Abstract
Chronic airway inflammation is a cardinal feature of chronic obstructive pulmonary disease (COPD), a destructive cigarette smoke-induced lung disease. Although it is apparent that dendritic cells (DCs) are an important constituent of the chronic inflammatory cell influx found in airways of COPD patients, the functional roles of DCs in the pathogenesis of smoking-induced emphysema are unknown. We postulated that DCs activated by cigarette smoke constituents directly participate in the chronic inflammation that characterizes COPD airways. Concordant with this hypothesis, we observed that incubation of DCs with cigarette smoke extract (CSE), and chronic exposure of mice to cigarette smoke, both augmented the generation of neutrophilic chemokines by immature and lipopolysaccharide (LPS) or CD40L-matured DCs. The generation of interleukin-8 (CXCL8/IL-8) by human DCs conditioned with CSE was suppressed by the anti-oxidant n-acetyl cysteine (NAC), implying the involvement of oxidant sensitive pathways as a primary mechanism involved in the enhanced CXCL8/IL-8 generation. Cigarette smoke extract and nicotine also augment the production of secreted prostaglandin E2 and intracellular cyclo-oxygenase-2 (COX-2) in maturing DCs. Whereas NAC suppressed production of CXCL8 by CSE-conditioned DCs, it augmented production of PGE2 and cellular COX-2 levels in maturing DCs. These studies indicate that the stimulation of DCs by cigarette smoke-induced oxidative stress and nicotine promote the generation of pro-inflammatory responses that promote chronic inflammation in smokers. Certain pharmacologic strategies such as anti-oxidant therapy may be only partially effective in mitigating cigarette smoke-induced pro-inflammatory DC-mediated responses in smokers.
Keywords: Smoking, dendritic cell, oxidative stress, neutrophil, chemokines, prostaglandins
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of chronic morbidity and is the fifth leading cause of death worldwide (Rabe et al., 2007). In the West, the disease is almost always caused by cigarette smoking, which induces chronic airway inflammation associated with irreversible airflow limitation and progressive decline in lung function (Barnes, 2003). The chronically inflamed airways of COPD patients contain several inflammatory cells that persist even in the absence of overt infection (Barnes, 2003). Among the cells know to infiltrate and persist in the lungs of COPD patients are neutrophils, macrophages, B and T lymphocytes, and dendritic cells (DCs) (Barnes, 2003; Demedts et al., 2007). The specific triggers that lead to recruitment and retention of these inflammatory cells are not entirely clear. In addition, the potential roles of inflammatory cells in the lungs of smokers in the development of COPD are not very well defined. Cigarette smokers are also predisposed to develop lung cancer, and COPD itself is an independent risk factor for lung cancer development (Turner et al., 2007), suggesting that smoking-induced chronic lung inflammation may promote tumor growth as well as COPD.
Lung DCs are potent antigen presenting cells distributed in an intra and sub-epithelial location in small airways and have the capacity to profoundly influence both innate and acquired immunity in the lung (Lambrecht et al., 2001). A recent study described significant increase in DC numbers in the small airways of patients with COPD, and found a positive correlation between DC infiltration and disease severity, suggesting a direct pathogenic role for these cells in the COPD lung (Demedts et al., 2007). Potential mechanisms by which DCs may participate in the chronic airway inflammation in COPD are not well understood. In prior studies, we demonstrated that cigarette smoke constituents suppress Th-1 stimulatory functions of DCs while preserving the capacity of DCs to generate interleukin-6 and interleukin-10 upon maturation with lipopolysaccharide (LPS) (Vassallo et al., 2005). The current study was designed to determine mechanisms by which cigarette smoke constituents activate DCs to produce soluble factors that promote local inflammation and immune-suppression in the airways. We sought to identify molecular mechanisms by which DC-mediated chronic inflammation induced by cigarette smoke constituents not only predisposes to chronic airway inflammation, but may additionally promote lung cancer development though epithelial cell transformation. We postulated that cigarette smoke induces the generation of the neutrophilic chemokine CXCL8 / IL-8, a central mediator of neutrophil recruitment in human airways (Baggiolini et al., 1989). We also postulated that cigarette smoke constituents induce the production of prostaglandin-E2 (PgE2), an arachidonic acid metabolite that profoundly influences DC function, and has been associated with epithelial cell transformation, an important step in new cancer formation (Yoshimatsu et al., 2001). Furthermore, we postulated that nicotinic stimulation and oxidative stress induced by cigarette smoke to be central mechanisms by which these pro-inflammatory mediators are generated in the airways of smokers, and utilized relevant models and inhibitors to define the relative contribution of oxidative stress and nicotinic stimulation on DCs in inflammatory cell recruitment and prostaglandin generation.
2. Materials and Methods
2.1. General reagents
Mecamylamine hydrochloride, N-acetyl cysteine (NAC), and bovine catalase were purchased from Sigma biochemicals. CD11c+ magnetic beads were purchased from Miltenyi Biotech. Human recombinant CD40L was purchased from Axxora Platform Biochemicals. Purified hamster anti-mouse CD40 ligating antibody (clone HM40) was purchased from BD Biosciences. Recombinant human and murine IFN-γ and IL-4 were obtained from R&D Systems, while recombinant human GM-CSF was obtained from Immunex. Nicotine was obtained from Sigma (catalog number 72290). Polymyxin B was purchased from Sigma biochemicals.
2.2. Generation of cigarette smoke extract (CSE)
Aqueous CSE was prepared from Kentucky research cigarettes 1RF4 as recently described (Vassallo et al., 2005). The nicotine levels in the CSE preparations were measured in the institutional clinical laboratory using liquid chromatography-tandem mass spectrometry: the mean nicotine content in 4 separate samples of 100% CSE was 21,460±2778 ng/ml. The final concentration of CSE used in the experiments described was 1–2% - final nicotine concentration in vitro of 1% CSE was equal to 214.6±27.8 ng/ml. The concentrations of <3% were chosen because of preliminary viability studies that demonstrated a lack of non-specific toxicity and >95% viability compared with controls, as determined by the XTT assay and AnnexinV/propridium iodide staining with these CSE preparations (Vassallo et al., 2005).
2.3. Human monocyte-derived DCs
Human monocytes were isolated from buffy coats obtained from healthy non-smoking adult blood donors following approval from the institutional review board. Monocytes were isolated using depleting antibody cocktails (StemCell laboratories) and monocyte-derived DCs were generated with GM-CSF and IL-4 as previously described (Vassallo et al., 2005). Maturation was induced by 18-hour culture with 100 ng/ml LPS [from E. coli; Sigma] or 1µg/ml soluble recombinant human CD40L [Axxora Platform].
2.4. Measurement of cytokines
Human CXCL8/IL-8 levels in supernatants were measured using commercially available ELISA according to the manufacturer’s instructions [Diaclone]. Murine KC and MIP-2 were measured using ELISA obtained from R&D Systems according to manufacturer instructions. Human IL-10 and IL-12p40 levels in supernatants were measured using a commercially available ELISA from eBioscience.
2.5. Flow cytometric analysis of CD86 experession on human monocyte-derived DCs
Flow cytometry was used to determine the expression surface CD86 on immature, CSE-stimulated and LPS-matured human DCs. FITC-conjugated anti-CD-86 was obtained from BD Pharmingen. Before staining with anti-CD86, DCs were incubated with an excess of human IgG (20 µg per sample) (Sigma-Aldrich) for 30 min to block nonspecific binding. Staining was performed on ice for 30 min. Ten thousand cells were acquired for each sample, and dead cells were gated out on the basis of light scatter properties. Acquisition and analysis of data was performed on a FACScan (BD Pharmingen).
