi: 10.1016/s0142-9612(03)00476-9. [DOI] [PubMed] [Google Scholar]
- 10.Demir MM, Yilgor I, Yilgor E, Erman B. Electrospinning of polyurethane fibers. Polymer. 2002;43:3303–9. [Google Scholar]
- 11.Lee KH, Kim HY, Ryu YJ, Kim KW, Choi SW. Mechanical behavior of electrospun fiber mats of poly(vinyl chloride)/polyurethane polyblends. J Polym Sci Polym Phys. 2003;41:1256–62. [Google Scholar]
- 12.Fujimoto K, Minato M, Miyamoto S, Kaneko T, Kikuchi H, Sakai K, Okada M, Ikada Y. Porous polyurethane tubes as vascular graft. J Appl Biomat. 1993;4:347–54. doi: 10.1002/jab.770040409. [DOI] [PubMed] [Google Scholar]
- 13.Fromstein JD, Woodhouse KA. Elastomeric biodegradable polyurethane blends for soft tissue application. J Biomater Sci Polymer Edn. 2002;13:391–406. doi: 10.1163/156856202320253929. [DOI] [PubMed] [Google Scholar]
- 14.Kowligi RR, von Maltzahn WW, Eberhart RC. Fabrication and characterization of small-diameter vascular prostheses. J Biomed Mater Res. 1988;22:245–56. doi: 10.1002/jbm.820221405. [DOI] [PubMed] [Google Scholar]
- 15.Doi K, Nakayama Y, Matsuda T. Novel compliant and tissue-permeable microporous polyurethane vascular prosthesis fabricated using an excimer laser ablation technique. J Biomed Mater Res. 1996;31:27–33. doi: 10.1002/(SICI)1097-4636(199605)31:1<27::AID-JBM4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 16.Liu SQ, Kodama M. Porous polyurethane vascular prostheses with variable compliances. J Biomed Mater Res. 1992;26:1489–94. doi: 10.1002/jbm.820261108. [DOI] [PubMed] [Google Scholar]
- 17.Saad B, Matter S, Ciardelli G, Uhlschmid GK, Welti M, Neuenschwander P, Suter UW. Interactions of osteoblasts and macrophages with biodegradable and highly porous polyesterur-ethane foam and its degradation products. J Biomed Mater Res. 1996;32:355–66. doi: 10.1002/(SICI)1097-4636(199611)32:3<355::AID-JBM8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 18.Nam YS, Park TG. Porous biodegradable polymeric scaffolds prepared by thermally induced phase separation. J Biomed Mater Res. 1999;47:8–17. doi: 10.1002/(sici)1097-4636(199910)47:1<8::aid-jbm2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 19.Tabor CW, Tabor H. Polyamines. AnnuRev Biochem. 1984;53:749–90. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- 20.Bogdanov B, Vidts A, Van Den Bulcke A, Verbeeck R, Schacht E. Synthesis and thermal properties of poly(ethylene glycol)—poly(ε-caprolactone) copolymers. Polymer. 1998;39:1631–6. [Google Scholar]
- 21.An JH, Kim HS, Chung DJ, Lee DS. Thermal behaviour of poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) tri-block copolymers. J Mater Sci. 1999;32:726–31. [Google Scholar]
- 22.Zhang RY, Ma PX. Poly(α-hydroxyl acids)/hydroxyapatite porous composites for bone-tissue engineering. I. Preparation and morphology. J Biomed Mater Res. 1999;44:446–55. doi: 10.1002/(sici)1097-4636(19990315)44:4<446::aid-jbm11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 23.Hsu YY, Gresser JD, Trantolo DJ, Lyons CM, Gangadharam PR, Wise DL. Effect of polymer foam morphology and density on kinetics of in vitro controlled release of isoniazid from compressed foam matrices. J Biomed Mater Res. 1997;35:107–16. doi: 10.1002/(sici)1097-4636(199704)35:1<107::aid-jbm11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 24.Ray JL, Leach R, Herbert JM, Benson M. Isolation of vascular smooth muscle cells from a single murine aorta. Methods Cell Sci. 2002;23:185–8. doi: 10.1023/a:1016357510143. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Ma T, Kniss DA, Lasky LC, Yang ST. Effects of filtration seeding on cell density, spatial distribution, and proliferation in nonwoven fibrous matrices. Biotechnol Prog. 2001;17:935–44. doi: 10.1021/bp0100878. [DOI] [PubMed] [Google Scholar]
