Abstract
The general features of neuroplasticity are developmentally regulated. Although it has been hypothesized that the loss of plasticity in mature neurons may be due to synaptic saturation and functional reduction of NMDA receptors (NMDAR), the molecular mechanisms remain largely unknown. We examined the effects of NMDAR activation and KCl-mediated membrane depolarization on ERK1/2 signaling following in vitro maturation of cultured cortical neurons. Although NMDA stimulated robust increase of intracellular calcium at both DIV (day in vitro) 3 and 14, the activation of ERK1/2 and CREB was impaired at DIV 14. Specifically, the phosphorylation of ERK1/2 was stimulated by both NMDA and KCl at DIV 3. However, at DIV 14, NMDA-, but not KCl-stimulated ERK1/2 and CREB phosphorylation was significantly diminished. Consistently, the NMDA-induced transcription of ERK/CREB-regulated genes Bdnf exon 4, Arc and zif268 was significantly attenuated at DIV 14. Moreover, compared to DIV 3 neurons, the basal level of phosphorylated-ERK1/2 in DIV 14 neurons increased tremendously following maturation, and was more susceptible to dephosphorylation. Blocking calcium channels by nifedipine or NMDAR by APV caused more dramatic ERK dephosphorylation in DIV 14 neurons. We further demonstrate that the loss of plasticity-related signaling is unrelated to NMDA-induced cell death of the DIV 14 neurons. Taken together, these results suggest that the attenuation of certain aspects of neuroplasticity following maturation may be due to the reduction of NMDAR-mediated gene transcription and a saturation of ERK1/2 activity.
Keywords: NMDA receptors, BDNF, ERK, CREB, Ca2+-stimulated signaling, neuronal maturation
INTRODUCTION
Neurons and their synapses undergo refinement during development. These changes lead to dramatic modifications in both synaptic and nuclear function, which are required for neuroplasticity, an important feature of the brain to accommodate neuronal activities. Although it has been strongly implicated that brain maturation and aging results in the loss of certain aspects of plasticity, the molecular and cellular mechanisms are largely unknown. Most of the cellular insights have been obtained by investigating long-term potentiation (LTP) and long-term depression (LTD) (Malenka and Bear 2004). Interestingly, NMDA receptor (NMDAR)-mediated, but not L-type voltage-gated calcium channel (L-VGCC)-mediated LTP, is significantly impaired in the aged hippocampus (Bach et al. 1999; Rosenzweig et al. 1997; Shankar et al. 1998). In contrast, LTD and depotentiation are more readily induced in the aged brains (Norris et al. 1996). Therefore, it has been postulated that the threshold for LTP is higher, and the threshold for LTD and depotentiation is lower following brain maturation (Foster and Norris 1997; Kemp and Bashir 2001; Rosenzweig and Barnes 2003). However, the molecular basis for such “thresholds” is not identified. Furthermore, there are few cellular studies on how maturation affects activity-dependent responses in stimulated neurons.
It has been hypothesized that, as the major regulatory event in stimulated neurons, the developmental changes in Ca2+ -stimulated signals may modulate the intrinsic properties of neurons during maturation (Ghosh and Greenberg 1995; Konur and Ghosh 2005). Upon neuronal stimulation, the elevation of intracellular Ca2+ ([Ca2+]i) initiates many signaling cascades, including a rapid modification of synaptic function and a delayed activation of plasticity-related gene transcription (Ghosh and Greenberg 1995; Ginty 1997; West et al. 2001). Due to the properties of these signaling pathways, it has been further suggested that the function of Ca2+-stimulated protein kinases play a major role in regulating plasticity in the stimulated neurons. For example, it is well documented that calcium influx through NMDAR or L-VGCC activates Ras-MEK-ERK1/2 signaling. The activation of ERK1/2 may further up-regulate the transcription factor CREB (cAMP responsive element binding protein) and CREB-mediated gene transcription. These molecular events in the nucleus are tightly coupled to, and required for certain aspects of neuronal function, such as neuroplasticity, differentiation and survival (Kitagawa 2007; Lopez de Armentia et al. 2007; Peltier et al. 2007; Waltereit and Weller 2003).
Activity-dependent transcription of immediate early genes (IEGs), such as Bdnf (Aid et al. 2007; West et al. 2002), zif268 (also known as egr1) (Bozon et al. 2002), and Arc (Lanahan and Worley 1998), is induced by neuronal activation, and depends on the Ca2+-stimulated activity of ERK1/2 and CREB (Impey et al. 1998). Importantly, suppression of ERK1/2 causes defective LTP, imparied memory formation, as well as attenuated neuronal survival (English and Sweatt 1997; Sweatt 2004; Winder et al. 1999; Ying et al. 2002). The nuclear function of ERK1/2 has been suggested by its regulation of the transcription factors CREB and SRF (serum responsive factor). Ca2+ stimulation of the proto-oncogene Ras significantly activates ERK1/2 through phosphorylation on T202/Y204, which in turn activates Rsk, phosphorylates CREB at S133, and promotes CREB-mediated transcription (Davis et al. 2000; Mazzucchelli and Brambilla 2000). The activation of ERK1/2 also stimulates Elk1 and leads to SRE (serum responsive element)-mediated transcription.
To investigate the molecular mechanisms underlying the plasticity-related signaling following maturation, we examined the phosphorylation of ERK1/2 (at T202/Y204) and CREB (at S133) as well as ERK/CREB-mediated transcription in developing cortical neurons in culture. We used culture conditions that allowed for the naturally occurring spontaneous activity. Our findings reveal a reduction of NMDAR-mediated signal transduction following in vitro neuronal maturation.
MATERIALS AND METHODS
Primary neuronal culture
Cortices and hippocampi were dissected from postnatal day 0 Sprague Dawley rats, and prepared for culturing as described previously (Zheng et al. 2008). After dissection, tissues were chopped into 1 mm3 pieces, and digested with 10 units/ml papain (Worthington, Freehold, NJ) and 100 units/ml DNase I (Roche) in dissociation buffer (82 mM Na2SO4, 30 mM K2SO4, 5.8 mM MgCl2, 0.25 mM CaCl2, 20 mM glucose, 0.001% phenol red, 0.45 mg/ml cysteine, and 1.5 mM HEPES, pH 7.6) at 37°C for 30–40 min. The digestion was washed with dissociation buffer and triturated with Neurobasal A medium (Invitrogen, Carlsbad, CA). The cells were seeded on poly-D-lysine (50ug/ml, Sigma, St. Louis, MO) coated plates. One hour after plating, Neurobasal A was replaced with growth media including Neurobasal A, 1x B27 supplement (Invitrogen), 100 units/ml penicillin, 0.1 mg/ml streptomycin, and 0.5 mM glutamine. One-third of the medium was replenished every 3 days during the culturing of neurons. We excluded tetrodotoxin from the culture medium to allow for endogenous synaptic activity. Therefore, the basal activity and induction of ERK1/2, and CREB phosphorylation represent the natural profiles for cultured neurons.
Neuronal stimulation
To induce membrane depolarization, cortical or hippocampal neurons were treated with KCl (50mM). We used bath incubation of NMDA (50 uM)/glycine (2 uM) to activate NMDAR. Because Ca2+-stimulated phosphorylation of ERK1/2 and CREB is mainly mediated by NMDAR and L-VGCC (West et al. 2001; West et al. 2002), we pre-treated neurons with nifedipine (an L-VGCC antagonist at 5 uM) for 30 min before NMDA/glycine application to eliminate the effects of L-VGCC. Similarly, a 30 min pre-treatment with APV (an NMDAR antagonist at 100 uM) was used before KCl stimulation to exclude the role of NMDAR. In all pre-treatments, CNQX (40 uM) was included to block non-NMDA type glutamate receptors. To address the role of protein phosphatases (PP) in Ca2+-stimulated ERK/CREB phosphorylation and ERK/CREB-mediated gene transcription, okadaic acid (1uM) was co-applied with NMDA/glycine. All drugs were added directly to the medium, and during incubation the neurons were kept in the 5% CO2 incubator.
