Abstract
The mammalian target of rapamycin (mTOR)/p70S6 kinase (S6K) pathway plays an important role in brain-derived neurotrophic factor (BDNF)-mediated protein synthesis and neuroplasticity. Although many aspects of neuronal function are regulated by intracellular calcium ([Ca2+]i) and calmodulin (CaM), their functions in BDNF-induced phosphorylation of p70S6K and protein synthesis are largely unknown. Here, we report that BDNF, via TrkB-dependent activation of mTOR, induces sustained phosphorylation of p70S6K at Thr389 and Thr421/Ser424. BDNF-induced phosphorylation at Thr389 was dependent on PI3 kinase, but independent of ERK-MAPK. The previously identified MAPK phosphorylation site at Thr421/Ser424 required both PI3K and MAPK in BDNF-stimulated neurons. Further, we found that the reduction in [Ca2+]i, but not extracellular calcium, blocked the BDNF-induced phosphorylation of p70S6K at both sites. Inhibition of CaM by W13 also blocked p70S6K phosphorylation. In correlation, W13 inhibited BDNF-induced local dendritic protein synthesis. Interestingly, sustained elevation of [Ca2+]i by membrane depolarization antagonized the BDNF-induced p70S6K phosphorylation. Finally, the BDNF-induced p70S6K phosphorylation did not require the increase of calcium level through either extracellular influx or PLC-mediated intracellular calcium release. Collectively, these results indicate that the basal level of intracellular calcium gates BDNF-induced activation of p70S6K and protein synthesis through CaM.
Keywords: p70S6 Kinase, BDNF, PI3 Kinase, mTOR, calmodulin
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family, and plays an essential role in regulating plasticity, such as long-term potentiation (LTP) (Korte et al. 1995) and long-term memory (LTM) (Bekinschtein et al. 2007a). The functional relevance of BDNF in neuroplasticity is, at least, partially due to its ability to increase both global and local protein synthesis (Sutton and Schuman 2005; Takei et al. 2004; Takei et al. 2001; Tanaka et al. 2008). It has been hypothesized that both PI3K/mTOR and ERK play important roles in activating translational machinery at multiple stages, including ribosomal biogenesis, initiation, and elongation (Gelinas et al. 2007; Inamura et al. 2005; Kelleher et al. 2004; Klann and Dever 2004; Schratt et al. 2004; Sutton and Schuman 2005; Takei et al. 2004; Takei et al. 2001), leading to an increase in mRNA translation. By activating the TrkB tyrosine kinase receptor, BDNF simultaneously stimulates multiple signaling cascades, including phosphoinositide 3-kinase (PI3K)/ mammalian target of rapamycin (mTOR) and Ras/ERK pathways, to enhance neuronal protein synthesis. Consequently, the up-regulation of PI3K/mTOR and ERK correlates with, and is required for LTP and LTM (Kelleher et al. 2004; Parsons et al. 2006; Tang et al. 2002; Tsokas et al. 2007; Ying et al. 2002).
p70S6K is a major kinase for the 40S ribosomal protein S6, whose phosphorylation often regulates the translation of 5′ TOP-containing mRNA transcripts and correlates with elevation in protein synthesis (Hay and Sonenberg 2004; Pfeiffer and Huber 2006). Among many potential phosphorylation sites (Pullen and Thomas 1997; Saitoh et al. 2002), the phosphorylation at both Thr389 and Thr421/Ser424 is required for the full activation of p70S6K (Lehman et al. 2003). Studies with non-neuronal neutrophils suggested that the phosphorylation at Thr389 is regulated by PI3K, and the phosphorylation at Thr421/Ser424 by MAPK (Lehman et al. 2003). In the BDNF-stimulated neurons, the up-regulation of p70S6K phosphorylation pairs with the enhancement of protein synthesis (Takei et al. 2004). p70S6K phosphorylation and protein synthesis can be blocked by inhibiting mTOR, which is a key translational regulator in both neuronal and non-neuronal tissues (Bekinschtein et al. 2007b; Hay and Sonenberg 2004; Schratt et al. 2004).
Given the important roles of mTOR/p70S6K in activity-dependent protein synthesis and plasticity (Costa-Mattioli et al. 2009; Parsons et al. 2006; Tang et al. 2002; Tsokas et al. 2007), this study aims to investigate the regulatory mechanisms of p70S6K phosphorylation in BDNF-stimulated neurons. We examined how distinct BDNF-activated signaling pathways function in the phosphorylation of p70S6K at distinct sites. We further tested whether the function of Ca2+ and its effecter molecule CaM play a central role in regulation at these sites for the following reasons: 1). Ca2+ impinges on the activation of both PI3K and ERK; 2). Ca2+ and Ca2+-binding proteins have been shown to play a role in gene expression via translation and transcription (Atkins et al. 2004; Gong et al. 2006; Iizuka et al. 2007; West et al. 2001), though little is known whether Ca2+ signaling regulates BDNF-mediated phosphorylation of translation factors. By using pharmacological manipulation on Ca2+ levels and CaM activity, we found that removal or elevation of intracellular Ca2+ ([Ca2+]i) leads to a decrease in BDNF-stimulated phosphorylation of p70S6K at both Thr389 and Thr421/Ser424 sites. We further show that CaM activity gates the phosphorylation of p70S6K at multiple sites, and that this regulation is mainly mediated through the PI3K, rather than through CaM-dependent kinase (CaMK) signaling. Finally, we show that the CaM-dependent regulation of BDNF signaling is important for BDNF to drive local protein synthesis in dendrites.
MATERIALS AND METHODS
Cell culture
Cortices or hippocampi from new born (P0-P2) Sprague Dawley rats were used for neuronal culture (Zheng et al. 2008). The tissues were first digested with papain (10 units/ml, Worthington, Freehold, NJ) and DNase I (Roche, 100 units/ml) at 37°C for 30min, and washed with Neurobasal A (Invitrogen, Carlsbad, CA). The mechanically separated neurons were plated on 12-well plates coated with PDL (50 μg/ml, Sigma, St. Louis, MO) at a density of 0.5 to 1 million cells/well. The cultures were maintained in Neurobasal A with B27 supplement, 0.5 mM glutamine and 1 X penicillin/streptomycin.
Neuronal treatments and stimulations
Primary neuronal cultures were stimulated with recombinant human BDNF (at 5 ng/ml, or at the indicated concentrations) (Calbiochem) or KCl (50 mM). To block the receptor tyrosine kinase TrkB, neurons were pre-incubated for 30 min before BDNF application with 0.2 μM K252a (a general Trk receptor inhibitor) or 0.4 μg/ml TrkB-IgG (an extracellular BDNF binding domain coupled to IgG). A 30 min pre-incubation with 100 μM APV or 10 μM nifedipine was used to block the NMDA receptors (NMDAR) or L-type voltage-gated calcium channels (L-VGCC), respectively. A 30 min pre-incubation with 10 μM U0126 was used for MEK inhibition. 30 μM LY294002 was used to inhibit PI3K activity. 5 μM KN93 or 10 μM KN62 was used to inhibit CaM K I/II/IV activity. U73122 (5, 10 or 25 μM) was used to inhibit PLC activity. 70 μM W13 (or as indicated) was used for CaM inhibition. EGTA (2.5 mM) and BAPTA-AM (33 μM or as indicated) was used to chelate extracellular calcium and intracellular calcium, respectively. XeC (5 μM) was used to block IP3-mediated calcium release from the intracellular store. Thapsigargin (1 μM) or dantrolene (40 μM) was used to deplete intracellular Ca2+ stores. All these inhibitors were purchased from EMD Bioscience. They were added to neuronal cultures 30 min before BDNF stimulation. Unless otherwise indicated, samples were harvested 15 min after BDNF treatment.
