Abstract
Background
This study examines the relationship among psychosocial factors, behavioral risks for abnormal cervical cytology, and abnormal cervical cytology.
Methods
A self-administered questionnaire was used to measure perceived stress, discrimination, lifetime stressful events, optimism, social support, and psychological state. Women with normal Pap smears attending a primary care clinic and women attending a colposcopy clinic because of an abnormal Pap smear were eligible. The scores between the two groups were compared.
Results
A total of 265 women participated in the study. There were no significant relationships between psychosocial factors and cervical cytology status. In a regression model, age (B = −0.057, p = 0.001) was predictive of having abnormal cervical cytology. Smoking was correlated with an increased family Apgar score (p = 0.021), Perceived Stress Scale (PSS) score (p = 0.049), and Revised Life Stressor Checklist score (p < 0.001). A higher mean number of lifetime male partners was related to increased family Apgar score (p = 0.012), Revised Life Stressor Checklist score (p < 0.001), and major event discrimination (p < 0.001). Earlier age at coitarche was associated with increased family Apgar score (p < 0.001).
Conclusions
These results do not support that psychosocial factors play a role in the risk of developing abnormal cervical cytology. Behavioral risks for developing abnormal cervical cytology are associated with life stressors, family function, and perceived discrimination.
Introduction
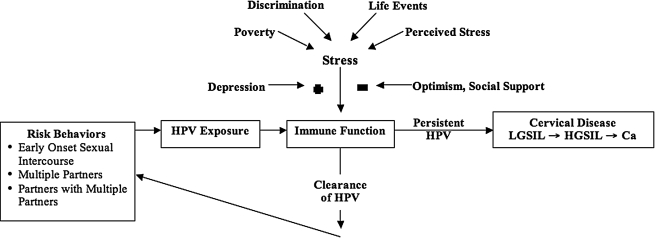
The presence of infection with high-risk types of human papillomavirus (HPV) in nearly all cervical cancer cases indicates that HPV is the primary biological cause of cervical cancer.1 HPV is a sexually transmitted virus, with prevalence highest in women <age 30.1 More than 50 types of HPV have been identified as infecting the genital tract and are classified as either high risk or low risk for development of cervical cancer. Persistent HPV infection places women at risk for developing cervical lesions,2 although most cervical HPV infections are transitory and are cleared before detection.3 As outlined in Figure 1, the psychoneuroimmunological model allows one to bring together the potential biobehavioral mechanisms of HPV-mediated disease.4
FIG. 1.
An overview of the psychoneuroimmunolgical model applied to cervical cancer.
Psychosocial factors may create a chronic state of immunosuppression, which may be conducive to HPV persistence and development of cervical lesions.4 Several studies have documented that immunosuppression, iatrogenic or autoimmune, increases the risk for cervical cancer.5–7 Increased stressful life events, intimate partner violence (IPV), stressful and uncontrollable life events, lack of social support, and passive/helpless coping style have been associated with increased risk for abnormal cervical cytology.8–13 Other sources of stress, such as discrimination, could also influence the risk of developing persistent HPV. Other factors, such as optimism and social support, could minimize the impact of stress on immune function. In addition, stress could cause depression, which would in turn heighten the impact of psychosocial factors on immune function.
The measures used in previous studies have been limited and did not fully explore the relationship between psychosocial factors and risk behaviors for developing abnormal cervical cytology, especially smoking, age at coitarche, and number of lifetime sexual partners.14,15 For this study, psychosocial factors were measured as poverty, perceived stress, discrimination, and lifetime stressful events. Discrimination was chosen based on our experience with women with abnormal cervical cytology, which suggests high rates of discriminatory events. Optimism and social support were measured as potential minimizers of the impact of the psychosocial factors. Depression would add to the impact of psychosocial factors on immune function. We hypothesized that women with normal cervical cytology would have significantly less stress as measured by these tools than women attending a colposcopy clinic because of abnormal cervical cytology. We chose abnormal cervical cytology as a surrogate of persistent HPV infection and ineffective immune response to clear it.
Materials and Methods
Participants
The study participants were women attending either a primary care clinic or a colposcopy clinic within an integrated academic health system in the Midwest. Eligibility for study participation required women to be ≥18 years of age. The women recruited from the primary care clinic had a history of normal Pap smears within the past 2 years. The women recruited from the colposcopy clinic had abnormal cervical cytology and were in various stages (e.g., new diagnosis, follow-up of previous colposcopy results) of evaluation for abnormal cervical cytology. Patients were excluded from the study if they had a hysterectomy or were unable to provide consent. The University of Michigan Institutional Review Board approved the study.
