Abstract
Heparin-binding EGF-like growth factor (HBEGF) is expressed by trophoblast cells throughout gestation. First-trimester cytotrophoblast cells are protected from hypoxia-induced apoptosis because of the accumulation of HBEGF through a posttranscriptional autocrine mechanism. Exogenous application of HBEGF is cytoprotective in a hypoxia/reoxygenation (H/R) injury model and initiates trophoblast extravillous differentiation to an invasive phenotype. The downstream signaling pathways induced by HBEGF that mediate these various cellular activities were identified using two human first-trimester cytotrophoblast cell lines, HTR-8/SVneo and SW.71, with similar results. Recombinant HBEGF (1 nM) induced transient phosphorylation of MAPK3/1 (ERK), MAPK14 (p38), and AKT within 15 min and JNK after 1–2 h. To determine which downstream pathways regulate the various functions of HBEGF, cells were treated with specific inhibitors of the ERK upstream regulator MEK (U0126), the AKT upstream regulator phosphoinositide-3 (PI3)-kinase (LY294002), MAPK14 (SB203580), and JNK (SP600125), as well as with inactive structural analogues. Only SB203580 specifically prevented HBEGF-mediated rescue during H/R, while each inhibitor attenuated HBEGF-stimulated cell migration. Accumulation of HBEGF at reduced oxygen was blocked only by a combination of U0126, SB203580, and SP600125. We conclude that HBEGF advances trophoblast extravillous differentiation through coordinate activation of PI3 kinase, ERK, MAPK14, and JNK, while only MAPK14 is required for its antiapoptotic activity. Additionally, hypoxia induces an autocrine increase in HBEGF protein levels through MAPK14, JNK or ERK. These experiments reveal a complexity of the intracellular signaling circuitry that regulates trophoblast functions critical for implantation and placentation.
Keywords: AKT, apoptosis, cell differentiation, cell migration, mitogen-activated protein kinases, phosphoinositide-3-kinase, pregnancy, signal transduction, trophoblast
Heparin-binding epidermal growth factor has numerous functions in human trophoblast cells, including supporting invasion and survival and induction of its own synthesis. These are to be mediated through independent downstream MAPK and AKT signaling pathways.
INTRODUCTION
Blastocyst implantation is a tightly regulated and dynamic process that establishes a pregnancy. Central to implantation are the trophoblast cells that populate the exterior of the blastocyst. These unique cells invade the endometrium interstitially and intravascularly [1] and can survive the changes in oxygen concentration that accompany early development of the placenta [2]. Early in this process, trophoblast cells function in a relatively hypoxic uterine environment, a condition that is drastically altered during the 10th week of pregnancy in humans when extravillous trophoblast cells occluding the maternal arteries dislodge, allowing highly oxygenated blood to enter the intervillous space within the developing placenta. Trophoblasts survive this oxidative challenge and accelerate the pace of invasion [3]. Oxygen fluctuations occur throughout pregnancy with great variation among individuals [2]. The elevation of oxygen after an ischemic episode can damage trophoblast cells because of the resulting oxidative stress [4], possibly precipitating pathological outcomes [5].
The epidermal growth factor (EGF) signaling system is capable of regulating diverse cellular activities, including survival, invasion and differentiation [6, 7]. EGF-related growth factors are expressed abundantly in the receptive endometrium [8, 9], with heparin-binding EGF-like growth factor (HBEGF) having a prominent role during peri-implantation development [10–12]. It is specifically expressed at the site of blastocyst attachment in mice, immediately prior to implantation [13], and appears cyclically in humans at the apical surface of luminal epithelial cells during the period when the endometrium is most receptive for embryo implantation [10]. Conditional excision of HBEGF in the murine uterus delays blastocyst implantation and reduces litter sizes [14], suggesting that HBEGF is important not only for timely attachment of the blastocyst but also for subsequent invasive events. Indeed, HBEGF accelerates the differentiation of mouse trophoblast cells to an adhesive phenotype [15], increasing the area over which they subsequently migrate [13]. Similar stimulatory effects of HBEGF have been reported for human embryos [16]. HBEGF is implicated in both the successful invasion and the survival of human cytotrophoblast cells [17–19]. Members of the EGF family, including HBEGF, EGF, and TGFA, are capable of inducing altered integrin expression and accelerating trophoblast migratory and invasive activity in first-trimester human cytotrophoblast cells [17, 20]. EGF is capable of preventing cytokine-induced apoptosis in term cytotrophoblast and syncytiotrophoblast [21, 22], and both EGF [23] and HBEGF [24] block apoptosis resulting from exposure to hypoxia. During the first trimester, trophoblast cells have the ability to survive and proliferate in the very low oxygen environment present at the implantation site [25, 26]. Investigation of their survival capacity revealed that cytoprotective activity is provided as HBEGF accumulates in cytotrophoblast cells exposed to low oxygen tension [18].
Failure of trophoblast cells to survive and invade maternal tissues interferes with the remodeling of uterine spiral arteries required to increase blood flow to the growing conceptus and is thought to contribute to pre-eclampsia [27–29], intrauterine growth restriction [30, 31], and spontaneous abortion [2]. The physiological interaction of trophoblast cells with oxygen during pregnancy is complex, with a preference for low levels during the first trimester. It has been hypothesized that fluctuations in oxygen during early pregnancy create hypoxia/reoxygenation (H/R) episodes that produce oxidative stress, which may compromise trophoblast survival [4, 5, 32]. Indeed, activation of the EGF signaling system with HBEGF or related growth factors can prevent apoptosis due to oxidative stress caused by exposing first-trimester cytotrophoblast cells to H/R [19]. Examination of placentas delivered by women with pre-eclampsia reveals a dramatic reduction in HBEGF expression [33] and suggests the important role of this signaling system.
Although HBEGF appears to have many important functions in trophoblast cells, the underlying mechanisms have not yet been assessed. To this end, we have initiated experiments to identify intracellular signaling pathways that are responsible for the multiple outcomes of HBEGF signaling. Using two immortalized, human, first-trimester cytotrophoblast cell lines, we have examined the downstream signaling circuitry that regulates the ability of HBEGF to autoregulate, induce migration, and inhibit apoptosis. As with all EGF family ligands, HBEGF initially binds to and activates members of the HER/ERBB receptor tyrosine kinase family [6, 7]. EGFR, ERBB2, and ERBB4 (HERs 1, 2, and 4) but not ERBB3 (HER3) possess functional intracellular tyrosine kinase activities that, on ligation and dimerization, induce cross phosphorylation of their intracellular domains. Phosphorylated tyrosine residues then serve as docking sites for intracellular proteins that direct downstream signaling pathways, including phosphoinositide-3-kinase (PIK3) and MAPK cascades. Using inhibitors of the most common MAPK pathways and PIK3, we have examined their roles downstream of HBEGF in trophoblast cell extravillous differentiation and survival and in the upregulation of HBEGF during hypoxia.