Western blot and quantification
Neurons were grown on 12-well plates. After KCl or NMDA/glycine stimulation, neurons (cortical or hippocampal) were extracted with 50 ul of SDS sample buffer (10 mM Tris-HCl buffer pH 6.8, 10% glycerol, 2% sodium dodecylsulfate, 0.01% bromophenol blue and 5% mercaptoethanol) per well, and boiled at 100°C for 10 min. The extracts (from 3 × 105 cells) were separated by 10% SDS-PAGE, transferred to nitrocellulose membranes (Pierce), and blocked with 5% skim milk in PBS-T (PBS, 0.1% triton X100) for 30min at room temperature. The membranes were then incubated with primary antibodies against phosphorylated ERK1/2 (p-ERK1/2) (1:1000, Cell Signaling Technology, Beverly, MA), and phosphorylated-CREB (p-CREB) (1:1000, Upstate, Lake Placid, NY) in PBS-T overnight at 4°C. After extensive washing and incubation with horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibodies (1:5000, Pierce, Rockford, IL), the signals were visualized by chemiluminescence methods (SuperSignal® West Pico, Pierce, Rockford, IL). For the purpose of normalization, the membranes were stripped, and re-probed with antibodies against total ERK1/2 (T-ERK1/2) (1:1000, Cell Signaling Technology, Beverly, MA), total CREB (T-CREB) (1:500, Upstate, Lake Placid, NY), or β-actin (1:10,000, Sigma, St. Louis, MO). Several exposure times were used to obtain signals in the linear range. The bands were quantified using Scion Image Beta 4.0.2 software (Scion Corp. Frederick, Maryland).
Semi-quantitative RT-PCR
To measure the activity-induced transcription of immediate early genes (Arc, zif268 and Bdnf exon 4), control and stimulated neurons were lysed, and total RNA was purified by the TRIzol method (Invitrogen). The cDNA was synthesized from 1 ug total RNA by the reverse transcription (RT) kit SuperScript (Invitrogen), and subjected to PCR. The amount of Arc, zif268, and Bdnf exon 4 mRNA was determined by semi-quantitative RT-PCR using gene-specific primers. The primers used for Arc are: AGACACAGCAGATCCAGCTG (forward) and TGGCTTGTCTTCACCTTCAG (reverse). These primers result in a 420bp PCR product. The primers used for Bdnf Exon 4 are: CTCCGCCATGCAATTTCCAC (forward), and GCCTTCATGCAACCGAAGTA (reverse). These primers result in a 274bp PCR product. The primers used for zif268 are: AACTGGAGGAGATGATGCTG (forward) and ATGAAGCAGCTGGAGAAGGC (reverse). These primers result in a 435bp PCR product. RT-PCR product of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal control. Primers, 5'-TCCATGACAACTTTGGCATTGTGG-3' and 5'-GTTGCTGTTGAAGTCG CAGGAGAC-3', were used for GAPDH. The cycle number for Arc and zif268 is 25. The cycle number for Bdnf exon 4 is 26. The cycle number for GAPDH is 20. The annealing temperature is 55 °C for all genes except for zif268 (62 °C). The melting temperature is 94 °C for all genes. The RT-PCR products were analyzed by agarose gel electrophoresis, and quantified by Scion Images.
Calcium imaging of cultured neurons
Cultured cortical neurons were used for calcium imaging on DIV 3 or DIV 14 as described previously (Nunez and McCarthy 2007). The cell permeable fluorescent indicator fura-2 acetoxymethyl ester (fura-2-AM) was used to measure intracellular calcium. Cells were loaded with fura-2-AM (3 uM) in DMSO (<0.5%) and incubated for 30 min. The fura-2-AM-loaded coverslips were placed in a perfusion chamber and mounted on a Nikon Eclipse TE-2000U inverted microscope. Cells were then rinsed for 30 min with physiological saline solution (PSS) (137 mM NaCl, 5 mM KCl, 1 mM MgCl2, 3 mM CaCl2, 10 mM HEPES, 25 mM Glucose) at 32–34°C to remove the extracellular dye, and to obtain the baseline value of [Ca2+]i. After the stable baseline was achieved, neurons were perfused with CNQX/APV (for 10 min) before the application of KCl/CNQX/APV (for 5 min), or perfused with CNQX/nifedipine (for 10 min) before the application of NMDA/glycine/CNQX/nifedipine (for 5 min). The perfusion buffer was PSS for calcium imaging. Illumination was provided by a Sutter DG-5 high-speed wavelength switcher while fluorescent images were acquired with a Roper Coolsnap Cascade 512B cooled CCD camera. Intracellular calcium concentrations were calculated from the ratio of background corrected fura-2 emission (520 nm) at two excitation wavelengths (340 nm/380 nm). Universal Imaging Metamorph/Metafluor software was used for all image acquisition and analysis.
Analysis of cell death
Cultured neurons were pre-treated with CNQX(40 uM) and nifedipine (5 uM) for 30 min, and then treated with NMDA (at 10 or 50 uM) along with glycine (2 uM), CNQX and nifedipine for 30 min. Twenty hours after the treatment, neurons were fixed with 6% PFA, permeabilized with PBS/0.5% Triton X-100, and stained with the DNA binding dye DAPI (1 ug/ml). Nuclei with condensed staining were used as an indication for cell death.
Data analysis
The results were analyzed among groups using the post hoc Student–Newman–Keuls procedure for multiple comparisons. Student's paired t-test was used to assess significance for data with two groups. Data are expressed as average +/− standard deviation (SD). Differences with p-values less than 0.05 were considered significant.
RESULTS
The NMDAR-mediated activation of ERK1/2 and CREB is ablated following in vitro neuronal maturation
Primary neuronal cultures display certain features of neural development. The in vitro development stages were experimentally defined (Dotti et al. 1988). Depending on the culturing conditions, neurons may show fully matured features (stage 5) as early as after 7 days of in vitro development (Dotti et al. 1988). However, significant synaptic formation extends beyond DIV 7. Sala et al. demonstrated significant differences in CREB activation between DIV 7 and DIV 14 cultured neurons (Sala et al. 2000). Under our culture conditions, we observed significant dendrite and axon formation without fully developed synapses in DIV 3 neurons. At DIV 14, significant spontaneous synaptic activity was apparent (see Fig. 5, Fig. 8). Therefore, we chose DIV 3 and 14 as two distinct development stages during in vitro maturation to study Ca2+-stimulated signaling.
Fig. 5.
Total expression and the phosphorylation of ERK1/2 are significantly up-regulated following in vitro maturation. Primary cortical cultures were examined for the level of p-ERK1/2 and total ERK1/2 at DIV 3, 6 and 14. Representative Western blots are shown in (a). The relative levels of total ERK1/2 (T-ERK1/2) were normalized to beta-actin and quantified (n=5 from each group) (b). To obtain the profile for ERK1/2 activation during the development, the level of p-ERK1/2 was normalized with T-ERK1/2 (c). The relative expression level at DIV 3 was arbitrarily set as 1. Data are average ± S.D. (d) and (e), DIV 14 neurons show more dephosphorylation of p-ERK1/2 when L-VGCC and NMDAR are blocked. Samples were collected from the control and nifedipine/CNQX- or APV/CNQX-treated neurons, and analyzed for the level of p-ERK1/2. The representative Western blots are shown in the upper panels, and quantification in the lower panels (average +/− SD, n=4). (f) and (g), traces of the F340/380 ratios (measured once every 5 sec) show [Ca2+]i levels in DIV 3 (f) and 14 (g) cortical neurons. Traces were selected randomly for 3 neurons. The duration of nifedipine/CNQX treatment is shown as indicated.
Fig. 8.
The transcriptional up-regulation of zif268, Arc, and bdnf exon 4 requires prolonged synaptic activation by bicuculline. DIV 3 or 14 neurons were treated with bicuculline (50 uM) and analyzed for p-ERK and p-CREB (a) 30 min after stimulation (n=3). For b and c, the transcription of zif268, Arc, and Bdnf exon 4 was analyzed by RT-PCR. Samples were collect 1 hr (b) or 4 hrs (c) after bicuculline stimulation (n=4). RT-PCR did not reveal any transcriptional up-regulation 1 hr after bicuculline stimulation. The representative Western blots or RT-PCR results are displayed in the left panels. The quantifications are shown in the right panels (average +/1 SD; *:p<0.05). The development stages (DIV 3 and DIV 14) and treatments are indicated in the figure.
To examine Ca2+-stimulated ERK1/2 activation, we used two simple stimulation approaches to induce Ca2+ influx. First, membrane depolarization was achieved by bath incubation with KCl. Second, bath incubation with NMDA/glycine was used to activate NMDAR. At DIV 3, cortical neurons displayed robust activation of p-ERK1/2 after both KCl-induced membrane depolarization and NMDA/glycine application (Fig. 1a, and data not shown). However, NMDA/glycine failed to activate p-ERK1/2 at DIV 14 (data not shown). In addition, we noticed that the fold increase of KCl-induced ERK1/2 phosphorylation was less in DIV 14 neurons than in DIV 3 neurons (Fig. 1a). Hippocampal neurons showed similar activation profile for p-ERK1/2 (supplementary Fig. 1).
