Abstract
New neurons are generated in the granule cell layer of the dentate gyrus (GCL) throughout adulthood. This process is modulated by many environmental and neurochemical factors. We previously observed that castrated mice, compared to sham-operated mice, perform poorly in the delayed matching to place water-maze task (DMTP). In this study we quantified the number of doublecortin expressing (DCX+) immature neurons and Ki-67 expressing (Ki-67+) proliferating progenitors in mice previously tested in a spatial DMTP task, a non-spatial DMTP, or that received equivalent amounts of handling only. Regardless of DMTP training experience castration reduced immature neuron number in the GCL but had no effect on proliferating progenitors. Compared to handling only, visible DMTP training reduced the immature neuron number but hidden DMTP training had no effect. Castration did not alter these environmental effects. Finally, performance on the spatial DMTP task did not correlate with immature neuron number. In addition, while the number of immature neurons was strongly reduced following cranial irradiation with 137Cs, this treatment did not affect spatial DMTP performance. Thus, in mice, castration disrupts spatial memory and reduces immature neuron number, but there is no strong link between these effects.
Keywords: Hippocampus, Spatial Memory, Irradiation, Androgens, Neurogenesis
1. Introduction
New neurons are produced throughout life in the hippocampal granule cell layer (GCL) in mice (Raber et al., 2004a), a brain region important for spatial learning and memory (Kresnor, 2007). This process proceeds through stages that include proliferation of progenitor cells, differentiation into neurons, migration of new neurons into the GCL, and maturation of connectivity with afferent and efferent targets (Ming and Song, 2005). Many factors have been identified that increase new neuron during these stages either singly or in combination such as environmental enrichment in mice (Kempermann et al., 2002) and hippocampus-dependent learning in rats (Shors, 2004). In contrast, stress (Mirescu and Gould, 2006) decreases the production of new neurons in the GCL of rats. Neurochemical factors such as growth factors (Zhao et al., 2007), neurotrophins (Lee et al., 2002) in mice, and neurotransmitters (Cameron et al., 1998) in rats, can likewise affect the rates of proliferation or survival of new neurons. In addition, steroid hormones such as corticosterone (Mayer et al., 2006; Mirescu et al., 2006), estrogens (Ormerod et al., 2003), and androgens have recently been suggested to affect hippocampal neurogenesis in rats (Spritzer and Galea, 2007). However, estrogens do not appear to effect neurogenesis in mice (Lagace et al., 2007). The effects of androgens on hippocampal neurogenesis have not been studied in mice.
Androgens also alter hippocampus-dependent memory formation (Bimonte-Nelson et al., 2003; Driscoll et al., 2005; Raber et al., 2002). The hippocampus is required for performance on spatial memory tasks in rats (Duva et al., 1997; Morris et al., 1982; Xavier et al., 1999) and mice (Gerlai et al., 2002). Although castration does not affect spatial ‘reference’ memory performance where the same information is learned repeatedly, castration disrupts newly formed spatial memories in a delay-dependent manner in rats (Sandstrom et al., 2006; Spritzer et al., 2007). Compared to intact rats, castrated rats show more working memory errors in the radial arm maze task (Spritzer et al., 2007), and they exhibit lower retention scores with increasing retention intervals in the delayed-matching to place water maze task (DMTP) (Sandstrom et al., 1998). We recently observed the same effect in mice (Benice and Raber, 2009). The mechanism for castration induced spatial memory deficits unknown, but some have suggested a role for changes in neurotransmitter levels (Leonard et al., 2007; Nakamura et al., 2002; Romeo et al., 2005), or structural changes such as modulation of spine synapse density in the hippocampus (Leranth et al., 2004a; Leranth et al., 2004b). It is also possible that hippocampal new neuron production mediates androgen effects on spatial memory. The production of new hippocampal neurons has been implicated in spatial memory performance in mice (Bizon et al., 2004; Kempermann et al., 2002; Raber et al., 2004b). However, the link between androgen-induced changes in the number of immature neurons and spatial memory has not been investigated.
In this study, we studied male C57BL/6J mice and asked whether: 1) castration modulates the number of immature neurons or proliferating neuronal progenitors in the GCL of male mice; 2) androgens are required to produce effects of DMTP training on immature neuron number of progenitor proliferation 3) changes in immature neuron number or progenitor proliferation might account for the effects of castration on spatial memory in the DMTP. Although androgens and DMTP testing experience mice also affect neurogenesis in the sub-ventricular zone, we chose to examine the GCL because the hippocampus is strongly implicated in performance on spatial memory tasks like the DMTP task (Xavier et al., 1999). To do this we used unbiased stereology to measure the number of DCX-expressing immature neurons (DCX+) and Ki-67-expressing proliferating progenitor cells (Ki-67+) in the dentate gyrus of castrated and intact male mice that were previously tested in either a spatial version of the DMTP task, a non-spatial version of the DMTP task, or that received equivalent amounts of handling only. Similar to rat studies, we previously found that in mice castration disrupted spatial memory following a 1-hour retention interval, but had no effect following a 1-minute retention interval and had no effect on visible DMTP performance (Benice and Raber, 2009). Here, we hypothesized that castration would reduce the number of immature neurons and proliferating progenitor cells in the GCL, and that the number of immature neurons and proliferating progenitor cells would correlate with spatial DMTP performance. We also hypothesized that hidden DMTP training would increase the number of immature neurons in the GCL compared to either visible DMTP training or handling only, and that castration would inhibit these effects. Finally, if the number of immature neurons mediated the castration-induced disruption of spatial DMTP performance, we hypothesized that inhibition of neurogenesis by cranial irradiation with 137Cs would have a similar effect.
2. Results
Castration disrupted hidden DMTP performance following a 1-hour retention interval
This data is presented elsewhere (Benice and Raber, 2009), and is summarized below. Whereas both sham-operated and castrated mice showed similar retention scores in the hidden target portion of the DMTP task following a 1-minute retention interval, this ability was disrupted in castrated mice following a 1-hour retention interval (Table 1) (Benice and Raber, 2009). This performance deficit was probably due to a spatial memory disruption in castrated mice since both castrated and sham-operated mice showed equivalent retention scores during the visible target portion of the test (Benice and Raber, 2009).
