Abstract
The present study describes the creation and characterization of a hepatoma cell line, n4mBid, that supports all stages of the hepatitis C virus (HCV) life cycle and strongly reports HCV infection by a cell-death phenotype. The n4mBid cell line is derived from the highly HCV-permissive Huh-7.5 hepatoma cell line and contains a modified Bid protein (mBid) that is cleaved and activated by the HCV serine protease NS3-4A. N4mBid exhibited a 10–20 fold difference in cell viability between the HCV-infected and mock-infected states, while the parental Huh-7.5 cells showed <2 fold difference under the same conditions. The pronounced difference in n4mBid cell viability between the HCV- and mock-infected states in a 96-well plate format points to its usefulness in cell survival-based high-throughput screens for anti-HCV molecules. The degree of cell death was found to be proportional to the intracellular load of HCV. HCV-low n4mBid cells, expressing an anti-HCV short hairpin RNA, showed a significant growth advantage over naïve cells and could be rapidly enriched after HCV infection, suggesting the possibility of using n4mBid cells for the cell survival-based selection of genetic anti-HCV factors.
Keywords: cytopathic effect, genetic selection, enrichment, plaque assay, n4mBid (hepatoma cell line)
Approximately 180 million people worldwide are infected with hepatitis C virus (HCV), with an incidence of 3–4 million each year (Alter and Seeff, 2000; Wasley and Alter, 2000). Hepatitis C virus is an enveloped, positive-sense RNA virus belonging to the Flaviviridae family. The 9.6-kb viral genome encodes a single large polyprotein that is processed by viral and cellular proteinases to produce the virion structural proteins (core and glycoproteins E1 and E2), P7, and nonstructural proteins (NS2, NS3, NS4A, NS4B, NS5A and NS5B) (Bukh et al., 2002; Ikeda et al., 2002; Pietschmann et al., 2002). Although HCV was first discovered two decades ago (Choo et al., 1989), our knowledge of the virus remains very limited. The recent development of a HCV cell culture system (HCVcc) (Lindenbach et al., 2005; Wakita et al., 2005; Zhong et al., 2005) that supports the entire HCV life cycle in vitro opens new doors to HCV antiviral research. The HCVcc system is based on the genotype 2a JFH-1 strain of HCV that can replicate efficiently without adaptive mutations in human Huh7 cells and derived hepatocellular carcinoma cell lines, such as Huh-7.5 (Blight et al., 2002). Transfection of these hepatocytes with in vitro-transcribed JFH-1, or chimeric J6/JFH-1 genomic RNA, results in the secretion of infectious viral particles.
Unfortunately, cultured cells infected with HCVcc do not show a conveniently identifiable phenotype. Currently, antibody staining is the most widely used assay for detecting HCV infection. The amount of infectious HCV particles in a sample is typically expressed as 50% cell culture infectious dose/ml (CCID50/ml) (Lindenbach and Rice, 2005) or focus forming units/ml (FFU/ml) (Zhong et al., 2005). Both methods for infectious virus titer determination entail immune staining of viral proteins by HCV-specific antibodies. Antibody-based detection methods are usually expensive, tedious and not easily adapted to high-throughput settings. The most easily observable cellular phenotype is cell death. The availability of many commercial assays for quantifying cell viability also makes the reporting of cell death easily adaptable to high-throughput applications. Although it has been reported that Huh-7.5 and derived cells infected with HCVcc demonstrate a cytopathic effect (Gottwein et al., 2007; Zhong et al., 2006), the amount of cell death is not sufficient for cell death-based in vitro assays.
In the present study, we describe the creation and characterization of a reporter cell line, n4mBid, that can effectively correlate HCV infection to a cell-death phenotype. N4mBid cells were generated from the highly HCV-permissive hepatoma cell line Huh-7.5 (Blight et al., 2002). To enhance the cytopathic effect of HCV, a modified version of the pro-apoptotic protein, Bid (Hsu et al., 2003), was introduced into Huh-7.5 cells. Apoptosis or programmed cell death is a highly regulated process triggered by genetically programmed signaling pathways, and is essential for normal cellular homeostasis and development (Thornberry and Lazebnik, 1998; Zimmermann et al., 2001). Bid is a member of the BH3-only family of apoptosis inducers (Strasser, 2005). Wild-type Bid is activated in the cell by cleavage of its leader peptide by caspases or granzyme B, exposing the BH3 domain. The exposed BH3 domain allows Bid to interact with the Bax protein, setting in motion an apoptotic cascade (Cory and Adams, 2002; Strasser, 2005). In the modified Bid (mBid) system, the endogenous cleavage site of Bid is replaced by the HCV NS5A/NS5B cleavage junction sequence (AEDVVCCSMSYS), making it susceptible to the HCV serine protease NS3-4A (Hsu et al., 2003). The level of mBid-mediated apoptosis is proportional to intracellular expression levels of NS3-4A. The mBid construct has been shown to induce apoptosis in rat fibroblasts in the presence of the HCV serine protease, and in Huh7 cells transfected with a HCV subgenomic replicon (Hsu et al., 2003).
