Abstract
Following myocardial infarction (MI), circulating blood monocytes respond to chemotactic factors, migrate into the infarcted myocardium, and differentiate into macrophages. At the injury site, macrophages remove necrotic cardiac myocytes and apoptotic neutrophils; secrete cytokines, chemokines, and growth factors; and modulate phases of the angiogenic response. As such, the macrophage is a primary responder cell type that is involved in the regulation of post-MI wound healing at multiple levels. This review summarizes what is currently known about macrophage functions post-MI and borrows literature from other injury and inflammatory models to speculate on additional roles. Basic science and clinical avenues that remain to be explored are also discussed.
Keywords: macrophage, myocardial infarction, matrix metalloproteinases, left ventricular remodeling, angiogenesis, fibrosis
Introduction
A myocardial infarction (MI) occurs when a coronary artery becomes occluded, resulting in an insufficient oxygen supply to the downstream myocardium.1 The myocardium reacts to an ischemic challenge with an intricate series of changes in cellular and extracellular components, characterized at the tissue level by altered wall structure, chamber geometry, and pump function, a process termed left ventricular remodeling.2–4 The lack of oxygen supply induces necrosis in the cardiac myocytes, which stimulates the complement cascade and initiates an inflammatory response.5 The inflammatory component is primarily composed of neutrophil and macrophage infiltration. Macrophages influence several wound healing events, including fibroblast activation necessary for scar formation and endothelial cell activation necessary for angiogenesis.6 The timing and rate of macrophage infiltration is orchestrated by a wide range of cytokines and chemokines.6, 7
Due to improvements in emergency percutaneous coronary interventions (reperfusion) and pharmacologic interventions such as inhibitors of the renin-angiotensin-aldosterone system, the 30-day survival rate in post-MI patients is >90%.4, 8–11 However, a significant number of MI patients (3–40%, depending on the study) will undergo adverse remodeling of the left ventricle (LV), leading to LV dilation and compromised LV function that drives the constellation of signs and symptoms indicative of congestive heart failure (CHF).8 In a subgroup of 412 post-MI patients from the Survival and Ventricular Enlargement (SAVE) trial12 (with ejection fractions <40%), 17% developed CHF by the 2 year follow-up.13 Lavine et al demonstrated a 29% incidence of late onset CHF in post-MI patients diagnosed in 1988–1992, when reperfusion strategies were in use.14 For MI patients evaluated in 1979–1998, CHF incidence was 41% within 6.6±5.0 years and median survival was 4 years after onset.15, 16 Of 3860 stable MI patients from the Cholesterol and Recurrent Events (CARE) study, 6.3% developed CHF within 5 years, at a linear rate of 1.3% per year.17 Because only the most stable post-MI patients were included, this is the most conservative rate reported. While the number of patients who progress to CHF declined over the past 25 years (current rates are approximately 3%), survival in patients diagnosed with CHF has not changed. Regardless of variable incidence rates among studies, MI predisposes patients to CHF, a significant long-term complication and leading cause of mortality in post-MI patients.3 Identifying vulnerable patients early in the pathogenesis, therefore, will allow better risk stratification post-MI.18–20 The macrophage regulates multiple aspects of the post-MI wound healing response, and as such is a likely candidate for investigation and intervention. Accordingly, this review summarizes the role of macrophages in the post-MI wound healing process and discusses outstanding issues that need to be addressed in the field. Ultimately, altering macrophage function may lead to the development of novel therapeutic approaches for LV remodeling.
Macrophage Activation
Macrophages belong to the mononuclear phagocyte system21, and are derived from CD34+ bone marrow progenitors.22 Monocytes within the peripheral blood include a heterogeneous mixture of different cell subpopulations.23 Commitment to the macrophage lineage gives rise to macrophage colony-forming cells, which are succeeded by monoblasts, the first characteristic phagocytic cells.21 Monocytes then enter the blood stream and extravasate into injured tissues in response to chemotactic signals.21, 22, 24
Monocyte adherence to extracellular matrix initiates the conversion to a macrophage, in part by inducing the expression of cytokines such as macrophage colony stimulating factor (M-CSF), tumor necrosis factor α(TNFα), platelet derived endothelial cell growth factor, transforming growth factors α and β (TGFα and β), interleukin-1 (IL-1), and insulin-like growth factor.25 M-CSF is a cytokine necessary for macrophage survival26, TNFα is a potent inflammatory cytokine27, and platelet derived growth factor serves as a potent chemoattractant and mitogen for fibroblasts.25 TGFβ1 release from macrophages also contributes to fibrosis, by stimulating extracellular matrix release (primarily collagen) in myocardial fibroblasts.28 Caspases, aspartate-specific cysteine proteases, are also important for the differentiation of blood monocytes into macrophages.29 In mice, the conditional deletion of caspase-8 in myelomonocytic precursors blocked the formation of macrophages but did not affect the differentiation of dendritic cells or granulocytes.29
Macrophage activation can be a heterogeneous process, resulting in the generation of different classes of cells that exhibit diverse immunological functions.30 There are two major macrophage activation patterns: classical (M1) and alternative (M2), which show distinct cell marker expression (Table 1). There is currently no literature available to assign post-MI macrophages to either pathway, and, because stimuli for both activation pathways are present at varying times post-MI, likely a heterotypic representation occurs. Porcheray and colleagues have shown that macrophage activation occurs first through the M1 pathway, and then shifts to the M2 pathway. Further shifts between pathways are reversible.31
Table 1.
Markers of Classical (M1) and Alternative (M2) Activation.
| Classical (M1) | Alternative (M2) |
|---|---|
| Pro-inflammatory: | Anti-Inflammatory: |
| Arginase I/II | |
| CD 163 | |
| Fas Ligand high | Fas Ligand low |
| Basic Fibroblast Growth Factor | |
| Interferon γ high | IFN γ low |
| Interleukin-1β high | Interleukin-4 Receptor I |
| Interleukin-6 high | IL-6 low |
| Interleukin-8 high | |
| Interleukin-10 low | IL-10 high |
| Interleukin-12 high | IL-12 low |
| Interleukin-23 high | IL-23 low |
| Matrix Metalloproteinases | MS-1-High Molecular Weight Protein |
| Inducible Nitric Oxide Synthase | Transforming Growth Factor β |
| Nitric Oxide | Vascular Endothelial Growth Factor |
| Tumor Necrosis Factor α high | TNF α low |
| Extracellular Matrix Destruction | Extracellular Matrix Reconstruction |
Differential macrophage activation affects cytokine secretion and downstream signaling. M1 macrophage activation is induced by lipopolysaccharide, Th1-related cytokines, or C-C chemokines.32–36 M1 macrophages promote inflammation and ECM destruction.37–41 IL-1β secretion in the M1 phase induces matrix metalloproteinase-9 (MMP-9), which activates TGFβ and stimulates fibroblast proliferation. M2 activation is induced by glucocorticoids or Th2-related cytokines.32–35 M2 macrophages facilitate ECM reconstruction, cell proliferation, and angiogenesis.42–44 Macrophage phagocytosis of apoptotic cells triggers TGFβ production, suggesting that phagocytosis may stimulate the conversion to the M2 phenotype.45 IL-10 inhibits production of IL-1β, IL-6, IL-12, IL-18, and TNF-α to downregulate M1 activation, while TGFβ feeds back to decrease MMP-9 activty by inducing tissue inhibitor of metalloproteinase-1 (TIMP-1). Inducible nitric oxide synthase and MMPs are considered specific for MI activated macrophages; and MS-1 high-molecular-weight protein (MS-1-HMWP)46 and CD163 are specific for M2 activated macrophages.47 The transition and dynamic balance between the two activation phases will determine the net LV remodeling process. However, this dynamic interaction loop has not been clearly defined, and the quantitative relationships among regulation factors have not been described. Because we now have true and reliable markers of macrophage activation (Table 1), studies that characterize activation patterns post-MI will be forthcoming.48
Macrophage activation upregulates phagocytic, chemotactic, secretory, and angiogenic functions, depending on the stimulus (Table 2). The macrophage undergoes reciprocal activation, such that an increase in one function is often accompanied by a decrease in another function.31 Using an in vitro rat bone marrow-derived macrophage model, for example, Erwig and colleagues demonstrated that initial cytokine exposure influences final macrophage function.49 They determined that macrophage activation by one cytokine can affect the response to a subsequent cytokine. Interferon-γ stimulated nitric oxide secretion was blocked if the macrophage was pretreated with IL-4, TGFβ, or TNFα. TNFα stimulated the uptake of apoptotic neutrophils, and this stimulation was blocked if the macrophage was pretreated with Interferon-γ, IL-4, or IL-10. TGFβ stimulated the expression of β glucuronidase, and this stimulation was blocked if the macrophage was pretreated with Interferon-γ. Thus, because the local environment determines which functions will be upregulated, the exact combination of cytokines, chemokines, adhesion molecules, and growth factors surrounding the cell will determine the precise activation phenotype for that particular macrophage. This provides a potential mechanism to explain how heterotypic macrophage differentiation can occur in the same LV.
Table 2.
