Abstract
Objectives/Methods
Elevated CRP levels predict increased incidence of cardiovascular events and poor outcomes following interventions. There is the suggestion that CRP is also a mediator of vascular injury. Transgenic mice carrying the human CRP gene (CRPtg) are predisposed to arterial thrombosis post-injury. We examined whether CRP similarly modulates the proliferative and hyperplastic phases of vascular repair in CRPtg when thrombosis is controlled with daily aspirin and heparin at the time of trans-femoral arterial wire-injury.
Results
Complete thrombotic arterial occlusion at 28 days was comparable for wild-type and CRPtg mice (14 and 19%, respectively). Neointimal area at 28d was 2.5 fold lower in CRPtg (4190 ± 3134 μm2, n = 12) compared to wild-types (10,157 ± 8890 μm2, n = 11, p < 0.05). Likewise, neointimal/media area ratio was 1.10 ± 0.87 in wild-types and 0.45 ± 0.24 in CRPtg (p < 0.05). Seven days post-injury, cellular proliferation and apoptotic cell number in the intima were both less pronounced in CRPtg than wild-type. No differences were seen in leukocyte infiltration or endothelial coverage. CRPtg mice had significantly reduced p38 MAPK signaling pathway activation following injury.
Conclusions
The pro-thrombotic phenotype of CRPtg mice was suppressed by aspirin/heparin, revealing CRP’s influence on neointimal growth after trans-femoral arterial wire-injury. Signaling pathway activation, cellular proliferation, and neointimal formation were all reduced in CRPtg following vascular injury. Increasingly we are aware of CRP multipotent effects. Once considered only a risk factor, and recently a harmful agent, CRP is a far more complex regulator of vascular biology.
Keywords: C-reactive protein, Neointima, Angioplasty, p38 MAPK
1. Introduction
The acute phase reactant C-reactive protein (CRP) is a powerful predictor for cardiovascular disease on the one hand, and supports an array of vascular biologic events on the other. CRP levels are associated with future cardiovascular events in seemingly healthy subjects and with worse prognosis in acute coronary patients [1]. CRP promotes thrombosis [2,3] and complement activation [4], and might impact lipoprotein metabolism [5]. The confluence of clinical and basic evidence suggests that CRP should predict outcome after vascular intervention. Indeed, several reports have demonstrated a worsened outcome, including high rates of angiographic restenosis, in patients with high pre-procedural CRP levels [6–8]. Others however, found no correlation between CRP and restenosis [9–11]. Dibra et al. reported on the outcome of percutaneous coronary interventions (PCI) in 1152 patients. High CRP levels (>5 mg/l) were not associated with excess restenosis, but myocardial infarction and death at 1 month were twice as prevalent as in patients with lower CRP [10]. Interestingly, such events can commonly be attributed to thrombotic complications. In a second study of more than 900 PCI patients, pre-procedural CRP levels correlated with post-PCI mortality and MI but not with 1-year revascularization, which reflects restenosis [11]. The results of both studies support a causal link between CRP and thrombosis but not with neointimal growth and restenosis.
We have previously shown, using a human CRP transgenic mouse (CRPtg) model, that human CRP accelerates thrombosis [3] induced by guide-wire denudation of the femoral artery or by carotid photochemical injury. The current study examined whether CRP affects neointima formation in the context of PCI independent of its ability to induce thrombosis. Male CRPtg underwent femoral arterial guide-wire injury while receiving drug therapy sufficient to prevent thrombosis. Proliferation and apoptosis were investigated as well as p38 mitogen activated protein kinase (MAPK) activation. Contrary to our expectations, and in contrast to CRP’s exacerbation of neointima formation after ligation injury of the carotid artery [12], the guide-wire injured femoral arteries of CRPtg receiving anti-thrombotics had reduced cellular proliferation and neointimal formation as compared to wild-type controls.
2. Methods
2.1. Wire injury model
Male CRPtg and age-matched congenic wild-type mice underwent bilateral wire injury of the femoral artery as described [13]. CRPtg mice are congenic with C57BL/6 and carry a 31-kb ClaI fragment of human genomic DNA comprised of the CRP gene, 17 kb of 5′-flanking sequence, and 11.3 kb of 3′-flanking sequence [14]. The study was approved by Hebrew University animal ethics committee.