2.6. Measurement of cellular COX-2
Human immature monocyte-derived DCs (day 6–7) were plated at a density of 1×106/ml in complete media [RPMI, 10% fetal bovine serum] and GM-CSF/IL-4 as described above. Cigarette smoke extract, nicotine, LPS (100ng/ml) and NAC (2.5mM) were added to the cells at the time points indicated. Protein lysates were prepared using RIPA buffer (150 mM NaCl, 1.0% Igepal CA-630, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris). Protein concentrations in respective extracts were determined using a commercially available Bradford assay (Pierce) referenced against an albumin standard. Cellular COX-2 protein levels were determined in equal amounts of whole cell protein lysates using a commercially available COX-2 ELISA (Assay Designs).
2.7. In vivo exposure of mice to cigarette smoke and isolation of murine lung and systemic DCs
In vivo studies were performed to determine the effect of chronic cigarette smoking on lung DC cytokine and PGE2 production. The system used to expose mice to cigarette smoke was the Teague TE-2 system (Witschi et al., 2000). This is a manually-controlled cigarette smoking machine that produces a combination of side-stream and mainstream cigarette smoke in a chamber, which is then transported to a collecting and mixing chamber where varying amounts of air is mixed with the smoke mixture (Witschi et al., 2000). The cigarette smoke/air mixture is then infused into a closed chamber containing mice. The mixture of air and cigarette smoke is manually regulated based on assessment of total suspended particulates captured in filters connected to the chamber [49.7 ± 3.7 mg/m3]. In this model, mice were exposed to regulated concentrations of cigarette smoke generated from 2 cigarettes every 10 minutes for three 45-minute periods each day, 5 days/week for a total of 4–8 weeks. In this model, mice do not require restraint and are very tolerant of the exposure to cigarette smoke. Inhalation of smoke by the mice was monitored by measuring serum nicotine levels at the time of sacrifice. Following 4–8 weeks in the smoking chamber, mice were sacrificed; blood was removed by right heart puncture and submitted for nicotine analyses, and lungs removed by dissection. A mean blood nicotine level of 112 ± 46 ng/ml was attained compared with undetectable levels in the controls at the time of sacrifice (7 separate experimental groups of mice: blood nicotine measured in the Mayo institutional clinical laboratory). Lung tissue was placed in RPMI, dissected [<2mm in size], digested with collagenase (Liberase Blendzyme type 3 from Roche used as a final concentration of 0.2 Units per ml of RPMI) and DNAse (Bovine Pancreatic DNAse from StemCell, final concentration 25µg/ml) for 45–60 minutes at 37°C, and CD11c positive lung DCs were isolated using CD11c+ magnetic beads over a magnetic column according to the manufacturers instructions (Miltenyi Biotech). Equal numbers of CD11c+ lung DCs from cigarette smoke (CS) and age-matched control mice were matured in complete media (RPMI supplemented with 10% FCS) for 18 hours with LPS (100ng/ml) or CD40 ligating antibody (HM40 – 1µg/ml in the presence of 50ng/ml murine recombinant IFNγ). An identical procedure was used to isolate splenic DCs, with the only variation that the collagenase concentration used was half that used in the lung DC preparation.
2.8. Statistical Analysis
All data are shown as the mean ± standard error. Comparisons between means were made using student’s t-test or ANOVA and Dunnett’s test were relevant. Statistical differences were considered to be significant if P was < 0.05. Statistical analysis was performed using GraphPad Prism 5 software.
3. Results
3.1. Cigarette smoke extract stimulates CXCL8 release from immature and maturing DCs
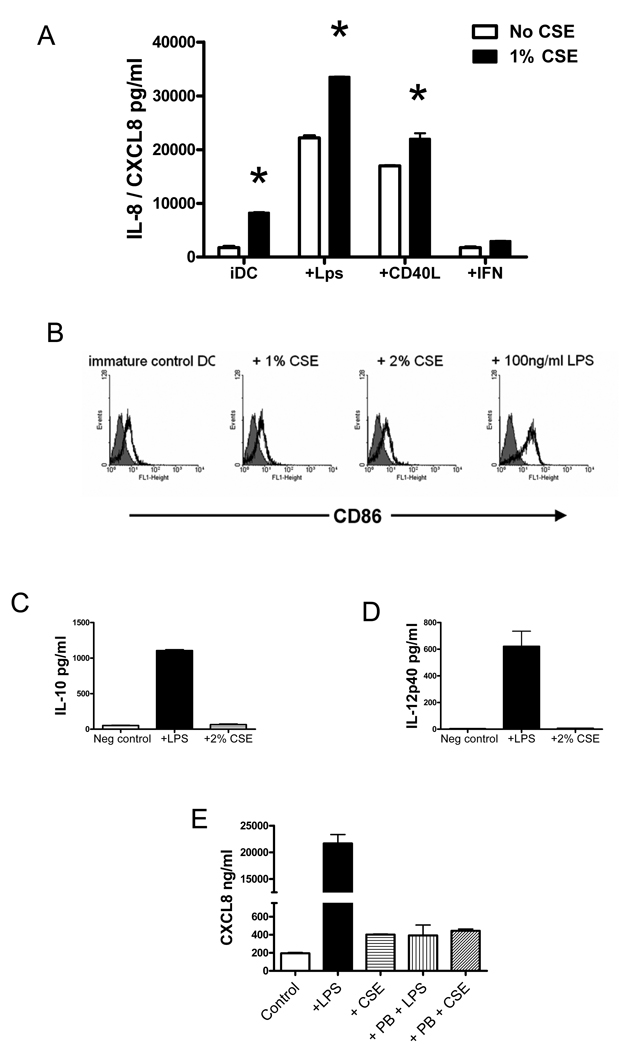
Lung DCs are distributed in sub and intra-epithelial regions of the airways, where neutrophilic influx occurs in COPD (Demedts et al., 2007). To determine whether cigarette smoke induces neutrophilic chemokine production from DCs, human monocyte-derived DCs were incubated with CSE, in the presence or absence of either 100ng/ml LPS, or 1µg/ml recombinant human CD40L as maturational agents. In these experiments, a CSE concentration of 1 – 2% was selected because prior studies demonstrated non-specific toxicity (cell death) in DCs exposed to CSE levels >3% (Vassallo et al., 2005). Following 18 hours of incubation, CXCL8/IL-8 levels produced by immature or maturing DCs were measured in the supernatants using ELISA. Cigarette smoke extract induced CXCL8/IL-8 from immature DCs and significantly augmented CXCL8/IL-8 production from maturing DCs (Figure 1A). Immature DCs cultured with GM-CSF and IL-4 produce a significant amount of basal CXCL8/IL-8 (Figure 1A). In the presence of 1% CSE, the amount secreted increased from 1.74 ± 0.2 to 8.3 ± 0.1 ng/ml (Figure 1A, p<0.01). As expected, maturation of DCs with LPS or CD40L substantially augmented CXCL8/IL-8 production when compared with immature DCs (Figure 1A), and the addition of 1% CSE significantly augmented CXCL8/IL-8 generation when compared with DC matured in the absence of CSE (Figure 1A, p<0.01). Contamination with bacterial endotoxin, a potent activator of DCs and inducer of CXCL8/IL-8 production, is highly unlikely to be the cause of the observed induction in CXCL8/IL-8. In parallel experiments and previously published data (Vassallo et al., 2005), we failed to observe other effects on DC function that would be expected if the active component in CSE were endotoxin (published in (Vassallo et al., 2005)). For instance, stimulation of immature DCs for 24 hours with CSE concentrations that significantly induce CXCL8/IL-8 production, failed to induce the expression of the co-stimulatory molecule CD86, which is abundantly upregulated in the presence of LPS (Figure 1B). Similarly, incubation of immature DCs with 2% CSE for 18 hours failed to induce either IL-10 or IL-12p40 cytokines compared with control DCs, whereas 100ng/ml LPS was a potent inducer of both cytokines (Figure 1 C and D). To definitively rule out the potential of LPS contamination as a source of CXCL8/IL-8 generation by CSE-stimulated DCs, immature DCs were incubated with either 2% CSE or 100ng/ml of LPS for 18 hours in the presence or absence of 10µg/ml of Polymyxin B, a potent antagonist of LPS (Jacobs and Morrison, 1977). Whereas Polymyxin B inhibited CXCL8/IL-8 generation from LPS-stimulated DCs by greater than 95% (Figure 1E - 21667±2887 vs 392.0±0.04 pg/ml by control DCs compared with Polymyxin-treated DCs respectively; p<0.001 by ANOVA), the production of CXCL8/IL-8 generation from CSE-stimulated DCs was not suppressed (401.8±10.31 vs 443.7±32.31 pg/ml for control DCs compared with Polymyxin-treated DCs; p=NS by ANOVA). Taken together these data convincingly demonstrate that CSE stimulates CXCL8/IL-8 generation from both immature and maturing human DCs employing a mechanism of action that cannot be explained by LPS contamination.