Primary Macrophage Functions
| Role Post-MI | |
|---|---|
| Phagocytosis | Remove necrotic myocytes and apoptotic neutrophils |
| Chemotaxis | Recruit additional macrophages to injury site to amplify response |
| Secretion | Regulate scar formation by secreting growth factors, angiogenic factors, and MMPs |
| Angiogenesis | Restore blood flow |
Activated macrophages also produce angiotensin converting enzyme (ACE), which induces local angiotensin II (Ang II) expression without activating the systemic renin-angiotensin-aldosterone system.50 While the exact role of macrophage-derived ACE on post-MI remodeling has not been studied, high-density ACE binding is found at the site of infarction from day 4 post-MI, and ACE activity is increased in the aneurysmal infarct.51–54 In addition, ACE inhibitors have been shown to reduce the risk of recurrent MI in patients with LV dysfunction.55 ACE inhibitors specifically reduce LV mass and decrease fibrosis in the viable myocardium, which indicate a direct connection between macrophages, macrophage-derived ACE, myofibroblast stimulation, and LV remodeling.56
Macrophage Roles in the Inflammatory Response in Wound Healing and Post-MI LV Remodeling
The three major components of the MI response are inflammation, scar formation, and scar remodeling, with overlapping time frames between individual components. Macrophages play roles in all three components. Similar to other tissues (e.g. atherosclerotic plaque, skin, lung, and tumors), macrophage infiltration into the LV regulates multiple wound healing functions, including phagocytosis and wound debridement, angiogenesis, fibroblast activation and proliferation, and collagen metabolism.57 Macrophage activation has been extensively studied in animal models of the development and progression of coronary atherosclerosis, and macrophages roles in atherosclerosis have been previously reviewed.58, 59 Because macrophage-depleted animals exhibit defective wound repair, macrophages are likely a primary source of growth factors necessary for scar formation.25, 60–66
The primary role of the macrophage in the post-MI LV is to facilitate wound healing through phagocytosis of necrotic cells and secretion of growth factors and angiogenic molecules. Macrophage migration is directed by signals from the injured myocardium, including signals from resident cells (myocytes) and acute inflammatory cells (neutrophils). The local synthesis of chemoattractants, adhesion molecules, and other proteins recruits and confines macrophages to the site of injury and nearby border zone.67 Activated macrophages, in turn, produce many cytokines, chemokines, and proteases such as MMPs.68, 69
Following MI, the inflammatory reaction is a prerequisite for healing and scar formation (Figure 1). A principle inflammatory cell type that modulates the inflammatory response to MI is the macrophage.70 The inflammatory response is both accelerated and augmented if the ischemic tissue is reperfused. Paradoxically, this enhancement of the inflammatory response actually improves tissue repair. Indeed, early clinical trials inhibiting the inflammatory component with methylprednisolone drastically increased mortality due to LV rupture.7, 71, 72 Several laboratories have recently shown that M-CSF treatment increases macrophage infiltration post-MI, resulting in improved function and accelerated infarct repair, while macrophage depletion using clodronate-containing liposomes impaired wound healing in a cryoinjury mouse model.73–75 Inflammation, therefore, is a necessary component of the healing response. Recently, Leor and colleagues showed that injecting activated macrophages into the ischemic myocardium, immediately after coronary artery ligation, improved myocardial healing, attenuated LV remodeling, and preserved LV function.76 This experiment mimicked reperfusion, except that neutrophils were not included in the equation. This experiment suggests that a major beneficial role of reperfusion is to increase the kinetics of macrophage infiltration.77
Figure 1.
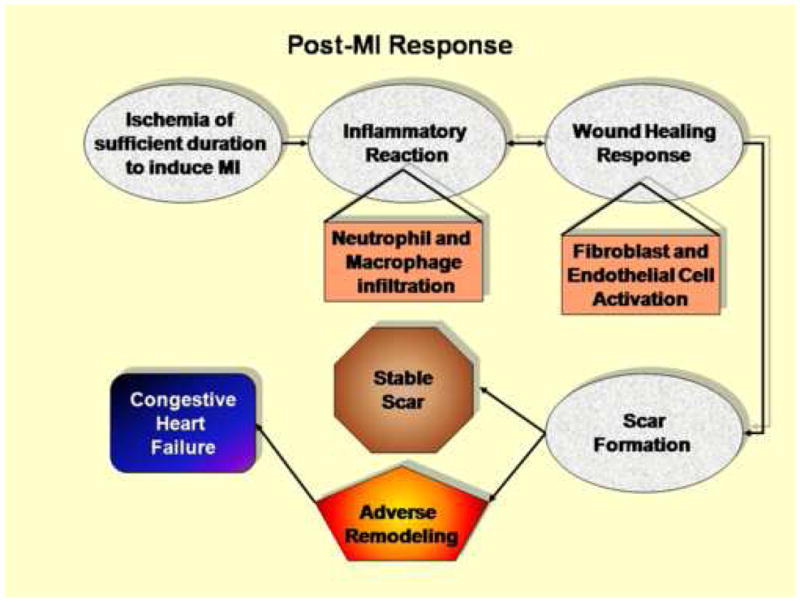
Ischemia of sufficient duration to induce myocardial infarction stimulates an inflammatory reaction that is characterized by infiltration of neutrophils during the first day and macrophages beginning at day 3. The influx of macrophage coordinates the wound healing response, which involves activation of both endothelial cells and cardiac fibroblasts. The coordination between inflammation and scar formation determines the fate post-MI.
The inflammatory response to MI is similar to the inflammatory response to wound healing processes in tissue types throughout the body.25 The primary difference between wound healing in other tissues and the post-MI response is that cardiac myocytes are generally considered to be terminally differentiated cells, which precludes significant tissue regeneration.78, 79 Similarities include a common mechanism of complement activation that mediates the upregulation of a series of cytokines, chemokines, and cell adhesion molecules that trigger the recruitment of leukocytes (predominantly neutrophils and monocytes) into the infarct region.5, 80 Neutrophil roles post-MI have been extensively reviewed previously and will not be considered in detail here.7 Inhibition of local complement activation improves LV function and increases perfusion of previously ischemic myocardium, indicating a role for acute complement activation in exacerbating reperfusion injury.81 Induction of chemokines by complement also initiates monocyte recruitment and macrophage activation, further implicating this pathway.36
No studies have evaluated post-MI macrophage function by directly isolating macrophages from the infarcted myocardium. Instead, peritoneal macrophages or blood monocytes are most frequently isolated and stimulated as surrogate cells to examine the role of cytokines and growth factors on macrophage activation.82–84 While these models do not entirely recapitulate the in vivo environment of the post-MI macrophage, immunohistochemical techniques have been applied to validate in vitro results in the in vivo setting.83
Macrophage infiltration into the myocardium post-MI involves upregulation of both β2 integrins on the blood monocyte and adhesion molecules on the endothelial cell, stimulated by the local generation of chemotactic factors, including apoptotic neutrophils that previously homed to the infarct region.85 Once activated, macrophages produce many cytokines, chemokines and growth factors. Over 150 secretory proteins have been identified from macrophage cultures, including those listed in Table 3.24, 86–89 Among the secreted proteins are IL-1 α and β, IL-6, TNFα, and macrophage inflammatory proteins 1α, 1β, 2α, and 2β 90, 91 Cytokines and growth factors secreted at the site of injury regulate the inflammatory component, angiogenesis, and fibroblast proliferation.7 In particular, monocyte chemotactic protein-1 (MCP-1) is a small molecular weight C-C chemokine that serves as a potent monocyte chemotactic factor in several models of inflammation92, including MI.70, 93 Produced by vascular endothelial cells, smooth muscle cells, and macrophages, MCP-1 heralds macrophage infiltration into the ischemic myocardium.94 No studies to date have examined whether any of the macrophage secreted factors listed in Table 3 could be used to discriminate between normal wound healing and adverse LV remodeling post-MI.
Table 3.
| Cytokines and Chemokines | Growth Factors |
|---|---|
| Interferon α | Angiotensinogen |
| Interleukin-1α and β-6, -8, -10 | Basic fibroblast growth factor |
| Macrophage inflammatory proteins | Endothelial cell inhibitory factor |
| Monocyte chemotactic protein-1 | Granulocyte colony stimulating factor |
| Tumor necrosis factor α | Granulocyte macrophage colony stimulating factor |
| Proteases and Protease Inhibitors | Insulin-like Growth Factor 1 |
| Angiotensin converting enzyme | Macrophage colony stimulating factor |
| ADAMTS-4, -7, -8, -9 | Monocyte chemotactic protein-1 |
| α2-macroglobulin | Substance P |
| Caspases 2, 3, 8, 9 | Thrombospondin-1 |
| Cathepsin B | Transforming growth factors and |
| MMP-1, -7, -8, -9, -12 | Vascular endothelial growth factor |
| Plasmin | |
| Plasminogen activator inhibitor 1 |
Macrophage Roles in Post-MI Phagocytosis and Wound Debridement
A primary function of the macrophage is to engulf apoptotic and necrotic cells within an injured tissue.95 Experiments involving the depletion of macrophages and circulating monocytes resulted in decreased wound debridement, reduced fibroblast activation, and reduced fibrotic response in a rat kidney injury model.96 Galectin-3 is an animal lectin abundantly expressed in macrophages that has been implicated in regulating phagocytosis. Cells deficient in galectin-3 display reduced phagocytosis of apoptotic cells both in vitro and in vivo.97 Phenotypic alterations in macrophage response can be evaluated by monitoring phagocytosis in isolated cells. Marée and colleagues elegantly monitored in vitro time-course assays of the number of engulfed apoptotic cells observed within macrophages between a diabetic and non-diabetic group to quantify rates of macrophage phagocytosis in terms of rates and percentage uptake of cells over time. They observed that macrophage defects can be a potential pre-disposition to diseases such as type 1 diabetes.98 Parameters of macrophage phagocytosis that can be evaluated include percentage phagocytosed, phagocytic index, densities of activated and unactivated macrophages, maximum number of engulfed cells, and several rate variables (Table 4).
Table 4.
