Abstract
Estrogen receptor-alpha (ESR1) is highly expressed in the efferent ductules of all species studied as well as in the epididymal epithelium in mice and other select species. Male mice lacking ESR1 (Esr1KO) are infertile, but transplantation studies demonstrated that Esr1KO germ cells are capable of fertilization when placed in a wild-type reproductive tract. These results suggest that extratesticular regions, such as the efferent ductules and epididymis, are the major source of pathological changes in Esr1KO males. Previous studies have shown alterations in ion and fluid transporters in the efferent duct and epididymal epithelia of Esr1KO males, leading to misregulation of luminal fluid pH. To determine the effect of an altered epididymal milieu on Esr1KO sperm, we assayed sperm morphology in the different regions of the epididymis. Sperm recovered from the epididymis exhibited abnormal flagellar coiling and increased incidence of spontaneous acrosome reactions, both of which are consistent with exposure to abnormal epididymal fluid. Analysis of the epididymal fluid revealed that the osmolality of the Esr1KO fluid was reduced relative to wild type, consistent with prior reports of inappropriate fluid absorption from the efferent ductules. This, along with the finding that morphological defects increased with transit through the epididymal duct, suggests that the anomalies in sperm are a consequence of the abnormal luminal environment. Consistent with this, incubating Esr1KO sperm in a more wild-type-like osmotic environment significantly rescued the abnormal flagellar coiling. This work demonstrates that Esr1KO mice exhibit an abnormal fluid environment in the lumen of the efferent ducts and epididymis, precluding normal sperm maturation and instead resulting in progressive deterioration of sperm that contributes to infertility.
Keywords: epididymis, estradiol receptor, male reproductive tract, sperm, sperm maturation
Estrogen receptor alpha knockoutmice have a hypo-osmotic epididymal environment that contributes to defects in sperm morphology and function.
INTRODUCTION
Spermatozoa exit the testis as highly differentiated but functionally immature cells that require further maturation within the extratesticular ducts to acquire the ability to recognize and fertilize an egg [1]. This essential developmental process requires sperm interaction with a progressively changing luminal milieu that is regulated by secretory and absorptive activities of the epididymal epithelium. Activities of this epithelium are controlled by androgens [2–4] as well as estrogen [5–8]. As sperm progress from the proximal to the distal regions of the epididymis, there is an effective concentration and compaction that occurs in parallel with substantial water reabsorption, occurring mainly within the efferent ductules [9]. In addition, there are several notable changes in luminal composition that occur along the length of the epididymis including net Na+, Cl− and  reabsorption, K+ secretion, and luminal acidification [10]. Another significant characteristic of the epididymal fluid is that it is hyperosmotic relative to serum. Although the osmolality of fluid-bathing spermatozoa in the testis approximates that in blood, as spermatozoa are transported through the epididymal duct, they encounter a slowly increasing osmotic environment that is a result of the secretion of low-molecular-weight, water-soluble compounds [10–13]. By the time spermatozoa enter the cauda epididymis, where they are stored before ejaculation, the osmolality is at least 1.3-fold higher than fluids in the testis or female reproductive tract [14–18].
reabsorption, K+ secretion, and luminal acidification [10]. Another significant characteristic of the epididymal fluid is that it is hyperosmotic relative to serum. Although the osmolality of fluid-bathing spermatozoa in the testis approximates that in blood, as spermatozoa are transported through the epididymal duct, they encounter a slowly increasing osmotic environment that is a result of the secretion of low-molecular-weight, water-soluble compounds [10–13]. By the time spermatozoa enter the cauda epididymis, where they are stored before ejaculation, the osmolality is at least 1.3-fold higher than fluids in the testis or female reproductive tract [14–18].
In the mouse, when sperm are ejaculated into the female reproductive tract, they immediately encounter an environment that is approximately 110 mmol/kg lower than that in the cauda epididymis [19]. Given that the release of sperm into a physiologically “hypotonic” environment is a normal event, it is feasible that it may serve as a physiological trigger of sperm activation after quiescence in the epididymis.
To cope with these constant environmental challenges, spermatozoa, like somatic cells, undergo a process of regulatory volume decrease (RVD). However, unlike somatic cells, spermatozoa have little cytoplasm and therefore rely mainly on the cytoplasmic droplet to mediate the bulk of water and osmolyte transport involved in RVD [15]. When exposed to hypo-osmotic conditions, water will enter the spermatozoa in an attempt to reach osmotic equilibrium. This inflow of water will increase sperm volume, and the plasma membrane will expand [20]. If volume regulation fails, spermatozoa assume a swollen state that is manifested by flagellar coiling or angulation, usually at the site of the cytoplasmic droplet. This change in morphology provides the smallest membrane area for the increased volume, limiting the damage done to the membrane through stretching [21].
Spermatozoa that are swollen or angulated as a result of failed volume regulation are disadvantaged during fertilization. There are several naturally occurring animal models of male infertility in which angulated spermatozoa are a defining characteristic [22–24]. One of the most notable tail defects is known as the “Dag defect,” first identified in a Jersey bull in which sperm exhibited a “corkscrew-like” coiling of the flagellum. In addition, there are several transgenic mouse models in which angulation occurs within the epididymis, including the c-ros (Ros1) knockout (Ros1tm1Cbm), LRG4 (GPCR) hypomorphic mutant (Lgr4Gt(pU-21)1Kymm) [25], ApoER2 knockout (Lrp8tm1Her) [26], GPX5Tag2 (Tg(Gpx5-TAg)2Mpo) [27], and Foxi1 (Foxi1tm1Sven ) [28] null mutants. Although a systematic analysis has not been performed to determine commonalities of these infertile males, the LRG4 mutant mice exhibited decreased Esr1 expression in the efferent ducts and epididymis [25], which resulted in a phenotype similar to that seen in the Esr1KO male [29].
The Esr1KO male is a useful animal model to study the interactions between maturing sperm and the epididymis. Animals that lack a functional Esr1 gene are infertile, and sperm recovered from the cauda epididymis exhibit low percent motility, beat less vigorously, and are ineffective at in vitro fertilization [30]. Testes of the Esr1KO show transient increase in weight due to fluid back pressure [29, 31], which is caused by a defect in ion transport and fluid reabsorption in the efferent ductules [29, 31]. In a companion article, loss of ESR1 is shown to lead to misregulation of acid/base transporters and a failure of epididymal acidification [32]. The infertile Ros1 and Foxi1 knockout males both harbor a similar physiological phenotype of elevated luminal pH, with a specific corresponding defect in sperm morphology: flagellar angulation. In both of these models, the flagellar angulation hinders the ability of spermatozoa to cross the uterotubular junction, serving as a block to fertilization. Furthermore, studies utilizing hamster and sea urchin sperm suggest a correlation between increased pH and spontaneous acrosome reactions [33–36].
To elucidate the relationship between the misregulation of fluid dynamics and infertility of Esr1KO mice, we systematically investigated sperm morphology as they transverse the Esr1KO epididymal duct. This article reports that Esr1KO spermatozoa have two distinct morphological defects: an increased propensity for spontaneous acrosome reactions and severe flagellar coiling. These defects appear to be at least partly due to the decreased osmolality of the Esr1KO luminal environment because when Esr1KO sperm are incubated in a more wild-type (WT)-like osmotic environment, the degree of coiling is minimized. Conversely, when WT sperm are exposed to an environment of high pH and low osmolality, the proportion of straight sperm flagella are significantly reduced. Together, the results indicate that the defect in flagellar coiling of Esr1KO sperm is not intrinsic to the axoneme but rather a consequence of development within an abnormal microenvironment.