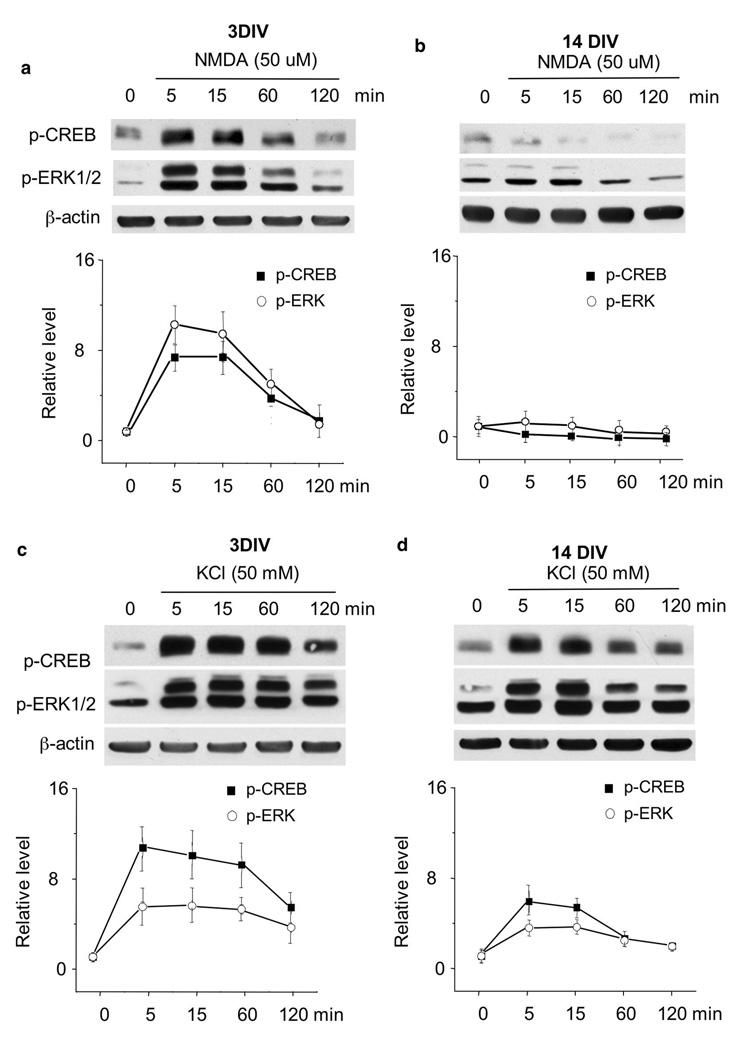
Fig. 1.
The activation of ERK1/2 and CREB declines following in vitro maturation. Cortical neurons were stimulated by 50 mM KCl (a, b, and c) or 50 uM NMDA2 uM glycine (b and c) at DIV3 and 14. (a). KCl stimulated ERK1/2 phosphorylation at both DIV 3 and 14. For (b) and (c), neurons were pretreated with CNQX and nifedipine, or CNQX and APV, as indicated, for 30min before the stimulation by KCl (along with APV and CNQX) and NMDA/glycine (along with nifedipine and CNQX). Samples were collected 15 min after stimulation. The signal of p-ERK1/2 was normalized with total ERK1/2 or beta-actin. The signal of p-CREB was normalized with beta-actin. Data are average +/− SD (n=5 for each group).
The activation of NMDAR and L-VGCC is strongly implicated in regulating many forms of plasticity, including activity-dependent gene transcription (West et al. 2001; West et al. 2002; Xia et al. 1996). To discriminate between the roles of NMDAR- and L-VGCC-mediated activation, we measured the activation of p-ERK1/2 in neurons pre-treated with APV (NMDAR antagonist) or nifedipine (L-VGCC blocker). We also included CNQX in the pre-treatment to eliminate the function of non-NMDA type glutamate receptors. NMDAR-mediated (with L-VGCC blocked by nifedipine) ERK1/2 phosphorylation was observed at DIV 3 but not DIV 14 (Fig. 1b and c). In contrast, membrane depolarization by KCl (with NMDAR blocked by APV) activated ERK1/2 phosphorylation at both DIV 3 and 14 (Fig. 1b and c).
One major downstream target of ERK1/2 signaling is the transcription factor CREB, which regulates many aspects of plasticity (such as LTP and memory formation), development, and neural survival (Kitagawa 2007; Lopez de Armentia et al. 2007; Peltier et al. 2007; Waltereit and Weller 2003). Here, we demonstrate a significant decline in NMDAR-mediated CREB phosphorylation following maturation. While membrane depolarization stimulated CREB phosphorylation at both DIV 3 and 14, the NMDAR-mediated p-CREB was observed in DIV 3 (Fig. 1b) but not DIV 14 neurons (Fig. 1c).
To determine how the ERK1/2 signaling pathway responds to NMDAR activation following neuronal maturation, we measured the phosphorylation of ERK1/2 and CREB at different time points after stimulation. As shown in Fig. 2, the activation of both p-ERK1/2 and p-CREB in DIV 3 neurons was very rapid. The peak activation occurred 5min after NMDA (with L-VGCC blocked by nifedipine) application. The ERK1/2 activation lasted for at least 120 min, and CREB activation lasted for at least 60 min (Fig. 2a). In contrast, neither transient nor persistent activation was observed for p-ERK1/2 or p-CREB in DIV 14 neurons after NMDA stimulation (Fig. 2b). We also noticed that, compared to control neurons, the level of p-ERK1/2 and p-CREB showed significant reduction at 120min in DIV 14 neurons (Fig. 2b, p<0.05).
Fig. 2.
Time course of ERK1/2 and CREB activation. DIV 3 (a and c) and 14 (b and d) cortical neurons were stimulated by NMDA or KCl as indicated. The samples were collected 5 min, 15 min, 60 min, and 120 min after stimulation, and the level of p-ERK1/2 and p-CREB was analyzed by Western blots. For (a) and (b), the neurons were pre-treated with CNQX and nifedipine for 30min before the application of NMDA/glycine (along with CNQX and nifedipine). For (c) and (d), neurons were pre-treated with CNQX and APV for 30 min before the application of KCl (along with CNQX and APV). The levels of p-ERK1/2 and p-CREB were normalized to beta-actin. The representative Western blots are shown in the upper panels, and the quantification in the lower panels. Data are average +/− SD (n=5 for each group).
Although membrane depolarization by KCl (with NMDAR blocked by APV) stimulated p-ERK1/2 and p-CREB in both DIV 3 and 14 neurons, neurons at DIV 14 were less responsive. First, the fold-increase in KCl-induced p-ERK1/2 and p-CREB was less for DIV 14 neurons than DIV 3 neurons at every time point (Fig. 2c and d). Secondly, the ERK1/2 and CREB phosphorylation started to decline at an earlier time point for DIV 14 neurons. For example, the peak level of ERK1/2 and CREB phosphorylation persisted for up to 60min after KCl stimulation in DIV 3 neurons (Fig. 2c). In DIV 14 neurons, the level of p-ERK1/2 and p-CREB declined significantly at 60min (Fig. 2d).
