Abstract
Because sphingosine 1-phosphate (S1P) is a potent stimulator of angiogenesis, we hypothesized that the S1P pathway is activated to stimulate endometrial/placental angiogenesis during pregnancy. We initially localized S1P signaling pathway members in the gravid and nongravid uterine horns of unilaterally pregnant ewes. Sphingosine kinase-1 expression was greater in gravid compared to nongravid horns. In situ hybridization revealed elevated expression of sphingosine 1-phosphate phosphatase (SGPP1) in gravid interplacentomal endometrial stroma on Days 20 and 40 compared to the nongravid uterine horn, but expression increased in endometrium of the nongravid uterine horn between Days 40 and 120. SGPP1 expression increased in placentomes late in gestation. Sphingosine 1-phosphate lyase mRNA was modestly expressed at Day 20 and then decreased. In contrast, sphingosine 1-phosphate receptor 1 (S1PR1) mRNA increased in endometrium and caruncular stroma of the gravid uterine horn. Treatment with FTY720 and VPC23019, S1P receptor antagonists, blocked human and ovine endothelial cell invasion using an in vitro model of sprouting angiogenesis. Knockdown of S1PR1 with siRNA reduced invasion responses as well. We previously reported that delta-like 4 (DLL4) and A disintegrin and metalloproteinase with thrombospondin-like repeats 1 (ADAMTS1) participate in endothelial cell invasion stimulated by S1P and growth factors in vitro, and thus investigated whether their expression correlated with areas undergoing angiogenesis in vivo. DLL4 expression was similar to S1PR1, while ADAMTS1 mRNA was expressed by endometria of both nongravid and gravid horns, as well as conceptus and placentomes. These results establish that S1P signaling pathway members and S1P- and growth factor-regulated genes are prominent in uterine and placental tissue and in some cases are correlated with areas undergoing angiogenesis. Thus, S1P signaling may be crucial for proper fetal-placental development.
Keywords: ADAMTS1, DLL4, endometrium, endothelial, FTY720, S1P, S1P1, siRNA, siRNA gene silencing, sphingosine 1-phosphate, unilateral pregnancy model, uterus
Sphingosine 1-phosphate signaling pathway members are prominent in uterine and placental tissues and, in some cases, associate with areas undergoing angiogenesis.
INTRODUCTION
The mammalian uterus undergoes dynamic morphological changes to facilitate pregnancy. Considerable angiogenesis occurs in the uterine endometrium and placenta to ensure the transfer of nutrients from the maternal to the fetal circulation in all mammalian species. Ruminants have a cotyledonary placenta that provides for the conveyance of hematotrophic nutrition from the uterus to the fetus primarily through placentomes, which are specialized structures formed through the interdigitation of fetal cotyledons and maternal caruncles [1]. These cotyledons are highly branched, villous, tree-like folds of fetal trophectoderm that protrude into maternal endometrial caruncular crypts (aglandular endometrial areas). In sheep, a single layer of epithelial syncytial plaques, which are composed of both trophectoderm and endometrium, separate extensive capillary networks within the placenta and uterus. By gestational Day 40 there is maximal juxtaposition of the endometrial and placental microvasculature [2]. Placentomes provide a critical source of hematotrophic nutrition for the fetus because the maternal and fetal blood vessels are in very close proximity for exchanging oxygen and micronutrients [1]. Failure of placentome development results in death of the fetus [3]. Additional nutritional support for the fetus is provided by the interplacentomal endometrium, which contains multiple coiled and branched exocrine glands that secrete histotroph, which is a mixture of nutrients, adhesion molecules, enzymes, growth factors, and cytokines taken up by specialized structures of the chorioallantois called areolae [4, 5]. Striking angiogenesis occurs in both placentomes and interplacentomal endometrium. In the ovine interplacentomal endometrium, the capillary density doubles by Day 24 of gestation [6–8]. As gestation progresses, there is increased organization of endothelial cells and formation of new blood vessels throughout the uterus and placenta [9].
Several key signaling pathways mediate angiogenesis, including integrin cell surface receptors, transmembrane metalloproteinases, growth factors, lipids, and extracellular matrix proteins. The role of classical angiogenic growth factors, vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (FGF2) in uterine angiogenesis during late gestation is well described [1, 9–14]. Additionally, there is much evidence that the lysosphingolipid sphingosine 1-phosphate (S1P) biochemical pathway influences various aspects of angiogenesis [15–22], but little is known about the role of S1P in angiogenesis during pregnancy.
Sphingosine 1-phosphate is generated following the phosphorylation of sphingosine by sphingosine kinase 1 (SPHK1) or sphingosine kinase 2 (SPHK2). Bioactive S1P can be recycled into sphingosine through the actions of sphingosine 1-phosphate phosphatase 1 (SGPP1) and sphingosine 1-phosphate phosphatase 2 (SGPP2) [15, 23]. S1P can also be irreversibly cleaved via sphingosine 1-phosphate lyase (SGPL) to generate ethanolamine phosphate and hexadecenal, which can exit the sphingolipid pathway [15, 23]. Bioactive S1P that has not been recycled or proteolytically cleaved to an inactive form is present in blood and tissues, where it can activate one or more of its five high-affinity G-protein-coupled receptors, S1PR1–S1PR5 [15]. Of the receptors, S1PR1, S1PR2, and S1PR3 are expressed in murine endometrium following implantation, and expression correlates with embryonic angiogenesis during development [24, 25]. Further, combining S1P treatment with angiogenic growth factors stimulates endothelial cell invasion of three-dimensional (3-D) collagen matrices approximately 5- to 10-fold compared to either S1P or growth factors alone [22, 26]. These endothelial invasion responses are mediated through the known angiogenic molecules delta-like 4 (DLL4) and A disintegrin and metalloproteinase with thrombospondin-type repeats-1 (ADAMTS1) [22].
This study was designed to test our hypothesis that during pregnancy, S1P is produced locally in the uterus in response to the conceptus and acts via its receptors to coordinate and regulate the expression of critical genes that mediate angiogenesis and facilitate vascular support and maintenance of nutrient exchange at the fetal-maternal interface. The objectives of this study were to 1) investigate the precise temporal and spatial localization of members of the S1P signaling pathway in a unilaterally pregnant model, 2) investigate expression of key genes in response to treatment with a combination of S1P and angiogenic growth factors within placentomal and interplacentomal endometrium, and 3) mechanistically determine whether S1P acts via S1P receptors to regulate endothelial cell invasion into 3-D collagen matrices.
MATERIALS AND METHODS
Animals and Experimental Design
Experimental and surgical procedures involving animals in the present studies were approved by the Institutional Animal Care and Use Committee of Texas A&M University.
Unilateral Pregnant Ewes
Ewes (Texas A&M, College Station, TX) were checked daily for estrous behavior using vasectomized rams. Following at least one estrous cycle of normal duration, the ovary ipsilateral to the right uterine horn was removed, and a double ligature was placed at the base of the right uterine horn at the bifurcation. At the following estrus (Day 0), ewes were mated to intact rams to generate a nongravid and gravid uterine horn in each bred ewe [27]. Ewes were hysterectomized on either Day 20, 40, 80, or 120 of gestation (n = 4 ewes per day). Several sections (thickness ∼1–1.5 cm) of placentomal (or caruncular) and interplacentomal (or intercaruncular) tissues from each uterine horn (nongravid and gravid) were placed in fresh 4% paraformaldehyde fixative for 24 h and then embedded in Paraplast Plus (Oxford Labware, St. Louis, MO).
Immunohistochemical Analysis
SPHK1 protein was localized by immunohistochemistry in paraffin-embedded ovine uterine tissues using rabbit anti-human polyclonal SPHK1 IgG (AB46719; Abcam, Cambridge, MA) at 2 μg/ml and the Rabbit Super ABC kit (Biomeda, Foster City, CA). Antigen retrieval was conducted using boiling citrate. Replacement of primary antibody with nonimmune rabbit IgG (Sigma Chemical Co., St. Louis, MO) at the same final concentration was used as the negative control and diaminobenzidine tetrachloride (Sigma) was used as the chromagen, with all steps conducted according to methods as previously described [28].
