Abstract
The effect of pulse pressure on arterial wall remodeling remains unclear, although remodeling of the arterial wall under hypertensive pressure and elevated flow has been well documented. The objective of this study was to evaluate matrix remodeling in arteries under nonpulsatile and hyperpulsatile pressure as compared to arteries under normal pulsatile pressure. Using a novel ex vivo organ culture model that allowed us to change pressure pulsatility without changing mean pressure or flow, arteries were cultured for 7 days under normal, nonpulsatile, and hyperpulsatile pressures with the same mean pressure and flow rate. Fenestrae in internal elastic lamina (IEL), collagen content, connexin 43, and fibronectin proteins were examined in these arteries using confocal microscopy, immunoblotting, and immunohistochemistry. Our results showed that the mean fenestrae size and area fraction of fenestrae to total area of IEL decreased 51 % and 45 % in arteries cultured under nonpulsatile pressure and decreased 45 % and 54 % under hyperpulsatile pressure, respectively, compared to arteries under normal pulsatile pressure. There was no difference in fibronectin (FN) and collagen III levels among the three pulse groups, while collagen I and connexin 43 expression increased 80.8% and 35.3% in the hyperpulsatile arteries, respectively, but not in nonpulsatile arteries. In conclusion, our results demonstrated, for the first time, that an increase or elimination in pulse pressure from its normal physiologic level stimulates arterial wall matrix structural changes. Hyperpulsatile pressure has a more pronounced effect than the diminished pulse pressure, which may provide a mechanism for increased wall stiffness in arteries under hyperpulsatile pressure.
Keywords: Pulsatile pressure, internal elastic lamina, collagen, Connexin 43, porcine artery, ex vivo, organ culture
INTRODUCTION
Arteries are subjected to pulsatile blood flows and pressures in vivo. Normal arterial pressure consists of a pulse pressure oscillating around a nonpulsatile mean pressure. Pulse pressure may elevate or diminish due to arterial stiffening, stenosis, or abnormal cardiac function [1, 2]. Pulse pressure may even be temporarily eliminated with the use of nonpulsatile cardiopulmonary bypass machines or ventricular assist devices [3]. Elevated pulse pressure in humans is associated with vascular stiffening and is increasingly recognized as an important risk factor for cardiovascular disease [2, 4, 5]. On the other hand, an important issue in using nonpulsatile heart-lung machines is how the nonpulsatile flow produced by the pumps negatively impacts organ function [3, 6, 7]. However, the effect of nonpulsatile pressure has not been clearly defined in arteries. It is also necessary to understand the effect of pulse pressure on cellular function, in order to select suitable pressure conditions in bioreactor design for tissue engineering of vascular grafts.
In addition to steady stretch and shear stress, pulsatility has been postulated as a crucial mechanical stimulus for vascular cells. In healthy and hypertensive human subjects, it was reported that carotid internal diameter and intima-media thickness were strongly influenced by carotid pulse pressure [8–10], although it is difficult to specify the direct effect of pulse pressure in human subjects or animal models due to the technical difficulty of separating changes in mean and pulse pressure. On the other hand, in vitro studies of smooth muscle and endothelial cells have demonstrated that cyclic stretch increased collagen and Connexin 43 expression and increased cell proliferation [11–13], suggesting a possible affect of pulse pressure. Recent studies indicate that pulse pressure/cyclic stretch activates focal adhesion kinase and ERK½ activity through pathways independent of steady pressure/stretch [14, 15]. Furthermore, it has been well-documented that elevations in mean pressure (hypertensive) and flow lead to significant wall remodeling in arteries [16–18]. The elevated circumferential stretch generated by hypertensive pressure increases collagen and elastin content [19–22]. However, the effect of pulse pressure on matrix remodeling remains unclear. Therefore, the matrix remodeling under different pulse pressure needs to be investigated.
The objective of this study was to determine matrix remodeling of arterial walls under nonpulsatile pressure and hyperpulsatile pressure as compared to normal pulsatile pressure. Weused a unique ex vivo porcine artery organ culture model, which allowed us to independently alter the pulse pressure without changing the mean pressure and flow rate.
MATERIALS AND METHODS
Ex vivo artery organ culture
Common porcine carotid arteries (n=42) were harvested at a local abattoir and transported to the lab in cold phosphate buffered saline solution. Arterial segments, 3–5cm in length, were prepared and mounted into an ex vivo artery organ culture system as previously described [23, 24]. The perfusion and bath media were DMEM supplemented with sodium bicarbonate (3.7g/L), antibiotic/antimycotic (14mL/L), L-glutamine (10mL/L), calf serum (100mL/L), and HEPES buffer (25mL/L). The perfusion medium was also supplemented with Dextran (50–55g/L) to increase its viscosity to the level of normal blood. The flow loops were placed in incubators for temperature and CO2 control (37°C, 5% CO2). Artery lengths were increased to a physiological stretch ratio of 1.5 [23]. The flow and pressure in all flow loops were increased gradually to physiological levels, by adjusting the pump speed and a resistance clamp. The pulse pressure was controlled by adjusting the length of a T-end tubing and the rigidity of the tubing of the flow loop [23]. To achieve nonpulsatile flow, a pulse dampener (Cole-Parmer) was added into the flow loop to fully damp down the pressure pulse.
Arteries were divided into three different pulse pressure groups. A pulsatile group was maintained at a mean pressure of 100 mmHg, with a pulsatile pressure of 30 mmHg and a mean flow rate of 160 ml/min. A hyperpulsatile group was maintained at the same mean pressure and flow rate, but the pulse pressure was increased to 50 mmHg. A nonpulsatile group was maintained at the same mean pressure and flow rate, with no pulse pressure. Arteries were cultured for up to 7 days and then harvested for histology and protein measurements.