The NMDAR-mediated transcription of plasticity-related genes is diminished following in vitro neuronal maturation
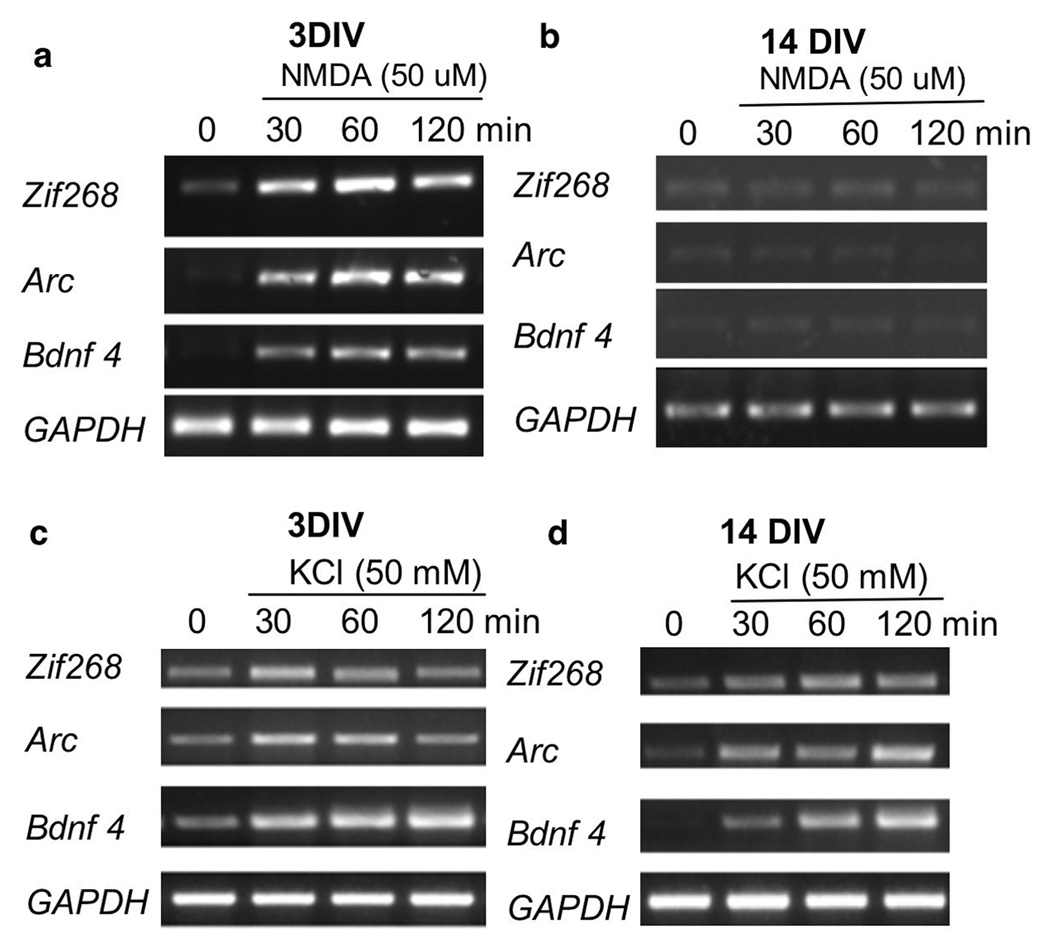
To determine the functional relevance of the impaired ERK/CREB signaling following maturation, we measured the transcription of several plasticity-related immediate early genes, including Arc, zif268, and Bdnf exon 4. Molecular studies on the promoter region of these genes implicate the regulatory role of ERK1/2 and CREB (Rolli et al. 1999; Tao et al. 1998; Waltereit et al. 2001). In fact, the transcription of these immediate early genes is tightly regulated by neuronal activity. Both in vivo experience (such as learning and seizure) and in vitro stimulation (such as NMDA and KCl treatment) lead to significant transcriptional up-regulation of Arc, zif268 and Bdnf exon 4 (Lanahan and Worley 1998).We analyzed the transcription profile of Arc, zif268 and Bdnf exon 4 at 30 min, 60 min and 120 min after KCl or NMDA/glycine stimulation in DIV 3 and 14 neurons. We pre-treated the neurons with nifedipine and CNQX (for 30 min) before NMDA/glycine application. We pre-treated the neurons with APV and CNQX (for 30 min) before KCl application. Because we could not detect any significant up-regulation at 15 min (data not shown), 30min was chosen for the earliest time point. Consistent with Fig. 1, NMDAR activation stimulated significant transcription at all time point in DIV 3 neurons (Fig. 3a; n=4, p<0.05), no NMDAR-mediated transcription was observed in DIV 14 neurons (Fig. 3b). In contrast, membrane depolarization (with the NMDAR blocked by APV) stimulated significant transcription of Arc, zif268, and Bdnf exon 4 in both DIV 3 and DIV 14 neurons (Fig. 3c and d; n=4, p<0.05).
Fig. 3.
NMDA-stimulated transcription of Arc, zif268, and Bdnf exon 4 declines following maturation. DIV 3 (a and c) and 14 (b and d) cortical neurons were stimulated by 50 mM KCl or 50 uM NMDA/2 uM glycine, as indicated. The samples were collected 30 min, 60 min, and 120 min after stimulation, and the mRNA levels of Arc, zif268, and Bdnf exon 4 were analyzed by semi-quantitative RT-PCR. For (a) and (b), the neurons were pre-treated with CNQX and nifedipine for 30 min before the application of NMDA/glycine (along with CNQX and nifedipine). For (c) and (d), neurons were pre-treated with CNQX and APV for 30 min before the application of KCl (along with CNQX and APV). Similar results were obtained from 4 independent experiments.
NMDAR activation and membrane depolarization lead to significant elevation of intracellular Ca2+ in mature neurons
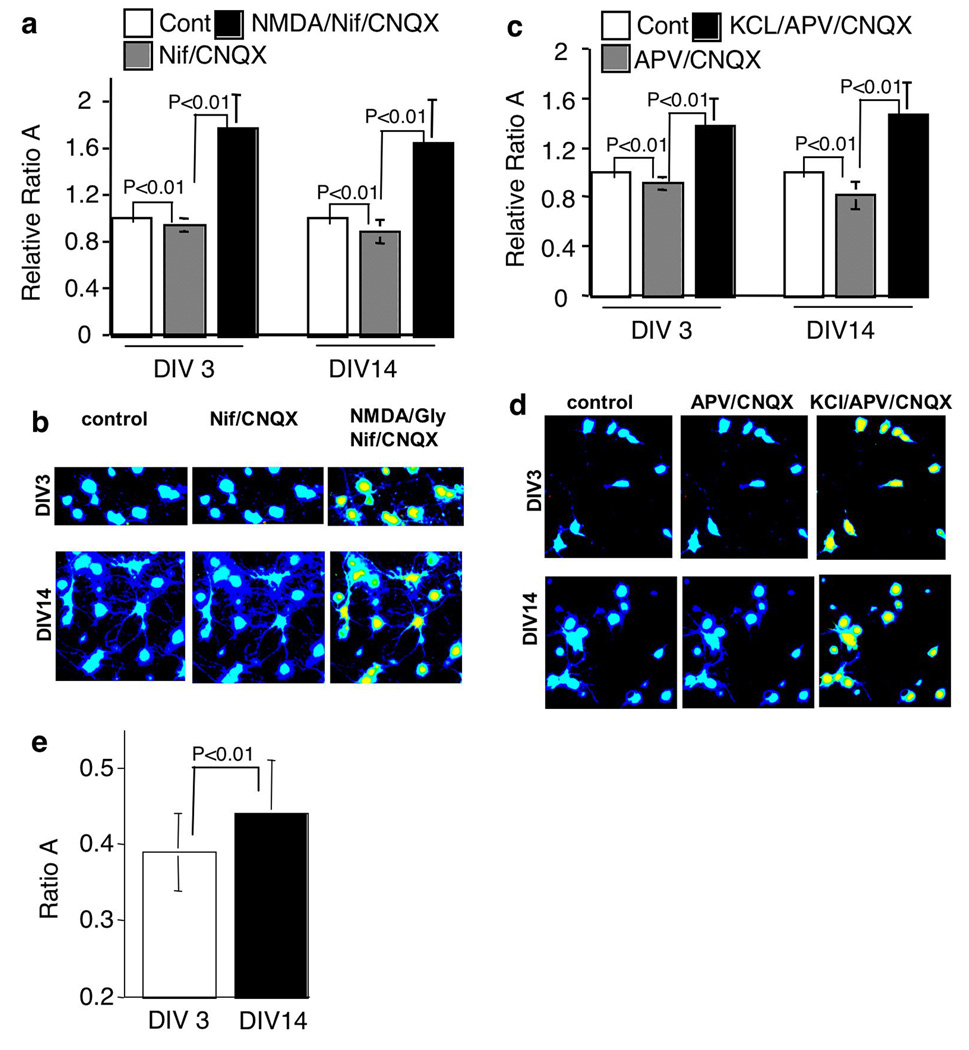
A simple possibility for the decline in Ca2+-stimulated signal transduction and gene expression is that the activity-dependent dynamics of [Ca2+]i is compromised following maturation. Therefore, we used intracellular Ca2+ imaging to further examine NMDA- and KCl-stimulated Ca2+ influx at different developmental stages. As shown in Fig. 4, we did not detect significant difference between DIV 3 and 14 neurons. Compared to the non-treated control neurons, NMDAR activation caused a 78 +/− 28% (average +/− SD) increase of the F340/380 signal in DIV3 neurons, and a 64 +/−37% (average +/− SD) increase in DIV 14 neurons. If normalized to the F340/380 signal in neurons pretreated with nifedipine/CNQX, the increase was 89 +/− 27% (average +/− SD) for DIV 3, and 86 +/− 38% (average +/− SD) for DIV 14 (Fig. 4a and b).
Fig. 4.
