Abstract
Recent decade has witnessed an extensive advancement in our understanding of transcriptional regulation, in part due to a rapid progress in technologies which allow studying physical proximities between various chromatin regions at a resolution beyond that offered by conventional microscopy techniques. However, these methods do not specifically identify the protein component(s) that might mediate such interactions. Here we discusses the detailed protocol for Combined 3C-ChIP-Cloning (6C) technology which combines multiple techniques to simultaneously identify physical proximities between chromatin elements as well as the proteins that mediate these interactions. We further explore how the 6C assay can be incorporated with other techniques for a complete, cell-type-specific mapping of all inter and intrachromosomal interactions mediated by specific proteins. Thus, 6C assay provides an indispensable tool to address the role of specific proteins in nuclear organization and advances our understanding about the relation of chromatin higher order organization with transcriptional regulation to the next level.
KEY TERMS: Transcriptional regulation, Chromatin folding, Nuclear organization
INTRODUCTION
Last few years have shed light on molecular mysteries of how chromatin folding into higher order domains and inter and intrachromosomal interactions may regulate many aspects of gene-specific transcription, genomic imprinting and X-inactivation. In past, evidences from microscopy studies already suggested that transcription events accompany changes at the chromosome level, sub chromosomal and gene-specific levels. However, the recent advancements in our understanding of transcriptional regulation were accomplished due to a rapid progress in technologies to address higher order chromatin conformation and long-range chromosomal interactions. These methods allow studying physical proximities between various chromatin regions and allow analyzing the folding of chromatin in vivo at a resolution beyond that offered by conventional microscopy techniques. However, these methods do not specifically identify the protein component(s) that might mediate such interactions. This article discusses the detailed protocol for Combined 3C-ChIP-Cloning (6C) technology, where we combine multiple techniques to simultaneously identify physical proximities between chromatin elements as well as the proteins that mediate these interactions. This method also allows determining if a candidate protein has potential to mediate inter- or intrachromosomal interactions. We further explore how the 6C assay can be incorporated with other techniques to discover all the chromatin regions in the nucleus that interact with a given chromatin element in a specific protein-dependent manner which would lead to a complete, cell-type-specific mapping of all inter and intrachromosomal interactions mediated by specific proteins. Thus, 6C assay provides an indispensable tool to address the role of specific proteins in nuclear organization and advances our understanding about the relation of chromatin higher order organization with transcriptional regulation to the next level. Here, we provide step-by-step protocol of the 6C methodology and explore how it can be used by scientists interested in studying the role of specific proteins in chromatin higher order folding and nuclear organization.
BASIC PROTOCOL: Combined 3C-ChIP-Cloning (6C) Technology
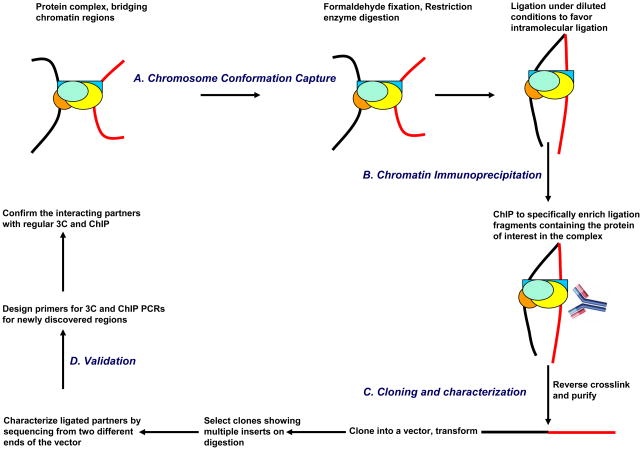
As the nomenclature “Combined 3C-ChIP-Cloning (6C) Technology” suggests, the 6C assay incorporates three established methodologies: chromosome conformation capture (3C) (Dekker et al. 2002), chromatin immunoprecipitation (ChIP) and cloning (Fig. 1). During the first steps, conventional 3C assay is performed where the chromatin is crosslinked, digested with restriction enzymes, and ligated under much diluted conditions to favor intramolecular ligation. This is immediately followed by immunoprecipitation using an antibody against the protein of interest (i.e., the suspected ‘bridging protein’ or the protein whose mediating physical proximities the scientist wishes to map). Thenceforth, the crosslinks are reversed, DNA is purified and the fragments are cloned into a vector that harbors the same restriction enzyme site overhangs that were generated in the enzyme digestion step of the 3C portion of the protocol. Then, these clones are digested with the same restriction enzyme for screening. In an ideal scenario, clones showing multiple inserts should represent physical proximities involving the protein targeted in the immunoprecipitation steps. These clones are then sequenced to uncover the identity of the partners. For a detailed discussion about some recent methods developed for studying chromatin long-range interactions, see Simonis et al. (Simonis et al, 2007). For additional experimental examples on the 6C assay discussed here, refer to Tiwari et al. (Tiwari et al, 2008). Below, we provide a more detailed outline of the experimental steps involved the 6C assay to allow a better appreciation of the potentials and limitations of the method. The entire procedure can be completed in six days.
