Abstract
Background
Composition of nonselective proteinuria includes several endogenous ligands of Toll-like receptors (TLRs) not normally present in Bowman's space, thus raising the possibility that TLRs are involved in proteinuria-mediated podocyte injury.
Methods
Kidneys of NEP25 mice, a model of glomerular sclerosis induced by podocyte-specific injury, were immunohistochemically evaluated for the presence of fibrin/fibrinogen, which are potent ligands for TLRs. A podocyte cell line was treated with fibrinogen or lipopolysaccharides and examined for expression of cytokines. siRNAs were used to knockdown components of TLR signaling.
Results
We found deposits of fibrin/fibrinogen only in the damaged podocytes of proteinuric kidneys, indicating that podocytes are exposed to these potent TLR ligands in proteinuric state. In cultured podocytes, we confirmed mRNA expressions of TLR2, TLR4, as well as their major TLR signal transducer, MyD88. Fibrinogen and lipopolysaccharides dose-dependently upregulated mRNA expressions of MCP-1, TNF-α and TLR2 in podocytes as well as increased the MCP-1 protein in the medium. Knockdown of TLR2 and TLR4 inhibited the fibrinogen-induced MCP-1 mRNA upregulation. Knockdown of MyD88 also inhibited the upregulation.
Conclusion
These results suggest that plasma macromolecules that appear in Bowman's space in proteinuric conditions have the capacity to induce podocyte cytokines through TLRs, and thereby accelerate podocyte injury.
Key Words: Podocyte injury, Toll-like receptors, Proteinuria
Introduction
Podocyte injury is now considered not only a cause of proteinuria, but also a key step in the progression of various glomerular diseases [1,2,3,4,5]. We recently developed a transgenic mouse strain, NEP25, which expresses human CD25 transgene selectively in podocytes [6]. Injection of an immunotoxin (LMB2) that binds specifically to human CD25 induces proteinuria and subsequently glomerular sclerosis, indicating that podocyte injury alone can trigger the progression of glomerular sclerosis. Analysis of chimeric mice made up of CD25+ and CD25– cells showed that not only CD25+ but also CD25– podocytes within the same glomerulus become damaged in association with proteinuria following LMB2 injection [5]. Of interest, marked reduction of glomerular filtration induced by ureteral ligation prevented podocyte injury and development of subsequent glomerular sclerosis in this model. These observations led us to hypothesize that plasma macromolecules, which appear in Bowman's space in proteinuric conditions, are toxic to the podocytes and contribute to progressive glomerular injury.
Toll-like receptors (TLRs) were originally identified to constitute a defense mechanism against invasion of pathogenic microorganisms by the innate immune system [7,8]. When TLRs are activated by ligand binding, the Toll/IL-1 domain binds to the adaptor molecule MyD88 and transduces extracellular signals to intracellular signals that activate NF-κB and induce subsequent cytokine expressions. Recent findings indicate that activation of TLRs by a variety of endogenous ligands is pivotal in the progression of primary vascular diseases such as myocardial infarction and atherosclerosis [9,10,11,12]. Endogenous ligands of TLR4 include type III repeat extra-domain A of fibronectin, oligosaccharides of hyaluronic acid, heat shock proteins and fibrinogen [12,13,14,15,16,17]. These ligands are often seen in the blood and urine of patients with progressive glomerular diseases. TLR4 has been suggested to be involved in the development of membranoproliferative glomerulonephritis in mice with overexpression of thymic stromal lymphopoietin in which the nephropathy is triggered by deposition of immune complexes [17]. We therefore examined the possibility that macromolecules present in nonselective proteinuria act as pathogenic ligands by activating podocyte TLRs which, in turn, leads to induction of proinflammatory cytokines, thereby accelerating podocyte injury.
Materials and Methods
Materials
Solutions and FBS used for cell culture were purchased from Invitrogen (Carlsbad, Calif., USA). Fibrinogen and lipopolysaccharides (LPS; from Escherichia coli 055:B5 purified by ion-exchange chromatography) were purchased from Sigma (St. Louis, Mo., USA). MCP-1 ELISA and TNF-α ELISA were purchased from Biosource International (Camarillo, Calif., USA). RNeasy Mini Kit and Hyperfect Transfection Reagent were purchased from Qiagen (Hilden, Germany). Probes for real-time PCR, TaqMan reverse transcription reagents, TaqMan Master Mix and siRNAs were purchased from Applied Biosystems (Foster City, Calif., USA). Polyclonal anti-fibrinogen antibody was purchased from Nordic Immunological Laboratories (Tilburg, The Netherlands). Monoclonal anti-synaptopodin antibody was purchased from Progen (Heidelberg, Germany). Polyclonal anti-podocalyxin antibody was a generous gift from Dr. Kurihara, Jyuntendo University, Tokyo, Japan.
