Abstract
Early life events influence vulnerability to psychiatric illness. This has been modelled in rats and it has been demonstrated that different durations of maternal separation shape adult endocrine and behavioural stress reactivity. One system through which maternal separation may act is the locus coeruleus (LC)–norepinephrine system that regulates emotional arousal. Here we demonstrate that different durations of maternal separation have distinct effects on LC physiology and dendritic morphology. Rat pups were separated from the dam for 15 min/d (HMS-15) or 180 min/d (HMS-180) from post-natal days 2–14. Others were either undisturbed (HMS-0) or were vendor-purchased controls. LC characteristics were compared at age 22–35 d using whole-cell recordings in vitro. Cells were filled with biocytin for morphological analysis. LC neurons of HMS-180 rats were tonically activated compared to HMS-15 and control rats, with firing rates that were 2-fold higher than these groups. Corticotrophin-releasing factor (CRF) application did not further activate LC neurons of HMS-180 rats but increased LC firing rate in HMS-0 and control rats. LC neurons of HMS-15 rats were resistant to excitation by CRF. Maternal separation also affected LC dendritic morphology. LC dendrites of HMS-15 rats exhibited less branching and decreased total dendritic length, an effect that could decrease the probability of contacting limbic afferents that terminate in the pericoerulear region. This effect may provide a structural basis for an attenuated magnitude of emotional arousal. Together, these results demonstrate long-term consequences of early life events on the LC–norepinephrine system that may shape adult behaviour.
Keywords: Development, norepinephrine, plasticity, stress
Introduction
Early life experiences have profound effects that endure into adulthood and can determine individual differences in predisposition to affective disorders (Brown & Anderson, 1991; Brown et al. 1987; Cui & Vaillant, 1996; Heim & Nemeroff, 2001; Kendler et al. 1995; Repetti et al. 2002). The early rearing environment, during which the mother–infant relationship is established, is a particularly potent influence that can shape behavioural and endocrine responses to stress and thereby vulnerability to mood disorders in adulthood (Essex et al. 2002; Levine, 1994; Luecken & Lemery, 2004). The neurobiological mechanisms by which adult behaviour and emotional responses are shaped by early rearing conditions have been studied in rodent models that involve separation of rat pups from the dam for varying periods of time, as well as variations in handling of litters (Ellenbroek et al. 1998; Lehmann et al. 1998; Meaney et al. 1991; Plotsky et al. 2005; Pryce & Feldon, 2003; Sanchez et al. 2001). Because experimental manipulations vary between studies and there is controversy over what manipulation constitutes an appropriate control group, a consensus has not been reached regarding the specific consequences of repeated handling vs. repeated long-term maternal separation. Nonetheless, an invariable finding is that manipulations of early rearing conditions impact on behavioural and endocrine responses to stressors in adulthood (Caldji et al. 2000; Feldon & Weiner, 1988; Lippmann et al. 2007; Liu et al. 2000; Plotsky et al. 2005).
Early rearing conditions can determine the direction and magnitude of adult endocrine and behavioural responses to challenges through effects on corticotrophin-releasing factor (CRF), a primary mediator of the stress response (Francis et al. 1999; Plotsky et al. 2005). One CRF circuit that is particularly sensitive to early rearing conditions is the limbic–locus coeruleus (LC) circuit that has been implicated in emotional arousal (Aston-Jones et al. 1996; Liddell et al. 2005; Sterpenich et al. 2006). CRF axon terminals from the central nucleus of the amygdala densely innervate LC dendrites in the peri-coerulear region and activate the LC during stress (Curtis et al. 2002). Separation of rat pups from the dam for 180 min from post-natal day (PND) 2 to PND 14 increases CRF levels and CRF receptor mRNA in the LC, and CRF protein and mRNA in the amygdala compared to short-term handling and separation (15 min), absence of handling or handling associated with animal facility caretaking (Plotsky et al. 2005). These effects are consistent with up-regulation of CRF in this limbic–coerulear circuit (Francis et al. 1999; Plotsky et al. 2005). In contrast, rats that underwent 15 min separation had evidence of CRF receptor down-regulation in the LC compared to all groups (Plotsky et al. 2005). By regulating components of CRF transmission in this circuit, early rearing conditions can determine the level of activity and stress-sensitivity of the LC–norepinephrine system, which in turn could influence the behavioural phenotype.
CRF, acting as a neuromodulator, increases LC firing rate and norepinephrine extracellular levels in forebrain targets during stress thereby facilitating arousal and a shift from focused to scanning attention (Valentino et al. 1983; Valentino & Van Bockstaele, 2005, 2008). In addition to its electrophysiological effects, CRF increases dendritic growth of LC neurons in organotypic cultures and of LC-like CATH cells (Cibelli et al. 2001; Swinny & Valentino, 2006). Because expansion of the LC dendritic tree increases the probability that LC dendrites will contact limbic afferents that terminate outside of the nuclear LC in the peri-coerulear region, this effect could provide a structural basis for heightened emotional arousal.
The present study examined the ability of different rearing conditions that have been reported previously to alter CRF levels in the amygdala and LC region to affect LC neuronal activity in slice preparations in vitro. Additionally, the effects of these conditions on LC dendritic morphology were compared.
