Abstract
Background
This study examined regional cortical volumes in 6-week abstinent men dependent on crack-cocaine only (Cr) or on both crack-cocaine and alcohol (CrA). Our goal was to test the a priori hypothesis of prefrontal cortical volume reduction, along with associated impairments in frontal mediated functions, and to look for differences between the Cr and CrA groups.
Methods
Structural magnetic resonance imaging (MRI) of the brain and neuropsychological assessment were performed on 17 6-week abstinent Cr subjects, 29 six-week abstinent CrA subjects, and 20 normal controls. Cortical volume was quantified in the prefrontal, parietal, temporal and occipital regions.
Results
Cr and CrA subjects showed comparable reductions in prefrontal gray matter volume compared to controls; this reduction was negatively associated with performance impairments in the executive function domain.
Conclusions
Dependence on Cr (with or without concomitant alcohol dependence) was associated with reduced prefrontal cortical volume. Cr dependence with concomitant alcohol dependence was not associated with greater prefrontal volume reductions than Cr dependence alone. The existence of these findings at 6-week abstinence indicates that they are not a result of acute cocaine or alcohol exposure. The association of reduced prefrontal cortical volume with cognitive impairments in frontal cortex mediated abilities suggests that this reduced cerebral volume has functional consequences.
Keywords: Cocaine, Alcoholism, MRI, Frontal lobe, Neurotoxicity
1. Introduction
There are very few studies of structural brain changes secondary to dependence on crack-cocaine (Cr), especially after a period of sustained abstinence. There is also little literature to date that examines the comorbid effects of chronic cocaine and alcohol abuse, even though a majority of cocaine abusers also abuse alcohol (Grant and Harford, 1990), and many individuals who present for substance abuse services exhibit both alcohol and cocaine dependence (Brown et al., 1994).
Preferential prefrontal gray matter atrophy associated with alcohol abuse was first suggested by Courville (1955), and continues as one of the strongest and most frequent findings in investigations of the cerebral effects of alcohol dependence (Harper and Kril 1990; Pfefferbaum et al., 1995, 1998). The advent of MRI has led to many imaging studies that document frontal lobe volume deficits in alcoholics and polysubstance dependent individuals (including individuals dependent on cocaine) (Bartzokis et al., 2000; Danos et al., 1988; Liu et al., 1998; O’Neill et al., 2001; Pascual-Leone et al., 1991; Pezawas et al., 1998; Pfefferbaum et al., 1998). Frontal lobe mediated functional impairments have also been documented in chronic alcoholism (reviewed by Fein et al. (1990) and by Oscar-Berman (2000)). The frontal (including fronto-limbic) system functions affected include emotional reactivity, behavioral inhibition, problem solving, abstraction, working memory, planning, and susceptibility to interference. Frontal-cerebellar circuits are also disrupted which affect problem solving, contextual memory, and execution and learning of procedures (Sullivan et al., 2000).
Our neuropsychological studies of subjects 6-weeks abstinent from Cr dependence or from both crack and alcohol (CrA) dependence found the greatest deficits in the executive function domain, which is mediated by the frontal cortex (Di Sclafani et al., in press). In earlier electrophysiological studies, we found that abstinent Cr and CrA dependent individuals had a delayed latency of the frontal cortex mediated orienting response to novel stimuli (the P3A component) (Biggins et al., 1997). Various other investigators have used PET and SPECT functional imaging to show deficits in cerebral blood flow in the prefrontal cortex of cocaine abusers (Tumeh et al., 1990; Volkow et al., 1988; Weber et al., 1993).