In Situ Hybridization Analysis
SGPP1, SGPL, S1PR1, DLL4, and ADAMTS1 mRNAs were localized in paraffin-embedded uterine tissue as previously described [27, 29]. Briefly, deparaffinized, rehydrated, and deproteinated uterine cross sections (∼5 μm; n = 4) were hybridized with radiolabeled antisense or sense ovine cRNA probes [27, 29, 30] generated from linearized plasmid DNA templates. Partial cDNAs for SGPP1, SGPL, S1PR1, DLL4, and ADAMTS1 were generated by RT-PCR as described previously [30, 31]. Primers for each target gene were derived from conserved sequences of human and bovine genes using Primer 3 (Version 2.0.0-alpha; http://primer3.sourceforge.net/) [32]. Total RNA isolated from endometria and placentomes collected on Days 40, 80, and 120 served as template for S1PR1, and cDNA clones for SGPP1, SGPL, DLL4, and ADAMTS1 were purchased from Open Biosystems (Huntsville, AL). Amplified PCR products were subcloned into the pCRII cloning vector using a T/A Cloning kit (Invitrogen, Carlsbad, CA) and sequenced in both directions using an ABI PRISM Dye Terminator Cycle Sequencing kit and an ABI PRISM automated DNA sequencer (Applied Biosystems, Foster City, CA) to confirm identity. Probes were synthesized by in vitro transcription with [α-35S] uridine 5-triphosphate (PerkinElmer Life Sciences, Wellesley, MA). After hybridization, washes, RNase A digestion, and autoradiography were performed using NTB-2 liquid photographic emulsion (Eastman Kodak, Rochester, NY). Slides were exposed at 4°C, developed in Kodak D-19 developer, counterstained with Harris modified hematoxylin (Fisher Scientific, Fairlawn, NJ), dehydrated, and protected with cover slips.
Endothelial Cell Invasion in 3-D Collagen Matrices
Primary human umbilical vein endothelial cells (HUVECs; Lonza Inc., Allendale, NJ) were grown and maintained as described [33]. Ovine uterine artery endothelial cells (oUAECs) were isolated and propagated as described [26]. Cell passages 3–6 were seeded on the upper surface of 3-D collagen matrices (2.5 mg/ml) containing 1 μM S1P (Avanti Polar Lipids, Alabaster, AL). Cell monolayers were allowed to attach for 30 min prior to addition of M199 (HUVECs) or MEM (oUAECs) containing a reduced serum II supplement, VEGF (40 ng/ml), FGF2 (40 ng/ml), and ascorbic acid (50 μg/ml) [22, 33].
Inhibition of Endothelial Invasion Using FTY720
Endothelial cells were seeded with indicated concentrations of FTY720 (0–50 nM; Husker Chemical, Shanghai, China) or vehicle control (dimethyl sulfoxide [DMSO], Sigma), and samples (three wells per treatment) were allowed to invade for 24 h, after which they were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) for 10 min and stained with 1.09 μM DAP1 (Molecular Probes, Carlsbad, CA). Eyepieces mounted with a reticle (displaying a 10 × 10 grid that covers an area of 1 mm2 at 10× magnification) were used for quantifying average numbers of invading cells per standardized field [22, 33]. For each data set, three separate fields for each treatment were recorded and averaged.
siRNA Transfection
siGENOME SMARTpool human S1PR1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control siRNAs were obtained from Dharmacon (Lafayette, CO). Endothelial cells were seeded into 25-cm2 flasks at 40%–50% confluency. The following day, cells were washed three times with serum- and antibiotic-free Dulbeco modified Eagle medium (DMEM) and transfected with 200 nM siRNA in 2.7 ml antibiotic-free DMEM using 20 μl siPORT Amine (Ambion, Austin, TX). Flasks were aspirated and supplemented with antibiotic-free growth medium at 8 h posttransfection. This transfection procedure was repeated 2 days after the first transfection. After the second transfection, cells were allowed to recover prior to testing in invasion assays. Lysates were collected by placing collagen matrices in boiling sample buffer for Western blot analysis using anti-human polyclonal antisera directed to S1PR1 (OPA1‐15004; Affinity Bioreagents, Golden, CO) and verification of gene knockdown as previously described [22].
Photomicrography
Digital photomicrographs of in situ hybridization (bright field and dark field images) and immunohistochemical staining were evaluated using an Axioplan 2 microscope (Carl Zeiss, Thornwood, NY) interfaced with an Axiocam HR digital camera and Axiovision 4.1 software (Carl Zeiss). Multicapture composite images were collected sequentially as individual overlapping frames that were subsequently assembled using Adobe Photoshop (version 6.0; Adobe Systems Inc., San Jose, CA). Digital photographs of HUVEC invasion assays were captured using an Olympus CKX41 microscope (Leeds Instruments Inc., Irving, TX). Photographic plates were assembled using Adobe Photoshop.
RESULTS
Temporal and Spatial Localization of S1P Signaling Pathway Members in the Uterus and Placenta During Pregnancy Supports a Role for S1P in Mediating Angiogenesis
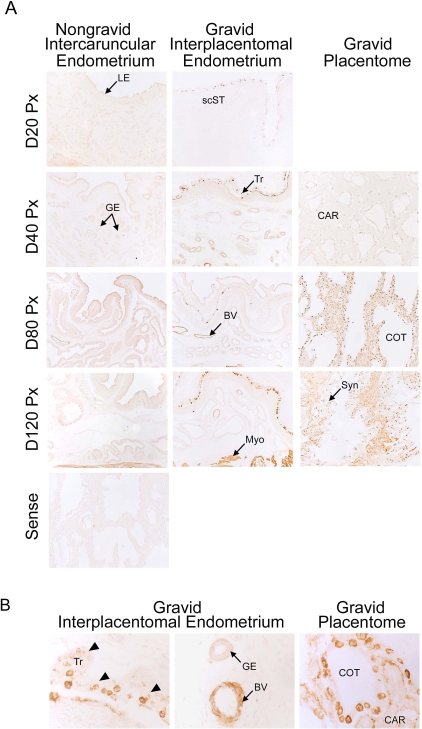
Immunohistochemistry localized SPHK1, an enzyme that phosphorylates sphingosine to SIP, to both uterine and placental tissues (Fig. 1). In interplacentomal endometrium, basal protein expression was low in nongravid uterine horns on all days of pregnancy. In contrast, SPHK1 was detected in blood vessels within the endometrium of gravid uterine horns by Day 80 and maintained through Day 120 of pregnancy (Fig. 1A). Higher magnification images allow for more detailed evaluation of SPHK1 expression within specific tissues. SPHK1 protein expression was highly upregulated in trophectoderm, uterine vasculature, cells within syncytia, and throughout caruncular tissue of the placentome (Fig. 1B). Additionally, the number of SPHK1-positive cells and the intensity of immunostaining increased from Days 20 through 120 of gestation in gravid tissues (Fig. 1A). In placentomes, SPHK1 was present in the epithelial syncytium from Days 20 through 120 of pregnancy. Interestingly, a dramatic increase of SPHK1 protein expression was observed in caruncular tissue between Days 40 and 80 of pregnancy, and high expression was maintained through Day 120 (Fig. 1A). SPHK1 was also detected in the myometrium of both gravid and nongravid uterine horns, with expression being greater in the gravid uterine horn (Fig. 1A).
FIG. 1.