Figure 1. Cigarette smoke extract stimulates CXCL8 release from immature and maturing DCs.
A) Human monocyte-derived DCs were incubated with (black bars) or without (open bars) 1% CSE, in the presence or absence of either 100ng/ml LPS, or 1µg/ml recombinant human CD40L for 18 hours. Supernatants were collected and CXCL8/IL-8 levels were measured using ELISA. *p<0.01 using ANOVA. Data shown is representative of three independent experiments. B) Human monocyte-derived DCs were incubated with CSE or LPS as indicated for 18 hours. Cells were stained using FITC-conjugated anti-human CD86 antibodies (open black histograms) or control non-immune FITC-conjugated antibody (gray histogram) as a control. Data is representative of 3 independent experiments. C) Human monocyte-derived DCs were incubated with either 100ng/ml LPS or 2% CSE for 18 hours. IL-10 levels were measured in supernatants using ELISA. D) Human monocyte-derived DCs were incubated with either 100ng/ml LPS or 2% CSE for 18 hours. IL-12p40 levels were measured in supernatants using ELISA. Data shown in C and D are representative of 2 independent experiments. E) Human monocyte-derived DCs were incubated with 2% CSE, 100ng/ml LPS, and either CSE or LPS in combination with 10ug/ml of Polymyxin B (PB). Following 18 hours of incubation, CXCL8/IL-8 levels were measured in supernatants using ELISA.
3.2. Oxidative stress, rather than nicotinic stimulation, is the prevalent mechanism by which CSE induces CXCL8/IL-8 in DCs
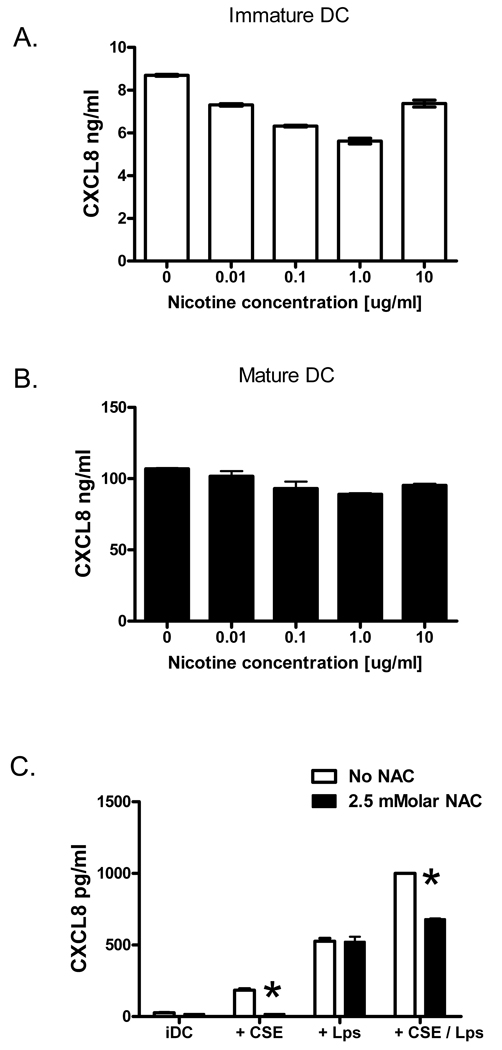
The observation that CSE promotes CXCL8/IL-8 production by immature and maturing DCs led us to search for specific cigarette smoke constituents capable of inducing chemokine generation. Since the alkaloid nicotine is a pharmacologically active component of CSE, we first tested if nicotine concentrations attained in CSE (or within the range of clinically-relevant nicotine levels (Lawson et al., 1998)) could stimulate CXCL8/IL-8 generation by human DCs. To determine this, immature human DCs were incubated with 0.01–10 µg/ml nicotine for 12–18 hours, and CXCL8 levels were subsequently determined by ELISA. Stimulation of immature DCs with a broad range of nicotine concentrations failed to induce CXCL8 production (Figure 2A). Indeed, rather that induce CXCL8/IL-8, treatment of DCs with nicotine concentrations of 0.01ug/ml or higher resulted in a small but statistically significant reduction in chemokine generation (Figure 2A, p<0.05 by Dunnett’s multiple comparison test). Nicotine also failed to augment CXCL8 production by maturing DCs (Figure 2B), suggesting that the observed induction of CXCL8 by CSE-stimulated immature and maturing DCs (Figure 1) could not be explained by the nicotine content in CSE. Cigarette smoke and CSE are also potent sources of oxidative stress, a constituent of cigarette smoke that has been demonstrated to induce inflammatory cytokine generation from a number of cell types (Yang et al., 2006). To determine whether oxidative stress is indeed responsible for the induction of CXCL8/IL-8 by CSE from human DCs, we determined the effect of pre-treating DCs in vitro with NAC, a potent anti-oxidant, prior to the addition of CSE and DC-activating factors. N-acetyl-cysteine (2.5mM) was added to the DC culture 60 minutes prior to the addition of either 2% CSE, 100ng/ml of LPS, or a combination of CSE and LPS (Figure 2C). Cigarette smoke extract induced a significant increase in CXCL8/IL8 production by immature DCs (Figure 2C, 27.3 ± 0.4 vs 183.9 ± 14.9 pg/ml of CXCL8 respectively, p<0.001). Pre-incubation with NAC effectively inhibited the CSE-induced CXCL8 production by DCs (Figure 2C, p<0.001). Pre-treatment of DCs with 2.5mM NAC also significantly suppressed CXCL8 production by DCs stimulated with a combination of LPS and 2% CSE (Figure 2C, p<0.001). In contrast, pre-treatment of DCs with NAC failed to suppress CXCL8/IL-8 generation by LPS-activated DCs. Taken together, these data imply that oxidative stress induced by CSE is an important mechanism by which CXCL8/IL-8 is induced in immature and maturing DCs, whereas nicotinic receptor stimulation is not.
Figure 2. Oxidative stress, rather than nicotinic stimulation, is the prevalent mechanism by which CSE induces CXCL8/IL-8 in DCs.