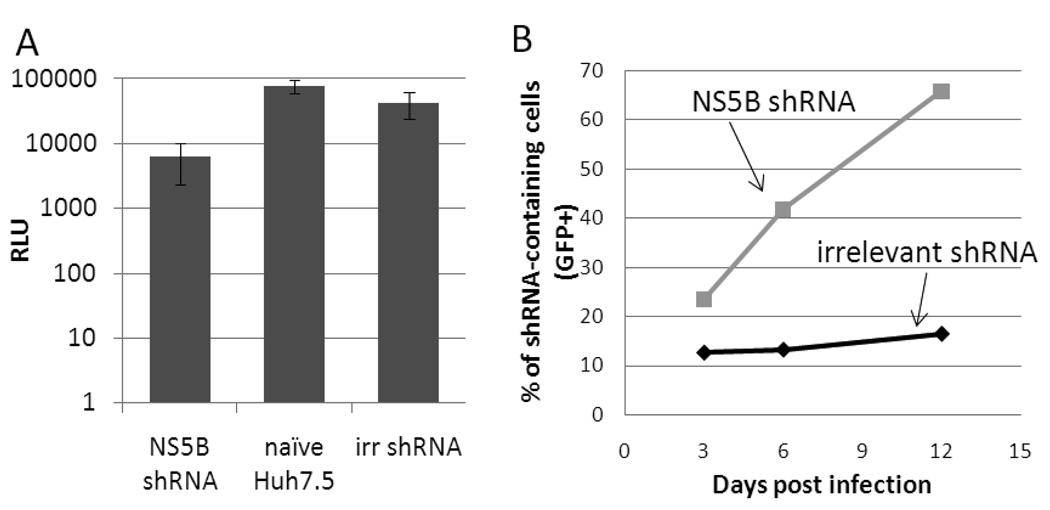
We first devised a competitive enrichment assay for evaluating cells for their ability to both support productive virus infection (virus spread) and undergo a significant cytopathic effect. We reasoned that, upon HCV infection, cells with a lower load of intracellular HCV, via expression of an anti-HCV factor for example, should experience weaker apoptosis than cells with a high HCV load. The survival advantage of HCV-low cells in the midst of a HCV challenge should in turn lead to their enrichment within a cell population subjected to HCV infection over time. In addition, when such a population of cells is infected with HCV at a multiplicity of infection (MOI) less than 1, cells supporting faster virus spread (i.e. able to produce more viruses) should generate a higher selection pressure for the survival of HCV-low cells. To probe the survival advantage experienced by HCV-low cells present in a population of cells infected with HCVcc, we first created a HCV-low sub-population using a shRNA targeting the HCV genome. An anti- NS5B shRNA harboring the targeting sequence CATTATGACTCAGTCTTAA was inserted into the lentiviral vector pLVTHM (Wiznerowicz and Trono, 2003) and introduced into Huh-7.5 cells via lentiviral transduction. The shRNA-bearing lentiviral pseudoparticles contain an independent EGFP reporter gene under the control of a EF1a promoter, allowing the shRNA-expressing cells to be monitored by flow cytometry. After infection with a Gaussia luciferase (Gluc) reporter HCVcc, Jc1FLAG(p7-nsGluc2A) (Marukian et al., 2008), Huh-7.5 cells expressing NS5B shRNA showed a ~10-fold lower supernatant Gluc activity relative to naïve Huh-7.5 cells and cells expressing an irrelevant shRNA, demonstrating the ability of the selected shRNA to suppress HCV replication inside a host cell (Figure 1A). To verify the growth advantage of the NS5B shRNA-expressing HCV-low cells over naïve cells after HCV infection, the shRNA-expressing Huh-7.5 cells were mixed with naïve Huh-7.5 cells at a ratio of ~1:10. This cell population was subsequently challenged with Jc1 HCVcc (Pietschmann et al., 2006) at a MOI of ~1 and the proportion of GFP+ cells was monitored using flow cytometry. The percentage of NS5B shRNA-expressing (GFP+) cells increased rapidly after infection, suggesting that HCV exerts a selective pressure for the survival and enrichment of HCV-low cells from within a cell population containing an excess of naïve cells (Figure 1B). The percentage of irrelevant shRNA-expressing cells remained relatively constant under the same conditions. The enrichment of NS5B shRNA-expressing cells continued for ~12 days post infection, resulting in a peak representation of ~66%. A decline in the percentage of NS5B shRNA-expressing cells was observed after 12 days (data not shown), perhaps due to the emergence over time of non-shRNA-containing cell populations resistant to HCV infection. Although these results demonstrate the growth advantage experienced by the HCV-low, NS5B shRNA-expressing Huh-7.5 cells over naïve cells in response to HCV infection, this growth advantage is only minor as the NS5B-shRNA expressing cells require 12 days to be significantly enriched for a cell population.