Histology and collagen content measurement
Arteries were harvested after organ culture and pressure-fixed in 10% formalin at 100 mmHg overnight, processed, and embedded in paraffin blocks. Serial cross-sections (5 µm) were cut and processed for hematoxylin and eosin staining and trichrome collagen staining as described previously [25]. For collagen staining, the slides were hydrated and soaked in Bouins solution for 5 min, washed, and transferred to Weigert’s Iron Hematoxylin for 2 min. The slides were rinsed in water and incubated in Biebrich Scarlet-Acid Fuchsin for 5 min, followed by a 10 min incubation in a phosphomolybdic-phosophotungstic acid solution. The slides were stained with Analine Blue for 2 min, washed in 1% aqueous acetic acid, dehydrated, and mounted. Collagen appears blue under the light microscope. The percent area of tissue stained positive for collagen was measured photometrically using Image-Pro Plus 4.5(Media Cybernetics, Silver Spring) and expressed as a percent of the total tissue area.
Western blotting
Cultured arteries were harvested, washed once with PBS, snap frozen in liquid nitrogen, and stored at −20°C. Samples were homogenized using the Reagent 4 protein extraction buffer (Sigma). Protein extracts (10 µg total protein) were separated by 4%–12% SDS-PAGE. Using specific primary antibodies for connexin 43 (Sigma at 1:2000), collagen type I and Type III (Sigma, dilution 1:1000), fibronectin (Chemicon International, dilution 1:1000) and β-actin (Sigma, dilution 1:5000), immunoreactive proteins were detected and then visualized with an enhanced chemiluminescence detection system (ECL) according to manufacturer directions. The relative intensities of the protein bands were quantified by scanning densitometry and normalized to the intensity of β–actin.
Laser scanning confocal microscopy
To examine IEL, arteries were stripped of adventitia, opened longitudinally, and cut transversely into 1×1 cm2 pieces, stretched to 1.5 cm×1.25 cm (the artery stretching ratio under systolic pressure in pigs), fixed in 4% formalin overnight, then treated with 0.5% triton X-100 for 15min, and stained with Hochest-33258 (1µg/ml Molecular Probes) for 30 min, and mounted under coverslips with glycerol/PBS (1:9) for en face morphological examination using confocal microscopy (LSM 510 META, Zeiss, Germany). From each vessel, 10 images were captured with a 40× oil immersion objective. The size and percentage of fenestrae in the images were quantified using Image-Pro Plus 4.5.
Immunohistochemistry
Arteries were rinsed in PBS and then frozen in OCT freezing medium before sectioning. Frozen 6-µm sections were fixed in acetone, followed by immunostaining. The tissue sections were blocked by 10% normal goat serum dissolved in PBS at 37°C for 15 min, then incubated with the primary antibody Cx43 (Sigma dilution 1:500) at room temperature for 1 h. An additional slide incubated with no primary antibody served as the negative control. All the sections were washed in PBS for 1 hour and incubated with the secondary antibody (Alexa488-conjugated goat anti-rabbit IgG, Molecular Probe, dilution 1:500) for 1 hour at room temperature. The samples were mounted with medium containing propidium iodide (Vector Laboratories) for counterstaining nuclei and examined by laser scanning confocal microscopy (Zeiss, LSM510)[23].
Statistical analysis
Data are given as mean ± SD. Significance was set at p<0.05 and determined by one-way ANOVA.
RESULTS
Effects of pulsatile pressure on internal elastic lamina remodeling
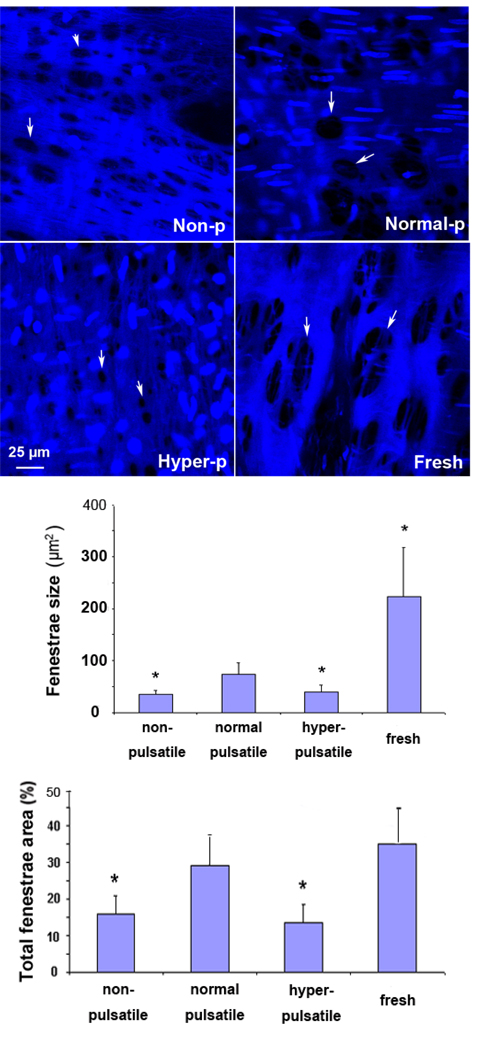
All arteries after 7 days in organ culture maintained IEL with fenestrae. Arteries cultured under the nonpulsatile and hyperpulsatile pressure, however, demonstrated reduced (smaller size) fenestrae compared to arteries cultured under normal pulsatile pressure (Fig. 1). The fenestrae in arteries cultured under normal pulsatile pressure were similar to the fenestrae in fresh arteries, indicating that IEL morphology was mainteained in organ culture.
Figure 1.
Top: Confocal microscope images of fenestrae (marked by arrows) in the IEL of porcine carotid arteries cultured under nonpulsatile pressure (non-p), normal pulsatile pressure (normal-p), and hyperpulsatile pressure (hyper-p) for 7 days as compared to a fresh artery. Middle & Bottom: Comparison of the average fenestrae size and the total fenestrae area ratio in arteries cultured under different pulse pressure. Data were plotted as means± SD. *p<0.05. Sample size n=3.