Animals were bled to obtain baseline CRP levels. To prevent thrombosis [3], aspirin treatment was started on the day prior to surgery (5 mg/kg, i.p. daily until artery harvest). A single heparin injection (100 U/kg, i.p.) was administered prior to surgery. All animals received bro-modeoxyuridine (BrdU) (50 mg/kg i.p.) 18 and 1 h before sacrifice. Femoral arteries were harvested for immunohistochemical and morphometric analyses on day 7 (n = 6/group) or day 28 after injury (n = 14–16/group). These were fixed in 4% paraformaldehyde, embedded in paraffin, and cut into three segments. Multiple sections (5 μm-thick) of each retrieved segment were prepared for VerHoeff tissue elastin and Carstair’s fibrin stains, and for immunohistochemical analysis. Microscopic images were captured and the dimensions of media and neointima were determined using Adobe Photoshop 5.0. The one arterial section with maximal neointimal area was chosen for further calculations. Standard avidin–biotin procedures for leukocytes (CD45, DAKO), endothelial cells (CD31, DAKO) and BrdU were performed. TACS 2 TdT-DAB immunostaining kit (Trevigen) was used for in situ detection of apoptotic cells.
2.2. Signaling pathway activation
Activation of p38 MAPK was measured in the injured arteries 6 h after vascular injury. Pools of 5 arteries from CRPtg mice and their WT littermates were lysed, homogenized under ultrasound, boiled for 5 min and protein content was quantified using the Bradford method. Twenty micrograms of protein were separated in 12% SDS-PAGE gels, blotted onto nitrocellulose and then incubated overnight with primary antibodies specific for mouse p38 (1:500, Cell Signaling) or P-p38 (1:500, Cell Signaling). Activation of p38 MAPK in pooled samples of lung tissue (5 mice per pool) was determined likewise before injury, 6 h after injury and at 24 h after injury. The ratio between the phosphorylated isoforms and the nonphosphorylated isoforms of each protein was calculated.
2.3. Data analysis
Group statistics are presented as the mean ± S.D. Group means were compared using the unpaired two-tailed Student’s t-test. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Arterial response to injury
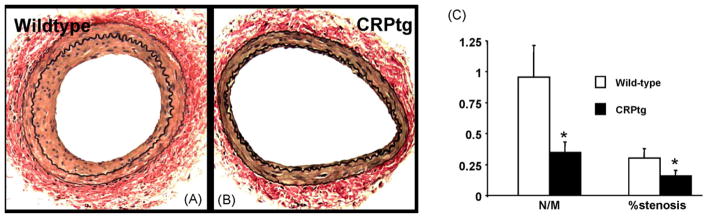
Serum levels of human CRP in CRPtg, measured by ELISA, were 24 ± 13 and 52 ± 8 mg/l at baseline and at 24 h after injury, respectively, Complete thrombotic occlusion was similar in both groups: observed in 2 of 14 wild-type (14%) and 3 of 16 CRPtg arteries (19%). Wild-type and CRPtg mice not treated with aspirin or heparin bolus had occlusion rates of 15% and 75%, respectively (data not shown), as reported earlier [3]. When mice were treated with aspirin, neointimal formation after guide-wire injury of the femoral artery was less profound in CRPtg compared to wild-type mice (Fig. 1A–C). Intimal area on day 28 was 2.5 fold lower in CRPtg; 10,157 ± 8890 μm2 in wild-type (2136–22,080 μm2, n = 12) and 4190 ± 3134 μm2 in CRPtg (2299–10,263 μm2, n = 13, p < 0.05). The neointima to media area ratio was similarly lower in CRPtg (1.10 ± 0.87 versus 0.45 ± 0.24, Fig. 1C). The arterial cross-sectional area, i.e. the area within the external elastic lamina, was identical in wild-type and CRPtg mice, 38,802 ± 11,097 and 40,444 ± 10,346 μm2, respectively, precluding a major difference in vascular remodeling following injury.
Fig. 1.
Photomicrographs of wild-type (A) and CRPtg (B) femoral arteries stained for elastin at 28 days after wire injury. C, bar graph of neointima to media ratio (N/M) and arterial stenosis in wild-type and CRPtg (n = 12–13/group, p < 0.05).