Figure 1.
Summary of the Combined 3C-ChIP-Cloning (6C) method. Briefly, the conventional 3C assay (Tolhuis et al. 2002) in performed till the ligation step, following which the chromatin is subjected to chromatin immunoprecipitation (ChIP) using an antibody against the protein of interest. Then, the purified DNA is ligated into a cloning vector bearing the sequence overhangs generated in the enzyme digestion used in the 3C assay to facilitate insert cloning and further screening. The screening of clones is done by digestion with the restriction enzyme that was used for the 3C assay, and the ones showing multiple inserts were subjected to sequencing from both directions in the vector to reveal the identity of the partners.
MATERIALS
5-bromo-4-chloro-3-indoyl-β-D-galactopyranoside (X-gal) (25 mg/ml stock)
Agarose gel (1%, UltraPure™; Invitrogen)
Antibody, specific for the protein of interest
Elution buffer (see recipe)
Bovine serum albumin (BSA; 0.5% in PBS; New England Biolabs)
Cell type of interest, grown under appropriate cell culture conditions
Cells, competent, high-efficiency (≥ 5 × 109 cfu/μg DNA)
XL10-Gold ultracompetent cells (Stratagene) have produced optimal results.
Cell lysis solution (see recipe)
ChIP dilution buffer (see recipe)
Cloning vector
DNA gel stain (SYBR® Safe, 10,000X concentrate in DMSO; Invitrogen)
DNA ladder, 100-bp and/or 1-kb (New England Biolabs)
DNA polymerase (REDTaq®; Sigma, D4309) and dNTP set (100 mM; Invitrogen) (for PCRs after ChIP step in the 6C assay (step 44) and for 3C PCRs to validate 6C interactions (see Discussion)
Ethanol (70 and 100%)
Fetal bovine serum (10% in PBS)
Formaldehyde solution (≥ 36.5%; Sigma-Aldrich, 33220)
Glycine (2.5 M; Fisher, BP381)
Glycogen (Roche)
Isopropyl-β-D-thio-galactopyranoside (IPTG) (200 mg/mL stock)
Wash buffers (high-salt and low-salt) (see recipe)
Prepare the high-salt and low-salt versions of this buffer separately.
LB agar
LB-ampicillin agar plates (optional; see Step 47)
LB-kanamycin agar plates (optional; see Step 47)
LB medium
Ligation buffer
Liquid nitrogen (optional; see Step 11)
Magnetic beads, Protein A-conjugated
Magnetic beads, Protein G-conjugated
PCR buffer (10X)
PCR primers for sequencing the plasmid as well as for validating physical interactions between captured chromatin elements by 3C assay and protein occupancy by ChIP assay
Phenol:chloroform:isoamyl alcohol, UltraPure™ (25:24:1 v/v/v; Invitrogen)
Phosphate-buffered saline (PBS; GIBCO, 20012)
Protease inhibitor cocktail (Sigma, P8340)
Proteinase K (10 mg/mL in TE buffer, pH 8.0; Invitrogen)
PureLink™ HQ 96 Mini Plasmid DNA Purification Kit (Invitrogen)
This kit facilitates the simultaneous growth of several bacterial colonies and yields high amounts of high-quality DNA from 96 different plasmids in the least amount of time, thus allowing large-scale screening of the plasmids. It is particularly useful for screening a number of plasmids in order to obtain clones having multiple inserts. Alternatively, standard plasmid preparation methods or kits can be used.
Restriction enzyme
Restriction enzyme buffer (10X)
RNase A, DNase-free (10 mg/mL; Sigma, R6513)
Sodium acetate (3 M, pH 5.2; Fisher, BP333)
Sodium dodecyl sulfate (SDS; 20% w/v; Fisher, BP166)
T4 DNA ligase (400 U/μL)
TBE buffer
TE buffer
Triton X-100 (20% v/v; VWR)
Trypsin-EDTA (0.05% Trypsin EDTAo4Na)(1X) liquid (Invitrogen, 25300120)
Water, deionized, autoclaved
Equipment
Aluminum foil
Cell scraper
Centrifuge, clinical
Centrifuge, high-speed, refrigerated, equipped with swinging bucket rotor for 15-mL tubes
DNA analysis software (Vector NTI)
DNA sequencer analysis software (Finch TV)
Gel electrophoresis tank, horizontal
Gel imaging system for quantifying PCR products
Hemocytometer
Incubator, humidified, equilibrated with 5% CO2, preset to 37°C
Magnetic stand for 1.5-mL Eppendorf tubes (Invitrogen)
Microcentrifuge, refrigerated
Microscope, inverted
Pipettes, Pasteur
Pipettors and tips, 5–1000 μL
Refrigerator or cold room, preset to 4°C
Rotating wheel/platform
Shaking incubator
Sonicator
Spectrophotometer
Thermal cycler, automated
Timer
Tubes, Eppendorf, 1.5-mL
Tubes, polypropylene, 15-mL (e.g., 17 × 100-mm)
Tubes, polypropylene, 50-mL
Tubes, polypropylene, conical, 15-mL (e.g., 17 × 120-mm)