Membrane depolarization and NMDAR activation induce significant calcium influx in mature neurons. The intracellular Ca2+ concentration, [Ca2+]i, was measured by the Fura ratio (F340/380) in cortical neurons at DIV 3 and 14. For (a) and (b), baseline level of [Ca2+]i was obtained when perfused with PPS, then the neurons were perfused with nifedipine and CNQX in PPS for 10min. The peak level of [Ca2+]i was measured 5 min after NMDA (50 uM)/glycine (2 uM) (in PPS containing nifedipine and CNQX) stimulation. For (c) and (d), neurons were perfused with APV and CNQX in PPS for 10 min. The peak value of [Ca2+]i was measured 5 min after KCl (in PPS containing APV and CNQX) stimulation. The ratio of F340/380 was arbitrarily set to 1 for the untreated control neurons, and the relative changes in F340/380 ratio with stimulated neurons was normalized to the control level. Quantifications are shown in (a) and (c) (expressed as average +/− SD). Pseudo-colored images of neurons before (control) and after treatments (as indicated) are shown in (b) and (d). The absolute ratios of F340/380 for untreated control neurons at DIV 3 and 14 are shown in (e) (expressed as average +/− SD).
Compared to the non-treated control neurons, membrane depolarization caused a 39 +/− 22% (average +/− SD) increase of F340/380 in DIV 3 neurons, and a 47 +/−26% (average +/− SD) increase in DIV 14 neurons. If normalized to the F340/380 value in neurons pretreated with APV/CNQX, the increase was 50 +/− 22% (average +/− SD) on DIV 3, and 82 +/− 27% (average +/− SD) on DIV 14 (Fig. 4c and d).
These data implicate that there is no direct correlation between the activation of ERK1/2 signaling and [Ca2+]i transient following neuronal stimulation. Thus, we speculate that the intrinsic properties of NMDAR-mediated ERK1/2 signaling may be altered following maturation.
The basal level of p-ERK1/2 elevates following maturation and is more susceptible to dephosphorylation
It has been hypothesized that the loss of neuronal response and plasticity may be attributed to signal saturation. The data in Fig. 1 and 2 suggest that the level of p-ERK1/2 at DIV 14 is higher than DIV 3. We also noticed that the basal level of [Ca2+]i in DIV 14 neurons was higher than DIV 3 neurons (Fig. 4e). Therefore, we examined the developmental profile of ERK1/2.
The level of activated ERK1/2, p-ERK1/2, was low in 3 DIV cortical neurons (Fig. 5a). It was significantly enhanced at 6 DIV, and further elevated at 14 DIV (Fig. 5a and c). Total ERK1/2 expression also gradually increased in the later developmental stages, T-ERK1/2 level roughly doubled in DIV 14 neurons (as compared to DIV 3 neurons) (Fig. 5a and b). A similar profile was observed in cultured hippocampal neurons (Supplementary Fig. 2). After normalization to total ERK1/2, the activated form (p-ERK1/2) was still significantly increased following maturation of both cortical (Fig. 5c) and hippocampal neurons (Supplementary Fig. 2).
On one hand, the elevation of basal p-ERK1/2 leaves less room for further activation following neuronal stimulation, which is implicated in Fig. 1 and supplementary Fig. 1. Membrane depolarization resulted in a 9.2 +/− 2.3 fold increase in p-ERK1/2 in DIV 3 neurons, and a significantly less increase in DIV 14 neurons (6 +/− 1.3 fold, Fig. 1a). On the other hand, it is possible that the elevated basal p-ERK1/2 level may be more susceptible to deactivation through dephosphorylation in mature neurons. Here, we found that blocking L-VGCC by nifedipine (Fig. 5d), as well as blocking NMDAR by APV (Fig. 5e), caused a more dramatic reduction in p-ERK1/2 in cortical neurons at DIV 14 than DIV 3. Similar results were observed with hippocampal neurons (Supplementary Fig. 2d and e).
The dramatic elevation of basal p-ERK1/2 following maturation may be related to the extensive synapse formation, which leads to significant spontaneous synaptic activity. As shown in Fig. 5f and g, the spontaneous activity-mediated [Ca2+]i transients were apparent in DIV 14 neurons, but were minimal in DIV 3 neurons. Blocking L-VGCC by nifedipine (Fig. 5f and g) or blocking NMDAR by APV (data not shown) significantly reduced the transients and the baseline [Ca2+]i level. Correlated with the p-ERK1/2 profile, blocking L-VGCC or NMDAR caused a more dramatic reduction in baseline [Ca2+]i level at DIV 14 than DIV 3. Treatment with nifedipine/CNQX resulted in a 6 +/− 1% (average +/− SEM) reduction in the F340/380 signal in DIV 3 neurons, and a 12 +/− 2% (average +/− SEM) reduction in DIV 14 neurons (Fig. 4a). Consistently, treatment with APV/CNQX resulted in a 8 +/−1% (average +/− SEM) reduction in DIV 3 neurons, and a 19 +/− 2% (average +/− SEM) reduction in DIV 14 neurons (Fig. 4c).
The reduction in NMDA-mediated ERK activation and gene transcription is unrelated to its role in excitotoxic neuronal cell death
It has been well established that, excessive NMDA application induces cell death counteracting its function in regulating plasticity-related signaling. It has been further suggested that bath NMDA application activated extra-synaptic NMDAR, resulting in the shut-off of CREB signaling and cell death (Hardingham et al. 2002). Therefore, it is important to know that the reduction in NMDAR-mediated plasticity-related signaling is not directly related to the toxic effects of NMDA at DIV 14. We first compared NMDA-induced neuronal cell death in DIV 3 and DIV 14 neurons. At 50 uM, NMDA (co-applied with glycine, CNQX, and nifedipine) did not cause any measurable cell death in DIV 3 neurons (Fig. 6). Consistent with the previous studies, NMDA at 50 uM induced significant cell death in DIV 14 neurons (Fig. 6). We next found that, at lower concentration (10 uM), NMDA did not induce significant cell death in either DIV 3 or DIV 14 neurons (Fig. 6). Our data support that bath NMDA incubation mainly activated extra-synaptic NMDARs in DIV 3 neurons, because bicuculline did not stimulate any significant up-regulation of p-ERK/p-CREB and the transcription of zif268, Arc and Bdnf 4 during the earlier developmental stages at DIV 3 (see below) and DIV 7 (data not shown). These data demonstrate that activation of extra-synaptic receptors does not necessarily induce cell death. Furthermore, the function of extra-synaptic NMDARs in cell death may be developmentally regulated. At DIV 14, 10 uM NMDA activates preferentially synaptic receptors (Soriano et al. 2006). The toxic effects of NMDA at 50 uM may be due to the activation of extra-synaptic receptors (in addition to synaptic receptor activation).
Fig. 6.
Bath incubation with NMDA at 10 uM does not cause significant excitotoxic cell death. The neuronal cell death was triggered by NMDA as described in Materials and Methods. DAPI staining demonstrated that NMDA (at 10 uM and 50 uM) did not induce any apparent cell death in DIV 3 neurons. Although high concentration of NMDA (at 50 uM) induced significant cell death in DIV 14 neurons (*: p<0.05), low concentration of NMDA (at 10 uM) did not. Representative DAPI staining is shown in A, and quantification (from 6 independent cultures for both DIV 3 and DIV 14) is shown in B.
To further rule out excitotoxic effects, we stimulated the neurons with NMDA at a non-toxic concentration. At 10 uM, NMDA (co-applied with glycine, CNQX, and nifedipine) stimulated significant p-ERK, p-CREB, as well as the transcription of plasticity-related genes zif268, Arc, and Bdnf exon 4, in DIV 3 neurons (Fig. 7). In contrast, much less activation of p-ERK and p-CREB was observed in DIV 14 neurons. There was no significant transcriptional up-regulation of zif268 and Arc on DIV 14 (Fig. 7). Attenuated up-regulation was observed for Bdnf 4 (Fig. 7).
Fig. 7.
The NMDA-stimulated plasticity-related signaling is significantly dampened following maturation. (a) The non-toxic concentration of NMDA (at 10 uM) stimulated significant more phosphorylation of ERK and CREB at DIV 3 than at DIV 14. Neurons were pre-treated with CNQX and nifedipine for 30 min, then stimulated with NMDA/glycine (in the presence of CNQX and nifedipine). The samples were harvested 15 min after stimulation, and analyzed by Western blots (n=4; *: p<0.05). (b) DIV 3 and DIV 14 neurons were treated as described in (a). The samples were collected 1 hr after the stimulation, the mRNA level of zif268, Arc, and Bdnf exon 4 was examined by semi-quantitative RT-PCR (n=4; *: p<0.05).