Table 1.
DMTP performance in castrated and sham-operated mice
| Retention Interval (mean ± SEM) |
||
|---|---|---|
| 1-min | 1-hour | |
| castrated | 25.2 ± 6.7 | 8.6 ± 4.7 * |
| sham-operated | 21.3 ± 6.0 | 30.2 ± 4.9 |
- p < 0.05 by Duncan's post-hoc test following ANOVA
N = 10 for each experimental group.
Castration and visible DMTP training reduced the number of DCX+, but not Ki-67+ cells, in the GCL
Both DCX+ and Ki-67+ cells in the hippocampus were found exclusively in the sub-granular zone of the GCL of the dentate gyrus, indicating specific staining for DCX and Ki-67 respectively (data not shown). For DCX+ cells, there was an effect of castration (F (1, 17) = 5.47, p = 0.03) with castrated mice having fewer DCX+ cells than sham-operated mice. There was also an effect of DMTP training type (F (2, 17) = 11.6, p = 0.001) with mice receiving visible DMTP training having fewer DCX+ cells compared to mice that received either hidden DMTP training or handling only (Figure 1D). However, there was no interaction between castration and DMTP training (F(2, 17) = 0.24, p = 0.79). For Ki-67+ cells, there was no effect of either castration or DMTP training type (Figure 1F). Neither castration nor DMTP training type affected the GCL volumes (Figure 1B).
Figure 1.
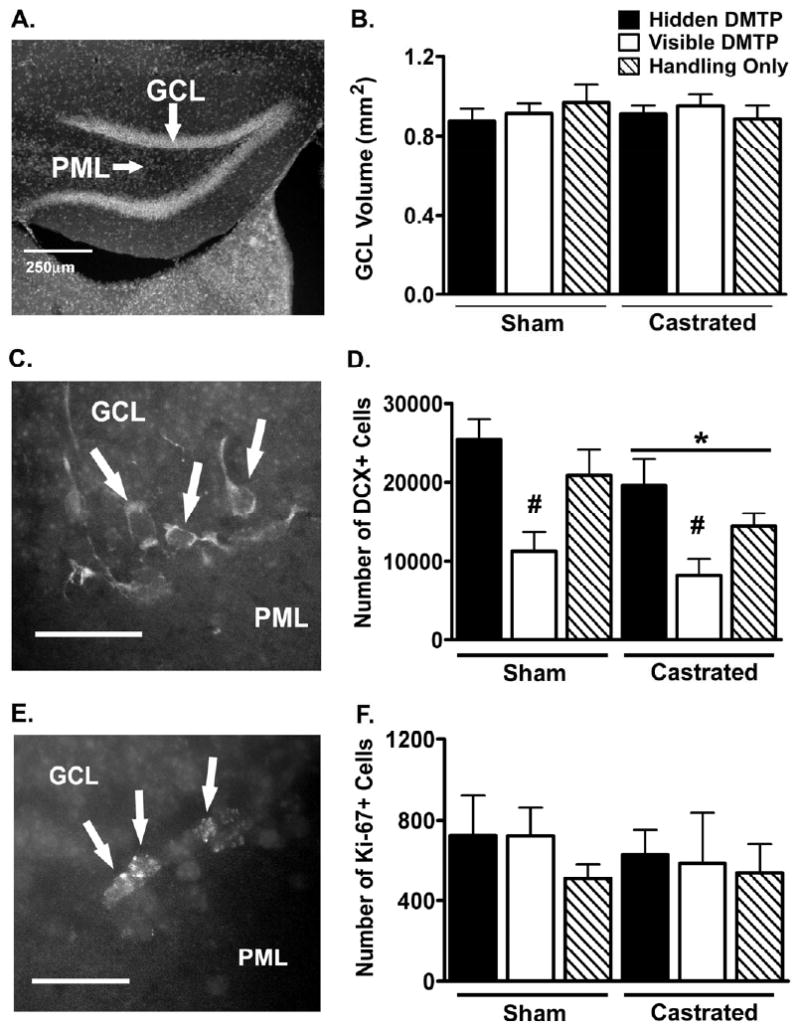
Effects of castration and DMTP training experience on the volume estimates (A, B) in the granule cell layer (GCL) of the dentate gyrus, number of DCX+ immature neurons (C,D), and number of Ki67+ proliferating progenitors (E,F). Arrows point to profiles of DCX+ immature neurons (C), Ki-67+ proliferating progenitors (E) in images taken in the region 250-500μm from the most rostral end of the GCL. DAPI counter-staining was used to visualize the granule cells in the dentate gyrus and measure the volume of the GCL (A). GCL = granule cell layer, PML = polymorph layer. In (C) and (D) the marker bar in the lower left corner indicates 25μm distance.
* significant effect of castration (p < 0.05) by two-way ANOVA
# p < 0.05 by Duncan's post-hoc test compared to either hidden DMTP or handling only.
Experimental group sizes were as follows:
For DCX: Hidden DMTP (n=4 sham-operated, n=3 castrated), Visible DMTP (n=4 sham-operated, n=4 castrated), Handling Only (n=4 sham-operated, n=4 castrated).
For Ki-67: Hidden DMTP (n=6 sham-operated, n=5 castrated), Visible DMTP (n=6 sham-operated, n=6 castrated), Handling Only (n=6 sham-operated, n=6 castrated).
No relationship between immature neuron number and hidden DMTP performance
Pearson's correlations revealed no significant relationship between the numbers of DCX+ immature neurons and hidden DMTP performance at either the 1-minute (r = 0.205, p = 0.66) or 1-hour (r = 0.31, p = 0.50) retention interval. Likewise, the numbers of Ki-67+ proliferating progenitors did not correlate with hidden DMTP performance at either the 1-minute (r = 0.122, p = 0.80) or 1-hour (r = 0.47, p = 0.29) retention interval.