The use of Cr has been associated with a high incidence of cocaine-related stroke. Daras et al. (1994) reported on 55 cases of neurovascular events (25 ischemic and 30 hemorrhagic) related to cocaine use. They suggest that a sudden cocaine-associated rise in systemic arterial pressure may cause hemorrhages, frequently in association with an underlying aneurysm or arterial venous malformation. Vasospasm, arteritis, myocardial infarction with cardiac arrhythmias and increased platelet aggregation may provoke infarcts. Konzen et al. (1995) presented three case descriptions documenting brain infarcts in cocaine users that appear to result from vasospasm of large arteries and secondary intravascular thrombosis. Bartzokis et al. (1999) hypothesized that the incidence of white matter signal hyperintensities (possibly symptomatic of cerebral ischemia in cocaine abusers) observed on T2-weighted MRI would be increased in asymptomatic crack cocaine dependent individuals. They compared 61 Cr dependent participants (age 25–66 years) with 57 normal controls of comparable age. The cocaine-dependent sample had four times the incidence of hyperintense lesions as controls. The incidence of lesions increased with age in the cocaine dependent sample, and this increase was not related to the number of years cocaine was used. The authors concluded that Cr dependent participants had a significantly increased age-related risk of white matter damage.
In the present study, we examined volumes of regional cortical tissue and white matter signal hyperintensities in 6-week abstinent Cr and CrA subjects compared to normal controls. We expected both the Cr and CrA samples to demonstrate prefrontal cortical volume deficits, with consequent frontal mediated functional impairments. We also expected the substance dependent samples to show an increased volume of white matter signal hyperintensities compared to normal controls. We hypothesized that the sample dependent on both cocaine and alcohol would show both greater structural and greater functional effects than the sample dependent on cocaine alone.
2. Methods
2.1. Subjects
Substance dependent male subjects (n = 46) were either inpatients in the Substance Abuse Inpatient Unit at the San Francisco Department of Veterans Affairs Medical Center or patients in substance abuse residential treatment centers in the San Francisco Bay area. All substance dependent subjects met DSM-IV criteria for Cr dependence (n = 17) or CrA dependence (n = 29). Although subjects were included who met DSM-IV criteria for abuse of other substances, no subject met DSM-IV criteria for dependence on any substance other than crack or alcohol. In addition, no subject in the Cr group met criteria for abuse or dependence on alcohol. Subjects were monitored via urine screens that tested for the presence of alcohol, cocaine, amphetamines, benzodiazapines, and opiates. Random urine screens were performed on a weekly basis by the residential treatment centers, and every other day on the inpatient unit. All but six of the subjects were in inpatient or residential treatment centers at the time of the MRI study and neuropsychological testing. On the day of the neuropsychological testing all individuals (including normal controls) had a urine screen, as well as a ‘breathalyzer’ for alcohol.
Self-report measures of crack and alcohol use were used to estimate dose and duration of peak use and average lifetime use (Table 1). Alcohol use was quantified in terms of standard drinks (4 oz. of wine, 12 oz. of beer, or 1 oz. of 80-proof liquor). We quantified crack use by average monthly cost of crack consumed (because of the difficulty in assessing the quantity and quality of an illicit drug).
Table 1.
Demographic and substance use variables
| Normal controls (n = 20) | Subjects (n = 46) at 6-week abstinence |
||
|---|---|---|---|
| Crack dependent only (n = 17) Crack and alcohol dependent (n = 29) | |||
| Age | 39±6 | 39±7 | 42±6 |
| Years of education | 14.6±1.9 | 13.4±1.3 | 12.8±2.0 |
| Ethnicitya | 12 AA, 7 C, 1 H | 16 AA, 1 C | 16 AA, 6 C, 7 H |
| Mean lifetime alcohol use (drinks per month) | 13±9 | 22±31 | 286±232 |
| Duration of mean lifetime alcohol use (months) | N/A | N/A | 288±89 |
| Peak alcohol use (drinks per month) | N/A | N/A | 544±289b |
| Duration of peak alcohol use (months) | N/A | N/A | 67±66b |
| Mean lifetime crack use (dollars per month) | N/A | 977±1178 | 909±753 |
| Duration of mean lifetime crack use (months) | N/A | 174±75 | 172±67 |
| Peak crack use (dollars per month) | N/A | 2739±2935 | 3005±5608 |
| Duration of peak crack use (months) | N/A | 26±25 | 45±36 |
AA, African American; C, Caucasian; H, Hispanic.
n = 22; about 25% of subjects dependent on both crack and alcohol could not accurately recall this information.