Parameters that quantify macrophage phagocytosis 98
|
Macrophage Roles in Post-MI Scar Formation
Myocardial fibroblasts actively regulate the post-MI response by influencing myocardial structural, biochemical, mechanical, and electrical properties of the myocardium.99 As such, fibroblasts sense, integrate, and functionally respond to multiple factors by altering ECM turnover.100 The source of the post-MI myofibroblast, whether from existing resident fibroblasts, bone marrow-derived precursors, or a combination of sources, remains controversial.101–106 Nonethless, fibroblast conversion to a myofibroblast phenotype is characterized by increased expression of α smooth muscle actin expression, proliferation, and ECM production (collagen I, collagen III, fibronectin, and laminin).107 Fibroblasts secrete collagen to form first a provisional scar and later a more advanced scar. Fibroblasts also synthesize many other factors relevant to LV remodeling, including MMPs, TIMPs, IL-1, IL-6, TGFβ, connective tissue growth factor, TNFα, Ang II, and endothelin I.108–116 Ang II stimulates fibroblasts transformation into myofibroblast and induces ECM production.117–120 TGFβ released from necrotic myocytes and activated macrophages is also important in the phenotypic transformation of interstitial fibroblast to myofibroblasts, which express receptors to Ang II, TGFβ, and endothelin-1.116, 121
There are multiple response factors that will amplify fibroblast activation, and the macrophage is a major source for these initiating factors. Interestingly, fibroblast activation also occurs at day 3 post-MI, in parallel with the observed changes in macrophage migration and activation. Yano and colleagues have shown that the level of collagen deposited post-MI is directly correlated with macrophage numbers.74 The cause and effect link between macrophage infiltration and concomitant fibroblast activation, however, remains to be explored.
Macrophage Roles in Post-MI Angiogenesis
The term angiogenesis describes the sprouting of new capillaries from postcapillary venules.122, 123 Angiogenesis stimulated by tissue hypoxia occurs via activation of hypoxia-inducible factor 1α gene expression and usually leads to the development of capillaries.124 In contrast, arteriogenesis refers to the process of maturation or de novo growth of collateral conduits122, 125, 126 and typically occurs outside the area of ischemia.122 Both are important components of inflammatory reactions and subsequent repair processes.21
Angiogenesis is tightly regulated and occurs within the context of a fine balance between conditions that facilitate (angiogenic) and those that inhibit (angiostatic) vessel formation.86 A diverse literature implicates macrophages in the angiogenic process, and, indeed, the single most extensively evaluated role for macrophages is with angiogenesis.23 The occurrence of angiogenesis correlates positively with the number of macrophages in various injury models, including MI, stroke, and skin wound healing.127 Manoonkitiwongsa and colleagues have proposed a “clean up hypothesis” to explain the relationship between macrophages and angiogenesis in the brain following stroke, in that new blood vessels are formed to promote macrophage infiltration and to remove necrotic cells.127 During wound healing, DNA synthesis increases in endothelial cells concomitant with macrophage infiltration, and macrophages isolated from such wound sites induce angiogenesis by in vitro assays.128 Culture media from activated macrophages also stimulates angiogenesis, while unstimulated peritoneal macrophage conditioned media does not.129
Activated macrophages have the capability to influence each phase of the angiogenic process, including alterations of the local extracellular matrix, induction of endothelial cells to migrate or proliferate, and inhibition of vascular growth with formation of differentiated capillaries (Figure 2).21 Macrophages are key angiogenic effector cells, producing a number of growth stimulators and inhibitors130, yet also help to turn off angiogenesis by releasing angiostatic factors such as thrombospondin-1 and various cytokines (e.g. interferon α, monocyte-derived endothelial cell inhibitory factor, IL-1, TNFα, and TGFβ).21
Figure 2.
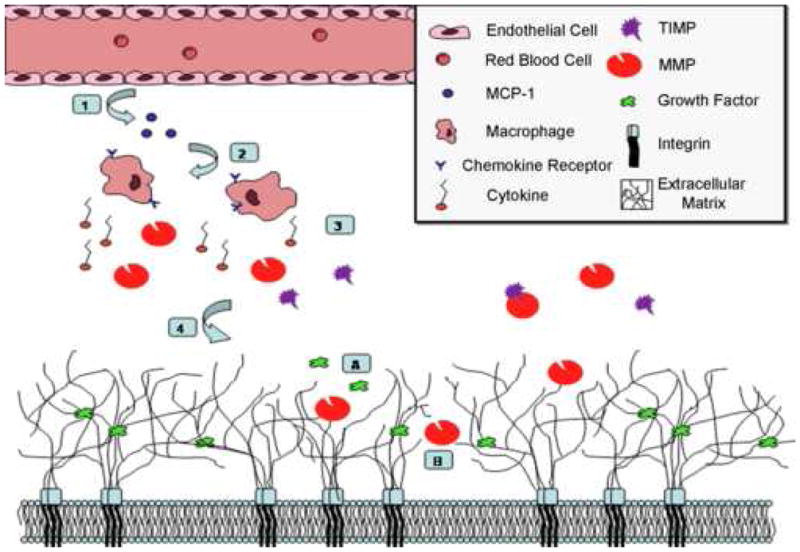
Macrophage regulation of angiogenesis in ischemic tissue. 1) Vascular endothelial cells release monocyte chemotactic protein-1 (MCP-1) into the extracellular space. 2) MCP-1 attracts macrophages with the MCP-1 receptor, C-C chemokine receptor. 3) Activated macrophages secrete a variety of substances, including cytokines and matrix metalloproteinases (MMPs). 4) MMPs and TIMPs may function in early angiogenesis through interactions with extracellular matrix components. A) Degradation of matrix can release and activate extracellular matrix-bound growth factors and angiogenic molecules. MMPs and TIMPs also stimulate proliferation of endothelial and smooth muscle cells. B) MMP migration through matrix can create a pathway in the extracellular matrix for endothelial or precursor cells for angiogenesis.
Macrophages Induce Angiogenesis via Matrix Metalloproteinases (MMPs)
MMPs are a family of zinc-dependent enzymes comprised of more than 25 individual members divided into specific classes based on in vitro extracellular matrix substrate assays.131 Interestingly, MMPs also show specificity for many non-matrix proteins such as cytokines, adhesion molecules, growth factors, and receptors.132 MMPs are produced by constitutive myocardial cell types (myocytes, fibroblasts, and endothelial cells), as well as by inducible cell types (neutrophils, macrophages, and myofibroblasts).2 MMP activation requires cleavage of an approximately 10 kD propeptide in the amino terminus through the cysteine switch mechanism.131 When the peptide domain is cleaved, the bond between a cysteine in the propeptide domain and the active site zinc is disrupted to expose the active site. MMPs are regulated at every level, including transcription, translation, secretion, and activation. Studies conducted by Shankavaram et al. indicate that agents that block any part of the signal transduction pathway will inhibit MMP synthesis. Although this inhibition mechanism is published, this strategy is not highly selective.2, 133
Induction of angiogenesis and subsequent migration of endothelial cells requires the dissolution of the capillary basement membrane and the local extracellular matrix.21, 134–136 Macrophages are a rich source of MMPs that can degrade extracellular matrix molecules, modulate mechanical architecture, and liberate extracellular matrix-bound growth factors.21 Thus, MMP and plasmin release by local macrophages may have profound effects on the formation of new vasculature as cell-matrix interactions are modified.24
Moldovan and colleagues demonstrated that macrophages also serve a structural role in angiogenesis by actively digging channels in the matrix that become inhabited with endothelial cells to form capillaries.23, 24 The sequence of events for endothelial cells begins with the destruction of the basement membrane and local degradation of the extracellular matrix, a process mediated by macrophage-derived MMPs. This allows endothelial cells to migrate by extending cytoplasmic buds in the direction of chemotactic factors.21 Initially, macrophages may modify the extracellular matrix by drilling a tunnel, which would thereafter be colonized either by capillary sprouts or by endothelial progenitor cells.86, 137 At the same time, macrophages potentially nurture and/or attract additional cells by secreting differentiation, growth, and angiogenic factors.86 Macrophage-derived MMPs also produce angiostatic factors. For example, MMP-7, -9, and -12 can generate angiostatin through cleavage of plasminogen.138, 139
MMP-Directed Extracellular Matrix Degradation Releases Pro-angiogenic Factors
Both pro-angiogenic and anti-angiogenic factors are bound to matrix components, and as such are functionally sequestered within the intact extracellular matrix. These factors include basic fibroblast growth factor, TGFβ, and granulocyte-macrophage colony-stimulating factor. Proteolytic modification of the matrix may facilitate their enzymatic release, making them available for endothelial cells.21, 24 Modification of the extracellular matrix also yields fragments of constituent proteins, which have potent effects on the angiogenic process.24 Matricryptin is the term first coined by Davis et al. to describe peptides generated from the degradation of ECM macromolecules.140–142 Matricryptins, also known as matrikines or cryptic fragments, are biologically active molecules with relevant biological roles, including effects on vascular myogenic response, angiogenesis or tumor formation.140, 141, 143–148 What role matricryptins play in regulating macrophage function, however, has not been explored.
Additional Roles of Macrophage-Derived MMPs
Macrophages synthesize several MMP types, including MMP-1, -7, -8, -9, and -12. These particular MMPs may facilitate and direct macrophage migration, amplify the inflammatory response, and mediate signaling processes through proteolysis of both extracellular matrix and non-matrix substrates, ultimately influencing the myocardial response to MI. McGavigan and colleagues monitored serological markers of collagen synthesis and degradation in the plasma of 51 post-MI patients. They estimated there was approximately a 25% net loss of collagen within the first 24 h post-MI, and that patients with the highest degradation rates had the highest remodeling as measured by echocardiographic indices.149 This experiment suggests that collagen degradation by MMPs is a highly relevant function early post-MI.