137Ce irradiation did not disrupt spatial memory in the DMTP task
If the number of immature neurons in the GCL mediated the effects of castration on hidden DMTP performance, we hypothesized that inhibiting neurogenesis by cranial irradiation would mimic the effects of castration (Figure 2). The irradiation treatment had a clear effect in reducing the number of DCX+ neurons in the GCL 3-months later (Figure 2A). In contrast, there was no effect of retention interval (F(2, 40) = 1.13, p = 0.33) or irradiation (F(1, 20) = 0.89, p = 0.35) on hidden DMTP performance (Figure 2B). However, the mice did show significant spatial memory in this task since the retention scores for each retention interval, collapsed across irradiation groups, were significantly greater than zero by one-sample Student's t-tests with Bonforroni corrections (p < 0.01 for each test).
Figure 2.
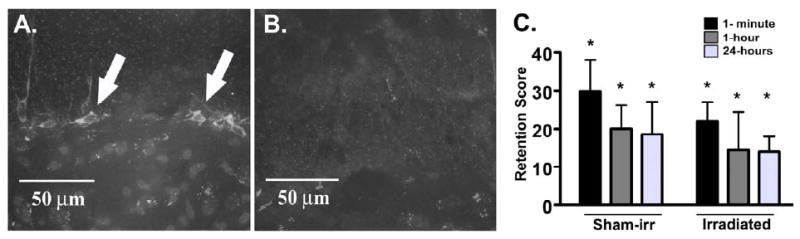
Effects of new neuron production inhibition by 137Cs irradiation on hidden DMTP performance. Irradiation drastically reduced the number of DCX+ immature neurons in the GCL (A) compared to sham irradiated controls (B). Both sham-irradiated controls (Sham-irr, n = 10) and irradiated mice (n = 10) showed significant retention scores following a 1-minute and 1-hour, but not 24-hour, retention interval (C). However, neither retention interval nor irradiation affected retention scores.
*p < 0.01 compared to zero by one-sample Student's t-test.
3. Discussion
There were three major findings in this study: 1) castration resulted in a significant reduction in the number of immature neurons in the GCL, 2) castration did not modulate the effects of DMTP training experience on the number of immature neurons or proliferating progenitors, 3) reducing the number of immature neurons by 137Cs irradiation had no significant effect on spatial DMTP task performance. These results suggest that castration-induced changes in the number of new hippocampal neurons may not play a major role in the cognitive impairment. Our data do not support a major role for immature hippocampal neurons in performance during the DMTP water maze task, consistent with the mixed results regarding the role of neurogenesis in spatial memory.
Effects of castration on the number of immature hippocampal neurons
Compared to sham-operated mice, castrated mice had fewer DCX+ immature neurons in the GCL of the dentate gyrus, though the effect was modest. In contrast, castration had no effect on the number of Ki-67+ proliferating progenitor cells. This result is consistent with the emerging idea that androgens provide some survival support to developing dentate granule cells, influence neuronal differentiation, change neuronal maturation rate, or a combination, without influencing precursor proliferation (Galea et al., 2006). Similar to this, castration in rats modestly reduced the number of bromodeoxyuridine (BrdU) labeled cells in the GCL 30-days, but not 24-hours following labeling, which was recovered by androgen replacement (Spritzer and Galea, 2007). However, a limitation of this study was that the neuronal lineage of the surviving BrdU-labeled cells could not be directly verified. Our study verifies that androgens affect the number of new neurons, since DCX is expressed only in immature neurons. However, since we did not measure the number of new glia produced during the experiment we cannot determine whether the effect was on survival or differentiation. In addition, since DCX is expressed during a limited period in neuronal development (Ming and Song, 2005), it is possible that castration increased the maturation rate of the immature neurons thereby reducing the number expressing DCX. The current data cannot conclude which of these alternatives is the case. In addition, we cannot rule out that the 3-month time between castration and testing might have allowed for some compensation to occur, leading to reduced castration effects on DCX+ and Ki-67+ cells. Nonetheless, our data adds to accumulating evidence that the effects of androgens on immature neurons are probably species independent. This effect has been observed in songbirds (Absil et al., 2003), meadow voles (Ormerod and Galea, 2003), rats (Spritzer and Galea, 2007), and now mice. This is important as it suggests that the enhancement of immature neuron number by androgens may also occur in humans.
Effects of DMTP training on the number of immature hippocampal neurons
The number of immature neurons found in the hippocampus was strongly influenced by the type of DMTP. The number of immature neurons was lower following visible DMTP training than following handling only or hidden DMTP training. Since DMTP training experience did not affect the proliferating progenitor population, the differences in immature neuron numbers are likely due to changes in neuronal differentiation, survival, or maturation rate, but not proliferation rate. From this we might conclude that handling and hidden DMTP training affected these processes equally, while visible DMTP training reduced either neuronal differentiation or survival. However, it is also possible that visible training increased maturation rate leading to reduced numbers of new neurons expressing DCX. This contrasts with previous studies in which, compared to naïve controls, hippocampus-dependent learning in a reference memory water maze increased the survival of cells that were labeled with BrdU one week prior to training (Gould et al., 1999; Prickaerts et al., 2004). However, it is not clear that the control rats in these studies experienced equivalent handling, thus handling by itself could increase survival of new neurons. Alternatively, prolonged DMTP training in the water maze might reduce the number of immature neurons in the hippocampus of rats by increasing corticosterone production (Mohapel et al., 2006). However, both the hidden and visible DMTP groups were exposed to the water maze for similar amounts of time over 9 days, so this explanation seems unlikely.