Normal controls (n = 20) were recruited by flyers posted in community centers, through newspaper advertisement, and by ‘word of mouth’. They were non-drinkers or light social drinkers, had never used cocaine, and had no history of other drug abuse. The University of California at San Francisco Committee on Human Research approved all procedures, and written consent was obtained from all individuals prior to study.
A trained clinician used the SCID (Structured Clinical Interview for DSM-IV, non-patient version) and a psychiatric resident performed a neurologic exam to assess all study participants. All individuals in the study were screened to exclude those with any neurologic or Axis 1 disorder unrelated to substance abuse, and those who were HIV-seropositive by polymerase chain reaction testing. Individuals were also excluded from the study for a history of any loss of consciousness that required medical attention.
2.2. Neuropsychological measures
All individuals were administered the computerized standard version of the MicroCog Assessment of Cognitive Functioning (Powell et al., 1993), which yields age and education adjusted scores. The MicroCog includes 18 subtests that assess performance in the domains of attention, executive function, spatial processing, immediate and delayed memory, calculation, and reaction time. The subtests included in each domain were: (1) Attention (Numbers Forward, Numbers Reversed, Alphabet, Word List 1), (2) Executive Function (Analogies, Object Match A and B), (3) Spatial Processing (Tic Tac 1 and 2, Clocks), (4) Immediate Memory (Story Immediate 1 and 2, Word List 2), (5) Delayed Memory (Story Delay 1 and 2, Address Delay), and (6) Reaction Time (Timers 1 and 2). The neuropsychological test methods and results were presented in detail previously (Di Sclafani et al., in press).
2.3. Magnetic resonance imaging
2.3.1. Image acquisition
All sequences (Fig. 1) were carried out on a whole body 1.5 T magnetom vision system equipped with a standard quadrature head coil. A vacuum-molded head holder was used to minimize motion of the subject’s head. The structural imaging protocol consisted of: (a) a sagittal T1-weighted localizer sequence; (b) an oblique axial double spin-echo sequence (TR/TE1/TE2 = 3000/20/80 ms), angulated at −10° from the planum sphenoidale. This study yielded both a T2-weighted and a proton-density (PD) weighted image, and covered the entire brain in contiguous 3 mm thick slices. These slices were obtained in an interleaved manner, with an in-plane resolution of 0.94 × 0.94 mm; (c) an oblique coronal T1-weighted gradient echo sequence (3D MP-RAGE, TR/TE = 9.7/4.6 ms), angulated perpendicular to the optic nerve, with a 1.5 mm section thickness, and an in-plane resolution of 1 × 1 mm.
Fig. 1.
Counter-clockwise from the top right: the same mid-ventricular axial MRI slice in the T2-weighted, T1-weighted, PD-weighted, and segmented forms for an abstinent Cr and alcohol dependent subject (age 52). The segmented image shows a moderate amount of white matter signal hyperintensity (white areas).
2.3.2. Image processing
The tissue volumes in the segmented image (Fig. 1) were obtained using computer-assisted methods. All of the image analysis procedures have been described previously (Fein et al., 2000). Cardenas et al. (2001), using repeat MRIs on 10 subjects, demonstrated intraclass correlation reliability coefficients for these procedures that were greater than 0.80 for all tissues, with cortical gray matter, white matter, sulcal cerebrospinal fluid (CSF), and ventricular CSF reliabilities greater than 0.92. The image-processing technician was blind to subject demographics and group membership. The T1-, PD-, and T2-weighted images were used for segmentation into tissue categories. The segmentation process began with automated stripping of the skull from the images, inhomogeneity filtering of the spin-echo images, and coregistration of the spin-echo data set to the T1-weighted images. In the next step, the operator chose very conservative samples of gray matter, white matter, and CSF as seeds for a K-Means cluster analysis (SAS, 1988) that assigned all of the voxels in the brain to these three tissue categories. This was followed by manual editing of the axial segmented images on a slice-by-slice basis to separate cortical from subcortical gray matter and ventricular from sulcal CSF. The technician also reclassified voxels as white matter signal hyperintensity that had been classified by the K-means procedure as either gray matter or CSF (due to their relative hyperintensity), but were clearly white matter by anatomic location.