Study design
Women were approached in the office waiting room with the consent of their healthcare provider. After explanation of the study, participants received a consent form with prescreening eligibility questions. If the participant was eligible for the study and interested in participating, the consent form was signed, and she was given the survey questionnaire. The participants completed and returned the survey questionnaire before leaving the clinic.
Survey questionnaire responses were entered into an SPSS database (SPSS, Inc., Chicago, IL) by the study identification number without any identifying information. Medical records were audited to verify the primary care clinic participants' history of normal Pap tests. The colposcopy clinic participants' pathology records were reviewed to determine cervical disease status.
Questionnaire
A single questionnaire compiled from several validated tools was used. The survey questionnaire had 117 questions and consisted of two sections, a demographic and behavior section and a psychosocial factors section. The demographic and behavior portion contained questions about sexual behavior, alcohol and tobacco use, medical and health history, and general demographic information. Questions regarding the common risk factors for cervical cancer were also included in this section. The primary risk factors for cervical cancer are history of or current cigarette smoking, high numbers of sexual partners, and early age of initiating sexual intercourse.
Perceived stress was evaluated using the Perceived Stress Scale (PSS), which measures recent general perceived stress using 14 items associated with feelings in the past month.16 The major discrimination scale consists of 11 yes/no questions pertaining to perceived discrimination in such areas as job promotion or housing. The score for this tool was obtained by summing the number of positive responses, with a higher score representing increased perceived discrimination.17 The daily discrimination tool consists of 9 questions about the perception of daily experiences of discrimination, such as being treated with less courtesy than other people or perceiving that other people act as if the individual is inferior or dishonest.17 The Revised Life Stressor Checklist assesses significant life events, which may be either chronic stressors or potential posttraumatic stress disorder (PTSD) symptoms.18 It lists 28 life events with yes/no responses for the participant to circle if they occurred. The score for this tool is obtained by summing the yes responses. Social support was assessed using the family Apgar, which measures the participant's satisfaction with current family function.19,20 The Life Orientation Test (LOT) was designed to examine the role played by optimism and dispositional self-consciousness in coping effectively or ineffectively with impediments encountered in the course of goal-directed activities.21 The LOT score was obtained by summing the responses to the 8 scored questions after reversing the responses to the 4 negatively phrased questions. Higher LOT scores reflect greater dispositional optimism. The Beck Depression Inventory for Primary Care (BDI-PC) is derived from the BDI and assesses the presence of depressive symptoms in primary care outpatients within the past 2 weeks, including the present day.22,23 BDI-PC scores were obtained by summing the response ratings for each of the seven items and may range from 0 to 21, with a cutoff of ≥4 for a positive score.
Statistical analysis
The data collected consisted of questionnaire responses and were used only if the response to a measure was complete. Survey measures with missing responses were not included in the analysis. After the data were cleaned and organized in an SPSS file, the study participants' demographic and risk information was reviewed and summarized using the relevant descriptive statistics. Correlations between the demographic variables were examined using the relevant comparative statistics. The individual scores for PSS, family Apgar, sum of stressful life events, daily discrimination, major event discrimination, LOT, and BDI-PC were calculated, and descriptive statistics were summarized. Correlations among the scores were examined using relevant comparative statistics. Comparison on each tool score was made between racial groups (white vs. African American), clinical site (primary care clinic vs. colposcopy), age, marital status (married vs. single), and risk behaviors for cervical cancer.
Multivariate analysis was performed using the cervical disease status (yes/no) as the dependent outcome. The variables that correlated with cervical disease status in the univariate analysis were used as possible independent variables in logistic regression. The possible independent variables included age, marital status, income, PSS score, major events and daily discrimination scales, Revised Life Stressor Checklist score, family Apgar score, LOT score, BDI-PC score, and the behavioral risk factors. If one independent variable was found associated with cervical disease, variables correlated with it were placed in the logistic regression model with an interactive term. For example, if age, marital status, or income was significantly correlated, all three were placed in the model with an interactive term. If there was no effect on the relationship, the other variables were dropped. We also considered a model with all independent variables compared to a reduced model.