The average sizes of the fenestrae in the nonpulsatile and hyperpulsatile groups were 36.2±6.4 µm2 and 40.4±13.0 µm2 respectively, corresponding to 51% and 45% reductions in the size from the fenestrae in the normal pulsatile group (73.9±21.5 µm2; p<0.05 for both). The fenestrae area to total area ratios were 15.8±6.2% and 13.3±2.8% in the nonpulsatile and hyperpulsatile groups respectively, which were significantly lower than that in the normal pulsatile group (29.0±7.7%; p<0.05 for both). The fenestrae area to total area ratio of the normal pulsatile group was similar to the ratio of the fresh artery group (p=n.s.).
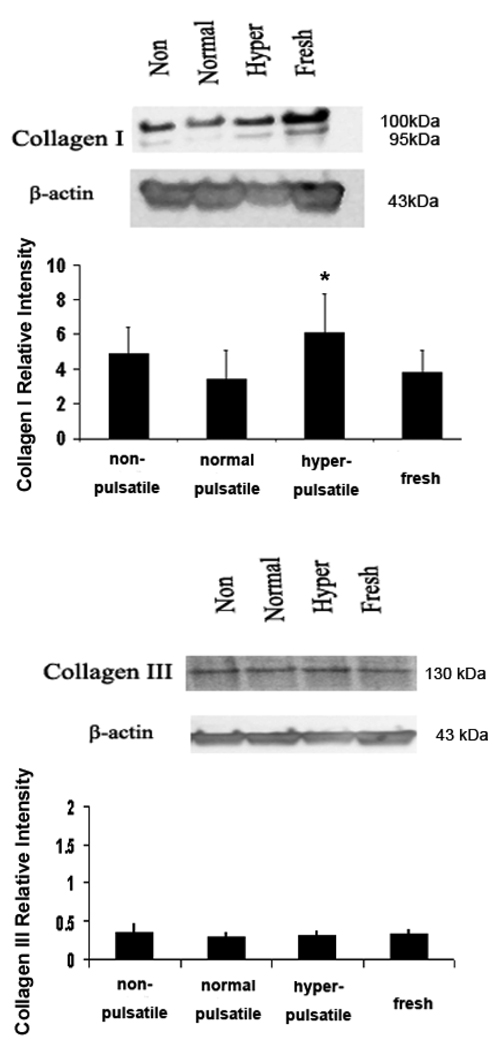
Fibronectin (FN) and collagen contents
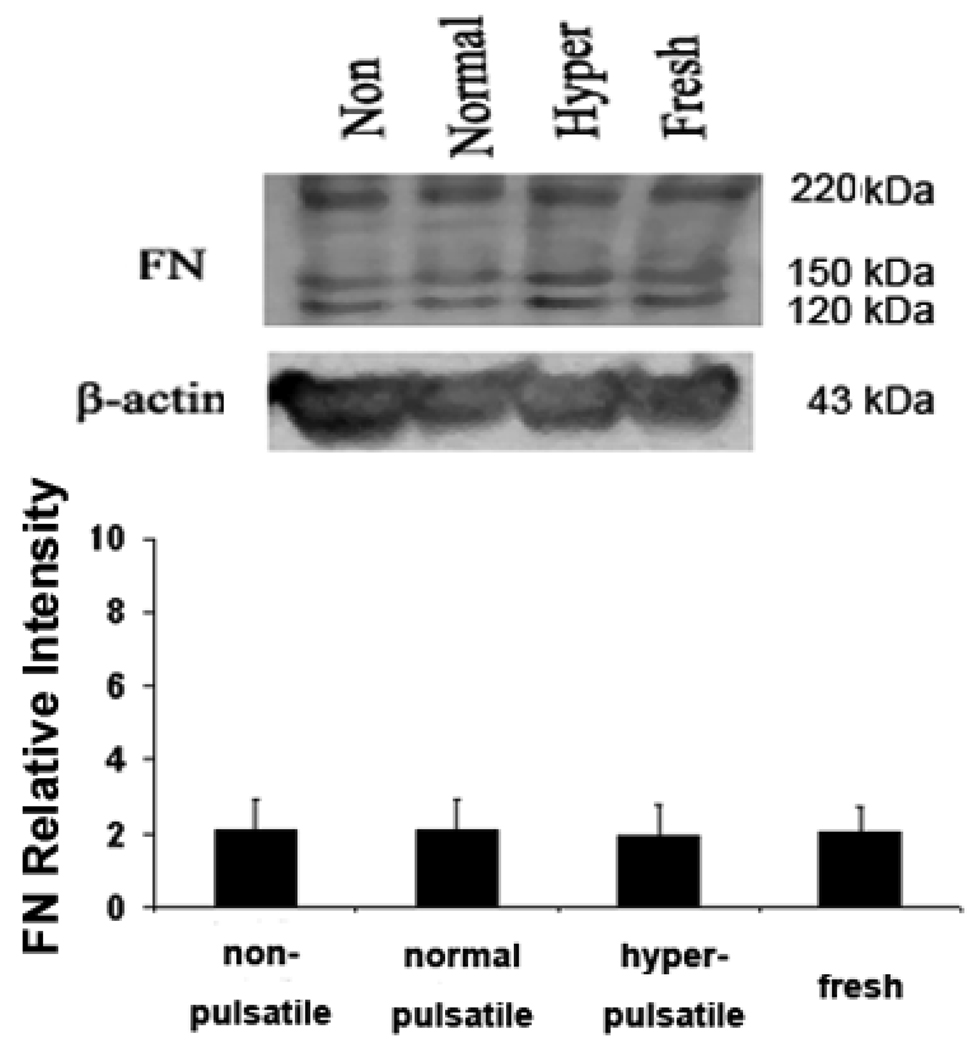
Figure 2 shows the collagen type I and type III contents in the artery cultured for 7 days under nonpulsatile, normal pulsatile, hyperpulsatile pressure. Increased collagen type I expression was seen in the hyperpulsatile group compared to the normal pulsatile group (p<0.05). However, the collagen type III and FN levels remained similar among all four groups.
Figure 2.
Western blot demonstrating the collagen I, collagen III and FN in arteries cultured under different pulse pressure for 7 days. Bargraphs illustrate the relative intensities obtained from densitomertric measurements (mean ± SD. *P < 0.01). Higher levels of collagen I were seen in the nonpulsatile and hyperpulsatile arteries as compared to normal pulse arteries. Collagen III and FN showed no measurable/significant difference among all groups.