While the mechanism underlying the effects of behavioral experience on adult hippocampal new neuron production are not completely clear, factors such stress (Mayer et al., 2006; Wong and Herbert, 2006), hippocampal activation (Smith et al., 2005; Toda et al., 2008), and neurotrophins certainly play a role, at least in rats (Lee et al., 2002; Scharfman et al., 2005). Androgens are known to modulate the hypothalamic-pituitary-adrenal axis response to stressful stimuli (McCormick et al., 2002). The neurosteroid metabolite of DHT, 3α-diol, is a potent positive modulator of GABA neurotransmission (Frye et al., 2001) and may thereby affect afferent activation of hippocampal circuits. Also, castration can reduce the expression of some neurotrophic peptides in the brain (Bimonte-Nelson et al., 2003; Katoh-Semba et al., 1994). In these ways, androgens have potential to modulate the effects of learning on immature neurons. However, we found that castration did not change the effect of DMTP testing experience on immature neuron number. This indicates that, although DMTP experience strongly modulated the number of immature neurons, androgens were not required for this effect. However, it is possible that androgens might play a role in the neurogenesis -modulating effects of other experiences such as environmental enrichment (Kempermann et al., 2002) or exercise (van Praag et al., 1999).
The role of immature neurons in the effect of castration on DMTP performance
The removal of androgens by castration was previously found to disrupt spatial memory in the DMTP task (Benice and Raber, 2009). Here, we found that castration also reduced the number of immature neurons in the GCL of the same mice. If the reduction in immature neuron number mediated the disruption of spatial memory, we hypothesized to find that a) the number of immature neurons in the GCL would correlate with hidden DMTP performance, and b) reduction of the number of immature neurons by irradiation would likewise disrupt spatial memory in the DMTP. Contrary to our hypotheses, DCX+ neuron number did not correlate with retention scores following either a 1-minute or a 1-hour retention interval in the hidden DMTP. In addition, irradiation did not affect spatial memory in the DMTP task following retention intervals that were sensitive to castration. In addition, we had found previously that hormonally intact mice showed similar retention scores in the DMTP task following a 1-minute, 1-hour, or 24-hour retention interval (Benice and Raber, 2009). The current results from both sham-irradiated and irradiated mice support this previous finding. From this we conclude that, at least for the water maze DMTP task used in this study, androgens likely affect spatial memory by other mechanisms apart from reducing immature neuron number. The evidence implicating hippocampal neurogenesis in performance on spatial memory tasks is complex. Many studies (Raber et al., 2004b; Rola et al., 2004; Shi et al., 2006; Snyder et al., 2005; Villasana et al., 2006), but not all (Wojtowicz et al., 2008), using irradiation to suppress new neuron production have reported disruption in spatial learning and memory in the reference memory version of the Morris water maze. In the same vein, manipulations such as environmental enrichment or voluntary exercise, which enhance the production of new hippocampal neurons, also improve spatial memory performance in both mice and rats (Alaei et al., 2007; Luo et al., 2007; Mello et al., 2008). However, inhibition of cellular proliferation in the brain prior to such manipulations suggest that new neuron production may not mediate the associated spatial memory improvements (Meshi et al., 2006). To complicate matters further, a recent study reported improved spatial ‘working memory’ in the radial arm maze following inhibition of neurogenesis in mice (Saxe et al., 2007). To our knowledge, ours is the first to report the effects of irradiation on spatial water maze DMTP performance in mice. In addition to not supporting a role for immature neuron number in castration-induced spatial memory deficits, our data is also consistent with the idea that the number of immature neurons is not directly related to spatial memory in the DMTP task. However, we cannot rule out the possibility that cranial irradiation might affect brain function in other ways to compensate for changes in immature neuron number.
Potential mechanisms for cognitive androgenic effects
During early development, manipulation of androgen levels alters the morphology of brain regions important for cognition, but these effects are more subtle in adulthood. Although not true for the hippocampus as a whole, the volume of the granule cell layer of the dentate gyrus (GCL) and the size of the pyramidal neurons in the CA1 and CA3 regions are larger in males than females, and administration of T to neonatal females has been shown to masculinize these traits (Isgor and Sengelaub, 1998). Similarly, neonatal castration of male rats reduces the GCL volume to a smaller female-typical size (Isgor and Sengelaub, 1998). In adult rats, changes in androgen levels appear to have little effect on GCL volume in the hippocampus (Isgor and Sengelaub, 1998). Our results support this finding in that castration of adult mice did not affect the GCL volume. However, in contrast to regional volumes, the density of CA1 apical dendritic spines in the stratum radiatum in the hippocampus in adult male rats is reduced by castration and normalized by replacement with either T or DHT (Leranth et al., 2003). The same is true in adult female ovariectomized rats (Leranth et al., 2004a). Although clearly androgen sensitive, it is not known at present how changes in hippocampal synaptic morphology contribute to cognitive performance.
Androgen manipulations might affect performance on some cognitive tasks by altering neurotransmitter systems. For example, administration of the neuro-active steroid 3α-diol recovers a castration-induced passive avoidance performance deficit in adult male rats (Frye and Seliga, 2001). Also, androgens might affect cognition by altering some of the electrophysiological characteristics of hippocampal N-methyl-D-aspartate type glutamate receptors (NMDAR), which has been shown in hippocampal slice preparations from adult male rats (Pouliot et al., 1996). Androgens might also interact with acetylcholinergic pathways since castration reduces brain levels of choline-acetyltransferase, the enzyme synthesizing acetylcholine (Nakamura et al., 2002), and worsens the negative effects of scopolamine treatment on errors in the radial arm maze (Daniel et al., 2003). In addition, increased dopaminergic signaling is implicated in the disruptive effect of castration on T-maze alternation performance (Kritzer et al., 2001). Finally, T, via aromatization to 17β-estradiol, maintains normal mRNA expression of the serotonin type 2A receptor in the cortex of male rats (Sumner and Fink, 1998). However, a direct link between this receptor and cognitive task performance has yet to be made.
Conclusion
In conclusion, this study adds to a growing literature supporting that changes in androgen status affect the number of immature neurons in the mammalian hippocampus. Although castration in mice disrupts performance in the spatial water maze DMTP task, reductions in the number of immature neurons in the GCL do not seem sufficient to cause this disruption. Further research is warranted to elucidate the biological mechanisms underlying the cognitive effects of androgens.