Materials and methods
Animals
Timed-pregnant Sprague–Dawley rats (Taconic Farms, USA) were delivered on gestation day 15. All dams were housed in standard animal cages with conventional bedding material and ad libitum access to food and water in a room with a 12-h light/dark cycle (lights on 06:00 hours). The variable rearing condition models and nomenclature were adopted according to Plotsky et al. (2005) as follows. The day of birth was considered PND 0. Litters were standardized to 6–8 male pups per dam on PND 2 and were assigned to one of the following rearing conditions from PND 2 to PND 14 inclusive: (1) non-handled [hours of maternal separation (HMS-0)] animals were not exposed to any handling or experimenter manipulation, including cage bedding changes, from PND 2 to PND 14, (2) handling with brief maternal separation for 15 min (HMS-15) from PND 2 to PND 14, (3) handling with longer maternal separation for 180 min (HMS-180) from PND 2 to PND 14 and (4) a control group which consisted of vendor-reared rats (control).
The HMS protocol was initiated in the morning between 09:00 and 11:00 hours. Each dam was removed from the home cage to an adjacent cage, and each litter was removed and placed as a group into a plastic cage (15 × 15 cm) that was transferred to an incubator in an adjacent room. These pup cages were lined with 3 cm of bedding material (wood shavings) and were placed into an incubator set to maintain an ambient temperature of 32 °C. At the conclusion of the separation period, pups were returned to the maternity cage, rolled in the soiled home cage bedding material, and the dam was returned. Rat pups were weaned on PND 21–22. Electrophysiological experiments were performed within the next 2 wk. Care and use of animals was approved by the Institutional Animal Care and Use Committee of the Children's Hospital of Philadelphia.
Electrophysiology
Rats ranging in age from PND 22 to PND 35 were rapidly decapitated and the head placed in ice-cold oxygenated artificial cerebrospinal fluid (aCSF) in which sucrose was substituted for sodium. The brain was rapidly removed and blocked. Following blocking of the tissue, horizontal 200-μm-thick slices of brainstem containing the LC were cut using a Leica microslicer (Leica, USA) and placed in a holding vial containing aCSF at 34–36 °C bubbled with 95% O2/5% CO2 for 1 h. The composition of the aCSF was (in mm): NaCl 124, KCl 3.0, NaH2PO4 1.25, MgSO4 2.0, CaCl2 2.5, dextrose 10 and NaHCO3 26. After 1 h the slices were kept at room temperature, aCSF bubbled with 95% O2/5% CO2 until transferred to the recording chamber. A single slice was placed in a recording chamber and continuously superfused with oxygenated aCSF at 1.5–2 ml/min at 32 °C maintained by an in-line solution heater (TC-324; Warner Instruments, USA). LC neurons were visualized using a Nikon E600 upright microscope (Optical Apparatus, USA) fitted with a 40× water-immersion objective, differential interference contrast and infrared filter. The image from the microscope was enhanced using a CCD camera and displayed on a monitor. Recording pipettes were fashioned on a P-97 micropipette puller (Sutter Instruments, USA) using borosilicate glass capillary tubing (1.2 mm o.d., 0.69 mm i.d.; Warner Instruments). Pipettes were filled with electrolyte of the following composition (in mm): K-gluconate 70, KCl 70, NaCl 2.0, EGTA 4.0, Hepes 10, MgATP 4.0, Na2GTP 0.3, and 0.1% biocytin (pH 7.3).
A visualized cell was approached with the electrode, a GΩ seal established and the cell membrane ruptured to obtain a whole-cell recording using a Multiclamp 700B amplifier (Molecular Devices, USA). Series resistance was monitored throughout the experiment. If the series resistance of the electrode was unstable or exceeded four times the electrode resistance, electrophysiological data from the cell were discarded. The main criteria for accepting a recording were an action potential amplitude of 65–70 mV, action potential shape characteristic of an LC neuron and membrane potential between −50 and −60 mV. If the cell retained a stable baseline and resistance and did not depolarize over time, the cell was retained for analysis. Signals were digitized by Digidata 1320-series analogue-to-digital converter and stored online using pClamp 9 software (Molecular Devices). Only one cell per slice was recorded. The experimental protocol involved recording baseline cell characteristics in current clamp, including firing rate (Hz), input resistance (derived from the linear portion of a voltage-current plot of hyperpolarizing current steps MΩ), resting membrane potential (mV), membrane time constant (τ, ms), action potential amplitude (mV) and duration (ms) and afterhyperpolarization (AHP) amplitude (mV) and AHP t1/2 duration (measured from the peak of the AHP to half the amplitude of the AHP, in ms) (Beck et al. 2004). After determining baseline characteristics, CRF (final concentration of 11 nm) was bath-applied for at least 3 min and then the cell characteristics were measured again. In some experiments, the selective CRF1 antagonist, antalarmin (final concentration of 3 μm) was bath-applied after determining baseline cell characteristics. After antalarmin was in the bath for at least 10 min, the firing rate and membrane properties in the presence of antalarmin were recorded. CRF was then bath-applied as described above and cell characteristics determined again in the presence of both CRF and antalarmin. Data were analysed with Clampfit software (Molecular Devices).
Immunohistochemistry
Following recordings, slices were transferred to a vial containing 4% paraformaldehyde in phosphate buffer (PB, 100 mm, pH 7.4) and kept overnight in fixative. Immunohistochemistry for tyrosine hydroxylase (to identify the LC) and visualization of biocytin (to identify the labelled cell) were performed as follows. Slices were permeabilized with Triton X-100 (0.3%), non-specific binding was blocked with normal donkey serum (5%) for 1 h and slices incubated overnight at 4 °C with a mouse monoclonal antibody against tyrosine hydroxylase (1:1000; ImmunoStar, USA). After washing, the slices were incubated with donkey anti-mouse IgG Alexa Fluor 488 (1:1000) and streptavidin conjugated to Alexa Fluor 594 (1:1000) (Molecular Probes, USA) for 2 h. The slices were mounted on glass slides with Fluoromount-G (SouthernBiotech, USA) and visualized by fluorescence microscopy using a Leica DMRXA microscope. Images were captured with a Hamamatsu ORCA-ER digital camera (Bridgewater, USA) using Open Laboratory software (Improvision, UK).