A) Human DCs were stimulated with nicotine (0–10ug/ml) for 18 hours, and CXCL8 levels were subsequently determined in the supernatants by ELISA. B) Human DCs were matured with 100ng/ml LPS while simultaneously treated with nicotine (0–10ug/ml) for 18 hours. CXCL8 levels were measured by ELISA in supernatants. C) Human DCs were pre-incubated with NAC [2.5mM] 60 minutes prior to the addition of either 2% CSE, 100ng/ml of LPS, or a combination of CSE and LPS. CXCL8 levels were measured in supernatants using ELISA. *p<0.001 with ANOVA. Figures A, B and C are representative of 3 independent observations.
3.3. Cigarette smoke extract induces PGE2 production and intra-cellular COX-2 levels in maturing DCs
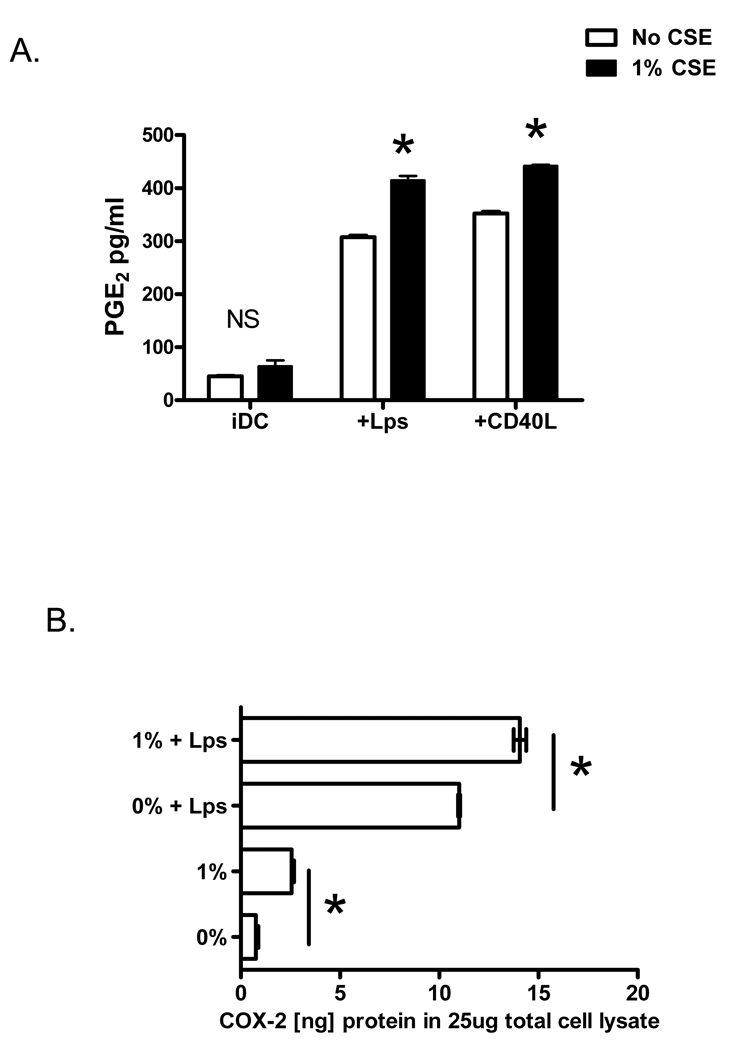
Prostaglandin-E2, an arachidonic acid metabolite that profoundly influences DC function and promotes inflammatory lung responses, is found in elevated concentrations in the exhaled breath of patients with COPD (Montuschi et al., 2003). To determine whether cigarette smoke alters PGE2 production by maturing DCs, immature and LPS or CD40L-matured human DCs were incubated for 18 hours in the presence or absence of CSE. When immature DCs were stimulated with 1% CSE, secreted PGE2 levels were unchanged compared to control DCs (Figure 3A). Production of PGE2 was however augmented from DCs matured with LPS or stimulation of the CD40 receptor by CD40L (Figure 3A, p<0.001). Cyclooxygenase-2 (COX-2) is the inducible, rate-limiting enzyme involved in prostaglandin synthesis (Krysan et al., 2006). To determine the effect of CSE on DC COX-2 protein, human immature and maturing DCs were incubated with CSE for 6 hours, placed on ice, and whole cell lysates were prepared. Quantitative determination of cellular COX-2 was performed on equal amounts of total cell protein using a COX-2 specific ELISA. Consistent with the observation that CSE induces DC secreted PGE2, a significant increase in cellular COX-2 protein levels was observed in CSE-stimulated maturing DCs (Figure 3B, p<0.001). Interestingly, although CSE did not induce PGE2 by immature DCs, CSE induced a small but statistically significant increase in cellular COX-2 protein in immature DCs (Figure 3B, p<0.001).
Figure 3. Cigarette smoke extract induces PGE2 production and intra-cellular COX-2 levels in maturing DCs.
Immature and LPS (100ng/ml) or CD40L-matured (1ug/ml) human DCs were incubated for 18 hours in the presence (black bars) or absence (open bars) of 1% CSE. Secreted PGE2 levels in the supernatants were measured using ELISA. *p<0.001 with ANOVA. Data is representative of 3 independent experiments. B) Human immature and LPS-matured (100ng/ml) DCs were incubated with CSE for 4–6 hours, and whole cell lysates were prepared. Quantitative determination of cellular COX-2 was performed using ELISA. *p<0.001 with ANOVA. Figure is representative of 3 independent experiments.
3.4. Anti-oxidants do not suppress PGE2 production by maturing DCs
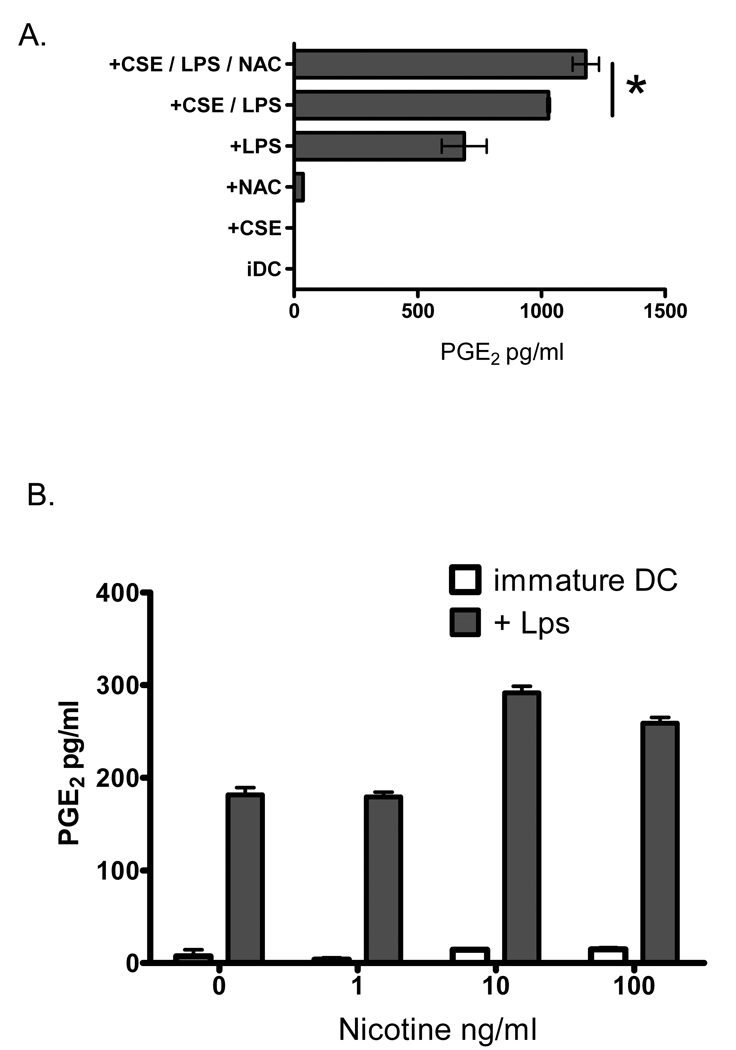
Having observed that oxidative stress induced by CSE is a primary mechanism by which DC CXCL8 is induced, we speculated that a similar mechanism may be responsible for the observation that CSE augments secreted PGE2 and cellular COX-2 levels in human DCs. To determine this, monocyte-derived DCs were pre-incubated with the anti-oxidant NAC (2.5mMolar) for 60 minutes prior to the addition of 1% CSE and LPS as a maturational agent. Following an 18-hour incubation, secreted PGE2 levels were measured using ELISA. In contrast to what was observed with CXCL8/IL-8, pre-incubation of DCs with NAC failed to suppress PGE2 release by CSE stimulated DCs (Figure 4A, ANOVA with Dunnett’s comparison test p=not significant). As expected, a significant increase in PGE2 production occurred when LPS-matured DCs were activated in the presence of 1% CSE (Figure 4A, 688 ± 64 compared with 1029 ± 3 pg/ml PGE2 respectively; ANOVA p<0.05). The addition of 2.5mM NAC to the DCs stimulated with both LPS and CSE resulted in a significant augmentation, rather than suppression, of PGE2 production (Figure 4A; ANOVA p<0.01). To determine if the nicotinic component in CSE could independently augment DC production of PGE2, immature and LPS-matured human DCs were incubated in the presence or absence of increasing concentrations of nicotine within the range of those found in our CSE preparation. Nicotine (1–100ng/ml) did not induce PGE2 from immature DCs but significantly augmented PGE2 production from LPS-matured DCs (Figure 4B; ANOVA p<0.001). Taken together these data imply that nicotinic receptor stimulation on human DCs is more relevant that oxidative stress as a mechanism by which CSE augments PGE2 production during DC maturation.