Polyacrylamide gel electrophoresis (PAGE) and Western blotting
After BDNF or KCl treatment, medium was removed from the culture. Neurons were lysed, harvested in SDS-PAGE loading buffer (10 mM Tris-HCl buffer pH 6.8, 10% glycerol, 2% sodium dodecylsulfate, 0.01% bromophenol blue and 5% mercaptoethanol), and boiled for 10 min. The collected samples were separated by 10% or 4-20% gradient SDS-PAGE, and transferred to nitrocellulose membrane. After blocking with 5% non-fat milk in PBST, the blots were incubated with primary antibodies at 4°C in PBS with 0.1% Triton X-100 and 5% non-fat milk for 12 to 16 hours. The blots were then washed 5 times with PBST (PBS with 0.1% Triton X-100), and incubated with HRP-conjugated secondary antibodies at room temperature for 1 hour. The blots were then extensively washed, and subjected to ECL detection (SuperSignal® West Pico, Pierce, Rockford, IL).
Polyclonal antibodies against phosphorylated-p70S6K at Thr389 (1:1000) and at Thr421/Ser424 (1:1000) were used to determine the phosphorylation of S6K at the corresponding sites. Polyclonal antibodies against phosphorylated-Akt at Ser473 (1:1000) and phosphorylated-ERK1/2 at Thr202/Tyr204 (1:1000) were used to determine the phosphorylation of Akt and ERK1/2, respectively. Polyclonal antibodies against total S6K (1:1000), total Akt (1:1000), and total ERK1/2 (1:2000) were used to determine protein loading. Monoclonal antibody against beta-actin (1:10,000) was also used to determine protein loading. Anti-beta-actin was purchased from Sigma. The secondary HRP-conjugated antibodies (1:5000) were from Pierce. All other antibodies were from Cell Signaling.
To avoid signal saturation, the x-ray films were exposed to the blots with several exposure durations. The signals within the linear range were chosen for further density analysis by Scion Image software (Scion Corp. Frederick, Maryland).
Analysis of global protein synthesis by metabolic labeling
The incorporation of S-35-methione was used to trace new protein synthesis as described (Takei et al. 2001) with minor modifications. Neurons were maintained in Neurobasal A/B27 media. S-35 methionine (10 μCi, with the specificity of 10 mCi/mmol, Perkin Elmer) was added directly to the culture for 15min. Then the neurons were stimulated with KCl or BDNF for 30 min. The reaction was stopped by 1 ml ice-cold PBS, and subsequently washed with 1 ml PBS. The S-35-labelled neurons were lysed with 500 μl 10 mg/ml casein in 0.5 M NaOH at 37°C for 30 min. The lysates were transferred to 1.5 ml micro-centrifuge tubes, and mixed with 500 μl 20% ice-cold trichloroacetic acid (TCA). After centrifugation (10,000 × g) for 10 min, the supernatants and pellets were separated. The radioactivity in the supernatant represents free S-35 methionine, and the radioactivity in the pellet represents the incorporated S-35 methionine. The pellets were then washed with ice-cold 5% TCA, and dissolved in 0.1 M NaOH. Protein synthesis was determined by the ratio of radioactivity in the pellet to the total radioactivity (pellet plus supernatant).
Analysis of local dendritic protein synthesis
To visualize dynamic dendritic protein synthesis, cultured hippocampal neurons were prepared at a density of 230-460 cells/mm2 and infected with Sindbis virus expressing a fluorescent translation reporter as previously described (Aakalu et al. 2001; Sutton et al. 2007). The Sindbis viral vector contains an expression cassette including the coding sequence for a myristoylated and destabilized GFP flanked by the 5′ and 3′ UTRs of α-CaMKII (Aakalu et al. 2001). At 8-9 hrs post-infection, the growth media was replaced by pre-warmed (37°C) HEPES-buffered saline (containing, in mM: 119 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 30 Glucose, 10 HEPES, pH 7.4) at least 60 min prior to imaging. Neurons with a pyramidal-like morphology were imaged with an Olympus FV1000 laser scanning confocal microscope using a Plan-Apochromat 40X/0.95 objective. GFP fluorescence was visualized by excitation with the 488 line of an argon ion laser, and emitted light was collected between 500-600 nm. After a baseline image was acquired, neurons were immediately challenged with BDNF (20 ng/ml) or vehicle (mock-treatment) and subsequently imaged at 30 min intervals. In experiments using W13 or anisomycin, neurons were pre-treated with these agents for 60 min prior to imaging. All images were acquired in 0.5 μm sections over a range that encompassed the entire dendritic volume. Image analysis was conducted on maximal intensity z-compressed stacks. The primary dendrite from each cell was linearized using NIH Image J, and fluorescence intensity was measured as a function of both time and distance from the cell soma. The dendritic compartment was divided into proximal and distal domains, defined by distances of less or greater than, respectively, 125 μm from the soma.
Data analysis
Student t-test was used to analyze data between two groups. ANOVA was used for data between multiple groups. The p value of less than 0.05 was considered statistically significant.
RESULTS
BDNF induces phosphorylation of p70S6K in a TrkB- and mTOR-dependent manner
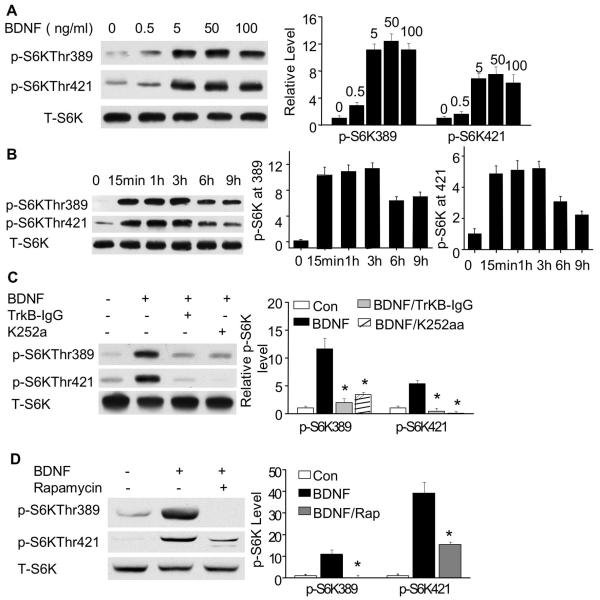
Previous studies have demonstrated that 100 ng/ml BDNF (about 4 nM for BDNF dimer) stimulated the phosphorylation of mTOR and p70S6K (Takei et al. 2004), which promotes the induction of protein synthesis. We explored this regulation in greater detail by assaying, in cultured cortical neurons, p70S6K phosphorylation at multiple sites over a range of BDNF concentrations and incubation times. Consistent with previous observations, we found that 100 ng/ml BDNF (a standard concentration used in many physiological studies) induced robust phosphorylation of p70S6K at both Thr389 and Thr421/Ser424 (Fig. 1A). Interestingly, we found that even a 200-fold lower BDNF concentration (0.5 ng/ml) stimulated mild but significant phosphorylation at both Thr389 and Thr421/Ser424 (Fig. 1A). The full-scale up-regulation of p70S6K phosphorylation was achieved by BDNF treatment at 5 ng/ml (0.2 nM), a concentration ~ 20-fold lower than that typically used. Consistently, 5 ng/ml BDNF also stimulated significant mTOR phosphorylation (data not shown).
Fig. 1.