4. Experimental Procedure
Mice
The mice used for the new neuron production studies were the same mice that were previously tested in the DMTP task (Benice and Raber, 2009). In addition, there was a separate group of mice that received an equivalent amount of handling without being tested in the DMTP test. These mice were both handled and euthanized at the same time as the other mice that received DMTP testing. For the castration, equal numbers of mice were either castrated or sham-operated in which the scrotum was opened under isofluorane anesthesia and either the testicles were removed (castration) or they were not (sham operation). The wounds were then sutured and healed normally. Mice were tested in the DMTP procedure 3 months following surgery. For the irradiation studies, additional male C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME) at 4-6 weeks of age. All mice were housed in groups of 5 mice with water and food (PicoLab Rodent Diet 20, #5053; PMI Nutrition International, St. Louis, MO) provided ad libitum, and with a 12h light:12h dark cycle (on 6:00 am, off 6:00 pm). All procedures conformed to the standards of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the Oregon Health and Science University.
Mice received ionizing radiation from a 137Cs source as described, with some modifications (Villasana et al., 2006). Following an i.p. injection of anesthesia (80 mg/kg ketamine (Sigma) and 20 mg/kg xylazine (Sigma), mice were placed in adjustable Plexiglas cylinder tubes (Fisher Scientific) containing multiple holes for breathing. The tubes were vertically placed inside 5 cm thick lead shielding with the back of the mouse head facing the source of the irradiator through a 2 cm diameter hole bored through the lead brick. Dosimetry was performed by putting dosimetry chips (n = 10 in total) in the Plexiglass cylinder tubes near the location of the heads of the mice. The obtained data was then used to calculate the required irradiation time. The mice were sham-irradiated or irradiated at 10 Gy in a Mark 1 Cesium Irradiator (Shepherd and Associates, San Fernando, CA) at a dose rate of 236.8 Rad/min. The cerebellum, eyes, and body were shielded with lead and the experimental procedure was identical between the sham-irradiated (n=10) and irradiated (n=10) animals. The mice were behaviorally tested starting 3 months following irradiation to match the time-course experienced by the castrated and sham operated mice (Benice and Raber, 2009). The castration procedures were detailed previously (Benice and Raber, 2009) in which the mice were tested 3 months following castration.
Delayed Matching to Place Test (DMTP) for Irradiated Mice
This test was used to assess the status of memory for a spatial location after increasing delays between learning and retrieval. All mice were experimentally naive prior to testing and were singly housed beginning 24 hours prior to the first behavioral test, to minimize the potential effects of social influences on behavioral performance. Mice learned to locate a submerged platform in a circular pool of opaque water (144 cm diameter), either by navigating using extra-maze cues located in the room (Hidden Training) or by swimming to a cue attached to the platform (Visible Training). Separate groups of mice underwent hidden training or visible training. In addition, a third group of mice simultaneously received similar amounts of handling as mice that received hidden or visible DMTP testing, however these mice were not tested.
Mice were trained in the DMTP procedure for 2 sessions per day (2-3 hour inter-session interval), each with a different platform location determined in pseudorandom order out of 12 possible platform locations. Each session consisted of a learning trial and a retrieval trial separated by a retention interval. There was a 7-day training phase during which the retention interval was 1 minute. Following the training phase, there was a test phase in which the retention interval was increased to either 1-hour or 24 hours. There were four sessions per retention interval in the test phase. During each trial, mice were placed into the water at one of 5 locations, against the wall of the pool, in a pseudo-random order. They swam for 120 seconds or until they found the platform. If they didn't find the platform within 120 seconds, they were led to it by the experimenter. They were allowed to remain on the platform for 10 seconds.
The swim pattern of the mice during each trial was recorded by a video tracking system (Ethovision v2.3, Noldus Information Technology, Wageningen Netherlands), and the latency to find the platform location, in seconds, was calculated since there was no difference in swim speed between the groups. Spatial memory was assessed by the retention score, which was calculated as the difference between the swim time in the learning trial and the swim time in the retrieval trial, divided by the total swim time for the session. In other words, the retention score reflected the improvement in swim time between the learning and retrieval trials as a percentage of the total swim time during the session. Thus, better spatial memory corresponded to higher retention scores. During the training phase, efficacy of training was assessed by examining the retention score averaged across the last four training sessions (Sandstrom et al., 2006). During the testing phase, the retention scores of each mouse were averaged across all sessions for a given retention interval.
Immunohistochemistry
Within 24 hours of the last DMTP trial mice were deeply anesthetized with a cocktail of 100 mg/kg ketamine, 10 mg/kg xylazine, and 2 mg/kg acepromazine and were then perfused transcardially with PBS followed by 4% paraformaldahyde pH 7.4. Brains were removed and post-fixed overnight in 4% paraformaldahyde at 4°C, transferred to 30% sucrose solution, and were then embedded in cryoprotectant and stored at -80°C until sectioning. Serial, coronal sections (50 μm) were collected through the entire dentate gyrus for each brain and ten to twelve sections per mouse, with a 200 μm inter-section distance comprising the entire dentate gyrus, were collected onto Superfrost microscope slides (Fisher Scientific). Sections were then stained for fluorescent visualization of either DCX, a marker of immature neurons, or Ki-67, a marker of dividing cells (Ming and Song, 2005). Due to the labor-intensive nature of this process, sections were stained in ‘batches’ each comprised of one animal each from the six experimental groups. This insured identical labeling of brain sections between experimental groups as sections from each group in a ‘batch’ received equal treatment. Following an antigen retrieval step according to manufacturer's instructions (H-3000, Vector Laboratories), sections were incubated for 2 hours at room-temperature in a blocking solution (5% normal donkey serum in PBS containing 0.05% Triton X-100 and 0.2% bovine serum albumen (PBT)), followed by incubation overnight at 4°C in either goat-anti-doublecortin (1:200, Santa Cruz Biotechnology) or mouse-anti-Ki67 (1:400, BD Pharmingen) primary antibodies. The DCX antibody shows staining only in neuronal cell lines and the antigenicity colocalizes with GFP expressed under control of the DCX promoter, indicating specificity of the antibody for DCX protein (Karl et al., 2005). Likewise, the Ki-67 antibody staining is completely blocked by preadsoption with a Ki-67 peptide in flow-cytometry experiments, proving specificity for the Ki-67 protein (personal communication from BD BioSciences). Following 4 washes with PBT followed by 1 wash with PBS (10 min each), sections were incubated for 2 hours at room temperature in either donkey-anti-goat-IgG or donkey-anti-mouse-IgG antibodies (both diluted at 1:100) conjugated to Texas-red for fluorescent visualization (Jackson Immunoresearch). Following 4 washes with PBT and 1 wash with PBS (10 min each), the sections were covered with anti-fade solution containing a DAPI counter-stain (Vectashield, Vector Laboratories), and were cover-slipped (Fisher Scientific). Slides were stored in the dark at 4°C until they were imaged.