Immunohistochemical localization of SPHK1 in sections of nongravid and gravid uteri collected from unilaterally pregnant ewes on Days 20, 40, 80, and 120 of gestation (A) and Day 80 of gestation (B). Rabbit IgG applied to a Day 80 placentome served as a negative control. GE, uterine glandular epithelium; Tr, placental trophectoderm/chorion; BV, blood vessels; Myo, myometrium; CAR, caruncular tissue; COT, cotyledonary tissue; scST, endometrial stratum compactum stroma; Syn, epithelial syncytia of the placentome. Arrowheads indicate placental trophectoderm in (B). Representative images are shown from independent samples (n = 4). Width of each field is 940 μm (A) and 220 μm (B).
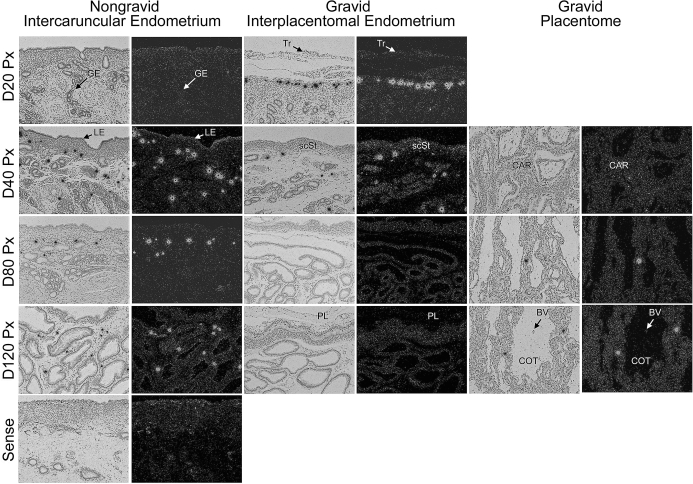
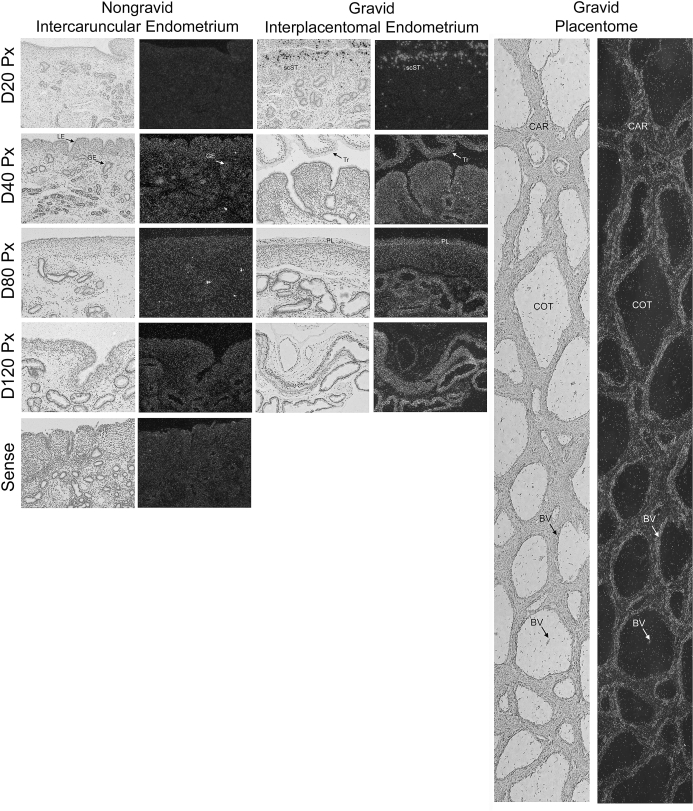
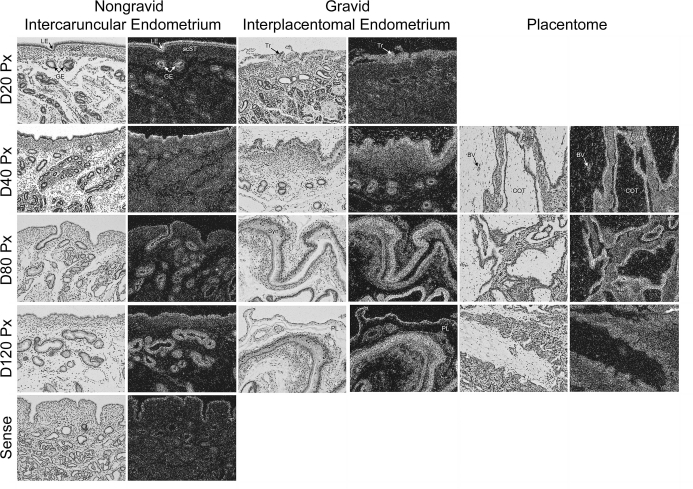
In situ hybridization was used to localize mRNAs for both SGPP1, the phosphatase that converts S1P to sphingosine, and SGPL, which is responsible for the irreversible cleavage of S1P and the exit from the sphingolipid cell signaling pathway. We did not observe widespread and robust hybridization; rather, expression of SGPP1 mRNA was selectively detectable in unidentified cells scattered within uterine stroma of both nongravid and gravid uterine horns (Fig. 2). In endometria of nongravid uterine horns, SGPP1-positive cells were initially detected on Day 40 and remained present through Day 120 of pregnancy (Fig. 2). In contrast, SGPP1-positive cells were prominent in the stratum compactum stroma (scST) directly underlying the conceptus on Day 20, became more sparsely distributed throughout the interplacentomal endometrium on Day 40, and were absent on Days 80 and 120 of pregnancy (Fig. 2). In the placentomes, SGPP1 was detected in only a few cells within the caruncular stroma on Days 80 and 120 of pregnancy (Fig. 2).
FIG. 2.
In situ hybridization analysis of SGPP1 mRNA in sections of nongravid and gravid uteri collected from unilaterally pregnant ewes on Days 20, 40, 80, and 120 of gestation. Corresponding bright field and dark field images of representative cross sections are shown. A section from Day 80 nongravid intercaruncular endometrium hybridized with radiolabeled sense cRNA probe served as a negative control. LE, endometrial luminal epithelium; GE, endometrial glandular epithelium; scSt, endometrial stratum compactum stroma; Tr, placental trophectoderm; PL, placental chorion; CAR, caruncular tissue; COT, cotyledonary tissue. Representative images are shown from independent samples (n = 4). Width of each field is 940 μm.
Under the conditions tested, SGPL mRNA expression was undetectable in nongravid uterine horns (Fig. 3). However in gravid uterine horns, a pattern of expression similar to SGPP1, albeit to a lesser extent, was observed. SGPL mRNA localized to a population of cells concentrated in the scST adjacent to attached trophectoderm on Day 20 of pregnancy, then decreased and became more dispersed from Days 40 to 120 of pregnancy (Fig. 3). Interestingly, as pregnancy progressed, cells expressing SGPL became less numerous in endometria and more numerous in myometria so that expression levels were high in the myometrium during late pregnancy (Fig. 4, right panel). An increase in SGPL mRNA expression levels over basal levels was not detectable in placentomes or trophectoderm on any day of pregnancy studied.
FIG. 3.
In situ hybridization analysis of SGPL mRNA in sections of nongravid and gravid uteri collected from unilaterally pregnant ewes on Days 20, 40, 80, and 120 of gestation. Corresponding bright field and dark field images of representative cross sections are shown. A section from Day 80 nongravid intercaruncular endometrium hybridized with radiolabeled sense cRNA probe served as a negative control. LE, endometrial luminal epithelium; GE, endometrial glandular epithelium; scST, endometrial stratum compactum stroma; Tr, placental trophectoderm; PL, placental chorion; CAR, caruncular tissue; COT, cotyledonary tissue. Representative images are shown from independent samples (n = 4). Width of each field is 940 μm.
FIG. 4.