2.3.3. Regional processing in the Talairach coordinate system
In the Talairach coordinate system, the brain is subdivided into a 12 (superior–inferior) × 9 (anterior–posterior) × 8 (lateral) grid, with a total of 864 voxels. Each of the 864 voxels is assigned to the appropriate Brodmann area in the 1988 Talairach atlas (Talairach and Tournoux, 1988). We defined four cortical regions of interest (ROI) by their corresponding Brodmann areas: prefrontal (6, 8, 9, 44–47), parietal (5, 7, 23, 31, 39, 40), occipital (17–19, 37), and temporal (20, 21, 34, 38, 41, 42). The ensuing transformation of each subject’s T1-weighted image to the Talairach coordinate system involved piecewise linear transformations of 12 compartments for each subject’s brain. The resulting ROIs were specific to each subject, but reflect a common Talairach definition. The final regional tissue volumes resulted from superimposing the subject-specific ROI on the subject’s segmented image (and counting the segmented voxels). This method of obtaining common ROIs across individuals’ brains has been described in detail elsewhere (Cardenas et al., 2001), and has been used in prior studies describing regional volume deficits (Fein et al., 2000, 2002; O’Neill et al., 2001).
2.4. Statistics
Our general analytic approach for all measures was to compare substance dependent subjects (the combined Cr and CrA groups) to normal controls, and then to compare the Cr and CrA groups to each other. Statistical analyses were performed using the ‘proc GLM’ routine in the SAS™ software package (SAS, 1988). All analyses of structural imaging variables controlled for inter-subject differences in head size using intracranial vault volume (ICV), either by removing the contribution of ICV via covariance analysis (ANCOVA), or by examining the imaging variables as a proportion of ICV. The group difference effect size (percent of variance of the imaging variable accounted for by group membership) was computed from the ANCOVA. Associations of imaging measures with age, neuropsychological domain scores, and with substance use variables were examined using Spearman correlations. White matter signal hyperintensities were compared across groups using the nonparametric Wilcoxon test, as the distribution of white matter signal hyperintensity volume is highly skewed toward zero.
3. Results
3.1. Demographic and substance use variables
The demographic and substance use variables for all subjects are presented in Table 1. The abstinent Cr and CrA samples did not differ in age from each other or from normal controls (t64 = 1.21, ns and t44 = 1.48 ns). The substance dependent group (combined Cr and CrA samples) did have less education than normal controls (t64 = 2.66, P = 0.01), but the Cr and CrA samples did not differ from each other (t44 = 1.06 ns). The Cr and CrA samples did not differ on average lifetime cocaine dose or duration or on peak dose (all P’s > 0.40). There was a trend for duration of peak cocaine use to be greater in the CrA compared to the Cr group (t35 = 1.78, P = 0.08).
3.2. Neuropsychological measures
Multivariate analysis of variance across the MC domain Z-scores revealed significant impairment in the substance dependent group versus normal controls (Wilks’ λ, F6, 59 = 3.11, P = 0.01). Comparisons for individual domains showed poorer performance by the substance dependent group in all domains except for immediate and delayed memory. The largest effect was in the executive function domain (with group membership accounting for 19.7% of the variance of the domain Z-score). The Cr and CrA groups did not differ from each other across MC domains (Wilks’ λ, F6,39 = 0.53, P = 0.78). The lack of a significant multivariate test did not allow for comparison of the Cr and CrA groups on individual neuropsychological domains; however, group membership in Cr versus CrA explained less than 4.1% of the variance in any neuropsychological domain.