Activation of p70S6K phosphorylation by BDNF depends on TrkB, and is inhibited by rapamycin. A: Cortical neurons were treated with (in ng/ml) 0.5, 5, 50 and 100 BDNF for 15 min. The phosphorylation of p70S6K at Thr389 and Thr421/Ser424 was measured by Western blot analysis using phospho-specific antibodies. The level of total p70S6K (T-p70S6K) was used for normalization. B: Cell lysates were collected 15 min, 1 h, 3 h, 6 h, and 9 h after BDNF-treatment, and analyzed for p70S6K phosphorylation. C: To address whether the Trk tyrosine kinase receptors mediate p70S6K phosphorylation, cortical neurons were pre-treated with K252a or TrkB IgG for 30 min before BDNF stimulation (5 ng/ml). The level of p-p70S6K at Thr389 and Thr421/Ser424 was analyzed by Western blots. D: Rapamycin blocked BDNF-induced p70S6K phosphorylation. Rapamycin was added to the neuronal culture 30 min before BDNF (5 ng/ml) treatment. The representative images are shown in the left panels, and quantifications are shown in the right panels (n=3 from separate experiments). Relative intensity of p-p70S6K was normalized to T-p70S6K. * indicates significant inhibition of BDNF-induced p70S6K phosphorylation (p<0.05).
Because BDNF-stimulated protein synthesis may lead to long-lasting functional modification on neurons (e.g., long-term potentiation; Kanhema et al. 2006; Ying et al. 2002), we tested whether low concentrations of BDNF could induce sustained p70S6K phosphorylation. We found that the up-regulation of p70S6K, at both Thr389 and Thr421/Ser424, by 5 ng/ml BDNF persisted for at least 9 hours with maximal phosphorylation observed between 15 min and 3 h (Fig. 1B). To test whether BDNF-induced p70S6K phosphorylation depends on the function of Trk receptor tyrosine kinases, we pre-treated neurons with the Trk inhibitor K252a before BDNF stimulation. We also pre-treated neurons with TrkB-IgG, which is an extracellular BDNF binding domain fused to IgG, to specifically block BDNF binding to TrkB. We found that both K252a and TrkB-IgG significantly blocked BDNF-stimulated p70S6K phosphorylation at both Thr389 and Thr421/Ser424 (Fig. 1C).
We next pre-treated neurons with rapamycin, a well-known inhibitor of mTOR, which is an upstream regulator of p70S6K in the PI3K pathway. Interestingly, we observed that BDNF-mediated phosphorylation of p70S6K at both Thr389 and Thr421/Ser424 was significantly blocked by rapamycin (Fig. 1D). Tsokas and colleagues previously reported that rapamycin pretreatment prevented phosphorylation at Thr389 induced by high frequency synaptic stimulation (HFS), but the HFS-induced phosphorylation at Thr421/Ser424 was not affected by rapamycin (Tsokas et al. 2007). Likewise, we found that rapamycin completely prevented BDNF-induced phosphorylation at Thr389, whereas the inhibition of BDNF-induced phosphorylation at Thr421/Ser424 was incomplete. Still, however, the ability of BDNF to drive Thr421/Ser424 phosphorylation was partially dependent on mTOR, suggesting that the regulation of Thr389 and Thr421/Ser424 phosphorylation by mTOR may be tailored to different neuronal stimuli, such as BDNF vs. HFS.
BDNF-induced p70S6K at Thr389 and Thr421/Ser424 is differentially regulated by PI3K and MAPK
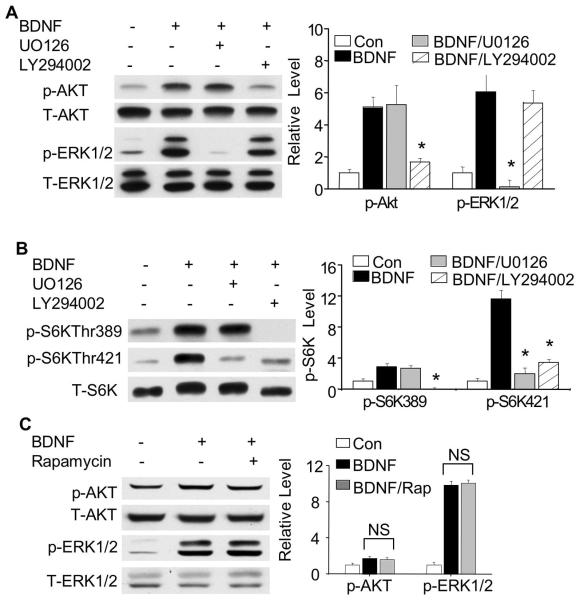
In BDNF-stimulated neurons, the activation of TrkB stimulates both Ras-Raf-MAPK and PI3K-Akt-mTOR signaling (Reichardt 2006), and each of these pathways plays a role in the activation of neuronal protein synthesis induced by BDNF (Takei et al. 2001). Previous work has shown that phosphorylation at Thr389 and Thr421/Ser424 depends on PI3K and MAPK, respectively. Here, we found that BDNF drove parallel activation of MAPK and PI3K pathways with little cross-talk (Fig. 2A). Inhibiting MEK by U0126 did not block BDNF-stimulated Akt phosphorylation, and inhibiting PI3K by LY294002 did not block BDNF-stimulated ERK phosphorylation (Fig. 2A). Consistent with the previous reports, blocking MEK by U0126 inhibited BNDF-stimulated phosphorylation at Thr421/Ser424 but not Thr389 (Fig. 2B). Surprisingly, blocking PI3K by LY294002 inhibited phosphorylation at both sites (Fig. 2B). These results suggest that while PI3K controls phosphorylation at Thr389 independent of MAPK, signaling through both pathways is necessary for maximal regulation at Thr421/Ser424. Finally, we found that rapamycin did not block BDNF-induced up-regulation of p-ERK or p-Akt (Fig. 2C), despite the fact that rapamycin blocks BDNF-induced phosphorylation at both Thr389 and Thr421/Ser424 (Fig. 1D). These results are consistent with the notion that mTOR acts downstream of PI3K to promote activation of p70S6K in response to BDNF, as co-activation of MAPK and PI3K without mTOR activity is not sufficient to stimulate pS6K phosphorylation.
Fig. 2.
PI3K and MAPK pathway differentially regulate p70S6K phosphorylation at Thr389 and Thr421/Ser424. A: LY294002 inhibits BDNF-stimulated p-Akt but not p-ERK; U0126 inhibits BDNF-stimulated p-ERK but not p-Akt. B: Western blots show effects of LY294002 and U0126 on p-p70S6K. C: Western blots show effects of rapamycin on p-ERK and p-Akt. Representative images are shown in the left panels, and quantification in the right panels (n=3 from separate experiments). The relative intensity of p-p70S6K, p-Akt and p-ERK was normalized to T-p70S6K, T-Akt and T-ERK. *: p<0.05 when comparison was made between BDNF-stimulated neurons and inhibitor-treated neurons. NS: not statistically different.
BDNF-induced p70S6K phosphorylation depends on intracellular calcium and CaM
Previous observations have indicated an important role of calcium and CaM in the regulation and activation of intracellular pathways related to neuronal plasticity. BDNF can alter intracellular calcium in a variety of ways via, for example, enhancement of glutamate release, modulation of NMDA and calcium channels, and stimulating calcium release from internal stores (Levine et al. 1998; Lin et al. 1998; Reichardt 2006; Rose et al. 2004). We thus investigated whether calcium-dependent processes impinge on BDNF-induced p70S6K activation.