We then investigated how neurons responded to the activation of synaptic NMDARs, which does not cause any measurable cell death (Hardingham et al. 2002). We used bicuculline to stimulate synaptic activity. Because DIV 3 neurons have not developed significant synapses, bicuculline did not stimulate any activation of ERK/CREB phosphorylation (Fig. 8a), as well as gene transcription (Fig. 8c). In DIV 14 neurons, bicuculline activated the phosphorylation of both ERK and CREB (Fig. 8a). However, the fold increase is less than that induced by NMDA (at both 10 and 50 uM) in DIV 3 neurons (Fig. 2 and Fig. 7). Although NMDA (at both 10 and 50 uM) stimulated significant gene transcription in DIV 3 neurons 1 hr after stimulation, we did not observe any transcriptional up-regulation in DIV 14 neurons 1 hr after bicuculline treatment (Fig. 8b). No significant transcription was observed 2 hrs after bicuculline stimulation either (data not shown). Consistent with previous studies, prolonged incubation with bicuculline did activate gene expression in DIV 14 neurons. We observed significant up-regulation of Arc, bdnf 4, and zif268 4 hrs after bicuculline treatment (Fig. 8c). These data further demonstrate that the plasticity-related signaling in mature neurons is less responsive to NMDAR activation.
We next co-treated neurons with okadaic acid (OA) and 50 uM NMDA (along with glycine, CNQX and nifedipine), both of which would cause significant neuronal cell death (Suuronen et al. 2000; Yi et al. 2008; Yoon et al. 2006). If the death-triggering stimulation is causal for the lack of NMDA-mediated ERK activation at DIV 14, then co-application of OA and NMDA would not stimulate p-ERK, p-CREB and gene transcription. Interestingly, co-application of OA (at 1uM) and NMDA (at 50 uM) significantly activated p-CREB, p-ERK, as well as the transcription of zif268, Arc, and Bdnf 4 in DIV 14 neurons (Fig. 9). Treatment with OA alone also activated these plasticity-related events (data not shown). These data suggest that cell death and Ca2+-stimulated signaling may be differentially regulated, and the reduction in NMDA-mediated ERK activation and gene transcription is unrelated to its role in excitotoxic neuronal cell death.
Fig. 9.
Co-application of okadaic acid and NMDA results in the activation of ERK, CREB, as well as the transcription of zif268, Arc, and Bdnf exon 4 in DIV 14 neurons. DIV 14 cortical neurons were pre-treated with a general protein phosphatase inhibitor okadaic acid (1uM), or PP2A inhibitor cantharidin (0.1uM), or PP2B inhibitor FK506 (1uM) for 30 min before NMDA (50 uM)/glycine (2 uM) stimulation. (a). To determine the level of p-ERK1/2 and p-CREB, samples were harvested 15 min after NMDA (50 uM)/glycine/nifedipine/CNQX stimulation, and analyzed by Western blots. (b). To determine the mRNA level of Arc, zif268 and Bdnf exon 4, samples were collected 60 min after NMDA (50 uM)/glycine/nifedipine/CNQX application. A 30 min pre-treatment with nifedipine and CNQX was included for all samples. Representative images are shown in the upper panels, and quantification in the lower panels (average +/− SD; n=4). The level of RT-PCR products of the individual genes, p-ERK, and p-CREB in the control neurons was set as 1.
The activity of okadaic acid (OA)-sensitive protein phosphatases, but not PP2A and PP2B, antagonize the stimulation of p-ERK, p-CREB, and gene transcription
Our data show that the general protein phosphatases (PPs) inhibitor OA caused ERK/CREB and transcriptional activation. It is consistent with the hypothesis that the level of phosphorylation is determined by the balance between protein kinases and protein phosphatases. It has been demonstrated that PPs are activated by neuronal activity. Here, we further examined the role of the PP2A and PP2B, which are major Ca2+-regulated PPs in neurons, in NMDA-stimulated DIV 14 neurons. In contrast to the effects of OA, inhibition of PP2A and PP2B did not elevate the phosphorylation of ERK and CREB (Fig. 9a). The level of Arc, bdnf 4, and zif268 mRNA was not affected by the inhibition of PP2A and PP2B either (Fig. 9b). These data demonstrate that OA-sensitive PPs, but not PP2A and 2B, may antagonize p-ERK, p-CREB, and gene transcription during maturation.
DISCUSSION
Plasticity is a remarkable mechanism by which the brain is able to react to environmental stimuli. Because the early postnatal period is critical for the animals to shape their neuronal circuits, it has been hypothesized that neurons during early development are more responsive. The loss of certain aspects of plasticity following development has been suggested for both the cerebral cortex (Akopian and Walsh 2006; Carmignoto and Vicini 1992) and hippocampus (Rosenzweig and Barnes 2003) during maturation. Here, we used cultured neurons to study Ca2+-stimulated signaling following in vitro maturation. Although our neuronal preparations are already highly differentiated by the time of plating, they undergo tremendous development of dendrites and axons as well as synaptogenesis during the 2 weeks following culturing. Therefore, the intrinsic signaling properties may be significantly different following in vitro maturation. For example, the mature DIV 14 neurons showed significant more spontaneous synaptic activity, potentially causing the more “experienced” synapses to be less sensitive to external stimuli. The major findings of this study are (i) NMDAR-mediated ERK/CREB activation and gene transcription are significantly diminished following maturation, and (ii) mature neurons maintain higher basal level of [Ca2+]i and ERK activity, and more susceptible to deactivation upon the blockage of NMDAR and L-VGCC. Furthermore, although DIV 3 neurons are more resistant to excitotoxic cell death than DIV 14 neurons, the reduction in NMDA-mediated signaling following maturation is not directly related to the excitotoxic effects of NMDA. Although the situation in our study may be quite different from in vivo maturation and aging, our results are consistent with some of the phenotypes implicated in age-related decline in plasticity.
There are discrepancies in the literature concerning the role of NMDAR and L-VGCC in gene expression. It has been observed that persistent phosphorylation of CREB was mediated by the activation of L-VGCC, but not NMDAR. Therefore, it has been concluded that NMDAR does not play a major role in regulating gene expression (West et al. 2001; West et al. 2002). However, a large body of evidence strongly supports the function of NMDAR in regulating numerous plasticity-related genes (Tabuchi et al. 2000; Xia et al. 1996). Our results suggest that the functional role of these two mechanisms depends on the age of the neurons. Furthermore, in numerous previous studies, the neurons were pre-treated with tetrodotoxin (TTX) for at least 4 to 8 hours before NMDA or KCl stimulation, so that the spontaneous neuronal activity was suppressed. Because DIV 14 neurons showed significant spontaneous activity-induced Ca2+ oscillation (as compared to DIV 3 neurons), long-term suppression with TTX may re-sensitize the neurons and lead to NMDA stimulation of p-ERK (Sala et al. 2000). In this study, we examined neurons under the condition that the basal neuronal activity was not suppressed. In our paradigm, the NMDAR-mediated gene expression at 14 DIV was significantly diminished.
Although the NMDAR-mediated ERK/CREB phosphorylation and immediate early gene transcription was ablated or decreased in DIV 14 neurons, we were surprised that NMDA (in the presence of CNQX and nifedipine) stimulated comparable calcium influx at DIV 3 and 14. The reduction in NMDAR-mediated signaling also does not correlate with the expression level of NMDARs following maturation. In cultured neurons, although NR2B remained constant, the expression of both NR1 and NR2A increased in mature neurons (Brewer et al. 2007). Interestingly, the NMDA-induced current amplitude and density (without the co-application of nifedipine and CNQX) significantly increased across in vitro maturation (Brewer et al. 2007). Consistently, we found that, without CNQX and nifedipine, NMDA induced a more pronounced increase in [Ca2+]i at DIV 14 (52% increase of F340/380 at DIV3 and 270% increase at DIV 14, data not shown). Although the NMDAR-mediated (with the co-application of nifedipine and CNQX) global changes in [Ca2+]i are indistinguishable between DIV 3 and 14, the local situation at the post-synaptic density (PSD) may be different. Because NMDA induces larger current in mature neurons (Brewer et al. 2007), proteins localized at the PSD may experience higher level calcium transients upon NMDAR activation. It is well accepted that normal NMDAR activation is required for plasticity and neuronal survival. In contrast, excessive NMDAR activation triggers excitotoxic cell death. However, our experimental set-up is not sufficient to test the possibility that excessive elevation of Ca2+ within the synapse fails to activate ERK1/2 signaling.