3.3. Structural imaging measures
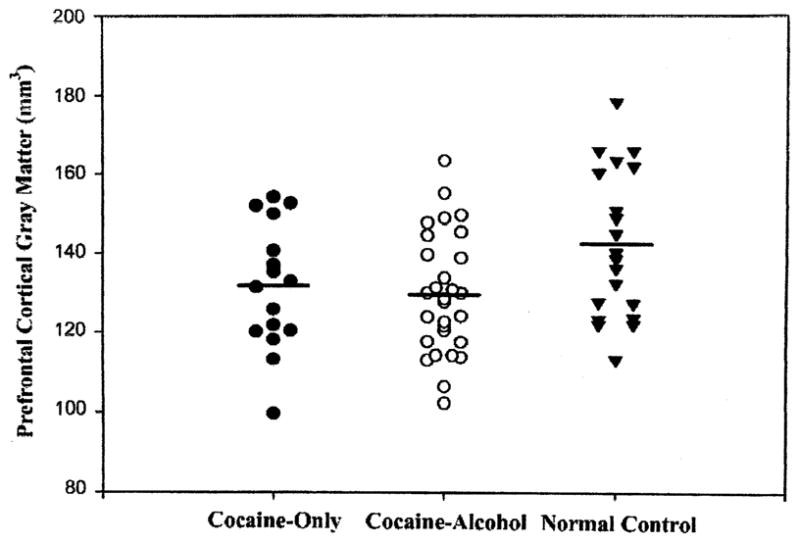
Normal controls, CrA and Cr did not differ on ICV (1453±148 cm3 versus 1429±132 cm3 versus 1369±87 cm3, ns). There were no differences among the groups in volumes of total cortical gray matter, white matter, ventricular or sulcal CSF (group membership accounted for less than 2.8% of the variance of any of these measures; all P’s > 0.18). Fig. 2 shows that the combined substance dependent samples had reduced regional gray matter in the prefrontal cortex only, with group membership accounting for 10.0% of the variance (P < 0.01), but less than 1.6% of the variance for parietal, temporal, and occipital cortices (all P’s > 0.32). The Wilcoxon test showed no difference in volume of white matter signal hyperintensities in substance dependent subjects versus normal controls (P = 0.34). The CrA and Cr groups did not differ on any of the above-mentioned structural imaging measures (all P’s > 0.35).
Fig. 2.

The abstinent substance dependent samples had reduced regional gray matter in the prefrontal cortex only, with group membership accounting for 10.0% of the variance (P < 0.01); this is compared to less than 1.6% of the variance for parietal, temporal, and occipital cortices (all P > 0.32).
For substance dependent subjects the prefrontal cortical volume showed a partial correlation with the executive function domain score (Spearman r = 0.31, P < 0.05). There was no association between any structural imaging variable and neurocognitive performance in normal controls. Prefrontal cortical volume had comparable negative correlations with age in both the substance dependent and normal control groups (r = −0.30, and r = −0.24). Prefrontal cortical volume was not associated with any cocaine or alcohol use variable.
4. Discussion
The primary finding in this study was a similar reduction in prefrontal gray matter volume in the CrA and Cr subjects. The existence of prefrontal volume reduction at 6-week abstinence establishes that it is not a result of acute cocaine exposure. The negative association of prefrontal cortical volume with performance on frontal cortex mediated abilities in the substance dependent samples suggests that the prefrontal volume deficits have functional consequences. Consistent with our a priori hypothesis, Cr dependence is associated with structural and functional damage to the prefrontal cortex. Contrary to our a priori hypothesis, however, white matter signal hyperintensity was not increased in the substance dependent samples.
There are only a few studies that examine regional volume changes in cocaine abusers. Liu et al. (1998) reported findings that mirror the results presented here. They studied 25 male polydrug abusers (age 21–44) compared to 14 normal male controls of comparable age. Twenty-three of the 25 individuals in the substance abuse group indicated that cocaine was their drug of choice. All subjects were housed on a closed research ward where at least 15 days abstinence from drugs and alcohol was documented. The substance dependent sample showed reduced normalized gray matter volume in the prefrontal lobe only.