UV lamp
Vacuum aspirator
Vortex mixer
Water bath, variable temperature
Day 1
Crosslinking Cells and Preparing Nuclei
1. Grow cell type of interest in the appropriate medium and under suitable conditions until they are 70–80% confluent.
-
2. Add 0.86 mL of 37% formaldehyde (to a final concentration of 2%) directly to a cell culture dish containing approximately 2 × 107 cells in 15 mL of media. Swirl gently to mix. Incubate for 10 min at room temperature.
Formaldehyde is an excellent cross-linking agent which reacts with amino and imino groups of proteins and nucleic acids to generate protein–protein and protein–nucleic acid bridges, but does not react with free double-stranded DNA. This action requires relatively short distances (2 A°) and therefore, is very selective for intimate interactions. Formaldehyde-mediated crosslinks can easily be reversed under certain conditions.
-
3. Add 1.057 mL of 2 M glycine (to a final concentration of 0.125 M) to the dish. Swirl gently to mix. Incubate for 5 min on ice.
The formaldehyde reaction is stopped by the addition of glycine (0.125 M final concentration) and transferring to 4 C.
4. Aspirate the media. Wash the cells with 5 mL of ice-cold PBS containing protease inhibitor cocktail.
-
5. Add 5 mL of 0.2X trypsin-EDTA (1X trypsin diluted five times in PBS). Incubate in the cell culture incubator (5% CO2/37°C) for 5 min.
A combination of Trypsin and EDTA is very efficient for detaching cells. Trypsin digests the adhesion proteins in cell-cell and cell-matrix interactions and EDTA is a calcium chelator, which integrins needs to interact with other proteins for cell adhesion.
6. Neutralize the trypsin with 10 mL of PBS containing 10% fetal bovine serum.
-
7. Scrape the cells gently from the plate and transfer them to 50 mL-tubes on ice.
Optionally, scrape a second time with ice-cold PBS containing protease inhibitor cocktail.
8. Collect the cells by centrifuging at 1300 rpm for 8 min at 4°C.
9. Wash twice with 10 mL ice-cold PBS containing protease inhibitor cocktail. Pipet gently to make a single-cell suspension, while working on ice. Collect the cells by centrifuging at 1300 rpm for 8 min at 4°C. Resuspend the cells second time in PBS and count before centrifugation.
-
10. Add 10 mL of cell lysis solution containing protease inhibitor cocktail to the cells. Mix gently by pipetting and incubate for 10 min on ice.
Monitor efficient lysis under a microscope. These conditions normally work well for most cell types. However, certain cell types may require different incubation times and/or different strengths of the buffer for efficient lysis.
-
11. Centrifuge at 1800 rpm for 5 min at 4°C. Discard the supernatant.
At this point, the nuclei can be snap-frozen in liquid nitrogen and stored at −80°C until use.
Restriction Enzyme Digestion and Intramolecular Ligation
12. Resuspend the pellet in 500 μL of 1.14X restriction enzyme buffer.
13. Pellet the nuclei by centrifugation at 1800 rpm for 5 min at 4°C. Resuspend in 500μL of 1.14X restriction enzyme buffer and transfer to 1.5-mL eppendorf tubes.
14. Add 7.5 μL of 20% SDS (to a final concentration of 0.3% SDS) and mix by gentle pipetting. Shake gently for 1 h at 37°C.
-
15. Add 50 μL of 20% Triton X-100 (to a final concentration of 1.8%). Shake gently for 1 h at 37°C.
Triton X-100 binds and effectively removes SDS. This is required for the restriction enzyme to digest efficiently in the subsequent steps.
16. Add 1200 units of restriction enzyme (i.e., 12 μL of 100 units/μL) and incubate overnight with gentle shaking at the temperature recommended by the manufacturer for the corresponding enzyme.
Day 2
-
17. Add 40 μL of 20% SDS (to a final concentration of 1.6%) and incubate for 30 min at 65°C with gentle shaking.
A higher concentration of SDS is added to make sure that the restriction enzyme is completely inactivated.
18. Transfer the sample to a 15-mL tube containing 7 mL of 1.15X ligation buffer.
19. Add 400 μL of 20% Triton X-100 (to a final concentration of 1%) and incubate for 1 h at 37°C with occasional shaking.
-
20. Add 50 μL of T4 DNA ligase and incubate for 4 h at 16°C followed by 30 min at room temperature.
Here, the T4 DNA Ligase serves to catalyze the formation of a phosphodiester bond between the 5′-phosphate and 3′-hydroxyl termini generated by the restriction digestion of the chromatin fragments which lie in close physical proximity.
