Abstract
Our objective in this work was to develop a flexible, biodegradable scaffold for cell transplantation that would incorporate a synthetic component for strength and flexibility and type I collagen for enzymatic lability and cytocompatibility. A biodegradable poly(ester urethane)urea was synthesized from poly(caprolactone), 1,4-diisocyanatobutane, and putrescine. Using a thermally induced phase separation process, porous scaffolds were created from a mixture containing this polyurethane and 0%, 10%, 20%, or 30% type I collagen. The resulting scaffolds were found to have open, interconnected pores (from 7 to >100 um) and porosities from 58% to 86% depending on the polyurethane/collagen ratio. The scaffolds were also flexible with breaking strains of 82–443% and tensile strengths of 0.97–4.11 MPa depending on preparation conditions. Scaffold degradation was significantly increased when collagenase was introduced into an incubating buffer in a manner that was dependent on the mass fraction of collagen present in the scaffold. Mass losses could be varied from 15% to 59% over 8 weeks. When culturing umbilical artery smooth muscle cells on these scaffolds higher cell numbers were observed over a 4-week culture period in scaffolds containing collagen. In summary, a strong and flexible scaffold system has been developed that can degrade by both hydrolysis and collagenase degradation pathways, as well as support cell growth. This scaffold possesses properties that would make it attractive for future use in soft tissue applications where such mechanical and biological features would be advantageous.
Keywords: Scaffold, Biodegradation, Collagen, Polyurethane
Introduction
Cell transplantation approaches are often dependent upon the use of a scaffold to provide temporary mechanical function to the application site or to aid in cellular retention and localization. In selecting a scaffold for such an application, a dichotomy can be made in the available scaffold types between those based on synthetic materials, usually biodegradable polymers, and those comprised of naturally occurring biomolecules, such as collagen, fibrin, and glycosaminoglycans. Generally speaking, the advantages of the synthetic materials are their well-defined and controllable mechanical and morphological properties and their lack of immunogenicity. Natural materials benefit from inherent bioactivity that may include cell adhesiveness, enzymatic lability, and an affinity for autologous growth regulatory molecules. The unmet goal in the field of scaffold development is to provide access to a mimetic extracellular matrix that combines the mechanical robustness of synthetic materials with the biological activities found in natural scaffolds.
One approach to creating a scaffold possessing both biological activity and attractive mechanical properties is to physically combine natural and synthetic polymers. The biocomposite thus formed could add cellular compatibility to the synthetic scaffold component while improving the mechanical properties of the natural material. The mechanical properties of such a composite might be varied by altering the nature and fraction of the synthetic component to match the mechanical requirement for different tissues. Furthermore, if the synthetic component were to be a thermoplastic polymer, a variety of solvent-based processing techniques might be used to process the two components into a scaffold micro- and macroarchitecture appropriate for the application.
Collagen has been widely employed in previous reports seeking to develop biocomposite materials. In the native extracellular matrix collagen provides the primary strength, but not elastic, component in tissues. Collagen is an attractive natural material for scaffolds in that it provides for cell adhesion, has inherent enzymatic lability, and has been widely used clinically in a processed scaffold format as a hemostatic agent and for soft tissue repair (18). Blends of water-soluble polymers such as poly(vinyl alcohol) (PVA) (1,22), poly(acrylic acid) (PAA) (2,4,16), and hyaluronic acid (20,24) with acid soluble collagen have been investigated where collagen incorporation provides putative improvements in cytocompatibility to the hydrophilic polymers. Collagen has also been incorporated with water-insoluble polymers such as polylactide (PLA) (5,7), polyglycolide (PGA), poly(dl-lactide-co-glycolide) (PLGA) (5), and polyca-prolactone (6). Given the mechanical properties of these latter polymers, the resulting biocomposites were relatively stiff and not as mechanically attractive for soft tissue applications.
Our objective in this report was to combine type I collagen in varying amounts with a thermoplastic, elastomeric, and biodegradable polymer to create a biocomposite scaffold that possessed collagenase sensitivity, supported cellular growth, and maintained attractive mechanical properties for soft tissue applications. To accomplish this, we blended a previously reported biodegradable poly(ester urethane)urea (9) and type I collagen fibers in a relatively mild organic solvent [dimethyl sulfoxide (DMSO)], then used the technique of thermally induced phase separation (10) to create microporous scaffolds. The effects of different collagen mass fractions, different quenching temperatures used in scaffold processing, and different net poly(ester urethane)urea (PEUU) concentrations on scaffold morphology (porosity, average pore size, and pore size range) were examined. Mechanical and thermal properties of the scaffolds were evaluated for those with and without collagen. Scaffold degradation in an aqueous buffer with or without collagenase added was measured to specifically demonstrate the impact of collagen fraction on collagenase sensitivity. Finally, as an assessment of cytocompatibility, human smooth muscle cell growth was measured in scaffolds with and without collagen present.
Materials and Methods
Polymer Synthesis
Polycaprolactone diol (PCL, MW = 2000) was obtained from Aldrich and dried under vacuum for 48 h to remove residual water before use. 1,4-Diisocyanatobutane (BDI, Fluka), putrescine (Aldrich), and stannous octoate (Sigma) were used as received. The solvent DMSO was dried over molecular sieves (3 Å). PEUU was synthesized as described previously using a two-step solution polymerization (9). Briefly, synthesis was carried out in a 250-ml round bottom flask under dry nitrogen with reactant stoichiometry of 2:1:1 (BDI/PCL/ putrescine). BDI at 15 wt% in DMSO was continuously stirred with 25 wt% PCL in DMSO followed by stannous octoate addition. The reaction was allowed to proceed for 3 h at 80°C followed by cooling to room temperature. Putrescine was then added dropwise with stirring and the reaction was continued at room temperature for 18 h. The resulting PEUU solution was precipitated in distilled water, the wet polymer was immersed for 24 h in isopropanol to remove unreacted monomers, and the polymer was dried under vacuum at 50°C for 24 h.