Animal Experiments
The institutional Animal Care and Use Committee at Vanderbilt University Medical Center and the Animal Experimentation Committee of Tokai University approved the protocol in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
NEP25 mice were injected with LMB2 (25 ng/g body weight) and sacrificed on the 5th day after the injection, as described earlier [6]. Kidneys were isolated and processed for histological analysis. Polyclonal anti-fibrinogen antibody (1:1,000 dilution) was used as the primary antibody to stain paraffin sections. Monoclonal anti-synaptopodin (1:1) antibody or polyclonal anti-podocalyxin (1:2,000) antibody was used to stain adjacent sections. We have tested two different antibodies (Santa Cruz Biotechnology, Santa Cruz, Calif., USA) against TLR2 or TLR4 to assess their in vivo expressions (n = 3). Although positive staining was observed in frozen sections of the kidney, the staining pattern was markedly different from in situ hybridization or immunohistochemistry previously reported [18]. Further, the staining patterns were not altered by ischemia-reperfusion (n = 3), findings that contradict previous reports of TLR2 and TLR4 upregulation under the same experimental condition [19,20,21,22]. Since we failed to verify that commercially available antibodies faithfully represent TLRs in vivo, we studied cultured podocytes.
Cell Culture
A conditionally immortalized mouse podocyte cell line [23] was the generous gift from Dr. Mundel, Mount Sinai School of Medicine, New York, N.Y., USA. Cells were cultured on laminin-coated dishes or tissue culture plates. Cells were maintained in Dulbecco's Modified Eagle Medium containing 10% FBS and 50 μg/ml IFN-γ at the permissive temperature of 33°C. Experiments were performed using differentiated cells. Cells were differentiated by incubating them at the nonpermissive temperature of 37°C in a medium without IFN-γ for at least 1 week.
MCP-1 Protein Expression
Differentiated cells were treated with several different concentrations of LPS or fibrinogen for 24 h. After 24 h of treatment, concentration of MCP-1 or TNF-α protein in culture supernatant was determined by ELISA.
TLR2, TLR4, MCP-1, TNF-α and MyD88 mRNA Expression
Differentiated cells were treated with several different concentrations of LPS or fibrinogen for 3 h. Total RNA was prepared using an RNeasy Mini Kit. 100 ng of total RNA were applied for cDNA synthesis using MMLV reverse transcriptase in a volume of 10 μl. The mRNA expression was assessed for TLR2, TLR4, MCP-1, TNF-α and MyD88 in 1 μl of cDNA by real-time PCR method (7300 Real-time PCR System; Applied Biosystems, or iCycler; Bio-Rad Laboratories, Hercules, Calif., USA). β-Actin expression served as a control. Assay IDs for each probe were TLR2: Mm00442346_m1, TLR4: Mm00445274_m1, MCP-1: Mm00441242_m1, TNF-α: Mm00443258_m1, MyD88: Mm00440338_m1, and β-actin: Mm00607939_s1. Data were calculated using the comparative Ct method. In some experiments, stock solutions of fibrinogen and LPS were heat-denatured for 15 min at 95°C.
Measurement of Endotoxin
Endotoxin contamination in fibrinogen preparation was assessed by the limulus amebocyte lysate test according to the manufacturer's instruction (Endspecy® tests; Seikagaku Kogyo, Tokyo, Japan). Control experiments with endotoxin-spiked samples showed more than 90% recovery of the spike.
Knockdown Experiments by RNAi
Cells were cultured in 6-well cell culture plates. 24 h prior to transfection, the medium in each well was replaced with 2 ml of fresh medium. Two microliters of siRNA solution (20 μM) were diluted with 100 μl of serum-free medium, and 12 μl of transfection reagent was added to the diluted siRNA. In double knockdown experiments, 1 μl of TLR2 siRNA and 1 μl of TLR4 siRNA were combined. The mixture was incubated at room temperature for 10 min and added dropwise onto the cells. Cell culture plates were gently swirled to ensure a uniform distribution of transfection complexes. The cells were incubated for 48 h and then stimulated with fibrinogen for 3 h. ID numbers for siRNA from Applied Biosystems (Ambion) were 187990 for TLR2, 188777 for TLR4, 156410 for MyD88, and AM4611 for negative control.