Confocal Microscopy and Unbiased Stereology Analysis
The total number of DCX+ and Ki-67+ cells in the bilateral granule cell layer (GCL) of the dentate gyrus was estimated, using the optical fractionator technique (West et al., 1991). Brains from a subgroup of mice that received either hidden DMTP training (sham-operated n = 4, castrated n = 3), visible DMTP training (sham-operated n = 4, castrated n = 4), or handling only (sham-operated n = 4, castrated n = 4) were selected at random for analysis. Due to higher inter-subject variability in the number of Ki-67+ cells, an additional 2 subjects were counted in each DMTP training group. For DCX, eight to ten stacks of 5 images each (dissectors), with a 2 μm inter-image distance, were taken bilaterally within the GCL of each brain section. Images were collected using the 40× objective lens of an Olympus spinning disc confocal microscope (IX81, Olympus Imaging Corp.), equipped with Slidebook software (Intelligent Imaging Solutions). A square counting frame (75 μm × 75 μm) was laid over the computer screen with the GCL visible within, and cells were counted when they appeared within the frame in one image of the 5-image stack but not in the preceding image. DCX+ cells were counted only when the staining appeared surrounding a distinctly labeled DAPI counter-stained nucleus. This way, a total of 80-120 individual dissectors were counted within each brain to obtain an unbiased and accurate average density of DCX+ cells/mm3 within the GCL of each mouse. Due to the very low density of Ki-67+ cells observed in each section, the total number of cells was counted bilaterally. Ki-67 cells were counted when the nuclear Ki-67 stain overlapped a DAPI counter-stained nucleus. Thus, the entire GCL for each section constituted the dissectors for approximating the density of Ki-67+ cells.
The Cavalieri principle (West et al., 1991) was used to estimate the total volume of the GCL, by outlining the DAPI counter-stained GCL in the Slidebook software to obtain the GCL area in each section bilaterally. The sum of these areas was multiplied by the inter-section distance (200 μm) and the total number of cells was then obtained by multiplying the average density measurement by the total volume estimate.
Statistical Analysis
The total number of DCX+ cells, total number of Ki-67+ cells, and total volume estimate were analyzed using two-way ANOVA to measure the effects of castration and DMTP training type. For the DMTP procedure, two-way mixed ANOVA was used to analyze the effects of irradiation (between-subjects factor) and retention interval (within-subjects factor) on retention scores. Duncan's post-hoc tests, which apply corrections for multiple comparisons, were used to assess differences between individual groups where appropriate. Pearson's correlation analysis was used to examine the relationships between DMTP performance measures and new neuron production markers. All statistical analyses were performed using SPSS software (SPSS Inc., Chicago, IL), and p < 0.05 was considered significant for all tests.
Acknowledgments
This work was supported by: NIH;R01-AG20904, NIH;T32-NS007466-05, NIH;5T32AG023477-02, NIH;RR-00163, NASA;NNJ05HE63G, EMF;AG-NS-0201, Alzheimer's Association IIRG-05-14021
Abbreviations
- GCL
Granule cell layer of the hippocampus
- DMTP
Delayed matching to place task
- DCX
Doublecortin
- NMDAR
N-methyl-d-aspartate receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Absil P, Pinxten R, Balthazart J, Eens M. Effect of age and testosterone on autumnal neurogenesis in male European starlings (Sturnus vulgaris) Behavioural Brain Research. 2003;143:15–30. doi: 10.1016/s0166-4328(03)00006-8. [DOI] [PubMed] [Google Scholar]
- Alaei H, Moloudi R, Sarkaki AR, Azizi-Malekabadi H, Hanninen O. Daily running promotes spatial learning and memory in rats. Journal of Sports Science and Medicine. 2007;6:429–433. [PMC free article] [PubMed] [Google Scholar]
- Benice TS, Raber J. Dihydrotestosterone modulates spatial working-memory performance in male mice. Journal of Neurochemistry. 2009 doi: 10.1111/j.1471-4159.2009.06183.x. In Press. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Nelson ME, Eckman CB, Barber J, Scott TY, Granholm AC. Testosterone, but not nonaromatizable dihydrotestosterone, improves working memory and alters nerve growth factor levels in aged male rats. Experimental Neurology. 2003;181:301–12. doi: 10.1016/s0014-4886(03)00061-x. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Lee HJ, Gallagher M. Neurogenesis in a rat model of age-related cognitive decline. Aging Cell. 2004;3:217–234. doi: 10.1111/j.1474-9728.2004.00099.x. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Hazel TG, McKay RD. Regulation of neurogenesis by growth factors and neurotransmitters. J Neurobiol. 1998;36:287–306. [PubMed] [Google Scholar]
- Daniel JM, Winsauer PJ, Moerschbaecher JM. Castration in rats impairs performance during acquisition of a working memory task and exacerbates deficits in working memory produced by scopolamine and mecamylamine. Psychopharmacology (Berl) 2003;170:294–300. doi: 10.1007/s00213-003-1537-4. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Hamilton DA, Yeo RA, Brooks WM, Sutherland RJ. Virtual navigation in humans: the impact of age, sex, and hormones on place learning. Hormones & Behavior. 2005;47:326–35. doi: 10.1016/j.yhbeh.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Duva CA, Floresco SB, Wunderlich GR, Lao TL, Pinel JP, Phillips AG. Disruption of spatial but not object-recognition memory by neurotoxic lesions of the dorsal hippocampus in rats. Behavioral Neuroscience. 1997;111:1184–1196. doi: 10.1037//0735-7044.111.6.1184. [DOI] [PubMed] [Google Scholar]