PEEU/Collagen Scaffold Fabrication
PEUU/collagen scaffolds were fabricated using a method described by Liu et al. (15) and previously reported by us for polyurethanes (10) with modification. PEUU was dissolved in DMSO to form solutions. Type I, insoluble collagen from bovine Achilles tendon (Sigma) was uniformly dispersed in DMSO by using a commercial blender (Waring) for 1 min at room temperature. The collagen fiber suspension was added into the PEUU solution at 80°C and the mixture was stirred for 1 min to disperse the collagen fibers in the PEUU solution. Mixtures with PEUU at final concentrations of 5% and 10% and PEUU/collagen ratios of 100:0, 90:10, 80: 20, and 70:30 were obtained. These mixtures were then injected into a glass cylinder mold equipped with two rubber stoppers. The mold was made of two glass tubes; the outer diameter of the inner tube was 5 mm, and the inner diameter of the outer tube was 10 mm. The mold was cooled to a temperature of 0°C, −20°C, or −80°C for 3 h and was then removed and placed into absolute alcohol at a temperature of −20°C for 7 days to extract the DMSO. The alcohol was changed daily. The scaffold was air dried and then vacuum dried for 24 h at room temperature.
Scaffold Characterization
Scaffold surfaces and cross sections were examined with scanning electron microscopy (SEM) with cross sections obtained by fracturing after freezing in liquid nitrogen. Pore size ranges were determined using digital image processing of SEM images. Scaffold porosity was determined using an ethanol displacement method similar to that reported by Zhang and Ma (25), Hsu et al. (11), and reported by our group previously (10). Scaffold samples were individually immersed in a cylinder containing a known volume of ethanol (V1). The sample was immersed for 5 min with air physically pressed from the scaffolds to induce pore filling. The total volume of ethanol and the ethanol-filled scaffold was recorded (V2). The ethanol-filled scaffold was then removed from the cylinder and the residual ethanol volume was recorded (V3). Scaffold porosity (p) was calculated as: p = (V1 − V3)/(V2 − V3).
Differential scanning calorimetry was performed in a DSC-60 (Shimadzu) differential scanning calorimeter. Scanning rates of 20°C/min were used over a temperature range of 0–200°C.
Scaffold tensile properties (n = 4 per scaffold type) were measured on an Instron testing device with a 5-lb load cell and a cross-head speed of 10 mm/min according to ASTM D638-98.
Scaffold Degradation
To quantify scaffold degradation, dry scaffolds (1.5 × 0.5 cm) were weighed and immersed in 10 ml phosphate-buffered saline (PBS, pH 7.4) with or without 1 U/ml type I collagenase (Sigma) added. The degradation was conducted at 37°C in a water bath. Scaffolds were retrieved at 1- or 2-week intervals and rinsed with water. Samples were weighed after drying in vacuum for 2 days. The weight remaining was calculated as the percent of the original dry scaffold weight found at each time point (n = 4 for each scaffold type at each time point).
Smooth Muscle Cell Culture
Scaffolds were cut and sterilized by immersion in 70% ethanol for 6 h, followed by rinsing with PBS and exposure to the ultraviolet light source in a laminar flow hood (Class II A/B3 Biological Safety Cabinet) for 2 h. Scaffolds were placed in a centrifuge tube with human umbilical artery smooth muscle cells (UASMCs, Bio Whittaker) at a density of 1 × 106 cells/ml in medium (SmGM-2 with 10% fetal bovine serum, BioWhittaker). Vacuum was applied to the centrifuge tube for 30 s followed by exposure to ambient air. This was repeated five times. Scaffolds were then cut to 6-mm discs and fit into the bottom of a 96-well tissue culture polystyrene plate. Another 50 μl of UASMCs at a density of 1 × 106 cells/ml were added and the plate was kept in an incubator for 3 h, then 150 μl medium (SmGM-2) was added to each well. To evaluate cell growth, the culture medium was replaced every second day. Randomly selected wells were destructively evaluated with the MTT cell viability assay each week for 4 weeks (n = 4 per scaffold type per time). The surfaces of cultured scaffold/cell constructs were examined by SEM. Samples were fixed in 2.5% glutaraldehyde, dehydrated in a graded series of ethanol/water solutions, dried, and then sputter coated with gold.
Statistical Methods
Data are expressed as mean ± SD. Single-factor analysis of variance (ANOVA) was employed to assess the statistical significance of data. Newman-Keuls testing was used for post hoc evaluations of differences between specific groups.
Results
PEUU/Collagen Scaffold Morphology
PEUU/collagen biocomposite scaffolds were created using thermally induced phase separation from DMSO solvent where three processing parameters were varied: the initial PEUU solution concentration, the PEUU/collagen weight ratio, and the quenching temperature. The notation used when referring to these scaffolds is CXX/ YYZZ, where XX refers to the collagen weight percent (either 0, 10, 20, or 30), YY refers to the PEUU solution concentration in wt% (either 5 or 10), and ZZ refers to the quenching temperature (−80, −20, or 0, but with the negative sign not shown). Table 1 summarizes the different scaffold types created together with the notation for each scaffold. Also presented in this table are the porosities, average pore sizes, and pore size ranges for each of the scaffold types.
Table 1.
PEUU/Collagen Scaffold Morphological Parameters
| Polymer | PEUU/Collagen Ratio | PEUU Concentration (%) | Quenching Temperature (°C) | Porosity (%) | Pore Size (μm)* | Pore Size Range (μm)* |
|---|---|---|---|---|---|---|
| C0/1080 | 100/0 | 10 | −80 | 86 ± 2 | 91 ± 42 | 26–175 |
| C10/1080 | 90/10 | 10 | −80 | 64 ± 4 | 87 ± 23 | 49–147 |
| C20/1080 | 80/20 | 10 | −80 | 64 ± 1 | 26 ± 10 | 10–45 |
| C30/1080 | 70/30 | 10 | −80 | 58 ± 10 | 18 ± 7 | 7–32 |
| C20/1020 | 80/20 | 10 | −20 | 71 ± 8 | 19 ± 10 | 7−48 |
| C20/1000 | 80/20 | 10 | 0 | 68 ± 6 | 107 ± 52 | 50–280 |
| C20/0580 | 80/20 | 5 | −80 | 85 ± 2 | 93 ± 36 | 36–178 |
Measured from cross section.