Western Blotting
Podocytes were treated with indicated siRNA(s) for 48 h in 12-well plates. Then, cells were trypsinized, lysed with 100 μl of 50 mM Tris-HCl pH 7.4, 0.5 mM PMSF, 2 mM CaCl2, 1% Triton-X100 and centrifuged at 14,000 g for 10 min. Protein concentration in each sample was determined by using a DC protein assay kit (Bio-Rad Laboratories). NuPAGE LDS sample buffer (Invitrogen) was added to each sample. Samples were boiled for 5 min. Five micrograms protein from each sample were loaded to NuPAGE Novex Bis-Tris 4–12% gradient gels (Invitrogen) and electrophoresed at 200 V for 35 min. Separated proteins were transferred to a sheet of immobilon-P membrane (Millipore, Bedford, Mass., USA). Transferred membranes were immunoblotted with goat anti-TLR4 antibody (sc-16240; Santa Cruz Biotechnology). Signal was developed by the ECL Western blotting detection system using anti-goat IgG (Amersham, Biosciences, Piscataway, N.J., USA) as a second antibody and detected by an image analyzer (CS analyzer; Atto, Tokyo, Japan). Membranes were stripped using a stripping buffer (Nacalai Tesque, Kyoto, Japan) and immunoblotted with mouse anti-β-actin antibody (Sigma). Two of the available antibodies for TLR2 did not detect any band in those blots.
Statistical Analysis
Values are expressed as means ± SD. Statistical difference was assessed by one-way ANOVA. A p-value <0.05 was considered significant.
Results
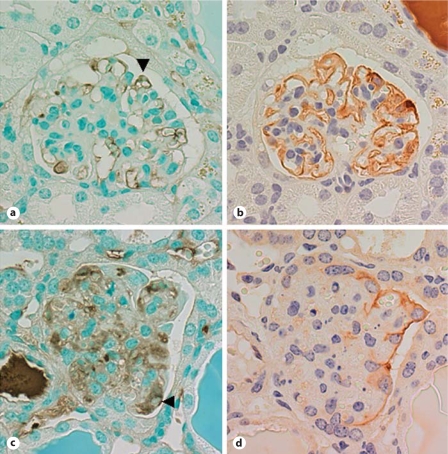
Using our model of podocyte-specific injury, we first examined whether fibrin/fibrinogen appears in Bowman's space when massive proteinuria is induced by podocyte injury. In addition to a marked staining pattern observed in capillaries and sclerotic mesangial regions, anti-fibrinogen antibody staining was demonstrated within podocytes and parietal epithelial cells in injured glomeruli (fig. 1). Fibrinogen-positive podocytes were found in glomeruli with mild injury with normal expression pattern of synaptopodin (fig. 1a, b) as well as in severely injured glomeruli (fig. 1c, d). These results indicate that podocytes take up fibrinogen, fibrin or its degradation product and were thus exposed to these plasma proteins.
Fig. 1.
Fibrinogen, fibrin or its degradation product deposits in the glomerulus with injured podocytes. NEP25 mice were injected with LMB2 (25 ng/g body weight) and kidneys were isolated on the 5th day after injection. Paraffin sections were stained with anti-fibrinogen antibody. a Fibrinogen, fibrin or its degradation products are detected in a podocyte (arrowhead) of a glomerulus with slightly damaged capillary tufts. b Synaptopodin staining confirmed the identity of podocytes in adjacent sections. c Positive staining for fibrinogen, fibrin or its degradation product is found in a podocyte (arrowhead) of a sclerotic glomerulus. d Since synaptopodin staining is absent in damaged podocytes, podocalyxin staining was used to identify podocytes.
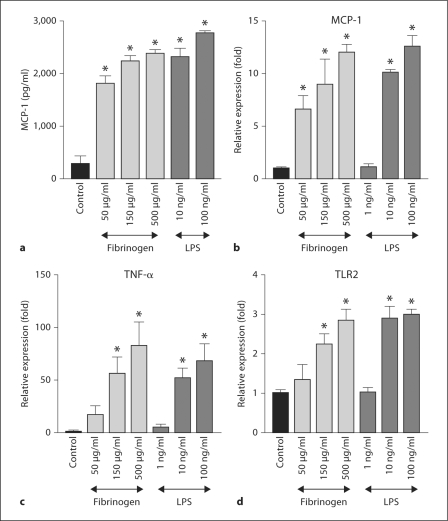
To explore the potential role of TLRs in podocyte injury, we then examined whether these cells respond to fibrinogen, a serum component. Fibrinogen treatment dramatically increased MCP-1 protein in the supernatant of cultured podocytes (fig. 2a). Similarly, LPS, a well-known ligand for TLR4, also increased MCP-1 protein in these cells. Although the amount of TNF-α protein in the medium of control cells was below detection limit and the data from fibrinogen- or LPS-treated cells were highly variable, a similar tendency was observed for TNF-α protein expression (data not shown). To examine whether induction of this cytokine in the podocyte is dependent on transcriptional regulation, we assessed the dose dependency of expression of MCP-1 mRNA. In addition to MCP-1 mRNA expression, fibrinogen treatment showed dose-dependent induction of TNF-α and TLR2 (fig. 2b–d). Similar results were observed when cells were treated with LPS. By contrast, neither LPS nor fibrinogen affected TLR4 mRNA expression (data not shown). Maximal induction of MCP-1, TNF-α and TLR2 mRNA expressions were observed 3 h after treatments (data not shown).