Morphological analysis
In preliminary experiments the morphological characteristics of LC neurons that were patched for different durations were compared and it was determined that after 10 min there was no difference. For all of the neurons for which morphological characteristics were reported, cells were recorded for at least 30 min. Labelled LC neurons were photographed with a high-resolution charge-coupled device camera at 200× magnification. The morphological analysis was performed by an investigator blind to the treatment group of the specimen. Only cells explicitly located within the LC were included for analysis. Well-established methods of analysis were used to characterize and quantify dendritic morphology of the recorded cells (Swinny et al. 2004; Swinny & Valentino, 2006). The entire contours of dendrites for each cell were traced using the morphometric program supplied by OpenLab (Improvision). Dendritic projections arising from the cell soma were counted as primary dendrites. The branch number was defined as the number of times these primary dendrites gave rise to side branches. The length of the longest process was defined as the distance between the cell body and the most distal dendritic ending. Finally, the total dendritic length was calculated by the addition of the lengths of all dendritic arbors.
Statistical tests
Data are presented as means±standard errors of the mean (s.e.m.) unless otherwise stated. Statistical differences between baseline electrophysiological characteristics and morphological parameters of the different treatment groups were assessed using parametric and non-parametric one-way analysis of variance (ANOVA) and Tukey–Kramer post-hoc test. A paired t test was used to test for significance of the CRF or antalarmin effect.
Solutions and drugs
All chemicals used for making the aCSF and electrolyte were obtained from Sigma (USA). Ovine CRF was supplied by Dr J. Rivier (The Salk Institute, San Diego, CA, USA) and used at a final concentration of 11 nm. Antalarmin (dissolved in DMSO; final DMSO concentration in bath, 0.015%) was provided by Kenner C. Rice (Laboratory of Medicinal Chemistry, NIH/NIDDK, Bethesda, MD, USA) and used at a final concentration of 3 μm.
Results
Intrinsic membrane properties
The spontaneous firing rate of LC neurons of control rats was within the previously reported range (0.5–5 Hz) (Jedema & Grace, 2004; Pan et al. 2002; Williams et al. 1984). Representative raw baseline tracings from different treatment groups are depicted in Fig. 1. There was an effect of rearing condition on mean LC spontaneous firing rate [F(3, 86)=5.7, p= 0.008]. The mean LC spontaneous firing rate was >2-fold greater in HMS-180 rats compared to control and HMS-15 rats (Table 1, Figs 1, 2). The mean spontaneous firing rate of HMS-0 rats was intermediate between HMS-180 and HMS-15 or the control group and not significantly different from any group. Most active and passive membrane characteristics of LC neurons were similar between the different treatment groups (Table 1). An exception to this was the action potential duration that was greater in HMS-0 rats compared to HMS-180 rats (Kruskal–Wallis ANOVA, (3)=10.8, p<0.05).
Fig. 1.

Early rearing environment affects locus coeruleus (LC) spontaneous firing rate. (a–d) Representative traces of whole-cell recordings from LC neurons in current clamp, showing the different baseline firing rates of spontaneously active LC neurons from (a) control, (b) HMS-0, (c) HMS-15 and (d) HMS-180 rats. On average cells from HMS-180 had increased spontaneous firing rates compared to LC neurons from controls and HMS-15 rats.
Table 1.
Baseline electrophysiological properties of locus coeruleus neurons across different treatment groupsa
| Control (n=20) |
HMS-0 (n=26) |
HMS-15 (n=25) |
HMS-180 (n=16) |
Statistics ANOVA | |
|---|---|---|---|---|---|
| Firing rate (Hz) | 1.05±0.15 | 1.47±0.23 | 1.12±0.16 | 2.33±0.34b | p=0.008 |
| Input resistance (mΩ) | 265±30 | 230±48 | 194±15 | 200±13 | p=0.216 |
| Resting membrane potential (mV) | −54.8±1.9 | −53.2±1.2 | −51±1.3 | −50±1.1 | p=0.115 |
| Tau (ms) | 34.9±2 | 35±3.3 | 27.9±2.1 | 34.3±2.8 | p=0.158 |
| AHP amplitude (mV) | 26.7±0.9 | 24.8±0.6 | 25.7±0.7 | 27.8±1.2 | p=0.073 |
| AHP duration (t½: ms) | 62.6±7.2 | 82.2±18.8 | 67.3±9.5 | 45.7±3.7 | p=0.084 |
| Action potential amplitude (mV) | 68.2±0.9 | 66.8±2.6 | 63.4±1.8 | 65.1±1.4 | p=0.164 |
| Action potential duration (ms) | 1.8±0.1 | 2.1±0.2c | 1.6±0.1 | 1.6±0.1 | p=0.013 |
| Action potential threshold (mV) | −29.1±1.2 | −27.9±2.5 | −29.9±1.6 | −30.7±2.1 | p=0.782 |
AHP, Afterhyperpolarization.
Values represent means±s.e.m.
All parameters were assessed at resting membrane potential except for action potential measurements which were conducted at a holding potential of −65 mV. Values in parentheses indicate the number of cells analysed in each group. For control, HMS-0, HMS-15 and HMS-180 groups the number of rats was 7, 15, 10, and 7, respectively.