In Figure 1 electron micrographs of the surface and cross-sectional morphologies of scaffolds are presented where the PEUU/collagen ratio was varied, but the PEUU solution concentration and the quenching temperature were maintained at 10% and −80°C, respectively. These images demonstrate that although pore sizes varied substantially, all of the scaffolds had open surface pores and interconnected pores in cross section. In general, the pores in cross section were found to be larger than those on the surface. The pore sizes of both the surface and cross section decreased with increasing collagen ratio. This trend can be seen in Table 1 for cross-sectional data and is apparent visually in Figure 1 for both surface and cross section images. The pore sizes of scaffolds with a PEUU/collagen ratio of 90:10 were not significantly smaller than in the scaffolds that did not contain collagen. This was in contrast to the more substantial decreases in pore sizes seen with 20% and 30% collagen content (p < 0.01 vs. 0% and 10% collagen). The porosity of the scaffolds with collagen was significantly reduced from that for scaffolds without collagen (p < 0.01). Collagen-containing scaffolds processed at 80 C and with 10% PEUU had porosities from 58% to 64%, whereas the 0% collagen scaffold was 86% porous.
Figure 1.
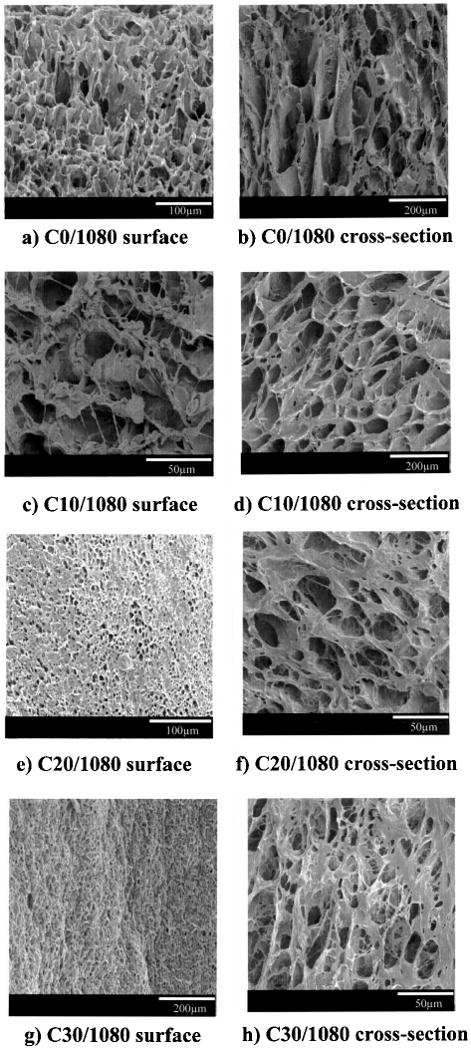
Scanning electron micrographs of PEUU/collagen scaffolds generated by varying the PEUU/collagen ratio. (a, b) 100:0 PEUU/collagen, (c, d) 90:10; (e, f) 80:20; (g, h) 70: 30. For polymer notation refer to Table 1. Scaffold surface morphologies are seen in (a), (c), (e), and (g). Cross-sectional morphologies are seen in (b), (d), (f), and (h). All scaffolds were prepared using a 10% PEUU solution and a quenching temperature of −80°C. Scale bars are presented in the lower right corner for each image.
The next scaffold processing parameter investigated was the quenching temperature. The PEUU/collagen ratio and PEUU concentration were fixed at 20% collagen and 10 wt% PEUU, respectively, while the quenching temperature was varied to examine −80°C, −20°C, and 0°C. The scaffolds quenched at −80°C (Fig. 1e, f) and −20°C (Fig. 2a, b) had comparable features with the pore sizes on the order of 20 μm on the surface and in cross section. When the quenching temperature was raised to 0°C the pore sizes increased significantly (p < 0.01 vs. −80°C and −20°C for cross section pores) as seen in Figure 2c and d. The porosities did not vary greatly when the quenching temperature was raised, with scaffolds exhibiting porosities between 64% and 71%.
Figure 2.
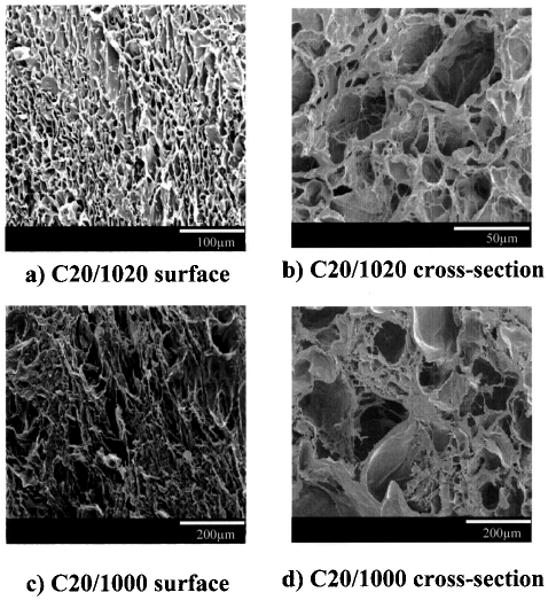
Micrographs of PEUU/collagen scaffolds generated by varying the quenching temperature. (a, b) −20°C, (c, d) 0°C. For polymer notation refer to Table 1. Scaffold surface morphologies are seen in (a) and (c). Cross section morphologies are seen in (b) and (d). All scaffolds were prepared from 10% PEUU solution and a PEUU/collagen ratio of 80:20. Micrographs of scaffolds made at a quenching temperature of −80°C with the same PEUU solution percent and PEUU/collagen ratio are found in Figure 1e and f.