MATERIALS AND METHODS
Animals and Tissue Preparation
The present study used homozygous Esr1KO males produced using ZP3-Cre-mediated excision of exon 3 as previously described [37, 38]. The Esr1KO male mice are infertile, whereas heterozygous mice show normal fertility and sperm motility. Comparisons were made between Esr1−/− and Esr1+/− heterozygous (HET) or Esr1+/+ WT littermates, as indicated. For all analyses, at least three age-matched mice of each genotype were used. Unless otherwise noted, adult mice used for these studies were 6–14 wk of age. The mice were euthanized by cervical dislocation. All experiments involving animals were conducted according to Emory University, University of Illinois and University of Kentucky institutional standards (IACUC) for the care and use of experimental animals. Testes from Esr1+/+ and Esr1−/− mice 8 wk of age were fixed in Bouin solution as previously described [39]. Epididymides were dissected clean of connective tissue and subdissected into four regions: the initial segment and the remaining caput, corpus, and cauda.
Sperm Collection
All reagents were purchased from Sigma Aldrich unless noted otherwise. Sperm were collected and incubated in either a modified Krebs-Ringer buffer (dmKBRT) [40] or Medium B [127 mM NaCl, 5.3 mM KCl, 18.2 mM HEPES, pH 7.4]. Initial segment and caput sperm were collected by puncturing tissue with a 27½-gauge needle and gently expressing fluid out of the perforations into the collection media. Cauda sperm were collected by shredding tissue in the appropriate medium and filtering through a nylon mesh (03–35/16 Nitex, Sefar Filtration Inc.). To collect sperm from testes, the tunica albugina was removed, and the testes were dissected and shredded in collection media. The resulting suspension was filtered through a nylon mesh (03–180/44 Nitex, Sefar Filtration Inc.) to remove tissue pieces and large cellular debris.
Acrosome Status
Coomassie Blue (Bio-Rad Laboratories) was used to stain the acrosome contents and score the status (acrosome reacted/not reacted) following the procedure previously described [41]. Sperm were fixed for 10 min in 4% paraformaldehyde at room temperature, washed, and resuspended in 0.1 M ammonium acetate (pH 9.0). A smear of the suspension was created onto positively charged glass slides (ProbeOn Plus; Fisher Scientific) and allowed to air-dry overnight. Smears were covered with Coomassie stain (0.22% Coomassie G250, 50% methanol, 10% glacial acetic acid) and allowed to incubate for 9 min. Slides were washed with running tap water, air-dried, and cover slipped using Aquatex (EMD Chemicals Inc.). Sperm were scored under 60× magnification (Nikon Eclipse E800), and the proportion of acrosome-reacted sperm is presented as the percent of the total sperm counted. The data presented are the average of at least three experiments. Means were compared using an unpaired Welch-corrected Student t-test.
Electron Microscopy
Sperm within the caput epididymides (with initial segment removed) were analyzed in situ via electron microscopy. Tissues were freshly dissected and submersion fixed in 2.5% glutaraldehyde buffered with 100 mM cacodylate. Fixed tissues were rinsed in cacodylate buffer, stained with osmium, and processed for thin Epon resin sections (60–70 nm) by the Emory University School of Medicine Electron Microscopy Core.
Sperm Flagellar Morphology
Flagellar morphology was assessed via light microscopy. Sperm were recovered and processed as previously described. Flagellar morphology was assessed from control and Esr1KO mice, and sperm were scored as normal (straight axonemes), loops (hairpin bend), midpiece coils (coils restricted to the midpiece region), and whole tail coils (coils that include the principal piece). All coils are presented as the sum of midpiece and whole-tail coils. Sperm were examined under 60× magnification. The proportion of each morphological phenotype was determined on the basis of the total number of sperm counted (≥400) from each region of each animal. The data presented are the average of at least three experiments. Differences between control and knockout mice were tested using an unpaired Student t-test.
Osmometry
Osmolality was measured using a Wescor 5500 Vapro Vapor Pressure Osmometer (Wescor Inc.) outfitted with a 2-μl sample holder to provide more accurate measurements of small sample volumes. Both caput epididymides (including initial segments) from a single animal were subdissected and pooled. Each testis was individually measured. To avoid contamination from blood, prominent vessels were cannulated with a needle and drained. The external capsule was removed, and internal tubules were penetrated in multiple locations with a 27½-gauge needle, and luminal fluid contents were gently released through the holes and collected using Wiretrol II microcapillary tubes (Drummond Scientific Company). The collected fluid was centrifuged at 16 000 × g for 5 min. The supernatant (2 μl) was used to saturate an eighth-inch round Kimwipe sample disc (Kimberly Clark Professional). Prior to each experimental run, the osmometer was calibrated using 2 μl of both a 290- and a 1000-mmol/kg standard. To increase sensitivity, the low-range switch was actuated for every sample measured. The epididymides and testes of at least three WT, HET and Esr1KO mice were measured in replicate. Comparisons of sample means were examined by an unpaired Student t-test.
Sperm Morphology Rescue
To assess the efficiency of Esr1KO sperm to recover from morphological defects, the basal dmKBRT medium was modified to create a series of solutions of increasing osmolality (300–430 mmol/kg) that bracketed a normal range for WT epididymal fluid. The standard dmKBRT (without NaCl) was modified by the addition of NaCl to reach desired osmolality measured by vapor pressure osmometry (Vapro 5500; Wescor Inc.). The caput (including initial segment) and cauda epididymides regions from each animal were divided in half, and each half was placed in a different osmolality for sperm collection. Spermatozoa were acclimated to the media for 30 min at 37°C and assessed for acrosome status and flagellar morphology as described previously. Means were compared to the standard dmKBRT osmolality using a paired Student t-test. The data presented are the average of at least three experiments.
Assessment of Sperm Response to Challenge Media
WT sperm were exposed to an in vitro hypo-osmolality environment. A simple HEPES-buffered medium (medium B) was created with NaCl concentrations adjusted to produce a series of solutions ranging in osmolality from 220 to 300 mmol/kg and buffered by the addition of 1 M sodium hydroxide to pH 7.8. Morphology and acrosome status of caput (including initial segment) and cauda sperm were assessed and compared to the standard medium B, pH 7.4 (300 mmol/kg) after incubation for 30 min at 37°C. The data presented are the average of at least three experiments. Means were compared using a paired Student t-test.