Enrichment of Ligation Fragments Containing the Protein of Interest in the Complex
21. Remove 10μL from the sample (from Step 20) to be used as the “input” sample for subsequent analysis and store at −20°C. Prepare one input sample for each treatment group or cell type.
-
22. Add 800-μL aliquot from the sample (from Step 20) to 7.2 mL of ChIP dilution buffer (to make a 10-fold dilution).
Thus, the same 3C ligation reaction can be split and used for ChIP analyses with different antibodies.
-
23. Add 4–10 μg of the antibody of interest per immunoprecipitation and rotate end-over-end overnight at 4°C.
In addition, run an IP in parallel without any antibody or IgG or as a negative control.
Day 3
-
24. Prepare the magnetic beads as below:
-
Mix Protein G: Protein A (1:3) (or only Protein G or Protein A depending on the antibody) in a 1.5-mL Eppendorf tube.
Each immunoprecipitation experiment requires 100 μL of this bead mix. The choice of beads strictly depends on the type of antibody being used.
Add 1 mL of 0.5% BSA in PBS and mix.
Place the tube in a magnetic stand for 1 min.
-
Remove the solution carefully by pipeting or by gentle vaccum suction.
Be careful not to aspirate the beads.
Remove the tube from the stand. Add 1 mL of 0.5% BSA in PBS and resuspend the beads by inverting the tube.
Repeat Steps 24. iii–iv.
Resuspend the beads in 1 mL of 0.5% BSA in PBS and rotate end-over-end overnight at 4°C.
-
25. Add 100 μL of the blocked magnetic bead solution to each immunoprecipitate sample (from Step 23) and incubate with end-over-end rotation for 3 hours at 4°C
26. Pre-chill one 1.5-mL tube on ice for each immunoprecipitate.
-
27. Transfer ~1.5 mL of each immunoprecipitate to the above pre-chilled tube and place the tubes in the magnetic stand to collect the beads. Remove the supernatant and add another aliquot of the remaining immunoprecipitate. Repeat until all the beads have been collected.
Alternatively, centrifuge at 2000 rpm for 5 min to pellet the magnetic beads. Aspirate some of the supernatant, leaving about 1 mL in the tube. Resuspend the pellet with the remaining supernatant and transfer to a chilled tube. Let the tube sit in the magnetic stand for 1 min to collect the beads. Remove the remaining supernatant.
28. Remove the tubes from the magnetic stand. Place on ice.
-
29. Wash the beads four times with 500 μL of low-salt wash buffer containing protease inhibitor cocktail. Agitate gently to resuspend the beads. Place the tubes in the magnetic stand to collect the beads. Remove the supernatant.
Always use buffers containing freshly added protease inhibitors for each wash. In case the beads are stuck at the bottom of the tube, place on the magnetic stand momentarily to move the beads to the side and continue to invert.
-
30. Wash the beads once with 500 μL of high-salt wash buffer containing protease inhibitor cocktail and collect the beads as in step 29.
The stringent washing conditions are necessary to reduce background coming from the non-specific sticking of chromatin to Protein-G or A beads.
-
31. Wash the beads once with 1 mL of TE buffer containing protease inhibitor cocktail and collect the beads as in step 29.
The beads are relatively loose in the TE buffer. Thus, care should be taken while removing the supernatant and manual pipetting is preferred over vacuum aspiration.
32. Centrifuge at 960g for 3 min at 4°C. Remove any residual TE buffer.
-
33. Add 210 μL of freshly prepared Elution buffer. Incubate for 15 min at 65°C. Vortex briefly every 2 min.
Alternatively, shake gently for 15 min in a shaking incubator preset to 65°C.
Use of freshly prepared elution buffer seems to increase the elution efficiency significantly.
34. Centrifuge at 16000g for 1 min at room temperature.
35. Transfer 200 μL of the supernatant to a new 1.5-mL tube carefully without touching the beads.
36. Thaw the inputs (from Step 21) and add 190 μL of Elution buffer.
-
37. Place the samples (from Step 35) and inputs (from Step 36) at 65°C overnight for reverse-crosslinking.
This step serves to reverse the DNA-Protein and Protein-Protein crosslinks generated by formaldehyde.
Day 4
-
38. Add 200 μL of TE buffer to dilute SDS in the elution buffer. Add 8 μL of RNase A (10 mg/mL) and incubate for 2 h at 37°C.
Ribonuclease A (RNase A) is an endonuclease that cleaves single-stranded RNA and thus helps to get rid of the RNA contamination.
-
39. Add 8 μL of Proteinase K (10 mg/mL) and incubate for 2 h at 50°C.
Proteinase K is a serine protease that exhibits very broad cleavage specificity. It cleaves peptide bonds adjacent to the carboxylic group of aliphatic and aromatic amino acids and is useful for general digestion of protein in the samples.