The effect of polymer solution concentration on scaffold structure was assessed by generating scaffolds from PEUU solutions with concentrations of 5 wt% and 10 wt%, while keeping the quenching temperature at −80°C and the PEUU/collagen ratio at 80:20. The scaffold made from 5% PEUU solution had larger surface and cross section pores (Fig. 3) than the scaffold derived from a 10% solution (Fig. 1e, f) (p < 0.01 for cross section pores). The porosity was also significantly higher (p < 0.05) with the lower PEUU concentration (85% vs. 64%) and approximated that of the scaffold made with no collagen present.
Figure 3.

Micrographs of PEUU/collagen scaffold (a) surface and (b) cross section, where the scaffold was made from a 5% PEUU solution, a PEUU/collagen ratio of 80:20, and a quenching temperature of −80°C. For comparison, in Figure 1e and f micrographs of a scaffold made from 10% PEUU solution are seen where the same PEUU/collagen ratio and quenching temperature was used.
Scaffold Thermal Properties
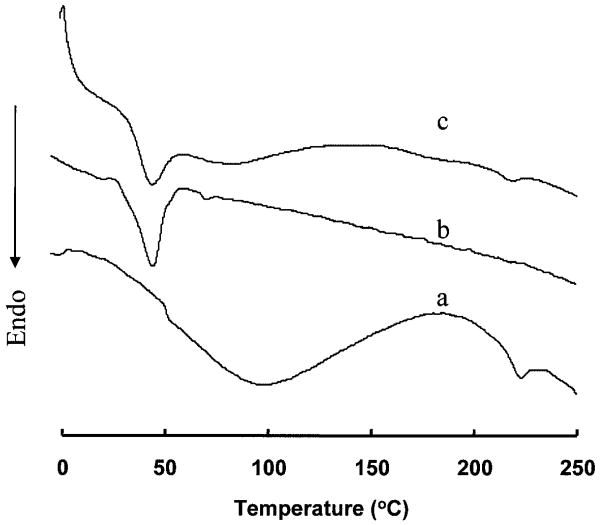
DSC was used to assess the thermal properties of collagen alone, a PEUU scaffold with no collagen, and a PEUU/collagen scaffold at an 80:20 ratio from 10% PEUU concentration and quenched at −80°C. It is seen in Figure 4 that collagen alone had a glass transition temperature (Tg) at 50.8°C and a broad and endothermic peak between 52°C and 170°C. An endothermic peak is also seen at 222.5°C, corresponding to the denaturation transition of collagen. The PEUU scaffold had a melting temperature (Tm) of 44.0°C and no other notable transitions. The PEUU/collagen composite scaffold showed characteristic thermal behaviors of both PEUU and collagen. A melting temperature was found at 43.9°C, attributable to PEUU, and an endothermic peak at 218.6°C, attributable to collagen denaturation. There also was a broad endothermic peak from 55°C to 150°C, comparable to that seen in collagen alone.
Figure 4.
Differential scanning calorimetry curves are presented for collagen (a), a PEUU scaffold (b), and a scaffold with a PEUU/collagen ratio of 80:20 (c). Each curve was obtained from the first experimental run.
Scaffold Mechanical Properties
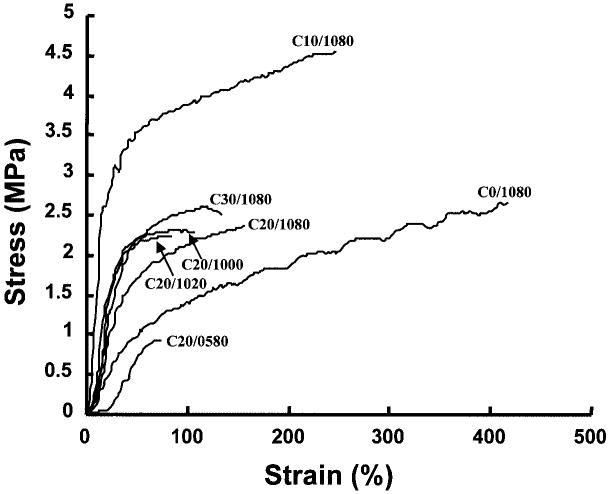
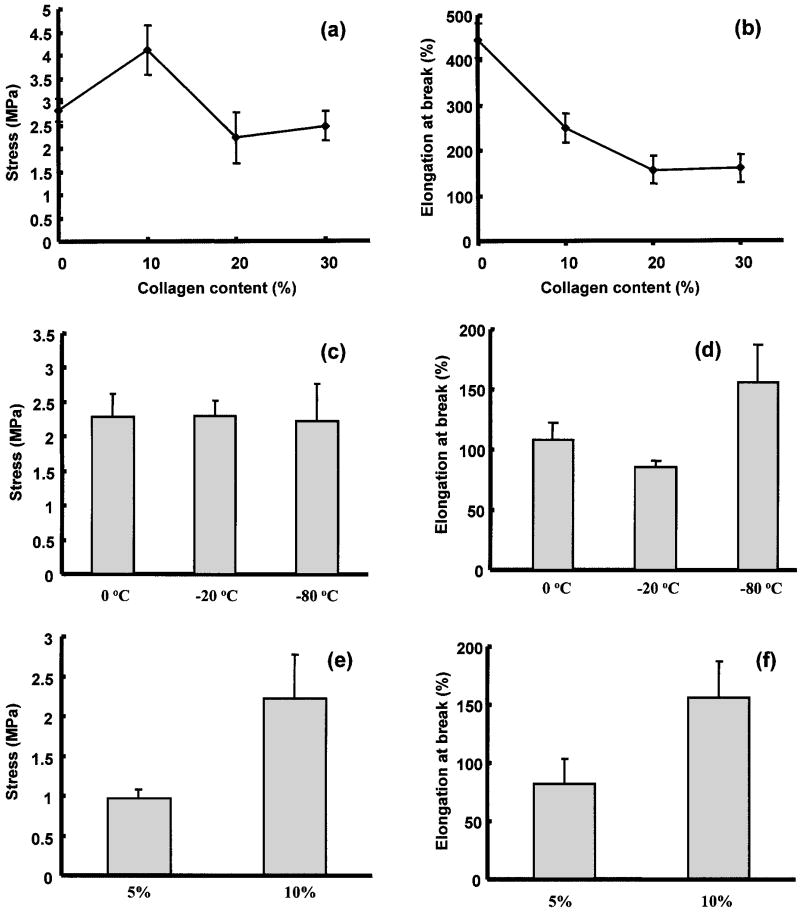
The mechanical properties of the formed scaffolds are presented in terms of representative stress-strain curves in Figure 5 and as average tensile strengths and elongations at break in Figure 6. Tensile strengths ranged from 0.97 to 4.11 MPa and elongations at break from 82% to 443% depending on the preparation conditions. The PEUU/collagen ratio effect on scaffold mechanical properties is seen in Figure 6a and b. When 10% collagen was added, the scaffold has significantly higher tensile strength (p < 0.01) than the scaffold without collagen prepared under the same conditions. When the scaffold collagen content