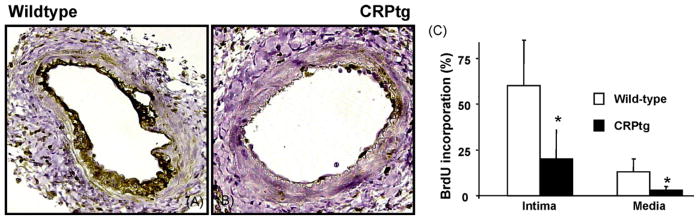
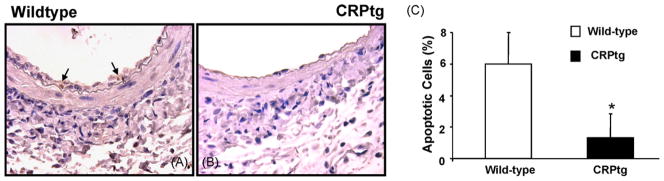
Sections of arteries harvested 7 days after injury were stained for fibrin to confirm the adequacy of thrombotic control. No fibrin was detected in either wild-type or CRPtg mice (not shown). At this time wild-type mice (Fig. 2) demonstrated substantial cell proliferation within the media (60 ± 26%) and newly formed intima (13 ± 7%, n = 5). These values were 3–4 fold reduced in CRPtg mice (20 ± 16% and 3 ± 2%, proliferation in media and intima, respectively, n = 5). BrdU incorporation and proliferation at 28 days were sharply reduced to less than 2% in both controls and CRPtg mice. Intimal cell apoptosis at 7 days in CRPtg mice was 1.3 ± 1.5%, more than four-fold reduced when compared to the number of apoptotic cells in wild-type mice (6 ± 2%, n = 4, p < 0.05, Fig. 3). In sections obtained at 28 days no apoptotic cells could be detected (not shown). Leukocyte accumulation, assessed by CD-45 immunostaining 7 days after injury, was no different in wild-type and CRPtg mice (16 ± 8 and 14 ± 11%, respectively, n = 4, not shown). Endothelialization, evaluated by staining for CD-31 was no different in the arteries of control and CRPtg mice (82 and 77% luminal coverage, respectively data not shown).
Fig. 2.
Photomicrographs of wild-type (A) and CRPtg (B) femoral arteries stained for and BrdU at day +7 after wire injury. C, bar graph denoting the reduction in the number of proliferating cells in CRPtg compared to wild-type arteries (n = 5, p < 0.05).
Fig. 3.
Photomicrographs of wild-type (A) and CRPtg (B) femoral arteries stained for apoptotic cells at day +7 after wire injury. C, bar graph denoting the reduction in the number of apoptotic cells in CRPtg compared to wild-type arteries (n = 4, p < 0.05).
3.2. Activation of signaling pathways following injury
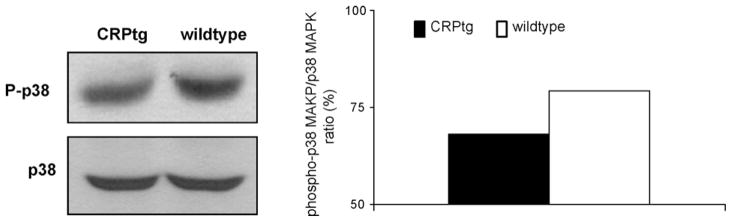
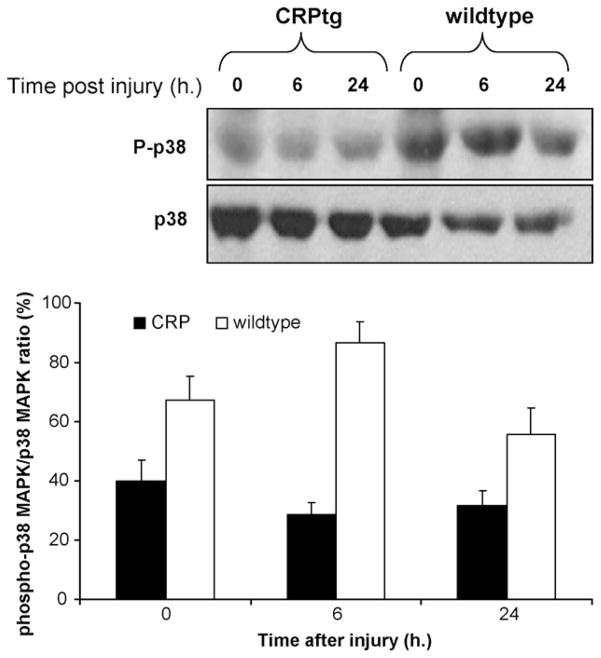
The activation of p38 MAPK at 6 h after injury was studied by Western Blot analysis of the phosphorylated and non-phosphorylated isoforms in the injured artery. Proteins were pooled from 5 arteries for each experiment as the amounts of proteins obtained from a single artery were too small to enable such evaluation on their own. Phospho p38 MAPK was reduced in CRPtg mice when compared with wild-type mice. Total p38 MAPK was similar in CRPtg and wild-types, and thus phospho-p38 MAPK normalized to total p38 MAPK remained reduced (Fig. 4, right). The reduction of p38 MAPK activation in CRPtg stands in contradiction to previous studies of this pathway [15]. To further clarify CRP-p38 MAPK inter-action we broadened our studies beyond the injured artery. p38 MAPK activation was studied in lung tissue prior to and at 6 and 24 h after vascular injury. Phopho-p38 MAPK levels, and the ratio between the phosphorylated isoform and total p38 MAPK were reduced at all 3 time points with the largest difference noted at 6 h after injury (Fig. 5, n = 5/each time point, p < 0.05).