Bartzokis et al. (2000) did not find a difference in frontal cortical volume in their study of age-related brain volume reductions in amphetamine and cocaine addicts compared to normal controls. The cocaine dependent sample (n = 10) showed a reduced temporal lobe volume and a negative age versus temporal gray matter association in comparison to the amphetamine (n = 9) and normal control (n = 16) samples. The disagreement in our results for the frontal cortex may reflect differing anatomical definitions of frontal versus prefrontal lobe. In addition, they did not address recent drug use in their samples (which consisted of recent admissions to inpatient and outpatient treatment centers); thus acute effects of cocaine or other drugs could have contributed to their findings.
We fully expected that concomitant cocaine and alcohol dependence would be associated with greater structural brain abnormalities than cocaine dependence alone, but such was not the case. However, O’Neill et al. (2001) had a similar result in their study of 16-week abstinent individuals dependent on cocaine only, alcohol only, and cocaine and alcohol. They found smaller normalized prefrontal gray matter volumes on quantified structural MRIs in the combined cocaine dependent samples. Within the prefrontal gray matter, significant volume loss was localized to the dorsolateral prefrontal and lateral prefrontal regions. Similar to our study, O’Neill et al., did not find a difference in frontal cortical volume between the sample dependent on cocaine only and the sample dependent on both cocaine and alcohol.
Impairments in prefrontal lobe mediated abilities have been noted in studies of alcohol abuse (Oscar-Berman, 2000), and in the scant literature on cocaine abuse (Di Sclafani et al., in press). MicroCog incorporates an Analogies and an Object Match test in the executive function domain. The Analogies test is based on Luria’s (1980) argument that patients with frontal lesions are impaired in the ability to transfer meaning (such as in the understanding and use of analogies, proverbs, metaphors, etc.). The Object Match test requires subjects to group the same objects according to different principles. Individuals with prefrontal lobe damage find it difficult to switch mental set once a concept has been derived, which may reflect rigidity, inflexibility, or perseveration (traits characteristic of substance abusers, particularly perseveration). In light of the documented effects of both chronic cocaine dependence and chronic alcohol dependence on pre-frontal lobe mediated abilities, it is surprising that concomitant cocaine and alcohol dependence in this sample was not associated with greater neuropsychological impairments on these prefrontal tests. However, the data consistently fail to indicate even additive effects of cocaine and alcohol abuse on frontal mediated functional impairments. This is consistent with our failure to find greater prefrontal cortical volume deficits in the subjects dependent on both cocaine and alcohol versus those dependent on cocaine only.
A cautionary note must be sounded vis-a-vis the attribution of abnormal brain structure and function to substance exposure. There is evidence of premorbid frontal lobe abnormalities in individuals at risk for substance abuse that may be associated with the predisposition to substance abuse. These structural and functional abnormalities are actually premorbid and comorbid, in that they are also associated with the presence of comorbid mood and externalizing disorder symptoms and traits (e.g. Berman and Noble, 1995; Hesselbrock et al., 1991; Pihl and Peterson, 1991; Poon et al., 2000). Moreover, the comorbidity of psychiatric disorders and substance use is substantial. Therefore, we cannot rule out a contribution from premorbid (and comorbid) differences to the prefrontal cortical volume reduction in the substance abusers versus normal controls.
Finally, we were surprised that we did not find an increase in the volume of white matter signal hyperintensities in the substance dependent samples. It may be that the relatively young age of our substance dependent sample was responsible for our failure to find evidence for ischemic brain disease in this group.
In summary, the current study suggests that chronic Cr dependence is associated with prefrontal cortex volume reductions that are present at 6-week abstinence (and thus are not acute effects of cocaine use). Comorbid alcohol dependence had no additional effect on prefrontal cortical volumes or on neuropsychological deficits in cocaine dependent individuals. The prefrontal cortex volume reductions are associated with poorer performance on tests of executive function, abilities known to be mediated by the frontal lobe.