was increased to 20% or 30%, the strength decreased to levels statistically equivalent to the scaffold with no collagen added. Varying the quenching temperature for scaffolds made from an 80:20 PEUU/collagen ratio did not have a significant impact on tensile strengths (Fig. 6c), while raising the quenching temperature to −20°C or 0°C from −80°C significantly decreased the elongation at break (p < 0.05) (Fig. 6d). In Figure 6e and f the mechanical properties of scaffolds made from a PEUU solution of 5% and 10% are presented. The PEUU/collagen ratio was fixed at 80: 20 and the scaffolds were quenched at −80°C. The scaffold made from 5% PEUU solution had a significantly lower tensile strength (p < 0.03) and elongation at break (p < 0.02) than the scaffold made from 10% PEUU solution.
Figure 5.
Typical stress–strain curves are presented for the scaffolds made from different PEUU/ collagen ratios, quenching temperatures, and PEUU solution concentrations. Table 1 provides the processing parameters for the different polymer notations seen in the figure.
Figure 6.
Effects of PEUU/collagen ratio (a, b), quenching temperature (c, d), and PEUU solution concentration (e, f), on tensile strength and elongation at break of PEUU/collagen scaffolds.
Scaffold Degradation and Sensitivity to Collagenase
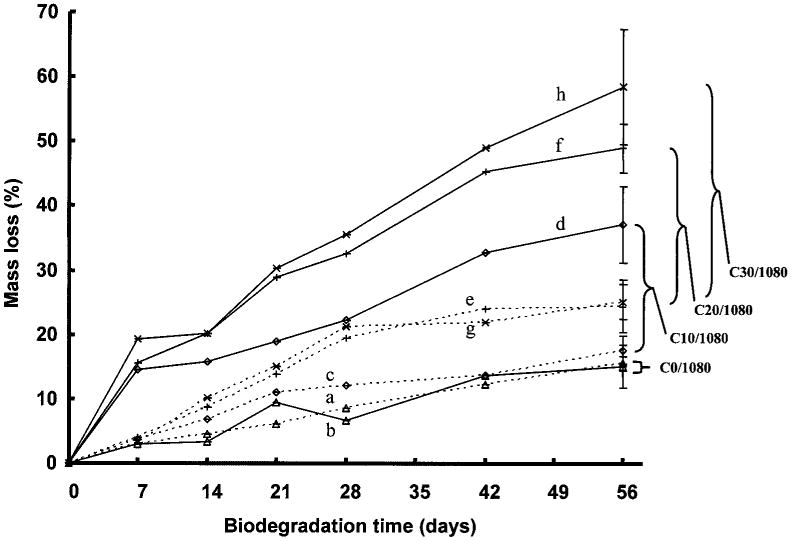
Scaffold degradation in buffer or buffer with collagenase added was assessed for scaffolds with 0%, 10%, 20%, and 30% collagen as summarized in Figure 7. Scaffold mass loss varied from 15.5% to 25.1% in buffer over an 8-week period, while the scaffolds in the collagenase solution had mass losses from 15.1% to 58.5%. In buffer alone, scaffolds with a collagen content of 20% and 30% had greater mass loss at 8 weeks than pure PEUU scaffolds and those with 10% collagen content (p < 0.02). No significant difference was found for PEUU scaffolds containing no collagen when they were incubated in buffer with or without collagenase. When collagen was present in the scaffolds, all scaffolds degraded faster with collagenase in the buffer (p < 0.001). Increasing the collagen content increased the degradation at 8 weeks in the collagenase solution (p < 0.05).
Figure 7.
Degradation of PEUU/collagen scaffolds with varying PEUU/collagen ratio in the presence (solid lines) or absence (dashed lines) of collagenase. All scaffolds were made from 10% PEUU solution and quenched at −80°C. Brackets represent differences in mass loss due to collagenase at 56 days for the four types of scaffolds examined. (a) PEUU/collagen 100:0 without collagenase, (b) PEUU/collagen 100:0 with collagenase, (c) PEUU/collagen 90:10 without collagenase, (d) PEUU/collagen 90:10 with collagenase, (e) PEUU/collagen 80:20 without collagenase, (f) PEUU/collagen 80:20 with collagenase, (g) PEUU/collagen 70:30 without collagenase, (h) PEUU/collagen 70:30 with collagenase.