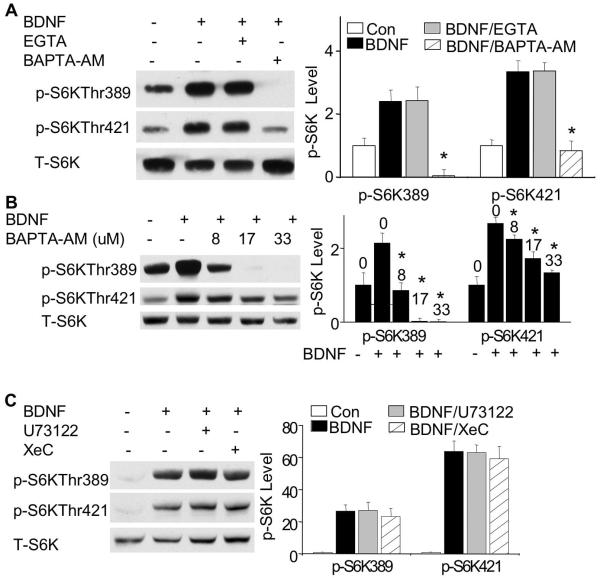
We first tested the role of NMDA receptors (NMDAR) and L-type voltage-gated,calcium channels (L-VGCC), both of which are strongly implicated in regulating synaptic plasticity. Blocking NMDAR and L-VGCC by APV and nifedipine, respectively, did not affect BDNF-induced p70S6K phosphorylation at both sites (data not shown). To inhibit overall calcium influx, we used EGTA (2.5 mM) to chelate extracellular calcium. Although the same concentration of EGTA blocked NMDA-induced ERK phosphorylation (data not shown), there was no effect of EGTA on BDNF-induced phosphorylation of p70S6K at either Thr389 or Thr421/Ser424 (Fig. 3A). In contrast, chelation of intracellular calcium by BAPTA-AM (33 μM) ablated BDNF-stimulated p70S6K phosphorylation at both Thr389 and Thr421/Ser424 (Fig. 3A). Further analysis determined that BDNF-stimulated p70S6K phosphorylation could be blocked by BAPT-AM at as low as 8 μM, and the phosphorylation at Thr389 was more sensitive to BAPTA-AM than Thr421/Ser424 (Fig. 3B). Our previous results showed that pre-treatment with BAPTA-AM only suppressed BDNF-stimulated PI3K activity but not ERK (Zheng et al. 2009; Zheng et al. 2008). Thus, we propose that intracellular calcium may regulate BDNF-induced p70S6K phosphorylation through PI3K rather than ERK.
Fig. 3.
Intracellular, but not extracellular calcium is required for BDNF-induced p70S6K phosphorylation. A: The BDNF-induced phosphorylation of p70S6K is blocked by BAPTA-AM, but not EGTA. Cultured neurons were pre-treated with BAPTA-AM (33 μM) to chelate intracellular calcium, or with EGTA (2.5 mM) to chelate extracellular calcium for 30 min before BDNF stimulation. The samples were collected 15 min after BDNF stimulation, and analyzed by Western blot. B: Neurons were pre-treated with BAPTA-AM at different concentrations as indicated (in μM) for 30 min, and then stimulated by BDNF. Samples were collected 15 min after BDNF stimulation, and p-p70S6K was analyzed by Western blots. C: Inhibition of PLC by U73122 or inhibition of Ca2+ release from intracellular Ca2+ stores by XeC does not block BDNF-induced p-p70S6K. Representative images are shown in the left panels and quantification (normalized to T-p70S6K) in the right panels (n=3 from separate samples). *: p<0.05 when comparison was made between BDNF-stimulated neurons and BAPTA-AM-treated neurons.
Stimulation of TrkB by BDNF can induce IP3-mediated calcium release from internal stores via activation of the PLC-γ pathway (Finkbeiner et al. 1997; Reichardt 2006). Therefore, it is possible that PLC-γ signaling is involved in BDNF-induced phosphorylation of p70S6K. To examine this possibility, we pretreated neurons with a potent PLC inhibitor U73122 (5 μM), and an IP3 receptor inhibitor XeC to prevent Ca2+ release from intracellular Ca2+ stores. Surprisingly, neither U73122 nor XeC blocked BDNF-induced p70S6K phosphorylation (Fig. 3C). Moreover, at higher concentration, U73122 (25 μM) also failed to suppress p70S6K phosphorylation (data not shown). When neurons were pre-treated with thapsigargin (1 μM) or dantrolene (40 μM), which deplete intracellular Ca2+ stores, no inhibition of BDNF-induced p70S6K phosphorylation was observed (data not shown). These results implicate that PLC-γ -mediated intracellular calcium release is not required for p70S6K activation. Combined with that BDNF-stimulated extracellular calcium influx (presumably through NMDAR and L-VGCC) are not necessary for p70S6K activation, we conclude that the basal level of intracellular calcium may gate BDNF-induced p70S6K phosphorylation.
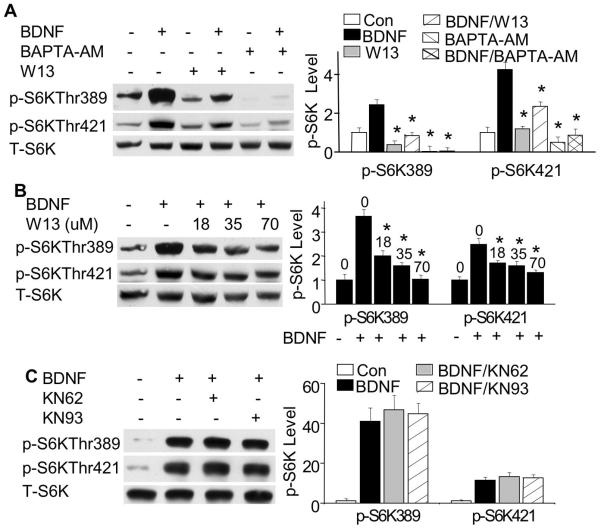
Because CaM is a major effector of intracellular calcium, and plays a key role in regulating neuronal function (Soderling 2000), we investigated the effects of CaM inhibition on p70S6K phosphorylation. Neurons were pre-treated for 30 min with W13 (70 μM), a specific CaM inhibitor. As shown in Fig. 4A, BDNF-induced phosphorylation of p70S6K at Thr389 was dramatically reduced by W13. A milder, but significant, suppression was observed for the phosphorylation at Thr421/Ser424 (Fig. 4A). In contrast to Thr389, the basal level of phosphorylation at Thr421/Ser424 was not affected by W13 in un-stimulated neurons (Fig. 4A). Further analysis showed that phosphorylation at both Thr389 and Thr421/Ser424 could be blocked by W13 at as low as 18 μM (Fig. 4B).
Fig. 4.
CaM activity is required for BDNF-induced p70S6K phosphorylation. A: Neurons pre-treated with CaM inhibitor W13 (70 μM) or BAPTA-AM (33 μM) show significant reduction of BDNF-stimulated phosphorylation at both Thr389 and Thr421/Ser424. B: Neurons were pre-treated with W13 at different concentrations as indicated (in μM) for 30 min, and then stimulated by BDNF. Samples were collected 15 min after BDNF stimulation, and p-p70S6K was analyzed by Western blots. C: Inhibition of CaM kinases I/II/IV by KN62 or KN93 does not inhibit BDNF-stimulated p70S6K phosphorylation. Representative images are shown in the left panels and quantification (normalized to T-p70S6K) in the right panels (n=3 from separate samples). *: p<0.05 when comparison was made between BDNF-stimulated neurons and W13- or BAPTA-treated neurons.
Previous reports implicate that CaM may regulate p70S6K through CaM-dependent protein kinases (CaMKs) (Griffith 2004; Soderling 2000). In this study, we also observed that the phosphorylation of α-CaMKII was ablated in W13 (at 30 μM)-treated neurons (data not shown). To further determine the role of CaMKs on BDNF-induced p70S6K phosphorylation, neurons were pre-treated for 30 min with CaMK I/II/IV inhibitors KN62 (10 μM) and KN93 (5 μM). We found that inhibition of CaMKs by either KN62 or KN93 had no effects on BDNF-induced p70S6K phosphorylation (Fig. 4C), while the two inhibitors effectively blocked the phosphorylation of α-CaMKII at Thr286 (data not shown). At higher concentration, KN62 (50 μM) and KN93 (25 μM) also failed to suppress BDNF-induced p70S6K phosphorylation (data not shown). These data suggest that the regulatory function of intracellular calcium and CaM on the phosphorylation of p70S6K is not mediated through CaMKI, II or IV.