- Frye CA, Park D, Tanaka M, Rosellini R, Svare B. The testosterone metabolite and neurosteroid 3alpha-androstanediol may mediate the effects of testosterone on conditioned place preference. Psychoneuroendocrinology. 2001;26:731–50. doi: 10.1016/s0306-4530(01)00027-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Seliga AM. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cognitive, Affective & Behavioral Neuroscience. 2001;1:371–81. doi: 10.3758/cabn.1.4.371. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Spritzer MD, Barker JM, Pawluski JL. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16:225–32. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- Gerlai RT, McNamara A, Williams S, Phillips HS. Hippocampal dysfunction and behavioral deficit in the water maze in mice: an unresolved issue? Brain Res Bull. 2002;57:3–9. doi: 10.1016/s0361-9230(01)00630-x. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nature Neuroscience. 1999;2:260–5. doi: 10.1038/6365. see comment. [DOI] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR. Prenatal gonadal steroids affect adult spatial behavior, CA1 and CA3 pyramidal cell morphology in rats. Horm Behav. 1998;34:183–98. doi: 10.1006/hbeh.1998.1477. [DOI] [PubMed] [Google Scholar]
- Karl C, Couillard-Despres S, Prang P, Munding M, Kilb W, Brigadski T, Plotz S, Mages W, Luhmann H, Winkler J, Bogdahn U, Aigner L. Neuronal precursor-specific activity of a human doublecortin regulatory sequence. J Neurochem. 2005;92:264–82. doi: 10.1111/j.1471-4159.2004.02879.x. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, Semba R, Kato H, Ueno M, Arakawa Y, Kato K. Regulation by androgen of levels of the beta subunit of nerve growth factor and its mRNA in selected regions of the mouse brain. Journal of Neurochemistry. 1994;62:2141–7. doi: 10.1046/j.1471-4159.1994.62062141.x. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Annals of Neurology. 2002;52:135–43. doi: 10.1002/ana.10262. see comment. [DOI] [PubMed] [Google Scholar]
- Kresnor RP. A behavioral analysis of dentate gyrus function An attractor network in the hippocampus: theory and neurophysiology. Prog Brain Res. 2007;163:567–76. doi: 10.1016/S0079-6123(07)63030-1. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, McLaughlin PJ, Smirlis T, Robinson JK. Gonadectomy impairs T-maze acquisition in adult male rats. Hormones & Behavior. 2001;39:167–74. doi: 10.1006/hbeh.2001.1645. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Fischer SJ, Eisch AJ. Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus. 2007;17:175–80. doi: 10.1002/hipo.20265. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–75. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Leonard ST, Moerschbaecher JM, Winsauer PJ. Testosterone potentiates scopolamine-induced disruptions of nonspatial learning in gonadectomized male rats. Exp Clin Psychopharmacol. 2007;15:48–57. doi: 10.1037/1064-1297.15.1.48. [DOI] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23:1588–92. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, MacLusky NJ. Androgens Increase Spine Synapse Density in the CA1 Hippocampal Subfield of Ovariectomized Female Rats. The Journal of Neuroscience. 2004a;24:495–499. doi: 10.1523/JNEUROSCI.4516-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Prange-Kiel J, Frick KM, Horvath TL. Low CA1 spine synapse density is further reduced by castration in male non-human primates. Cerebral Cortex. 2004b;14:503–10. doi: 10.1093/cercor/bhh012. [DOI] [PubMed] [Google Scholar]
- Luo CX, Jiang J, Zhou QG, Zhu XJ, Wang W, Zhang ZJ, Han X, Zhu DY. Voluntary exercise-induced neurogenesis in the postischemic dentate gyrus is associated with spatial memory recovery from stroke. J Neurosci Res. 2007;85:1637–46. doi: 10.1002/jnr.21317. [DOI] [PubMed] [Google Scholar]
- Mayer JL, Klumpers L, Maslam S, de Kloet ER, Joels M, Lucassen PJ. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalises the corticosterone-induced reduction of adult hippocampal neurogenesis. J Neuroendocrinol. 2006;18:629–31. doi: 10.1111/j.1365-2826.2006.01455.x. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Linkroum W, Sallinen BJ, Miller NW. Peripheral and central sex steroids have differential effects on the HPA axis of male and female rats. Stress. 2002;5:235–47. doi: 10.1080/1025389021000061165. [DOI] [PubMed] [Google Scholar]
- Mello PB, Benetti F, Cammarota M, Izquierdo I. Effects of acute and chronic physical exercise and stress on different types of memory in rats. An Acad Bras Cienc. 2008;80:301–9. doi: 10.1590/s0001-37652008000200008. [DOI] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–31. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- Ming Gl, Song H. Adult neurogenesis in the mammalian central nervous system. Annual Review of Neuroscience. 2005;28:223–50. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–8. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Noiman L, Gould E. Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proc Natl Acad Sci U S A. 2006;103:19170–5. doi: 10.1073/pnas.0608644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapel P, Mundt-Petersen K, Brundin P, Frielingsdorf H. Working memory training decreases hippocampal neurogenesis. Neuroscience. 2006;142:609–13. doi: 10.1016/j.neuroscience.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Fujita H, Kawata M. Effects of gonadectomy on immunoreactivity for choline acetyltransferase in the cortex, hippocampus, and basal forebrain of adult male rats. Neuroscience. 2002;109:473–85. doi: 10.1016/s0306-4522(01)00513-9. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Galea LA. Reproductive status influences the survival of new cells in the dentate gyrus of adult male meadow voles. Neurosci Lett. 2003;346:25–8. doi: 10.1016/s0304-3940(03)00546-9. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Lee TTY, Galea LAM. Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rats. Journal of Neurobiology. 2003;55:247–60. doi: 10.1002/neu.10181. [DOI] [PubMed] [Google Scholar]