Smooth Muscle Cell Growth in Scaffolds
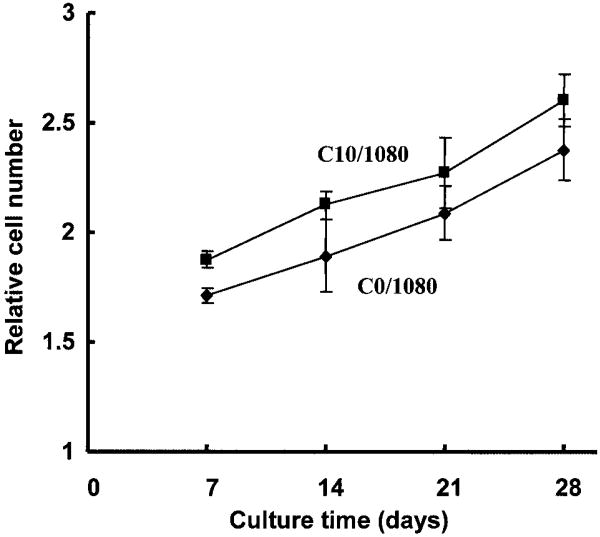
UASMCs were cultured on PEUU scaffolds or on scaffolds with a PEUU/collagen ratio of 90:10 for up to 4 weeks. The number of cells in the scaffolds as quantified by mitochondrial activity assay is presented in Figure 8. The cell number continued to increase during the culture period. The number of cells on PEUU/collagen scaffold was higher than PEUU scaffold at 2, 3, and 4 weeks (p < 0.05). Scanning electron micrographs (Fig. 9a and b) showed that after 4 weeks in culture, UASMCs on both surfaces showed an elongated morphology. Cells on both the PEUU and PEUU/collagen scaffolds formed multilayers, although electron micrographs consistently demonstrated a more consistent cellular coverage on surfaces of the collagen containing scaffolds.
Figure 8.
Growth curves for scaffolds made from PEUU (C0/ 1080) and PEUU with 10% collagen (C10/1080) seeded with umbilical artery smooth muscle cells and cultured for up to 28 days. The relative cell number is based upon MTT absorption and reflects mitochondrial activity.
Figure 9.
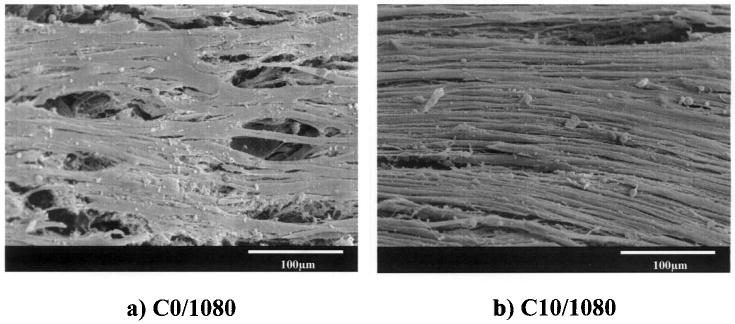
Electron micrographs of PEUU and PEUU/collagen (90:10) scaffold surfaces after 28 days of culture with umbilical artery smooth muscle cells.
Discussion
In this report we have demonstrated the physical combination of a recently reported biodegradable polyurethane with type I collagen, followed by a thermally induced phase separation process to create composite scaffolds that possess properties attractive for supporting cellular transplantation. In examining the resulting morphology of the formed scaffolds it was generally found that the cross-sectional pores were larger than those on the surface (Figs. 1–3). This effect can be explained by the physics of the phase separation process, where cooling occurs more rapidly on the cooling vessel surfaces. A faster cooling period to the point of DMSO crystallization will result in smaller DMSO-rich domains, which later become pores upon solvent extraction. A slower cooling period allows more time for DMSO-rich and PEUU-rich regions to coalesce and form larger pore structures. Thus, the faster cooling surface region has smaller pores. This effect can also be seen when comparing cross-sectional pore sizes from scaffolds quenched at 0°C to those quenched at lower temperatures (Table 1), and was observed in our previous studies where thermally induced phase separation was used to make pure polyurethane scaffolds (10).
Mixing collagen into the PEUU solution had the effect of decreasing scaffold porosity and, at higher collagen content, decreasing pore size. This effect may have been due to interactions between the amide bonds of collagen and the urethane/urea bonds restricting polyurethane and DMSO phase separation, resulting in smaller DMSO-rich regions and thus smaller pores. The additional mass added to the PEUU mixture from the collagen may also have contributed to reductions in scaffold porosity, although a trend was not evident beyond the addition of 10% collagen.
Pore size and porosity for scaffolds containing an 80: 20 ratio of PEUU/collagen could be returned to approximate the values for pure PEUU scaffolds by decreasing the PEUU solution concentration from 10% to 5%. This effect is attributable to having relatively more solvent present for pore creation and may also be a result of lower solution viscosity with lower PEUU content, which could facilitate more rapid phase separation. The resulting porosity of 85% and mean pore size of 93 μm for the C20/0580 scaffold would be attractive as a scaffold where high cell densities and mass transfer are desired.
In Figure 4, comparing the thermal properties of collagen, PEUU, and the PEUU/collagen composite, it is seen that the melting temperature of the composite was almost identical to the pure PEUU scaffold, and that the composite did not exhibit a glass transition temperature, as found with collagen. The latter effect was probably due to the overlap of the melting transition of PEUU and the glass transition of collagen. The denaturation temperature for the PEUU/collagen scaffold was lower than for collagen alone. This could be due to hydrogen bond interactions between collagen and PEUU decreasing collagen chain interactions, which would facilitate the conformational change at denaturation relative to collagen alone. The existence of the endothermic denaturation peak in the composite implies that collagen in the composite maintained a triple helical structure and did not substantially change conformation during scaffold processing (21). Further evidence that would strengthen this conclusion in future studies might include transmission electron imaging to demonstrate characteristic collagen banding or analysis of circular dichroism patterns from the composite scaffolds.