RESULTS
Esr1KO Spermatozoa Have Increased Rates of Spontaneous Acrosome Reaction
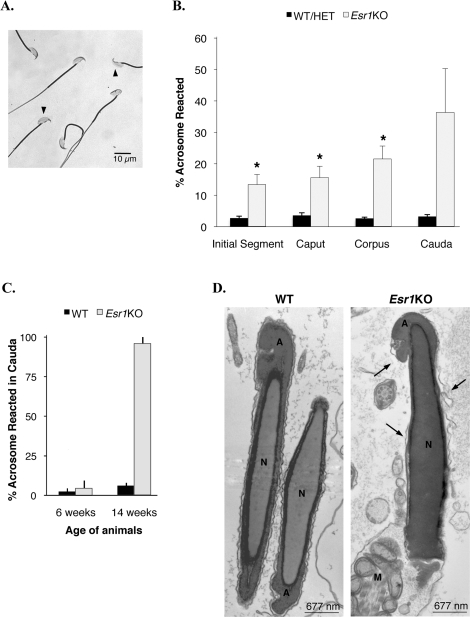
Previous studies revealed that the Esr1KO epididymis fails to appropriately express several water/ion transporters, which leads to a more alkaline epididymal environment [32]. To determine the effect that this altered fluid milieu had on sperm, we evaluated acrosome integrity via light microscopy (Fig. 1A). Sperm from animals 6–14 wk of age were collected from the four main epididymal regions (initial segment, caput, corpus, and cauda) into a simple HEPES-buffered salt solution to eliminate the contribution from capacitation factors, and the basal frequency of spontaneous acrosome reactions was determined (Fig. 1B). Approximately 3% of sperm from control mice exhibited spontaneous acrosome reactions, which remained constant through all segments of the epididymis. In comparison to control sperm, Esr1KO mice had a significantly increased proportion of acrosome-reacted sperm in every segment of the epididymis, except for the cauda region. The frequency of acrosome-reacted sperm increased dramatically as they progressed from the proximal to the distal regions of the epididymal duct (initial segment: 13 ± 3%; caput: 16 ± 4%; corpus: 22 ± 4%; cauda: 36 ± 14%). To examine the large variation in acrosome status of Esr1KO sperm recovered from the cauda epididymis, specific ages of 6 and 14 wk were compared (Fig. 1C); however, only two animals at each age were available for evaluation. The mean difference in acrosome reaction between 6 and 14 wk was large, suggesting that the acrosome phenotype progressively worsened with age and was nearly 100% penetrant at 14 wk. Interestingly, evidence of this phenotype was seen in situ via electron microscopy, where Esr1KO caput sperm were often observed with vesiculated, irregular, and/or broken plasma membranes that overlaid the acrosome vesicle (Fig. 1D).
FIG. 1.
Esr1KO sperm have increased frequency of spontaneous acrosome reactions. A) Sperm from the initial segment, caput, corpus, and cauda were assessed for acrosome status under light microscopy by Coomassie Blue staining. Arrowheads indicate sperm loss of acrosomal contents. B) Wild-type (WT) and heterozygote (HET) mice have a basal level of spontaneous acrosome reactions that remain stable in all regions of the epididymis (3 ± 1%). In comparison, Esr1KO mice have a significantly increased proportion of spontaneous reactions in the initial segment, caput, and corpus. Each bar represents the mean ± SEM. C) Acrosomal status of cauda sperm at 6 and 14 wk of age (two animals per group). The large variability in acrosome status in the Esr1KO cauda epididymis may be associated with an age-dependent defect that progressively worsens and approaches 100% in 14-wk-old mice. Each bar represents the mean ± SD. D) Electron microscopy of WT sperm reveals normal intact plasma membranes surrounding the nucleus (N) and acrosomic vesicle (A). Esr1KO sperm revealing a phenotype of vesiculated, irregular, or broken plasma membranes (arrows) that overlie the acrosomic vesicle (A) and nucleus (N). M, mitochondria in the midpiece. *Denotes significant difference from WT/HET within an epididymal segment (P ≤ 0.01).
Esr1KO Sperm Exhibit Severe Flagellar Coiling
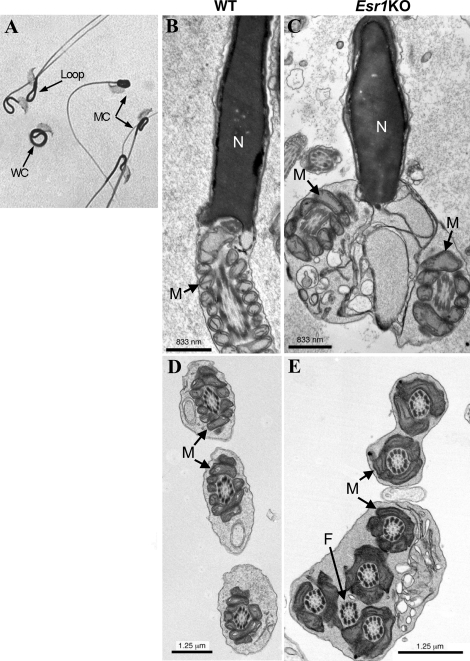
Light microscopy revealed that Esr1KO spermatozoa exhibited a flagellar defect characterized by varying degrees of severity (Fig. 2A). In the mildest form, hairpin loops were observed, the occurrence of which was highly variable and often found in sperm of control mice. Often these loops were associated with bends or acute angulation in the area of the cytoplasmic droplet. More severe forms of Esr1KO flagellar defects included coiling confined to just the midpiece of the flagellum, with the remaining tail free from obstruction, or coiling that encompasses the entire length of the sperm tail (i.e., Dag defect). Electron microscopic analysis confirmed the incidence of this phenotype in situ (Fig. 2B; Esr1KO). While components of the axoneme and cytoskeletal elements were present and appeared normal, often two or more cross sections of the same sperm flagellum were found enclosed in one plasma membrane, a phenotype not observed in WT or HET mice.
FIG. 2.
Esr1KO sperm exhibit flagellar angulation and coiling. A) Light microscopy revealed varying degrees of coiling in Esr1KO sperm. Hairpin loops (loop) were the mildest form observed and were frequently found in control animals. Coils isolated to the tail midpiece region (MC) and coils that encompassed the entire tail length (WC) were more severe. B) Electron microscopy (TEM) of wild-type (WT) sperm within the caput epididymis showing normal nucleus (N) and midpiece region with mitochondria (M). C) TEM of Esr1KO sperm within the caput epididymis showing the coiling phenotype in situ, where sperm were frequently imaged with two or more midpiece or flagellar cross sections enclosed in one membrane. M, mitochondria. D) TEM of WT sperm showing midpiece cross sections with normal classical axonemal architecture. M, mitochondria. E) TEM of Esr1KO sperm showing the coiled phenotype with two or more cross sections of the midpiece with mitochondria (M) and flagellar axonemal complex (F).
Esr1KO Sperm Coiling Increases with Transit Through the Epididymal Duct
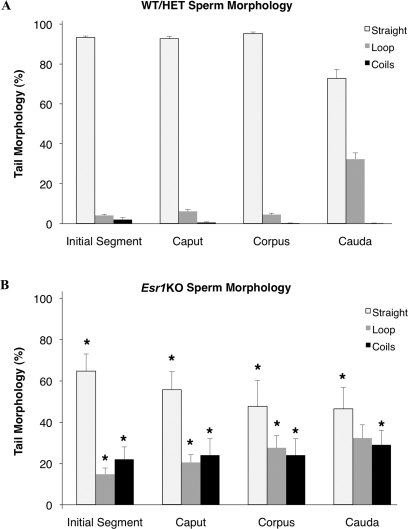
Quantification of the incidence of sperm coiling as a function of epididymal transit verified that sperm from control mice had a consistent morphology of straight tails (94 ± 1%) in the initial segment through the corpus epididymis when freshly released and dispersed in basal medium. Straight tails were decreased (to 73 ± 4%) in the cauda as a result of a substantial increase in hairpin loops (Fig. 3A). This flagellar angulation, or looping, of the sperm tail has been previously described in mammalian sperm from several species as a phenomenon of sperm swelling [42, 43]. In contrast to control mice, sperm recovered from all segments of the Esr1KO epididymis had a significant decrease in the proportion with straight-tail morphology, the incidence of which became more pronounced as sperm traversed the epididymis (initial segment: 65 ± 8%; caput: 56 ± 9%; corpus 48 ± 13%; cauda 46 ± 10%) (Fig. 3B). These data indicate that the morphology of Esr1KO sperm progressively deteriorates as sperm remain longer in the abnormal luminal environment.