Fig. 2.
Fibrinogen or LPS induces MCP-1 protein and mRNA expression in podocytes. a MCP-1 protein induction. Cultured differentiated podocytes were treated with fibrinogen or LPS for 24 h. MCP-1 in culture supernatant was assessed by an ELISA. Data are representative of 5 wells. * p < 0.01 vs. control. b–d Dose-response of fibrinogen- and LPS-induced gene upregulation in podocytes. Cultured differentiated podocytes were treated with fibrinogen or LPS for 3 h at the indicated concentrations (n = 3). Expressions of MCP-1, TNF-α or TLR2 mRNA were assessed by real-time RT PCR. * p < 0.01 vs. control.
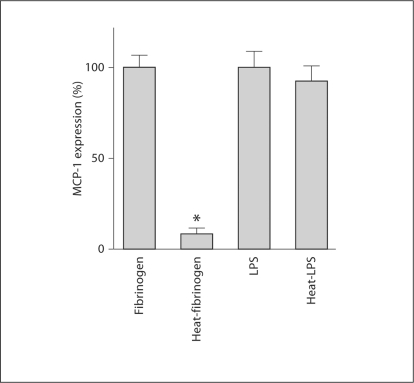
Since contamination of LPS, if any, in the fibrinogen preparation may affect these results, we assessed the endotoxin content in fibrinogen and LPS preparations. While the endotoxin content in 10 ng/ml of LPS preparation was estimated to be 22,670 EU/l, in 500 μg/ml of fibrinogen preparation it was 1,186 EU/l. The latter value is, therefore, approximately 1/20 of 10 ng/ml LPS preparation. Heat denaturation of fibrinogen has been reported to diminish activation of TLR4 [17]. In concert with this previous report, heat denaturation of fibrinogen reduced fibrinogen-induced MCP-1 mRNA upregulation (fig. 3) in our study. In contrast, heat-treated LPS was capable of inducing MCP-1 mRNA upregulation in a manner similar to untreated LPS.
Fig. 3.
Effect of heat treatment on fibrinogen- and LPS-induced MCP-1 mRNA upregulation in podocytes. Fibrinogen or LPS was heat-treated at 95°C for 15 min. Cultured differentiated podocytes were treated with 500 μg/ml fibrinogen, 500 μg/ml heat-treated fibrinogen, 10 ng/ml LPS or 10 ng/ml heat-treated LPS for 3 h at the indicated concentrations (n = 3). Expression of MCP-1 was assessed by real-time RT PCR. * p < 0.01 vs. control.