Of the MMPs identified in the macrophage, MMP-9 may be the most relevant to post-MI remodeling. MMP-9 readily digests denatured collagens and gelatins150, and additionally can process multiple cytokines and adhesion molecule (e.g., endothelin-1, intercellular adhesion molecule-1, IL-1β and IL-8).151–153 MMP-9 is prominently expressed during macrophage differentiation and over-expressed post-MI.154 MMP-9 null mice display attenuated LV remodeling during the early healing process post-MI, suggesting a prominent role for MMP-9 in the post-MI response.155 MMP-9 may also play a specific role in angiogenesis. Johnson and colleagues demonstrated that MMP-9 was necessary for capillary branching, as MMP-9 null mice showed a blunted angiogenic response following skeletal muscle ischemia.156 In the myocardium, however, MMP-9 deletion promoted post-MI angiogenesis.157 Together, the above studies provide rationale for examining MMP inhibitors that target particular MMPs, including MMP-9. Currently, there are no individual MMP type inhibitors, and clinical trials using broad spectrum MMP inhibitors in cancer studies have been plagued with side effects attributed to the non-selective nature of these protease inhibitors.158–161 Future studies evaluating the use of MMP-9 specific inhibitors may yield promising results. Targeted drug delivery strategies that selectively inhibit MMP-9 in the cardiac tissue, rather than systemic inhibition, may also need to be considered.
In addition to MMPs, macrophages also express TIMPs. Activated MMPs are inhibited by binding of the TIMP C-terminal domain.150 TIMPs play a role in a variety of disease processes, including post-MI remodeling. Previous studies reveal that endogenous MMP inhibitory control is lost early in the post-MI period, and this loss of TIMP was a major factor in LV remodeling.162, 163 In addition to MMP blocking functions, TIMPs also regulate cell growth and signaling.164 Little is known about the role of macrophage-derived TIMPs in LV remodeling, and the net balance between macrophage-derived MMP activity and TIMP inhibition remains to be further clarified.
Future Directions
While many studies correlate macrophage infiltration with changes in LV remodeling parameters, direct causal links between macrophages and many of the above-mentioned processes remains to be experimentally established. The exciting results already seen in the macrophage biology arena reveal avenues for basic science research, in regards to macrophage function in the post-MI setting specifically and the wound-healing environment generally. A sampling of questions that remain to be explored include:
How do macrophage roles in the LV post-MI compare and contrast with macrophage roles in other tissues? Are macrophages in the post-MI myocardium identical to macrophages that infiltrate into other injury sites? What are features in common and what are distinct characteristics of myocardial macrophage activation? How do these changes affect macrophage function?
What are the complete functions of macrophages post-MI? Which components of the macrophage response are beneficial and/or necessary for wound healing, and which components are detrimental? Can detrimental components be selectively inhibited therapeutically?
What is the complete secretory catalogue of macrophages, both in the basal state and following activation? Using genomic and proteomic techniques to identify the complete catalogue of secretory proteins expressed in macrophages, both in the unactivated and activated states, will help to assign previously unknown roles for macrophages in remodeling.
Can macrophages be used to target drug therapy to the MI site? Several groups have proposed using macrophages to target drug delivery, and whether this strategy will be effective remains to be determined.6, 90, 95, 165, 166
How do macrophages regulate the function of other cell types post-MI, particularly endothelial cells and myofibroblasts? What role will influencing macrophage function have on these other cell types?
From a clinical research view, there are also several outstanding issues to be resolved. The recent results of the Assessment of Pexelizumab in Acute Myocardial Infarction (APEX-AMI) trial indicate that further mechanistic insight is still required.167 Patients were randomized to receive either placebo or Pexelizumab, a C5a inhibitor, 10 min prior to primary percutaneous transluminal coronary intervention (PCI), and treatment was continued for 24 h. At 30 days follow up, there was no difference in survival between the two groups, indicating that PCI already provided maximal benefit for short-term mortality. Whether Pexelizumab prevented the long-term progression to heart failure was not measured. Future trials evaluating the effects of short-term anti-inflammatory strategies on long-term outcomes is warranted. In addition, improved imaging modalities, including contrast echocardiography and magnetic resonance imaging, now provide improved monitoring opportunities to evaluate infarct scar development and remodeling. In conclusion, inhibition studies using anti-inflammatory strategies have taught us the importance of discerning beneficial and detrimental roles for the macrophage post-MI. The ability of isolate these opposing functions could potentially be used to derive novel therapeutic strategies.
Acknowledgments
Funding
The authors gratefully acknowledge support from the National Institutes of Health (National Heart Lung and Blood Institute, HL-75360). JML was a recipient of the UTHSCSA Medical Student Research Stipend Program and EFL was the recipient of a NIH research supplement (HL-75360S1).
References
- 1.Fishbein MC, Maclean D, Maroko PR. The Histopathologic Evolution of Myocardial Infarction. Chest. 1978;73:843–9. doi: 10.1378/chest.73.6.843. [DOI] [PubMed] [Google Scholar]
- 2.Lindsey ML. MMP induction and inhibition in myocardial infarction. Heart Fail Rev. 2004;9:7–19. doi: 10.1023/B:HREV.0000011390.44039.b7. [DOI] [PubMed] [Google Scholar]
- 3.Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367:356–67. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 4.Pfeffer MA, Braunwald E. Ventricular Remodeling After Myocardial Infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–72. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 5.Rossen RD, Michael LH, Kagiyama A, Savage HE, Hanson G, Reisberg MA, Moake JN, Kim SH, Self D, Weakley S. Mechanism of complement activation after coronary artery occlusion: evidence that myocardial ischemia in dogs causes release of constituents of myocardial subcellular origin that complex with human C1q in vivo. Circulation research. 1988;62:572–84. doi: 10.1161/01.res.62.3.572. [DOI] [PubMed] [Google Scholar]
- 6.Ren G. Inflammatory mechanisms in myocardial infarction. Current Drug Targets -Inflammation & Allergy. 2003;2:242–56. doi: 10.2174/1568010033484098. [DOI] [PubMed] [Google Scholar]
- 7.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovascular research. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 8.Solomon SD, Glynn RJ, Greaves S, Ajani U, Rouleau J-L, Menapace F, Arnold JMO, Hennekens C, Pfeffer MA. Recovery of Ventricular Function after Myocardial Infarction in the Reperfusion Era: The Healing and Early Afterload Reducing Therapy Study. Ann Intern Med. 2001;134:451–8. doi: 10.7326/0003-4819-134-6-200103200-00009. [DOI] [PubMed] [Google Scholar]
- 9.Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ, Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–77. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 10.Pfeffer JM, Fischer TA, Pfefer MA. Angiotensin-Converting Enzyme Inhibition and Ventricular Remodeling After Myocardial Infarction. Annu Rev Physiol. 1995;57:805–26. doi: 10.1146/annurev.ph.57.030195.004105. [DOI] [PubMed] [Google Scholar]
- 11.Solomon SD, Pfeffer MA. The decreasing incidence of left ventricular remodeling following myocardial infarction. Basic research in cardiology. 1997;92:61–5. doi: 10.1007/BF00805561. [DOI] [PubMed] [Google Scholar]
- 12.Sutton MSJ, Pfeffer MA, Moye L, Plappert T, Rouleau JL, Lamas G, Rouleau J, Parker JO, Arnold MO, Sussex B, Braunwald E. Cardiovascular Death and Left Ventricular Remodeling Two Years After Myocardial Infarction: Baseline Predictors and Impact of Long-term Use of Captopril: Information From the Survival and Ventricular Enlargement (SAVE) Trial. Circulation. 1997;96:3294–9. doi: 10.1161/01.cir.96.10.3294. [DOI] [PubMed] [Google Scholar]
- 13.Solomon SD, Sutton MSJ, Lamas GA, Plappert T, Rouleau JL, Skali H, Moye L, Braunwald E, Pfeffer MA for the Survival And Ventricular Enlargement (SAVE) Investigators. Ventricular Remodeling Does Not Accompany the Development of Heart Failure in Diabetic Patients After Myocardial Infarction. Circulation. 2002;106:1251–5. doi: 10.1161/01.cir.0000032313.82552.e3. [DOI] [PubMed] [Google Scholar]
- 14.Lavine SJ. Prediction of heart failure post myocardial infarction: comparison of ejection fraction, transmitral filling parameters, and the index of myocardial performance. Echocardiography. 2003;20:691–701. doi: 10.1111/j.0742-2822.2003.02156.x. [DOI] [PubMed] [Google Scholar]