Figure 1.
NS5B shRNA expression provides a survival advantage to host Huh-7.5 cells. A) HCV replication at 36 h after infection. Expression of NS5B shRNA resulted in a ~10-fold decrease in HCV replication levels, as reported by Gluc activity of Jc1FLAG(p7-nsGluc2A) HCVcc (Marukian et al., 2008). RLU, relative luciferase units. B) NS5B shRNA-expressing cells (squares), but not irrelevant shRNA-expressing cells (diamonds), were quickly enriched from naïve Huh-7.5 cells after HCVcc infection. This experiment was repeated once with similar results.
To generate a cell line that is able to more efficiently report HCV infection via cell death and thus able to support the more rapid enrichment of HCV-low cells from within a HCV-infected cell population, we introduced the mBid construct into Huh-7.5 cells. For generating a HCV-permissive cell line harboring mBid, HIV lentiviral particles harboring the mBid gene and pseudotyped with the envelope glycoprotein from vesicular stomatitis virus were produced from 293T cells using the pTRIP expression vector (Sirven et al., 2001; Zennou et al., 2000) and used to transduce Huh-7.5 cells to obtain a population of mBid-expressing cells. Ten different clones from this population were isolated and evaluated using our competitive enrichment assay, as described below.
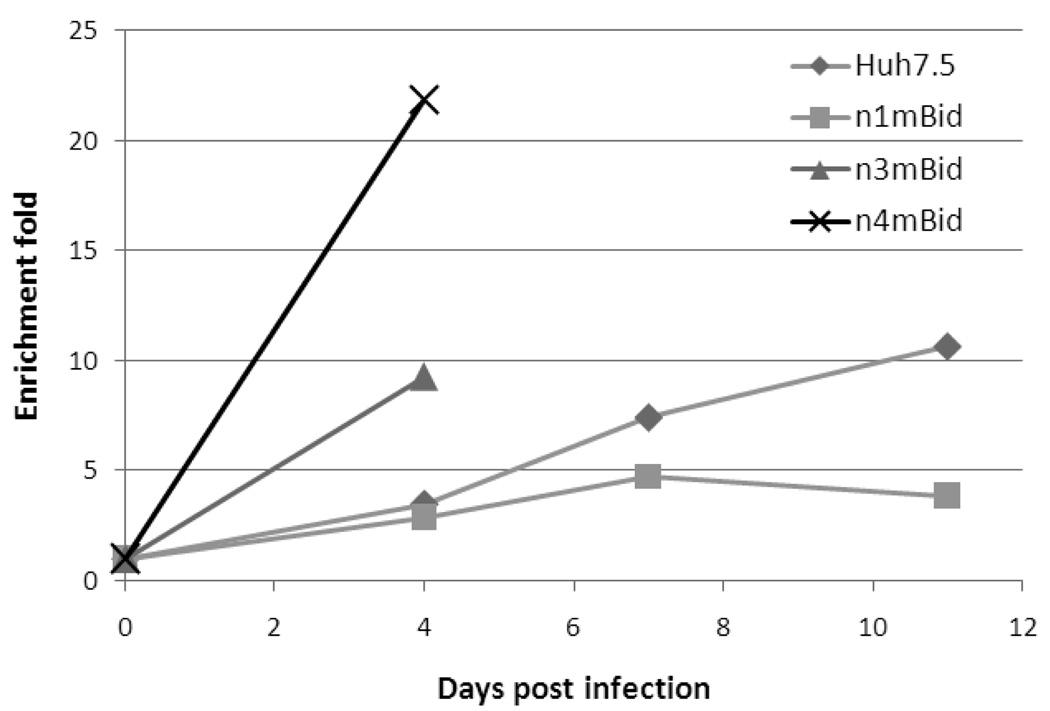
A fraction (~0.5%) of each of the 10 clonally isolated mBid-expressing, Huh-7.5-derived cell lines was transduced with the above-described NS5B shRNA. These cell populations were subsequently challenged with Jc1 HCVcc at MOI <1. Naïve mBid-expressing Huh-7.5 cells underwent more pronounced apoptosis than cells expressing the NS5B shRNA, leading to a rapid increase in the percentage of NS5B shRNA-expressing (GFP+) cells over time. Different rates of enrichment were obtained for different mBid clones after HCV infection. Data obtained from 3 representative mBid clones and naïve Huh-7.5 cells are shown in Figure 2. The observed difference in enrichment rates is likely due to differences in the mBid expression levels and the ability to support HCV spread between different cell lines. The best clone, n4mBid, showed 22-fold enrichment of the HCV-low NS5B shRNA-expressing cells four days post HCV infection (initial representation of NS5B shRNA-containing cells in the population was 0.3%, final representation was 6.6%), in contrast to naïve Huh-7.5 cells which showed only 3.5-fold enrichment over the same time period (0.7% initial representation, 2.4% final). The increased enrichment rate of the HCV-low cells in n4mBid relative to Huh-7.5 is likely due to high levels of mBid expression in n4mBid, leading to high sensitivity to cell death via HCV infection. No cytopathic effect was observed for n4mBid cells in the absence of HCV infection. Some cell clones, especially those showing rapid enrichment of NS5B shRNA-containing cells, were only followed for 4 days (Figure 2) due to the massive cell death induced by HCV infection in these clones, resulting in a scarcity of surviving cells for subsequent analysis.
Figure 2.
Comparison of the fold enrichment of NS5B shRNA-expressing (GFP+) Huh-7.5 and Huh-7.5-derived mBID clones from naïve cells of the same cell line.
The ability of n4mBid cells to support the rapid enrichment of HCV-low cells after HCV infection makes the cell line a candidate for the cell survival-based selection of genetic elements targeting the HCV life cycle. Screening for HCV genetic suppressor elements derived from fragmented HCV genomic RNA is currently under investigation. The pronounced cell-death phenotype exhibited by n4mBid cells upon HCV infection is also valuable for reporting HCV infection in solid- and liquid-based cell assays.
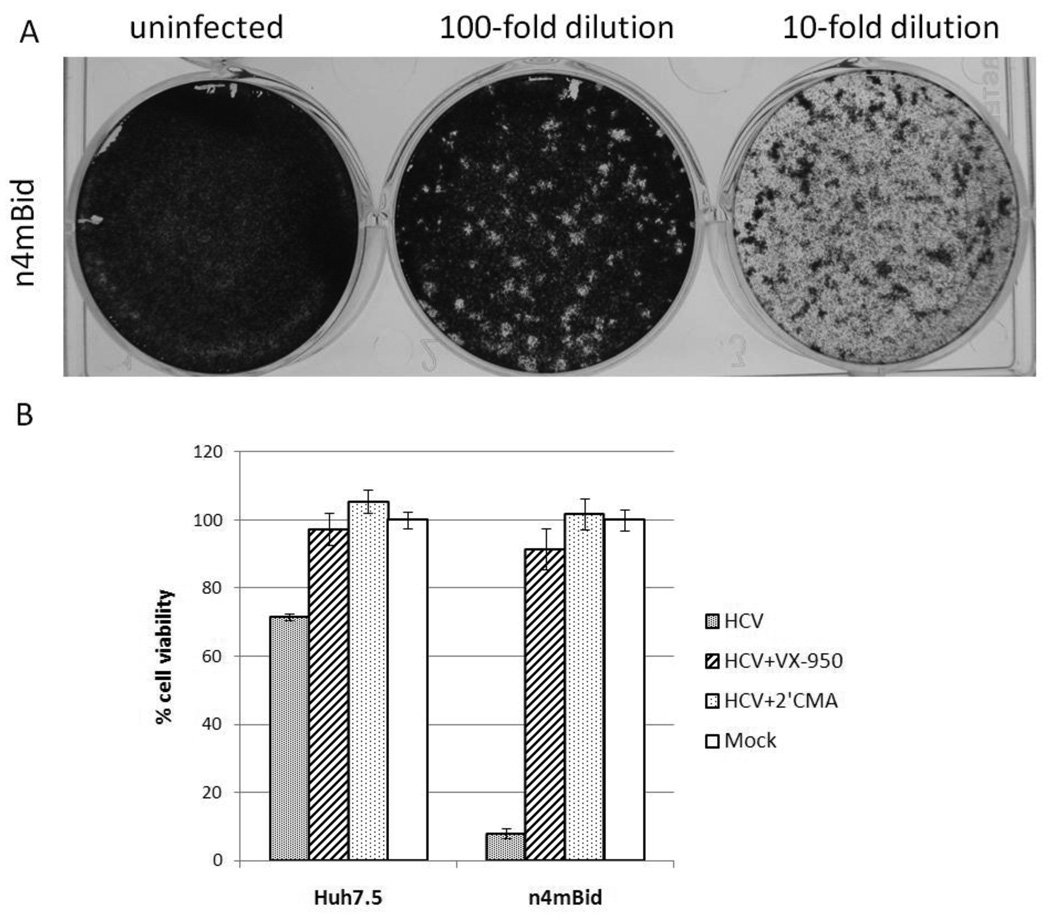
The plaque assay is one of the most widely used techniques for determining infectious virus titers. It is based on the ability of a single infectious virus to form a “plaque” on a confluent monolayer culture of cells. A plaque is formed when one cell is productively infected by a single virus particle leading to the death of the cell. The newly released viral particles can infect and kill surrounding cells. When stained with a dye that only adheres to viable but not dead cells, virus-infected monolayer cultures can display plaques as colorless “halos” against a colored background. We sought to probe the ability of n4mBid cells to undergo plaque formation in response to infection by HCVcc. N4mBid were seeded in 20-mm-diameter plates at a density of 4×105 