40. Add an equal volume of phenol:chloroform:isoamyl alcohol to the samples and shake well to mix. Centrifuge at 10000 rpm for 15 min and transfer the upper phase to a new eppendorf tube. Repeat this step with the upper phase.
41. Precipitate the DNA by adding a one-tenth volume of 3 M sodium acetate, pH 5.2, 1 μL glycogen and two volumes of cold absolute ethanol.
42. Wash with 0.4 volumes of 70% ethanol. Vortex gently after adding.
-
43. Dissolve the pellet in 15 μL of nuclease-free H2O.
The samples can be stored at −20 until use.
44. Test the success of the ChIP reaction using 1μL of undiluted immunoprecipitate (or an “input” sample diluted 1:100) by PCR with primers specific for a genomic region known to be targeted by the protein of interest in the cell type under investigation.
Cloning of 3C-Ligated Immunoprecipitated Fragments
-
45. Using established cloning protocols, clone the 3C-ChIP products (from Step 43) into a vector that has enzyme overhangs similar to those generated in the 3C assay.
Typically, this step should involve cloning an “IgG/no antibody 3C-ChIP product” and “Plus antibody 3C-ChIP product,” in addition to regular cloning controls.
-
46. Use the ligated vector to transform high-efficiency competent bacterial cells.
Using high efficiency bacterial cells is important to increase the number of transformants. The ligated vectors will vary in size and can range up to several kilo bases which can reduce the transformation efficiency.
47. Plate the transformed cells on LB-agar plates containing the antibiotic of choice (e.g., ampicillin or kanamycin), with X-gal and IPTG in the experimental plates. Place the bacterial plates (inverted) in a 37°C incubator for overnight.
Day 5
-
48. The following day, count the number of blue and white colonies in the bacterial plates from samples immunoprecipitated with the specific antibody as well as the controls (i.e., no antibody or IgG).
In this standard blue-white selection, white colonies represent bacteria-harboring plasmids with inserts, whereas blue colonies represent bacteria with plasmids without the insert. In a 6C assay, the number of white colonies obtained should be several-fold higher in samples immunoprecipitated with the specific antibody than in controls. This indicates that the pull-down with a specific antibody led to enrichment of specific protein-occupied genomic regions, including intramolecularly ligated genomic regions that contain the protein of interest in the complex.
The bacterial plates can be wrapped with Parafilm and stored in 4°C for a few days until inoculation.
-
49. Pick several white colonies (i.e., containing the insert) from the bacterial plates and inoculate LB media containing the appropriate antibiotic using each such colony. Incubate in a shaking incubator at 220 rpm overnight at 37°C.
The bacterial cultures can be stored in 4°C for a few days until plasmid preparation.
Day 6
Plasmid Purification and Screening for Ligated Partners
50. Purify the plasmids from the above inoculates. This can be done using the PureLinkHQ 96 Mini Plasmid DNA Purification Kit according to the manufacturer’s instructions or using standard plasmid preparation protocols or kits.
51. Digest the purified plasmids with the same restriction enzyme used in Step 16 for screening, by following standard restriction digestion protocols.
52. Separate the resulting DNA fragments by electrophoresis in a 1% agarose gel containing SYBR Safe. Use an appropriately sized DNA ladder for reference.
53. Select the plasmids showing more than one insert and sequence the inserts using primers from two different ends of the vector (e.g., T3 and T7 promoter-specific primers for pBluescript® II RI Predigested Vector).
Ideally, multiple inserts mean interacting partners that were possibly ligated due to them being part of the same protein containing complex against which the antibody was used for immunoprecipitation during the procedure.
If the fragments are too big and/or there are more than two inserts in the plasmids, subclone the fragments into a new restriction enzyme-digested vector before proceeding with sequencing.
REAGENTS AND SOLUTIONS
Elution buffer
NaHCO3 (0.1 M)
Sodium dodecyl sulfate (1%)
This solution should be prepared freshly each time before use.
Cell lysis solution
NaCl (10 mM)
Nonidet P-40 (0.2%)
Tris (10 mM, pH 8.0)
Store at 4°C for upto 1 year.
ChIP dilution buffer
EDTA (1.2 mM)
NaCl (167 mM)
Sodium dodecyl sulfate (0.01%)
Tris-HCl (16.7 mM, pH 8.1)
Triton X-100 (1.1%)
Store at 4°C for upto 1 year.
High-salt wash buffer, high-salt
EDTA (2 mM)
NaCl (500 mM)
Sodium dodecyl sulfate (0.1%)
Tris-HCl (20 mM, pH 8.1)
Triton X-100 (1%)
Store at 4°C for upto 1 year.
Low-salt wash buffer, low-salt
EDTA (2 mM)
NaCl (150 mM)
Sodium dodecyl sulfate (0.1%)
Tris-HCl (20 mM, pH 8.1)
Triton X-100 (1%)
Store at 4°C for upto 1 year.