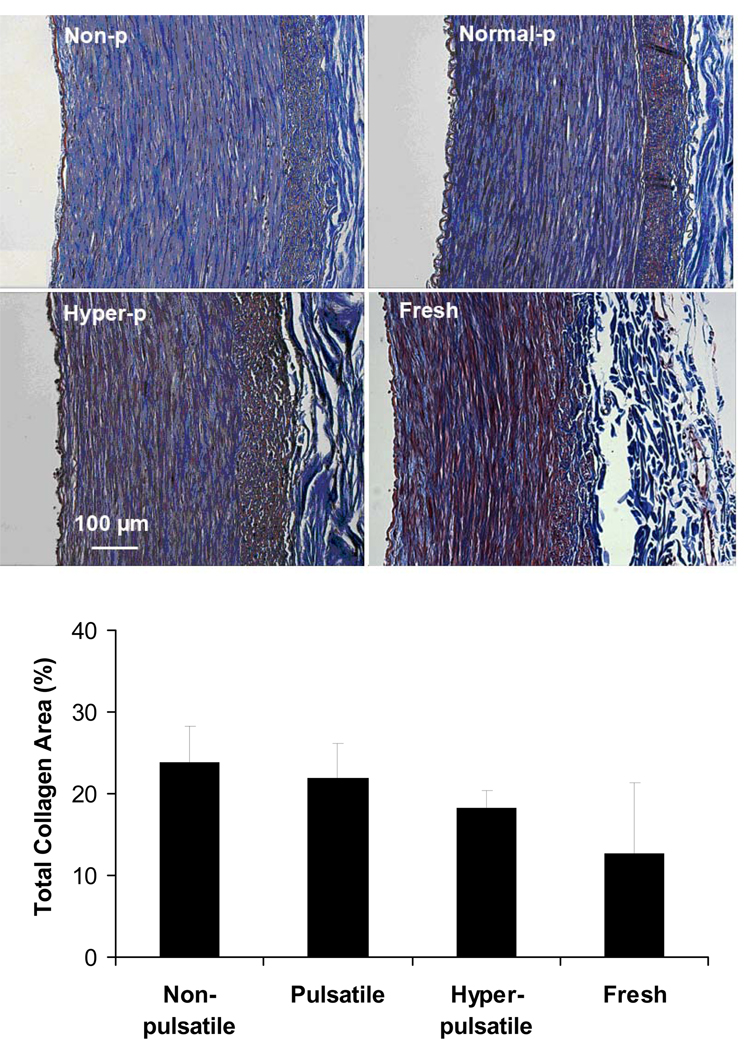
Trichrome staining demonstrated that all arteries had dense collagen in the media and adventitia layers (Fig.3 top). Photometric measurement showed no statistical difference in the collagen levels between groups. (Fig. 3 bottom)
Figure 3.
Top: Light microscope images of trichrome stained porcine carotid arteries cultured under nonpulsatile pressure (non-p), normal pulsatile pressure (normal-p), and hyperpulsatile pressure (hyper-p) for 7 days as compared to a fresh artery. The trichrome stain colors collagen blue and elastin black. Bottom: Percent area of collagen calculated using photometric measurement in arteries cultured under different pulse pressures. Data were plotted as means± SD. Sample size n=3, 4, 6, and 5, respectively.
Effects of pulsatile pressure on Connexin43 expression
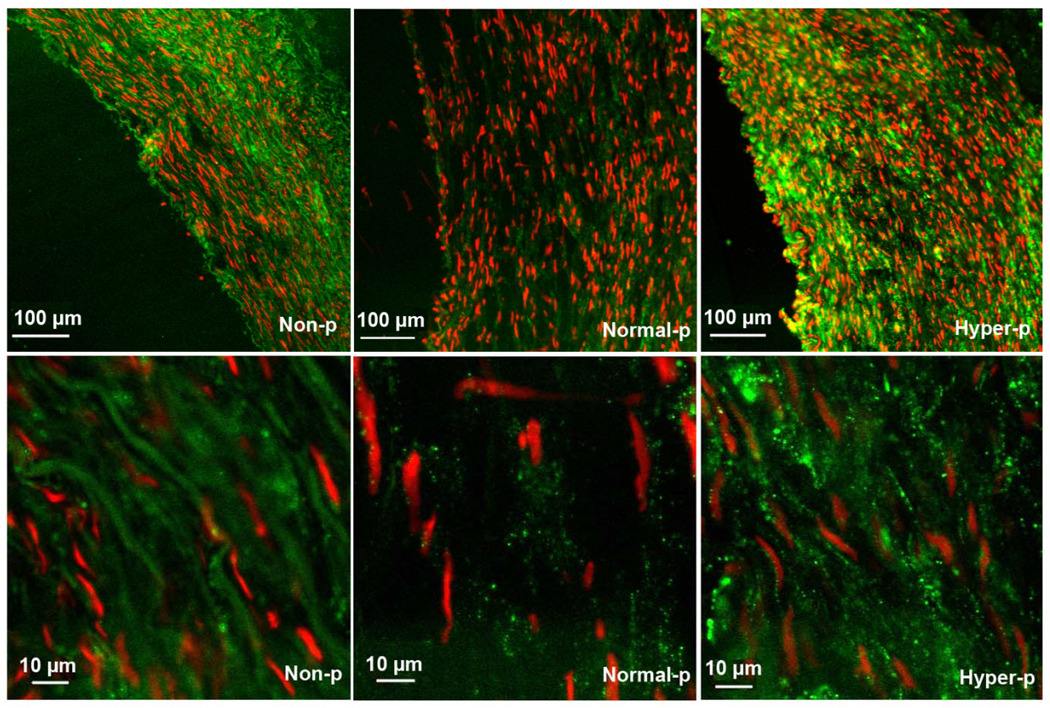
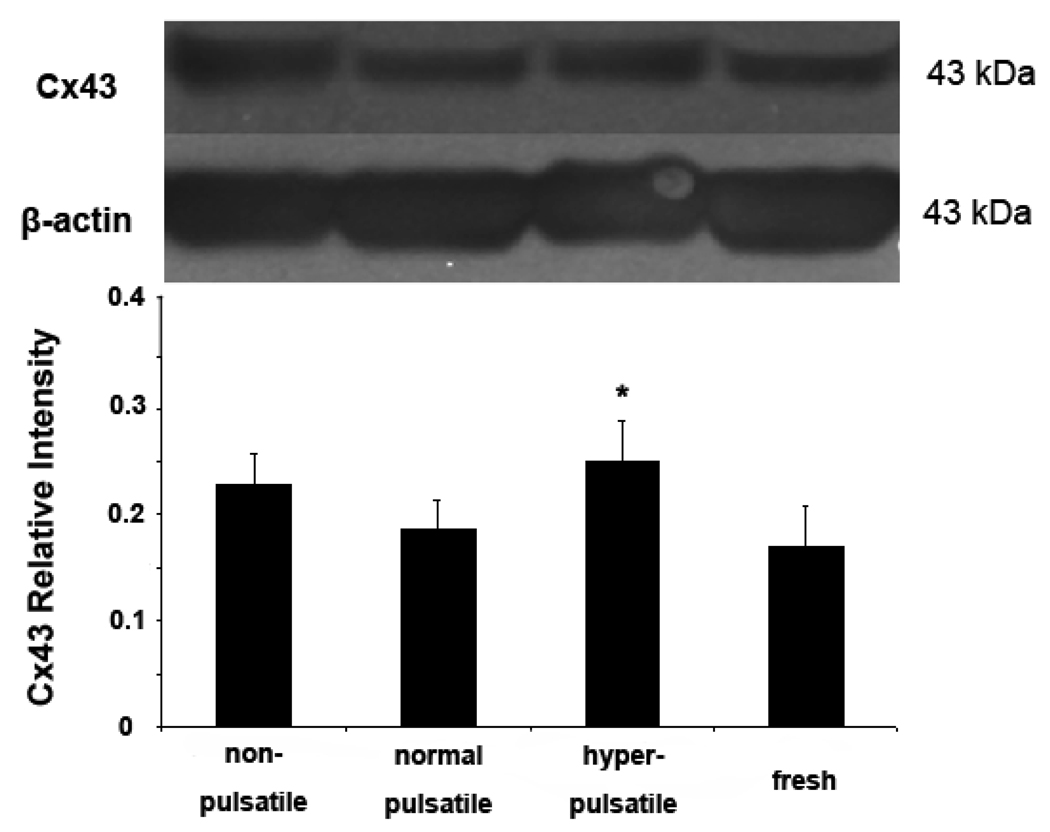
Immunohistochemistry staining demonstrated that the gap junction protein Cx43 is increased by both nonpulsatile and hyperpulsatile pulse pressure compared to normal pulse pressure while hyperpulsatile arteries demonstrated the highest level of Cx43 (FIG. 4). Immunoblotting showed that the expression of Cx43 protein was higher in the hyperpulsatile group compared to the normal pulsatile group (Fig. 5). The normal pulsatile group showed no difference from the fresh arteries.
Figure 4.
Distribution of connexin 43 in arteries cultured under nonpulsatile pressure (non-p), normal pulsatile pressure (normal-p), and hyperpulsatile pressure (hyper-p) conditions for 7 days. The connexin 43 was stained green while the nuclei were stained red by propidium iodide.
Figure 5.
Comparison of connexin 43 and β-actin expression in arteries cultured for 7 days under nonpulsatile, pulsatile, and hyperpulsatile pressure conditions and in fresh arteries. The top panel shows a typical blotting image and the bottom panel summarizes densitometric measurements (mean ± SD) *p<0.05. Sample size n=4.
DISCUSSION
Using an ex vivo organ culture model, we were able to achieve independent alteration of pulse pressure without altering the mean pressure and flow rate. Using our novel model, we have demonstrated, for the first time, increased extracellular matrix remodeling in the arterial wall due to alterations in pulse pressure alone. Our results demonstrated that an increase or elimination in pulse pressure from its normal physiologic level decreases the fenestrae size in the internal elastic lamina (IEL). Increases in pulsatile pressures increase collagen I and connexin 43 expression. These results indicate that alterations in pulse pressure stimulate wall matrix remodeling.