Fig. 4.
Activation of p38 MAPK signaling pathway in the iliofemoral artery at 6 h after wire injury in wild-type and CRPtg mice. On the left panel are representative Western Blots showing the specific bands detected using antibodies against phospho-p38 and p38 MAPK (protein pooled of 5 mice in each Blot). The right panel is a bar graph showing the density ratio between the phopshorylated and total isoforms.
Fig. 5.
Temporal activation of p38 MAPK in the lung prior to and after iliofemoral wire injury. Representative Western Blot showing the specific band detected using antibodies against phosphorylated-p38 and p38 MAPK, and a bar graph showing the density ratio between the phopshorylated and total p38 MAPK isoforms (n = 5, mean ratio ± S.D., p < 0.05 for all time points).
4. Discussion
It is increasingly evident that CRP is not simply a marker of cardiovascular events but a participant in cardiovascular biology. High CRP levels are predictive of acute cardiovascular events and of worse outcome following myocardial infarction and percutaneous coronary interventions, but do not correlate with the burden of atherosclerosis [16,17]. In a transgenic mouse model human CRP clearly predisposed to thrombosis [3], but most reports do not support a causal role in atherogenesis [18–21]. The present study examined the effect of CRP on vascular repair following wire injury in the femoral artery of CRP transgenic mice in which thrombosis was pharmacologically suppressed. In this particular circumstance cell signaling, cell proliferation, and neointima formation were all reduced compared with wild-type controls subjected to the same therapy and injury regimen. This finding is surprising given an earlier report that neointima formation is exacerbated in CRPtg subjected to carotid artery ligation [45], but the two outcomes are not necessarily at odds. Rather, taken together, the results of the two studies might indicate that some or all of the CRP effect on neointima generation might depend on its ability to promote thrombosis, which itself induces cell proliferation. The present study is the first to investigate the influence of CRP on vascular repair in the context of anti-thrombotic therapy.
In vitro data suggest that CRP has vast vascular pro-inflammatory effects (for review [22] icluding production of adhesion molecules, MCP-1 and ET-1 from endothelial cells, blunts endothelial vasoreactivity, reduces NO production and eNOS activity and upregulates the expression of complement inhibitory factors on endothelial cells. Yet, as most of the experiments were in tissue culture with cause–effect exhibited following the use of commercial CRP preparations concern has been raised for spurious effects of contaminants. When impurities, in particular the preservative sodium azide and the contaminant endotoxin, are removed from commercial CRP preparations the putative direct endothelial activity is questioned [23–28]. Transgenic animals that express human CRP, or mice that express rabbit CRP [20], can be used to resolve the issue of this suspected spurious artifactual results. We used CRPtg mice to examine the effects of CRP in vascular injury. This, in spite of less neointima and neointima to media ratio that is observed in mice following vascular injury (as compared with larger species) that warrants larger animals groups to demonstrate discernible differences in repair. CRPtg male mice possess constitutively high and constant CRP expression. CRPtg mice do not reproduce the inflammatory situation observed in high-CRP patients, but can specifically help in studying CRP-associated effects. Consistently elevated levels are achieved without repeated injections that might introduce conformational changes in CRP [29] or contaminants like endotoxin that could affect neointimal formation [30].
In a previous study with CRPtg we found that CRP promotes an arterial pro-thrombotic phenotype [3]. As 75% of the injured arteries in CRPtg mice were occluded by thrombi, further analysis of the effect of CRP on vascular repair was precluded in those studies. To enable such an analysis CRPtg mice were treated with an anti-thrombotic regimen that consisted of heparin at the time of injury, and aspirin beginning a day before injury and continuing for the duration of the study. This empirical regimen suppressed vascular occlusion in CRPtg to the level observed in control wild-type mice. Both heparin [31] and aspirin [32] can provide an anti-proliferative effect in addition to their anti-thrombotic effect. However, suppression of neointimal formation following experimental vascular injury requires substantially higher doses and far longer durations of administration (of heparin) than applied here [32–35]. The decrease in intimal hyperplasia with increased CRP though contrary to expectations may not be surprising in retrospect. While blood CRP is increased in response to inflammatory stimuli, CRP per se has anti-inflammatory properties (for review [4,14]). CRP binds and promotes the clearance of apoptotic cells, sustaining an anti-inflammatory response [36]. While apoptosis is generally associated with suppression of neointimal formation [37,38], CRP-induced clearance of apoptotic cells could explain the reduction in apoptotic cells observed in the CRPtg injured vessel. CRP suppresses neutrophil chemotaxis [39] and superoxide generation [40] that are associated with neointimal formation. CRP confers protection against autoimmune diseases: CRPtg are relatively insensitive to murine systemic lupus [41], and experimental allergic encephalomyelitis [42]. Furthermore, CRP upregulated the expression of complement inhibitory factors on endothelial cells [43], suggesting that CRP may protect from complement-mediated vascular injury implicated in intimal hyperplasia [44].