Acknowledgments
This work was supported by NIDA grant DA09453 (George Fein).
References
- Bartzokis G, Beckson M, Lu P, Edwards N, Rapoport R, Wiseman E, Bridge P. Age-related brain volume reductions in amphetamine and cocaine addicts and normal controls: implications for addiction research. Psychiat Res: Neuroimag. 2000;98:93–102. doi: 10.1016/s0925-4927(99)00052-9. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Goldstein IB, Hance DB, Beckson M, Shapiro D, Lu PH, Edwards N, Mintz J, Bridge P. The incidence of T2-weighted MR imaging signal abnormalities in the brain of cocaine-dependent patients is age-related and region-specific. AJNR Am J Neuroradiol. 1999;20 (9):1628–1635. [PMC free article] [PubMed] [Google Scholar]
- Berman S, Noble E. Reduced visuospatial performance in children with the D2 dopamine receptor A1 allele. Behav Genet. 1995;25 (1):45–58. doi: 10.1007/BF02197241. [DOI] [PubMed] [Google Scholar]
- Biggins C, Fein G, MacKay S. ERP evidence for frontal cortex effects of chronic cocaine abuse. Biol Psychiat. 1997;42 (6):472–485. doi: 10.1016/S0006-3223(96)00425-8. [DOI] [PubMed] [Google Scholar]
- Brown TG, Seraganian P, Tremblay J. Alcoholics also dependent on cocaine in treatment: do they differ from ‘pure’ alcoholics? Addict Behav. 1994;19 (1):105–112. doi: 10.1016/0306-4603(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Ezekiel F, Di Sclafani V, Gomberg B, Fein G. Reliability of tissue volumes and their spatial distribution for segmented magnetic resonance images. Psychiat Res: Neuroimag. 2001;106 (3):193–205. doi: 10.1016/s0925-4927(01)00075-0. [DOI] [PubMed] [Google Scholar]
- Courville CB. Effects of Alcohol on the Nervous System of Man. San Lucas Press; Los Angeles: 1955. [Google Scholar]
- Danos P, Van Roos D, Kasper S, Bromel T, Broich K, Krappel C, Solymosi L, Moller H. Enlarged cerebrospinal fluid spaces in opiate-dependent male patients: a stereological CT study. Neuropsychobiology. 1988;38:80–83. doi: 10.1159/000026521. [DOI] [PubMed] [Google Scholar]
- Daras M, Tuchman AJ, Koppel BS, Samkoff LM, Weitzner I, Marc J. Neurovascular complications of cocaine. Acta Neurol Scand. 1994;90 (2):124–129. doi: 10.1111/j.1600-0404.1994.tb02691.x. [DOI] [PubMed] [Google Scholar]
- Di Sclafani V, Tolou-Shams M, Price LJ, Fein G. Neuropsychological performance of individuals dependent on crack-cocaine or crack-cocaine and alcohol at six weeks and six months abstinence. Drug Alcohol Depend. 2002;66:161–171. doi: 10.1016/s0376-8716(01)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Bachman L, Fisher S, Davenport L. Cognitive impairments in abstinent alcoholics. West J Med. 1990;152:531–537. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Tanabe J, Cardenas V, Weiner MW, Jagust WJ, Reed BR, Norman D, Schuff N, Kusdra L, Greenfield T, Chui H. Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology. 2000;55:1626–1635. doi: 10.1212/wnl.55.11.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol-dependent individuals. Alcohol Clin Exp Res. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Harford TC. Concurrent and simultaneous use of alcohol with cocaine: results of national survey. Drug Alcohol Depend. 1990;25:97–104. doi: 10.1016/0376-8716(90)90147-7. [DOI] [PubMed] [Google Scholar]
- Harper CG, Kril JJ. Neuropathology of alcoholism. Alcohol Alcoholism. 1990;25 (23):207–216. doi: 10.1093/oxfordjournals.alcalc.a044994. [DOI] [PubMed] [Google Scholar]