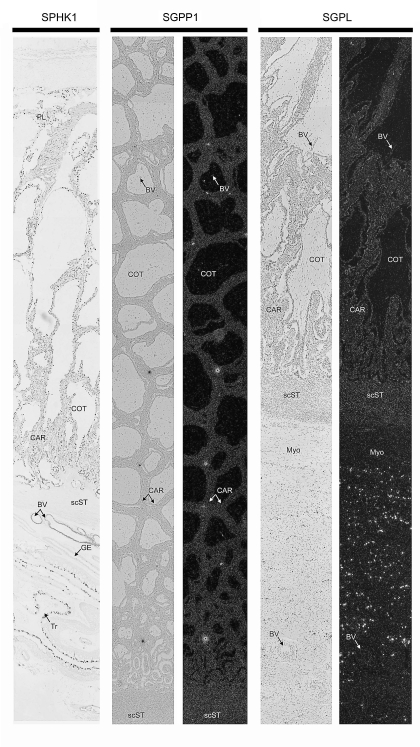
Composite images of immunohistochemical localization of SPHK1 protein and in situ hybridization analyses of SGPP1 as well as SGPL mRNAs within placentomes from Day 80 of pregnancy. GE, endometrial glandular epithelium; scST, endometrial stratum compactum stroma; BV, blood vessel; CAR, caruncular tissue; COT, cotyledonary tissue; Myo, myometrium. Representative images are shown from independent samples (n = 4). Width of each field is 940 μm.
Multicapture composite images of Day 80 placentomes, the highly vascularized structures responsible for hematotrophic support of the conceptus, demonstrated cell type-specific expression patterns for SPHK, SGPP1, and SGPL (Fig. 4). Immunoreactive SPHK1 was detectable throughout the epithelial syncytia and caruncular stroma, with particularly high expression in blood vessels and trophectoderm of the interplacentomal endometria. In situ hybridization revealed mRNA expression of the phosphatase SGPP1, which was detected intermittently in caruncular stroma. The lyase, SGPL, was abundant in the myometrium, but otherwise not significant in caruncular and cotyledonary tissue (Fig. 4). Collectively, these results suggest that immunoreactive SPHK1 is abundantly expressed in placentomes, where it may serve to phosphorylate sphingosine and form S1P, whereas phosphatase or lyase mRNA expression levels are low to undetectable under the conditions used. These data suggest that increased levels of bioactive S1P may be available to promote angiogenesis within placentomes.
S1PR1 Is Expressed in Vascularized Regions of the Pregnant Uterus and Placenta, and Inhibition of S1PR1 Blocks Endothelial Cell Sprouting Responses
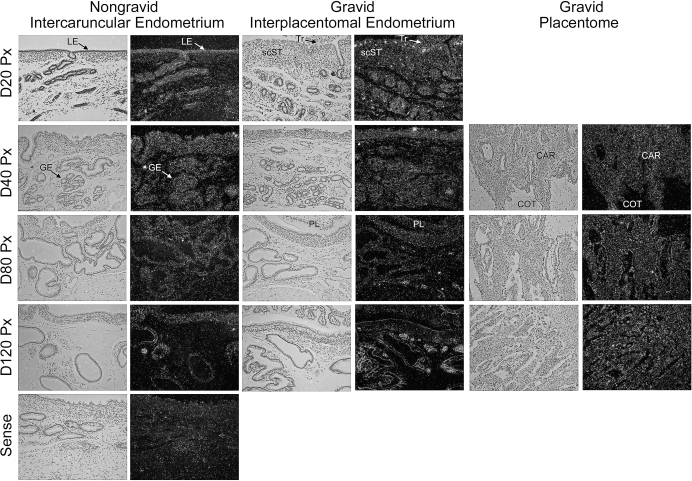
S1P signaling is mediated through S1PR1, S1PR2, S1PR3, S1PR4, and S1PR5 receptors. Because transgenic mice lacking S1P1 fail to form a functional vasculature [34], we investigated the pattern of S1PR1 expression in unilaterally pregnant ewes. In situ hybridization revealed that S1PR1 mRNA was present in both the nongravid and gravid uterine horns (Fig. 5). In the stroma of the nongravid uterine horn, S1PR1 levels were highest on Day 40 and then steadily declined throughout pregnancy. In interplacentomal regions of gravid uterine horns, S1PR1 mRNA was expressed within the scST, glandular epithelium (GE), and conceptus trophectoderm. On Day 20, S1PR1 mRNA expression was detectable in scattered cells within scST. As pregnancy progressed, this expression pattern waned; however, there was a marked increase in S1PR1 mRNA in all cell types at the fetal-maternal interface (trophectoderm and scST) and within the uterine glands at Day 80 (Fig. 5). Within placentomes, S1PR1 mRNA was localized to cotyledonary blood vessels as well as small terminal vessels of the maternal caruncle underlying the epithelial syncytia at Day 80 in composite images (Fig. 5, composite image). Expression in blood vessels of placentomes was highest at Day 40 and then decreased gradually through Day 120 of gestation (data not shown).
FIG. 5.
In situ hybridization analysis of S1PR1 mRNA in sections of nongravid and gravid uteri collected from unilaterally pregnant ewes on Days 20, 40, 80, and 120 of gestation. A composite image of a Day 80 placentome is shown (right panel). Corresponding bright field and dark field images of representative endometrial cross sections are shown. A section from Day 80 nongravid intercaruncular endometrium hybridized with radiolabeled sense cRNA probe served as a negative control. LE, endometrial luminal epithelium; GE, endometrial glandular epithelium; scST, endometrial stratum compactum stroma; Tr, placental trophectoderm; PL, placental chorion; CAR, caruncular tissue; COT, cotyledonary tissue; BV, blood vessel. Representative images are shown from independent samples (n = 4). Width of each field is 940 μm.
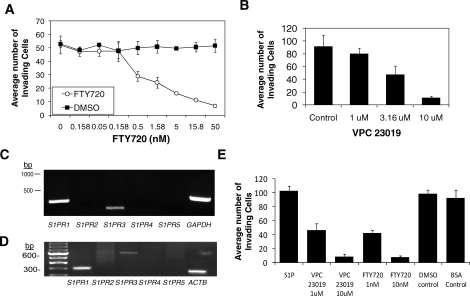
To demonstrate that S1P functionally stimulates angiogenic sprouting through the S1PR1, we utilized a previously described assay [22, 33] in which primary endothelial cells form sprouting structures and invade into 3-D collagen matrices in response to combined treatment with 1 μM S1P, 40 ng/ml of angiogenic growth factors, VEGF and FGF2, and 50 μg/ml of ascorbic acid. Initial experiments tested the effects of FTY720 and the S1PR1, S1PR2, and S1PR5 antagonist [35]. FTY720 decreased S1P and growth factor-stimulated HUVEC invasion dose-dependently, whereas DMSO, the vehicle control, had no effect on invasion (Fig. 6A). Further, a role for S1PR1 in the 3-D sprouting response was conducted using VPC23019, a functional S1PR1 and S1PR3 antagonist. Like FTY720, VPC23019 similarly interfered with S1P and growth factor-induced invasion of HUVEC in 3-D collagen matrices (Fig. 6B). RT-PCR analyses to determine expression of S1P receptors in HUVECs were carried out and revealed expression of S1PR1 and S1PR3 (Fig. 6C). A similar expression pattern was seen in oUAECs (Fig. 6D). These data demonstrate the S1P receptor expression profile is comparable in primary HUVEC and ovine endothelial cell lines. The expression of S1PR1 was confirmed in HUVECs and oUAECs, but we were unable to successfully demonstrate expression of S1PR3 by Western blot analyses (data not shown). Further, invasion of oUAECs stimulated by S1P and growth factors was inhibited by comparable doses of FTY720 and VPC23019 (Fig. 6E), demonstrating that HUVECs and oUAECs 1) invade similarly, 2) exhibit a similar S1P receptor expression profile, and 3) are sensitive to FTY720 and VPC23019.
FIG. 6.