LB agar
NaCl (10 g/L; Sigma-Aldrich, S9625)
Tryptone (10 g/L; BD, 211705)
Yeast extract (5 g/L; BD, 212750)
Agar (20 g/L)
Add deionized H2O to a final volume of 1 liter. Adjust the pH to 7.0 with 5 N NaOH. Autoclave. Pour into Petri dishes (~25 mL/100-mm plate).
Store at 4°C
LB-ampicillin agar plates
Ampicillin, filter-sterilized (10 mg/mL)
LB agar
Autoclave 1 liter of LB agar. Cool to 55°C. Add 10 mL of ampicillin. Pour into Petri dishes (~25mL/100-mm plate).
Store at 4°C
LB-kanamycin agar plates
Kanamycin, filter-sterilized
LB agar
Autoclave 1 liter of LB agar. Cool to 55°C. Add 50 mg of kanamycin. Pour into Petri dishes (~25 mL/100-mm plate).
Store at 4°C
LB medium
NaCl (10 g/L; Sigma-Aldrich, S9625)
Tryptone (10 g/L; BD, 211705)
Yeast extract (5 g/L; BD, 212750)
Add deionized H2O to a final volume of 1 liter. Adjust the pH to 7.0 with 5 N NaOH. Autoclave.
Store at 4°C
Ligation buffer
ATP (1 mM)
Dithiothreitol (10 mM)
MgCl2 (10 mM)
Tris-HCl (30 mM)
Adjust the pH to 7.8.
Store at −20°C until use.
TE buffer (pH 8.0)
EDTA (1 mM)
Tris-HCl (10 mM)
Store at RT for upto one year.
TBE buffer
Boric acid (0.89 M; J.T. Baker, 0084)
EDTA (0.02 M, pH 8.0)
Tris base (0.89 M)
Store at RT for upto one year.
Background Information
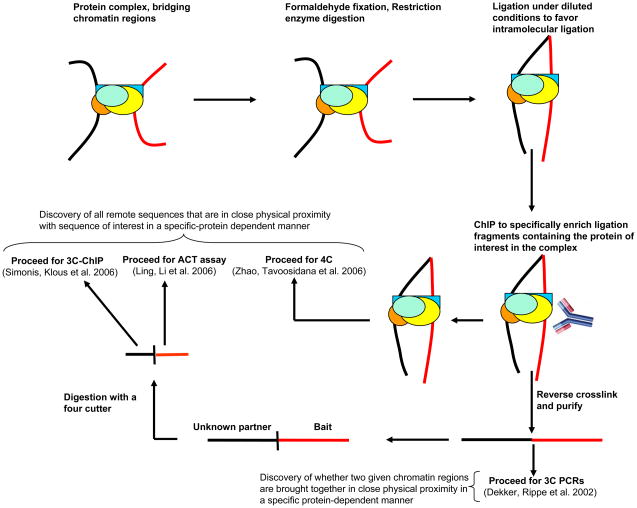
In 3C, cells are treated with formaldehyde which crosslinks proteins to other neighboring proteins and DNA which lie in very close physical proximity (<2 Å). This follows restriction digestion and then ligation under much diluted concentration. Under these conditions, ligation between cross-linked DNA fragments, which is intramolecular, is strongly favored over ligation between random fragments, which is intermolecular. Thereafter, the cross-links are reversed and ligation products are detected and quantified by polymerase chain reaction (PCR) using primers from the two remote sequences that are now joined due to ligation. The amount of corresponding ligation product detected is proportional to the cross-linking frequency of the two specific restriction fragments which in turn reflects the frequency with which these two genomic sites interact in the nucleus. Thus, 3C (Chromosome Conformation Capture) assay generates a population-average measurement of juxtaposition frequency between any two (given) chromatin elements, thus assessing their relative physical proximity in the nucleus (Dekker et al, 2002). All other techniques to study long-range chromatin interactions have evolved from this original 3C assay. 5C (Chromosome Conformation Capture Carbon Copy) technology has potential to screen a genomic region that interact with another genomic region of choice, such as a gene promoter and could be used to probe into the conformational fine structure of selected chromatin regions in the genome (Dostie et al, 2006). Both 4C assays (“Circular Chromosome Conformation Capture” and “3C-chip”) have unprecedented potential to capture all the genomic regions in the nucleus that come in close physical proximity to a DNA element or genomic region of interest in an unbiased and high-throughput fashion (Simonis et al, 2006; Zhao et al, 2006). However, none of these methods identify protein components that may mediate such physical proximities between chromatin elements and separate experiments must be complemented with separated assay carried out to address this question. The Combined 3C-ChIP-Cloning (6C) technology combines multiple techniques to simultaneously identify physical proximities between chromatin elements as well as the proteins that mediate these interactions. The 6C technique can be combined with these recently published techniques to discover all the chromatin regions in the nucleus that are brought in close physical proximity to the gene (or any other chromatin region of interest) in a specific protein-dependent manner (Fig. 2). Following immunoprecipitation with the antibody of interest, the samples can be processed for 4C (Circular Chromosome Conformation Capture) analysis (Zhao et al, 2006) or reverse-crosslinked, purified, digested with a four-cutter of choice and processed further for either 3C-chip (also called ‘4C’) (Simonis et al, 2006) or the ACT (Associated Chromosome Trap) assay (Ling et al, 2006). The 6C procedure can also be used to investigate whether two known chromatin regions are brought into close physical proximity by a specific protein of interest by following the amplification criteria used in the original 3C assay, subsequent to the reversal of crosslinking and purification (Dekker et al. 2002).