Vascular injury in human CRP transgenic mice on aspirin induced less cellular proliferation, reduced neointimal apoptotic cells and limited neointimal formation. These findings reinforce our earlier proposal that CRP is a potent determinant of cardiovascular biology, but they also reveal that the protein contribution is unexpectedly complex. In the case of neointima formation, the effect of CRP is profoundly influenced by the thrombotic process. The association of high CRP levels with worse outcome after PCI may therefore be derived from vascular pathologies other than proliferation and hyperplasia. Excessive thrombosis alone can induce the pathologies observed in high-CRP patients after vascular intervention [10]. Indeed, the dramatic reduction in thrombotic occlusion after wire injury further supports the extensive use of anti-thrombotics in patients with high-CRP levels. Further studies are warranted to determine whether some drugs are superior to others in suppressing CRP prothrombotic phenotype and if combination therapy should be applied.
The complex cellular interactions initiated by vascular injury are coordinated and modulated by the elaboration of cytokines and growth factors. The production and transduction of many of these mediators require phosphorylation of p38 mitogen-activated protein kinase (MAPK). Activation of p38 MAPK plays an important role in the vascular response, increasing smooth muscle proliferation and neointimal formation. Inhibition of p38 MAPK was previously shown to suppress neointimal formation following vascular injury [45]. The suppressed in vivo p38 MAPK activation reported in this study is of special interest. Previous study reported that CRP attains pro-inflammatory activity in peripheral blood mononuclear cells, via the activation of p38 MAPK [15]. Our studies, both in the injured artery and the distant lung tissue, indicate that CRP exerts an opposite effect, with suppression of p38 MAPK. These contradictory findings may result from the in vitro methodology which exposes the study to the risk of contamination with preservatives and endotoxin [23–28]. Another explanation is a possible differential effect of CRP on blood mononuclear cells and arterial and alveolar cells. Nevertheless, p38 MAPK activation appears to be reduced in the wounded artery as well as the distant lung and based on the reported roles of p38 MAPK signaling in vascular reparation we suggest its suppression by CRP as a pathway in the observed CRP anti-proliferative effect.
In the only other comparable study, Wang et al. reported that neointimal formation in the carotid artery of female mice (injured by ligation in mice not given anti-thrombotic therapy) was augmented in CRPtg compared to wild-type [12]. That proliferative effect was suppressed by estradiol administration. The enhanced reparative response observed in that study can be attributed to its contrasting methodology. The current results were obtained using male CRPtg that expressed >15 μg/ml of blood CRP at baseline and underwent denudation of the iliofemoral artery. Due to the high rate of complete thrombotic occlusion mice were treated with an anti-thrombotic regimen. In contrast, no thrombotic occlusion was seen in the carotid artery ligation experiments, but these were performed in females with <0.6 μg/ml of baseline blood CRP. Thus, vascular injury likely was exerted by a different mechanism, injury was induced in different arteries, and CRP blood levels differed by almost two-fold. The enhanced thrombosis that is observed in male CRPtg [3], which is capable of promoting proliferation and neointimal growth and thus was pharmacologically treated was not observed in Wang female mice and was not treated.
4.1. Study limitations
The CRPtg mouse model is devoid of some of the inflammatory changes that are associated with high-CRP levels in human, especially those that stimulate CRP expression such as increased IL-6 levels. Thus, the CRPtg model specifically studies CRP-associated actions, but does not fully reproduce the inflammatory changes observed in high-CRP subjects. Moreover, vascular repair following denudation differs from plaque formation; especially in view of CRP enhanced LDL oxidation [5]. The lesions formed in our model were sufficient to examine the question raised but are clearly relatively simple. Even the extent of endothelial recovery was higher than reported by others [46], perhaps suggesting that we had induced somewhat lesser degree of injury in our model system, but certainly without a difference between our animals or a difference that can itself account for the decreased hyperplasia seen in the CRPtg. Our results remain to be confirmed in more complex animal systems and more injurious vascular injury models such as the ApoE−/− CRPtg model or Watanabe heritable hyperlipidemic rabbits.