- 15.Hellermann JP, Jacobsen SJ, Redfield MM, Reeder GS, Weston SA, Roger VL. Heart failure after myocardial infarction: clinical presentation and survival. Eur J Heart Fail. 2005;7:119–25. doi: 10.1016/j.ejheart.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Hellermann JP, Goraya TY, Jacobsen SJ, Weston SA, Reeder GS, Gersh BJ, Redfield MM, Rodeheffer RJ, Yawn BP, Roger VL. Incidence of heart failure after myocardial infarction: is it changing over time? Am J Epidemiol. 2003;157:1101–7. doi: 10.1093/aje/kwg078. [DOI] [PubMed] [Google Scholar]
- 17.Lewis EF, Moye LA, Rouleau JL, Sacks FM, Arnold JM, Warnica JW, Flaker GC, Braunwald E, Pfeffer MA. Predictors of late development of heart failure in stable survivors of myocardial infarction: the CARE study. J Am Coll Cardiol. 2003;42:1446–53. doi: 10.1016/s0735-1097(03)01057-x. [DOI] [PubMed] [Google Scholar]
- 18.Anavekar NS, McMurray JJV, Velazquez EJ, Solomon SD, Kober L, Rouleau J-L, White HD, Nordlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, et al. Relation between Renal Dysfunction and Cardiovascular Outcomes after Myocardial Infarction. N Engl J Med. 2004;351:1285–95. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 19.Sanz G. Risk stratification in acute coronary syndromes: an unresolved issue. Rev Esp Cardiol. 2007;60 (Suppl 3):23–30. [PubMed] [Google Scholar]
- 20.Bhatheja R, Mukherjee D. Acute Coronary Syndromes: Unstable Angina/Non-ST Elevation Myocardial Infarction. Critical Care Clinics. 2007;23:709–35. doi: 10.1016/j.ccc.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol. 1994;55:410–22. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 22.Lewis CE, Pollard JW. Distinct Role of Macrophages in Different Tumor Microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 23.Moldovan NI, Goldschmidt-Clermont PJ, Parker-Thornburg J, Shapiro SD, Kolattukudy PE. Contribution of Monocytes/Macrophages to Compensatory Neovascularization: The Drilling of Metalloelastase-Positive Tunnels in Ischemic Myocardium. Circulation research. 2000;87:378–84. doi: 10.1161/01.res.87.5.378. [DOI] [PubMed] [Google Scholar]
- 24.Crowther M, Brown NJ, Bishop ET, Lewis CE. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J Leukoc Biol. 2001;70:478–90. [PubMed] [Google Scholar]
- 25.Singer AJ, Clark RAF. Cutaneous Wound Healing. New England Journal of Medicine. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 26.Frangogiannis NG, Mendoza LH, Ren G, Akrivakis S, Jackson PL, Michael LH, Smith CW, Entman ML. MCSF expression is induced in healing myocardial infarcts and may regulate monocyte and endothelial cell phenotype. Am J Physiol Heart Circ Physiol. 2003;285:H483–92. doi: 10.1152/ajpheart.01016.2002. [DOI] [PubMed] [Google Scholar]
- 27.Mann DL. STRESS-ACTIVATED CYTOKINES AND THE HEART: From Adaptation to Maladaptation. Annual Review of Physiology. 2003;65:81–101. doi: 10.1146/annurev.physiol.65.092101.142249. [DOI] [PubMed] [Google Scholar]
- 28.Jaffer FA, Sosnovik DE, Nahrendorf M, Weissleder R. Molecular imaging of myocardial infarction. Journal of molecular and cellular cardiology. 2006;41:921–33. doi: 10.1016/j.yjmcc.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Cathelin S, Rebe C, Haddaoui L, Simioni N, Verdier F, Fontenay M, Launay S, Mayeux P, Solary E. Identification of Proteins Cleaved Downstream of Caspase Activation in Monocytes Undergoing Macrophage Differentiation. J Biol Chem. 2006;281:17779–88. doi: 10.1074/jbc.M600537200. [DOI] [PubMed] [Google Scholar]
- 30.Van Ginderachter JA, Movahedi K, Hassanzadeh Ghassabeh G, Meerschaut S, Beschin A, Raes G, De Baetselier P. Classical and alternative activation of mononuclear phagocytes: Picking the best of both worlds for tumor promotion. Immunobiology. 2006;211:487–501. doi: 10.1016/j.imbio.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Porcheray F. Macrophage activation switching: an asset for the resolution of inflammation. Clinical and Experimental Immunology. 2005;142:481–9. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. The Journal of experimental medicine. 1992;176:287–92. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kodelja V, Muller C, Politz O, Hakij N, Orfanos C, Goerdt S. Alternative macrophage activation-associated CC-chemokine-1, a novel structural homologue of macrophage inflammatory protein-1 alpha with a Th2-associated expression pattern. Journal of Immunology. 1998;160:1411–8. [PubMed] [Google Scholar]
- 34.Tormey V, Faul J, Leonard C, Burke C, Dilmec A, Poulter L. T-cell cytokines may control the balance of functionally distinct macrophage populations. Immunology. 1997;90:463–9. doi: 10.1046/j.1365-2567.1997.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goerdt S, Politz O, Schledzewski K, Birk R, Gratchev A, Guillot P, Hakiy N, Klemke C, Dippel E, Kodelja V, Orfanos C. Alternative versus classical activation of macrophages. Pathobiology. 1999;67:222–6. doi: 10.1159/000028096. [DOI] [PubMed] [Google Scholar]
- 36.Frangogiannis NG. Chemokines in ischemia and reperfusion. Thrombosis and haemostasis. 2007;97:738–47. [PubMed] [Google Scholar]
- 37.Hart P, Vitti G, Burgess D, Whitty G, Piccoli D, Hamilton J. Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:3803–7. doi: 10.1073/pnas.86.10.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hart P, Whitty G, Burgess D, Croatto M, Hamilton J. Augmentation of glucocorticoid action on human monocytes by interleukin-4. Lymphokine Research. 1990;9:147–53. [PubMed] [Google Scholar]
- 39.Chizzolini C, Rezzonico R, De Luca C, Burger D, Dayer J. Th2 cell membrane factors in association with IL-4 enhance matrix metalloproteinase-1 (MMP-1) while decreasing MMP-9 production by granulocyte-macrophage colony-stimulating factor-differentiated human monocytes. Journal of Immunology. 2000;164:5952–60. doi: 10.4049/jimmunol.164.11.5952. [DOI] [PubMed] [Google Scholar]
- 40.Gibbs DF. Role of matrix metalloproteinases in models of macrophage-dependent acute lung injury. Evidence for alveolar macrophage as source of proteinases. American Journal of Respiratory Cell and Molecular Biology. 1999;20:1145–54. doi: 10.1165/ajrcmb.20.6.3482. [DOI] [PubMed] [Google Scholar]
- 41.Gibbs DF. Characterization of matrix metalloproteinases produced by rat alveolar macrophages. American Journal of Respiratory Cell and Molecular Biology. 1999;20:1136–44. doi: 10.1165/ajrcmb.20.6.3483. [DOI] [PubMed] [Google Scholar]
- 42.Song E, Ouyang N, Horbelt M, Antus B, Wang M, Exton M. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cellular Immunology. 2000;204:19–28. doi: 10.1006/cimm.2000.1687. [DOI] [PubMed] [Google Scholar]
- 43.Cao B. The potential role of PDGF, IGF-1, TGF-beta expression in idiopathic pulmonary fibrosis. Chinese Medical Journal (English Edition) 2000;113:776–82. [PubMed] [Google Scholar]
- 44.Sunderkotter C, Goebeler M, Schulze-Osthoff K, Bhardwaj R, Sorg C. Macrophage-derived angiogenesis factors. Pharmacol Ther. 1991;51:195–216. doi: 10.1016/0163-7258(91)90077-y. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X, Mosser D. Macrophage activation by endogenous danger signals. J of Pathology. 2008;214:161–78. doi: 10.1002/path.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goerdt S, Bhardwaj R, Sorg C. Inducible expression of MS-1 high-molecular-weight protein by endothelial cells of continuous origin and by dendritic cells/macrophages in vivo and in vitro. American Journal of Pathology. 1993;142:1409–22. [PMC free article] [PubMed] [Google Scholar]
- 47.Zwadlo G, Voegeli R, Osthoff K, Sorg C. A monoclonal antibody to a novel differentiation antigen on human macrophages associated with the down-regulatory phase of the inflammatory process. Experimental Cell Biology. 1987;55:295–304. doi: 10.1159/000163432. [DOI] [PubMed] [Google Scholar]
- 48.Mosser DM. The many faces of macrophage activation. J Leukocyte Biol. 2003;73:209–12. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 49.Erwig L-P, Kluth DC, Walsh GM, Rees AJ. Initial Cytokine Exposure Determines Function of Macrophages and Renders Them Unresponsive to Other Cytokines. J of Immunology. 1998;161:1983–8. [PubMed] [Google Scholar]
- 50.Weber KT. Extracellular matrix remodeling in heart failure: a role for de novo angiotensin II generation. Circulation. 1997;96:4065–82. doi: 10.1161/01.cir.96.11.4065. [DOI] [PubMed] [Google Scholar]
- 51.Hokimoto S, Yasue H, Fujimoto K, Sakata R, Miyamoto E. Increased angiotensin converting enzyme activity in left ventricular aneurysm of patients after myocardial infarction. Cardiovasc Res. 1995;29:664–9. [PubMed] [Google Scholar]
- 52.Hirsch A, Talsness C, Schunkert H, Paul M, Dzau V. Tissue-specific activation of cardiac angiotensin converting enzyme in experimental heart failure. Circulation Research. 1991;69:475–82. doi: 10.1161/01.res.69.2.475. [DOI] [PubMed] [Google Scholar]
- 53.Sun Y, Cleutjens J, Diaz-Arias A, Weber K. Cardiac angiotensin converting enzyme and myocardial fibrosis in the rat. Cardiovascular Research. 1994;28:1423–32. doi: 10.1093/cvr/28.9.1423. [DOI] [PubMed] [Google Scholar]