Figure 4. Anti-oxidants do not suppress PGE2 production by maturing DCs.
Human DCs were pre-incubated with NAC (2.5mM) for 60 minutes prior to the addition of either 1% CSE, 100ng/ml LPS, or combinations of the three. Secreted PGE2 levels were measured using ELISA following an 18 hour incubation. *p<0.05 with ANOVA. Data is representative of 3 independent experiments. B) Human DCs were incubated in the presence or absence of nicotine (1–100ng/ml) and incubated for 18 hours either in the presence of 100ng/ml LPS (grey bars) or without LPS (open bars). Secreted PGE2 levels were measured using ELISA. *p<0.001 with ANOVA.
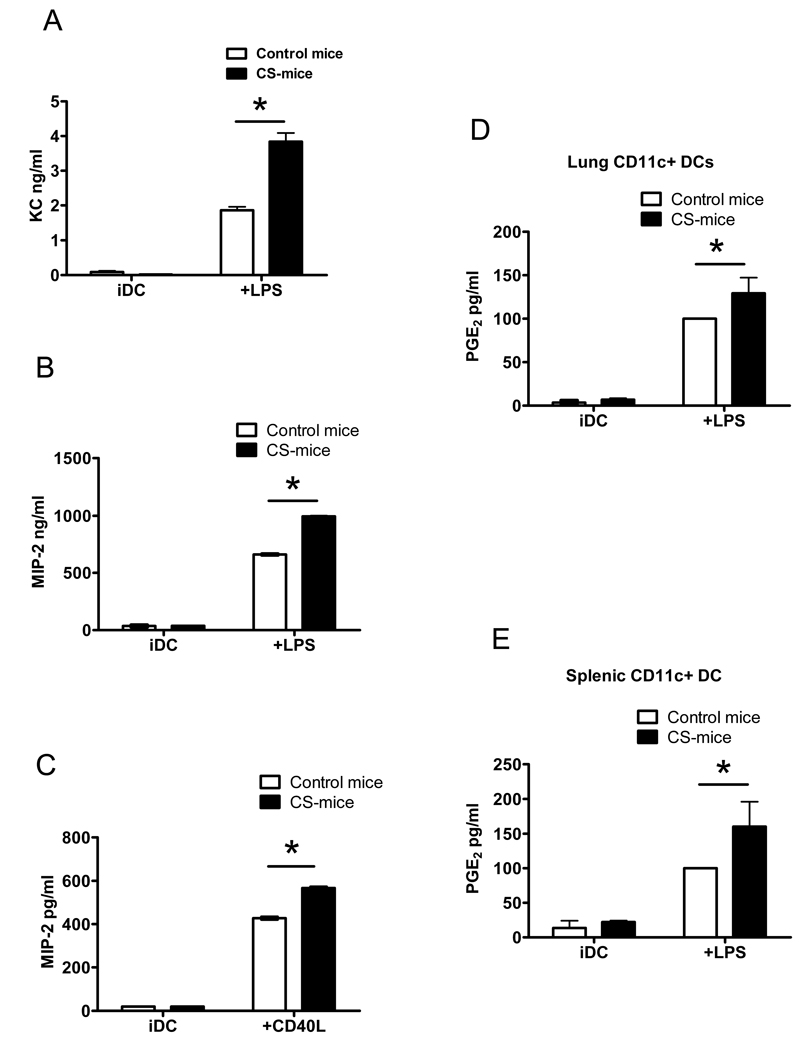
3.5. Cigarette smoking enhances neutrophil chemokine and PGE2 production by lung and systemic DCs
The biological relevance of CSE as a model system to study the effect of cigarette smoking on cellular functions has been a matter of debate (Shapiro, 2004). To validate and extend the in vitro observations, we tested the effect of cigarette smoking on the production of neutrophilic chemokines and PGE2 by maturing lung DCs. To test this, we utilized a manual smoking machine that allows exposure of mice to high concentrations of mixed environmental and mainstream cigarette smoke (Witschi et al., 1998). Following 4–8 weeks in the smoking chamber, mice were sacrificed and CD11c+ DCs were isolated from lungs as described in the methods section. Eight mice were included in the cigarette smoke (CS) group and controls. The average numbers of lung CD11c+ DCs extracted were 2.79±0.07×105 from each CS mouse compared with 2.3±0.03×105 from each control mouse [N=5 separate estimates]. Equal numbers of lung CD11c+ DCs (0.5×106/ml) extracted from cigarette smoke (CS), and control wild type (WT) mice were incubated with LPS (100ng/ml) or agonistic CD40 antibody (HM40, 1µg/ml) for an additional 14–18 hours and chemokine levels were measured in the supernatants. Although no structural homologue of CXCL8/IL-8 has been identified in mice, keratinocyte chemoattractant (KC) and macrophage inflammatory protein-2 (MIP-2) share many functional properties with CXCL8 and are considered functional murine homologs (Bozic et al., 1995). In accordance with the data in Figure 1, lung DCs extracted from CS-mice produced significantly more KC and MIP-2 following maturation by LPS (Figure 5A and B). In a separate experiment, we observed a parallel response when lung DCs from CS-mice were matured with an agonistic CD40 antibody (Figure 5C), indicating that augmentation of MIP-2 release occurs not only following innate toll receptor stimulation, but also following activation of DCs by T cell stimulatory molecules. Similar findings were obtained when KC levels were measured in supernatants from mouse lung DCs stimulated with agonistic CD40 antibodies: lung DCs from CS-mice produced 207.83±16.94 vs 68.19±4.43 pg/ml from control lung DCs – p=0.001 with t-test analysis (data not shown). Lung DCs from CS-mice also generated significantly greater amounts of PGE2 following overnight maturation with LPS (Figure 5D). In addition, systemic DCs extracted from spleens of CS-mice produced significantly higher amounts of PGE2 following maturation, indicating that the effect of smoking on DC PGE2 production is systemic and not restricted to lung DCs (Figure 5E). There were no significant differences in splenic DC numbers extracted from CS and control mice − 2.42 ±1.0×106 vs 2.84 ±1.3×106 per individual mouse respectively [N=4 separate estimates]. These data extend the in vitro findings by demonstrating that lung DCs from mice chronically exposed to cigarette smoke in sufficient quantities that effectively mimic a heavy cigarette smoker, produce significantly greater amounts of pro-inflammatory chemokines and PGE2.