MATERIALS AND METHODS
Cell Culture and Reoxygenation Injury
Two immortalized, first-trimester, human cytotrophoblast cell lines, HTR-8/SVneo [34] (provided by Dr. Charles Graham of Queens University) and SW.71 [35] (provided by Dr. Gil Mor of Yale University), were cultured at 2% or 20% O2, as previously described [17, 19]. The HTR-8/SVneo cell line originates from first-trimester villous explants and is immortalized by stably expressing the large T viral antigen [34]. The SW.71 cell line also originates from first-trimester villous explants but is immortalized by overexpressing the telomerase enzyme [35]. SW.71 cells resemble extravillous trophoblasts, including their expression of hCG, vimentin, cytokeratin-7, and their invasion of Matrigel.
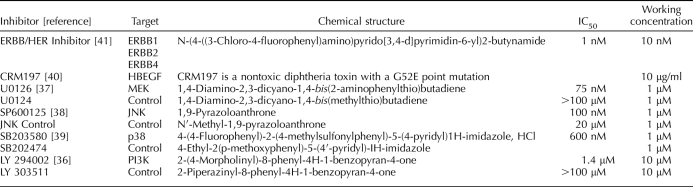
Exposure to H/R has been previously described [19]. Briefly, cells were cultured at 2% O2 for 2 h, and then media was replaced with fresh media pre-equilibrated at 20% O2 for an additional 6 h of culture at 5% CO2 and ambient O2. Cells cultured at 2% O2 for 8 h served as a control. Where indicated, cells were cultured in the presence of 1 nM recombinant human HBEGF (R&D Systems) with or without addition of inhibitors. Inhibitors and their inactive structural analogues (Table 1 [36–41]) were purchased from Calbiochem (EMD) and were specific for JNK (JNK Inhibitor II and negative control), MAPK14 (p38) (SB203580 and SB202474), PIK3 (PI3K) (LY294002 and LY303511), and MEK (U0126 and U0124). Inhibitors of EGFR/ERBB2/ERBB4 (ERBB/HER Inhibitor) tyrosine kinase activity (catalog no. 324840) and HBEGF signaling (cross-reacting material 197; CRM197) were also purchased from Calbiochem.
TABLE 1.
Inhibitors and inactive structural analogs used in this study.
Cell Death Assay
Cells fixed with 4% paraformaldehyde for 20 min were permeabilized with 0.1% Triton-X100 for 15 min and terminal deoxynucleotidyl transferase-mediated deoxyuridine 5-triphosphate nick end-labeled (TUNEL) using a kit from Roche Applied Science, as previously described [18]. Briefly, cell nuclei were counterstained with 1 mg/ml DAPI to obtain a “TUNEL Index” by calculating a ratio of TUNEL-positive nuclei to DAPI-positive nuclei. Previously, we determined that cell death in cytotrophoblast exposed to H/R or hypoxia alone in the absence of HBEGF signaling was due to apoptosis rather than necrosis [18, 19].
Migration Assay
A modified Boyden chamber assay was conducted using sterile transwell inserts with polycarbonate membrane filters containing 8-mm pores (Corning) to examine the extravillous differentiation of trophoblast cells to a migratory phenotype. Transwell inserts were coated top and bottom with 10 μg/ml human plasma fibronectin (Invitrogen) in sterile PBS at 4°C overnight. Fibronectin was removed from each well, and 500 μl of prewarmed serum-free media were added to the lower chamber. Treatments were carried out prior to conducting the migration assays. For each treatment, cells were first serum starved for 24 h by culturing in DMEM/F-12 containing 5 mg/ml BSA. Media was then exchanged for either fresh serum-free media (vehicle control) or serum-free media containing 10 nM recombinant HBEGF without (positive control) or with (experimental groups) inhibitor. After 4 h of culture, cells were washed twice with 2 ml of serum-free media, and culture was continued for an additional 20 h. After their pretreatments, 50 000 cells were added to the upper chamber of triplicate transwell inserts in a final volume of 200 μl. Transwell plates were incubated at 37°C for 9 h. Cells migrating to the underside of the membrane were trypsinized into the lower well, combining with cells that had detached from the underside of the membrane during culture. The cells were fixed with 10% formalin and mixed by pipetting. After allowing the cells to settle for 15 min, they were counted using a phase-contrast inverted light microscope at 100×, viewing 10 different fields in each well. From the average number of cells per field, the total number of cells in the lower well was calculated.
Western Blotting
Western blots were performed as previously described [42]. Cellular lysates were diluted in SDS sample buffer containing 5% β-mercaptoethanol, run on precast 4%–20% Tris-HCl gradient gels (BioRad), and blotted with primary antibodies. Antibodies against AKT1/2/3 (monoclonal rabbit), phospho-AKT1/2/3 (pAKT; Ser473; monoclonal rabbit), JNK1/2/3 (polyclonal rabbit), phospho-JNK1/2/3 (pJNK; Thr183/Tyr185; monoclonal mouse), MEK1/2 (polyclonal rabbit), and phospho-MEK1/2 (pMEK; Ser217/221; polyclonal rabbit) were purchased from Cell Signaling Technologies. Antibodies against HBEGF (polyclonal goat), MAPK14 (polyclonal rabbit), phospho-MAPK14 (pMAPK14; Thr180/Tyr182; polyclonal rabbit), MAPK3/1 (ERK; monoclonal mouse), and phospho-ERK (pERK; pERK1 at Thr202/Tyr204 and pERK2 at Thr185/Tyr187; monoclonal rabbit) were purchased from R&D Systems. Secondary anti-rabbit, anti-goat, and anti-mouse antibodies purchased from Cell Signaling Technologies were detected by enhanced chemiluminescence (Amersham Pharmacia Biotech). Bands were observed for MEK/pMEK, ERK/pERK, MAPK14/pMAPK14, AKT/pAKT, and JNK/pJNK at 45, 40/45, 40/45, 60, and 46/54 kDa, respectively.
Immunocytochemistry
Cells were fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton-X100, and stained for the presence of HBEGF protein, as previously described [17, 42]. For secondary antibody labeling, a horseradish peroxidase-conjugated anti-mouse/anti-rabbit kit was used (Dako EnVision System-HRP), as described by Armant et al. [18]. Image analysis was performed according to published procedures [33].