For proper brain functioning to occur, neuronal activity need to be regulated bi-directionally. Such plasticity has been demonstrated in LTP, LTD and depotentiation. Compared to LTP, which is an activity-dependent increase in synaptic efficacy, LTD shows an activity-dependent decrease in synaptic strength (Lynch 2004; Malenka and Bear 2004). Furthermore, LTP can be reversed, or depotentiated by low frequency stimulation. Although the functional relevance is not clear, it has been hypothesized that LTP may be responsible for memory formation, and LTD and depotentiation may be involved in forgetting/memory loss and active suppression of pre-established memories. Interestingly, aging-related memory impairments correlate well with defective LTP. The higher susceptibility of forgetting/memory loss also correlates with the lower threshold for LTD in aged animals (Rosenzweig and Barnes 2003). The in vivo studies suggest calcium dysregulation as a potential mechanism for the altered plasticity during normative aging (Foster and Kumar 2002). The overall increase in NMDAR (Brewer et al. 2007) and L-VGCC (Porter et al. 1997) expression, along with the significant synaptogenesis and the elevation of glutamate release following maturation (Fogal et al. 2005), may leads to higher basal [Ca2+]i. The altered Ca2+ homeostasis and Ca2+ dysregulation may modulate the intrinsic factors involved in NMDAR-mediated signaling. In our study, we found that the basal level of [Ca2+]i and p-ERK1/2 increased significantly following in vitro maturation. Hence, the mature neurons are more difficult to further activate, and consequently easier to deactivate. Blocking NMDAR and L-VGCC had more dramatic effects on the dephosphorylation of ERK1/2 following maturation. Although KCl-induced membrane depolarization did stimulate p-ERK and gene transcription, the fold increase was compromised in DIV 14 neurons. A similar synaptic saturation theory has been proposed for the reduction of plasticity (McNaughton et al. 1986; Moser et al. 1998). For example, artificial synaptic enhancement and potentiation blocked spatial memory (McNaughton et al. 1986).
In addition to protein kinases, it is strongly implicated that protein phosphatase (PP) activity plays an important role in regulating p-ERK1/2 and p-CREB, as well as LTP and memory formation. Previous studies demonstrated the differential role of PP1, PP2A and PP2B. For example, PP1 and PP2B were implicated in regulating MAPK signaling (Tian and Karin 1999) and CREB phosphorylation (Bito et al. 1996; Hagiwara et al. 1992), respectively. PP1 and PP2A, but not PP2B, regulate glutamate-induced phosphorylation of calmodulin-dependent protein kinase II (Kasahara et al. 1999). Furthermore, inhibition of PP2A, but not PP1, facilitated mGluR5-induced ERK1/2 phosphorylation in striatal neurons (Mao et al. 2005). A study by Sala et al. (Sala et al. 2000) has demonstrated that PP1 activity is coupled to NMDAR and de-phosphorylated p-CREB, but not p-ERK1/2, in mature neurons at DIV 14. In our experimental setting, we also observed general antagonizing effects of PP on ERK/CREB activation, as well as gene transcription. When DIV 14 neurons were co-treated with NMDA and OA, the level of p-ERK, p-CREB, zif268, Arc, and Bdnf exon 4 was significantly higher than in the controls. However, due to the non-specific inhibition effects of OA on PP, more rigorous molecular approaches are required to identify which specific PP is involved. Interestingly, genetic inhibition of PP1 by over-expressing I1 rescued spatial memory deficits in aged mice (Genoux et al. 2002).
Does the reduction in NMDAR-stimulated signaling result from the excitotoxic effects of NMDA following maturation? Numerous previous studies demonstrated that bath incubation with NMDA caused the shut-off of CREB activation (measured within 2 hrs after NMDA incubation) and neuronal cell death (measured about 24 hrs after NMDA incubation). Although the later events (cell death) may not be causal for the earlier events (CREB de-activation), it is possible that the NMDA-triggered cell death signaling antagonizes the plasticity-related signaling. Indeed, our own data show an inverse relation between NMDA-stimulated ERK/CREB activation and NMDA-induced cell death. DIV 3 neurons displayed more ERK/CREB activation but no apparent cell death after NMDA stimulation. In contrast, DIV 14 neurons displayed less ERK/CREB activation and more cell death. However, we demonstrated that cell death-triggering stimulation (by co-application of 50 uM NMDA and OA) did not necessarily shut off ERK/CREB and transcription (Fig. 9). It is also important to point out that activation of extra-synaptic NMDAR by bath NMDA incubation does not always deactivate ERK/CREB signaling and promote cell death. Our data clearly suggest neuronal maturation as an important regulator for both ERK/CREB signaling and cell death.
In summary, we documented a reduction in NMDAR-mediated ERK/CREB signaling and the transcription of several plasticity-related genes following in vitro maturation. Our study suggests that the loss of certain aspects of plasticity following brain maturation may be due to a combination of calcium dysregulation, elevation of basal ERK activity, and decrease in NMDAR-mediated signaling. Although we need to be cautious in extrapolating our in vitro data, previous reports do suggest declined CREB activation in aged animals. For example, compared to young adult mice (5- to 6-month old), the learning-induced p-CREB activation and c-Fos expression is significantly reduced in aged animals (23- to 24-month old) (Porte et al. 2008). In another animal model, the SAMP8 (senescence-accelerated mouse P8) mice show deficits in passive avoidance memory. The memory deficits are correlated with the reduction in learning-induced p-CREB activation (Tomobe et al. 2007). We suggest further investigations on the in vivo function of L-VGCC and NMDAR for activity-dependent ERK/CREB activation, as well as gene transcription, in aged animals.
Supplementary Material
Supplementary Fig. 1 The activation of ERK1/2 and CREB declines following in vitro maturation. Hippocampal neurons were stimulated by 50 mM KCl (a) or 50 uM NMDA/2 uM glycine (b) at DIV 3 and 14. (a). KCl stimulated ERK1/2 phosphorylation at both DIV 3 and 14. (b). NMDA stimulated ERK1/2 phosphorylation at DIV 3, but failed at DIV 14. Samples were collected 15 min after stimulation. The signal of p-ERK1/2 was normalized with total ERK1/2. Data are average +/− SD (n=5 for each group).
Supplementary Fig. 2 Total expression and the phosphorylation of ERK1/2 are significantly up-regulated following in vitro maturation. Primary hippocampal cultures were examined for the level of p-ERK1/2 and total ERK1/2 at DIV 3, 6 and 14. Representative Western blots are shown in (a). The relative levels of total ERK1/2 (T-ERK1/2) were normalized to beta-actin and quantified (n=5 from each group) (b). To obtain the profile for ERK1/2 activation during development, the level of p-ERK1/2 was normalized with total ERK1/2 (c). The relative expression level at DIV 3 was arbitrarily set as 1. Data are average ± S.D. (d) and (e), DIV 14 neurons show more dephosphorylation of p-ERK1/2 when L-VGCC and NMDAR are blocked. Samples were collected from the control and nifedipine/CNQX- or APV/CNQX-treated neurons, and analyzed for the level of p-ERK1/2. The representative Western blots are shown in the upper panels, and quantification in the lower panels (average +/− SD, n=4).
ACKNOWLEDGMENTS
We thank Dr. Yukun Yuan for critical reading of the manuscript, and suggestive comments. This work was supported by NIH grant MH076906 (to HW), and MH068347 (to JN). DS was supported by the NIH predoctoral NRSA (F31DA02378).