Figure 5. Cigarette smoking enhances neutrophil chemokine and PGE2 production by murine DCs.
Lung CD11c+ DCs were extracted from adult C57B6 mice placed in a Teague manual smoking chamber for 4–8 weeks. Following sacrifice, murine lungs from cigarette smoke (CS-) mice and control wild type (N=8 in each group) were digested with collagenase and DNAse, and lung DCs purified by magnetic cell isolation using CD11c+ magnetic beads. Equal numbers of lung CD11c+ DCs (0.5×106/ml) were incubated with a maturational agent for 18 hours prior to measurement of chemokines and PGE2. A) KC production measured by ELISA in supernatants of lung DCs following 18 hours incubation with (mDC) or without (iDC) 500ng/ml. B) Shows MIP-2 production by lung DCs stimulated in a identical manner as in A. Data shown in A and B are representative of 3 independent experimental runs each including 8 CS and control mice. C) Shows MIP-2 levels measured in supernatants using ELISA following maturation of lung DCs with 18 hours of agonistic CD40 antibody (HM40, 1µg/ml) as a maturational stimulus. Data is representative of one experimental run with 8 CS and 8 control mice. D) Lung DCs were stimulated with 500ng/ml LPS and PGE2 measured in supernatants using ELISA following 18 hour maturation. Pooled data from 4 independent experimental runs is shown, with levels of PGE2 expressed as a ratio of control DCs stimulated with LPS (arbitrarily assigned as 100%). E) PGE2 production by systemic DCs isolated from spleens of CS and control mice incubated for 18 hours with LPS (500ng/ml). Pooled data from 3 independent experimental runs is shown, with levels of PGE2 expressed as a ratio of control DCs stimulated with LPS (arbitrarily assigned as 100%). *p<0.05; 2-way ANOVA.
4. Discussion
Although appropriate mucosal inflammation is essential in the protection against pathogens, protracted recruitment of inflammatory cells leads to chronic inflammation, tissue damage and remodeling. Chronic obstructive pulmonary disease (COPD) is a lung disease characterized by persistent airway inflammation associated with irreversible airflow limitation, excessive mucus production, and progressive decline in lung function (Barnes, 2003). The chronically inflamed airways of COPD patients contain several inflammatory cells including neutrophils, macrophages, B and T lymphocytes, and DCs (Barnes, 2003; Demedts et al., 2007; Turato et al., 2002). The relative contributions of these various inflammatory cells to airway injury and remodeling are not entirely clear. In particular, the potential role of DCs as mediators of inflammation in the smoker’s airways is poorly understood. In the current study we analyzed the potential role of cigarette smoke-activated DCs as contributors to neutrophil recruitment in the airways, and on generation of prostaglandins that modulate immunity in the airways and potentially promote airway epithelial cell transformation and lung cancer development in smokers (Harris, 2007; Sandler and Dubinett, 2004). Human myeloid DCs and murine myeloid DCs obtained from a murine model of chronic tobacco exposure produce significantly higher amounts of neutrophil chemokines and PGE2 than control DCs. Whereas the generation of neutrophil-attracting chemokines by cigarette smoke-activated DCs could be mitigated by anti-oxidants, the production of PGE2, and endogenous DC COX-2 levels were actually augmented with NAC treatment. These data indicate that anti-oxidant therapy with agents like NAC may only partially effect, and may even exacerbate, chronic airway inflammation in cigarette smokers.
In the current study we describe an important role for cigarette smoke activated lung DCs as producers of neutrophilic chemokines. In COPD, neutrophils play a key role in tissue injury and remodeling by shifting the balance between protease and anti-protease in the lung favoring tissue destruction. Since airway DCs are distributed in a sub and intra-epithelial locations in the airways, the generation of neutrophilic chemokines by these cells facilitates the development of a chemokine gradient that promotes neutrophil trafficking from the vascular compartment towards the airway lumen. Cigarette smoke and CSE are potent sources of oxidative stress, a constituent of cigarette smoke that has been demonstrated to induce inflammatory cytokine generation from a number of cell types (Kirkham et al., 2003; Kode et al., 2006; Muller et al., 1997; Shi et al., 1996). Oxidative Stress is a term used to describe the generation of reactive oxygen and nitrogen species that have the capacity to alter multiple aspects of cellular and organ function. Endogenous oxidative stress is an inevitable consequence of our dependence on oxygen as a source of mitochondrial activity. In addition to endogenous sources of oxidative stress, cellular systems also have to cope with a variety of exogenous sources of oxidative stress including that imposed by either smoking or exposure to second-hand cigarette smoke. Several other studies have alluded to the importance of cigarette smoke-induced oxidative stress in the pathogenesis of chronic airway inflammation in smokers (Barnes, 2003; Kluchova et al., 2007; Pierrou et al., 2007; Tkacova et al., 2007). Oxidative stress induces CXCL8/IL-8 by macrophages and epithelial cells (Mio et al., 1997; Yang et al., 2006), and has been implicated in the induction of adhesion molecules involved in the recruitment of monocytes and neutrophils to sites of inflammation (Shen et al., 1996). The current study implies that cigarette smoke-induced oxidative stress on myeloid DCs results in an additional major contribution to the generation of neutrophilic airway inflammation in smokers. The current study also provides further support to the contention that generation of reactive oxidant species are a key mechanism by which smoking induces neutrophilic chemokine production, and suggests that anti-oxidant strategies may have a potential role as a means to limit cigarette-smoke mediated neutrophilic airway inflammation.
Lipid metabolites of arachidonic acid, including prostaglandins, are important endogenous modulators of innate and acquired immunity in the lung by virtue of their effect on several immune cells. Chronic obstructive pulmonary disease is associated with increased PGE2 production (Montuschi et al., 2003). The precise sources of PGE2 production in the lungs of COPD patients are unclear, although it is very probable that other cellular sources contribute to the elevated levels reported to occur in COPD (Martey et al., 2004). Herein we report lung DCs as a source of PGE2 in a chronic model of cigarette smoke exposure. The induction of COX-2 and secreted PGE2 is independent of the oxidative capacity of cigarette smoke, indicating that not all pro-inflammatory cellular responses induced by cigarette smoke are mediated by oxidative stress. This is an important observation that may have implications in the design of chemoprevention trials for smokers at risk of lung cancer. COPD is an established independent risk factor for lung cancer development, and for any level of tobacco exposure, patients with COPD are at greater risk for lung cancer development than smokers without COPD (Skillrud et al., 1986). Several studies have reported aberrant over-expression of the COX-2 protein in lung and other cancers, while therapy with inhibitors of COX-2 is associated with reduced occurrence of certain cancers (reviewed by (Sandler and Dubinett, 2004) and (Harris, 2007)). Furthermore, increased PGE2 levels are known to abrogate activation of tumor suppressors such as p53, are implicated in angiogenesis, tumor growth and invasion, and promote increased activity of oncogenic proteins such as K-ras (Dohadwala et al., 2002; Krysan et al., 2006; Lee et al., 2007; Shao et al., 2003). These data suggest that the COX-2 pathway and downstream PGE2 production are relevant targets for chemoprevention of lung cancer in COPD patients. Although anti-oxidants may seem an obvious choice as inhibitors of chronic inflammation in COPD, the current study suggests that certain anti-oxidants like NAC are ineffective and may actually exacerbate COX-2 up-regulation induced by cigarette smoke exposure in certain resident lung cells. The observation that NAC augments cigarette smoke-mediated DC COX-2 and secreted PGE2 may offer some insight into the perplexing clinical observation reported in primary prevention trials of patients at high risk of lung cancer, in whom the use of anti-oxidants either failed to reduce the occurrence of lung cancer compared to placebo, or was associated with increased mortality in current smokers (reviewed in (Soria et al., 2003)).