Compared to control and HMS-15.
Compared to HMS-180.
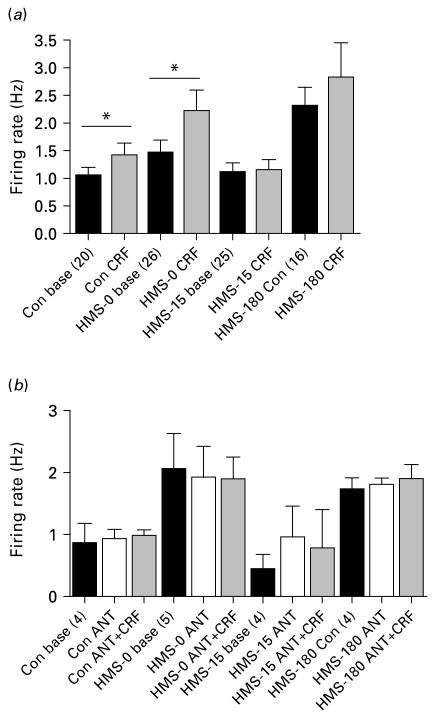
Fig. 2.
Early rearing environment differentially affects the sensitivity of locus coereleus (LC) neurons to the excitatory effects of corticotrophin-releasing factor (CRF) via CRF1. (a) Graphical representation of the differential effects of CRF on the mean firing rate of LC neurons. Bath application of 11 nm CRF significantly increased the firing rate of cells from control and HMS-0 animals (paired t test). In contrast, CRF did not elevate the mean firing rate of cells from HMS-15 or HMS-180 rats. For control, HMS-0, HMS-15 and HMS-180 groups the number of rats used was 7, 15, 10, and 7, respectively. (b) The selective CRF1 antagonist, antalarmin (ANT), blocked the excitatory effects of CRF. The number of cells analysed in each group is indicated in parentheses (* p<0.05).
Sensitivity to CRF
CRF (11 nm) significantly increased mean LC neuronal firing rate in control slices and in slices from HMS-0 rats. In contrast, LC neurons of HMS-15 rats, which had a similar basal firing rate as controls, were resistant to the excitatory effects of CRF (Table 2, Fig. 2). In slices from HMS-180 rats, CRF did not significantly increase firing rate above the already elevated rate (Table 2, Fig. 2). Application of the specific CRF receptor 1 (CRF1) antagonist, antalarmin, in a subset of cells, prior to CRF prevented the effects of CRF, confirming that they were mediated via the CRF1 (Fig. 2).
Table 2.
Effects of bath-applied CRF on selected electrophysiological properties of locus coeruleus neurons across different groupsa
| Control (n=20) | HMS-0 (n=26) | HMS-15 (n=25) | HMS-180 (n=16) | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | CRF | Baseline | CRF | Baseline | CRF | Baseline | CRF | |
| Firing rate (Hz) | 1.05±0.15 | 1.43±0.2 | 1.47±0.23 | 2.23 ±0.37 | 1.12±0.16 | 1.16±0.20 | 2.33±0.34 | 2.83±0.6 |
| p=0.02 | p=0.01 | p=0.48 | p=0.23 | |||||
| Resting membrane potential (mV) | −54.8±1.9 | −48.2±1.3 | −53.2±1.2 | −50.4±1.1 | −50.7±1.3 | −45.6±1.4 | −50.3±1.1 | −48.5±1.0 |
| p=0.002 | p=0.028 | p=0.002 | p=0.056 | |||||
| Input resistance (mΩ) | 265±30 | 273 ±22 | 230±48 | 264±54 | 194±14 | 213±19 | 200±13 | 197±18 |
| p=0.58 | p=0.029 | p=0.09 | p=0.845 | |||||
| AHP duration (t½: ms) | 62.6±7.2 | 51.9±6.2 | 82.2±18.8 | 55.2±14.9 | 67.3±9.5 | 63.8±11.0 | 45.7±3.7 | 34.6±2.5 |
| p=0.014 | p=0.022 | p=0.702 | p=0.002 | |||||
CRF, Corticotrophin-releasing factor; AHP, afterhyperpolarization.
The paired t test was used to test for significance.
Table 2 shows the effects of CRF on some of the active and passive membrane characteristics that were altered for each group. The duration of the AHP was decreased by CRF in all groups except the HMS-15 group (Table 2). Resting membrane potential was depolarized by CRF in all groups, although the effect in neurons from HMS-180 rats barely missed statistical significance (Table 2). CRF increased input resistance in HMS-0 and HMS-15 rats only (Table 2).
Morphological analysis
Figure 3 shows representative biocytin-filled LC neurons. The number of primary dendrites, the number of branch points, the length of the longest dendrite and the total dendritic length per cell were quantified (Fig. 4). The rearing condition did not influence the number of primary dendrites [F(3, 90)=0.13, p=0.94] or length of longest dendrite [F(3, 90)=1.3, p=0.3] (Fig. 4). There were statistically significant differences in the degree of dendritic branching between the experimental groups [F(3, 90)=4.3, p=0.007]. Tukey–Kramer post-hoc analysis revealed that the HMS-15 group showed less branching compared to the control or HMS-0 groups (Fig. 4). Additionally, there was a significant group effect on total dendritic length [F(3, 90)= 4.6, p<0.005]. Tukey–Kramer post-hoc analysis revealed that the main effect was the result of decreased total dendritic length in the HMS-15 group compared to the HMS-0 and HMS-180 groups (Fig. 4).
Fig. 3.