An important result of this study was the finding that the strength of PEUU/collagen composite scaffolds could generally be maintained relative to pure PEUU scaffolds in the face of added collagen (Figs. 5 and 6). Scaffold distensibility did decrease substantially with added collagen, although strains near the PEUU/collagen breaking strains would not be expected for most physiologic applications. The loss in distensibility with increasing collagen content might be due to collagen fibers serving as foci for tensile stress concentration and precipitation of failure. Increasing the collagen content might also weaken the tensile strength of the scaffold, but this effect may have been offset by the reduced porosities found in the PEUU/collagen scaffolds. Reduced porosity should generally strengthen the scaffold. When a lower concentration of PEUU was used in the PEUU/collagen scaffold there was a significant drop in both tensile strength and distensibility, due to less overall mass being present to bear the load. Although the 5% PEUU scaffold (C20/0580) was weaker, the properties of 0.97 MPa strength and 82% elongation at break are comparable to canine thoracic aorta (0.9 MPa strength) and human femoral arteries (approximately 90% breaking strain) (8,19). The morphological analysis and mechanical testing results indicate that it is possible to achieve attractive mechanical features even with relatively high collagen content in the composite scaffold. Tradeoffs can be made in the processing to balance the important parameters of scaffold porosity and pore size, strength and distensibility, and collagen content.
An attractive characteristic of many natural scaffolds is their sensitivity to cell-directed enzymatic degradation, whereas synthetic polymer scaffolds usually rely upon hydrolysis of ester or other labile bonds. A composite scaffold would ideally possess both activities. In Figure 7 an increasing sensitivity to degradation in collagenase-containing buffer is seen with increasing collagen content, and a lack of collagenase sensitivity is seen for PEUU scaffolds devoid of collagen. The enzymatic degradation did not appear to occur with a substantial burst within the first week and the average slopes of the degradation curves with collagenase present appeared higher than with buffer alone. There was no evidence that collagen was simply dissolving out of the scaffolds, but rather that there was a coupled degradation process involving PEUU hydrolysis and collagen enzymatic degradation. There was some evidence of synergistic interactions, because the mass loss of the 10% collagen scaffold at 56 days in collagenase was greater than 10% higher than for the scaffold mass loss in buffer. This might be explained by considering that the carboxylic acid groups of the water-soluble collagen degradation products could act to accelerate polyester degradation (9,17). Examining the degradation curves for PEUU/collagen scaffolds in buffer alone, it is seen that scaffolds with 20% and 30% collagen had more mass loss at 56 days than scaffolds with 0% or 10% collagen. This may be a result of higher collagen concentrations acting to loosen the PEUU structure and increase water access for hydrolytic attack. Although these in vitro data demonstrate the sensitivity of the scaffold to different degradation mechanisms, an in vivo study would be necessary to capture the effect of relevant matrix metalloproteinase concentrations and activity as well as other phenomena such as phagocytic activity that are not readily mimicked in vitro.
Demonstrating composite scaffold cytocompatibility and the benefit of adding collagen to the scaffold, smooth muscle cell numbers reflected in terms of mitochondrial activity were higher in the PEUU/collagen scaffold relative to the PEUU scaffold after 2 weeks of cell culture (Fig. 8). This occurred despite a significantly reduced porosity for the PEUU/collagen scaffold, which one might hypothesize would be a disadvantage in supporting cell adhesion and growth. The growth rates seen over the 4-week culture period appear comparable and suggest that there might be an early advantage in supporting cell adhesion for the PEUU/collagen scaffold. A limitation of this evaluation was the static cell culture method employed. If such scaffolds were to be seeded and grown to facilitate cell transplantation, more sophisticated culture techniques would likely be employed, such as spinner flask culture or transmural scaffold per-fusion to improve mass transfer characteristics (3).
This report extends earlier work in which we demonstrated the ability of the thermally induced phase separation technique to form highly porous polyurethane scaffolds from PEUU and a poly(ether ester urethane)urea, the latter polymer having generally faster hydrolytic degradation properties (10). The value in forming the PEUU/collagen composite is the introduced enzymatic sensitivity and improved cell adhesion discussed above, although the PEUU alone is relatively supportive of cell adhesion in serum-based culture medium (9). Pure collagen scaffolds, on the other hand, do not possess elastomeric behavior and are generally weak at high porosity without chemical cross-linking (13). Cross-linking of collagen scaffolds is often performed to increase tensile strength and reduce enzymatic lability (18), but common chemical cross-linking techniques can reduce the cytocompatibility of the scaffold (12,14). Earlier reported composites of collagen with polymers involved collagen integration into hydrogel materials (1,4) and combinations with thermoplastic polymers that do not possess the elastic mechanical properties of polyurethanes (6).
We have previously combined collagen with PEUU in an electrospinning processing technique to create a fibrous mesh with submicron diameter fibers (23). This report varies from that work in that the thermally induced phase separation technique utilizes an organic solvent that is milder than that required for electrospinning, so the collagen denaturation observed in that study at low collagen mass fractions would not be expected. Second, with the electrospinning technique it is a challenge to create strong scaffolds that also possess large pores, similar to those achievable with thermally induced phase separation. Finally, with the thermally induced phase separation technique it is easier to create scaffolds in complex geometries, because the process involves pouring solution into a mold that is then cooled, albeit with limitations following from even heat transfer.
In conclusion, we have created and characterized a biocomposite scaffold possessing collagenase sensitivity, supporting cellular growth, and exhibiting attractive mechanical properties for soft tissue applications by combining type I collagen in varying amounts with a thermoplastic, elastomeric, and biodegradable polymer. This scaffold type may prove useful as a vehicle to facilitate tissue development in vitro prior to transplantation, or as a means of providing temporary mechanical function and aiding in cellular retention and localization in cell transplantation.
Acknowledgments
This work was supported by the National Institutes of Health (HL069368).