- Hesselbrock V, Bauer LO, Hesselbrock MN, Gillen R. Neuropsychological factors in individuals at high risk for alcoholism. Recent Dev Alcohol. 1991;9:21–40. [PubMed] [Google Scholar]
- Konzen JP, Levine SR, Garcia JH. Vasospasm and thrombus formation as possible mechanisms of stroke related to alkaloidal cocaine. Stroke. 1995;26 (6):1114–1118. doi: 10.1161/01.str.26.6.1114. [DOI] [PubMed] [Google Scholar]
- Liu X, Matochik J, Cadet JL, London ED. Smaller volume of prefrontal lobe in polysubstance abusers: a magnetic resonance imaging study. Neuropsychopharmacology. 1998;18:243–252. doi: 10.1016/S0893-133X(97)00143-7. [DOI] [PubMed] [Google Scholar]
- Luria A. Higher Cortical Functions in Man. Basic Books; New York: 1980. [Google Scholar]
- O’Neill J, Cardenas V, Meyerhoff D. Separate and inter-active effects of cocaine and alcohol dependence on brain structures and metabolites: Quantitative MRI and proton MR spectroscopic imaging. Addict Biol. 2001;6:347–361. doi: 10.1080/13556210020077073. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M. Neuropsychological vulnerabilities in chronic alcoholism. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA’s Neuroscience and Behavioral Research Portfolio. NIAAA Publications; Bethesda: 2000. pp. 437–471. [Google Scholar]
- Pascual-Leone A, Dhuna A, Anderson D. Cerebral atrophy in habitual cocaine abusers: a planimetric CT study. Neurology. 1991;4:34–38. doi: 10.1212/wnl.41.1.34. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Fisher G, Diamant K, Schneider C, Schindler S, Thurnher M, Ploechl W, Eder H, Kasper S. Cerebral CT findings in male opioid-dependent patients: stereological, planimetric and linear measurements. Psychiat Res: Neuroimag. 1998;83:139–147. doi: 10.1016/s0925-4927(98)00028-6. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19 (5):1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a five year interval. Arch Gen Psychiat. 1998;55:905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- Pihl RO, Peterson JB. A biobehavioural model for inherited predisposition to alcoholism. Alcohol Suppl. 1991;1:151–156. [PubMed] [Google Scholar]
- Poon E, Ellis D, Fitzgerald H, Zucker R. Intellectual, cognitive, and academic performance among sons of alcoholics, during the early school years: differences related to subtypes of familial alcoholism. Alcohol Clin Exp Res. 2000;24 (7):1020–1027. [PubMed] [Google Scholar]
- Powell DH, Kaplan EF, Whitla D, Weinstraub S, Catlin R, Funkenstein HH. MicroCog Assessment of Cognitive Functioning. The Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- Sas II. SASSAT User’s Guide, Release 6.03. SAS Institute; Cary, NC, USA: 1988. [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000;24 (5):611–621. [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Brain. Theime Medical Publishers; New York, NY: 1988. [Google Scholar]
- Tumeh S, Nagel J, English R, Moore M, Holman B. Cerebral abnormalities in cocaine abusers: Demonstration by SPECT perfusion brain scintigraphy. Radiology. 1990;176:821–824. doi: 10.1148/radiology.176.3.2389042. [DOI] [PubMed] [Google Scholar]
- Volkow N, Mullani N, Gould K, Adler S, Krajewski K. Cerebral blood flow in chronic cocaine users: A study with positron emission tomography. Brit J Psychiat. 1988;152:641–648. doi: 10.1192/bjp.152.5.641. [DOI] [PubMed] [Google Scholar]
- Weber D, Franceschi D, Ivanovic M, Atkins H, Cabahug C, Wong C, Susskind H. SPECT and planar brain imaging in crack abuse: Iodine-123-iodoamphetamine uptake and localization. J Nucl Med. 1993;34:899–907. [PubMed] [Google Scholar]