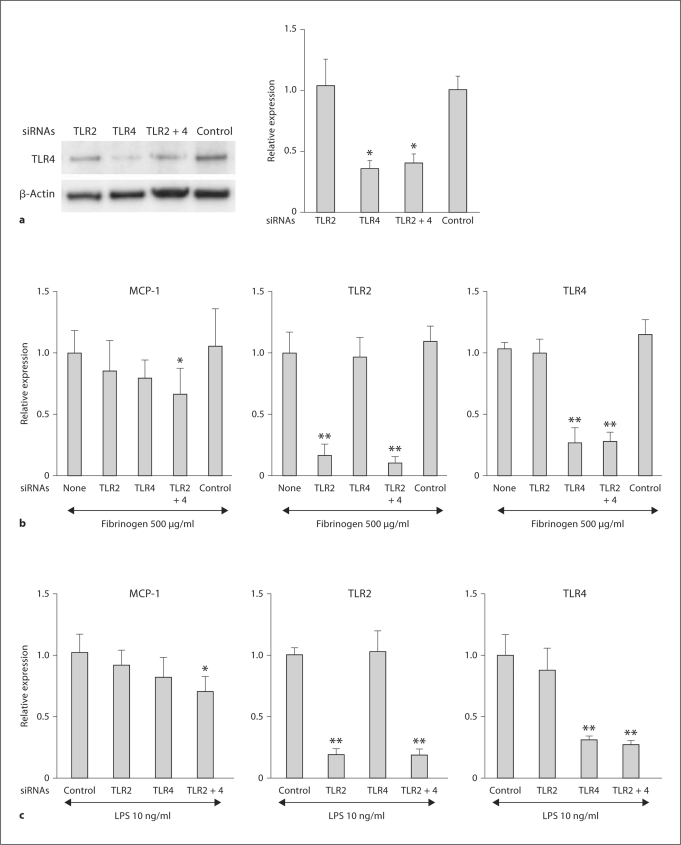
To test whether TLR signaling mediates the MCP-1 mRNA induction by fibrinogen, we utilized the RNAi technology. Effects of siRNA were assessed in podocytes treated with siRNA for 48 h and then fibrinogen for 3 h. Western blot analysis showed a 60–65% reduction in TLR4 expression in TLR4 siRNA-treated cells or TLR2 siRNA + TLR4 siRNA-treated cells (fig. 4a). Unfortunately, two available antibodies to TLR2 did not detect any band on those blots. When treated with siRNA for TLR2, immortalized podocytes had a 80–90% attenuation in the fibrinogen-induced upregulation of TLR2 mRNA expression (fig. 4b). Similarly, treatment with siRNA for TLR4 attenuated TLR4 mRNA expression by more than 70%. When compared to fibrinogen-induced expression of MCP-1 mRNA in control experiments, the mRNA induction was 15–25% lower in cells treated with siRNA for TLR2 or TLR4, and significant inhibition was observed when cells were treated with both siRNAs. The negative control siRNA was without effect on the expression of MCP-1, TLR2 or TLR4 in fibrinogen-treated cells. LPS-treated cells showed a response similar to that of fibrinogen-treated cells (fig. 4c). TLR2 siRNA was required to detect a significant reduction as in the case of fibrinogen. Therefore, it is not possible to exclude the possibility that contaminated TLR2 ligands were involved in the full induction of MCP-1 in the LPS treatment.
Fig. 4.
Effects of TLR knockdown on fibrinogen or LPS-induced MCP-1 mRNA upregulation in podocytes. a Effects of siRNAs on TLR4 protein expression. Cultured differentiated podocytes were treated with siRNA for TLR2 and/or TLR4 for 48 h (n = 4). Expression of TLR4 protein was assessed by Western blotting. * p < 0.05 vs. negative control siRNA-treated cells. b Effects of TLR knockdown on fibrinogen-induced MCP-1 mRNA upregulation in podocytes. Cultured differentiated podocytes were treated with siRNA for TLR2 and/or TLR4 for 48 h, then with 500 μg/ml fibrinogen for 3 h (n = 6). Expression of MCP-1, TLR2 or TLR4 mRNA was assessed by real-time RT PCR. * p < 0.05 and ** p < 0.01 vs. expression in fibrinogen-treated cells (none: no siRNA). c Effects of TLR knockdown on LPS-induced MCP-1 mRNA upregulation in podocytes. Podocytes treated with siRNA for TLR2 and/or TLR4 were subsequently treated with 10 ng/ml LPS for 3 h (n = 6).
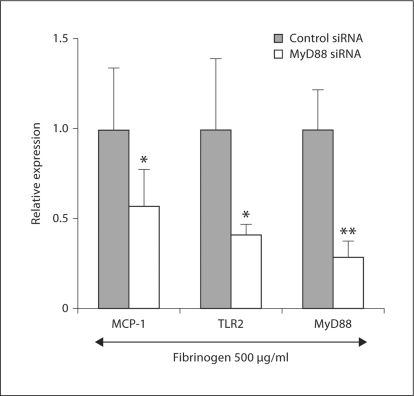
Finally, we examined whether the signal transduced by MyD88 was involved in TLR2- and TLR4-mediated MCP-1 induction. Treatment with siRNA for MyD88 led to a reduction in its expression by 80% (fig. 5). Under these experimental conditions, fibrinogen-induced MCP-1 expression was significantly attenuated, on average by 40%, and fibrinogen-induced TLR2 expression was attenuated on average by 60%, when compared to the negative control siRNA treatment.
Fig. 5.
Effects of MyD88 knockdown on fibrinogen-induced MCP-1 and TLR2 mRNA upregulation in podocytes. Cultured differentiated podocytes were treated with siRNA for MyD88 for 48 h, then with 500 μg/ml fibrinogen for 3 h (n = 6). Expression of MCP-1, TLR2 and MyD88 mRNA was assessed by real-time RT PCR. * p < 0.05 and ** p < 0.01 vs. expression in the negative control siRNA and fibrinogen-treated cells.