ELISA
ELISA was carried out using the HBEGF DuoSet ELISA Development kit (R&D Systems), as previously described [18, 19]. The optical density of the final reaction product was determined at 450 nm using a programmable multiplate spectrophotometer (Power Wave Workstation; Bio-Tek Instruments) with automatic wavelength correction.
Statistics
All assays were performed in triplicate, and all experiments were repeated at least three times and are reported as mean ± SEM. Statistical significance was determined at P < 0.05 by analysis of variance with the Student-Newman-Keuls post hoc test, using SPSS version 12.0 statistics software (SPSS).
RESULTS
HBEGF Activates Multiple Signaling Pathways
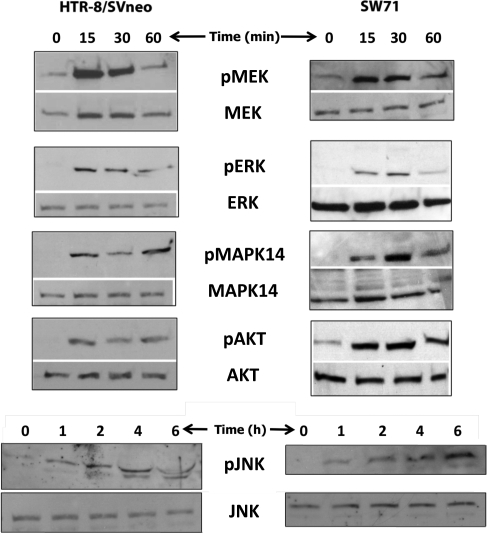
In order to identify signaling pathways targeted by HBEGF in human cytotrophoblast cell lines, cells were treated for 15 min to 6 h with 10 nM recombinant HBEGF. After 15 min, Western blotting and immunocytochemistry revealed a marked phosphorylation of MEK, MAPK14, ERK, and AKT (Fig. 1 and Supplemental Fig. S1 [all Supplemental Data are available online at www.biolreprod.org], respectively). Phosphorylation was maintained for at least 45 min during treatment with HBEGF, then declined to levels observed before treatment. Phosphorylation of JNK, however, was not significant after 1 h of HBEGF treatment but occurred shortly thereafter and remained phosphorylated for up to 6 h (Fig. 1 and Supplemental Fig. S1). We conclude that HBEGF induces a rapid, transient activation of the MAPK14 and ERK and PIK3 pathways but a slower or delayed activation of the JNK pathway downstream of ERBB/HER receptor tyrosine kinases.
FIG. 1.
Identification of signaling pathways activated by HBEGF. Extracts were prepared from HTR-8/SVneo (left panels) or SW.71 (right panels) cell lines at the indicated times after treatment with 1 nM HBEGF and analyzed by Western blotting. Each lane contained 30 μg of protein extract and was labeled with antibodies against the indicated proteins (lower panels) or their phosphorylated forms (upper panels). Images shown are representative of at least three experiments.
The rapid phosphorylations of ERK, MAPK14, and AKT were each attenuated by an inhibitor of ERBB/HER tyrosine kinase (Table 1) in a dose-dependent manner (Fig. 2A), establishing that the activity of HBEGF was mediated through its cognate receptors. If individual pathways were blocked with their respective inhibitor, HBEGF-induced phosphorylation of the specific target kinase was blocked but not the other kinases (Fig. 2, B–D). To validate JNK inhibition, an inhibitor of JNK blocked its phosphorylation when induced by H/R injury (Fig. 2E). Treating with the inactive structural analogues of these inhibitors had no effect on the phosphorylation status of any of the target proteins. None of the treatments altered the total levels of any protein (data not shown).
FIG. 2.
Characterization of kinase inhibitors by Western blotting. Extracts of HTR-8/SVneo cells were analyzed by Western blotting after (A–D) culture for 30 min in the absence (control) or presence of 1 nM HBEGF. Where indicated, cells were also treated with HBEGF plus (A) 1–100 nM ErbB/HER tyrosine kinase inhibitor (HER Inh), (B) 1 μM SB203580 (p38 Inh), (C) 10 μM LY294002 (PI3K Inh), or (D) 1 μM U0126 (MEK Inh) or their inactive structural analogues (NC Inh), as indicated. In E, cells were cultured for 8 h at 20% O2 (control) or subjected to hypoxia/reoxygenation (H/R), as described in the Materials and Methods section, in the absence or presence of 1 μM SP600125 (JNK Inh) or its inactive structural analogue (NC Inh). All samples were labeled with antibody against the indicated phosphoproteins. Images shown are representative of at least three experiments.
HBEGF Induces Differentiation Using Multiple Signaling Pathways
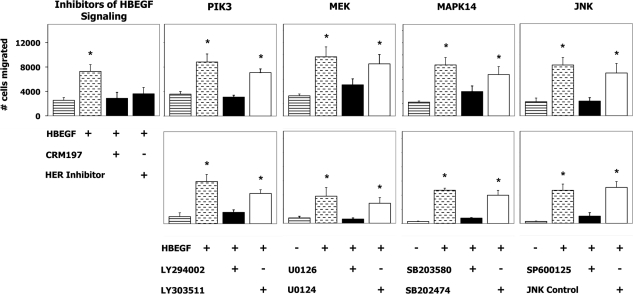
HBEGF has previously been shown to induce the extravillous differentiation of trophoblasts from first-trimester villous explants as evidenced by an increase in cell migration [17]. Pharmacological inhibitors were used, with inactive structural analogues as controls (Table 1), to delineate the signaling pathways downstream of HBEGF that mediate this differentiation. A 4-h treatment with HBEGF 20 h prior to assay was found in preliminary experiments (Supplemental Fig. S2) to be optimal for stimulation of cell migration. As displayed in Figure 3, HBEGF induced an increase (P < 0.05) in migration that was blocked by inhibiting either ERBB/HER tyrosine kinase activity or HBEGF signaling. When cells were cultured in the presence of HBEGF and an inhibitor of any of the three MAPK pathways (MAPK14, MEK, or JNK) or the PIK3 inhibitor, the increase in migration was blocked (Fig. 3). The inactive structural analogues of each inhibitor were without effect. Therefore, all four pathways were utilized by HBEGF to initiate trophoblast extravillous differentiation.
FIG. 3.
Signaling pathways required for HBEGF induction of extravillous differentiation. The number of SW.71 (upper panels) or HTR-8/SVneo (lower panels) cells migrating through a fibronectin-coated transwell membrane insert and into the lower chamber were measured after culture in the absence (striped bars) or presence (stippled bars) of 1 nM HBEGF, with kinase inhibitors (black bars) or the corresponding inactive structural analogue (white bars), as indicated. Values represent the average ± SEM of at least three experiments; *P < 0.05.