Advantage of using organ culture model
Reductions or elevations in pulse pressure in vivo are frequently superimposed with reductions or elevations in mean blood pressure, respectively, making it difficult to isolate pulse and steady components and specify the responses. In contrast, the organ culture model allows us to control pressure independent of flow rate [23] and to alter the pulse pressure without changing the mean pressure and flow rate. The organ culture model with whole vessel preparation and perfusion has demonstrated great advantages in preserving cells in their natural matrix and hemodynamic environment [23, 26]. Arterial structure and vasomotor function are well maintained under physiologic flow in organ culture [21, 23, 27, 28]. The model has been used extensively as an alternative model for studying vascular biology and remodeling by us and others [21, 23, 29–34]. Here we extend the advantage of this model to study the isolated effect of pulse pressure.
Physiologic implications
We and others have demonstrated that the arterial wall remodels differently at an elevation or diminution of mean pressure [18, 30]. Increased blood flow and decreased blood flow lead to different patterns of remodeling through different mechanisms [35–37]. It has been reported that response to flow decrease show a much slower kinetics than the response to flow increase, and that both responses are endothelium dependent [37]. Response to increased pressure may also be faster than the response to decreased pressure [17, 38, 39]. Similarly, here we see that elevated pulse pressure has a more robust effect than the diminished pulse pressure.
An important finding of this study was the decrease in the average size and total area ratio of the fenestration in nonpulsatile and hyperpulsatile arteries compared with normal pulsatile pressure. The decrease in fenestrae size may affect the mass transport through the IEL and thus affect vascular function [40]. Jackson et al. previously observed an increase of fenestrae size in rabbit carotid arteries under elevated or diminished axial strain [41, 42]. Possible explanations for this difference in results may lie in the difference in the direction of stress evaluated (circumferential in the previous studies vs. longitudinal in our study) and style of the strain (steady in the previous studies vs. cyclic in our study) as well the species (rabbit in the previous studies vs. pig in our study). Further study is needed to determine the causes.