Results
A total of 265 women participated in the study, with 141 women from the primary care clinic (with a history of normal Pap smears) and 124 women from the colposcopy clinic. The demographic data between the two groups are compared in Table 1. The normal cervical cytology participants were older than the colposcopy participants (p = 0.002) and were more likely to be married (p = 0.001). Additionally, a greater proportion of colposcopy participants reported annual household incomes of <$10,000 (p = 0.011). Age, marital status, and income were significantly correlated (p = 0.001) such that older women were more likely to be married and have higher annual household incomes.
Table 1.
Demographic Data of Normal Pap Test Group Compared with Colposcopy Group
| Normal Pap test group (n = 141) | Colposcopy group (n = 124) | |
|---|---|---|
| Age in years, mean (SD) | 33.5* (7.0) | 30.3 (9.5) |
| Race/ethnicity, n (%) | ||
| African American | 31 (22.0) | 22 (17.7) |
| White | 89 (63.1) | 94 (75.8) |
| Latina | 5 (3.5) | 3 (2.4) |
| Native American | 4 (2.8) | 3 (2.4) |
| Asian | 7 (5.0) | 2 (1.6) |
| Other | 7 (5.0) | 2 (1.6) |
| Marital status, n (%) | ||
| Divorced/separated | 19 (13.5) | 17 (13.7) |
| Married | 59 (41.8) | 32 (25.8)** |
| Unmarried partner | 17 (12.1) | 7 (5.6) |
| Single | 45 (31.9) | 68 (54.8)** |
| Widowed | 1 (0.7) | 0 |
| Education, n (%) | ||
| High school (incomplete) | 8 (5.7) | 6 (4.9) |
| Completed high school | 25 (17.7) | 17 (13.8) |
| College incomplete | 49 (34.7) | 42 (34.1) |
| College complete or beyond | 59 (41.8) | 58 (47.2) |
| Missing | 0 | 1 (0.7) |
| Income, n (%) | ||
| <$10,000 | 15 (10.6)* | 32 (25.8) |
| $10,000–<$20,000 | 23 (16.3) | 13 (10.5) |
| $20,000–<$40,000 | 34 (24.1) | 23 (18.5) |
| ≥$40,000 | 65 (46.1) | 56 (45.2) |
| Missing | 4 (2.8) | 0 |
| HPV status, n (%) | ||
| Negative | 141 (100) | 16 (12.9) |
| Positive | 0 | 106 (85.5) |
| Disease status, n (%) | ||
| Normal | 141 (100) | 43 (34.7) |
| ASCUS/AGUSa | 0 | 3 (2.4) |
| LGSIL/CIN I | 0 | 42 (33.9) |
| HGSIL/CIN II, III | 0 | 35 (28.2) |
| Missing | 0 | 1 (0.8) |
| Mean time since last Pap test in months, mean (SD) | 6.40 (6.0) | NAb |
Significant at the 0.01 level.
Significant at the 0.001 level.
ASCUS: atypical squamous cells of uncertain significance; AGUS: atypical glandular cells of uncertain significance; LGSIL: low-grade squamous intraepithelial lesions; HGSIL: high-grade squamous intraepithelial lesions.
NA: data not collected.
Risk behaviors of the normal cervical cytology and colposcopy groups are compared in Table 2. The number of lifetime sexual partners, number of sexual partners in last year, and number of new sexual partners in the last year were significantly skewed in a positive direction; therefore, these variables were log transformed to assure normal distribution for comparison. The colposcopy group had a significantly higher number of male partners in the past year (p = 0.038). There were no other significant differences between the groups on any of the other risk behaviors. Table 3 shows the comparison of psychosocial factor scores between the normal cervical cytology participants and the colposcopy study participants. None of the scores were significantly different between the normal cervical cytology and colposcopy groups.
Table 2.
Risk Behaviors of Normal Pap Test Group Compared with Colposcopy Group
| Normal Pap test group (n = 141) | Colposcopy group (n = 124) | |
|---|---|---|
| Smoking status, n (%) | ||
| Current | 26 (18.4) | 30 (24.2) |
| Former | 34 (24.1) | 34 (27.4) |
| Never | 81 (57.4) | 60 (48.4) |
| Lifetime number of male partners, mean (SD) | 8.54 (8.9) | 8.4 (10.2) |
| Range | 1–50 | 1–100 |
| Age at coitarche, mean (SD) | 17.1 (4.3) | 16.6 (2.5) |
| Range | Never–37 | 5–25 |
| Number of male partners in last year, mean (SD) | 1.04 (0.7) | 1.3* (1.2) |
| Range | 0–5 | 0–10 |
| Number of new male partner(s) in last year, mean (SD) | 0.49 (1.1) | 0.48 (1.2) |
| Range | 0–9 | 0–10 |
Correlation is significant at the 0.05 level (2-tailed).