The increase in intracellular calcium level and MAPK activation are not sufficient to induce p70S6K phosphorylation
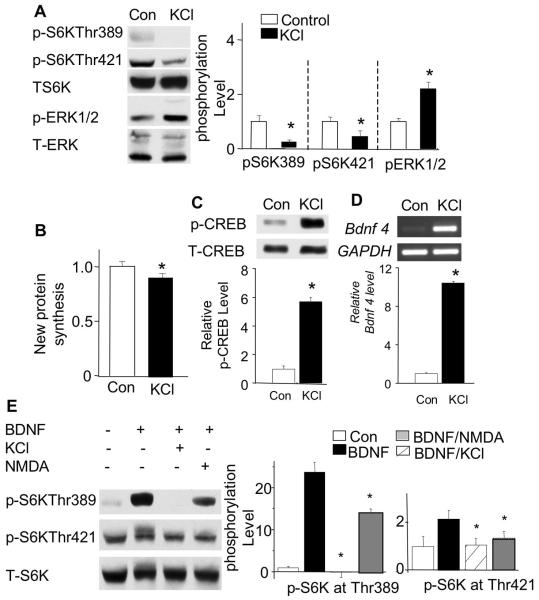
To obtain further experimental support, we next tested whether elevation in intracellular calcium is sufficient to cause p70S6K phosphorylation. To trigger activity-dependent calcium influx, we depolarized neurons by elevating the extracellular K+ concentration to 50 mM (with KCl; Dolmetsch et al. 2001). Consistent with other studies, we observed significant up-regulation of p-ERK1/2 (Fig. 5A). However, p70S6K phosphorylation at Thr421/Ser424, the previously identified MAPK site, was down-regulated in KCl-treated neurons (Fig. 5A). This result implicates that the activation of ERK alone is not sufficient to stimulate phosphorylation at Thr421/Ser424. The phosphorylation at Thr389 was also dramatically decreased in KCl-treated neurons (Fig. 5A). Consistent with the decrease in p70S6K phosphorylation, membrane depolarization resulted in a mild, but significant, reduction (11.4 +/−3%, p<0.05) in global protein synthesis (Fig. 5B). In contrast, membrane depolarization activated the transcription factor CREB (Fig. 5C) and transcriptional up-regulation of Bdnf exon 4 (Fig. 5D).
Fig. 5.
Increase in intracellular calcium antagonizes the BDNF-stimulated p70S6K phosphorylation. To increase the level of intracellular calcium, neurons were depolarized by KCl. A: Membrane depolarization by KCl (50 mM) for 15min suppresses p70S6K phosphorylation at both Thr389 and Thr421/Ser424 (n=3). B: Membrane depolarization causes a reduction in global protein synthesis. New protein synthesis was determined by S-35 labeling as described in “Materials and Methods” (n=5). C: Membrane depolarization activates transcription factor CREB (n=3). D: Membrane depolarization activates the transcription of immediate early gene Bdnf (n=3). The relative level of exon 4-containing Bdnf mRNA was determined by semi-quantitative RT-PCR using gene-specific primers. E: Co-application of KCl or NMDA (50 μM) with BDNF causes a significant reduction of p70S6K phosphorylation at both Thr389 and Thr421/Ser424 (n=3). Neurons were treated with either BDNF alone or with BDNF plus KCl (or NMDA) for 15 min, and then analyzed by Western blots. Quantifications are shown in the right (A and E) or lower (C and D) panels. *: p<0.05.
We next examined how membrane depolarization-induced intracellular calcium elevation affects BDNF-stimulated p70S6K phosphorylation. We co-applied KCl (50 mM) and BDNF to cultured neurons. We found that the BDNF-induced phosphorylation of p70S6K at Thr389 and Thr421/Ser424 was completely blocked in co-stimulated neurons (Fig. 5E). Because NMDA also can trigger a sustained [Ca2+]i elevation by stimulating NMDA receptors (Dolmetsch et al., 2001), we next co-applied NMDA (50 μM) and BDNF to cultures. NMDA significantly suppressed BDNF-induced p70S6K phosphorylation at Thr389 and Thr421/Ser424 (Fig.5E). Although these results do not identify the function of specific KCl- and NMDA-responsive molecules, they demonstrate that elevation of intracellular calcium decreases the phosphorylation of p70S6K, further supporting that the basal level of intracellular calcium is required for gating BDNF-induced p70S6K phosphorylation.
CaM is required for BDNF-induced protein synthesis
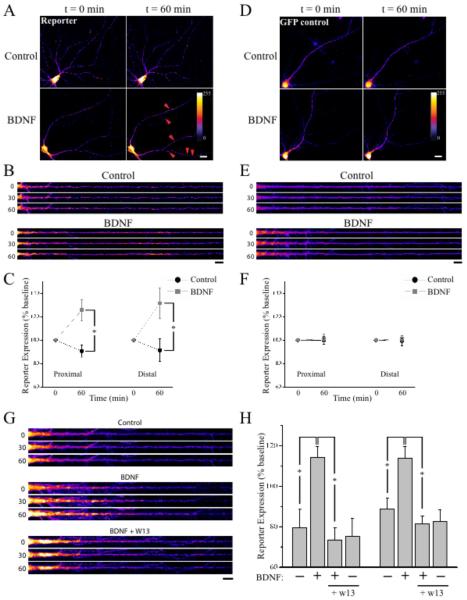
In addition to a stimulatory role on overall neuronal translation (Takei et al. 2001), BDNF is known to enhance local protein synthesis in dendrites (Aakalu et al. 2001; Yin et al. 2002). First, we confirmed that BDNF stimulated global protein translation by metabolic labeling with S35-methionine. At 5 ng/ml, BDNF caused a mild but significant increase in overall protein synthesis (11.7 +/− 4% increase comparing to un-treated neurons, p<0.05). A larger degree of overall protein synthesis (29 +/− 7% increase, p<0.05) was observed in neurons treated with BDNF at 20 ng/ml. To examine whether the requirement for CaM-dependent regulation of p70S6K phosphorylation by BDNF applies to dendritic synthesis, we examined regulation of a fluorescent translation reporter known to be responsive to BDNF (Aakalu et al. 2001). This construct uses a destabilized, myristoylated GFP that is flanked by the 5′ and 3′ untranslated regions (UTRs) of α-CaMKII. Using this same construct, Aakalu et al. (Aakalu et al. 2001) demonstrated that BDNF induced rapid reporter synthesis at specific hotspots along dendrites. Consistent with these earlier observations, we similarly found that BDNF application (20 ng/ml) rapidly stimulated reporter synthesis in dendrites (Fig. 6A-C), with particularly strong increases in reporter expression at specific hotspots (Fig. 6A and B). In contrast, reporter expression in mock-treated control neurons typically remained stable or even decreased over the course of imaging. These differential effects of BDNF were prevented by pre-treatment with the protein synthesis inhibitor anisomycin (40 μM; data not shown). To control for the effects of viral delivery and any potential post-translational processing that could alter GFP fluorescence, we also examined a control construct encoding GFP that lacked α-CaMKII UTRs. BDNF did not alter GFP expression of this control construct (Fig. 6D-F). Finally, pre-treatment with the calmodulin inhibitor W13 (30 μM) abolished the increase in reporter synthesis induced by BDNF, but did not alter reporter expression in control neurons (Fig. 6G-H). Taken together, these results suggest that local dendritic synthesis induced by BDNF is CaM-dependent.
Fig. 6.