cells per plate and incubated at 37°C under 5% CO2. Five hours post seeding, Jc1 HCVcc was serially diluted and added to the cell monolayer. The supernatants were removed after overnight incubation and the cell monolayers were overlaid with 3 mL of culture medium (DMEM, 6% FBS, 1× non-essential amino acid, 1× penicillin/streptomycin) containing 0.6 % ultrapure agarose. After an additional 5 days of incubation under normal culture conditions, the cells were fixed with 7% paraformaldehyde followed by staining with 0.02% crystal violet solution for visualization of plaques. Formation of plaques could be seen in wells infected with appropriate dilutions of virus (Figure 3A). No plaques were seen in mock-infected n4mBid cells or HCVcc-infected naïve Huh-7.5 cells (data not shown).
Figure 3.
Application of the n4mBid cell line in solid- or liquid-based assays. A) In a solid-based assay, n4mBid cells showed the formation of individual plaques following HCV infection. B) In a liquid assay, n4mBid cells showed a 10–20 fold difference in viability between the HCV- and mock-infected states, while the parental Huh-7.5 cells showed <2 fold difference during the same 4-day post infection time period, as measured by the CellTiter-Glo assay (Promega). Treatment with the HCV polymerase inhibitor 2’CMA (1 µM) or protease inhibitor VX-950 (1 µM) rescued n4mBid cells from the HCV-induced cytopathic effect. Cell viability is expressed as a percentage of mock-infected cells.
A major drawback of the use of n4mBid cells in a plaque-based method for quantifying HCV titers is the length of the assay - our assay gives results 5 days after infection, while histochemistry-based limiting dilution assays typically provide results at 2–3 days after infection. In addition, as seen in Figure 3A, the plaques in our system are not always clearly defined, making accurate determination of titers difficult. Moreover, since plaque formation relies on the spread of HCVcc, HCV isolates with less rapid spreading kinetics than Jc1 are less likely to form plaques. Given these considerations, we believe that a n4mBid-based plaque assay, although quantitative, should complement, rather than replace, current histochemistry-based limiting dilution assay for virus quantification.