FIG. 3.
Sperm tail coiling is more pronounced in the Esr1KO and increases with transit through the epididymis. A) Quantification of sperm tail morphology via light microscopy revealed that control wild-type (WT) and heterozygote (HET) mice have consistently straight-tail morphology when collected from the initial segment, caput, and corpus epididymis (93 ± 3%). In the control cauda, the percentage of straight flagella decreased to 75 ± 4%, reflecting an increase in hairpin loops. B) In comparison, Esr1KO mice had a significantly reduced proportion of straight sperm, relative to the control mice, recovered from the initial segment, caput, corpus, and cauda that reflects an increase in the proportion of both loops and more severe forms of tail coiling (midpiece and whole tail coils described in Fig. 2). Each bar represents the mean ± SEM. *Denotes significant difference from WT/HET within an epididymal segment (P ≤ 0.04).
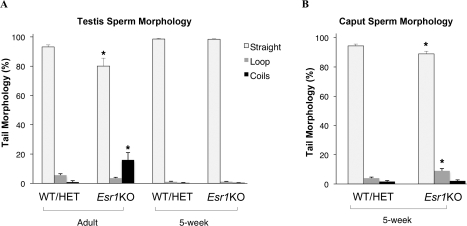
To determine if the sperm coiling observed in the Esr1KO epididymis was due to a primary effect on spermatogenesis, Esr1KO testicular sperm were evaluated for morphological abnormalities (Fig. 4A). Analysis of adult Esr1KO testes revealed that there was a small but significant decrease in straight morphology of sperm recovered from the testes relative to that seen in control mice (Esr1KO: 80 ± 5%; control: 93 ± 1%). Previous studies of the Esr1KO male have shown that these mice exhibit an age-dependent accumulation of fluid within the lumen of the reproductive tract that causes fluid back pressure and swelling of the testis [29]. This was evident in sections of 8-wk-old Esr1KO testes, which showed excessive accumulation of fluid and dilation of the seminiferous tubules along with coiled sperm in the lumens (Fig. 5B); age-matched WT testes exhibited no dilation of the seminiferous tubules and no coiled sperm (Fig. 5A). In the Esr1KO testes, coiled sperm were never observed within the seminiferous epithelium, only within the lumen. To test the hypothesis that age-associated testicular swelling was the cause of testicular sperm coiling, 5-wk-old Esr1KO animals were examined, as testicular swelling had not as yet begun at this age. In these younger animals, sperm from Esr1KO and control testes showed no difference in the percentage of sperm that were straight. Nevertheless, there was a slight but statistically significant reduction in the percentage of straight sperm recovered from the 5-wk-old Esr1KO caput compared to WT/HET (89 ± 2% vs. 94 ± 1%, respectively) (Fig. 4B). These results further indicate that Esr1KO sperm coiling is not an intrinsic spermatozoa defect but rather a morphological result of an abnormal epididymal physiology.
FIG. 4.
Esr1KO sperm tail coiling is a function of epididymal transit. A) Analysis of adult Esr1KO testes showed an approximate 10% decrease in straight-tail morphology, relative to control wild-type (WT) and heterozygote (HET) mice. However, sperm recovered from young (5-wk-old) Esr1KO testes that had not yet developed the characteristic fluid accumulation in the testes showed no difference in the percentage of straight flagella when compared to control mice (98 ± 1%). B) Nevertheless, sperm recovered from 5-wk-old Esr1KO caput had a small but statistically significant reduction in straight flagella. Each bar represents the mean ± SEM. *Denotes significant difference from corresponding sperm morphology type in WT/HET (P ≤ 0.05).
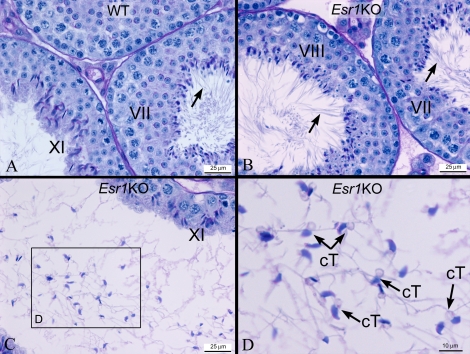
FIG. 5.
Esr1KO testis exhibits sperm flagellar angulation and coiling only in the lumen. A) Light microscopy of wild-type (WT) seminiferous tubules reveals normal development of germ cells in stages XI and VII. Flagella of elongated spermatids extend straight into the lumen (arrow). B) In Esr1KO testis, spermatids in the stage VII–VIII epithelia appear normal, with straight tails projecting into the lumen. C) When the lumen becomes dilated because of fluid back pressure, the sperm display coiled tails and angulation. D) Higher magnification of the luminal area in C. Sperm with coiled tails (cT) were observed in the lumen.
Hypo-Osmotic Pressure of the Esr1KO Luminal Fluids
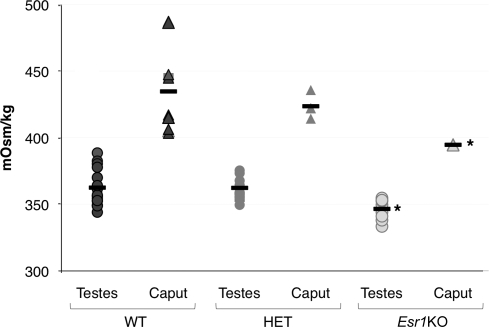
Other model systems have suggested that acute angulation and coiling of spermatozoa occur as result of hypo-osmotic challenges. Using vapor pressure osmometry, luminal fluid from the testes and proximal region of the epididymis (initial segment and caput) were measured (Fig. 6). As has been described in other species, as sperm enter the epididymis, they are exposed to an environment of increased osmolality [18]. WT and HET mice had a testes fluid osmolality of 362 ± 3 mmol/kg, which increased in the caput epididymis to 435 ± 11 mmol/kg for WT and 424 ± 4 mmol/kg for HET mice. In contrast, Esr1KO fluid collected from the testes (346 ± 2 mmol/kg) and caput epididymis (395 ± 1 mmol/kg) was significantly hypo-osmotic in comparison to WT and HET mice.
FIG. 6.
Osmolality of Esr1KO luminal fluids is reduced in comparison to control. Osmolality was measured from luminal fluids collected from the testes and whole caput region (including initial segment) of wild-type (WT), heterozygote (HET), and Esr1KO mice. The testicular fluid of WT and HET mice was 362 ± 3 mmol/kg and increased in the caput to 435 ± 11 and 424 ± 4 mmol/kg, respectively. In comparison, the luminal contents of the Esr1KO were significantly hypo-osmotic in the testes (346 ± 2 mmol/kg) and caput (395 ± 1 mmol/kg) relative to both WT and HET animals. Each point in the histogram represents an individual measurement. The three determinations for Esr1KO caput luminal fluid overlie one another. Bars = mean. *Denotes significant difference from corresponding regions in WT/HET (P ≤ 0.01).