Table 3.
Psychosocial Factor Scores of Normal Pap Test Group Compared with Colposcopy Group
| |
Normal Pap test group (n = 141) |
Colposcopy group (n = 124) |
|---|---|---|
| Tool | Mean (SD) | Mean (SD) |
| PSS | 25.9 (8.9) | 24.7 (8.7) |
| LOT | 28.2 (6.3) | 28.9 (5.8) |
| Family Apgar | 7.6 (2.8) | 7.6 (3.2) |
| Revised Life Stressor Checklist | 6.6 (4.6) | 6.9 (4.7) |
| Daily perceived discrimination | 45.3 (8.2) | 45.4 (7.6) |
| Discrimination major events | 0.9 (1.2) | 0.8 (1.0) |
| BDI-PC | 3.3 (4.0) | 2.9 (3.5) |
Current smoking status was significantly associated with increased PSS score (Pearson r = 0.173, p = 0.005), higher family Apgar score (Pearson r = 0.181, p = 0.003), more stressful life events on the Revised Life Stressor Checklist (Pearson r = 0.297, p < 0.001), and greater major events discrimination score (Pearson r = 0.179, p = 0.003). History of regular smoking was correlated with an increased family Apgar score (Pearson r = 0.142, p = 0.021), greater PSS score (Pearson r = 0.123, p = 0.049), and higher Revised Life Stressor Checklist score (Pearson r = 0.271, p < 0.001). Greater number of lifetime male partners was related to increased family Apgar score (Pearson r = 0.156, p = 0.012), higher Revised Life Stressor Checklist score (Pearson r = 0.310, p < 0.001), and more major event discrimination (Pearson r = 0.217, p < 0.001). Earlier age at coitarche was associated with increased family Apgar score (Pearson r = −0.236, p < 0.001), greater Revised Life Stressor Checklist score (Pearson r = −0.376, p < 0.001), and increased major event discrimination score (Pearson r = −0.191, p = 0.002).
A regression model was developed to test the effects of cervical cancer risk factors and psychosocial factor scores on the dependent variable, abnormal Pap smear. Only age (B = −0.057, p = 0.001) was a significant predictor of women with an abnormal Pap smear. None of the cervical cancer risk factors, other demographic features, or psychosocial factors were significant or modified the relationship of age.
Discussion
The findings from this study do not support that psychosocial factors have a relationship to the risk for cervical disease. Our findings are similar to those of other studies that have reported no association between cervical intraepithelial neoplasia (CIN) stage and negatively rated life events, lack of social support, coping style, and distress.24,25 This is in contrast to several studies that have documented elevated stress scores among women at greater risk for cervical cancer.8,26
Our findings may be attributed to the features of the study population. Overall, participants were highly educated, with 78% of the women (81% within the colposcopy group) being educated beyond high school, compared with 37.7% reported in another study.8 These highly educated women may have more effective coping mechanisms and responses than a less educated population to manage psychosocial factors. Poverty or annual income was not associated with abnormal cervical cytology, but only about 30% of the population reported an income considered below the poverty line.
In comparing the risk behaviors between the normal cervical cytology and colposcopy groups, only the mean number of male partners in the past year differed significantly. This finding replicates the results from previous studies.24 Unexpectedly, none of the other risk behaviors differed between the two groups. In general, the study participants with normal Pap smears had high rates of risk behaviors. This includes 75% of women in the normal cervical cytology group having had three or more lifetime sexual partners. This reflects that the integrated health system serves a high-risk population. Other studies have recruited women from larger and more diverse geographic areas for the control or normal cervical cytology comparison group.8,25,27
The psychosocial factors were significantly associated with a number of behavioral risk factors for exposure to HPV and possible development of abnormal cervical cytology. These relationships have not been examined previously. Such tools as family Apgar, discrimination, and LOT might be useful to identify young women at risk for engaging in behaviors that allow exposure to HPV.
This study had several limitations. A primary limitation is the racial/ethnic diversity in the sample population. The participants reporting white race/ethnicity made up nearly 70% of the sample, making generalization of these findings to other populations difficult. Furthermore, there may be additional relationships between minority women and psychosocial factors that were not captured in this study because of the small sample size. Second, an HPV test was not performed for the participants in the normal Pap test group. We assumed this group to be negative for HPV because of their history of normal cervical cytology; however, it is possible that some of these women may have current or past HPV infection.