- 54.Ou R, Sun Y, Ganjam V, Weber K. In situ production of angiotensin II by fibrosed rat pericardium. Journal of Molecular and Cellular Cardiology. 1996;28:1319–27. doi: 10.1006/jmcc.1996.0122. [DOI] [PubMed] [Google Scholar]
- 55.Napoleone E, Di Santo A, Camera M, Tremoli E, Lorenzet R. Angiotensin-converting enzyme inhibitors downregulate tissue factor synthesis in monocytes. Circulation Research. 2000;86:139–43. doi: 10.1161/01.res.86.2.139. [DOI] [PubMed] [Google Scholar]
- 56.Dean RG, Balding LC, Candido R, Burns WC, Cao Z, Twigg SM, Burrell LM. Connective Tissue Growth Factor and Cardiac Fibrosis after Myocardial Infarction. J Histochem Cytochem. 2005;53:1245–56. doi: 10.1369/jhc.4A6560.2005. [DOI] [PubMed] [Google Scholar]
- 57.Luikart SD, Levay-Young B, Hinkel T, Shearer J, Mills C, Caldwell MD, Gyetko MR, Oegema TR. Mactinin treatment promotes wound-healing-associated inflammation in urokinase knockout mice. Wound Repair Regen. 2006;14:123–8. doi: 10.1111/j.1743-6109.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- 58.Antohe F. Endothelial cells and macrophages, partners in atherosclerotic plaque progression. Vol. 112. Informa Healthcare; 2006. pp. 245–53. [DOI] [PubMed] [Google Scholar]
- 59.Boyle JJ. Macrophage Activation in Atherosclerosis: Pathogenesis and Pharmacology of Plaque Rupture. Current Vascular Pharmacology. 2005;3:63–8. doi: 10.2174/1570161052773861. [DOI] [PubMed] [Google Scholar]
- 60.Danenberg HD, Fishbein I, Gao J, Monkkonen J, Reich R, Gati I, Moerman E, Golomb G. Macrophage Depletion by Clodronate-Containing Liposomes Reduces Neointimal Formation After Balloon Injury in Rats and Rabbits. Circulation. 2002;106:599–605. doi: 10.1161/01.cir.0000023532.98469.48. [DOI] [PubMed] [Google Scholar]
- 61.Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, Cousins SW. Macrophage Depletion Diminishes Lesion Size and Severity in Experimental Choroidal Neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3586–92. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- 62.Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J. Macrophage Depletion Inhibits Experimental Choroidal Neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3578–85. doi: 10.1167/iovs.03-0097. [DOI] [PubMed] [Google Scholar]
- 63.Takayama J, Koyamada N, Abe T, Hatsugai K, Usuda M, Ohkohchi N, Satomi S. Macrophage depletion prevents accelerated rejection and results in long-term survival in hamster to rat cardiac xenotransplantation. Transplantation Proceedings. 2000;32:1016. doi: 10.1016/s0041-1345(00)01090-3. [DOI] [PubMed] [Google Scholar]
- 64.Liu T, van Rooijen N, Tracey DJ. Depletion of macrophages reduces axonal degeneration and hyperalgesia following nerve injury. Pain. 2000;86:25–32. doi: 10.1016/s0304-3959(99)00306-1. [DOI] [PubMed] [Google Scholar]
- 65.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duffield JS, Tipping PG, Kipari T, Cailhier J-F, Clay S, Lang R, Bonventre JV, Hughes J. Conditional Ablation of Macrophages Halts Progression of Crescentic Glomerulonephritis. The American journal of pathology. 2005;167:1207–19. doi: 10.1016/S0002-9440(10)61209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin Shedding of Syndecan-1 Regulates Chemokine Mobilization and Transepithelial Efflux of Neutrophils in Acute Lung Injury. Cell. 2002;111:635–46. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 68.Suzuki T, Hashimoto S, Toyoda N, Nagai S, Yamazaki N, Dong HY, Sakai J, Yamashita T, Nukiwa T, Matsushima K. Comprehensive gene expression profile of LPS-stimulated human monocytes by SAGE. Blood. 2000;96:2584–91. [PubMed] [Google Scholar]
- 69.Aikawa M, Rabkin E, Sugiyama S, Voglic SJ, Fukumoto Y, Furukawa Y, Shiomi M, Schoen FJ, Libby P. An HMG-CoA Reductase Inhibitor, Cerivastatin, Suppresses Growth of Macrophages Expressing Matrix Metalloproteinases and Tissue Factor In Vivo and In Vitro. Circulation. 2001;103:276–83. doi: 10.1161/01.cir.103.2.276. [DOI] [PubMed] [Google Scholar]
- 70.Kakio T, Matsumori A, Ono K, Ito H, Matsushima K, Sasayama S. Roles and Relationship of Macrophages and Monocyte Chemotactic and Activating Factor/Monocyte Chemoattractant Protein-1 in the Ischemic and Reperfused Rat Heart. Lab Invest. 2000;80:1127–36. doi: 10.1038/labinvest.3780119. [DOI] [PubMed] [Google Scholar]
- 71.Mannisi JA, Weisman HF, Bush DE, Dudeck P, Healy B. Steroid administration after myocardial infarction promotes early infarct expansion. A study in the rat. The Journal of clinical investigation. 1987;79:1431–9. doi: 10.1172/JCI112971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hammerman H, Kloner RA, Hale S, Schoen FJ, Braunwald E. Dose-dependent efects of short-term methylprednisolone on myocardial infarct extent, scar formation and ventricular function. Circulation. 1983;68:446–52. doi: 10.1161/01.cir.68.2.446. [DOI] [PubMed] [Google Scholar]
- 73.Okazaki T, Ebihara S, Asada M, Yamanda S, Saijo Y, Shiraishi Y, Ebihara T, Niu K, Mei H, Arai H, Yambe T. Macrophage Colony-Stimulating Factor Improves Cardiac Function after Ischemic Injury by Inducing Vascular Endothelial Growth Factor Production and Survival of Cardiomyocytes. The American journal of pathology. 2007;171:1093–103. doi: 10.2353/ajpath.2007.061191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yano T, Miura T, Whittaker P, Miki T, Sakamoto J, Nakamura Y, Ichikawa Y, Ikeda Y, Kobayashi H, Ohori K, Shimamoto K. Macrophage Colony-Stimulating Factor Treatment After Myocardial Infarction Attenuates Left Ventricular Dysfunction by Accelerating Infarct Repair. Journal of the American College of Cardiology. 2006;47:626–34. doi: 10.1016/j.jacc.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 75.van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJA. Macrophage Depletion Impairs Wound Healing and Increases Left Ventricular Remodeling after Myocardial Injury in Mice. The American journal of pathology. 2007;170:818–29. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leor J, Rozen L, Zuloff-Shani A, Feinberg MS, Amsalem Y, Barbash IM, Kachel E, Holbova R, Mardor Y, Daniels D, Ocherashvilli A, Orenstein A, et al. Ex Vivo Activated Human Macrophages Improve Healing, Remodeling, and Function of the Infarcted Heart. Circulation. 2006;114:I-94–100. doi: 10.1161/CIRCULATIONAHA.105.000331. [DOI] [PubMed] [Google Scholar]
- 77.Dobaczewski M, Bujak M, Zymek P, Ren G, Entman ML, Frangogiannis NG. Extracellular matrix remodeling in canine and mouse myocardial infarcts. Cell and Tissue Research. 2006;V324:475–88. doi: 10.1007/s00441-005-0144-6. [DOI] [PubMed] [Google Scholar]
- 78.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. The Journal of clinical investigation. 2001;107:1395–402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anversa P, Kajstura J. Ventricular Myocytes are not Terminally Differentiated in the Adult Mammalian Heart. Circulation research. 1998;83:1–14. doi: 10.1161/01.res.83.1.1. [DOI] [PubMed] [Google Scholar]
- 80.Rossen RD. Complement Activation in Cardiac Disease. In: Curtis MJ, editor. The Handbook of Immunopharmacologyed. 1993. pp. 75–86. [Google Scholar]
- 81.Horstick G. C1-esterase inhibitor in ischemia and reperfusion. Immunobiology. 2002;205:552–62. doi: 10.1078/0171-2985-00154. [DOI] [PubMed] [Google Scholar]
- 82.Deodato B, Altavilla D, Squadrito G, Campo GM, Arlotta M, Minutoli L, Saitta A, Cucinotta D, Calapai G, Caputi AP, Miano M, Squadrito F. Cardioprotection by the phytoestrogen genistein in experimental myocardial ischaemia-reperfusion injury. Br J Pharmacol. 1999;128:1683–90. doi: 10.1038/sj.bjp.0702973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsujita K, Kaikita K, Hayasaki T, Honda T, Kobayashi H, Sakashita N, Suzuki H, Kodama T, Ogawa H, Takeya M. Targeted Deletion of Class A Macrophage Scavenger Receptor Increases the Risk of Cardiac Rupture After Experimental Myocardial Infarction. Circulation. 2007;115:1904–11. doi: 10.1161/CIRCULATIONAHA.106.671198. [DOI] [PubMed] [Google Scholar]
- 84.Squadrito F, Altavilla D, Squadrito G, Campo GM, Arlotta M, Arcoraci V, Minutoli L, Serrano M, Saitta A, Caputi AP. 17Beta-oestradiol reduces cardiac leukocyte accumulation in myocardial ischaemia reperfusion injury in rat. Eur J Pharmacol. 1997;335:185–92. doi: 10.1016/s0014-2999(97)01201-6. [DOI] [PubMed] [Google Scholar]
- 85.Frangogiannis NG. The Mechanistic Basis of Infarct Healing. Antioxidants & Redox Signaling. 2006;8:1907–39. doi: 10.1089/ars.2006.8.1907. [DOI] [PubMed] [Google Scholar]
- 86.Moldovan L, Moldovan NI. Role of monocytes and macrophages in angiogenesis. Exs. 2005:127–46. doi: 10.1007/3-7643-7311-3_9. [DOI] [PubMed] [Google Scholar]
- 87.Ganz T. Macrophage function. New Horiz. 1993;1:23–7. [PubMed] [Google Scholar]
- 88.Rappolee DA, Werb Z. Secretory products of phagocytes. Curr Opin Immunol. 1988;1:47–55. doi: 10.1016/0952-7915(88)90050-7. [DOI] [PubMed] [Google Scholar]