Effects of Osmolality on Esr1KO Flagellar Coiling
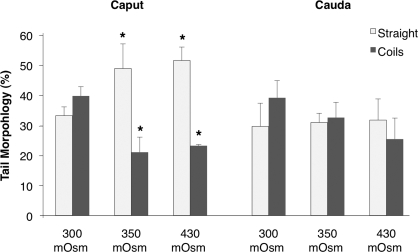
Given that flagellar coiling is believed to be a phenomenon of hypotonic swelling of spermatozoa, we determined whether exposure of Esr1KO sperm to a more WT-like tonicity could rescue the increase in flagellar angulation (Fig. 7). Flagellar coiling in these “hyperosmotic” conditions were compared to the standard sperm collection media, dmKBRT, of 300 mmol/kg. Similar to that described previously, approximately 35% of the Esr1KO caput sperm possessed a straight morphology when released into 300 mmol/kg dmKBRT. The proportion of straight sperm increased with increasing osmolality. When cauda sperm were assayed, although the trend was similar, the degree of morphological rescue was negligible, reflecting the irreversible damage done to sperm as they are stored in the abnormal epididymal environment over prolonged time periods.
FIG. 7.
Increased tonicity partially rescues the coiling defect of Esr1KO sperm. Tail morphology was assessed after sperm were released into a more wild-type-like tonicity, with 300 mmol/kg being the standard osmolality of sperm collection media. Sperm recovered from the Esr1KO caput responded with an approximately 20% increase in straight-tail morphology when placed into media of 350 and 430 mmol/kg. Although there was a similar trend in sperm recovered from the Esr1KO cauda, the degree of rescue was minimal. Each bar represents the mean ± SEM. *Denotes significant difference from corresponding sperm morphology in 300 mmol/kg (P ≤ 0.05).
Mimic of the Esr1KO Flagellar Phenotype
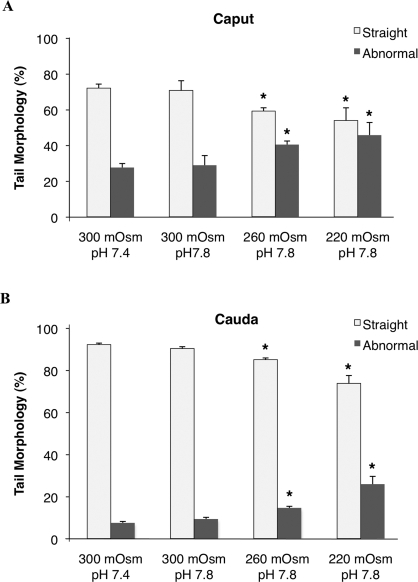
Data thus far suggest that defects in Esr1KO sperm are a result of storage in an abnormal luminal environment that is low in osmolality and high in pH. This would suggest that WT sperm exposed to an abnormal hypo-osmotic and/or alkaline environment would show an increased propensity for coiling. An in vitro assay was developed to test the response of WT sperm to increased pH and hypo-osmotic challenges. Caput and cauda sperm collected from WT mice were incubated in three osmotic media: 300, 260, and 220 mmol/kg with pH 7.8 (Fig. 8). Morphology was assessed and compared to a routine sperm collection medium of 300 mmol/kg, pH 7.4.
FIG. 8.
Decreased osmolality with increased pH produces Esr1KO-like morphological defects in wild-type sperm. Wild-type sperm were recovered from either the caput or cauda and introduced into media of osmolality 300, 260, and 220 mmol/kg, pH 7.8. Morphology was assessed and compared to basal medium (300 mmol/kg, pH 7.4). A) Although the increase in pH alone did not affect tail morphology, decreasing osmolality with increased pH resulted in a decreased proportion of sperm with straight flagellar morphology and increased coiling. B) Cauda sperm exposed to a similar media, with decreasing tonicity and increased pH, led to a decrease of straight flagella. Each bar represents the mean ± SEM. *Denotes significant difference from corresponding sperm morphology in 300 mmol/kg, pH 7.8 (P ≤ 0.03).
Normal caput sperm are immature [15] and unable to appropriately regulate cellular volume. Therefore, WT caput sperm incubated in 300 mmol/kg for 30 min showed an increased percentage of coiled and looped sperm tails (Fig. 8) compared to sperm examined immediately after removal from the epididymis (Fig. 3). When incubated in the more extreme hypo-osmotic media, in combination with alkaline pH, the caput sperm responded with cellular shape changes that mimicked those observed in Esr1KO mice. As a result, there was a significant decrease in the proportion of straight sperm flagella, which correlated with an increased proportion of looped and coiled tails (Fig. 8A). No additional effect on sperm morphology was observed with pH change alone in 300 mmol/kg. Cauda sperm responded less drastically to hypo-osmotic conditions, with a decrease in straight sperm flagella of approximately 20% in the most hypo-osmotic media (Fig. 8B). Although the recapitulation did not approach levels observed in the most severe Esr1KO phenotype, these results confirm that a combination of alkalinity and hypo-osmolality contributes to the Esr1KO flagellar defects.
DISCUSSION
In the Esr1KO mouse, efferent ductules fail to execute their absorptive function [29], which, in conjunction with alterations in ion and fluid transporters within the epididymis, results in significant changes in the luminal fluid composition. One of these consequent defects, the failure of luminal acidification, has been recently documented [32]. In this study, we show that the Esr1KO luminal fluid is more hypo-osmotic than its WT counterpart. This is likely due to the loss of efferent ductule function, leading to a decreased rate of fluid transport and consequent dilution of downstream luminal fluid. The systematic evaluation of Esr1KO sperm during epididymal transit revealed two classes of morphological defects: spontaneous acrosome reactions and severe flagellar coiling. Both of these defects were prominent within the initial segment and increased in frequency as sperm traveled into distal regions of the epididymis as well as with increased ages of the animals. The increase in severity of these phenotypes with age and transit further support the conclusion that functional defects in spermatozoa were a result of the abnormal fluid milieu created in part by the dysfunctional efferent ducts.
The acrosome is a membrane-bound structure that covers the anterior portion of the sperm nucleus and contains a large array of hydrolyzing enzymes. Functionally, the acrosome aids penetration of the egg coat by releasing its contents through a process known as the acrosome reaction (acrosomal exocytosis), which occurs on sperm binding to the egg coat. However, nonphysiological or false acrosome reactions can occur under various conditions, such as exposure to alkaline media [44]. Often these spontaneous acrosome reactions occur in moribund or dead spermatozoa when their membranes weaken, releasing hydrolyzing enzymes and causing autodigestion of the acrosome [45]. This can cause detachment of the outer acrosomal membrane and the overlying plasma membrane. In WT/HET mice, spermatozoa had a low proportion of spontaneous reactions throughout all epididymal regions. In contrast, Esr1KO sperm had a substantially higher rate of premature acrosome reactions, the frequency of which increased in the more distal epididymal regions. As these mice aged, the phenotype became nearly 100% penetrant in the cauda epididymis by 14 wk. Since spontaneously reacted spermatozoa are functionally incompetent, the increased incidence of these degenerative modifications in the Esr1KO epididymis would largely contribute to the reduced fertilizing ability of sperm in these animals [30]. Furthermore, it is reasonable to assume that these compromised cells likely constitute all or a portion of the nonmotile cells recovered from the cauda.