Figure 2.
Further applications and the future of Combined 3C-ChIP-Cloning (6C) methodology. 6C method can be combined with other techniques to identify the entire “interactome” in the nucleus for any gene or chromatin region of choice that is mediated by a specific protein of interest. Following the ChIP, the samples could be processed for 4C analysis (Zhao, Tavoosidana, Sjolinder, Gondor, Mariano, Wang, Kanduri, Lezcano, Sandhu, Singh et al. 2006) or reverse crosslinked, purified, digested with a four cutter of choice and further subjected to either 3C-chip (Simonis, Klous, Splinter, Moshkin, Willemsen, de Wit, van Steensel and de Laat 2006) or ACT assay (Ling, Li, Hu, Vu, Chen, Qiu, Cherry and Hoffman 2006). This technique could also be used to investigate whether two known chromatin regions are brought in close physical proximity by a protein of interest. For this purpose, subsequent to the reversal of crosslinking and purification, the amplification criteria used in the original 3C assay should be followed (Dekker, Rippe, Dekker and Kleckner 2002).
Critical Parameters and Troubleshooting
The 6C-captured interactions between chromatin elements should always be validated by performing independent 3C assays (Tolhuis et al, 2002) where one performs 3C-PCRs using multiple primer combinations from two different remote sequences. The primers for this purpose are designed similar to as described previously (Splinter et al, 2004). Importantly, one must also establish, by using separate assays, that the interacting regions captured in the 6C assay are truly occupied by the protein of interest in the cells being studied. To this end, one should carry out ChIP assays (Tiwari et al, 2008) using antibodies specific for the protein of interest. The immunoprecipitated DNA can then be amplified using primers spanning the restriction enzyme site found to be involved in the 3C ligation with other partner(s) in each of the 6C clones.
Many factors play a role to make a 6C assay successful. A 6C experiment should always involve careful planning, use of high quality reagents and utmost care at each of the steps with proper controls running alongside. Mammalian cells should be exponentially growing in the appropriate media so as to exhibit healthy growth curves appropriate to the cell type before they are processed for a 6C experiment. The restriction enzyme selection, a good ChIP-grade antibody and a good primer design are very crucial determinants of a successful 6C assay. The choice of restriction enzyme is at user’s discretion. However, it is important that the restriction enzyme harbors high digestion efficiency on the crosslinked chromatin. The selection of the restriction enzyme is a critical step for 6C methodology for at least two reasons. First, it affects both the 3C step and the later steps of cloning and screening. Second, it determines the resolution of interaction maps that is generated using the 6C assay with respect to defining the precise genomic regions engaged in physical pairing. Enzymes that produce cohesive ends from palindromic recognition sites are preferred in 3C assays due to their relatively much higher efficiency of ligation. For initial studies, six-cutters such as EcoRI, HindIII and BglII have been commonly used that cut every 4 kb along the genomic DNA. One must test a few regions of the genome for digestion efficiency either by Southern or PCR-based analysis using an aliquot of the restriction enzyme-digested nuclei before beginning to perform the entire 6C assay. It is advisable to obtain a digestion efficiency of at least 80% on the crosslinked chromatin before proceeding for 3C part of the 6C assay. Further, once the 6C interaction discovers an interaction, the results should be confirmed using a different restriction enzyme. Once the interaction is confirmed, frequent cutters such as MseI (predicted to cut every 256 bp) can be used to obtain higher-resolution maps. The reagents for use in a 6C assay should be stored at the appropriate conditions and no longer stored than prescribed, e.g. the crosslinking agent- formaldehyde should be no more than 1 year old as the efficiency of crosslinking will directly affect the frequency with which ligation products are formed and will thus impact the reliability of the entire procedure. A positive control should also be designed for every ligation product being detected in 3C PCRs during validation steps as described previously (Kurukuti et al, 2006) and PCR products obtained with the 3C and control templates should be run side by side on the same gel. The cloning vector should harbor enzyme overhangs similar to those generated in the 3C assay. Such vectors can be custom-constructed or obtained commercially. For example, for cloning fragments containing EcoRI ends, we used pBluescript® II RI Predigested Vector (Stratagene) in a recent study (Tiwari et al, 2008).