- 26.Wong WH, Mooney DJ. Synthesis and properties of biodegradable polymers used as synthetic matrices for tissue engineering. In: Atala A, Mooney DJ, editors. Synthetic biodegradable polymer scaffolds. Boston: Birkhauser; 1996. pp. 51–84. [Google Scholar]
- 27.Lelah MD, Cooper SL. Polyurethanes in medicine. Boca Raton: CRC Press; 1986. pp. 5–20. [Google Scholar]
- 28.Spaans CJ, de Groot JH, Dekens FG, Pennings AJ. High molecular weight polyurethanes and a polyurethane urea based on 1,4-butanediisocyanate. Polym Bull. 1998;41:131–8. [Google Scholar]
- 29.Ma PX, Zhang RY. Microtubular architecture of biodegradable polymer scaffolds. J Biomed Mater Res. 2001;56:469–77. doi: 10.1002/1097-4636(20010915)56:4<469::aid-jbm1118>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 30.Martz H, Beaudoin G, Paynter R, King M, Marceau D, Guidoin R. Physicochemical characterization of a hydrophilic microporous polyurethane vascular graft. J Biomed Mater Res. 1987;21:399–412. doi: 10.1002/jbm.820210311. [DOI] [PubMed] [Google Scholar]
- 31.Yamada H. Strength of biological materials. Baltimore: Williams & Wilkins; 1980. pp. 115–29. [Google Scholar]
- 32.Lu LC, Susan JP, Lyman MD, Lai HL, Leite SM, Tamada JA, Vacanti JP, Langer R, Mikos AG. In vitro degradation of porous poly(l-lactic acid) foams. Biomaterials. 2000;21:1595–605. doi: 10.1016/s0142-9612(00)00048-x. [DOI] [PubMed] [Google Scholar]
- 33.Agrawal CM, McKinney JS, Lanctot D, Athanasiou KA. Effects of fluid flow on the in vitro degradation kinetics of biodegradable scaffolds for tissue engineering. Biomaterials. 2000;21:2443–52. doi: 10.1016/s0142-9612(00)00112-5. [DOI] [PubMed] [Google Scholar]
- 34.Gomes ME, Ribeiro AS, Malafaya PB, Reis RL, Cunha AM. A new approach on injection moulding to produce biodegradable starch-based polymeric scaffolds: morphology, mechanical and degradation behaviour. Biomaterials. 2001;22:883–9. doi: 10.1016/s0142-9612(00)00211-8. [DOI] [PubMed] [Google Scholar]
- 35.van Tienen TG, Heijkants RGJC, Buma P, de Groot JH, Pennings AJ, Veth RPH. Tissue ingrowth and degradation of two biodegradable porous polymers with different porosities and pore sizes. Biomaterials. 2002;23:1731–8. doi: 10.1016/s0142-9612(01)00280-0. [DOI] [PubMed] [Google Scholar]
- 36.Storey RF, Wiggins JS, Puckett AD. Hydrolyzable poly(ester-urethane) networks from L-lysine diisocyanate and D,L-lactide, ε-caprolactone homopolyester and copolyester triols. J Polym Sci Part A: Polym Chem. 1994;32:2345–63. [Google Scholar]
- 37.Endean E, Toursarkissian B, Buckmaster M, Aziz S, Gellin G, Hill B. Regulation of polyamine synthesis and transport by fibroblast growth factor in aortic smooth muscle cells. Growth Factors. 1996;13:229–42. doi: 10.3109/08977199609003224. [DOI] [PubMed] [Google Scholar]
- 38.Wang JY, Viar MJ, Li J, Shi HJ, McCormack SA, Johnson LR. Polyamines are necessary for normal expression of the transforming growth factor-beta gene during cell migration. Am J Physiol. 1997;272:G713–20. doi: 10.1152/ajpgi.1997.272.4.G713. [DOI] [PubMed] [Google Scholar]
- 39.Milovic V, Turhanowa L, Fares FA, Lerner A, Caspary WF, Stein J. S-adenosylmethionine decarboxylase activity and utilization of exogenous putrescine are enhanced in colon cancer cells stimulated to grow by EGF. Z Gastroenterol. 1998;36:947–54. [PubMed] [Google Scholar]