In conclusion, following suppression of thrombosis in CRPtg mice neointimal formation after arterial injury was not accelerated but rather decreased. These findings are preceded by suppression of p38 MAPK pro-proliferative signaling, reduced proliferation and reduced number of apoptotic cells. While further studies are called for the role of CRP in vascular repair and atherothrombosis and its predictive value for post-PCI restenosis, the present study suggests a modulating role for CRP on the inflammatory effects of vascular repair. The findings we now report are in line with new data on the multidimensional nature of CRP. Once thought to be only a risk factor, and more recently as only a harmful agent, it is now increasingly understood that CRP is a far more complex regulator of vascular biology.
Acknowledgments
This work was supported in part by the Israel Sciences Foundation Grant (ISF #655/05) to HDD, and NIH grant (HL40309) to ERE.
References
- 1.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 2.Devaraj S, Xu DY, Jialal I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implications for the metabolic syndrome and atherothrombosis. Circulation. 2003;107:398–404. doi: 10.1161/01.cir.0000052617.91920.fd. [DOI] [PubMed] [Google Scholar]
- 3.Danenberg H, Szalai A, Swaminathan R, et al. Increased thrombosis following injury in human C-reactive protein transgenic mice. Circulation. 2003;108:512–5. doi: 10.1161/01.CIR.0000085568.13915.1E. [DOI] [PubMed] [Google Scholar]
- 4.Volanakis JE. Human C-reactive protein: expression, structure, and function. Mol Immunol. 2001;38:189–97. doi: 10.1016/s0161-5890(01)00042-6. [DOI] [PubMed] [Google Scholar]
- 5.Zwaka TP, Hombach V, Torzewski J. C-reactive protein-mediated low density lipoprotein uptake by macrophages: implications for atherosclerosis. Circulation. 2001;103:1194–7. doi: 10.1161/01.cir.103.9.1194. [DOI] [PubMed] [Google Scholar]
- 6.Buffon A, Liuzzo G, Biasucci LM, et al. Preprocedural serum levels of C-reactive protein predict early complications and late restenosis after coronary angioplasty. J Am Coll Cardiol. 1999;34:1512–21. doi: 10.1016/s0735-1097(99)00348-4. [DOI] [PubMed] [Google Scholar]
- 7.Walter DH, Fichtlscherer S, Sellwig M, et al. Preprocedural C-reactive protein levels and cardiovascular events after coronary stent implantation. J Am Coll Cardiol. 2001;37:839–46. doi: 10.1016/s0735-1097(00)01193-1. [DOI] [PubMed] [Google Scholar]
- 8.Versaci F, Gaspardone A, Tomai F, et al. Immunosuppressive therapy for the prevention of restenosis after coronary artery stent implantation (IMPRESS study) J Am Coll Cardiol. 2002;40:1935–42. doi: 10.1016/s0735-1097(02)02562-7. [DOI] [PubMed] [Google Scholar]
- 9.Horne BD, Muhlestein JB, Strobel GG, et al. Greater pathogen burden but not elevated C-reactive protein increases the risk of clinical restenosis after percutaneous coronary intervention. Am Heart J. 2002;144:491–500. doi: 10.1067/mhj.2002.125010. [DOI] [PubMed] [Google Scholar]
- 10.Dibra A, Mehilli J, Braun S, et al. Association between C-Reactive protein levels and subsequent cardiac events among patients with stable angina treated with coronary artery stenting. Am J Med. 2003;114:715–22. doi: 10.1016/s0002-9343(03)00183-9. [DOI] [PubMed] [Google Scholar]
- 11.Chan AW, Bhatt DL, Chew DP, et al. Relation of inflammation and benefit of statins after percutaneous coronary interventions. Circulation. 2003;107:1750–6. doi: 10.1161/01.CIR.0000060541.18923.E9. [DOI] [PubMed] [Google Scholar]
- 12.Wang D, Oparil S, Chen Y, et al. Estrogen treatment abrogates neointima formation in human C-reactive protein transgenic mice. Arterioscler Thromb Vasc Biol. 2005;25:2094–100. doi: 10.1161/01.ATV.0000179602.85797.3f. [DOI] [PubMed] [Google Scholar]
- 13.Reis E, Smyth S, Coller B. Mouse model of transluminal femoral artery injury. In: Simon D, Rogers C, editors. Contemporary Cardiology: Vascular Disease and Injury: Preclinical Research. Totowa, NJ: Humana Press; 2001. pp. 103–13. [Google Scholar]