A large percentage of Esr1KO sperm also exhibited flagellar angulation and coiling. The severe coiling observed in these animals is similar to the Dag defect previously described in several large domesticated animals [22, 23, 46, 47]. Although epididymal tissue and fluid properties were not measured for all of these “sterile studs,” there is correlative evidence from several ejaculation studies that suggests that the defect is of epididymal origin. For example, exhaustive ejaculations in a bull with Dag-defective sperm resulted in a dramatic reduction in the percent abnormal sperm [48]. In many of these cases there was a genetic linkage but no discovered etiology. Given the similar morphological defect and the hereditary link, it is plausible that mutations in the Esr1 gene or cofactors regulating its function [49] could be involved.
Significant knowledge of axoneme function has been derived from studies using chlamydomonas and tetrahymyna, two ciliated protists. From these model organisms and subsequent studies on sperm, it is understood that flagellar coiling and angulation that occurs in nonisotonic conditions can be a result of osmotic shock. Although it is routine to refer to common sperm preparation media as “isotonic” (300 mmol/kg), in reality the epididymal fluid provides a hyperosmotic microenvironment relative to common biological media and serum. As a result, under both in vitro and in vivo conditions, sperm experience several osmotic challenges. In order to counteract the influx of water that occurs under hypo-osmotic conditions, sperm undergo a process of regulatory volume decrease, which utilizes a number of different membrane channels localized mainly to the cytoplasmic droplet [11, 43]. While the mechanisms underlying flagella angulation in sperm are obscure, it may involve failure to properly undergo RVD.
Assessment of Esr1KO morphology revealed that there was a substantial reduction in the presence of straight (or normal) flagella, in comparison to WT/HET mice, with a trend for increased abnormalities in the distal regions. Esr1KO mice also exhibited a slight but significant reduction in the frequency of straight flagella recovered from adult testes. However, a testicular derivation of this phenotype was ruled out by assaying young animals that had not yet acquired the characteristic fluid back pressure and testicular swelling that is common in older Esr1KO mice. In young Esr1KO mice, although there was an absence of morphological defects within the testes, there was a significant increase in flagellar coiling in the caput epididymis. Also, in the current study, coiled sperm were observed only in the lumen of seminiferous tubules after fluid back pressure was present in the testis. These data further indicate that the sperm phenotype develops as a response to interactions with an abnormal epididymal environment. Treatment with the antiestrogen ICI 182,780 also causes cauda sperm acrosome and flagella abnormalities in situ, similar to what is described here [50]. Although ESR1 functional ablation was produced during puberty in the antiestrogen experimental model, ICI 182,780 treatment induced nearly all the phenotypic changes associated with the loss of Esr1 mRNA expression [50, 51]. However, in contrast to the Esr1KO mouse, there was no increase in testicular weight, an observation that further supports the conclusion that the sperm defect is posttesticular in nature.
Coiling of Esr1KO sperm typically occupied the flagellum, without involving the head, which differs from several knockout and mutant mouse models in which coiling encompasses the head (Gopc−/− [52, 53], Spem1−/− [54], Hook1−/− [55], Capza3−/− [56]). In these other animal models, abnormal sperm identified as “heads in the coils” [57] begin their development during spermatogenesis, and abnormal morphology is detectable within the seminiferous epithelium. In Gopc−/− mice, the defect is intrinsic to the germ cell within the seminiferous epithelium, but the tail disorganization worsens as sperm pass through the epididymis [52, 53]. In Spem1−/− mice, sperm coil defects observed in the epididymis were found within stage VIII of the seminiferous epithelium [54]. In contrast to these genetic models, the Esr1KO seminiferous epithelium appears relatively normal until fluid begins to accumulate in the testis, which results in dilation of the seminiferous tubules. Stretching of the seminiferous epithelium was associated with subsequent degeneration and ultimately tubular atrophy [29, 30]. Coiled tails were observed only in the lumen of dilated tubules and never within the Esr1KO seminiferous epithelium.
It is well known that sperm flagellar coiling and angulation occur in response to hypo-osmotic conditions [15, 19, 42, 58–60]. Studies by Drevius [58] and Lindahl and Drevius [60] found that hypotonic-induced coiling and bending of the tails was also correlated with abnormal sperm motility. Electron microscopy of these coiled spermatozoa revealed that the coiled tails were enclosed in a common cell membrane [42], which is similar to that observed in Esr1KO mice. Others have reported coiled sperm in the ejaculate, without evidence of osmolality changes [15, 48, 57]. However, whereas those studies determined osmolality of the ejaculate fluid, they did not examine for potential correlations between sperm coiling and osmolality defects upstream in the epididymal lumen [15].
The present study documents for the first time the osmolality of WT mouse caput epididymal fluid as 435 ± 11 mmol/kg. In contrast, the Esr1KO caput epididymis was significantly hypo-osmotic in comparison to WT/HET mice. This hypo-osmotic environment would account for the flagellar coiling that occurs within the epididymal lumen. In an attempt to rescue the coiled phenotype, we released Esr1KO spermatozoa into a more WT-like osmotic environment. As osmolality of the external media increased, the proportion of coiled flagella decreased and straight flagella increased. Although there was an improvement in the proportion of straight spermatozoa after incubation in a hypertonic media, the levels did not approach what is normally observed in control sperm. This most likely represents the heterogeneity of cells, some of which were unable to respond. The inability of some Esr1KO sperm to straighten in response to changes in osmolality could be due to the normal maturational sulfydryl oxidation of the outer dense fibers and fibrous sheath, which may “fix” spermatozoa in their swollen configurations [61, 62]. The fact that morphological rescue was notable only in sperm recovered from the caput epididymis and that sperm residing longer in the epididymal duct (cauda sperm) were refractory supports this hypothesis. Alternatively, the cells that did not respond could represent a proportion of cells with severely damaged membranes or those that were metabolically dead and could not, therefore, activate ion transport.
We also investigated the response of WT sperm exposed to a hypotonic environment. The combination of increased pH and hypo-osmolality but not increased pH alone substantially increased the proportion of spermatozoa that were similar in appearance to those found in the Esr1KO. Although there was a tendency for this to also occur with cauda sperm, the findings were not significant. Thus, the significant increase in flagellar angulation and coiling of WT caput sperm when incubated at relatively low osmolalities (<420 mmol/kg) supports the hypothesis that very high luminal fluid osmotic pressure is required for healthy sperm maturation in the epididymis.
This is the first account of severe sperm coiling (Dag defect) that is correlated with hypo-osmolality of the epididymal fluid. The results of this study suggest that coiling of Esr1KO sperm was in response to an abnormal luminal environment and demonstrate that ESR1 activity in the efferent ducts and epididymis is essential for maintaining a luminal environment that is suitable for proper sperm maturation and function. Recent work suggests that ESR1 activity is also supported by ligand-independent pathways [63]. Compromising the balance of luminal constituents has detrimental effects on sperm morphology and fertilizing capability, which may offer opportunities for contraceptive development [57]. The Esr1KO male can therefore serve as a unique model to better understand the endocrine regulation of luminal fluid physiology and subsequent effects on sperm function.
Acknowledgments
We would like to thank Susan-Hudgins Spivey for her excellent technical support in generating the mice used in these studies.