Photomicrographs of filled cells from a HMS-15 rat and HMS-180 rat. The top panels show biocytin-labelled cells visualized with a rhodamine filter. Middle panels show tyrosine hydroxylase (TH) immunoreactivity in same section visualized using a fluorescein filter. Bottom panels show the merged image indicating that the biocytin-labelled cells are TH expressing and within the nucleus locus coereleus. Top is dorsal and medial is to the left. Scale bar, 60 μm.
Fig. 4.
Quantification of different morphological parameters of recorded cells. The mean number of primary dendrites per cell (a) and the length of the longest dendrite (c) were not significantly different across animal groups. In contrast, there was a significant difference in dendritic branching (b) between the four groups [F(3, 90)=4.3, p=0.007]. Post-hoc comparison between groups revealed significant differences in branch number between the control and HMS-15 groups as well as the HMS-0 and HMS-15 groups (* p<0.01, ** p<0.005). In addition, the total dendritic length (d) differed significantly [F(3, 90)=4.6, p<0.005] between the HMS-0 and HMS-15 groups as well as the HMS-180 and HMS-15 groups (** p<0.005). The number of cells analysed in each group are indicated in parentheses in panel (a). For control, HMS-0, HMS-15 and HMS-180 groups the number of rats used was 7, 15, 10 and 7, respectively.
Discussion
Previous studies provided evidence that early life events govern the levels of CRF and/or CRF receptors in a limbic–LC circuit that regulates the magnitude of emotional arousal (Francis et al. 1999; Plotsky et al. 2005). Using the same conditions of varying maternal separation, the present study provides the first examination of the direct impact of early life events on LC structure and function. We propose that the variations in emotional arousal predicted by the early life environment are due in part to the enduring changes in both the physiology and the morphology of LC neurons and that these may result from the respective neuromodulatory and trophic roles of CRF in this region.
A model of CRF dysregulation in a limbic–LC circuit
Early life stress is associated with increased incidence of numerous psychiatric disorders including anxiety, depression, post-traumatic stress disorder (PTSD) and attentional disorders (Kaufman et al. 2000). The paradigm of varying degrees of maternal separation of rat pups used in the present study is thought to model vulnerability or resistance to these disorders seen in humans that have been exposed to early life trauma (Heim & Nemeroff, 2001). Importantly, the condition of a relatively long duration of maternal separation (HMS-180) mimics certain neurobiological changes that have been documented in these disorders. For example, the hypothalamic–pituitary–adrenal axis is dysregulated in depressed subjects in a manner indicative of CRF overactivity (Gold & Chrousos, 2002; Nemeroff et al. 1992; Raadsheer et al. 1994, 1995) and this is reproduced by the HMS-180 condition (Francis et al. 1999; Lippmann et al. 2007; Plotsky et al. 2005). This condition also reproduces the increase in CRF levels in the LC (Plotsky et al. 2005) that has been reported by multiple groups in depressed humans (Austin et al. 2003; Bissette et al. 2003). Amygdalar CRF levels are also elevated in HMS-180 rats. These findings, taken together, are significant because CRF acts as a neurotransmitter to activate the LC–norepinephrine system during stress and the amygdala is a primary source of CRF afferents to LC dendrites (Valentino & Van Bockstaele, 2008; Van Bockstaele et al. 1998). This circuit is thought to underlie emotional arousal (Aston-Jones et al. 1996; Liddell et al. 2005; Sterpenich et al. 2006; Valentino & Van Bockstaele, 2008). CRF up-regulation within this circuit could account for overactivity of the brain norepinephrine system that has been postulated to occur in affective disorders and PTSD (Charney et al. 1993; Ordway & Klimek, 2001; Southwick et al. 1999).
Impact of rearing conditions on LC physiology
CRF, acting as a neuromodulator, increases LC firing rate in vivo and in vitro with a maximum magnitude that approximates a doubling of the pre-CRF discharge rate (Curtis et al. 1997; Jedema & Grace, 2004). A major finding of the present study is that the HMS-180 condition that has been reported to increase CRF levels and CRF receptor binding in the LC resulted in spontaneous firing rates that were approximately twice those of control and HMS-15 rats. The inability of antalarmin to decrease LC firing rates of HMS-180 rats to control levels suggests that the elevated firing rates in this group are not due to ongoing occupation of CRF receptors in the slice preparation. Rather, the elevated CRF levels seen in vivo may have engaged the CRF receptor-effector cascade leading to an extensive duration of response that outlasts receptor occupation perhaps in the face of receptor internalization (Aldenhhoff et al. 1983; Reyes et al. 2006). In this regard, LC activation by CRF is present long after the majority of receptors are internalized (Reyes et al. 2006). Alternatively, the increased LC firing rates in this group may be unrelated to changes in CRF.
The cellular mechanisms underlying the elevated discharge are as yet unknown as membrane characteristics of LC neurons were not significantly altered in HMS-180 rats. However, the duration of the AHP that contributes to the duration of the interspike interval appeared to be smaller in the HMS-180 group compared to the others, an effect that would translate to higher firing rates.
It is noteworthy that LC discharge rates of HMS-0 rats were intermediate and not different from HMS-180. Certain studies suggest that HMS-0 rats are not a control group but represent an experimental treatment group that exhibits abnormal behaviour as evidenced by impairments in latent inhibition (Feldon & Weiner, 1988; Pryce & Feldon, 2003; Weiner et al. 1985). The absence of handling has been suggested to represent an impoverished environment that may also be adverse (Plotsky et al. 2005).