BDNF-induced translation in dendrites is CaM-dependent. Neurons expressing either the protein synthesis reporter (A-C) or control GFP (D-F) were either mock-treated (control) or treated with 20 ng/ml BDNF immediately following acquisition of a baseline image (t = 0). Representative full-frame examples (A, D) and time-lapse montages of straightened dendrites (B, E) are shown; fluorescence intensity is indicated by the color look-up table; scale bar = 20 μm in (A, D) and 10 μm in (B, E). Red arrowheads in (A) highlight hotspots of reporter synthesis evident after BDNF treatment. C and F: Mean (+/− SEM) GFP expression (relative to baseline) in the proximal (< 125 μm from soma) and distal dendritic compartment (> 125 μm from soma) for mock-treated (n = 10) and BDNF-treated neurons (n = 8). BDNF significantly (*p < 0.05 by t-test) enhanced GFP expression from the reporter, but not from a control construct lacking the α-CAMKII UTRs (n = 12/group). G: Time-lapse montages of straightened dendrites from mock-treated neurons (n = 12), or neurons treated with 20 ng/ml BDNF (n = 12) either alone or after pre-treatment (60 min) with 30 μM CaM inhibitor W13 (n = 12). Another group of neurons were treated with W13 alone (n = 9); fluorescence intensity is indicated by the color look-up table in (A), scale bar = 10 μm. H: Mean (+/− SEM) reporter expression (relative to baseline) in proximal (left columns) and distal dendrites (right columns) in each of the treatment conditions described in (G). The increase in dendritic reporter expression induced by BDNF was blocked by W13; *p < 0.05 by Fisher’s LSD.
DISCUSSION
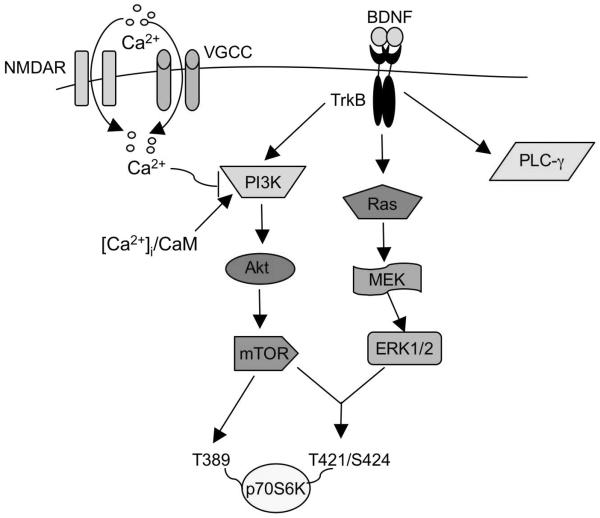
In this study, we examined the regulatory mechanisms for BNDF-induced phosphorylation of p70S6K in cultured neurons. As summarized in Fig. 7, we identified novel functions of PI3K, which regulates p70S6K phosphorylation at both Thr389 and Thr421/Ser424 through mTOR. Activation of MEK-ERK1/2 alone is not sufficient to support the phosphorylation at Thr421/Ser424, which may also require co-activation of mTOR in BDNF-stimulated neurons. The basal level of intracellular calcium, rather than elevation of the intracellular calcium, is required for the TrkB- and mTOR-dependant phosphorylation at both Thr389 and Thr421/Ser424. Furthermore, CaM activity is a key component for p70S6K phosphorylation and the BDNF-stimulated local protein synthesis in dendrites. Finally, we suggest that basal level of intracellular calcium and CaM may function through PI3K rather than CaM kinases.
Fig. 7.
Regulation of BDNF/TrkB-dependent p70S6K phosphorylation. Stimulation of receptor tyrosine kinase TrkB by BDNF leads to activation of Ras-MEK-ERK1/2, PI3K-Akt-mTOR, and PLCγ pathways. It appears that intracellular calcium and CaM impinge on PI3K-Akt-mTOR signaling and regulate p70S6K phosphorylation at both Thr389 and Thr421/Ser424. Phosphorylation at Thr421/Ser424, the previously identified MEK-ERK1/2 site, requires co-activation of Ras-MEK-ERK1/2 and mTOR. Extensive calcium influx, presumably through NMDA receptors and VGCC, may antagonize BDNF-stimulated PI3K signaling and in turn suppress p70S6K phosphorylation.
The phosphorylations at Thr389 and Thr421/Ser424 are differentially regulated in BDNF-stimulated neurons
Previous studies have demonstrated that BDNF at 100 ng/ml (about 4 nM for BDNF dimmer) stimulates robust p70S6K phosphorylation and protein synthesis (Takei et al. 2004). Here we demonstrate that subnanomolar concentration of BDNF (5ng/ml, about 0.2 nM) is sufficient to induce persistent phosphorylation of p70S6K at Thr389 and Thr421/Ser424, as well as global synthesis in cultured neurons. However, 20 ng/ml BDNF does stimulate more protein synthesis than 5 ng/ml, presumably due to that 5 ng/ml BDNF only partially stimulates other translational components, such as 4EBP, eIF-4E, and eEF2 (Costa-Mattioli et al. 2009; Klann and Dever 2004).
As described earlier, the phosphorylation of both sites at Thr389 and Thr421/Ser424 accounts for full strength of p70S6K activity. Previous studies with non-neuronal cells have implicated that Thr389 is the target of PI3K, and Thr421/Ser424 is mainly phosphorylated by ERK (Lehman et al. 2003). Because PI3K and ERK are the two major signaling pathways activated by BDNF/TrkB and involved in many aspects of plasticity, the activity of p70S6K is potentially tightly coupled to neuronal stimulation. It is apparent that the regulatory function of PI3K and ERK on these two sites depends on how neurons are stimulated. For example, when neurons are stimulated by adenylyl cyclase activator forskolin, inhibiting PI3K only blocks Thr389, but not Thr421/Ser424 phosphorylation (Gobert et al. 2008). Inhibiting MEK suppresses phosphorylation at both Thr389 and Thr421/Ser424 (Gobert et al. 2008). In addition, rapamycin only blocks phosphorylation at Thr389 but not Thr421/424 (Gobert et al. 2008). Another study has reported similar regulation by ERK and rapamycin (Tsokas et al. 2007). In the report, Tsokas and colleagues stimulated the CA1 hippocampal neurons with high frequency stimulation (HFS), and observed that rapamycin only blocked phosphorylation at Thr389 but not Thr421/Ser424, and U0126 blocked phosphorylation at both sites (Tsokas et al. 2007). Because MEK inhibition not only blocked p-ERK, but also blocked HFS-stimulated p-Akt, the MEK inhibition on Thr389/Ser424 may be indirect, and mediated through PI3K (Tsokas et al. 2007). In our study, we show that PI3K and MEK/ERK do not cross talk in BDNF-stimulated neurons. Inhibition of PI3K did not block p-ERK, and inhibition of MEK did not block p-Akt (Fig. 2A). We found that, in BDNF-stimulated neurons, the phosphorylation of Thr389 was regulated only by PI3K, and the phosphorylation of Thr421/Ser424 was regulated by both PI3K and MEK. Consistently, an inhibitor (rapamycin) of mTOR, which is down-stream of PI3K and up-stream of S6K, blocked the phosphorylation of both sites in BDNF-stimulated neurons. Moreover, the regulatory role of PI3K and ERK in p70S6K phosphorylation is reflected in protein synthesis. Inhibition of PI3K and MEK blocks activity-induced protein synthesis (Kelleher et al. 2004; Takei et al. 2001). It has been reported that inhibition of MEK impairs BDNF-mediated LTP in the dentate gyrus (Ying et al. 2002). However, the functional relevance of PI3K in BDNF-mediated LTP has not been addressed.