HBEGF Prevents Apoptosis Using the MAPK14 Pathway
It was recently reported that HBEGF prevents H/R-induced apoptosis in human cytotrophoblasts by signaling through its cognate receptors, EGFR and ERBB4 [19]. Cell death detected by TUNEL was found to be associated with several criteria for apoptosis. When trophoblast cells were exposed to H/R and monitored by TUNEL, there was a marked increase in apoptosis, as compared to cells cultured continuously at 2% O2 (Fig. 4 and Supplemental Fig. S3). Supplementation with recombinant HBEGF attenuated the increase in apoptosis. To identify the pathways utilized by HBEGF to inhibit apoptosis, cells were cultured with pharmacological inhibitors of PIK3, MEK, MAPK14, JNK, or their inactive structural analogues (Table 1). Inhibition of MAPK14 but not the other kinases blocked the cytoprotective effects of HBEGF during H/R injury (Fig. 4). The inactive structural analogue of the MAPK14 inhibitor had no effect. Using the same set of inhibitors, we found that the MAPK14 pathway is also required for cytotrophoblast survival at 2% O2 (Supplemental Fig. S4), having previously found that apoptosis is specifically prevented by autocrine HBEGF signaling during hypoxia [18]. Therefore, HBEGF signaling through the MAPK14 pathway appears to abrogate trophoblast apoptosis induced by H/R injury and hypoxia.
FIG. 4.
Signaling pathways required for HBEGF inhibition of apoptosis. The apoptotic indices were calculated in HTR-8/SVneo (A) or SW.71 (B) cell lines after exposure to H/R in the absence (striped bars) or presence (stippled bars) of 1 nM HBEGF, with kinase inhibitors (black bars) or the corresponding inactive structural analogues (white bars), as indicated. Significance was determined with reference to the apoptosis index observed in control cells cultured continuously at 2% O2 (dotted line). Values represent the average ± SEM of at least three experiments; *P < 0.05.
Hypoxia Increases Synthesis of HBEGF Through Autocrine Induction of MAPK14, ERK, or JNK
HBEGF cellular and secreted protein levels are significantly increased in cytotrophoblast cells after 4 h of culture at 2% O2 [18], but the downstream pathways responsible for its upregulation have not been identified. Using the HBEGF-specific antagonist CRM197, it was confirmed by immunohistochemical staining of HBEGF that HBEGF signaling is required for its upregulation during exposure to hypoxia (Fig. 5). Individual inhibitors of downstream signaling pathways did not alter the increase in HBEGF observed at 2% O2 (data not shown), so cytotrophoblast cells were treated with combinations of the inhibitors during hypoxic culture. By treating with all possible combinations of inhibitors or inactive structural analogues, it was determined that the three MAPK pathways but not PIK3 were each capable of mediating the increase in HBEGF protein levels at 2% O2 (Fig. 5 and Supplemental Fig. S5). HBEGF accumulation was prevented only when all three MAPK inhibitors were simultaneously applied. These findings were confirmed by quantifying HBEGF concentrations in cell lysates and using a specific ELISA (Supplemental Table S1).
FIG. 5.
Signaling pathways required for increased synthesis of HBEGF during hypoxia. HTR-8/SVneo (A) or SW.71 (B) cells were labeled with an antibody against HBEGF after culturing at 20% (gray bars) or 2% O2 (black bars) in the presence of kinase inhibitors or their inactive structural analogues (inactive analogues), as indicated by their target pathway. The HBEGF-specific antagonist, CRM197, was also used to block HBEGF signaling. All possible combinations of inhibitors or their inactive structural analogues were assessed, but only the most relevant combinations are shown here. Image analysis was used to quantify the relative stain intensity, which is shown in arbitrary units on the horizontal axis. Values represent the average ± SEM of at least three experiments; *P < 0.05.
DISCUSSION
The present investigation demonstrated that HBEGF transiently activates the MAPK14, JNK, and ERK MAPK pathways as well as the PIK3/AKT pathway downstream of the ERBB/HER tyrosine kinases in human cytotrophoblast cells. Only the MAPK14 pathway appeared to be utilized to prevent apoptosis induced by oxygen fluctuations. However, it functioned in combination with the ERK, JNK, and PIK3 pathways to induce trophoblast extravillous differentiation. HBEGF signaling is required to increase HBEGF protein levels when O2 is decreased to 2% [18], and both immunohistochemical and ELISA data indicated that HBEGF upregulation can be mediated by any one of the three MAPK pathways. These data confirm prior reports that HBEGF inhibits apoptosis [18, 19, 24, 43] and promotes differentiation of trophoblast cells toward a migratory, extravillous phenotype [15, 17, 44]. Moreover, the new findings identify separate downstream signaling pathways mediated by HBEGF-induced ERBB/HER activation that are responsible for each functional outcome.
Although HBEGF utilizes different pathways to mediate its diverse effects in trophoblasts, much remains to be learned about the downstream effectors that are involved. Several factors, in addition to HBEGF, that induce trophoblast migration include the ubiquitin-type plasminogen activator (uPA)/uPA receptor (uPAR) system, insulin-like growth factor (IGF), IGF-binding protein 1 (IGFBP1), hepatocyte growth factor, and endothelin 1 [45]. The uPA/uPAR system induces migration in two ways. It activates matrix metalloproteinases to initiate extracellular matrix degradation and induces intracellular Ca2+ signaling to activate phospholipase C (PLC), PIK3, and ERK [46]. Metalloproteinases are necessary for the shedding and secretion of HBEGF [6], which activated PIK3 and ERK in HTR-8SVneo cells, and can induce intracellular Ca2+ signaling (Jessmon and Armant, unpublished observation). IGF signaling also utilizes ERK to induce trophoblast migration, acting through the IGF type 2 receptor [47]. IGFBP1 induces migration by binding the α5β1 integrin, which leads to activation of focal adhesion kinase and ERK [48]. HBEGF also affects integrin signaling but through integrin switching rather than direct ligation [17]. TNF-alpha (TNF) also can induce integrin switching, with upregulation of integrin α1 and downregulation of integrin α6 [49], as well as increased expression of vascular integrins (αv and β3) in an immortalized trophoblast cell line, TCL1 [50]. Endothelin 1 activates two pathways: one involving PLC and intracellular Ca2+ signaling and the other involving ERK [51]. EGF also increases migratory activity in both HTR-8/SVneo cells [17, 52, 53] and freshly isolated first-trimester human cytotrophoblasts [20] through the coordinated activation of PIK3/AKT and ERK pathways [52]. Signaling through the PIK3/AKT pathway requires p70S6K and MTOR activation but increases migration only if the ERK pathway is simultaneously activated [52]. It remains to be ascertained whether EGF and HBEGF operate through the same intracellular signaling pathways in first-trimester cytotrophoblast cells. In the human extravillous cytotrophoblast cell line, SGHPL-4, EGF stimulates cell motility through the PIK3/AKT, MAPK14, and ERK pathways [54]. Interestingly, blocking the MAPK14 pathway with SB203580 also inhibited activation of AKT, suggesting that the pathways cross talk [54]. In contrast to EGF, the present study found that the PIK3, MAPK14, ERK, and JNK pathways mediated HBEGF induction of trophoblast migration without cross talk. Useful insights would be gained by identifying potential downstream effectors common to these four pathways.