- 89.Nathan CF. Secretory products of macrophages. J Clin Invest. 1987;79:319–26. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ito T, Ikeda U. Inflammatory cytokines and cardiovascular disease. Curr Drug Targets Inflamm Allergy. 2003;2:257–65. doi: 10.2174/1568010033484106. [DOI] [PubMed] [Google Scholar]
- 91.Kobusiak-Prokopowicz Mg. Kinetics of chemokines in acute myocardial infarction. Kardiologia Polska. 2005;62:301–14. discussion 15–6. [PubMed] [Google Scholar]
- 92.Dewald O. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circulation Research. 2005;96:881–9. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 93.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/Monocyte Chemoattractant Protein-1 Regulates Inflammatory Responses Critical to Healing Myocardial Infarcts. Circulation research. 2005;96:881–9. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 94.Kumar AG, Ballantyne CM, Michael LH, Kukielka GL, Youker KA, Lindsey ML, Hawkins HK, Birdsall HH, MacKay CR, LaRosa GJ, Rossen RD, Smith CW, et al. Induction of Moncyte Chemoattractant Protein-1 in the Small Veins of the Ischemic and Reperfused Canine Myocardium. Circulation. 1997;95:693–700. doi: 10.1161/01.cir.95.3.693. [DOI] [PubMed] [Google Scholar]
- 95.Condeelis J, Pollard JW. Macrophages: Obligate Partners for Tumor Cell Migration, Invasion, and Metastasis. Cell. 2006;124:263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 96.Duffield JS, Tipping PG, Kipari T, Cailhier JF, Clay S, Lang R, Bonventre JV, Hughes J. Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am J Pathol. 2005;167:1207–19. doi: 10.1016/S0002-9440(10)61209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sano H, Hsu DK, Apgar JR, Yu L, Sharma BB, Kuwabara I, Izui S, Liu F-T. Critical role of galectin-3 in phagocytosis by macrophages. J Clin Invest. 2003;112:389–97. doi: 10.1172/JCI17592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maree AFM, Komba M, Dyck C, Labecki M, Finegood DT, Edelstein-Keshet L. Quantifying macrophage defects in type 1 diabetes. Journal of Theoretical Biology. 2005;233:533–51. doi: 10.1016/j.jtbi.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 99.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovascular research. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 100.MacKenna D, Summerour SR, Villarreal FJ. Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovascular research. 2000;46:257–63. doi: 10.1016/s0008-6363(00)00030-4. [DOI] [PubMed] [Google Scholar]
- 101.Keane MP, Strieter RM, Lynch JP, 3rd, Belperio JA. Inflammation and angiogenesis in fibrotic lung disease. Seminars in respiratory and critical care medicine. 2006;27:589–99. doi: 10.1055/s-2006-957331. [DOI] [PubMed] [Google Scholar]
- 102.Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proceedings of the American Thoracic Society. 2006;3:377–82. doi: 10.1513/pats.200601-004TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–46. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated Production of Type I Collagen and Inflammatory Cytokines by Peripheral Blood Fibrocytes. J Immunol. 1998;160:419–25. [PubMed] [Google Scholar]
- 105.Quan TE, Cowper S, Wu S-P, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. The International Journal of Biochemistry & Cell Biology. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 106.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci USA. 2006;103:18284–9. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bouzegrhane F. Is angiotensin II a proliferative factor of cardiac fibroblasts? Cardiovascular Research. 2002;53:304–12. doi: 10.1016/s0008-6363(01)00448-5. [DOI] [PubMed] [Google Scholar]
- 108.Booz GW, Baker KM. Molecular signalling mechanisms controlling growth and function of cardiac fibroblasts. Cardiovasc Res. 1995;30:537–43. [PubMed] [Google Scholar]
- 109.Sun Y, Weber KT. Infarct scar: a dynamic tissue. Cardiovascular research. 2000;46:250–6. doi: 10.1016/s0008-6363(00)00032-8. [DOI] [PubMed] [Google Scholar]
- 110.Tyagi SC, Lewis K, Pikes D, Marcello A, Mujumdar VS, Smiley LM, Moore CK. Stretch-Induced Membrane Type Matrix Metalloproteinase and Tissue Plasminogen Activator in Cardiac Fibroblast Cells. J Cell Physiology. 1998;176:374–82. doi: 10.1002/(SICI)1097-4652(199808)176:2<374::AID-JCP16>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 111.Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol. 2001;280:C53–60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- 112.Tummalapalli CM, Heath BJ, Tyagi SC. Tissue inhibitor of metalloproteinase-4 instigates apoptosis in transformed cardiac fibroblasts. J Cell Biochem. 2001;80:512–21. doi: 10.1002/1097-4644(20010315)80:4<512::aid-jcb1005>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 113.Ahmed MS, Oie E, Vinge LE, Yndestad A, Oystein Andersen G, Andersson Y, Attramadal T, Attramadal H. Connective tissue growth factor--a novel mediator of angiotensin II-stimulated cardiac fibroblast activation in heart failure in rats. Journal of molecular and cellular cardiology. 2004;36:393–404. doi: 10.1016/j.yjmcc.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 114.Lin J, Liliensiek B, Kanitz M, Schimanski U, Bohrer H, Waldherr R, Martin E, Kauffmann G, Ziegler R, Nawroth PP. Molecular cloning of genes differentially regulated by TNF-[alpha] in bovine aortic endothelial cells, fibroblasts and smooth muscle cells. Cardiovascular research. 1998;38:802–13. doi: 10.1016/s0008-6363(98)00055-8. [DOI] [PubMed] [Google Scholar]
- 115.Gurantz D, Cowling R, Villarreal F, Greenberg B. Tumor necrosis factor-alpha upregulates angiotensin II type 1 receptors on cardiac fibroblasts. Circulation Research. 1999;85:272–9. doi: 10.1161/01.res.85.3.272. [DOI] [PubMed] [Google Scholar]
- 116.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–11. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sutton M, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–8. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 118.Sun Y. Fibrous tissue and angiotensin II. Journal of Molecular and Cellular Cardiology. 1997;29:2001–12. doi: 10.1006/jmcc.1997.0451. [DOI] [PubMed] [Google Scholar]
- 119.Weber KT. Tissue repair and angiotensin II generated at sites of healing. Basic Research in Cardiology. 1997;92:75–8. doi: 10.1007/BF00805565. [DOI] [PubMed] [Google Scholar]
- 120.Falkenhahn M, Franke F, Bohle R, Zhu Y, Stauss H, Bachmann S, Danilov S, Unger T. Cellular distribution of angiotensin-converting enzyme after myocardial infarction. Hypertension. 1995;25:219–26. doi: 10.1161/01.hyp.25.2.219. [DOI] [PubMed] [Google Scholar]
- 121.Guarda E, Katwa LC, Myers PR, Tyagi SC, Weber KT. Effects of endothelins on collagen turnover in cardiac fibroblasts. Cardiovasc Res. 1993;27:2130–4. doi: 10.1093/cvr/27.12.2130. [DOI] [PubMed] [Google Scholar]
- 122.Simons M. Angiogenesis: Where Do We Stand Now? Circulation. 2005;111:1556–66. doi: 10.1161/01.CIR.0000159345.00591.8F. [DOI] [PubMed] [Google Scholar]
- 123.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 124.Murdoch C, Lewis CE. Macrophage migration and gene expression in response to tumor hypoxia. International Journal of Cancer. 2005;117:701–8. doi: 10.1002/ijc.21422. [DOI] [PubMed] [Google Scholar]
- 125.Helisch A, Schaper W. Arteriogenesis: the development and growth of collateral arteries. Microcirculation. 2003;10:83–97. doi: 10.1038/sj.mn.7800173. [DOI] [PubMed] [Google Scholar]
- 126.de Muinck ED, Simons M. Re-evaluating therapeutic neovascularization. J Mol Cell Cardiol. 2004;36:25–32. doi: 10.1016/j.yjmcc.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 127.Manoonkitiwongsa PS, Jackson-Friedman C, McMillan PJ, Schultz RL, Lyden PD. Angiogenesis after stroke is correlated with increased numbers of macrophages: the clean-up hypothesis. J Cereb Blood Flow Metab. 2001;21:1223–31. doi: 10.1097/00004647-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 128.Ogawa K, Suzuki J, Narasaki M, Mori M. Healing of focal injury in the rat liver. Am J Pathol. 1985;119:158–67. [PMC free article] [PubMed] [Google Scholar]
- 129.Kobayashi S, Nagaura T, Kimura I, Kimura M. Interferon-gamma-activated macrophages enhance angiogenesis from endothelial cells of rat aorta. Immunopharmacology. 1994;27:23–30. doi: 10.1016/0162-3109(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 130.Ono M, Torisu H, Fukushi J-i, Nishie A, kuwano M. Biological implications of macrophage infiltration in human tumor angiogenesis. Cancer Chemother Pharmacol. 1999;43:S69–S71. doi: 10.1007/s002800051101. [DOI] [PubMed] [Google Scholar]
- 131.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovascular research. 2006;69:562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 132.Overall CM, Kleifeld O. Towards third generation matrix metalloproteinase inhibitors for cancer therapy. Br J Cancer. 2006;94:941–6. doi: 10.1038/sj.bjc.6603043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shankavaram UT, DeWitt DL, Wahl LM. Lipopolysaccharide induction of monocyte matrix metalloproteinase is regulated by the tyrosine phosphorylation of cytosolic phospholipase A2. J Leukoc Biol. 1998;64:221–7. doi: 10.1002/jlb.64.2.221. [DOI] [PubMed] [Google Scholar]