Footnotes
Supported by National Institutes of Health grants F31 HD 54330 (A.J.), RO1 HD 23479 (B.D.S.), P20 RR 15592 (C.K.), and NIH T32 ES07326 (R.A.H., A.J.).
REFERENCES
- Cooper TG.Role of the epididymis in mediating changes in the male gamete during maturation. Adv Exp Med Biol 1995; 377: 87–101. [DOI] [PubMed] [Google Scholar]
- Moore HD, Bedford JM.Short-term effects of androgen withdrawal on the structure of different epithelial cells in the rat epididymis. Anat Rec 1979; 193: 293–311. [DOI] [PubMed] [Google Scholar]
- Moore HD, Bedford JM.The differential absorptive activity of epithelial cells of the rat epididymus before and after castration. Anat Rec 1979; 193: 313–327. [DOI] [PubMed] [Google Scholar]
- Robaire B, Seenundun S, Hamzeh M, Lamour SA.Androgenic regulation of novel genes in the epididymis. Asian J Androl 2007; 9: 545–553. [DOI] [PubMed] [Google Scholar]
- Meistrich ML, Hughes TH, Bruce WR.Alteration of epididymal sperm transport and maturation in mice by oestrogen and testosterone. Nature 1975; 258: 145–147. [DOI] [PubMed] [Google Scholar]
- Orgebin-Crist MC, Eller BC, Danzo BJ.The effects of estradiol, tamoxifen, and testosterone on the weights and histology of the epididymis and accessory sex organs of sexually immature rabbits. Endocrinol 1983; 113: 1703–1715. [DOI] [PubMed] [Google Scholar]
- Shayu D, Hardy MP, Rao AJ.Delineating the role of estrogen in regulating epididymal gene expression. Soc Reprod Fertil Suppl 2007; 63: 31–43. [PubMed] [Google Scholar]
- Carreau S.Estrogens—male hormones? Folia Histochem Cytobiol 2003; 41: 107–111. [PubMed] [Google Scholar]
- Hess RA.The efferent ductules: structure and functions. Robaire B, Hinton B.The Epididymis: From Molecules to Clinical Practice New York:Kluwer Academic/Plenum Publishers;2002: 49–80. [Google Scholar]
- Turner TT.Necessity's potion: inorganic ions and small organic molecules in the epididymal lumen. Robaire B, Hinton BT.The Epididymis: From Molecules to Clincal Practice New York:Kluwer Academic/Plenum Publishers;2002: 49–80. [Google Scholar]
- Cooper TG, Yeung CH.Involvement of potassium and chloride channels and other transporters in volume regulation by spermatozoa. Curr Pharm Des 2007; 13: 3222–3230. [DOI] [PubMed] [Google Scholar]
- Turner TT.On the epididymis and its function. Invest Urol 1979; 16: 311–321. [PubMed] [Google Scholar]
- Hinton BT, Pryor JP, Hirsh AV, Setchell BP.The concentration of some inorganic ions and organic compounds in the luminal fluid of the human ductus deferens. Int J Androl 1981; 4: 457–461. [DOI] [PubMed] [Google Scholar]
- Acott TS, Carr DW.Inhibition of bovine spermatozoa by caudal epididymal fluid: II. Interaction of pH and a quiescence factor. Biol Reprod 1984; 30: 926–935. [DOI] [PubMed] [Google Scholar]
- Cooper TG, Yeung CH.Acquisition of volume regulatory response of sperm upon maturation in the epididymis and the role of the cytoplasmic droplet. Microsc Res Tech 2003; 61: 28–38. [DOI] [PubMed] [Google Scholar]
- Johnson AL, Howards SS.Intratubular hydrostatic pressure in testis and epididymis before and after long-term vasectomy in the guinea pig. Biol Reprod 1976; 14: 371–376. [DOI] [PubMed] [Google Scholar]
- Johnson AL, Howards SS.Hyperosmolality in intraluminal fluids from hamster testis and epididymis: a micropuncture study. Science 1977; 195: 492–493. [DOI] [PubMed] [Google Scholar]
- Levine N, Marsh DJ.Micropuncture studies of the electrolyte aspects of fluid and electrolyte transport in individual seminiferous tubules in the epididymis and the vas deferens in rats. J Physiol 1971; 213: 557–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung CH, Sonnenberg-Riethmacher E, Cooper TG.Infertile spermatozoa of c-ros tyrosine kinase receptor knockout mice show flagellar angulation and maturational defects in cell volume regulatory mechanisms. Biol Reprod 1999; 61: 1062–1069. [DOI] [PubMed] [Google Scholar]
- Jeyendran RS, Van der Ven HH, Perez-Pelaez M, Crabo BG, Zaneveld LJ.Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil 1984; 70: 219–228. [DOI] [PubMed] [Google Scholar]
- Cooper TG.Sperm maturation in the epididymis: a new look at an old problem. Asian J Androl 2007; 9: 533–539. [DOI] [PubMed] [Google Scholar]
- Blom E.A new sterilizing and hereditary defect (the “Dag defect”) located in the bull sperm tail. Nature 1966; 209: 739–740. [DOI] [PubMed] [Google Scholar]
- Rota A, Manuali E, Caire S, Appino S.Severe tail defects in the spermatozoa ejaculated by an English bulldog. J Vet Med Sci 2008; 70: 123–125. [DOI] [PubMed] [Google Scholar]
- van Duijn C.Ultrastructural mid-piece defects in spermatozoa from the subfertile Great Yorkshire boar. Proceeding of the 7th International Congress Anim Reprod and AI; 1972. 1
- Hoshii T, Takeo T, Nakagata N, Takeya M, Araki K, Yamamura K.LGR4 regulates the postnatal development and integrity of male reproductive tracts in mice. Biol Reprod 2007; 76: 303–313. [DOI] [PubMed] [Google Scholar]
- Andersen OM, Yeung CH, Vorum H, Wellner M, Andreassen TK, Erdmann B, Mueller EC, Herz J, Otto A, Cooper TG, Willnow TE.Essential role of the apolipoprotein E receptor-2 in sperm development. J Biol Chem 2003; 278: 23989–23995. [DOI] [PubMed] [Google Scholar]
- Sipila P, Cooper TG, Yeung CH, Mustonen M, Penttinen J, Drevet J, Huhtaniemi I, Poutanen M.Epididymal dysfunction initiated by the expression of simian virus 40 T-antigen leads to angulated sperm flagella and infertility in transgenic mice. Mol Endocrinol 2002; 16: 2603–2617. [DOI] [PubMed] [Google Scholar]
- Blomqvist SR, Vidarsson H, Soder O, Enerback S.Epididymal expression of the forkhead transcription factor Foxi1 is required for male fertility. EMBO J 2006; 25: 4131–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, Lubahn DB.A role for oestrogens in the male reproductive system. Nature 1997; 390: 509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, Korach KS.Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinol 1996; 137: 4796–4805. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Clarke L, Nie R, Carnes K, Lai LW, Lien YH, Verkman A, Lubahn D, Fisher JS, Katzenellenbogen BS, Hess RA.Estrogen action and male fertility: Roles of the sodium/hydrogen exchanger-3 and fluid reabsorption in reproductive tract function. Proc Natl Acad Sci U S A 2001; 98: 14132–14137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph A, Hess RA, Schaeffer DJ, Ko C, Hudgin-Spivey S, Chambon P, Shur BD.Absence of estrogen receptor alpha leads to physiological alterations in the mouse epididymis and consequent defects in sperm function. Biol Reprod 2010; 82: 948–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker GL, Joseph DB, Lennarz WJ.A study of factors involved in induction of the acrosomal reaction in sperm of the sea urchin, Arbacia punctulata. Dev Biol 1976; 53: 115–125. [DOI] [PubMed] [Google Scholar]