Discussion
Our observations that glomeruli of NEP25 mice have extensive staining with anti-fibrinogen antibody indicate that when podocytes are injured, considerable amounts of fibrinogen and/or fibrin degradation products appear in the ultrafiltrate. These findings fit well with the clinical demonstration of fibrinogen and fibrin degradation products in the urine of patients with nonselective proteinuria that was associated with progression of glomerular disease [24]. Recent suggestions that endogenous ligands, including fibrinogen/fibrin, promote vascular diseases through TLRs raise the possibility that this mechanism is also important in glomerular capillary diseases that characterize proteinuric states. The present study shows that functionally responsive TLR2 and TLR4 are expressed on podocytes. Thus, an immortalized podocyte cell line was found to be positive for TLR2 and TLR4 at mRNA level. Assuming that efficiencies of real-time PCR for TLR2 and TLR4 are comparable, TLR2 mRNA expression is almost four times higher than that of TLR4 (data not shown). Our studies also found that exposing the podocyte cell line to LPS, a well-known ligand of TLR4, induces MCP-1, TNF-α and TLR2. LPS has also been reported to induce podocyte granulocyte macrophage colony-stimulating factor [25] and B7-1 [26] in podocytes. Taken together, these data suggest that podocytes possess TLRs which may be upregulated by ligands present in glomerular ultrafiltrate in proteinuric states together with increase in proinflammatory cytokines. In this connection, it has been shown that TLR4 on podocytes was linked to the development of glomerular injury in a mouse model of cryoglobulinemic membranoproliferative glomerulonephritis [17]. Our study further indicates that TLRs are involved in proteinuric conditions in general, not limited to a specific immune-mediated glomerular injury. It is possible that other candidate ligands for TLRs potentially present in proteinuria include degradation products of the extracellular matrix. Increased matrix turnover during the development of glomerular sclerosis may expose TLRs on podocytes to endogenous ligands such as heparan sulfate, fibronectin extra-domain A and/or hyaluronic acid [12,13,14,15,16,17]. Recently, biglycan has been reported to trigger signals from TLR2 and TLR4 [27].
While previous studies in cultured mouse tubular epithelial cells exposed to LPS showed upregulated MCP-1, RANTES, TLR2 and TLR4 [28], we found no change in TLR4 mRNA expression in podocytes. Our data show that fibrinogen-induced upregulation of MCP-1, TNF-α and TLR2 are comparable to the patterns induced by LPS, raising the possibility that LPS and fibrinogen share common receptors. Our results that fibrinogen upregulated TLR2, but not TLR4, prompted us to test the involvement of TLR2 in fibrinogen-induced MCP-1 mRNA upregulation. In the present study, siRNA for both TLR2 and TLR4 showed additive inhibitory effects on the induction of MCP-1 mRNA. TLR2 siRNA or TLR4 siRNA alone did not show significant inhibition of MCP-1 mRNA induction by fibrinogen. This is likely due to insufficient knockdown of each TLR. Thus, the effects of fibrinogen on MCP-1 were, at least in part, mediated by TLR2 and TLR4. In contrast, since siRNA for TLR4 did not show any effect on TLR2 induction, TLR2 induction may be mediated by TLR2 alone. In podocytes, siRNA for TLR4 was shown to inhibit fibrinogen-induced chemokine expression [17]. In our study, involvement of TLR signal in fibrinogen-induced MCP-1 and TLR2 upregulation was further confirmed with the use of siRNA for the major signal transducer of TLRs, MyD88. Thus, MyD88 siRNA alone inhibited the induction of MCP-1 and TLR2 in podocytes, indicating that both TLR2 and TLR4 signaling cascades utilize MyD88 as their signal transducer.
It has been reported that podocyte injury increased expression of MCP-1 in various proteinuric conditions [29], while neutralization of MCP-1 showed antiproteinuric and renoprotective effects in crescentic glomerulonephritis [30,31]. Moreover, recent studies demonstrated an expression of CC chemokine receptor 2 in podocytes, and increased motility of podocytes after treatment with MCP-1 [32,33]. Therefore, overexpressed MCP-1 in podocytes may change the phenotype of podocytes and mediate propagation of podocyte injury.
An increasing body of experimental and clinical evidence suggests a role for plasma macromolecules within the glomerular ultrafiltrate in the progression of glomerular injury leading to chronic renal failure [34,35]. TLR 2 and/or TLR4 expressed in podocytes, activated by serum macromolecules such as fibrinogen in glomerular ultrafiltrate, may accelerate podocyte damage and, thereby, promote the progression of glomerular injury through induction of inflammatory cytokines. Although the results from the present study need to be confirmed in independent cell lines and primary cultures, the present study supports the notion of an involvement of serum macromolecules and potential role of TLRs in the progression of podocyte injury.