Given reports that the daily handling associated with the 15-min separation procedure (HMS-15) results in an anxiolytic phenotype, this group might be predicted to have lower basal LC firing rates (Caldji et al. 2000). The finding that LC firing rates were comparable in this group compared to controls indicates that the repetitive handling and stimulation involved in the HMS-15 condition does not have an opposing effect to tonically inhibit LC neuronal activity.
CRF increases LC firing rate in vitro in slice preparations (Jedema & Grace, 2004) and this effect was reproduced in control and HMS-0 groups in the present study. The increase in firing rate was accompanied by a membrane depolarization, and decrease in AHP duration. The lack of a statistically significant increase in LC discharge by CRF in HMS-180 rats may signify a pharmacological ceiling effect whereby CRF may not further increase firing rate above the already elevated baseline. Supporting this, CRF dose–response curves for LC activation in vivo plateau at an effect that is between a 100% and 135% increase above baseline (Curtis et al. 1997), similar to the percentage increase in LC spontaneous discharge seen in HMS-180 rats vs. controls.
A significant finding of the present study was that the firing rate of the LC neurons of HMS-15 rats was unaffected by CRF. This suggests that the neonatal stimulation associated with this condition may result in an enduring attenuated sensitivity of this arousal system to stressors. Unlike the situation with HMS-180 rats, the inability of CRF to activate LC neurons of HMS-15 rats could not be attributed to a pharmacological ceiling effect. The lack of effect of CRF on LC neurons in this group is consistent with decreased total CRF binding that has been reported (Plotsky et al. 2005) and may be related to the decreased length of their dendritic trees (see below). The lack of effect on firing rate is somewhat surprising in light of the finding that CRF significantly depolarized the resting membrane potential in the HMS-15 group. However, a primary distinction of this group was the inability of CRF to decrease the half-time of the AHP. The membrane depolarization is due to a decrease in potassium conductance, whereas the ionic mechanism underlying the change in duration of the AHP or the inter-spike interval by CRF is unknown. Therefore, it appears that even though CRF can depolarize the membrane and decrease potassium conductance, the primary effect underlying the change in firing rates is the decrease in the inter-spike interval (Jedema & Grace, 2004).
Impact of rearing conditions on LC dendritic morphology
The acute neuromodulatory effects of CRF have been well documented and are thought to mediate rapid responses to challenges in a dynamic environment. More recently, trophic effects of CRF on the morphology of LC neurons have been identified that suggest a more subtle mechanism by which CRF can regulate the brain norepinephrine system. Initial studies in LC-like CATH cells demonstrated that CRF increases neurite growth through MAP kinase signalling (Cibelli et al. 2001). A subsequent study demonstrated that CRF increased LC dendritic length in organotypic cultures via MAP kinase signalling and a mechanism involving the Rho GTPase, Rac 1 (Swinny & Valentino, 2006). These findings are significant because LC dendrites are known to extend for hundreds of microns outside of the cluster of cell bodies that make up the nucleus LC, into the peri-coerulear region. Indeed, afferents from limbic regions, such as the central nucleus of the amygdala and bed nucleus of the stria terminalis terminate not in the nuclear core, but in this peri-coerulear region where they contact LC dendrites (Van Bockstaele et al. 1998, 1999). The more extensive the LC dendritic tree, the higher the probability of contacting limbic afferents. As the limbic system conveys emotionally related information to the LC this could be another mechanism by which early life environment, by regulating the level of CRF, could shape a structure that determines the magnitude of emotional arousal. The present findings partially support this hypothesis in that cells from HMS-15 rats, the group that generally has been shown to exhibit dampened responses to stressors, showed significantly less dendritic branching and total dendritic length compared with other groups. This is consistent with a diminished trophic effect of CRF that parallels the attenuated physiological effect seen in this study and both of these changes could result from the reported decreased total binding (Plotsky et al. 2005). A less extensive LC dendritic field in HMS-15 rats would be predicted to result in decreased communication with limbic afferents that terminate in the peri-LC and this could contribute to the lower degree of emotional arousal of these subjects. The converse prediction, that the HMS-180 group and possibly the HMS-0 group would have longer dendrites was unfounded. Although the total dendritic length of cells from both HMS-0 and HMS-180 groups were on average greater than control values, this difference did not reach statistical significance. The lack of morphological effects in these groups could be explained by adaptive processes that occur in vivo but not in the explants. Alternatively, the magnitude of elevation in CRF in the LC region produced by the HMS-180 condition may not be sufficient to produce detectable morphological effects in vivo. Finally, it may be that the trophic effect of CRF is the default and only its absence impacts on LC dendritic structure in vivo. Nonetheless, differences in LC morphology between the groups suggest that by the varying levels of CRF and thus its trophic influence, early rearing conditions provide the structural foundations for determining emotional reactivity later on in life.
Caveats
Technical limitations of the whole-cell patch method necessitated the use of young rats for this study, so that it cannot be known for certain whether the electrophysiological and morphological changes produced by neonatal rearing conditions persist into adulthood as do some of the endocrine and behavioural consequences. Nonetheless, it should be noted that the adult LC electrophysiological phenotype is evident from PND 26 (Williams & Marshall, 1987). Finally, although the present data, taken with findings in the literature, suggest a role for CRF in the consequences of neonatal rearing conditions on the LC, absolute confirmation of this would require continuous blockade of CRF receptors during the time of the neonatal manipulation.