- Gregg KW, Metz CB.Physiological parameters of the sea urchin acrosome reaction. Biol Reprod 1976; 14: 405–411. [DOI] [PubMed] [Google Scholar]
- Hoshi M, Matsui T, Nishiyama I, Amano T, Okita Y.Physiological inducers of the acrosome reaction. Cell Differ Dev 1988; 25(suppl):19–24. [DOI] [PubMed] [Google Scholar]
- Hyne RV, Garbers DL.Requirement of serum factors for capacitation and the acrosome reaction of guinea pig spermatozoa in buffered medium below pH 7.8. Biol Reprod 1981; 24: 257–266. [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M.Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 2000; 127: 4277–4291. [DOI] [PubMed] [Google Scholar]
- Gieske MC, Kim HJ, Legan SJ, Koo Y, Krust A, Chambon P, Ko C.Pituitary gonadotroph estrogen receptor-alpha is necessary for fertility in females. Endocrinology 2008; 149: 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA.Quantitative and qualitative characteristics of the stages and transitions in the cycle of the rat seminiferous epithelium: light microscopic observations of perfusion-fixed and plastic-embedded testes. Biol Reprod 1990; 43: 525–542. [DOI] [PubMed] [Google Scholar]
- Rodeheffer C, Shur BD.Sperm from beta1,4-galactosyltransferase I-null mice exhibit precocious capacitation. Development 2004; 131: 491–501. [DOI] [PubMed] [Google Scholar]
- Larson JL, Miller DJ.Simple histochemical stain for acrosomes on sperm from several species. Mol Reprod Dev 1999; 52: 445–449. [DOI] [PubMed] [Google Scholar]
- Drevius LO, Eriksson H.Osmotic swelling of mammalian spermatozoa. Exp Cell Res 1966; 42: 136–156. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Sonnenberg-Riethmacher E, Cooper TG.Receptor tyrosine kinase c-ros knockout mice as a model for the study of epididymal regulation of sperm function. J Reprod Fertil Suppl 1998; 53: 137–147. [PubMed] [Google Scholar]
- Working PK, Meizel S.Correlation of increased intraacrosomal pH with the hamster sperm acrosome reaction. J Exp Zool 1983; 227: 97–107. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R.Mammalian fertilization: acrosome and the acrosome reaction. Knobil E, Neill JD.The Physiology of Reproduction, vol. 1 New York:Raven Press;1994: 206–214. [Google Scholar]
- Hellander JC, Samper JC, Crabo BG.Fertility of a stallion with low sperm motility and a high incidence of an unusual sperm tail defect. Vet Rec 1991; 128: 449–451. [DOI] [PubMed] [Google Scholar]
- Holt WV.Epididymal origin of a coiled-tail sperm defect in a boar. J Reprod Fertil 1982; 64: 485–489. [DOI] [PubMed] [Google Scholar]
- Gustafsson B, Einarsson S, Nicander L, Holtman M, Soosalu O.Morphological, physical and chemical examination of epididymal contents and semen in a bull with epididymal dysfunction. Andrologia 1974; 6: 321–331. [Google Scholar]
- Chang EC, Charn TH, Park SH, Helferich WG, Komm B, Katzenellenbogen JA, Katzenellenbogen BS.Estrogen receptors alpha and beta as determinants of gene expression: influence of ligand, dose, and chromatin binding. Mol Endocrinol 2008; 22: 1032–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HW, Nie R, Carnes K, Zhou Q, Sharief NA, Hess RA.The antiestrogen ICI 182,780 induces early effects on the adult male mouse reproductive tract and long-term decreased fertility without testicular atrophy. Reprod Biol Endocrinol 2003; 1: 57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Hess RA, Bahr JM, Lubahn DB, Taylor J, Bunick D.Estrogen receptor alpha has a functional role in the mouse rete testis and efferent ductules. Biol Reprod 2000; 63: 1873–1880. [DOI] [PubMed] [Google Scholar]
- Suzuki-Toyota F, Ito C, Toyama Y, Maekawa M, Yao R, Noda T, Iida H, Toshimori K.Factors maintaining normal sperm tail structure during epididymal maturation studied in Gopc-/- mice. Biol Reprod 2007; 77: 71–82. [DOI] [PubMed] [Google Scholar]
- Suzuki-Toyota F, Ito C, Toyama Y, Maekawa M, Yao R, Noda T, Toshimori K.The coiled tail of the round-headed spermatozoa appears during epididymal passage in GOPC-deficient mice. Arch Histol Cytol 2004; 67: 361–371. [DOI] [PubMed] [Google Scholar]
- Zheng H, Stratton CJ, Morozumi K, Jin J, Yanagimachi R, Yan W.Lack of Spem1 causes aberrant cytoplasm removal, sperm deformation, and male infertility. Proc Natl Acad Sci U S A 2007; 104: 6852–6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida K, Tres LL, Kierszenbaum AL.Structural and biochemical features of fractionated spermatid manchettes and sperm axonemes of the azh/azh mutant mouse. Mol Reprod Dev 1999; 52: 434–444. [DOI] [PubMed] [Google Scholar]
- Geyer CB, Inselman AL, Sunman JA, Bornstein S, Handel MA, Eddy EM.A missense mutation in the Capza3 gene and disruption of F-actin organization in spermatids of repro32 infertile male mice. Dev Biol 2009; 330: 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung CH, Tuttelmann F, Bergmann M, Nordhoff V, Vorona E, Cooper TG.Coiled sperm from infertile patients: characteristics, associated factors and biological implication. Hum Reprod 2009; 24: 1288–1295. [DOI] [PubMed] [Google Scholar]
- Drevius LO.Spiralization in tails of mammalian spermatozoa in hypotonic media. Nature 1963; 197: 1123–1124. [Google Scholar]
- Drevius LO.Bull spermatozoa as osmometers. J Reprod Fertil 1972; 28: 29–39. [DOI] [PubMed] [Google Scholar]
- Lindahl PE, Drevius LO.Observations on bull spermatozoa in a hypotonic medium related to sperm mobility mechanisms. Exp Cell Res 1964; 36: 632–646. [DOI] [PubMed] [Google Scholar]
- Cooper TG, Yeung CH, Wagenfeld A, Nieschlag E, Poutanen M, Huhtaniemi I, Sipila P.Mouse models of infertility due to swollen spermatozoa. Mol Cell Endocrinol 2004; 216: 55–63. [DOI] [PubMed] [Google Scholar]
- Cornwall GA, Vindivich D, Tillman S, Chang TS.The effect of sulfhydryl oxidation on the morphology of immature hamster epididymal spermatozoa induced to acquire motility in vitro. Biol Reprod 1988; 39: 141–155. [DOI] [PubMed] [Google Scholar]
- Sinkevicius KW, Laine M, Lotan TL, Woloszyn K, Richburg JH, Greene GL.Estrogen-dependent and -independent estrogen receptor-alpha signaling separately regulate male fertility. Endocrinology 2009; 150: 2898–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]