Last, our implementation of the psychoneuroimmunological model in Figure 1 may have contributed to the negative findings. We used abnormal cervical cytology as a surrogate of persistent HPV, as others have done. As highlighted in Table 1, the colposcopy clinic participants' cervical cytological abnormalities ranged from ASCUS (atypical squamous cells of uncertain significance) to HGSIL (high-grade squamous intraepithelial lesions). Only the HGSIL participants are likely to have had persistent HPV, whereas the other abnormalities may represent only transient HPV infections. Limiting our analysis to HGSIL is of little value, given the limited sample size. In addition, the cross-sectional nature of the study limits understanding about whether the HPV infection is persistent. Future studies should consider limiting the study population to either women with HGSIL or women with evidence of persistent HPV. In addition, we do not have a measure of immune function specific to HPV. A recent publication on perceived stress in women with cervical dysplasia examined T cell response to HPV-16.13 Higher levels of perceived stress were associated with impaired HPV-specific immune response in women with cervical dysplasia. Similar to our study, life events were not associated with T cell response to HPV-16.13 More studies are needed to implement the psychoneuroimmunological data with longitudinal data before any definitive conclusion can be made about the relationship of psychosocial factors to abnormal cervical cytology.
Acknowledgments
Support for this study was provided in part by the National Institutes of Health, K24 CA080846, Cancer Chemoprevention and Mentoring Clinical Researchers, and by the Centers for Disease Control and Prevention, 200-2005-14735, Early Detection Research Network: Clinical Validation of Molecular Signatures of Cervical Cancer: Biorepository for Biomarker Studies of Cervical Cancer.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Schiffman MH. Bauer HM. Hoover RN, et al. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J Natl Cancer Inst. 1993;85:958–964. doi: 10.1093/jnci/85.12.958. [DOI] [PubMed] [Google Scholar]
- 2.Dalstein V. Riethmuller D. Pretet JL, et al. Persistence and load of high-risk HPV are predictors for development of high-grade cervical lesions: A longitudinal French cohort study. Int J Cancer. 2003;106:396–403. doi: 10.1002/ijc.11222. [DOI] [PubMed] [Google Scholar]
- 3.Moscicki AB. Shiboski S. Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998;132:277–284. doi: 10.1016/s0022-3476(98)70445-7. [DOI] [PubMed] [Google Scholar]
- 4.Waller J. McCaffery KJ. Forrest S. Wardle J. Human papillomavirus and cervical cancer: Issues for biobehavioral and psychosocial research. Ann Behav Med. 2004;27:68–79. doi: 10.1207/s15324796abm2701_9. [DOI] [PubMed] [Google Scholar]
- 5.Jensen SE. Lehman B. Antoni MH. Pereira DB. Virally mediated cervical cancer in the iatrogenically immunocompromised: Applications for psychoneuroimmunology. Brain Behav Immun. 2007;21:758–766. doi: 10.1016/j.bbi.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Daneshpouy M. Socie G. Clavel C, et al. Human papillomavirus infection and anogenital condyloma in bone marrow transplant recipients. Transplantation. 2001;71:167–169. doi: 10.1097/00007890-200101150-00030. [DOI] [PubMed] [Google Scholar]
- 7.Harris TG. Burk RD. Palefsky JM, et al. Incidence of cervical squamous intraepithelial lesions associated with HIV serostatus, CD4 cell counts, and human papillomavirus test results. JAMA. 2005;293:1471–1476. doi: 10.1001/jama.293.12.1471. [DOI] [PubMed] [Google Scholar]
- 8.Coker AL. Bond S. Madeleine MM. Luchok K. Pirisi L. Psychosocial stress and cervical neoplasia risk. Psychosom Med. 2003;65:644–651. doi: 10.1097/01.psy.0000041471.57895.08. [DOI] [PubMed] [Google Scholar]
- 9.Coker AL. Bond SM. Pirisi LA. Life stressors are an important reason for women discontinuing follow-up care for cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2006;15:321–325. doi: 10.1158/1055-9965.EPI-05-0148. [DOI] [PubMed] [Google Scholar]