S1P1 receptor signaling is required for human and ovine endothelial cell sprouting responses in 3-D collagen matrices. In all experiments, endothelial invasion was stimulated with 1 μM S1P, 40 ng/ml VEGF, and 40 ng/ml FGF2. A) FTY720, an S1P receptor antagonist, blocked HUVEC invasion in a dose-dependent manner. B) HUVEC invasion was conducted with increasing doses of the S1PR1 and S1PR3 antagonist VPC23019. RT-PCR reactions using primer sets designed for human (C) and ovine (D) S1PR1–S1PR5 sequences using HUVEC and oUAEC cDNA as a template, respectively. Also pictured are GAPDH and beta actin (ACTB) positive controls. Primer sequences are available upon request. E) oUAEC invasion cultures were established in the presence of S1P in combination, with doses of FTY720 and VPC23019 indicated. DMSO and bovine serum albumin served as vehicle controls for FTY720 and VPC23019, respectively. Dose-responses were conducted four times, and duplicate experiments were performed on endothelial cell lines isolated from separate individuals.
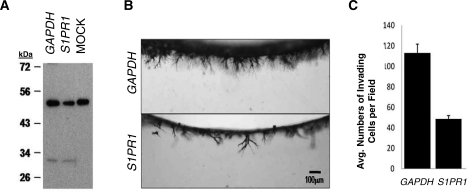
To demonstrate a functional requirement for S1PR1, HUVECs were treated with small interfering RNA (siRNA) directed to human S1PR1 and GAPDH control. Specificity of the siRNAs for S1PR1 was determined using Western blotting, which revealed a successful, albeit partial, knockdown of S1PR1 in extracts isolated from invading cultures (Fig. 7A). Although S1PR1 protein expression was not completely silenced by siRNA knockdown, invasion responses at 24 h were decreased (P < 0.05) compared to GAPDH control (Fig. 7, B and C). Thus, this in vitro model confirms a functional requirement for S1PR1 in endothelial sprouting responses that is consistent with the expression of S1PR1 and SPHK1 at areas of new blood vessel growth in the uterus and placenta during pregnancy.
FIG. 7.
Silencing of S1PR1 using custom-pooled siRNA significantly reduced HUVEC invasion. A) Extracts taken from 3-D invading cultures (16 h) were probed with antisera directed to S1P1 in Western Blot analysis. B) Invading cultures were fixed, stained, and photographed (side-view) at 24 h. C) Invasion density was quantified by counting the average numbers of invading cells per standardized field (n = 3 fields; mean ± SEM). P < 0.05. Results are representative of duplicate experiments.
The S1P- and Growth Factor-Regulated Genes, DLL4 and ADAMTS1, Display Different Patterns of Expression in the Uterus and Placenta, Which Suggests Complementary Roles in Support of Pregnancy
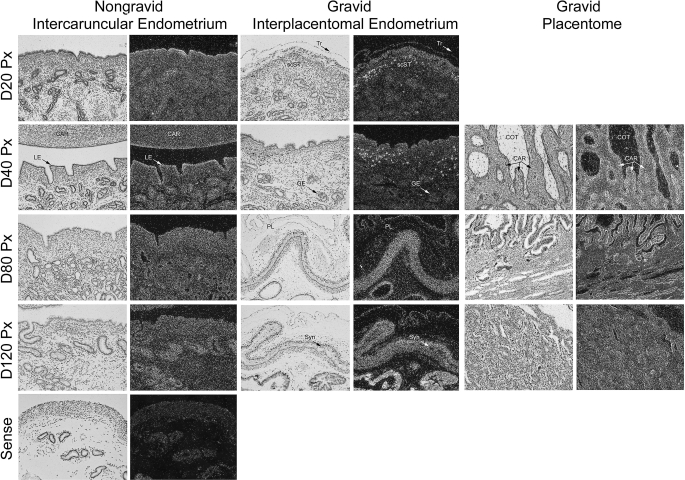
Previously, we demonstrated in vitro that DLL4 and ADAMTS1 expression is regulated at the mRNA level by S1P and angiogenic growth factors. Further, both DLL4 and ADAMTS1 were required for HUVEC sprouting responses in 3-D collagen matrices [22]. Based on these results, we used in situ hybridization to investigate whether DLL4 and ADAMTS1 mRNA were regulated in ovine uteri and placentae during pregnancy and whether the expression correlated with angiogenesis. DLL4 is a receptor for the Notch signaling pathway and a known marker of newly sprouting endothelial cells [36, 37]. In interplacentomal endometria, DLL4 mRNA expression was low to nondetectable in the nongravid uterine horn on all days of pregnancy (Fig. 8, left panels). In contrast, DLL4 mRNA was specifically expressed by a discrete population of cells in the scST of the gravid interplacentomal endometrium on Days 20 and 40 of gestation. By gestational Day 80, DLL4 mRNA was detectable throughout the fetal-maternal interface (trophectoderm, luminal epithelia [LE], and scST), and there was robust expression by GE on Days 80 and 120 (Fig. 8, center panels). Within the placentome, DLL4 mRNA was expressed specifically in the small terminal vessels of the maternal caruncular stroma underlying the epithelial syncytia that lines the caruncular crypts of the placentome (Fig. 8, right panels).
FIG. 8.
In situ hybridization analysis of DLL4 mRNA in sections of nongravid and gravid uteri collected from unilaterally pregnant ewes on Days 20, 40, 80, and 120 of gestation. Corresponding bright field and dark field images of representative endometrial cross sections are shown. A section from Day 80 nongravid intercaruncular endometrium hybridized with radiolabeled sense cRNA probe served as a negative control. LE, endometrial luminal epithelium; GE, endometrial glandular epithelium; scST, endometrial stratum compactum stroma; Tr, placental trophectoderm; PL, placental chorion; CAR, caruncular tissue; COT, cotyledonary tissue; Syn, epithelial syncytia of the placentome. Representative images are shown from independent samples (n = 4). Width of each field is 940 μm.
In situ hybridization analysis revealed that ADAMTS1 mRNA is expressed in a number of uterine and placental cell types of both nongravid and gravid uterine horns (Fig. 9). In endometria of nongravid uterine horns, ADAMTS1 mRNA was expressed by LE, stroma, and superficial GE on Day 20 of gestation. Moderate expression of ADAMTS1 was maintained in the nongravid LE and stroma, while mRNA in the GE increased to maximum levels by Day 120 of pregnancy (Fig. 9). In the interplacentomal endometria of the gravid uterine horn, ADAMTS1 mRNA was expressed by conceptus trophectoderm/chorion, as well as endometrial LE, scST, and GE on Day 20 of gestation. Expression in all of these cell types increased steadily to very high levels through Day 120 of pregnancy (Fig. 9). There was also abundant ADAMTS1 mRNA expressed within placentomes in the cotyledonary vasculature, the caruncular stroma, and the epithelial syncytia (Fig. 9).
FIG. 9.
In situ hybridization analysis of ADAMTS1 mRNA in sections of nongravid and gravid uteri collected from unilaterally pregnant ewes on Days 20, 40, 80, and 120 of gestation. Corresponding bright field and dark field images of representative endometrial cross sections are shown. A section from Day 80 nongravid intercaruncular endometrium hybridized with radiolabeled sense cRNA probe served as a negative control. LE, endometrial luminal epithelium; GE, endometrial glandular epithelium; scST endometrial stratum compactum stroma; Tr, placental trophectoderm; BV, blood vessel; CAR, caruncular tissue; COT, cotyledonary tissue; PL, placental chorion; Syn, epithelial syncytia of the placentome. Representative images are shown from independent samples (n = 4). Width of each field is 940 μm.