Nicotine has been demonstrated to have a number of effects on immune cell function, most of which seem to be inhibitory (Guinet et al., 2004; Kalra et al., 2004; Middlebrook et al., 2002; Nouri-Shirazi et al., 2007). The current study identifies nicotine as the likely primary constituent in cigarette smoke involved in cigarette smoke-induced COX-2 and PGE2 production by maturing DCs. Our findings are consistent with the work of others showing induction of COX-2 and secreted PGE2 by nicotinic stimulation of microglia (De Simone et al., 2005), fibroblasts (Ho and Chang, 2006), monocytes (Payne et al., 1996), and whole blood (Saareks et al., 1998). The biologic effects of nicotine on DCs are mediated through the alpha7-nicotinic acetylcholine receptor, which is expressed on both immature and mature DCs (data not shown) and has been shown to negatively regulate synthesis and release of tumor necrosis factor (TNF)-alpha in macrophages. Aberrant over-activation of the COX-2 pathway in activated DCs from smokers may participate in new cancer formation by secreting excessive PGE2 that may function in an autocrine fashion on adjacent epithelial cells to facilitate new tumor formation. It is therefore seems reasonable to postulate that a novel chemoprevention approach for COPD patients at risk for lung cancer could include selective nicotinic receptor antagonists.
In conclusion, we demonstrate in this study that DCs activated with oxidants from cigarette smoke produce significant amounts of neutrophilic chemokines, while nicotine present in CSE and cigarette smoke augments the production of secreted prostaglandin E2 and intracellular cyclo-oxygenase-2 (COX-2) in maturing DCs. These studies are important because they indicate the complex effects of cigarette smoke on DC activation, and indicate that certain pharmacologic strategies, while effective in controlling chemokine generation, may actually exacerbate prostanoid production and augment lung cancer risk in smokers.
Footnotes
Supported by funding from the Parker B Francis Fellowship Foundation, American Lung Association, American Histiocytosis Association, Flight Attendant Medical Research Institute, and Mayo Clinic institutional funds to RV.
References
- Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. New concepts in chronic obstructive pulmonary disease. Annu Rev Med. 2003;54:113–129. doi: 10.1146/annurev.med.54.101601.152209. [DOI] [PubMed] [Google Scholar]
- Bozic CR, Kolakowski LF, Jr., Gerard NP, Garcia-Rodriguez C, von Uexkull-Guldenband C, Conklyn MJ, Breslow R, Showell HJ, Gerard C. Expression and biologic characterization of the murine chemokine KC. J Immunol. 1995;154:6048–6057. [PubMed] [Google Scholar]
- De Simone R, Ajmone-Cat MA, Carnevale D, Minghetti L. Activation of alpha7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures. J Neuroinflammation. 2005;2:4. doi: 10.1186/1742-2094-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demedts IK, Bracke KR, Van Pottelberge G, Testelmans D, Verleden GM, Vermassen FE, Joos GF, Brusselle GG. Accumulation of dendritic cells and increased CCL20 levels in the airways of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:998–1005. doi: 10.1164/rccm.200608-1113OC. [DOI] [PubMed] [Google Scholar]
- Dohadwala M, Batra RK, Luo J, Lin Y, Krysan K, Pold M, Sharma S, Dubinett SM. Autocrine/paracrine prostaglandin E2 production by non-small cell lung cancer cells regulates matrix metalloproteinase-2 and CD44 in cyclooxygenase-2-dependent invasion. J Biol Chem. 2002;277:50828–50833. doi: 10.1074/jbc.M210707200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinet E, Yoshida K, Nouri-Shirazi M. Nicotinic environment affects the differentiation and functional maturation of monocytes derived dendritic cells (DCs) Immunol Lett. 2004;95:45–55. doi: 10.1016/j.imlet.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Harris RE. Cyclooxygenase-2 (cox-2) and the inflammogenesis of cancer. Subcell Biochem. 2007;42:93–126. doi: 10.1007/1-4020-5688-5_4. [DOI] [PubMed] [Google Scholar]
- Ho YC, Chang YC. Regulation of nicotine-induced cyclooxygenase-2 protein expression in human gingival fibroblasts. Acta Pharmacol Sin. 2006;27:409–413. doi: 10.1111/j.1745-7254.2006.00286.x. [DOI] [PubMed] [Google Scholar]
- Jacobs DM, Morrison DC. Inhibition of the mitogenic response to lipopolysaccharide (LPS) in mouse spleen cells by polymyxin B. J Immunol. 1977;118:21–27. [PubMed] [Google Scholar]
- Kalra R, Singh SP, Pena-Philippides JC, Langley RJ, Razani-Boroujerdi S, Sopori ML. Immunosuppressive and anti-inflammatory effects of nicotine administered by patch in an animal model. Clin Diagn Lab Immunol. 2004;11:563–568. doi: 10.1128/CDLI.11.3.563-568.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham PA, Spooner G, Ffoulkes-Jones C, Calvez R. Cigarette smoke triggers macrophage adhesion and activation: role of lipid peroxidation products and scavenger receptor. Free Radic Biol Med. 2003;35:697–710. doi: 10.1016/s0891-5849(03)00390-3. [DOI] [PubMed] [Google Scholar]
- Kluchova Z, Petrasova D, Joppa P, Dorkova Z, Tkacova R. The association between oxidative stress and obstructive lung impairment in patients with COPD. Physiol Res. 2007;56:51–56. doi: 10.33549/physiolres.930884. [DOI] [PubMed] [Google Scholar]
- Kode A, Yang SR, Rahman I. Differential effects of cigarette smoke on oxidative stress and proinflammatory cytokine release in primary human airway epithelial cells and in a variety of transformed alveolar epithelial cells. Respir Res. 2006;7:132. doi: 10.1186/1465-9921-7-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysan K, Reckamp KL, Sharma S, Dubinett SM. The potential and rationale for COX-2 inhibitors in lung cancer. Anticancer Agents Med Chem. 2006;6:209–220. doi: 10.2174/187152006776930882. [DOI] [PubMed] [Google Scholar]
- Lambrecht BN, Prins JB, Hoogsteden HC. Lung dendritic cells and host immunity to infection. Eur Respir J. 2001;18:692–704. [PubMed] [Google Scholar]
- Lawson GM, Hurt RD, Dale LC, Offord KP, Croghan IT, Schroeder DR, Jiang NS. Application of serum nicotine and plasma cotinine concentrations to assessment of nicotine replacement in light, moderate, and heavy smokers undergoing transdermal therapy. J Clin Pharmacol. 1998;38:502–509. doi: 10.1002/j.1552-4604.1998.tb05787.x. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Choi EM, Kim SR, Park JH, Kim H, Ha KS, Kim YM, Kim SS, Choe M, Kim JI, Han JA. Cyclooxygenase-2 promotes cell proliferation, migration and invasion in U2OS human osteosarcoma cells. Exp Mol Med. 2007;39:469–476. doi: 10.1038/emm.2007.51. [DOI] [PubMed] [Google Scholar]