- 10.Antoni MH. Goodkin K. Host moderator variables in the promotion of cervical neoplasia—I. Personality facets. J Psychosom Res. 1988;32:327–338. doi: 10.1016/0022-3999(88)90075-x. [DOI] [PubMed] [Google Scholar]
- 11.Antoni MH. Goodkin K. Host moderator variables in the promotion of cervical neoplasia—II. Dimensions of life stress. J Psychosom Res. 1989;33:457–467. doi: 10.1016/0022-3999(89)90007-x. [DOI] [PubMed] [Google Scholar]
- 12.Goodkin K. Antoni MH. Helder L. Sevin B. Psychoneuroimmunological aspects of disease progression among women with human papillomavirus-associated cervical dysplasia and human immunodeficiency virus type 1 co-infection. Int J Psychiatry Med. 1993;23:119–148. doi: 10.2190/F8F0-4UK8-XV79-EC6G. [DOI] [PubMed] [Google Scholar]
- 13.Fang CY. Miller SM. Bovbjerg DH, et al. Perceived stress is associated with impaired T-cell response to HPV16 in women with cervical dysplasia. Ann Behav Med. 2008;35:87–96. doi: 10.1007/s12160-007-9007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajeevan MS. Swan DC. Nisenbaum R, et al. Epidemiologic and viral factors associated with cervical neoplasia in HPV-16-positive women. Int J Cancer. 2005;115:114–120. doi: 10.1002/ijc.20894. [DOI] [PubMed] [Google Scholar]
- 15.Plummer M. Herrero R. Franceschi S, et al. Smoking and cervical cancer: Pooled analysis of the IARC multi-centric case-control study. Cancer Causes Control. 2003;14:805–814. doi: 10.1023/b:caco.0000003811.98261.3e. [DOI] [PubMed] [Google Scholar]
- 16.Cohen S. Kamarck T. Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 17.Kessler RC. Mickelson KD. Williams DR. The prevalence, distribution, and mental health correlates of perceived discrimination in the United States. J Health Soc Behav. 1999;40:208–230. [PubMed] [Google Scholar]
- 18.Wolfe J. Kimerling R. Gender issues in the assessment of posttraumatic stress disorder. In: Wilson J, editor; Keane T, editor. Assessing psychological trauma and PTSD. New York: The Guilford Press; 1997. pp. 192–238. [Google Scholar]
- 19.Smilkstein G. The family Apgar: A proposal for a family function test and its use by physicians. J Fam Pract. 1978;6:1231–1239. [PubMed] [Google Scholar]
- 20.Smilkstein G. Ashworth C. Montano D. Validity and reliability of the family Apgar as a test of family function. J Fam Pract. 1982;15:303–311. [PubMed] [Google Scholar]
- 21.Scheier MF. Carver CS. Optimism, coping, and health: Assessment and implications of generalized outcome expectancies. Health Psychol. 1985;4:219–247. doi: 10.1037//0278-6133.4.3.219. [DOI] [PubMed] [Google Scholar]
- 22.Beck AT. Guth D. Steer RA. Ball R. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behav Res Ther. 1997;35:785–791. doi: 10.1016/s0005-7967(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 23.Steer RA. Cavalieri TA. Leonard DM. Beck AT. Use of the Beck Depression Inventory for Primary Care to screen for major depression disorders. Gen Hosp Psychiatry. 1999;21:106–111. doi: 10.1016/s0163-8343(98)00070-x. [DOI] [PubMed] [Google Scholar]
- 24.Tiersma ES. van der Lee ML. Garssen B, et al. Psychosocial factors and the course of cervical intra-epithelial neoplasia: A prospective study. Gynecol Oncol. 2005;97:879–886. doi: 10.1016/j.ygyno.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Tiersma ES. van der Lee ML. Peters AA, et al. Psychosocial factors and the grade of cervical intra-epithelial neoplasia: A semi-prospective study. Gynecol Oncol. 2004;92:603–610. doi: 10.1016/j.ygyno.2003.10.046. [DOI] [PubMed] [Google Scholar]
- 26.Pereira DB. Antoni MH. Danielson A, et al. Life stress and cervical squamous intraepithelial lesions in women with human papillomavirus and human immunodeficiency virus. Psychosom Med. 2003;65:427–434. doi: 10.1097/01.psy.0000041620.37866.89. [DOI] [PubMed] [Google Scholar]
- 27.Coker AL. Preventing intimate partner violence: How we will rise to this challenge. Am J Prev Med. 2006;30:528–529. doi: 10.1016/j.amepre.2006.03.002. [DOI] [PubMed] [Google Scholar]