Functional significance
Long-term maternal separation used in the present study is thought to model early life adversity and the dysfunctions in CRF–norepinephrine interactions that occur in affective disorders. Here we show that this results in tonically elevated LC discharge. A high tonic mode of LC discharge is thought to be adaptive in a dynamic environment because it facilitates going off-task and searching the environment for alternative strategies, an effect that would be acutely adaptive in stress (Aston-Jones & Cohen, 2005). However, the persistence of high tonic LC activity would have maladaptive consequences, resulting in hyperarousal, inability to concentrate and disruption of tasks requiring focused attention, symptoms that are associated with anxiety, PTSD and depression. In contrast, the daily predictable handling of rats associated with the HMS-15 condition resulted in a system that was tonically ‘normal’, insensitive to CRF and structurally suppressed. The structural consequences of this condition would reinforce an attenuated arousal response to emotional stimuli. Although this would be associated with a non-anxious phenotype, the lack of an appropriate arousal response in the face of an acute stressor is also maladaptive. Together the results underscore the potential impact that rearing conditions during the critical neonatal period can have on behaviour via effects on the brain norepinephrine system.
Acknowledgments
Supported by PHS Grants MH40008 and ONR (N00014-03-1-0311). Dr Swinny's current address is School of Pharmacy and Biomedical Sciences, University of Portsmouth, Portsmouth, UK.
Footnotes
Statement of Interest: None.
References
- Aldenhhoff JB, Gruol DL, Rivier J, Vale W, Siggins GR. Corticotropin-releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science. 1983;221:875–877. doi: 10.1126/science.6603658. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual Review of Neuroscience. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Valentino RJ, Shipley MT. Role of the locus coeruleus in emotional activation. Progress in Brain Research. 1996;107:380–402. doi: 10.1016/s0079-6123(08)61877-4. [DOI] [PubMed] [Google Scholar]
- Austin MC, Janosky JE, Murphy HA. Increased corticotropin-releasing hormone immunoreactivity in monoamine-containing pontine nuclei of depressed suicide men. Molecular Psychiatry. 2003;8:324–332. doi: 10.1038/sj.mp.4001250. [DOI] [PubMed] [Google Scholar]
- Beck SG, Pan YZ, Akanwa AC, Kirby LG. Median and dorsal raphe neurons are not electrophysiologically identical. Journal of Neurophysiology. 2004;91:994–1005. doi: 10.1152/jn.00744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissette G, Klimek V, Pan J, Stockmeier C, Ordway G. Elevated concentrations of CRF in the locus coeruleus of depressed subjects. Neuropsychopharmacology. 2003;28:1328–1335. doi: 10.1038/sj.npp.1300191. [DOI] [PubMed] [Google Scholar]
- Brown GR, Anderson B. Psychiatric morbidity in adult inpatients with childhood histories of sexual and physical abuse. American Journal of Psychiatry. 1991;148:55–61. doi: 10.1176/ajp.148.1.55. [DOI] [PubMed] [Google Scholar]
- Brown GW, Bifulco A, Harris TO. Life events, vulnerability and onset of depression: some refinements. British Journal of Psychiatry. 1987;150:30–42. doi: 10.1192/bjp.150.1.30. [DOI] [PubMed] [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Charney DS, Deutch AY, Krystal JH, Southwick SM, Davis M. Psychobiologic mechanisms of posttraumatic stress disorder. Archives of General Psychiatry. 1993;50:294–305. doi: 10.1001/archpsyc.1993.01820160064008. [DOI] [PubMed] [Google Scholar]
- Cibelli G, Corsi P, Diana G, Vitiello F, Thiel G. Corticotropin-releasing factor triggers neurite outgrowth of a catecholaminergic immortalized neuron via cAMP and MAP kinase signalling pathways. European Journal of Neuroscience. 2001;13:1339–1348. doi: 10.1046/j.0953-816x.2001.01510.x. [DOI] [PubMed] [Google Scholar]
- Cui XJ, Vaillant GE. Antecedents and consequences of negative life events in adulthood: a longitudinal study. American Journal of Psychiatry. 1996;153:21–26. doi: 10.1176/ajp.153.1.21. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bello NT, Connally KR, Valentino RJ. Corticotropin-releasing factor neurons of the central nucleus of the amygdala mediate locus coeruleus activation by cardiovascular stress. Journal of Neuroendocrinology. 2002;14:667–682. doi: 10.1046/j.1365-2826.2002.00821.x. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Florin-Lechner SM, Pavcovich LA, Valentino RJ. Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. Journal of Pharmacology and Experimental Therapeutics. 1997;281:163–172. [PubMed] [Google Scholar]
- Ellenbroek BA, van den Kroonenberg PT, Cools AR. The effects of an early stressful life event on sensorimotor gating in adult rats. Schizophrenia Research. 1998;30:251–260. doi: 10.1016/s0920-9964(97)00149-7. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biological Psychiatry. 2002;52:776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Feldon J, Weiner I. Long-term attentional deficit in nonhandled males: possible involvement of the dopaminergic system. Psychopharmacology (Berlin) 1988;95:231–236. doi: 10.1007/BF00174515. [DOI] [PubMed] [Google Scholar]
- Francis DD, Caldji C, Champagne F, Plotsky PM, Meaney MJ. The role of corticotropin-releasing factor – norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biological Psychiatry. 1999;46:1153–1166. doi: 10.1016/s0006-3223(99)00237-1. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Molecular Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Grace AA. Corticotropin-releasing hormone directly activates noradrenergic neurons of the locus ceruleus recorded in vitro. Journal of Neuroscience. 2004;24:9703–9713. doi: 10.1523/JNEUROSCI.2830-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Plotsky PM, Nemeroff CB, Charney DS. Effects of early adverse experiences on brain structure and function: clinical implications. Biological Psychiatry. 2000;48:778–790. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Walters EE, MacLean C, et al. Stressful life events, genetic liability, and onset of an episode of major depression in women. American Journal of Psychiatry. 1995;152:833–842. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Stohr T, Schuller J, Domeney A, et al. Long-term effects of repeated maternal separation on three different latent inhibition paradigms. Pharmacology Biochemistry and Behavior. 1998;59:873–882. doi: 10.1016/s0091-3057(97)00529-7. [DOI] [PubMed] [Google Scholar]