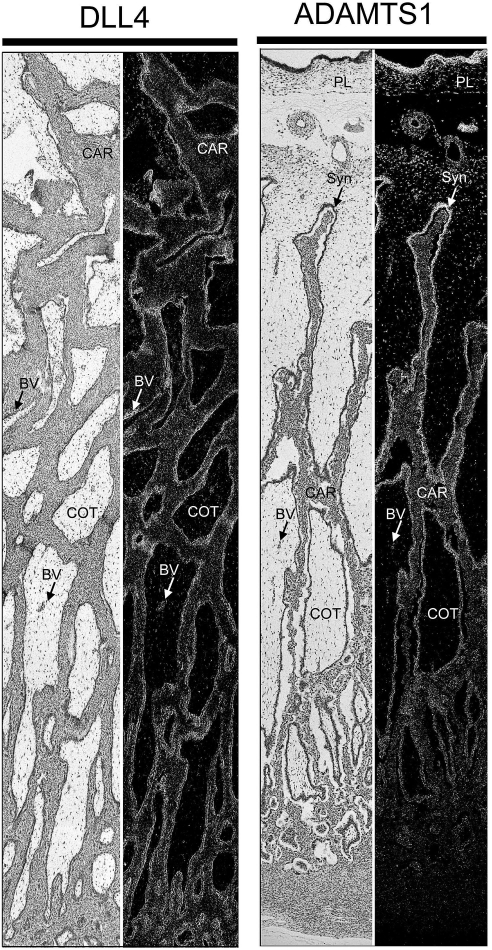
Multicapture composite images of Day 80 placentomes, the highly vascularized structures responsible for hematotrophic support of the conceptus, revealed distinct expression patterns for DLL4 and ADAMTS1 (Fig. 10). Significantly, DLL4 mRNA was expressed throughout the placentome and was localized to cotyledonary blood vessels and small terminal vessels of the maternal caruncle underlying the epithelial syncytia of the placentome. This closely resembled S1PR1 mRNA expression (Fig. 5). Separate evaluation of an entire Day 80 placentome revealed that ADAMTS1 mRNA expression was most abundant in the chorioallantois and proximal edges of the cotyledonary villi lining distal portions of the caruncular crypts (Fig. 10). Levels of ADAMTS1 decreased in the syncytia near the base of the caruncular crypts, and expression was lost upon reaching the placentomal capsule (Fig. 10). Thus, the expression of these S1P- and growth factor-regulated genes at key sites within the developing placentome overlaps with angiogenesis in some cases and suggests S1P might synergize with growth factors or other stimuli to support growth and development of the conceptus during pregnancy.
FIG. 10.
Composite images of in situ hybridization analyses for DLL4 and ADAMTS1 mRNA within placentomes collected from unilaterally pregnant ewes on Day 80 of pregnancy. Corresponding bright field and dark field images of representative placentomes are shown. BV, blood vessel; CAR, caruncular tissue; COT, cotyledonary tissue; PL, chorion; Syn, epithelial syncytia of the placentome. Representative images are shown from independent samples (n = 4). Width of each field is 940 μm.
DISCUSSION
Angiogenesis within the pregnant uterus occurs rapidly and is critical to provide hematotrophic support for fetal-placental development [6, 7, 9]. Insufficient endometrial/placental vascularization can compromise pregnancy in pathological situations such as preeclampsia. Considerable attention has been paid to regulation of uterine/placental angiogenesis by classical angiogenic factors such as VEGF and FGF. In addition, members of the S1P signaling pathway are expressed within mammalian uteri [24, 25], and we recently reported that S1P is a potent angiogenic factor that stimulates endothelial cell invasion and sprouting within 3-D collagen matrices [22]. Results of the present study include a comprehensive examination of S1P signaling pathway members in the ovine uterus and placenta throughout gestation in a unilaterally pregnant model and establish the ability to determine the molecular mechanism(s) by which S1P affects fetal-placental development. Our results clearly indicate that 1) robust expression of SPHK and moderate levels of SGPP1 and SGPL are associated with uterine and placental angiogenesis; 2) S1PR1 is present in endometrial and placental tissues undergoing angiogenesis, and antagonism of S1PR1 blocks S1P and growth factor-induced invasion responses in human and ovine endothelial cells; and 3) S1P- and angiogenic growth factor-regulated genes, DLL4 and ADAMTS1, are expressed in a cell-specific manner to optimally influence angiogenic events at the uterine-placental interface.
The present study utilized the unilaterally pregnant ewe to assess effects of the conceptus on regulation of S1P signaling pathway to better understand the role of S1P in mediating angiogenesis during pregnancy. The unilaterally pregnant model provides two distinct environments for assessment of angiogenic changes. The nongravid uterine horn does not form placentomes and lacks a placenta and fetus. Because the placenta exerts profound physical and paracrine/endocrine effects at the uterine-placental interface, including effects on angiogenesis, the nongravid uterine horn exhibits only moderate angiogenesis in response to maternal factors during pregnancy. In contrast, the gravid uterine horn exhibits the full angiogenic response to paracrine and endocrine factors of a normal pregnancy. This study is the first to use a unilaterally pregnant animal model for investigation of pregnancy-associated regulation of S1P signaling pathway members. It is important to note that the majority of genes investigated in this study, including SPHK, S1PR1, DLL4, and ADAMTS1, were expressed at higher levels in the gravid compared to nongravid uterine horn of unilaterally pregnant ewes. Our data are consistent with Skaznik-Wikiel et al. [25], who demonstrated specific upregulation of S1PR1 by the embryo using an oil-induced deciduoma model.
The enzymes SPHK1 and SPHK2 are necessary for the generation of S1P. Results of the present study strongly support a role for SPHK1 in uterine/placental angiogenesis. SPHK1 expression was detected in the trophectoderm, raising the possibility that S1P could be produced locally and secreted towards the interplacentomal endometrium to support a doubling of the vasculature during pregnancy. More importantly, SPHK was highly expressed in the placentomes that provide the major vascular network for high throughput transfer of molecules from the mother to fetus. SPHK was present in the epithelial syncytium that directly separates the terminal tips of uterine and placental capillaries. In addition, SPHK increased dramatically in the stroma of caruncular (endometrial) tissue, where a logarithmic increase in angiogenesis occurs during pregnancy. Our results further support that SPHK is important for fetal development. In the rat uterus, Sphk1 is localized to the GE, vasculature, and myometrium [38] and, in a pattern similar to that for sheep, steadily increases throughout gestation. In the mouse, Sphk1 mRNA is elevated in the decidua (an structure analogous to the sheep placentome) compared to the interimplantation sites [24]. Decidua of pregnant Sphk1 knockout mice exhibit an abnormal accumulation of dihydrosphingosine and sphingosine, and decidual blood vessels degenerate in proximity to the embryo [24]. Interestingly, inside-out signaling of S1P can be induced by receptor tyrosine kinase activation by various growth factors (e.g., VEGF, transforming growth factor-beta, and insulin-like growth factor). This leads to SPHK activation and translocation to the membrane, which induces local production of S1P to stimulate G protein-coupled receptors [39]. Collectively, our work and that of others suggest that SPHK1 is critical in maintaining the uterine environment that may signal in concert with various growth factor receptor pathways during pregnancy.
Although expression is comparable in the nongravid and gravid uterine horns of ewes, both SGPP1 and SGPL were abundantly expressed by cells located intermittently within the stratum compactum stroma, just beneath the basal lamina of the endometrial LE on Day 20 of pregnancy, which overlaps with the attachment phase of implantation in sheep. SGPP1 recycles S1P into sphingosine; conversely, SGPL irreversibly cleaves S1P to exit the lysosphingolipid pathway. One interpretation of this finding is that removal of bioactive S1P is necessary for conceptus implantation. Consistent with this possibility, localization of SGPP1 within the scST beneath the LE at the site of conceptus attachment would allow S1P produced in response to elevated SPHK1 secreted by the trophectoderm to be efficiently recycled. Further, relatively lower SGPP1 expression levels in gravid versus nongravid tissue suggest increased availability of S1P occurs in gravid tissue as pregnancy proceeds. Similar to SGPP1, SGPL mRNA levels declined in the gravid endometrium and became restricted to the myometrium and supporting serosa. Placentomes exhibited high levels of SPHK1, which phosphorylates sphingosine to form S1P, whereas low-level increases of SGPP1 and SGPL were detectable within placentomes as pregnancy progressed. The net result may be high levels of S1P that stimulate extensive angiogenesis within the placentome. However, we must cautiously interpret these findings because protein expression is not a reliable indicator of enzymatic activity, and mRNA may not accurately reflect protein expression levels of SGPP1 and SGPL. The expression patterns for both SGPP1 and SGPL within uteri of sheep differ from that in mice, in which both Sgpp1 and Sgpl1 increase in decidua versus interimplantation sites of the uterus [24]. The scattered expression patterns of SGPP1 and SGPL in multiple species warrants further investigation because S1P is a known mediator of lymphocyte trafficking, which is increased in the uterus during implantation and placentation [40]. Likewise, we have not investigated lipid phosphate phosphatases, which may also serve to modulate S1P bioavailability [41, 42].