The function of CaM in p70S6K phosphorylation and local protein synthesis
Although BDNF stimulates both extracellular Ca2+ influx and intracellular Ca2+ release, it is not clear how these dynamic changes in calcium regulate the mTOR-dependent phosphorylation of p70S6K. Our data show that the BDNF-mediated Ca2+ elevation may not be required. We further demonstrate that removal of [Ca2+]i by BAPTA-AM or persistent elevation of [Ca2+]i by membrane depolarization or activation of NMDAR causes p70S6K dephosphorylation, and antagonizes the effects of BDNF. Together, we suggest that the basal level [Ca2+]i is necessary, and may gate p70S6K phosphorylation. It is important to note that the basal level [Ca2+]i is an outcome of calcium homeostasis, which may be handled differently under specific environmental conditions. It was documented that the basal level [Ca2+]i increases during in vitro neuronal maturation, possible due to significant synaptogenesis (Zhou et al.....reffff). Disregulation of calcium homeostasis and increase [Ca2+]i are also observed in aged animals (refffffff). Further investigation is needed to clarify the crosstalk between basal [Ca2+]i and BDNF in vivo. The changes of intracellular calcium level not only affect p70S6K phosphorylation by BDNF, but also other translational factors, such as 4EBP and eIF4E. Previous reports show that sustained increase of [Ca2+]i by glutamate (Marin et al. 1997) or KCl (Iizuka et al. 2007) leads to inhibition of eukaryotic elongation factor-2 (eEF2) activity and in turn suppresses mRNA translation. It is important to note that membrane depolarization and bath incubation of NMDA trigger massive calcium influx in our culturing conditions. The massive calcium elevation may mimic the situations during brain injury and stroke (Sattler, R. & Tymianski, M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol Neurobiol 24, 107-29 (2001).Nishizawa, Y. Glutamate release and neuronal damage in ischemia. Life Sci 69, 369-81 (2001).refffff). However, mild elevation of calcium upon physiological activation of L-VGCC or NMDA receptors in vivo (e.g. during learning and exploration) may have different effects on BDNF-induced p70S6K activation and protein synthesis.
Further analysis on the major BDNF-stimulated pathways implicates that intracellular Ca2+ may regulate p70S6K phosphorylation through PI3K, but not ERK and CaM kinases. First, we have observed that the BNDF-mediated phosphorylation of Akt is similarly regulated by [Ca2+]i (Zheng et al. 2008). BAPTA-AM suppresses p-Akt, but not p-ERK in BDNF stimulated neurons; KCl leads to p-Akt dephosphorylation and ERK phosphorylation; co-application of KCl with BDNF causes Akt dephosphorylation without affecting BDNF-stimulated p-ERK (Zheng et al. 2008). Second, inhibition of CaM kinases by either KN62 or KN93 has no effects on BDNF-stimulated p70S6K phosphorylation.
Our results reveal that the regulatory function of [Ca2+]i is mediated, at least partially through CaM. There is evidence that CaM binds to PI3K in vitro, and W13 disrupts the binding (Fischer et al. 1998; Perez-Garcia et al. 2004). Thus, it is possible that the effects of [Ca2+]i reduction and CaM inhibition on phosphorylation of p70S6K may be attributed to the reduction of PI3K signaling. Previous studies have also suggested that the basal level of intracellular calcium and CaM plays an important role in activation of Akt (or PKB) and neuronal survival mediated by neurotrophic factors (Cheng et al. 2003; Egea et al. 2001). Although the function of protein synthesis in neuronal survival and neuronal death is not extensively investigated, there is evidence that transient focal ischemia leads to massive glutamate release and causes a decrease in PI3K activity and p70S6K phosphorylation (Janelidze et al. 2001). It is conceivable that the apoptotic effects of CaM inhibition suppress BDNF-regulated elevation in protein synthesis, which may be required for survival. Interestingly, double knockout mice for both S6K1 and S6K2 are perinatally lethal (Pende et al. 2004).
Although we demonstrate correlated responses of p70S6K phosphorylation and protein synthesis to CaM inhibition, the functional role of p70S6K in dendritic synthesis remains unclear. On one hand, partial phosphorylation of S6 and mitogen-stimulated translation of 5′ TOP-containing mRNA (as demonstrated by EF1A mRNA) are still present in S6K1/S6K2 double knockout cells (Pende et al. 2004). On the other hand, memory acquisition or retention for contextual fear conditioning, Morris water maze, and conditioned taste aversion is impaired in either S6K1 or S6K2 knockout mice (Antion et al. 2008b). Further analysis with the cellular plasticity models has demonstrated that the late phase LTP as well as the translation- and mGluR-dependent LTD are fairly normal in the S6K1 or S6K2 knockout mice (Antion et al. 2008a; Antion et al. 2008b). However, it is worthwhile to note that the basal level of EF1A was elevated in S6K knockout mice, and the activation of group 1 mGluR fails to further stimulate EF1A translation (Antion et al. 2008a). These studies suggest that the function of S6K is, at least, relevant to certain aspects of neuroplasticity. As far as we know, our data are the first to suggest that the CaM regulation of p70S6K phosphorylation may be involved in BDNF-stimulated dendritic protein translation. Future experiments with molecular approaches are needed to address the function of S6K in BDNF-mediated protein synthesis and plasticity.
Acknowledgments
This work was supported by grant from the National Institutes of Health (MH076906 to HW).
REFERENCES
- Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM. Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron. 2001;30(2):489–502. doi: 10.1016/s0896-6273(01)00295-1. [DOI] [PubMed] [Google Scholar]
- Antion MD, Hou L, Wong H, Hoeffer CA, Klann E. mGluR-dependent long-term depression is associated with increased phosphorylation of S6 and synthesis of elongation factor 1A but remains expressed in S6K-deficient mice. Mol Cell Biol. 2008a;28(9):2996–3007. doi: 10.1128/MCB.00201-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antion MD, Merhav M, Hoeffer CA, Reis G, Kozma SC, Thomas G, Schuman EM, Rosenblum K, Klann E. Removal of S6K1 and S6K2 leads to divergent alterations in learning, memory, and synaptic plasticity. Learn Mem. 2008b;15(1):29–38. doi: 10.1101/lm.661908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CM, Nozaki N, Shigeri Y, Soderling TR. Cytoplasmic polyadenylation element binding protein-dependent protein synthesis is regulated by calcium/calmodulin-dependent protein kinase II. J Neurosci. 2004;24(22):5193–5201. doi: 10.1523/JNEUROSCI.0854-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LR, Izquierdo I, Medina JH. Persistence of long-term memory storage requires a late protein synthesis- and BDNF-dependent phase in the hippocampus. Neuron. 2007a;53(2):261–277. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Katche C, Slipczuk LN, Igaz LM, Cammarota M, Izquierdo I, Medina JH. mTOR signaling in the hippocampus is necessary for memory formation. Neurobiol Learn Mem. 2007b;87(2):303–307. doi: 10.1016/j.nlm.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Cheng A, Wang S, Yang D, Xiao R, Mattson MP. Calmodulin mediates brain-derived neurotrophic factor cell survival signaling upstream of Akt kinase in embryonic neocortical neurons. J Biol Chem. 2003;278(9):7591–7599. doi: 10.1074/jbc.M207232200. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61(1):10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294(5541):333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- Egea J, Espinet C, Soler RM, Dolcet X, Yuste VJ, Encinas M, Iglesias M, Rocamora N, Comella JX. Neuronal survival induced by neurotrophins requires calmodulin. J Cell Biol. 2001;154(3):585–597. doi: 10.1083/jcb.200101023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19(5):1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- Fischer R, Julsgart J, Berchtold MW. High affinity calmodulin target sequence in the signalling molecule PI 3-kinase. FEBS Lett. 1998;425(1):175–177. doi: 10.1016/s0014-5793(98)00225-7. [DOI] [PubMed] [Google Scholar]