- 14.Szalai AJ, McCrory MA. Varied biologic functions of C-reactive protein: lessons learned from transgenic mice. Immunol Res. 2002;26:279–87. doi: 10.1385/IR:26:1-3:279. [DOI] [PubMed] [Google Scholar]
- 15.Lim MY, Wang H, Kapoun AM, et al. p38 Inhibition attenuates the pro-inflammatory response to C-reactive protein by human peripheral blood mononuclear cells. J Mol Cell Cardiol. 2004;37:1111–4. doi: 10.1016/j.yjmcc.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Folsom AR, Pankow JS, Tracy RP, et al. Association of C-reactive protein with markers of prevalent atherosclerotic disease. Am J Cardiol. 2001;88:112–7. doi: 10.1016/s0002-9149(01)01603-4. [DOI] [PubMed] [Google Scholar]
- 17.Khera A, de Lemos JA, Peshock RM, et al. Relationship between C-reactive protein and subclinical atherosclerosis: the dallas heart study. Circulation. 2006;113:38–43. doi: 10.1161/CIRCULATIONAHA.105.575241. [DOI] [PubMed] [Google Scholar]
- 18.Hirschfield GM, Gallimore JR, Kahan MC, et al. Transgenic human C-reactive protein is not proatherogenic in apolipoprotein E-deficient mice. PNAS. 2005;102:8309–14. doi: 10.1073/pnas.0503202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul A, Ko KW, Li L, et al. C-reactive protein accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109:647–55. doi: 10.1161/01.CIR.0000114526.50618.24. [DOI] [PubMed] [Google Scholar]
- 20.Reifenberg K, Lehr H-A, Baskal D, et al. Role of C-reactive protein in atherogenesis: can the apolipoprotein E knockout mouse provide the answer? Arterioscler Thromb Vasc Biol. 2005;25:1641–6. doi: 10.1161/01.ATV.0000171983.95612.90. [DOI] [PubMed] [Google Scholar]
- 21.Tennent GA, Hutchinson WL, Kahan MC, et al. Transgenic human CRP is not pro-atherogenic, pro-atherothrombotic or pro-inflammatory in apoE(−/−) mice. Atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Verma S, Devaraj S, Jialal I. Is C-reactive protein an innocent bystander or proatherogenic culprit? C-reactive protein promotes atherothrombosis. Circulation. 2006;113:2135–50. [PubMed] [Google Scholar]
- 23.Taylor KE, Giddings JC, van den Berg CW. C-reactive protein-induced in vitro endothelial cell activation is an artefact caused by azide and lipopolysaccharide. Arterioscler Thromb Vasc Biol. 2005;25:1225–30. doi: 10.1161/01.ATV.0000164623.41250.28. [DOI] [PubMed] [Google Scholar]
- 24.Lafuente N, Azcutia V, Matesanz N, et al. Evidence for sodium azide as an artifact mediating the modulation of inducible nitric oxide synthase by C-reactive protein. J Cardiovasc Pharmacol. 2005;45:193–6. doi: 10.1097/01.fjc.0000154371.95907.bd. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, Wang S, Deb A, et al. Proapoptotic, anti-migratory, anti-proliferative, and anti-angiogenic effects of commercial C-Reactive protein on various human endothelial cell types in vitro: implications of contaminating presence of sodium azide in commercial preparation. Circ Res. 2005;97:135–43. doi: 10.1161/01.RES.0000174612.90094.fd. [DOI] [PubMed] [Google Scholar]
- 26.Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106:1439–41. doi: 10.1161/01.cir.0000033116.22237.f9. [DOI] [PubMed] [Google Scholar]
- 27.Verma S, Wang CH, Li SH, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–9. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 28.Verma S, Kuliszewski MA, Li S-H, et al. C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation. 2004;109:2058–67. doi: 10.1161/01.CIR.0000127577.63323.24. [DOI] [PubMed] [Google Scholar]
- 29.Khreiss T, Jozsef L, Potempa LA, Filep JG. Conformational rearrangement in C-reactive protein is required for proinflammatory actions on human endothelial cells. Circulation. 2004;109:2016–22. doi: 10.1161/01.CIR.0000125527.41598.68. [DOI] [PubMed] [Google Scholar]
- 30.Danenberg H, Welt F, Walker M, et al. Systemic inflammation induced by lipopolysaccharide increases neointimal formation following balloon and stent injury in rabbits. Circulation. 2002;105:2917–22. doi: 10.1161/01.cir.0000018168.15904.bb. [DOI] [PubMed] [Google Scholar]