Our study delved into S1P1 receptor expression patterns in a unilaterally pregnant model. Of the five known G protein-coupled receptors (S1P1‐5), S1PR1 and S1PR3 are expressed in both embryonic and uterine tissues [25, 38], and ablation of S1PR1 is lethal to the embryo due to hemorrhaging [34]. Further, expression of S1PR1, S1PR3, and S1PR5 has been detected within human chorionic plate arteries [43]; however, we were unable to detect significant levels of S1PR5 mRNA or protein (data not shown), nor could we detect S1PR3 protein despite positive expression at the mRNA level (Fig. 6, C and D). Thus, although we cannot rule out a role for S1PR3 in the present study, we elected to focus on S1PR1. Similar to other species, S1PR1 was expressed by the trophectoderm and endometrium at the maternal-fetal interface of interplacentomal regions on all days of pregnancy. However, a major finding of this study was localization of S1PR1 to the capillary tips of the caruncular vasculature of placentomes, which are areas of active angiogenesis. These vessels supply the epithelial syncytium that separates maternal from fetal tissues and are directly responsible for hematotrophic support of fetal development. These results support data from mice, where S1PR1 is upregulated in the subluminal stromal compartment of the antimesometrial pole of the decidua by Day 5, and in the microvasculature of the mesometrial pole by Day 7 of pregnancy [25]. S1PR1 is also present at the maternal/fetal interface, as it is expressed by placental vascular stem cells and fetal connective tissues [25]. In humans it is hypothesized that sources of S1P affecting the fetal vasculature likely include the syncytiotrophoblast or cytotrophoblast cells, placental stromal cells, and fetal circulatory cells [43]. Collectively, results from mice, humans, and now sheep indicate that S1PR1 is an excellent candidate molecule for stimulating angiogenesis. To confirm a functional requirement for S1PR1 in mediating angiogenic sprouting events, an established in vitro 3-D endothelial cell invasion assay was used to demonstrate that FTY720, an S1P receptor antagonist, as well as siRNA specific for S1PR1, significantly decreased endothelial cell invasion and sprouting responses in vitro. These results clearly indicate that S1PR1 is required for S1P and growth factor-induced endothelial sprouting responses, and these results are also consistent with a role for S1PR1 in regulating angiogenic responses during pregnancy.
Appropriate regulation of gene expression is necessary for maintenance of pregnancy. We previously demonstrated that DLL4 and ADAMTS1 were differentially regulated by S1P, VEGF, and FGF2 [22], motivating in situ hybridization studies conducted here. In the pregnant ewe, DLL4 displayed a complex pattern of expression that appeared to be driven by the presence of placental/fetal tissues, because DLL4 expression is relatively low in the nongravid uterine horn of unilaterally pregnant ewes. In gravid uterine horns, DLL4 mRNA was detected throughout the maternal-fetal interface and uterine glands during later stages of gestation. Relevant to angiogenesis, DLL4 and S1PR1 mRNA expression patterns were similar. DLL4, like S1PR1, was observed in the maternal vasculature in placentomes. Interestingly, DLL4 also increased in the endometrial GE of gravid ewes during the later stages of pregnancy. DLL4 protein is expressed in human endometrium [50], where both cytoplasmic and apical membrane staining were observed in LE and GE with moderate diffuse staining in the cytoplasm of stromal cells [44]. Similarly, ADAMTS1 expression at the uterine-placental interface of sheep agreed with results from other species. In the pregnant human uterus, ADAMTS1 is associated with decidualization of endometrial stroma and localized specifically in the stroma surrounding the spiral arterioles of the secretory endometrium that are the primary source of hematotrophic support for fetal development [45]. Interestingly, similar to sheep, the endometrial GE of women also expresses high levels of ADAMTS1. In mice, Adamts1 was not present in the stroma, but rather detectable in uterine LE and GE [46]. Coordinate and complementary expression of DLL4 and ADAMTS1 suggest necessary roles for each in regulating the morphological changes within interplacentomal and placentomal endometrium to support pregnancy. Thus, S1P may be acting through S1P1 receptors to promote angiogenesis within placentomes, but whether DLL4 and ADAMTS1 expression patterns are directly regulated by S1P remains to be demonstrated.
Results of the present study demonstrate coordinated temporal regulation of SPHK, SGPP1, and SGPL, which support increased availability of S1P to stimulate placentomal angiogenesis and provide hematotrophic support for pregnancy in sheep. Antagonism of S1PR1 with pharmacological compounds and siRNA blocked S1P- and growth factor-stimulated human and sheep endothelial cell invasion, which was further supported by detection and localization of S1PR1 to the caruncular capillary tips of placentomes. Thus, S1P may be acting through S1PR1 to promote angiogenic responses. However, effects on other cell types and tissues remain to be defined. Collectively, the spatial expression of these members of the S1P signaling pathway at key sites of angiogenesis within the uterus suggests an important role in supporting growth and development of the conceptus during pregnancy. Future studies antagonizing the S1P pathway in vivo will determine key signals elicited by S1P and determine whether S1P is required for angiogenic responses, vascular and glandular development, and coordinate expression of ADAMTS1 and DLL4.
Footnotes
Supported by National Research Initiative Competitive Grants Program, Cooperative State Research, Education, and Extension Service, U.S. Department of Agriculture awards 2009-35203-05725 to K.J.B. and 2008-35203-18830 to K.A.D. along with National Institutes of Health Grant HL49210 to R.R.M.