Acknowledgements
This study was supported by National Institutes of Health Grants DK037868 and DK447577, and a Grant-in Aid for Scientific Research of the Japan Society for the Promotion of Science, 16109005.
We thank Dr. P. Mundel for the generous gift of immortalized podocytes.
Footnotes
Part of this study was presented in abstract form at the American Society of Nephrology Meeting (San Diego, Calif., USA, 2006) and the American Society of Nephrology Meeting (Philadelphia, Pa., USA, 2005).
References
- 1.Schwartz MM. The role of podocyte injury in the pathogenesis of focal segmental glomerulosclerosis. Ren Fail. 2000;22:663–684. doi: 10.1081/jdi-100101955. [DOI] [PubMed] [Google Scholar]
- 2.Kerjaschki D. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest. 2001;108:1583–1587. doi: 10.1172/JCI14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kriz W. Podocyte is the major culprit accounting for the progression of chronic renal disease. Microsc Res Tech. 2002;57:189–195. doi: 10.1002/jemt.10072. [DOI] [PubMed] [Google Scholar]
- 4.Asanuma K, Mundel P. The role of podocytes in glomerular pathobiology. Clin Exp Nephrol. 2003;7:255–259. doi: 10.1007/s10157-003-0259-6. [DOI] [PubMed] [Google Scholar]
- 5.Ichikawa I, Ma J, Motojima M, Matsusaka T. Podocyte damage damages podocytes: autonomous vicious cycle that drives local spread of glomerular sclerosis. Curr Opin Nephrol Hypertens. 2005;14:205–210. doi: 10.1097/01.mnh.0000165884.85803.e1. [DOI] [PubMed] [Google Scholar]
- 6.Matsusaka T, Xin J, Niwa S, Kobayashi K, Akatsuka A, Hashizume H, Wang QC, Pastan I, Fogo AB, Ichikawa I. Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol. 2005;16:1013–1023. doi: 10.1681/ASN.2004080720. [DOI] [PubMed] [Google Scholar]
- 7.Anders HJ, Banas B, Schlondorff D. Signaling danger: Toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol. 2004;15:854–867. doi: 10.1097/01.asn.0000121781.89599.16. [DOI] [PubMed] [Google Scholar]
- 8.Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 9.Frantz S, Ertl G, Bauersachs J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat Clin Pract Cardiovasc. 2007;4:444–454. doi: 10.1038/ncpcardio0938. [DOI] [PubMed] [Google Scholar]
- 10.Vink A, Schoneveld AH, van der Meer JJ, van Middelaar BJ, Sluijter JP, Smeets MB, Quax PH, Lim SK, Borst C, Pasterkamp G, de Kleijn DP. In vivo evidence for a role of toll-like receptor 4 in the development of intimal lesions. Circulation. 2002;106:1985–1990. doi: 10.1161/01.cir.0000032146.75113.ee. [DOI] [PubMed] [Google Scholar]
- 11.Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 12.Schoneveld AH, Hoefer I, Sluijter JP, Laman JD, de Kleijn DP, Pasterkamp G. Atherosclerotic lesion development and Toll like receptor 2 and 4 responsiveness. Atherosclerosis. 2008;197:95–104. doi: 10.1016/j.atherosclerosis.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Asea A. Heat shock proteins and toll-like receptors. Handb Exp Pharmacol. 2008;183:111–127. doi: 10.1007/978-3-540-72167-3_6. [DOI] [PubMed] [Google Scholar]
- 14.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 15.Chen K, Huang J, Gong W, Iribarren P, Dunlop NM, Wang JM. Toll-like receptors in inflammation, infection and cancer. Int Immunopharmacol. 2007;7:1271–1285. doi: 10.1016/j.intimp.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 16.Kuhns DB, Priel DA, Gallin JI. Induction of human monocyte interleukin (IL)-8 by fibrinogen through the toll-like receptor pathway. Inflammation. 2007;30:178–188. doi: 10.1007/s10753-007-9035-1. [DOI] [PubMed] [Google Scholar]
- 17.Banas MC, Banas B, Hudkins KL, Wietecha TA, Iyoda M, Bock E, Hauser P, Pippin JW, Shankland SJ, Smith KD, Stoelcker B, Liu G, Gröne HJ, Krämer BK, Alpers CE. TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol. 2008;19:704–713. doi: 10.1681/ASN.2007040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolfs TG, Buurman WA, van Schadewijk A, de Vries AB, Daemon MA, Hiemstra PS, van't Veer C. In vivo expression of toll-like receptor 2 and 4 by renal epithelial cells: IFN-γ and TNF-α mediated up-regulation during inflammation. J Immunol. 2002;168:1286–1293. doi: 10.4049/jimmunol.168.3.1286. [DOI] [PubMed] [Google Scholar]