The ability of the n4mBid cell line to report HCV infection in liquid culture was also investigated, since liquid culture assays can be easily extended to high-throughput formats. N4mBid cells were seeded in a 96-well plate at a density of 2.4×104 cells/well and incubated at 37°C under 5% CO2. Simultaneously, these cells were infected with Jc1 HCVcc alone or in the presence of either 1 µM HCV polymerase inhibitor 2’-C-methyladenosine (2’CMA) or HCV protease inhibitor VX-950 (Carroll et al., 2003; Chen and Tan, 2005), or mock-infected. Ninety-six hours post HCV infection, the number of viable cells in each well was quantified using the CellTiter-Glo assay (Promega). Depending on the titer of the infectious Jc1 HCVcc supernatant, a 10–20 fold difference in cell viability between the mock- and HCVcc-infected n4mBid cells was observed, demonstrating a strong HCV-induced cell-death phenotype. Huh-7.5 cells showed <2 fold difference in cell viability between the mock- and HCVcc-infected states under the same conditions (Figure 3B). The cell-death phenotype in both cell types could be rescued by the addition of either 2’CMA or VX-950, further confirming that the observed cell death is due to HCV infection. The significant difference in cell viability between infected and uninfected n4mBid cells demonstrates the potential of the n4mBid cell line to be used in high-throughput screens for HCV antivirals.
In conclusion, we engineered a cell line – n4mBid – that can support the entire life cycle of HCV and exhibit a pronounced death phenotype following HCV infection. N4mBid was generated from the HCV-permissive hepatocellular carcinoma cell line Huh-7.5 and contains a modified version of the pro-apoptotic factor Bid that can be activated by the HCV protease NS3-4A. N4mBid cells demonstrate a far more pronounced cytopathic effect following HCV infection than the parental Huh-7.5 cells, making n4mBid suited to multiple applications, including the enrichment of genetic inhibitors of HCV in a cell survival-based selection system, the quantification of infectious titers of HCV in a plaque assay, and the screening of anti-HCV molecules in a high-throughput format.
Acknowledgements
We thank John Law, Christina Rosas, Cynthia de la Fuente, Patricia Holst and Anesta Webson for laboratory support and technical assistance and Christopher Richardson for providing the modified BID constructs. Financial support for work conducted at RU came from NIH grants (CA057973, AI072613), generous gifts from the Greenberg Medical Research Institute, the Starr Foundation, and the Ellison Medical Foundation. Support for work conducted at TAMU came from new faculty start-up funds from the Artie McFerrin Department of Chemical Engineering and Texas Engineering Experiment Station, and from NIH grant 1R21AI083965-01.
Abbreviations
- HCV
hepatitis C virus
- HCVcc
HCV cell culture
- shRNA
small hairpin RNA
- Gluc
Gaussia luciferase
- 2’CMA
2’-C-methyladenosine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: A perspective on long-term outcome. Semin. Liver Dis. 2000;20:17–35. doi: 10.1055/s-2000-9505. [DOI] [PubMed] [Google Scholar]
- Blight KJ, McKeating JA, Rice CM. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J. Virol. 2002;76:13001–13014. doi: 10.1128/JVI.76.24.13001-13014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukh J, Pietschmann T, Lohmann V, Krieger N, Faulk K, Engle RE, Govindarajan S, Shapiro M, St Claire M, Bartenschlager R. Mutations that permit efficient replication of hepatitis C virus RNA in Huh-7 cells prevent productive replication in chimpanzees. Proc. Natl. Acad. Sci. USA. 2002;99:14416–14421. doi: 10.1073/pnas.212532699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SS, Tomassini JE, Bosserman M, Getty K, Stahlhut MW, Eldrup AB, Bhat B, Hall D, Simcoe AL, LaFemina R, Rutkowski CA, Wolanski B, Yang Z, Migliaccio G, De Francesco R, Kuo LC, MacCoss M, Olsen DB. Inhibition of hepatitis C virus RNA replication by 2'-modified nucleoside analogs. J. Biol. Chem. 2003;278:11979–11984. doi: 10.1074/jbc.M210914200. [DOI] [PubMed] [Google Scholar]
- Chen SH, Tan SL. Discovery of small-molecule inhibitors of HCV NS3-4A protease as potential therapeutic agents against HCV infection. Curr. Med. Chem. 2005;12:2317–2342. doi: 10.2174/0929867054864769. [DOI] [PubMed] [Google Scholar]
- Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- Gottwein JM, Scheel TK, Hoegh AM, Lademann JB, Eugen-Olsen J, Lisby G, Bukh J. Robust hepatitis C genotype 3a cell culture releasing adapted intergenotypic 3a/2a (S52/JFH1) viruses. Gastroenterology. 2007;133:1614–1626. doi: 10.1053/j.gastro.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Hsu EC, Hsi B, Hirota-Tsuchihara M, Ruland J, Iorio C, Sarangi F, Diao J, Migliaccio G, Tyrrell DL, Kneteman N, Richardson CD. Modified apoptotic molecule (BID) reduces hepatitis C virus infection in mice with chimeric human livers. Nat. Biotechnol. 2003;21:519–525. doi: 10.1038/nbt817. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Yi M, Li K, Lemon SM. Selectable subgenomic and genome-length dicistronic RNAs derived from an infectious molecular clone of the HCV-N strain of hepatitis C virus replicate efficiently in cultured Huh7 cells. J. Virol. 2002;76:2997–3006. doi: 10.1128/JVI.76.6.2997-3006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbach BD, Evans MJ, Syder AJ, Wölk B, Tellinghuisen TL, Liu CC, Maruyama T, Hynes RO, Burton DR, McKeating JA, Rice CM. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- Lindenbach BD, Rice CM. Unravelling hepatitis C virus replication from genome to function. Nature. 2005;436:933–938. doi: 10.1038/nature04077. [DOI] [PubMed] [Google Scholar]
- Marukian S, Jones CT, Andrus L, Evans MJ, Ritola KD, Charles ED, Rice CM, Dustin LB. Cell culture-produced hepatitis C virus does not infect peripheral blood mononuclear cells. Hepatology. 2008;48:1843–1850. doi: 10.1002/hep.22550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietschmann T, Kaul A, Koutsoudakis G, Shavinskaya A, Kallis S, Steinmann E, Abid K, Negro F, Dreux M, Cosset FL, Bartenschlager R. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc. Natl. Acad. Sci. USA. 2006;103:7408–7413. doi: 10.1073/pnas.0504877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietschmann T, Lohmann V, Kaul A, Krieger N, Rinck G, Rutter G, Strand D, Bartenschlager R. Persistent and transient replication of full-length hepatitis C virus genomes in cell culture. J. Virol. 2002;76:4008–4021. doi: 10.1128/JVI.76.8.4008-4021.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirven A, Ravet E, Charneau P, Zennou V, Coulombel L, Guétard D, Pflumio F, Dubart-Kupperschmitt A. Enhanced transgene expression in cord blood CD34(+)-derived hematopoietic cells, including developing T cells and NOD/SCID mouse repopulating cells, following transduction with modified trip lentiviral vectors. Mol. Ther. 2001;3:438–448. doi: 10.1006/mthe.2001.0282. [DOI] [PubMed] [Google Scholar]
- Strasser A. The role of BH3-only proteins in the immune system. Nat. Rev. Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao ZJ, Murthy K, Habermann A, Kräusslich HG, Mizokami M, Bartenschlager R, Liang TJ. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 2005;11:791–905. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasley A, Alter MJ. Epidemiology of hepatitis C: Geographic differences and temporal trends. Semin. Liver Dis. 2000;20:1–16. doi: 10.1055/s-2000-9506. [DOI] [PubMed] [Google Scholar]
- Wiznerowicz M, Trono D. Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J. Virol. 2003;77:8957–8961. doi: 10.1128/JVI.77.16.8957-8961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zennou V, Petit C, Guetard D, Nerhbass U, Montagnier L, Charneau P. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell. 2000;101:173–185. doi: 10.1016/S0092-8674(00)80828-4. [DOI] [PubMed] [Google Scholar]
- Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, Wieland SF, Uprichard SL, Wakita T, Chisari FV. Robust hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. USA. 2005;102:9294–9299. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Gastaminza P, Chung J, Stamataki Z, Isogawa M, Cheng G, McKeating JA, Chisari FV. Persistent hepatitis C virus infection in vitro: coevolution of virus and host. J. Virol. 2006;80:11082–11093. doi: 10.1128/JVI.01307-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann KC, Bonzon C, Green DR. The machinery of programmed cell death. Pharmacol. Ther. 2001;92:57–70. doi: 10.1016/s0163-7258(01)00159-0. [DOI] [PubMed] [Google Scholar]