- 134.Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977;14:53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- 135.Ingber DE, Folkman J. How does extracellular matrix control capillary morphogenesis? Cell. 1989;58:803–5. doi: 10.1016/0092-8674(89)90928-8. [DOI] [PubMed] [Google Scholar]
- 136.Mignatti P, Tsuboi R, Robbins E, Rifkin DB. In vitro angiogenesis on the human amniotic membrane: requirement for basic fibroblast growth factor-induced proteinases. J Cell Biol. 1989;108:671–82. doi: 10.1083/jcb.108.2.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bendeck MP. Mining the myocardium with macrophage drills: A novel mechanism for revascularization. Circ Res. 2000;87:341–3. doi: 10.1161/01.res.87.5.341. [DOI] [PubMed] [Google Scholar]
- 138.Falcone DJ, Khan KMF, Layne T, Fernandes L. Macrophage Formation of Angiostatin during Inflammation. J of Biological Chemistry. 1998;273:31480–5. doi: 10.1074/jbc.273.47.31480. [DOI] [PubMed] [Google Scholar]
- 139.Patterson BC, Sang QXA. Angiostatin-converting Enzyme Activities of Human Matrilysin (MMP-7) and Gelatinase B/Type IV Collagenase (MMP-9) The Journal of biological chemistry. 1997;272:28823–5. doi: 10.1074/jbc.272.46.28823. [DOI] [PubMed] [Google Scholar]
- 140.Davis GE, Bayless KJ, Davis MJ, Meininger GA. Regulation of Tissue Injury Responses by the Exposure of Matricryptic Sites within Extracellular Matrix Molecules. Am J of Pathology. 2000;156:1489–98. doi: 10.1016/S0002-9440(10)65020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Maquart F-X, Pasco S, Ramont L, Hornebeck W, Monboisse J-C. An introduction to matrikines: extracellular matrix-derived peptides which regulate cell activity: Implication in tumor invasion. Critical Reviews in Oncology/Hematology. 2004;49:199–202. doi: 10.1016/j.critrevonc.2003.06.007. [DOI] [PubMed] [Google Scholar]
- 142.Schellings MWM, Pinto YM, Heymans S. Matricellular proteins in the heart: possible role during stress and remodeling. Cardiovascular research. 2004;64:24–31. doi: 10.1016/j.cardiores.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 143.Clark RA, Wikner NE, Doherty DE, Norris DA. Cryptic chemotactic activity of fibronectin for human monocytes resides in the 120-kDa fibroblastic cell-binding fragment. J of Biological Chemistry. 1988;263:12115–23. [PubMed] [Google Scholar]
- 144.Xu J, Rodriguez D, Petitclerc E, Kim JJ, Hangai M, Moon YS, Davis GE, Brooks PC. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154:1069–79. doi: 10.1083/jcb.200103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hangai M, Kitaya N, Xu J, Chan CK, Kim JJ, Werb Z, Ryan SJ, Brooks PC. Matrix Metalloproteinase-9-Dependent Exposure of a Cryptic Migratory Control Site in Collagen is Required before Retinal Angiogenesis. The American journal of pathology. 2002;161:1429–37. doi: 10.1016/S0002-9440(10)64418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Khan KMF, Laurie GW, McCaffrey TA, Falcone DJ. Exposure of cryptic domains in the alpha 1-chain of laminin-1 by elastase stimulates macrophages urokinase and MMP-9 expression. The Journal of biological chemistry. 2002:M111290200. doi: 10.1074/jbc.M111290200. [DOI] [PubMed] [Google Scholar]
- 147.Schenk S, Quaranta V. Tales from the crypt[ic] sites of the extracellular matrix. Trends in Cell Biology. 2003;13:366–75. doi: 10.1016/s0962-8924(03)00129-6. [DOI] [PubMed] [Google Scholar]
- 148.Bellon G, Martiny L, Robinet A. Matrix metalloproteinases and matrikines in angiogenesis. Critical Reviews in Oncology/Hematology. 2004;49:203–20. doi: 10.1016/j.critrevonc.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 149.McGavigan AD, Maxwell PR, Dunn FG. Serological evidence of altered collagen homeostasis reflects early ventricular remodeling following acute myocardial infarction. International Journal of Cardiology. 2006;111:267–74. doi: 10.1016/j.ijcard.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 150.Visse R, Nagase H. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases: Structure, Function, and Biochemistry. Circulation research. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 151.Egeblad M, Werb Z. New Functions for the Matrix Metalloproteinases in Cancer Progression. Nat Rev Cancer. 2002;2:163–76. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 152.Shapiro SD. Diverse roles of macrophage matrix metalloproteinases in tissue destruction and tumor growth. Thrombosis and haemostasis. 1999;82:846–9. [PubMed] [Google Scholar]
- 153.Sellebjerg F, Sorensen TL. Chemokines and matrix metalloproteinase-9 in leukocyte recruitment to the central nervous system. Brain Research Bulletin. 2003;61:347–55. doi: 10.1016/s0361-9230(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 154.Lindsey M, Wedin K, Brown MD, Keller C, Evans AJ, Smolen J, Burns AR, Rossen RD, Michael L, Entman M. Matrix-Dependent Mechanism of Neutrophil-Mediated Release and Activation of Matrix Metalloproteinase 9 in Myocardial Ischemia/Reperfusion. Circulation. 2001;103:2181–7. doi: 10.1161/01.cir.103.17.2181. [DOI] [PubMed] [Google Scholar]
- 155.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. The Journal of clinical investigation. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Johnson C, Sung HJ, Lessner SM, Fini ME, Galis ZS. Matrix Metalloproteinase-9 Is Required for Adequate Angiogenic Revascularization of Ischemic Tissues: Potential Role in Capillary Branching. Circulation research. 2004;94:262–8. doi: 10.1161/01.RES.0000111527.42357.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lindsey ML, Escobar GP, Dobrucki LW, Goshorn DK, Bouges S, Mingoia JT, McClister DM, Jr, Su H, Gannon J, MacGillivray C, Lee RT, Sinusas AJ, et al. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;290:H232–9. doi: 10.1152/ajpheart.00457.2005. [DOI] [PubMed] [Google Scholar]
- 158.Corbitt CA, Lin J, Lindsey ML. Mechanisms to Inhibit Matrix Metalloproteinase Activity: Where are we in the Development of Clinically Relevant Inhibitors. Recent Patents on Anti-Cancer Drug Discovery. 2007;2:135–42. doi: 10.2174/157489207780832423. [DOI] [PubMed] [Google Scholar]
- 159.Peterson JT, Li H. Matrix Metalloproteinase Inhibitor Development for the Treatment of Heart Failure. Drug Dev Res. 2002;55:29–44. [Google Scholar]
- 160.Peterson JT. Matrix Metalloproteinase Inhibitor Development and the Remodeling of Drug Discovery. Heart Fail Rev. 2004;9:63–79. doi: 10.1023/B:HREV.0000011395.11179.af. [DOI] [PubMed] [Google Scholar]
- 161.Peterson JT. The importance of estimating the therapeutic index in the development of matrix metalloproteinase inhibitors. Cardiovascular research. 2006;69:677–87. doi: 10.1016/j.cardiores.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 162.Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, Clark LT, Pinsky DJ, Marmur JD. Tissue inhibitor of metalloproteinase-1 (TIMP-1) is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction. American Heart Journal. 2006;151:1101, e1–e8. doi: 10.1016/j.ahj.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 163.Peterson JT, Li H, Dillon L, Bryant JW. Evolution of matrix metalloprotease and tissue inhibitor expression during heart failure progression in the infarcted rat. Cardiovascular research. 2000;46:307–15. doi: 10.1016/s0008-6363(00)00029-8. [DOI] [PubMed] [Google Scholar]
- 164.Chirco R, Liu X-W, Jung K-K, Kim H-R. Novel functions of TIMPs in cell signaling. Cancer and Metastasis Reviews. 2006;25:99–113. doi: 10.1007/s10555-006-7893-x. [DOI] [PubMed] [Google Scholar]
- 165.Eue I. Growth inhibition of human mammary carcinoma by liposomal hexadecylphosphocholine: Participation of activated macrophages in the antitumor mechanism. Int J Cancer. 2001;92:426–33. doi: 10.1002/ijc.1201. [DOI] [PubMed] [Google Scholar]
- 166.Fujiwara N, Kobayashi K. Macrophages in Inflammation. Current Drug Targets -Inflammation & Allergy. 2005;4:281–6. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- 167.Investigators TAAMI. Pexelizumab for Acute ST-Elevation Myocardial Infarction in Patients Undergoing Primary Percutaneous Coronary Intervention: A Randomized Controlled Trial. JAMA. 2007;297:43–51. doi: 10.1001/jama.297.1.43. [DOI] [PubMed] [Google Scholar]
- 168.Kitty CM, Verhoeckx SB, de Groene Els M, Witkamp Renger F, van der Greef Jan, Rodenburg Richard JT. A combination of proteomics, principal component analysis and transcriptomics is a powerful tool for the identification of biomarkers for macrophage maturation in the U937 cell line. Proteomics. 2004;4:1014–28. doi: 10.1002/pmic.200300669. [DOI] [PubMed] [Google Scholar]
- 169.Wagsater D, Bjork H, Zhu C, Bjorkegren J, Valen G, Hamsten A, Eriksson P. ADAMTS-4 and -8 are inflammatory regulated enzymes expressed in macrophage-rich areas of human atherosclerotic plaques. Atherosclerosis. 2008;196:514–22. doi: 10.1016/j.atherosclerosis.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 170.Droin N, Cathelin S, Jacquel A, Guery L, Garrido C, Fontenay M, Hermine O, Solary E. A role for caspases in the differentiation of erythroid cells and macrophages. Biochimie. 2008;90:416–22. doi: 10.1016/j.biochi.2007.08.007. [DOI] [PubMed] [Google Scholar]