REFERENCES
- Reynolds LP, Biondini ME, Borowicz PP, Vonnahme KA, Caton JS, Grazul-Bilska AT, Redmer DA.Functional significance of developmental changes in placental microvascular architecture. Endothelium 2005; 12: 11–19. [DOI] [PubMed] [Google Scholar]
- Cross JC, Baczyk D, Dobric N, Hemberger M, Hughes M, Simmons DG, Yamamoto H, Kingdom JC.Genes, development and evolution of the placenta. Placenta 2003; 24: 123–130. [DOI] [PubMed] [Google Scholar]
- Mellor DJ, Mitchell B, Matheson IC.Reductions in lamb weight caused by pre-mating carunclectomy and mid-pregnancy placental ablation. J Comp Pathol 1977; 87: 629–633. [DOI] [PubMed] [Google Scholar]
- Roberts RM, Bazer FW.The functions of uterine secretions. J Reprod Fertil 1988; 82: 875–892. [DOI] [PubMed] [Google Scholar]
- Bazer FW.Uterine protein secretions: Relationship to development of the conceptus. J Anim Sci 1975; 41: 1376–1382. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA.Utero-placental vascular development and placental function. J Anim Sci 1995; 73: 1839–1851. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Borowicz PP, Vonnahme KA, Johnson ML, Grazul-Bilska AT, Wallace JM, Caton JS, Redmer DA.Animal models of placental angiogenesis. Placenta 2005; 26: 689–708. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Grazul-Bilska AT, Redmer DA.Angiogenesis in the female reproductive organs: pathological implications. Int J Exp Pathol 2002; 83: 151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowicz PP, Arnold DR, Johnson ML, Grazul-Bilska AT, Redmer DA, Reynolds LP.Placental growth throughout the last two thirds of pregnancy in sheep: vascular development and angiogenic factor expression. Biol Reprod 2007; 76: 259–267. [DOI] [PubMed] [Google Scholar]
- Charnock-Jones DS, Kaufmann P, Mayhew TM.Aspects of human fetoplacental vasculogenesis and angiogenesis, I: molecular regulation. Placenta 2004; 25: 103–113. [DOI] [PubMed] [Google Scholar]
- Dunk C, Shams M, Nijjar S, Rhaman M, Qiu Y, Bussolati B, Ahmed A.Angiopoietin-1 and angiopoietin-2 activate trophoblast Tie-2 to promote growth and migration during placental development. Am J Pathol 2000; 156: 2185–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girling JE, Rogers PA.Recent advances in endometrial angiogenesis research. Angiogenesis 2005; 8: 89–99. [DOI] [PubMed] [Google Scholar]
- Hildebrandt VA, Babischkin JS, Koos RD, Pepe GJ, Albrecht ED.Developmental regulation of vascular endothelial growth/permeability factor messenger ribonucleic acid levels in and vascularization of the villous placenta during baboon pregnancy. Endocrinology 2001; 142: 2050–2057. [DOI] [PubMed] [Google Scholar]
- Ahmed A, Dunk C, Ahmad S, Khaliq A.Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen—a review. Placenta 2000; 21(suppl A):S16–S24. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S.Exogenous and intracellularly generated sphingosine 1-phosphate can regulate cellular processes by divergent pathways. Biochem Soc Trans 2003; 31: 1216–1219. [DOI] [PubMed] [Google Scholar]
- English D, Brindley DN, Spiegel S, Garcia JG.Lipid mediators of angiogenesis and the signalling pathways they initiate. Biochim Biophys Acta 2002; 1582: 228–239. [DOI] [PubMed] [Google Scholar]
- Karliner JS.Lysophospholipids and the cardiovascular system. Biochim Biophys Acta 2002; 1582: 216–221. [DOI] [PubMed] [Google Scholar]
- Hla T.Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol 2004; 15: 513–520. [DOI] [PubMed] [Google Scholar]
- Langlois S, Gingras D, Beliveau R.Membrane type 1-matrix metalloproteinase (MT1-MMP) cooperates with sphingosine 1-phosphate to induce endothelial cell migration and morphogenic differentiation. Blood 2004; 103: 3020–3028. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Yoo HS, Suh PG, Oh S, Lim JS, Lee YM.Sphingosine mediates FTY720-induced apoptosis in LLC-PK1 cells. Exp Mol Med 2004; 36: 420–427. [DOI] [PubMed] [Google Scholar]
- Wang F, Van Brocklyn JR, Hobson JP, Movafagh S, Zukowska-Grojec Z, Milstien S, Spiegel S.Sphingosine 1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor. Potential involvement in angiogenesis. J Biol Chem 1999; 274: 35343–35350. [DOI] [PubMed] [Google Scholar]
- Su SC, Mendoza EA, Kwak HI, Bayless KJ.Molecular profile of endothelial invasion of three-dimensional collagen matrices: insights into angiogenic sprout induction in wound healing. Am J Physiol Cell Physiol 2008; 295: C1215–C1229. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM.Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 2008; 9: 139–150. [DOI] [PubMed] [Google Scholar]
- Mizugishi K, Li C, Olivera A, Bielawski J, Bielawska A, Deng CX, Proia RL.Maternal disturbance in activated sphingolipid metabolism causes pregnancy loss in mice. J Clin Invest 2007; 117: 2993–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaznik-Wikiel ME, Kaneko-Tarui T, Kashiwagi A, Pru JK.Sphingosine-1-phosphate receptor expression and signaling correlate with uterine prostaglandin-endoperoxide synthase 2 expression and angiogenesis during early pregnancy. Biol Reprod 2006; 74: 569–576. [DOI] [PubMed] [Google Scholar]
- Bayless KJ, Davis GE.Sphingosine-1-phosphate markedly induces matrix metalloproteinase and integrin-dependent human endothelial cell invasion and lumen formation in three-dimensional collagen and fibrin matrices. Biochem Biophys Res Commun 2003; 312: 903–913. [DOI] [PubMed] [Google Scholar]
- Dunlap KA, Erikson DW, Burghardt RC, White FJ, Reed KM, Farmer JL, Spencer TE, Magness RR, Bazer FW, Bayless KJ, Johnson GA.Progesterone and placentation increase secreted phosphoprotein one (SPP1 or osteopontin) in uterine glands and stroma for histotrophic and hematotrophic support of ovine pregnancy. Biol Reprod 2008; 79: 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Spencer TE, Bazer FW.Cathepsins in the ovine uterus: regulation by pregnancy, progesterone, and interferon tau. Endocrinology 2005; 146: 4825–4833. [DOI] [PubMed] [Google Scholar]
- Joyce MM, Gonzalez JF, Lewis S, Woldesenbet S, Burghardt RC, Newton GR, Johnson GA.Caprine uterine and placental osteopontin expression is distinct among epitheliochorial implanting species. Placenta 2005; 26: 160–170. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Spencer TE.WNT pathways in the neonatal ovine uterus: potential specification of endometrial gland morphogenesis by SFRP2. Biol Reprod 2006; 74: 721–733. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Spencer TE, Burghardt RC, Bazer FW.Ovine osteopontin, I: cloning and expression of messenger ribonucleic acid in the uterus during the periimplantation period. Biol Reprod 1999; 61: 884–891. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H.Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 2000; 132: 365–386. [DOI] [PubMed] [Google Scholar]
- Bayless KJ, Kwak HI, Su SC.Investigating endothelial invasion and sprouting behavior in three-dimensional collagen matrices. Nat Protocols 2009; 4: 1888–1898. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest 2000; 106: 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graler MH, Goetzl EJ.The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J 2004; 18: 551–553. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 2007; 445: 776–780. [DOI] [PubMed] [Google Scholar]
- Dufraine J, Funahashi Y, Kitajewski J.Notch signaling regulates tumor angiogenesis by diverse mechanisms. Oncogene 2008; 27: 5132–5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M, Allende ML, Proia RL.Sphingosine-1-phosphate regulation of mammalian development. Biochim Biophys Acta 2008; 1781: 435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebman DA, Spiegel S.Cross-talk at the crossroads of sphingosine-1-phosphate, growth factors, and cytokine signaling. J Lipid Res 2008; 49: 1388–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen PJ.Regulation of immune cells in the uterus during pregnancy in ruminants. J Anim Sci 2007; 85: E30–E31. [DOI] [PubMed] [Google Scholar]
- Brindley DN.Lipid phosphate phosphatases and related proteins: signaling functions in development, cell division, and cancer. J Cell Biochem 2004; 92: 900–912. [DOI] [PubMed] [Google Scholar]
- Brindley DN, Pilquil C.Lipid phosphate phosphatases and signaling. J Lipid Res 2009; 50(suppl):S225–S230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings DG, Hudson NK, Halliday D, O'Hara M, Baker PN, Davidge ST, Taggart MJ.Sphingosine-1-phosphate acts via rho-associated kinase and nitric oxide to regulate human placental vascular tone. Biol Reprod 2006; 74: 88–94. [DOI] [PubMed] [Google Scholar]
- Mazella J, Liang S, Tseng L.Expression of Delta-like protein 4 in the human endometrium. Endocrinology 2008; 149: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng YH, Zhu H, Pallen CJ, Leung PC, MacCalman CD.Differential effects of interleukin-1beta and transforming growth factor-beta1 on the expression of the inflammation-associated protein, ADAMTS-1, in human decidual stromal cells in vitro. Hum Reprod 2006; 21: 1990–1999. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim H, Lee SJ, Choi YM, Lee JY.Abundance of ADAM-8, ‐9, ‐10, ‐12, ‐15 and ‐17 and ADAMTS-1 in mouse uterus during the oestrous cycle. Reprod Fertil Dev 2005; 17: 543–555. [DOI] [PubMed] [Google Scholar]