Anticipated Results
The 6C assay combines multiple techniques to simultaneously identify interaction between chromatin elements and the proteins that mediate such physical proximities. This method also allows testing whether a candidate protein has potential to mediate inter- or intrachromosomal interactions. Using a possible combination of 6C with other techniques such as 4C, it may be possible to obtain a complete, cell-type-specific map of all inter and intrachromosomal interactions mediated by specific proteins. It is important to discuss that in our first application of the 6C assay (Tiwari et al, 2008), while screening the clones by restriction digestion, we found that only a very low frequency of clones had multiple inserts (five out of 352) and many reasons may account for it. First, the rest of the clones that had a single insert probably represent distinct genomic sites that are targets of the specific protein (in this study, EZH2) but are not engaged in any long-range associations. Interestingly, this may also be a reflection of the frequency at which specific protein-dependent (in this study, EZH2) long-range associations take place in the nucleus. Second, the low number of clones with multiple inserts may also result from the difficulty in cloning (ligation and transformation) and sequencing of large size fragments that might result from the ligation of a six cutter restriction enzyme (EcoRI in this study) digestion generated fragments. Third, intramolecular ligation after crosslinking and digestion will lead to a fraction of circular DNA that cannot be cloned and may further reduce the number of clones in such an assay. Finally, since this particular study used EcoRI which is a DNA methylation– sensitive enzyme, we might have also missed some interactions involving partners that bear DNA methylation at the EcoRI site.. Future work should involve developing strategies to tackle each of these issues to improve the overall efficiency of the method.
Acknowledgments
This work was supported by ES011858 (National Institute of Environmental Health Sciences) and CA116160 (National Institute of Health) grants to S.B.B. This work was also supported in part by Marie Curie International Incoming Fellowship Award of the FP7 to V.K.T at the FMI in Basel.
LITERATURE CITED
- Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295(5558):1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- Dostie J, Richmond TA, Arnaout RA, Selzer RR, Lee WL, Honan TA, Rubio ED, Krumm A, Lamb J, Nusbaum C, Green RD, Dekker J. Chromosome Conformation Capture Carbon Copy (5C): a massively parallel solution for mapping interactions between genomic elements. Genome research. 2006;16(10):1299–1309. doi: 10.1101/gr.5571506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurukuti S, Tiwari VK, Tavoosidana G, Pugacheva E, Murrell A, Zhao Z, Lobanenkov V, Reik W, Ohlsson R. CTCF binding at the H19 imprinting control region mediates maternally inherited higher-order chromatin conformation to restrict enhancer access to Igf2. Proc Natl Acad Sci U S A. 2006;103(28):10684–10689. doi: 10.1073/pnas.0600326103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling JQ, Li T, Hu JF, Vu TH, Chen HL, Qiu XW, Cherry AM, Hoffman AR. CTCF mediates interchromosomal colocalization between Igf2/H19 and Wsb1/Nf1. Science. 2006;312(5771):269–272. doi: 10.1126/science.1123191. [DOI] [PubMed] [Google Scholar]
- Simonis M, Klous P, Splinter E, Moshkin Y, Willemsen R, de Wit E, van Steensel B, de Laat W. Nuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture-on-chip (4C) Nat Genet. 2006;38(11):1348–1354. doi: 10.1038/ng1896. [DOI] [PubMed] [Google Scholar]
- Simonis M, Kooren J, de Laat W. An evaluation of 3C-based methods to capture DNA interactions. Nat Methods. 2007;4(11):895–901. doi: 10.1038/nmeth1114. [DOI] [PubMed] [Google Scholar]
- Splinter E, Grosveld F, de Laat W. 3C technology: analyzing the spatial organization of genomic loci in vivo. Methods Enzymol. 2004;375:493–507. doi: 10.1016/s0076-6879(03)75030-7. [DOI] [PubMed] [Google Scholar]
- Tiwari VK, Cope L, McGarvey KM, Ohm JE, Baylin SB. A novel 6C assay uncovers Polycomb-mediated higher order chromatin conformations. Genome research. 2008;18(7):1171–1179. doi: 10.1101/gr.073452.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhuis B, Palstra RJ, Splinter E, Grosveld F, de Laat W. Looping and interaction between hypersensitive sites in the active beta-globin locus. Mol Cell. 2002;10(6):1453–1465. doi: 10.1016/s1097-2765(02)00781-5. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Tavoosidana G, Sjolinder M, Gondor A, Mariano P, Wang S, Kanduri C, Lezcano M, Sandhu KS, Singh U, Pant V, Tiwari V, Kurukuti S, Ohlsson R. Circular chromosome conformation capture (4C) uncovers extensive networks of epigenetically regulated intra- and interchromosomal interactions. Nat Genet. 2006;38(11):1341–1347. doi: 10.1038/ng1891. [DOI] [PubMed] [Google Scholar]