- 19.Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest. 2005;115:2894–2903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shigeoka AA, Holscher TD, King AJ, Hall FW, Kiosses WB, Tobias PS, Mackman N, McKay DB. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J Immunol. 2007;178:6252–6258. doi: 10.4049/jimmunol.178.10.6252. [DOI] [PubMed] [Google Scholar]
- 21.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patole PS, Pawar RD, Lech M, Zecher D, Schmidt H, Segerer S, Ellwart A, Henger A, Kretzler M, Anders HJ. Expression and regulation of Toll-like receptors in lupus-like immune complex glomerulonephritis of MRL-Fas(lpr) mice. Nephrol Dial Transplant. 2006;21:3062–3073. doi: 10.1093/ndt/gfl336. [DOI] [PubMed] [Google Scholar]
- 23.Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- 24.Kamitsuji H, Whitworth JA, Dowling JP, Kincaid-Smith P. Urinary crosslinked fibrin degradation products in glomerular disease. Am J Kidney Dis. 1986;7:452–455. doi: 10.1016/s0272-6386(86)80183-4. [DOI] [PubMed] [Google Scholar]
- 25.Greiber S, Muller B, Daemisch P, Pavenstadt H. Reactive oxygen species alter gene expression in podocytes: induction of granulocyte macrophage-colony stimulating factor. J Am Soc Nephrol. 2002;13:86–95. doi: 10.1681/ASN.V13186. [DOI] [PubMed] [Google Scholar]
- 26.Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe Schwarz HK, Faul C, Kretzler M, Davidson A, Sugimoto H, Kalluri R, Sharpe AH, Kreidberg JA, Mundel P. Induction of B7-1 in podocytes is associated with nephritic syndrome. J Clin Invest. 2004;113:1390–1397. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Götte M, Malle E, Schaefer RM, Gröne HJ. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuboi N, Yoshikai Y, Matsuo S, Kikuchi T, Iwami K, Nagai Y, Takeuchi O, Akira S, Matsuguchi T. Roles of toll-like receptors in C-C chemokine production by renal tubular cells. J Immunol. 2002;169:2026–2033. doi: 10.4049/jimmunol.169.4.2026. [DOI] [PubMed] [Google Scholar]
- 29.Prodjosudjadi W, Gerritsma JS, van Es LA, Daha MR, Bruijn JA. Monocyte chemoattractant protein-1 in normal and diseased human kidneys: an immunohistochemical analysis. Clin Nephrol. 1995;44:148–155. [PubMed] [Google Scholar]
- 30.Lloyd CM, Dorf ME, Proudfoot A, Salant DJ, Gutierrez-Ramos JC. Role of MCP-1 and RANTES in inflammation and progression to fibrosis during murine crescentic nephritis. J Leukoc Biol. 1997;62:676–680. doi: 10.1002/jlb.62.5.676. [DOI] [PubMed] [Google Scholar]
- 31.Wada T, Yokoyama H, Furuichi K, Kobayashi KI, Harada K, Naruto M, Su SB, Akiyama M, Mukaida N, Matsushima K. Intervention of crescentic glomerulonephritis by antibodies to monocyte chemotactic and activating factor MCAF/MCP-1. FASEB J. 1996;10:1418–1425. [PubMed] [Google Scholar]
- 32.Rao VH, Meehan DT, Delimont D, Nakajima M, Wada T, Gratton MA, Cosgrove D. Role for macrophage metalloelastase in glomerular basement membrane damage associated with Alport syndrome. Am J Pathol. 2006;169:32–46. doi: 10.2353/ajpath.2006.050896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burt D, Salvidio G, Tarabra E, Barutta F, Pinach S, Dentelli P, Camussi G, Perin PC, Gruden G. The monocyte chemoattractant protein-1/cognate CC chemokine receptor 2 system affects cell motility in cultured human podocytes. Am J Pathol. 2007;171:1789–1799. doi: 10.2353/ajpath.2007.070398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17:2974–2984. doi: 10.1681/ASN.2006040377. [DOI] [PubMed] [Google Scholar]
- 35.Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116:288–296. doi: 10.1172/JCI27699. [DOI] [PMC free article] [PubMed] [Google Scholar]