- Pouliot WA, Handa RJ, Beck SG. Androgen modulates N-methyl-D-aspartate-mediated depolarization in CA1 hippocampal pyramidal cells. Synapse. 1996;23:10–9. doi: 10.1002/(SICI)1098-2396(199605)23:1<10::AID-SYN2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, Koopmans G, Blokland A, Scheepens A. Learning and adult neurogenesis: survival with or without proliferation? Neurobiology of Learning & Memory. 2004;81:1–11. doi: 10.1016/j.nlm.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Raber J, Bongers G, LeFevour A, Buttini M, Mucke L. Androgens protect against apolipoprotein E4-induced cognitive deficits. Journal of Neuroscience. 2002;22:5204–9. doi: 10.1523/JNEUROSCI.22-12-05204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Fan Y, Matsumori Y, Liu Z, Weinstein PR, Fike JR, Liu J. Irradiation attenuates neurogenesis and exacerbates ischemia-induced deficits. Annals of Neurology. 2004a;55:381–9. doi: 10.1002/ana.10853. [DOI] [PubMed] [Google Scholar]
- Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiation Research. 2004b;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Experimental Neurology. 2004;188:316–30. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Staub D, Jasnow AM, Karatsoreos IN, Thornton JE, McEwen BS. Dihydrotestosterone increases hippocampal N-methyl-D-aspartate binding but does not affect choline acetyltransferase cell number in the forebrain or choline transporter levels in the CA1 region of adult male rats. Endocrinology. 2005;146:2091–7. doi: 10.1210/en.2004-0886. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Kaufman J, Huettel SA. Males and females use different distal cues in a virtual environment navigation task. Cognitive Brain Research. 1998;6:351–60. doi: 10.1016/s0926-6410(98)00002-0. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Kim JH, Wasserman MA. Testosterone modulates performance on a spatial working memory task in male rats. Hormones & Behavior. 2006;50:18–26. doi: 10.1016/j.yhbeh.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MV, Kandel ER, Hen R. Paradoxical influence of hippocampal neurogenesis on working memory. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4642–6. doi: 10.1073/pnas.0611718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–56. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Shi L, Adams MM, Long A, Carter CC, Bennett C, Sonntag WE, Nicolle MM, Robbins M, D'Agostino R, Brunso-Bechtold JK. Spatial learning and memory deficits after whole-brain irradiation are associated with changes in NMDA receptor subunits in the hippocampus. Radiat Res. 2006;166:892–9. doi: 10.1667/RR0588.1. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Memory traces of trace memories: neurogenesis, synaptogenesis and awareness. Trends in Neurosciences. 2004;27:250–6. doi: 10.1016/j.tins.2004.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PD, McLean KJ, Murphy MA, Turnley AM, Cook MJ. Seizures, not hippocampal neuronal death, provoke neurogenesis in a mouse rapid electrical amygdala kindling model of seizures. Neuroscience. 2005;136:405–15. doi: 10.1016/j.neuroscience.2005.07.055. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–52. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Galea LAM. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev Neurobiol. 2007;67:1321–33. doi: 10.1002/dneu.20457. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Gill M, Weinberg A, Galea LA. Castration Differentially Affects Spatial Working and Reference Memory in Male Rats. Arch Sex Behav. 2007 doi: 10.1007/s10508-007-9264-2. [DOI] [PubMed] [Google Scholar]
- Sumner BE, Fink G. Testosterone as well as estrogen increases serotonin2A receptor mRNA and binding site densities in the male rat brain. Brain Res Mol Brain Res. 1998;59:205–14. doi: 10.1016/s0169-328x(98)00148-x. [DOI] [PubMed] [Google Scholar]
- Toda H, Hamani C, Fawcett AP, Hutchison WD, Lozano AM. The regulation of adult rodent hippocampal neurogenesis by deep brain stimulation. J Neurosurg. 2008;108:132–8. doi: 10.3171/JNS/2008/108/01/0132. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience. 1999;2:266–70. doi: 10.1038/6368. see comment. [DOI] [PubMed] [Google Scholar]
- Villasana L, Acevedo S, Poage C, Raber J. Sex- and APOE isoform-dependent effects of radiation on cognitive function. Radiat Res. 2006;166:883–91. doi: 10.1667/RR0642.1. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anatomical Record. 1991;231:482–97. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Askew ML, Winocur G. The effects of running and of inhibiting adult neurogenesis on learning and memory in rats. Eur J Neurosci. 2008;27:1494–502. doi: 10.1111/j.1460-9568.2008.06128.x. [DOI] [PubMed] [Google Scholar]
- Wong EY, Herbert J. Raised circulating corticosterone inhibits neuronal differentiation of progenitor cells in the adult hippocampus. Neuroscience. 2006;137:83–92. doi: 10.1016/j.neuroscience.2005.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier GF, Oliveira-Filho FJ, Santos AM. Dentate gyrus-selective colchicine lesion and disruption of performance in spatial tasks: difficulties in “place strategy” because of a lack of flexibility in the use of environmental cues? Hippocampus. 1999;9:668–81. doi: 10.1002/(SICI)1098-1063(1999)9:6<668::AID-HIPO8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Zhao M, Li D, Shimazu K, Zhou YX, Lu B, Deng CX. Fibroblast growth factor receptor-1 is required for long-term potentiation, memory consolidation, and neurogenesis. Biol Psychiatry. 2007;62:381–90. doi: 10.1016/j.biopsych.2006.10.019. [DOI] [PubMed] [Google Scholar]