References
- 1.Alexy P, Bakos D, Crkonova G, Kolomaznik K, Krsiak M. Blends of polyvinylalcohol with collagen hydrolysate: Thermal degradation and processing properties. Macromol Symp. 2001;170:41–49. [Google Scholar]
- 2.Barbani N, Lazzeri L, Cristallini C, Cascone MG, Polacco G, Pizzirani G. Bioartificial materials based on blends of collagen and poly(acrylic acid) J Appl Polym Sci. 1999;72:971–976. [Google Scholar]
- 3.Carrier RL, Rupnick M, Langer R, Schoen FJ, Freed LE, Vunjak-Novakovic G. Effects of oxygen on engineered cardiac muscle. Biotechnol Bioeng. 2002;78(6):617–625. doi: 10.1002/bit.10245. [DOI] [PubMed] [Google Scholar]
- 4.Cascone MG. Dynamic-mechanical properties of bioartificial polymeric materials. Polym Int. 1997;43:55–69. [Google Scholar]
- 5.Chen GP, Ushida T, Tateishi T. Hybrid biomaterials for tissue engineering: A preparative method for PLA or PLGA-collagen hybrid sponges. Adv Mater. 2000;12:455–457. [Google Scholar]
- 6.Coombes AGA, Verderio E, Shaw B, Li X, Griffin M, Downes S. Biocomposites of non-crosslinked natural synthetic polymers. Biomaterials. 2002;23:2113–2118. doi: 10.1016/s0142-9612(01)00341-6. [DOI] [PubMed] [Google Scholar]
- 7.Dunn MG, Bellincampi LD, Tria AJ, Zawadsky JP. Preliminary development of a collagen-PLA composite for ACL reconstruction. J Appl Polym Sci. 1997;63:1423–1428. [Google Scholar]
- 8.Fung YC. Biomechanics: Mechanical properties of living tissues. New York: Springer-Verlag; 1993. [Google Scholar]
- 9.Guan JJ, Sacks MS, Beckman EJ, Wagner WR. Synthesis, characterization, and cytocompatibility of elastomeric, biodegradable poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. J Biomed Mater Res. 2002;61:493–503. doi: 10.1002/jbm.10204. [DOI] [PubMed] [Google Scholar]
- 10.Guan JJ, Fujimoto KL, Sacks MS, Wagner WR. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials. 2005;26(18):3961–3971. doi: 10.1016/j.biomaterials.2004.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu YY, Gresser JD, Trantolo DJ, Lyons CM, Gangadharam PR, Wise DL. Effect of polymer foam morphology and density on kinetics of in vitro controlled release of isoniazid from compressed foam matrices. J Biomed Mater Res. 1997;35:107–116. doi: 10.1002/(sici)1097-4636(199704)35:1<107::aid-jbm11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 12.Jayakrishnan A, Jameela SR. Glutaraldehyde as a fixative in bioprostheses and drug delivery matrices. Biomaterials. 1996;17:471–484. doi: 10.1016/0142-9612(96)82721-9. [DOI] [PubMed] [Google Scholar]
- 13.Khor E. Methods for the treatment of collagenous tissues for bioprostheses. Biomaterials. 1997;18:95–105. doi: 10.1016/s0142-9612(96)00106-8. [DOI] [PubMed] [Google Scholar]
- 14.Koob TJ, Willis TA, Hernandez DJ. Biocompatibility of NDGA polymerized collagen fibers. I. Evaluation of cytotoxicity with tendon fibroblasts in vitro. J Biomed Mater Res. 2001;56:31–39. doi: 10.1002/1097-4636(200107)56:1<31::aid-jbm1065>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 15.Liu SQ, Kodama M. Porous polyurethane vascular prostheses with variable compliances. J Biomed Mater Res. 1992;26:1489–1494. doi: 10.1002/jbm.820261108. [DOI] [PubMed] [Google Scholar]
- 16.Nezu T, Winnik FM. Interaction of water-soluble collagen with poly(acrylic acid) Biomaterials. 2000;21(4):415–419. doi: 10.1016/s0142-9612(99)00204-5. [DOI] [PubMed] [Google Scholar]
- 17.Oh HJ, Kim WY, Lee DS, Lee YS. Polyurethane anionomers based on poly(butylene succinate), 4,4-meth-ylenebis(phenyl isocyanate), and 2,2-bis(hydroxymethyl) propionic acid. J Ind Eng Chem. 2000;6:425–430. [Google Scholar]
- 18.Pachence JM. Collagen-based devices for soft tissue repair. J Biomed Mater Res. 1996;33:35–40. doi: 10.1002/(SICI)1097-4636(199621)33:1<35::AID-JBM6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 19.Park JB, Lakes RS. Biomaterials: An introduction. 2. New York: Plenum; 1992. [Google Scholar]
- 20.Rehakova M, Bakos D, Vizarova K, Soldan M, Jurickova M. Properties of collagen and hyaluronic acid composite materials and their modification by chemical crosslinking. J Biomed Mater Res. 1996;30(3):369–372. doi: 10.1002/(SICI)1097-4636(199603)30:3<369::AID-JBM11>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 21.Samouillan V, Dandurand J, Lacabanne C, Thoma RJ, Adams A, Moore M. Comparison of chemical treatments on the chain dynamics and thermal stability of bovine pericardium collagen. J Biomed Mater Res. 2003;64A:330–338. doi: 10.1002/jbm.a.10326. [DOI] [PubMed] [Google Scholar]
- 22.Sarti B, Scandola M. Viscoelastic thermal-properties of collagen poly(vinyl alcohol) blends. Biomaterials. 1995;16:785–792. doi: 10.1016/0142-9612(95)99641-x. [DOI] [PubMed] [Google Scholar]
- 23.Stankus JJ, Guan JJ, Wagner WR. Fabrication of biodegradable, elastomeric scaffolds with sub-micron morphologies. J Biomed Mater Res. 2004;70A(4):603–614. doi: 10.1002/jbm.a.30122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taguchi T, Ikoma T, Tanaka J. An improved method to prepare hyaluronic acid and type II collagen composite matrices. J Biomed Mater Res. 2002;61:330–336. doi: 10.1002/jbm.10147. [DOI] [PubMed] [Google Scholar]
- 25.Zhang RY, Ma PX. Poly(α-hydroxyl acids)/hydroxy-apatite porous composites for bonetissue engineering. I. Preparation and morphology. J Biomed Mater Res. 1999;44:446–455. doi: 10.1002/(sici)1097-4636(19990315)44:4<446::aid-jbm11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]