Several intermediates have been implicated in the regulation of trophoblast apoptosis. In contrast to our finding that MAPK14 mediates the cytoprotective activity of HBEGF, it has been reported that H/R induces apoptosis through MAPK14 activation of the JNK pathway in trophoblasts from term villous explants [55]. It was recently discovered that H/R activates ASK1, leading to activation of both MAPK14 and JNK [56]. This supports the notion that first- and third-trimester trophoblast cells engage different signaling mechanisms in response to oxidative stress. In another study, JNK was responsible for inducing apoptosis in human first-trimester placental trophoblasts exposed to hyperosmolar stress [57]. In agreement, we have observed activation of JNK by H/R in the same cell line.
Currently, only a few antiapoptotic factors are known in trophoblasts. When exposed to reactive oxygen species (H2O2), BeWo cells undergo apoptosis, concomitant with an increase in the tumor suppressor gene, TP53, and a decrease in its inhibitor, MDM2 [58]. Interestingly, MDM2 is expressed in trophoblasts throughout early gestation but disappears from cytotrophoblast cells by the third trimester [59, 60]. Cytotrophoblast cells also express nuclear TP53 more strongly in the first trimester than at term [61, 62]. Taken together, it is likely that MDM2 suppresses the proapoptotic influence of TP53 in first-trimester trophoblasts, while TP53 becomes prominent late in gestation. Indeed, trophoblast apoptosis is relatively low in the first trimester, even in the face of oxidative stress, but increases toward term as trophoblast cells become less tolerant to changes in oxygen [22, 24]. Therefore, MDM2 is a potential intermediate in the antiapoptotic pathway downstream of MAPK14 signaling.
In addition to the EGF signaling system component HBEGF, EGF is cytoprotective for trophoblasts. It can inhibit apoptosis in term placental explants [63] and isolated term cytotrophoblasts [22] through the PIK3/AKT pathway [63]. Studies suggest that EGF does not utilize BCL2 to block cytokine-induced apoptosis in term cytotrophoblasts [64] but may work by decreasing the amount of ceramide produced during a proapoptotic signaling event [65]. EGF can activate PIK3 ERK, JNK, and sphingosine kinase 1 (SPHK1) in these cells, all of which are required subsequently to inhibit apoptosis [23, 66]. Interestingly, PIK3 and ERK are needed to block apoptosis [23], and the activation of SPHK1 is partially downstream of PIK3 [66]. EGF does activate MAPK14, but, in contrast to our findings, this pathway is not involved in the cytoprotective effects of EGF or the apoptotic pathway induced by cytokines [66]. This could indicate a difference between pathways activated by EGF and HBEGF but more likely reflects another difference between term and first-trimester trophoblasts. However, EGF increases proliferation of cytotrophoblasts in term villous explants, as assessed by MKI67 immunostaining [63], while HBEGF is a weak mitogen for first-trimester cytotrophoblasts [17] and term trophoblast cells [24]. Although EGF is cytoprotective, it is not upregulated with HBEGF in first-trimester trophoblast cells in response to hypoxia and thus is less likely to be part of a cytoprotective mechanism during early gestation [18].
Previous work demonstrated that the upregulation of HBEGF in first-trimester cytotrophoblast cells cultured at low oxygen is unique among the EGF ligand family [18]. It should be noted that this increase in protein is not accompanied by any change in its mRNA, indicating that HBEGF is posttranscriptionally regulated by oxygen. The upregulation of HBEGF protein during hypoxia is downstream of ERBB/HER tyrosine kinase signaling and metalloproteolytic shedding of HBEGF from the cell surface, suggesting that newly secreted HBEGF activates its own translation through autocrine signaling. The present study confirmed that the HBEGF-specific antagonist CRM197 blocks upregulation of HBEGF and further indicated that this positive feedback loop can utilize any one of the three major MAPK pathways but not PIK3. While this is the first report that HBEGF is posttranscriptionally regulated through MAPK signaling during hypoxia, other gene products are similarly regulated downstream of MAPK. For example, MAPK14 enhances translation of interleukin-8 in airway epithelial cells [67], activates translation of TNF in Kupffer cells and macrophages [68, 69], and stabilizes mRNA for interleukin 6 [70] and CCAAT enhancer binding protein-δ [71]. The JNK pathway is involved in the posttranscriptional regulation of TNF [72] and angiopoietin 2 [73]. The ERK and PIK3 pathways are both involved in translation of the Na+/K+ exchanger 1 [74] in cervical cancer cells and cyclooxygenase 2 in ovarian cancer cells [75]. In addition, microRNA (miRNA) is well known to regulate the translation and stability of mRNA in a gene-specific fashion [76] and has been shown to vary in HTR-8/SVneo cells in response to changing oxygen concentration [77]. The potential role of miRNA in the translational regulation of HBEGF by oxygen warrants future exploration.
This investigation has identified several intracellular signaling pathways activated by HBEGF in first-trimester trophoblast cells and has linked them to its numerous physiological effects. Different pathways are utilized by HBEGF to induce extravillous trophoblast differentiation, block apoptosis, and autoregulate HBEGF protein levels. This information provides a foundation for delineating the intracellular circuitry and transcriptional activity linking HBEGF signaling through its cognate receptors to physiological outcomes necessary for trophoblast function during implantation and placentation in humans.
Supplementary Material
Acknowledgments
The authors wish to than Dr. Charles Graham of Queens University for providing the HTR-8/SVneo cell line and Dr. Gil Mor of Yale University for providing the SW.71 cell line. They would also like to thank Mr. Michael Kruger for assistance with statistical analyses.