- Levine S. The ontogeny of the hypothalamic-pituitary-adrenal axis. The influence of maternal factors. Annals of the New York Academy of Science. 1994;746:275–288. doi: 10.1111/j.1749-6632.1994.tb39245.x. discussion 289-293. [DOI] [PubMed] [Google Scholar]
- Liddell BJ, Brown KJ, Kemp AH, Barton MJ, et al. A direct brainstem-amygdala-cortical ‘alarm’ system for subliminal signals of fear. Neuroimage. 2005;24:235–243. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. European Journal of Neuroscience. 2007;25:3091–3098. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nature Neuroscience. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Luecken LJ, Lemery KS. Early caregiving and physiological stress responses. Clinical Psychology Review. 2004;24:171–191. doi: 10.1016/j.cpr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Mitchell JB, Aitken DH, Bhatnagar S, et al. The effects of neonatal handling on the development of the adrenocortical response to stress: implications for neuropathology and cognitive deficits in later life. Psychoneuroendocrinology. 1991;16:85–103. doi: 10.1016/0306-4530(91)90072-2. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Krishnan KR, Reed D, Leder R, et al. Adrenal gland enlargement in major depression. A computed tomographic study. Archives of General Psychiatry. 1992;49:384–387. doi: 10.1001/archpsyc.1992.01820050048008. [DOI] [PubMed] [Google Scholar]
- Ordway GA, Klimek V. Noradrenergic pathology in psychiatric disorders: postmortem studies. CNS Spectrum. 2001;6:697–703. doi: 10.1017/s1092852900001395. [DOI] [PubMed] [Google Scholar]
- Pan YZ, Li DP, Chen SR, Pan HL. Activation of delta-opioid receptors excites spinally projecting locus coeruleus neurons through inhibiition of GABAergic inputs. Journal of Neurophysiology. 2002;88:2675–2683. doi: 10.1152/jn.00298.2002. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, et al. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Feldon J. Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neuroscience and Biobehavioral Reviews. 2003;27:57–71. doi: 10.1016/s0149-7634(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60:436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJ, et al. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer's disease and depression. American Journal of Psychiatry. 1995;152:1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psychology Bulletin. 2002;128:330–366. [PubMed] [Google Scholar]
- Reyes BA, Fox K, Valentino RJ, Van Bockstaele EJ. Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. European Journal of Neuroscience. 2006;23:2991–2998. doi: 10.1111/j.1460-9568.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Developmental Psychopathology. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Bremner JD, Rasmusson A, Morgan CA, 3rd, et al. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biological Psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- Sterpenich V, D'Argembeau A, Desseilles M, Balteau E, et al. The locus ceruleus is involved in the successful retrieval of emotional memories in humans. Journal of Neuroscience. 2006;26:7416–7423. doi: 10.1523/JNEUROSCI.1001-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinny JD, Metzger F, Ijkema-Paassen J, Gounko NV, et al. Corticotropin-releasing factor and urocortin differentially modulate rat Purkinje cell dendritic outgrowth and differentiation in vitro. European Journal of Neuroscience. 2004;19:1749–1758. doi: 10.1111/j.1460-9568.2004.03279.x. [DOI] [PubMed] [Google Scholar]
- Swinny JD, Valentino RJ. Corticotropin-releasing factor promotes growth of brain norepinephrine neuronal processes through Rho GTPase regulators of the actin cytoskeleton in rat. European Journal of Neuroscience. 2006;24:2481–2490. doi: 10.1111/j.1460-9568.2006.05129.x. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL, Aston-Jones G. Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Research. 1983;270:363–367. doi: 10.1016/0006-8993(83)90615-7. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. European Journal of Pharmacology. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele EJ. Functional interactions between stress neuromediators and the locus coeruleus-noradrenaline system. In: Steckler T, Kalin N, editors. Handbook of Stress and the Brain. Amsterdam: Elsevier; 2005. pp. 465–486. [Google Scholar]
- Van Bockstaele EJ, Colago EEO, Valentino RJ. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the coordination of emotional and cognitive limbs of the stress response. Journal of Neuroendocrinology. 1998;10:743–757. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Peoples J, Valentino RJ. Differential regulation of the rostrolateral peri-locus coeruleus region by limbic afferents. Biological Psychiatry. 1999;46:1352–1363. doi: 10.1016/s0006-3223(99)00213-9. [DOI] [PubMed] [Google Scholar]
- Weiner I, Schnabel I, Lubow RE, Feldon J. The effects of early handling on latent inhibition in male and female rats. Developmental Psychobiology. 1985;18:291–297. doi: 10.1002/dev.420180402. [DOI] [PubMed] [Google Scholar]
- Williams JT, Marshall KC. Membrane properties and adrenergic responses in locus coeruleus neurons of young rats. Journal of Neuroscience. 1987;7:3687–3694. doi: 10.1523/JNEUROSCI.07-11-03687.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, North RA, Shefner SA, Nishi S, Egan TM. Membrane properties of rat locus coeruleus neurons. Neuroscience. 1984;13:137–156. doi: 10.1016/0306-4522(84)90265-3. [DOI] [PubMed] [Google Scholar]