- Gelinas JN, Banko JL, Hou L, Sonenberg N, Weeber EJ, Klann E, Nguyen PV. ERK and mTOR signaling couple beta-adrenergic receptors to translation initiation machinery to gate induction of protein synthesis-dependent long-term potentiation. J Biol Chem. 2007;282(37):27527–27535. doi: 10.1074/jbc.M701077200. [DOI] [PubMed] [Google Scholar]
- Gobert D, Topolnik L, Azzi M, Huang L, Badeaux F, Desgroseillers L, Sossin WS, Lacaille JC. Forskolin induction of late-LTP and up-regulation of 5′ TOP mRNAs translation via mTOR, ERK, and PI3K in hippocampal pyramidal cells. J Neurochem. 2008;106(3):1160–1174. doi: 10.1111/j.1471-4159.2008.05470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong R, Park CS, Abbassi NR, Tang SJ. Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J Biol Chem. 2006;281(27):18802–18815. doi: 10.1074/jbc.M512524200. [DOI] [PubMed] [Google Scholar]
- Griffith LC. Calcium/calmodulin-dependent protein kinase II: an unforgettable kinase. J Neurosci. 2004;24(39):8391–8393. doi: 10.1523/JNEUROSCI.2888-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18(16):1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Iizuka A, Sengoku K, Iketani M, Nakamura F, Sato Y, Matsushita M, Nairn AC, Takamatsu K, Goshima Y, Takei K. Calcium-induced synergistic inhibition of a translational factor eEF2 in nerve growth cones. Biochem Biophys Res Commun. 2007;353(2):244–250. doi: 10.1016/j.bbrc.2006.11.150. [DOI] [PubMed] [Google Scholar]
- Inamura N, Nawa H, Takei N. Enhancement of translation elongation in neurons by brain-derived neurotrophic factor: implications for mammalian target of rapamycin signaling. J Neurochem. 2005;95(5):1438–1445. doi: 10.1111/j.1471-4159.2005.03466.x. [DOI] [PubMed] [Google Scholar]
- Janelidze S, Hu BR, Siesjo P, Siesjo BK. Alterations of Akt1 (PKBalpha) and p70(S6K) in transient focal ischemia. Neurobiol Dis. 2001;8(1):147–154. doi: 10.1006/nbdi.2000.0325. [DOI] [PubMed] [Google Scholar]
- Kanhema T, Dagestad G, Panja D, Tiron A, Messaoudi E, Havik B, Ying SW, Nairn AC, Sonenberg N, Bramham CR. Dual regulation of translation initiation and peptide chain elongation during BDNF-induced LTP in vivo: evidence for compartment-specific translation control. J Neurochem. 2006;99(5):1328–1337. doi: 10.1111/j.1471-4159.2006.04158.x. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116(3):467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Klann E, Dever TE. Biochemical mechanisms for translational regulation in synaptic plasticity. Nat Rev Neurosci. 2004;5(12):931–942. doi: 10.1038/nrn1557. [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92(19):8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman JA, Calvo V, Gomez-Cambronero J. Mechanism of ribosomal p70S6 kinase activation by granulocyte macrophage colony-stimulating factor in neutrophils: cooperation of a MEK-related, THR421/SER424 kinase and a rapamycin-sensitive, m-TOR-related THR389 kinase. J Biol Chem. 2003;278(30):28130–28138. doi: 10.1074/jbc.M300376200. [DOI] [PubMed] [Google Scholar]
- Levine ES, Crozier RA, Black IB, Plummer MR. Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-D-aspartic acid receptor activity. Proc Natl Acad Sci U S A. 1998;95(17):10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Wu K, Levine ES, Mount HT, Suen PC, Black IB. BDNF acutely increases tyrosine phosphorylation of the NMDA receptor subunit 2B in cortical and hippocampal postsynaptic densities. Brain Res Mol Brain Res. 1998;55(1):20–27. doi: 10.1016/s0169-328x(97)00349-5. [DOI] [PubMed] [Google Scholar]
- Marin P, Nastiuk KL, Daniel N, Girault JA, Czernik AJ, Glowinski J, Nairn AC, Prémont J. Glutamate-dependent phosphorylation of elongation factor-2 and inhibition of protein synthesis in neurons. J Neurosci. 1997;17(10):3445–3454. doi: 10.1523/JNEUROSCI.17-10-03445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci. 2006;26(50):12977–12983. doi: 10.1523/JNEUROSCI.4209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J, Mueller M, Fumagalli S, Kozma SC, Thomas G. S6K1(−/−)/S6K2(−/−) mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cell Biol. 2004;24(8):3112–3124. doi: 10.1128/MCB.24.8.3112-3124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Garcia MJ, Cena V, de Pablo Y, Llovera M, Comella JX, Soler RM. Glial cell line-derived neurotrophic factor increases intracellular calcium concentration. Role of calcium/calmodulin in the activation of the phosphatidylinositol 3-kinase pathway. J Biol Chem. 2004;279(7):6132–6142. doi: 10.1074/jbc.M308367200. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BE, Huber KM. Current advances in local protein synthesis and synaptic plasticity. J Neurosci. 2006;26(27):7147–7150. doi: 10.1523/JNEUROSCI.1797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen N, Thomas G. The modular phosphorylation and activation of p70s6k. FEBS Lett. 1997;410(1):78–82. doi: 10.1016/s0014-5793(97)00323-2. [DOI] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Blum R, Kafitz KW, Kovalchuk Y, Konnerth A. From modulator to mediator: rapid effects of BDNF on ion channels. Bioessays. 2004;26(11):1185–1194. doi: 10.1002/bies.20118. [DOI] [PubMed] [Google Scholar]
- Saitoh M, Pullen N, Brennan P, Cantrell D, Dennis PB, Thomas G. Regulation of an activated S6 kinase 1 variant reveals a novel mammalian target of rapamycin phosphorylation site. J Biol Chem. 2002;277(22):20104–20112. doi: 10.1074/jbc.M201745200. [DOI] [PubMed] [Google Scholar]
- Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME. BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci. 2004;24(33):7366–7377. doi: 10.1523/JNEUROSCI.1739-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling TR. CaM-kinases: modulators of synaptic plasticity. Curr Opin Neurobiol. 2000;10(3):375–380. doi: 10.1016/s0959-4388(00)00090-8. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. Local translational control in dendrites and its role in long-term synaptic plasticity. J Neurobiol. 2005;64(1):116–131. doi: 10.1002/neu.20152. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron. 2007;55(4):648–661. doi: 10.1016/j.neuron.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24(44):9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei N, Kawamura M, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor enhances neuronal translation by activating multiple initiation processes: comparison with the effects of insulin. J Biol Chem. 2001;276(46):42818–42825. doi: 10.1074/jbc.M103237200. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GC, Kasai H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319(5870):1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99(1):467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsokas P, Ma T, Iyengar R, Landau EM, Blitzer RD. Mitogen-activated protein kinase upregulates the dendritic translation machinery in long-term potentiation by controlling the mammalian target of rapamycin pathway. J Neurosci. 2007;27(22):5885–5894. doi: 10.1523/JNEUROSCI.4548-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci U S A. 2001;98(20):11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Edelman GM, Vanderklish PW. The brain-derived neurotrophic factor enhances synthesis of Arc in synaptoneurosomes. Proc Natl Acad Sci U S A. 2002;99(4):2368–2373. doi: 10.1073/pnas.042693699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22(5):1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Luo Y, Wang H. Regulation of brain-derived neurotrophic factor-mediated transcription of the immediate early gene Arc by intracellular calcium and calmodulin. J Neurosci Res. 2009;87(2):380–392. doi: 10.1002/jnr.21863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Soellner D, Nunez J, Wang H. The basal level of intracellular calcium gates the activation of phosphoinositide 3-kinase-Akt signaling by brain-derived neurotrophic factor in cortical neurons. J Neurochem. 2008;106(3):1259–1274. doi: 10.1111/j.1471-4159.2008.05478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]