Footnotes
Supported, in part, by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, DHHS and NIH grant R01HD045966, as well as through NICHD cooperative agreement U54 HD40093 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
REFERENCES
- Pijnenborg R, Robertson WB, Brosens I, Dixon G.Review article: trophoblast invasion and the establishment of haemochorial placentation in man and laboratory animals. Placenta 1981; 2: 71–91. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E.Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Invest 2004; 11: 342–352. [DOI] [PubMed] [Google Scholar]
- Norwitz ER, Schust DJ, Fisher SJ.Implantation and the survival of early pregnancy. N Engl J Med 2001; 345: 1400–1408. [DOI] [PubMed] [Google Scholar]
- Hung TH, Skepper JN, Burton GJ.In vitro ischemia-reperfusion injury in term human placenta as a model for oxidative stress in pathological pregnancies. Am J Pathol 2001; 159: 1031–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung TH, Skepper JN, Charnock-Jones DS, Burton GJ.Hypoxia-reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res 2002; 90: 1274–1281. [DOI] [PubMed] [Google Scholar]
- Holbro T, Hynes NE.ErbB receptors: directing key signaling networks throughout life. Annu Rev Pharmacol Toxicol 2004; 44: 195–217. [DOI] [PubMed] [Google Scholar]
- Riese DJ, Stern DF.Specificity within the EGF family/ErbB receptor family signaling network. Bioessays 1998; 20: 41–48. [DOI] [PubMed] [Google Scholar]
- Hofmann GE, Scott RT, Jr, Bergh PA, Deligdisch L.Immunohistochemical localization of epidermal growth factor in human endometrium, decidua, and placenta. J Clin Endocrinol Metab 1991; 73: 882–887. [DOI] [PubMed] [Google Scholar]
- Horowitz GM, Scott RT, Jr, Drews MR, Navot D, Hofmann GE.Immunohistochemical localization of transforming growth factor-alpha in human endometrium, decidua, and trophoblast. J. Clin. Endocrinol. Metab 1993; 76: 786–792. [DOI] [PubMed] [Google Scholar]
- Leach RE, Khalifa R, Ramirez ND, Das SK, Wang J, Dey SK, Romero R, Armant DR.Multiple roles for heparin-binding epidermal growth factor-like growth factor are suggested by its cell specific expression during the human endometrial cycle and early placentation. J Clin Endocrinol Metab 1999; 84: 3355–3363. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Gui Y, Apparao KB, Young SL, Mulholland J.Regulated expression of heparin-binding EGF-like growth factor (HB-EGF) in the human endometrium: a potential paracrine role during implantation. Mol Reprod Dev 2002; 62: 446–455. [DOI] [PubMed] [Google Scholar]
- Yoo HJ, Barlow DH, Mardon HJ.Temporal and spatial regulation of expression of heparin-binding epidermal growth factor-like growth factor in the human endometrium: a possible role in blastocyst implantation. Dev Genet 1997; 21: 102–108. [DOI] [PubMed] [Google Scholar]
- Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK.Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development 1994; 120: 1071–1083. [DOI] [PubMed] [Google Scholar]
- Xie H, Wang H, Tranguch S, Iwamoto R, Mekada E, Demayo FJ, Lydon JP, Das SK, Dey SK.Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proc Natl Acad Sci U S A 2007; 104: 18315–18320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Mayernik L, Schultz JF, Armant DR.Acceleration of trophoblast differentiation by heparin-binding EGF-like growth factor is dependent on the stage-specific activation of calcium influx by ErbB receptors in developing mouse blastocysts. Development 2000; 127: 33–44. [DOI] [PubMed] [Google Scholar]
- Martin KL, Barlow DH, Sargent IL.Heparin-binding epidermal growth factor significantly improves human blastocyst development and hatching in serum-free medium. Hum Reprod 1998; 13: 1645–1652. [DOI] [PubMed] [Google Scholar]
- Leach RE, Kilburn BA, Wang J, Liu Z, Romero R, Armant DR.Heparin-binding EGF-like growth factor regulates human extravillous cytotrophoblast development during conversion to the invasive phenotype. Dev Biol 2004; 266: 223–237. [DOI] [PubMed] [Google Scholar]
- Armant DR, Kilburn BA, Petkova A, Edwin SS, Duniec-Dmuchowski ZM, Edwards HJ, Romero R, Leach RE.Human trophoblast survival at low oxygen concentrations requires metalloproteinase-mediated shedding of heparin-binding EGF-like growth factor. Development 2006; 133: 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach RE, Kilburn BA, Petkova A, Romero R, Armant DR.Diminished survival of human cytotrophoblast cells exposed to hypoxia/reoxygenation injury and associated reduction of heparin-binding epidermal growth factor-like growth factor. Am J Obstet Gynecol 2008; 198: 471.e1–471.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass KE, Morrish D, Roth I, Bhardwaj D, Taylor R, Zhou Y, Fisher SJ.Human cytotrophoblast invasion is up-regulated by epidermal growth factor: evidence that paracrine factors modify this process. Dev Biol 1994; 164: 550–561. [DOI] [PubMed] [Google Scholar]
- Garcia-Lloret MI, Yui J, Winkler-Lowen B, Guilbert LJ.Epidermal growth factor inhibits cytokine-induced apoptosis of primary human trophoblasts. J Cell Physiol 1996; 167: 324–332. [DOI] [PubMed] [Google Scholar]
- Smith S, Francis R, Guilbert L, Baker PN.Growth factor rescue of cytokine mediated trophoblast apoptosis. Placenta 2002; 23: 322–330. [DOI] [PubMed] [Google Scholar]
- Mackova M, Kilani RT, Davidge ST, Guilbert LJ.The effect of oxygen tension on intracellular survival signalling in primary villous trophoblasts. Placenta 2003; 24: 627–637. [DOI] [PubMed] [Google Scholar]
- Imudia AN, Kilburn BA, Petkova A, Edwin SS, Romero R, Armant DR.Expression of heparin-binding EGF-like growth factor in term chorionic villous explants and its role in trophoblast survival. Placenta 2008; 29: 784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ.Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest 1996; 97: 540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genbacev O, Zhou Y, Ludlow JW, Fisher SJ.Regulation of human placental development by oxygen tension. Science 1997; 277: 1669–1672. [DOI] [PubMed] [Google Scholar]
- Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA.Placental apoptosis in preeclampsia. Obstet Gynecol 2000; 96: 271–276. [DOI] [PubMed] [Google Scholar]
- DiFederico E, Genbacev O, Fisher SJ.Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol 1999; 155: 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosens IA, Robertson WB, Dixon HG.The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu 1972; 1: 177–191. [PubMed] [Google Scholar]
- Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T.Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol 2002; 186: 158–166. [DOI] [PubMed] [Google Scholar]
- Khong TY, De Wolf F, Robertson WB, Brosens I.Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol 1986; 93: 1049–1059. [DOI] [PubMed] [Google Scholar]
- Hung TH, Burton GJ.Hypoxia and reoxygenation: a possible mechanism for placental oxidative stress in preeclampsia. Taiwan J Obstet Gynecol 2006; 45: 189–200. [DOI] [PubMed] [Google Scholar]
- Leach RE, Romero R, Kim YM, Chaiworapongsa T, Kilburn BA, Das SK, Dey SK, Johnson A, Qureshi F, Jacques S, Armant DR.Pre-eclampsia and expression of heparin-binding EGF-like growth factor. Lancet 2002; 360: 1215–1219. [DOI] [PubMed] [Google Scholar]
- Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK.Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res 1993; 206: 204–211. [DOI] [PubMed] [Google Scholar]
- Aplin JD, Straszewski-Chavez SL, Kalionis B, Dunk C, Morrish D, Forbes K, Baczyk D, Rote N, Malassine A, Knofler M.Trophoblast differentiation: progenitor cells, fusion and migration—a workshop report. Placenta 2006; 27(suppl A):S141–S143. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF.A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 1994; 269: 5241–5248. [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 1998; 273: 18623–18632. [DOI] [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A 2001; 98: 13681–13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW.A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 1994; 372: 739–746. [DOI] [PubMed] [Google Scholar]
- Mitamura T, Higashiyama S, Taniguchi N, Klagsbrun M, Mekada E.Diphtheria toxin binds to the epidermal growth factor (EGF)-like domain of human heparin-binding EGF-like growth factor/diphtheria toxin receptor and inhibits specifically its mitogenic activity. J Biol Chem 1995; 270: 1015–1019. [DOI] [PubMed] [Google Scholar]
- Klutchko SR, Zhou H, Winters RT, Tran TP, Bridges AJ, Althaus IW, Amato DM, Elliott WL, Ellis PA, Meade MA, Roberts BJ, Fry DW, et al. Tyrosine kinase inhibitors. 19. 6-Alkynamides of 4-anilinoquinazolines and 4-anilinopyrido[3,4-d]pyrimidines as irreversible inhibitors of the erbB family of tyrosine kinase receptors. J Med Chem 2006; 49: 1475–1485. [DOI] [PubMed] [Google Scholar]
- Kilburn BA, Wang J, Duniec-Dmuchowski ZM, Leach RE, Romero R, Armant DR.Extracellular matrix composition and hypoxia regulate the expression of HLA-G and integrins in a human trophoblast cell line. Biol Reprod 2000; 62: 739–747. [DOI] [PubMed] [Google Scholar]
- Wolff GS, Chiang PJ, Smith SM, Romero R, Armant DR.Epidermal growth factor-like growth factors prevent apoptosis of alcohol-exposed human placental cytotrophoblast cells. Biol Reprod 2007; 77: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JJ, Lee DR, Song HS, Kim KS, Yoon TK, Gye MC, Kim MK.Heparin-binding epidermal growth factor (HB-EGF) may improve embryonic development and implantation by increasing vitronectin receptor (integrin alphanubeta3) expression in peri-implantation mouse embryos. J Assist Reprod Genet 2006; 23: 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lala PK, Chakraborty C.Factors regulating trophoblast migration and invasiveness: possible derangements contributing to pre-eclampsia and fetal injury. Placenta 2003; 24: 575–587. [DOI] [PubMed] [Google Scholar]
- Liu J, Chakraborty C, Graham CH, Barbin YP, Dixon SJ, Lala PK.Noncatalytic domain of uPA stimulates human extravillous trophoblast migration by using phospholipase C, phosphatidylinositol 3-kinase and mitogen-activated protein kinase. Exp Cell Res 2003; 286: 138–151. [DOI] [PubMed] [Google Scholar]
- McKinnon T, Chakraborty C, Gleeson LM, Chidiac P, Lala PK.Stimulation of human extravillous trophoblast migration by IGF-II is mediated by IGF type 2 receptor involving inhibitory G protein(s) and phosphorylation of MAPK. J Clin Endocrinol Metab 2001; 86: 3665–3674. [DOI] [PubMed] [Google Scholar]
- Gleeson LM, Chakraborty C, McKinnon T, Lala PK.Insulin-like growth factor-binding protein 1 stimulates human trophoblast migration by signaling through alpha 5 beta 1 integrin via mitogen-activated protein kinase pathway. J Clin Endocrinol Metab 2001; 86: 2484–2493. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Miyamoto S, Komatsu H, Tsukimori K, Kobayashi H, Seki H, Takeda S, Nakano H.TNFalpha-induced apoptosis and integrin switching in human extravillous trophoblast cell line. Biol Reprod 2003; 68: 1771–1778. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Miyamoto S, Tsukimori K, Kobayashi H, Seki H, Takeda S, Kensuke E, Ohtani K, Shibuya M, Nakano H.Tumor necrosis factor and vascular endothelial growth factor induce endothelial integrin repertories, regulating endovascular differentiation and apoptosis in a human extravillous trophoblast cell line. Biol Reprod 2005; 73: 172–179. [DOI] [PubMed] [Google Scholar]
- Chakraborty C, Barbin YP, Chakrabarti S, Chidiac P, Dixon SJ, Lala PK.Endothelin-1 promotes migration and induces elevation of [Ca2+]i and phosphorylation of MAP kinase of a human extravillous trophoblast cell line. Mol Cell Endocrinol 2003; 201: 63–73. [DOI] [PubMed] [Google Scholar]
- Qiu Q, Yang M, Tsang BK, Gruslin A.EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves activation of both PI3K and MAPK signalling pathways. Reproduction 2004; 128: 355–363. [DOI] [PubMed] [Google Scholar]