Our results showed that total collagen content and collagen III did not change after elevation or diminished pulse pressure for 7 days, though collagen I protein was increased by elevated pulse pressure. This may be interpreted to mean that pulse pressure does not affect collagen content. On the other hand, it may take longer for the collagen to respond to pulse pressure. In fact, previous studies have showed that arterial wall thickening in human and rats increases after prolonged exposure to elevated pulse pressure lasting for weeks and months [8, 43, 44]. Additionally, cyclic stretch is known to increase collagen content and synthesis in cell cultures in vitro [45–47], though it is possible that isolating cells from their matrix produces an additional stimulus for the cell to secrete matrix components as it tries to rebuild its foundation. In the absence of this stimulus, the change may be too small to detect. Another possible explanation is that the Masson’s trichrome stain reflects the total collagen content but not the collagen synthesis. So it is possible that varying level of pulse pressure may increase collagen turnover without altering total collagen content. Another possibility is that other collagens, such as type IV and V, may decrease while collagen I increases, such that the net levels remain the same. In any regard, increased collagen I indicates wall remodeling under altered pulse pressure.
Increased gap junction protein Cx43 has been observed in vascular smooth muscle cells cultured in vitro under a 20% static stretch [12] and in endothelial cells under cyclic stretch [48]. Our results demonstrated an increase in Cx43 expression in hyperpulsatile arteries, indicating that increased cyclic stretch also increases Cx43 expression in arteries. Combined, these results indicated that increased pulse pressure affects cell communication and stimulated arterial wall remodeling.
Clinical relevance
Understanding the effect of pulse pressure on vascular function and matrix remodeling will help us to understand vascular aging and associated vascular disease. Pulse pressure significantly increases in aged populations [2, 4]. Matrix remodeling occurs during development, aging, and vascular disease [29, 49, 50]. Matrix remodeling due to alteration in pulse pressure may be a prominent factor in vascular wall stiffening in response to disease.
Patients with implantable heart pumps may experience nonpulsatile pressure for 7 to 10 days before regaining some pulsatility in blood pressure [6]. An important question related to the use of heart pumps and ventricular assist devices (e.g. centrifugal and axial flow rotary pumps) is how nonpulsatile flow produced by the pumps affects organ function [3, 6, 7]. The observed changes in IEL fenestrae and Cx43 expression indicate that arterial wall matrix could be altered after 7 days exposure to nonpulsatile flow. However, it remains unclear whether these changes can be reversed with the regain of pulsatile pressure and the issue needs further investigation.
In addition, the effect of pulse pressure on matrix remodeling indicates that the proper pulse pressure should be considered in the design of bioreactors in order to achieve the desired matrix structure for tissue engineering vascular grafts.
ACKNOWLEDGMENTS
This work was supported by the National Science Foundation under award number CBET-0602834 (HCH) and the National Institutes of Health HL-075360 (MLL). We thank Dr. Colleen Witt and the UTSA Research Center in Minority Institution (RCMI) Advanced Imaging Core for providing us the access and technical support for confocal microscopy. We also thank Dr. Yong-Ung Lee for his help in organ culture experiments.
References
- 1.Safar ME, Boudier HS. Vascular development, pulse pressure, and the mechanisms of hypertension. Hypertension. 2005;46(1):205–209. doi: 10.1161/01.HYP.0000167992.80876.26. [DOI] [PubMed] [Google Scholar]
- 2.Dart AM, Kingwell BA. Pulse pressure--a review of mechanisms and clinical relevance. J Am Coll Cardiol. 2001;37(4):975–984. doi: 10.1016/s0735-1097(01)01108-1. [DOI] [PubMed] [Google Scholar]
- 3.DeBakey ME. Development of mechanical heart devices. Ann Thorac Surg. 2005;79(6):S2228–S2231. doi: 10.1016/j.athoracsur.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 4.Izzo JL, Jr., Levy D, Black HR. Clinical Advisory Statement. Importance of systolic blood pressure in older Americans. Hypertension. 2000;35(5):1021–1024. doi: 10.1161/01.hyp.35.5.1021. [DOI] [PubMed] [Google Scholar]
- 5.Haider AW, Larson MG, Franklin SS, Levy D. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2003;138(1):10–16. doi: 10.7326/0003-4819-138-1-200301070-00006. [DOI] [PubMed] [Google Scholar]
- 6.Potapov EV, Loebe M, Nasseri BA, Sinawski H, Koster A, Kuppe H, Noon GP, DeBakey ME, Hetzer R. Pulsatile flow in patients with a novel nonpulsatile implantable ventricular assist device. Circulation. 2000;102(19) Suppl 3:III183–III187. doi: 10.1161/01.cir.102.suppl_3.iii-183. [DOI] [PubMed] [Google Scholar]
- 7.Saito S, Nishinaka T, Westaby S. Hemodynamics of chronic nonpulsatile flow: implications for LVAD development. Surg Clin North Am. 2004;84(1):61–74. doi: 10.1016/S0039-6109(03)00220-2. [DOI] [PubMed] [Google Scholar]