- 31.Woods TC, Blystone CR, Yoo J, Edelman ER. Activation of EphB2 and its ligands promotes vascular smooth muscle cell proliferation. J Biol Chem. 2002;277:1924–7. doi: 10.1074/jbc.M108189200. [DOI] [PubMed] [Google Scholar]
- 32.Voisard R, Fischer R, Osswald M, et al. Aspirin (5 mmol/l) inhibits leukocyte attack and triggered reactive cell proliferation in a 3D human coronary in vitro model. Circulation. 2001;103:1688–94. doi: 10.1161/01.cir.103.12.1688. [DOI] [PubMed] [Google Scholar]
- 33.Lovich MA, Edelman ER. Tissue concentration of heparin, not administered dose, correlates with the biological response of injured arteries in vivo. Proc Natl Acad Sci USA. 1999;96:11111–6. doi: 10.1073/pnas.96.20.11111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swafford AN, Jr, Bratz IN, Knudson JD, et al. C-reactive protein does not relax vascular smooth muscle: effects mediated by sodium azide in commercially available preparations. Am J Physiol Heart Circ Physiol. 2005;288:H1786–95. doi: 10.1152/ajpheart.00996.2004. [DOI] [PubMed] [Google Scholar]
- 35.Napoli C, Cirino G, Del Soldato P, et al. Effects of nitric oxide-releasing aspirin versus aspirin on restenosis in hypercholesterolemic mice. Proc Natl Acad Sci USA. 2001;98:2860–4. doi: 10.1073/pnas.041602898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gershov D, Kim S, Brot N, Elkon KB. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an anti-inflammatory innate immune response: implications for systemic autoimmunity. J Exp Med. 2000;192:1353–64. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Z, Fukutomi T, Zago AC, et al. Simvastatin reduces neointimal thickening in low-density lipoprotein receptor-deficient mice after experimental angioplasty without changing plasma lipids. Circulation. 2002;106:20–3. doi: 10.1161/01.cir.0000022843.76104.01. [DOI] [PubMed] [Google Scholar]
- 38.Shibata R, Kai H, Seki Y, et al. Inhibition of STAT3 prevents neointima formation by inhibiting proliferation and promoting apoptosis of neointimal smooth muscle cells. Hum Gene Ther. 2003;14:601–10. doi: 10.1089/104303403321618128. [DOI] [PubMed] [Google Scholar]
- 39.Kew R, Hyers T, Webster R. Human C-reactive protein inhibits neutrophil chemotaxis in vitro: possible implications for the adult respiratory distress syndrome. J Lab Clin Med. 1990;115:339–45. [PubMed] [Google Scholar]
- 40.Dobrinich R, Spagnuolo P. Binding of C-reactive protein to human neutrophils. Inhibition of respiratory burst activity. Arthritis Rheum. 1991;34:1031–8. doi: 10.1002/art.1780340813. [DOI] [PubMed] [Google Scholar]
- 41.Szalai AJ, Weaver CT, McCrory MA, et al. Delayed lupus onset in (NZB × NZW)F1 mice expressing a human C-reactive protein trans-gene. Arthritis Rheum. 2003;48:1602–11. doi: 10.1002/art.11026. [DOI] [PubMed] [Google Scholar]
- 42.Szalai AJ, Nataf S, Hu XZ, Barnum SR. Experimental allergic encephalomyelitis is inhibited in transgenic mice expressing human C-reactive protein. J Immunol. 2002;168:5792–7. doi: 10.4049/jimmunol.168.11.5792. [DOI] [PubMed] [Google Scholar]
- 43.Li SH, Szmitko PE, Weisel RD, et al. C-reactive protein upregulates complement-inhibitory factors in endothelial cells. Circulation. 2004;109:833–6. doi: 10.1161/01.CIR.0000117087.27524.0E. [DOI] [PubMed] [Google Scholar]
- 44.Sawada M, Yanamoto H, Nagata I, et al. Prevention of neointimal formation by a serine protease inhibitor, FUT-175, after carotid balloon injury in rats. editorial comment. Stroke. 1999;30:644–50. doi: 10.1161/01.str.30.3.644. [DOI] [PubMed] [Google Scholar]
- 45.Ju H, Nerurkar S, Sauermelch C, et al. Sustained activation of p38 mitogen-activated protein kinase contributes to the vascular response to injury. J Pharmacol Exp Ther. 2002;301:15–20. doi: 10.1124/jpet.301.1.15. [DOI] [PubMed] [Google Scholar]
- 46.Hutter R, Sauter BV, Reis ED, et al. Decreased reendothelialization and increased neointima formation with endostatin overexpression in a mouse model of arterial injury. Circulation. 2003;107:1658–63. doi: 10.1161/01.CIR.0000058169.21850.CE. [DOI] [PubMed] [Google Scholar]