- Qiu Q, Yang M, Tsang BK, Gruslin A.Both mitogen-activated protein kinase and phosphatidylinositol 3-kinase signalling are required in epidermal growth factor-induced human trophoblast migration. Mol Hum Reprod 2004; 10: 677–684. [DOI] [PubMed] [Google Scholar]
- LaMarca HL, Dash PR, Vishnuthevan K, Harvey E, Sullivan DE, Morris CA, Whitley GS.Epidermal growth factor-stimulated extravillous cytotrophoblast motility is mediated by the activation of PI3-K, Akt and both p38 and p42/44 mitogen-activated protein kinases. Hum Reprod 2008; 23: 1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cindrova-Davies T, Spasic-Boskovic O, Jauniaux E, Charnock-Jones DS, Burton GJ.Nuclear factor-kappa B, p38, and stress-activated protein kinase mitogen-activated protein kinase signaling pathways regulate proinflammatory cytokines and apoptosis in human placental explants in response to oxidative stress: effects of antioxidant vitamins. Am J Pathol 2007; 170: 1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cindrova-Davies T.Gabor Than Award Lecture 2008: pre-eclampsia—from placental oxidative stress to maternal endothelial dysfunction. Placenta 2009; 30(suppl A):S55–S65. [DOI] [PubMed] [Google Scholar]
- Zhong W, Xie Y, Wang Y, Lewis J, Trostinskaia A, Wang F, Puscheck EE, Rappolee DA.Use of hyperosmolar stress to measure stress-activated protein kinase activation and function in human HTR cells and mouse trophoblast stem cells. Reprod Sci 2007; 14: 534–547. [DOI] [PubMed] [Google Scholar]
- Heazell AE, Taylor NN, Greenwood SL, Baker PN, Crocker IP.Does altered oxygenation or reactive oxygen species alter cell turnover of BeWo choriocarcinoma cells? Reprod Biomed Online 2009; 18: 111–119. [DOI] [PubMed] [Google Scholar]
- Heazell AE, Crocker IP.Live and let die—regulation of villous trophoblast apoptosis in normal and abnormal pregnancies. Placenta 2008; 29: 772–783. [DOI] [PubMed] [Google Scholar]
- Fulop V, Mok SC, Genest DR, Gati I, Doszpod J, Berkowitz RS.p53, p21, Rb and mdm2 oncoproteins: expression in normal placenta, partial and complete mole, and choriocarcinoma. J Reprod Med 1998; 43: 119–127. [PubMed] [Google Scholar]
- Marzusch K, Ruck P, Horny HP, Dietl J, Kaiserling E.Expression of the p53 tumour suppressor gene in human placenta: an immunohistochemical study. Placenta 1995; 16: 101–104. [DOI] [PubMed] [Google Scholar]
- Roncalli M, Bulfamante G, Viale G, Springall DR, Alfano R, Comi A, Maggioni M, Polak JM, Coggi G.C-myc and tumour suppressor gene product expression in developing and term human trophoblast. Placenta 1994; 15: 399–409. [DOI] [PubMed] [Google Scholar]
- Moll SJ, Jones CJ, Crocker IP, Baker PN, Heazell AE.Epidermal growth factor rescues trophoblast apoptosis induced by reactive oxygen species. Apoptosis 2007; 12: 1611–1622. [DOI] [PubMed] [Google Scholar]
- Ho S, Winkler-Lowen B, Morrish DW, Dakour J, Li H, Guilbert LJ.The role of Bcl-2 expression in EGF inhibition of TNF-alpha/IFN-gamma-induced villous trophoblast apoptosis. Placenta 1999; 20: 423–430. [DOI] [PubMed] [Google Scholar]
- Payne SG, Brindley DN, Guilbert LJ.Epidermal growth factor inhibits ceramide-induced apoptosis and lowers ceramide levels in primary placental trophoblasts. J Cell Physiol 1999; 180: 263–270. [DOI] [PubMed] [Google Scholar]
- Johnstone ED, Mackova M, Das S, Payne SG, Lowen B, Sibley CP, Chan G, Guilbert LJ.Multiple anti-apoptotic pathways stimulated by EGF in cytotrophoblasts. Placenta 2005; 26: 548–555. [DOI] [PubMed] [Google Scholar]
- Berube J, Bourdon C, Yao Y, Rousseau S.Distinct intracellular signaling pathways control the synthesis of IL-8 and RANTES in TLR1/TLR2, TLR3 or NOD1 activated human airway epithelial cells. Cell Signal 2009; 21: 448–456. [DOI] [PubMed] [Google Scholar]
- Wang YY, Dahle MK, Steffensen KR, Reinholt FP, Collins JL, Thiemermann C, Aasen AO, Gustafsson JA, Wang JE.Liver X receptor agonist GW3965 dose-dependently regulates lps-mediated liver injury and modulates posttranscriptional TNF-alpha production and p38 mitogen-activated protein kinase activation in liver macrophages. Shock 2009; 32: 548–553. [DOI] [PubMed] [Google Scholar]
- Yun JH, Koo JE, Koh YS.Mitogen-activated protein kinases are involved in tumor necrosis factor alpha production in macrophages infected with Orientia tsutsugamushi. Microbiol Immunol 2009; 53: 349–355. [DOI] [PubMed] [Google Scholar]
- Zhao W, Liu M, Kirkwood KL.p38alpha stabilizes interleukin-6 mRNA via multiple AU-rich elements. J Biol Chem 2008; 283: 1778–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Si J, DeWille JW.Ultraviolet radiation (UVR) activates p38 MAP kinase and induces post-transcriptional stabilization of the C/EBPdelta mRNA in G0 growth arrested mammary epithelial cells. J Cell Biochem 2008; 103: 1657–1669. [DOI] [PubMed] [Google Scholar]
- Wang D, Zuehlsdorff S, Larson AC.Rapid 3D radiofrequency field mapping using catalyzed double-angle method. NMR Biomed 2009; 22: 882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps ED, Updike DL, Bullen EC, Grammas P, Howard EW.Transcriptional and posttranscriptional regulation of angiopoietin-2 expression mediated by IGF and PDGF in vascular smooth muscle cells. Am J Physiol Cell Physiol 2006; 290: C352–C361. [DOI] [PubMed] [Google Scholar]
- Chiang Y, Chou CY, Hsu KF, Huang YF, Shen MR.EGF upregulates Na+/H+ exchanger NHE1 by post-translational regulation that is important for cervical cancer cell invasiveness. J Cell Physiol 2008; 214: 810–819. [DOI] [PubMed] [Google Scholar]
- Cao Z, Liu LZ, Dixon DA, Zheng JZ, Chandran B, Jiang BH.Insulin-like growth factor-I induces cyclooxygenase-2 expression via PI3K, MAPK and PKC signaling pathways in human ovarian cancer cells. Cell Signal 2007; 19: 1542–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R.Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev 2006; 20: 515–524. [DOI] [PubMed] [Google Scholar]
- Donker RB, Mouillet JF, Nelson DM, Sadovsky Y.The expression of Argonaute2 and related microRNA biogenesis proteins in normal and hypoxic trophoblasts. Mol Hum Reprod 2007; 13: 273–279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.