- 8.Laurent S, Tropeano AI, Lillo-Lelouet A, Jondeau G, Laloux B, Boutouyrie P. Local pulse pressure is a major determinant of large artery remodelling. Clin Exp Pharmacol Physiol. 2001;28(12):1011–1014. doi: 10.1046/j.1440-1681.2001.03569.x. [DOI] [PubMed] [Google Scholar]
- 9.Boutouyrie P, Bussy C, Lacolley P, Girerd X, Laloux B, Laurent S. Association between local pulse pressure, mean blood pressure, and large-artery remodeling. Circulation. 1999;100(13):1387–1393. doi: 10.1161/01.cir.100.13.1387. [DOI] [PubMed] [Google Scholar]
- 10.Baguet JP, Mallion JM, Moreau-Gaudry A, Noirclerc M, Peoc'h M, Siche JP. Relationships between cardiovascular remodelling and the pulse pressure in never treated hypertension. J Hum Hypertens. 2000;14(1):23–30. doi: 10.1038/sj.jhh.1000933. [DOI] [PubMed] [Google Scholar]
- 11.Smith JD, Davies N, Willis AI, Sumpio BE, Zilla P. Cyclic stretch induces the expression of vascular endothelial growth factor in vascular smooth muscle cells. Endothelium. 2001;8(1):41–48. doi: 10.3109/10623320109063156. [DOI] [PubMed] [Google Scholar]
- 12.Cowan DB, Lye SJ, Langille BL. Regulation of vascular connexin43 gene expression by mechanical loads. Circ Res. 1998;82(7):786–793. doi: 10.1161/01.res.82.7.786. [DOI] [PubMed] [Google Scholar]
- 13.Gupta V, Grande-Allen KJ. Effects of static and cyclic loading in regulating extracellular matrix synthesis by cardiovascular cells. Cardiovasc Res. 2006;72(3):375–383. doi: 10.1016/j.cardiores.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Lehoux S, Esposito B, Merval R, Tedgui A. Differential regulation of vascular focal adhesion kinase by steady stretch and pulsatility. Circulation. 2005;111(5):643–649. doi: 10.1161/01.CIR.0000154548.16191.2F. [DOI] [PubMed] [Google Scholar]
- 15.Lehoux S, Esposito B, Merval R, Loufrani L, Tedgui A. Pulsatile stretch-induced extracellular signal-regulated kinase 1/2 activation in organ culture of rabbit aorta involves reactive oxygen species. Arterioscler Thromb Vasc Biol. 2000;20(11):2366–2372. doi: 10.1161/01.atv.20.11.2366. [DOI] [PubMed] [Google Scholar]
- 16.Bayer IM, Adamson SL, Langille BL. Atrophic remodeling of the artery-cuffed artery. Arterioscler Thromb Vasc Biol. 1999;19(6):1499–1505. doi: 10.1161/01.atv.19.6.1499. [DOI] [PubMed] [Google Scholar]
- 17.Courtman DW, Cho A, Langille L, Wilson GJ. Eliminating arterial pulsatile strain by external banding induces medial but not neointimal atrophy and apoptosis in the rabbit. Am J Pathol. 1998;153(6):1723–1729. doi: 10.1016/S0002-9440(10)65687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han HC, Marita S, Ku DN. Changes of opening angle in hypertensive and hypotensive arteries in three-day organ culture. J Biomech. 2006;39:2410–2418. doi: 10.1016/j.jbiomech.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Lehoux S, Tedgui A. Signal transduction of mechanical stresses in the vascular wall. Hypertension. 1998;32(2):338–345. doi: 10.1161/01.hyp.32.2.338. [DOI] [PubMed] [Google Scholar]
- 20.Lehoux S, Tedgui A. Cellular mechanics and gene expression in blood vessels. J Biomech. 2003;36(5):631–643. doi: 10.1016/s0021-9290(02)00441-4. [DOI] [PubMed] [Google Scholar]
- 21.Bardy N, Karillon GJ, Merval R, Samuel JL, Tedgui A. Differential effects of pressure and flow on DNA and protein synthesis and on fibronectin expression by arteries in a novel organ culture system. Circ Res. 1995;77(4):684–694. doi: 10.1161/01.res.77.4.684. [DOI] [PubMed] [Google Scholar]
- 22.Bardy N, Merval R, Benessiano J, Samuel JL, Tedgui A. Pressure and angiotensin II synergistically induce aortic fibronectin expression in organ culture model of rabbit aorta. Evidence for a pressure-induced tissue renin-angiotensin system. Circ Res. 1996;79(1):70–78. doi: 10.1161/01.res.79.1.70. [DOI] [PubMed] [Google Scholar]
- 23.Han HC, Ku DN. Contractile responses in arteries subjected to hypertensive pressure in seven-day organ culture. Ann Biomed Eng. 2001;29(6):467–475. doi: 10.1114/1.1376391. [DOI] [PubMed] [Google Scholar]
- 24.Han HC, Ku DN, Vito RP. Arterial wall adaptation under elevated longitudinal stretch in organ culture. Ann Biomed Eng. 2003;31(4):403–411. doi: 10.1114/1.1561291. [DOI] [PubMed] [Google Scholar]
- 25.Davis NP, Han HC, Wayman B, Vito R. Sustained axial loading lengthens arteries in organ culture. Ann Biomed Eng. 2005;33(7):867–877. doi: 10.1007/s10439-005-3488-x. [DOI] [PubMed] [Google Scholar]
- 26.Gleason RL, Gray SP, Wilson E, Humphrey JD. Department of Mechatronics and Precision Engineering, Faculty of Engineering. 6. Vol. 126. Sendai, Japan: Tohoku University; 2004. A multiaxial computer-controlled organ culture and biomechanical device for mouse carotid arteries; pp. 787–795. [DOI] [PubMed] [Google Scholar]
- 27.Labadie RF, Antaki JF, Williams JL, Katyal S, Ligush J, Watkins SC, Pham SM, Borovetz HS. Pulsatile perfusion system for ex vivo investigation of biochemical pathways in intact vascular tissue. Am J Physiol. 1996;270(2 Pt 2):H760–H768. doi: 10.1152/ajpheart.1996.270.2.H760. [DOI] [PubMed] [Google Scholar]