- Martey CA, Pollock SJ, Turner CK, O'Reilly KM, Baglole CJ, Phipps RP, Sime PJ. Cigarette smoke induces cyclooxygenase-2 and microsomal prostaglandin E2 synthase in human lung fibroblasts: implications for lung inflammation and cancer. Am J Physiol Lung Cell Mol Physiol. 2004;287:L981–L991. doi: 10.1152/ajplung.00239.2003. [DOI] [PubMed] [Google Scholar]
- Middlebrook AJ, Martina C, Chang Y, Lukas RJ, DeLuca D. Effects of nicotine exposure on T cell development in fetal thymus organ culture: arrest of T cell maturation. J Immunol. 2002;169:2915–2924. doi: 10.4049/jimmunol.169.6.2915. [DOI] [PubMed] [Google Scholar]
- Mio T, Romberger DJ, Thompson AB, Robbins RA, Heires A, Rennard SI. Cigarette smoke induces interleukin-8 release from human bronchial epithelial cells. Am J Respir Crit Care Med. 1997;155:1770–1776. doi: 10.1164/ajrccm.155.5.9154890. [DOI] [PubMed] [Google Scholar]
- Montuschi P, Kharitonov SA, Ciabattoni G, Barnes PJ. Exhaled leukotrienes and prostaglandins in COPD. Thorax. 2003;58:585–588. doi: 10.1136/thorax.58.7.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T, Haussmann HJ, Schepers G. Evidence for peroxynitrite as an oxidative stress-inducing compound of aqueous cigarette smoke fractions. Carcinogenesis. 1997;18:295–301. doi: 10.1093/carcin/18.2.295. [DOI] [PubMed] [Google Scholar]
- Nouri-Shirazi M, Tinajero R, Guinet E. Nicotine alters the biological activities of developing mouse bone marrow-derived dendritic cells (DCs) Immunol Lett. 2007;109:155–164. doi: 10.1016/j.imlet.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Payne JB, Johnson GK, Reinhardt RA, Dyer JK, Maze CA, Dunning DG. Nicotine effects on PGE2 and IL-1 beta release by LPS-treated human monocytes. J Periodontal Res. 1996;31:99–104. doi: 10.1111/j.1600-0765.1996.tb00470.x. [DOI] [PubMed] [Google Scholar]
- Pierrou S, Broberg P, O'Donnell RA, Pawlowski K, Virtala R, Lindqvist E, Richter A, Wilson SJ, Angco G, Moller S, Bergstrand H, Koopmann W, Wieslander E, Stromstedt PE, Holgate ST, Davies DE, Lund J, Djukanovic R. Expression of genes involved in oxidative stress responses in airway epithelial cells of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:577–586. doi: 10.1164/rccm.200607-931OC. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. Global Strategy for the Diagnosis, Management, and Prevention of COPD - 2006 Update. Am J Respir Crit Care Med. 2007 doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- Saareks V, Mucha I, Sievi E, Vapaatalo H, Riutta A. Nicotine stereoisomers and cotinine stimulate prostaglandin E2 but inhibit thromboxane B2 and leukotriene E4 synthesis in whole blood. Eur J Pharmacol. 1998;353:87–92. doi: 10.1016/s0014-2999(98)00384-7. [DOI] [PubMed] [Google Scholar]
- Sandler AB, Dubinett SM. COX-2 inhibition and lung cancer. Semin Oncol. 2004;31:45–52. doi: 10.1053/j.seminoncol.2004.03.045. [DOI] [PubMed] [Google Scholar]
- Shao J, Lee SB, Guo H, Evers BM, Sheng H. Prostaglandin E2 stimulates the growth of colon cancer cells via induction of amphiregulin. Cancer Res. 2003;63:5218–5223. [PubMed] [Google Scholar]
- Shapiro SD. Smoke gets in your cells. Am J Respir Cell Mol Biol. 2004;31:481–482. doi: 10.1165/rcmb.F285. [DOI] [PubMed] [Google Scholar]
- Shen Y, Rattan V, Sultana C, Kalra VK. Cigarette smoke condensate-induced adhesion molecule expression and transendothelial migration of monocytes. Am J Physiol. 1996;270:H1624–H1633. doi: 10.1152/ajpheart.1996.270.5.H1624. [DOI] [PubMed] [Google Scholar]
- Shi MM, Godleski JJ, Paulauskis JD. Regulation of macrophage inflammatory protein-1alpha mRNA by oxidative stress. J Biol Chem. 1996;271:5878–5883. doi: 10.1074/jbc.271.10.5878. [DOI] [PubMed] [Google Scholar]
- Skillrud DM, Offord KP, Miller RD. Higher risk of lung cancer in chronic obstructive pulmonary disease. A prospective, matched, controlled study. Ann Intern Med. 1986;105:503–507. doi: 10.7326/0003-4819-105-4-503. [DOI] [PubMed] [Google Scholar]
- Soria JC, Kim ES, Fayette J, Lantuejoul S, Deutsch E, Hong WK. Chemoprevention of lung cancer. Lancet Oncol. 2003;4:659–669. doi: 10.1016/s1470-2045(03)01244-0. [DOI] [PubMed] [Google Scholar]
- Tkacova R, Kluchova Z, Joppa P, Petrasova D, Molcanyiova A. Systemic inflammation and systemic oxidative stress in patients with acute exacerbations of COPD. Respir Med. 2007;101:1670–1676. doi: 10.1016/j.rmed.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Turato G, Zuin R, Miniati M, Baraldo S, Rea F, Beghe B, Monti S, Formichi B, Boschetto P, Harari S, Papi A, Maestrelli P, Fabbri LM, Saetta M. Airway inflammation in severe chronic obstructive pulmonary disease: relationship with lung function and radiologic emphysema. Am J Respir Crit Care Med. 2002;166:105–110. doi: 10.1164/rccm.2111084. [DOI] [PubMed] [Google Scholar]
- Turner MC, Chen Y, Krewski D, Calle EE, Thun MJ. Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. Am J Respir Crit Care Med. 2007;176:285–290. doi: 10.1164/rccm.200612-1792OC. [DOI] [PubMed] [Google Scholar]
- Vassallo R, Tamada K, Lau JS, Kroening PR, Chen L. Cigarette smoke extract suppresses human dendritic cell function leading to preferential induction of Th-2 priming. J Immunol. 2005;175:2684–2691. doi: 10.4049/jimmunol.175.4.2684. [DOI] [PubMed] [Google Scholar]
- Witschi H, Espiritu I, Yu M, Willits NH. The effects of phenethyl isothiocyanate, N-acetylcysteine and green tea on tobacco smoke-induced lung tumors in strain A/J mice. Carcinogenesis. 1998;19:1789–1794. doi: 10.1093/carcin/19.10.1789. [DOI] [PubMed] [Google Scholar]
- Witschi H, Uyeminami D, Moran D, Espiritu I. Chemoprevention of tobacco-smoke lung carcinogenesis in mice after cessation of smoke exposure. Carcinogenesis. 2000;21:977–982. doi: 10.1093/carcin/21.5.977. [DOI] [PubMed] [Google Scholar]
- Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291:L46–L57. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]
- Yoshimatsu K, Altorki NK, Golijanin D, Zhang F, Jakobsson PJ, Dannenberg AJ, Subbaramaiah K. Inducible prostaglandin E synthase is overexpressed in non-small cell lung cancer. Clin Cancer Res. 2001;7:2669–2674. [PubMed] [Google Scholar]