- 28.Clerin V, Gusic RJ, O'Brien J, Kirshbom PM, Myung RJ, Gaynor JW, Gooch KJ. Mechanical environment, donor age, and presence of endothelium interact to modulate porcine artery viability ex vivo. Ann Biomed Eng. 2002;30(9):1117–1127. doi: 10.1114/1.1519262. [DOI] [PubMed] [Google Scholar]
- 29.Chesler NC, Ku DN, Galis ZS. Transmural pressure induces matrixdegrading activity in porcine arteries ex vivo. Am J Physiol. 1999;277(5 Pt 2):H2002–H2009. doi: 10.1152/ajpheart.1999.277.5.H2002. [DOI] [PubMed] [Google Scholar]
- 30.Zulliger MA, Montorzi G, Stergiopulos N. Biomechanical adaptation of porcine carotid vascular smooth muscle to hypo and hypertension in vitro. J Biomech. 2002;35(6):757–765. doi: 10.1016/s0021-9290(02)00020-9. [DOI] [PubMed] [Google Scholar]
- 31.Vorp DA, Peters DG, Webster MW. Gene expression is altered in perfused arterial segments exposed to cyclic flexure ex vivo. Ann Biomed Eng. 1999;27(3):366–371. doi: 10.1114/1.158. [DOI] [PubMed] [Google Scholar]
- 32.Conklin BS, Zhong DS, Zhao W, Lin PH, Chen C. Shear stress regulates occludin and VEGF expression in porcine arterial endothelial cells. J Surg Res. 2002;102(1):13–21. doi: 10.1006/jsre.2001.6295. [DOI] [PubMed] [Google Scholar]
- 33.Nichol JW, Petko M, Myung RJ, Gaynor JW, Gooch KJ. Hemodynamic conditions alter axial and circumferential remodeling of arteries engineered ex vivo. Ann Biomed Eng. 2005;33(6):721–732. doi: 10.1007/s10439-005-4494-8. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto T, Okumura E, Miura Y, Sato M. Mechanical and dimensional adaptation of rabbit carotid artery cultured in vitro. Med Biol Eng Comput. 1999;37(2):252–256. doi: 10.1007/BF02513295. [DOI] [PubMed] [Google Scholar]
- 35.Langille BL. Arterial remodeling: relation to hemodynamics. Can J Physiol Pharmacol. 1996;74(7):834–841. [PubMed] [Google Scholar]
- 36.Ku DN. Blood flow in arteries. Ann Rev Fluid Mech. 1997;29:399–434. [Google Scholar]
- 37.Langille BL. Remodeling of developing and mature arteries: endothelium, smooth muscle, and matrix. J Cardiovasc Pharmacol. 1993;21 Suppl 1:S11–S17. doi: 10.1097/00005344-199321001-00003. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto T, Hayashi K. Department of Mechatronics and Precision Engineering, Faculty of Engineering. 3. Vol. 116. Sendai, Japan: Tohoku University; 1994. Mechanical and dimensional adaptation of rat aorta to hypertension; pp. 278–283. [DOI] [PubMed] [Google Scholar]
- 39.Liu SQ, Fung YC. Department of Mechatronics and Precision Engineering, Faculty of Engineering. 4. Vol. 111. Sendai, Japan: Tohoku University; 1989. Relationship between hypertension, hypertrophy, and opening angle of zero-stress state of arteries following aortic constriction; pp. 325–335. [DOI] [PubMed] [Google Scholar]
- 40.Hayman DM, Sprague EA, Han HC. Pulse pressure alters arterial function. BMES Annual Fall Meeting; Los Angels, CA. 2005. [Google Scholar]
- 41.Jackson ZS, Dajnowiec D, Gotlieb AI, Langille BL. Partial off-loading of longitudinal tension induces arterial tortuosity. Arterioscler Thromb Vasc Biol. 2005;25(5):957–962. doi: 10.1161/01.ATV.0000161277.46464.11. [DOI] [PubMed] [Google Scholar]
- 42.Jackson ZS, Gotlieb AI, Langille BL. Wall tissue remodeling regulates longitudinal tension in arteries. Circ Res. 2002;90(8):918–925. doi: 10.1161/01.res.0000016481.87703.cc. [DOI] [PubMed] [Google Scholar]
- 43.Boutouyrie P, Bussy C, Lacolley P, Girerd X, Laloux B, Laurent S. Association between local pulse pressure, mean blood pressure, and large-artery remodeling. Circulation. 1999;100:1387–1393. doi: 10.1161/01.cir.100.13.1387. [DOI] [PubMed] [Google Scholar]
- 44.Safar ME, Laurent P. Pulse pressure and arterial stiffness in rats: comparison with humans. Am J Physiol Heart Circ Physiol. 2003;285(4):H1363–H1369. doi: 10.1152/ajpheart.00513.2003. [DOI] [PubMed] [Google Scholar]
- 45.Schlumberger W, Thie M, Rauterberg J, Robenek H. Collagen synthesis in cultured aortic smooth muscle cells. Modulation by collagen lattice culture, transforming growth factor-beta 1, and epidermal growth factor. Arterioscler Thromb. 1991;11(6):1660–1666. doi: 10.1161/01.atv.11.6.1660. [DOI] [PubMed] [Google Scholar]
- 46.Li Q, Muragaki Y, Hatamura I, Ueno H, Ooshima A. Stretch-induced collagen synthesis in cultured smooth muscle cells from rabbit aortic media and a possible involvement of angiotensin II and transforming growth factor-beta. J Vasc Res. 1998;35(2):93–103. doi: 10.1159/000025570. [DOI] [PubMed] [Google Scholar]
- 47.Leung DY, Glagov S, Mathews MB. Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science. 1976;191(4226):475–477. doi: 10.1126/science.128820. [DOI] [PubMed] [Google Scholar]
- 48.Kwak BR, Silacci P, Stergiopulos N, Hayoz D, Meda P. Shear stress and cyclic circumferential stretch, but not pressure, alter connexin43 expression in endothelial cells. Cell Commun Adhes. 2005;12(5–6):261–270. doi: 10.1080/15419060500514119. [DOI] [PubMed] [Google Scholar]
- 49.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90(3):251–262. [PubMed] [Google Scholar]
- 50.Lehoux S, Lemarie CA, Esposito B, Lijnen HR, Tedgui A. Pressure-induced matrix metalloproteinase-9 contributes to early hypertensive remodeling. Circulation. 2004;109(8):1041–1047. doi: 10.1161/01.CIR.0000115521.95662.7A. [DOI] [PubMed] [Google Scholar]