Abbreviations
- BDNF
brain-derived neurotrophic factor
- CREB
cAMP responsive element binding protein
- ERK
extracellular signal-regulated kinase
- DIV
days in vitro
- LTD
long term depression
- LTP
long term potentiation
- NMDA
N-methyl-D-aspartic acid
- NMDAR
NMDA receptor
- VGCC
voltage-gated calcium channel
- L-VGCC
L-type VGCC
REFERENCES
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85(3):525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian G, Walsh JP. Pre- and postsynaptic contributions to age-related alterations in corticostriatal synaptic plasticity. Synapse. 2006;60(3):223–238. doi: 10.1002/syn.20289. [DOI] [PubMed] [Google Scholar]
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci U S A. 1999;96(9):5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87(7):1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- Bozon B, Davis S, Laroche S. Regulated transcription of the immediate-early gene Zif268: mechanisms and gene dosage-dependent function in synaptic plasticity and memory formation. Hippocampus. 2002;12(5):570–577. doi: 10.1002/hipo.10100. [DOI] [PubMed] [Google Scholar]
- Brewer LD, Thibault O, Staton J, Thibault V, Rogers JT, Garcia-Ramos G, Kraner S, Landfield PW, Porter NM. Increased vulnerability of hippocampal neurons with age in culture: temporal association with increases in NMDA receptor current, NR2A subunit expression and recruitment of L-type calcium channels. Brain Res. 2007;1151:20–31. doi: 10.1016/j.brainres.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258(5084):1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci. 2000;20(12):4563–4572. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8(4):1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English JD, Sweatt JD. A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. J Biol Chem. 1997;272(31):19103–19106. doi: 10.1074/jbc.272.31.19103. [DOI] [PubMed] [Google Scholar]
- Fogal B, Trettel J, Uliasz TF, Levine ES, Hewett SJ. Changes in secondary glutamate release underlie the developmental regulation of excitotoxic neuronal cell death. Neuroscience. 2005;132(4):929–942. doi: 10.1016/j.neuroscience.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Foster TC, Kumar A. Calcium dysregulation in the aging brain. Neuroscientist. 2002;8(4):297–301. doi: 10.1177/107385840200800404. [DOI] [PubMed] [Google Scholar]
- Foster TC, Norris CM. Age-associated changes in Ca(2+)-dependent processes: relation to hippocampal synaptic plasticity. Hippocampus. 1997;7(6):602–612. doi: 10.1002/(SICI)1098-1063(1997)7:6<602::AID-HIPO3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002;418(6901):970–975. doi: 10.1038/nature00928. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268(5208):239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- Ginty DD. Calcium regulation of gene expression: isn't that spatial? Neuron. 1997;18(2):183–186. doi: 10.1016/s0896-6273(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Hagiwara M, Alberts A, Brindle P, Meinkoth J, Feramisco J, Deng T, Karin M, Shenolikar S, Montminy M. Transcriptional attenuation following cAMP induction requires PP-1-mediated dephosphorylation of CREB. Cell. 1992;70(1):105–113. doi: 10.1016/0092-8674(92)90537-m. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5(5):405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21(4):869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Kasahara J, Fukunaga K, Miyamoto E. Differential effects of a calcineurin inhibitor on glutamate-induced phosphorylation of Ca2+/calmodulin-dependent protein kinases in cultured rat hippocampal neurons. J Biol Chem. 1999;274(13):9061–9067. doi: 10.1074/jbc.274.13.9061. [DOI] [PubMed] [Google Scholar]
- Kemp N, Bashir ZI. Long-term depression: a cascade of induction and expression mechanisms. Prog Neurobiol. 2001;65(4):339–365. doi: 10.1016/s0301-0082(01)00013-2. [DOI] [PubMed] [Google Scholar]
- Kitagawa K. CREB and cAMP response element-mediated gene expression in the ischemic brain. Febs J. 2007;274(13):3210–3217. doi: 10.1111/j.1742-4658.2007.05890.x. [DOI] [PubMed] [Google Scholar]
- Konur S, Ghosh A. Calcium signaling and the control of dendritic development. Neuron. 2005;46(3):401–405. doi: 10.1016/j.neuron.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Lanahan A, Worley P. Immediate-early genes and synaptic function. Neurobiol Learn Mem. 1998;70(1–2):37–43. doi: 10.1006/nlme.1998.3836. [DOI] [PubMed] [Google Scholar]
- Lopez de Armentia M, Jancic D, Olivares R, Alarcon JM, Kandel ER, Barco A. cAMP response element-binding protein-mediated gene expression increases the intrinsic excitability of CA1 pyramidal neurons. J Neurosci. 2007;27(50):13909–13918. doi: 10.1523/JNEUROSCI.3850-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84(1):87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mao L, Yang L, Arora A, Choe ES, Zhang G, Liu Z, Fibuch EE, Wang JQ. Role of protein phosphatase 2A in mGluR5-regulated MEK/ERK phosphorylation in neurons. J Biol Chem. 2005;280(13):12602–12610. doi: 10.1074/jbc.M411709200. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli C, Brambilla R. Ras-related and MAPK signalling in neuronal plasticity and memory formation. Cell Mol Life Sci. 2000;57(4):604–611. doi: 10.1007/PL00000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, Rao G, Baldwin J, Rasmussen M. Long-term enhancement of hippocampal synaptic transmission and the acquisition of spatial information. J Neurosci. 1986;6(2):563–571. doi: 10.1523/JNEUROSCI.06-02-00563.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EI, Krobert KA, Moser MB, Morris RG. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281(5385):2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- Norris CM, Korol DL, Foster TC. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J Neurosci. 1996;16(17):5382–5392. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Evidence for an extended duration of GABA-mediated excitation in the developing male versus female hippocampus. Dev Neurobiol. 2007;67(14):1879–1890. doi: 10.1002/dneu.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier J, O'Neill A, Schaffer DV. PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev Neurobiol. 2007;67(10):1348–1361. doi: 10.1002/dneu.20506. [DOI] [PubMed] [Google Scholar]
- Porte Y, Buhot MC, Mons N. Alteration of CREB phosphorylation and spatial memory deficits in aged 129T2/Sv mice. Neurobiol Aging. 2008;29(10):1533–1546. doi: 10.1016/j.neurobiolaging.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Porter NM, Thibault O, Thibault V, Chen KC, Landfield PW. Calcium channel density and hippocampal cell death with age in long-term culture. J Neurosci. 1997;17(14):5629–5639. doi: 10.1523/JNEUROSCI.17-14-05629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolli M, Kotlyarov A, Sakamoto KM, Gaestel M, Neininger A. Stress-induced stimulation of early growth response gene-1 by p38/stress-activated protein kinase 2 is mediated by a cAMP-responsive promoter element in a MAPKAP kinase 2-independent manner. J Biol Chem. 1999;274(28):19559–19564. doi: 10.1074/jbc.274.28.19559. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69(3):143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Rao G, McNaughton BL, Barnes CA. Role of temporal summation in age-related long-term potentiation-induction deficits. Hippocampus. 1997;7(5):549–558. doi: 10.1002/(SICI)1098-1063(1997)7:5<549::AID-HIPO10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Sala C, Rudolph-Correia S, Sheng M. Developmentally regulated NMDA receptor-dependent dephosphorylation of cAMP response element-binding protein (CREB) in hippocampal neurons. J Neurosci. 2000;20(10):3529–3536. doi: 10.1523/JNEUROSCI.20-10-03529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar S, Teyler TJ, Robbins N. Aging differentially alters forms of long-term potentiation in rat hippocampal area CA1. J Neurophysiol. 1998;79(1):334–341. doi: 10.1152/jn.1998.79.1.334. [DOI] [PubMed] [Google Scholar]
- Soriano FX, Papadia S, Hofmann F, Hardingham NR, Bading H, Hardingham GE. Preconditioning doses of NMDA promote neuroprotection by enhancing neuronal excitability. J Neurosci. 2006;26(17):4509–4518. doi: 10.1523/JNEUROSCI.0455-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suuronen T, Kolehmainen P, Salminen A. Protective effect of L-deprenyl against apoptosis induced by okadaic acid in cultured neuronal cells. Biochem Pharmacol. 2000;59(12):1589–1595. doi: 10.1016/s0006-2952(00)00282-3. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14(3):311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Tabuchi A, Nakaoka R, Amano K, Yukimine M, Andoh T, Kuraishi Y, Tsuda M. Differential activation of brain-derived neurotrophic factor gene promoters I and III by Ca2+ signals evoked via L-type voltage-dependent and N-methyl-D-aspartate receptor Ca2+ channels. J Biol Chem. 2000;275(23):17269–17275. doi: 10.1074/jbc.M909538199. [DOI] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20(4):709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Tian J, Karin M. Stimulation of Elk1 transcriptional activity by mitogen-activated protein kinases is negatively regulated by protein phosphatase 2B (calcineurin) J Biol Chem. 1999;274(21):15173–15180. doi: 10.1074/jbc.274.21.15173. [DOI] [PubMed] [Google Scholar]
- Tomobe K, Okuma Y, Nomura Y. Impairment of CREB phosphorylation in the hippocampal CA1 region of the senescence-accelerated mouse (SAM) P8. Brain Res. 2007;1141:214–217. doi: 10.1016/j.brainres.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Waltereit R, Dammermann B, Wulff P, Scafidi J, Staubli U, Kauselmann G, Bundman M, Kuhl D. Arg3.1/Arc mRNA induction by Ca2+ and cAMP requires protein kinase A and mitogen-activated protein kinase/extracellular regulated kinase activation. J Neurosci. 2001;21(15):5484–5493. doi: 10.1523/JNEUROSCI.21-15-05484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltereit R, Weller M. Signaling from cAMP/PKA to MAPK and synaptic plasticity. Mol Neurobiol. 2003;27(1):99–106. doi: 10.1385/MN:27:1:99. [DOI] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci U S A. 2001;98(20):11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nat Rev Neurosci. 2002;3(12):921–931. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- Winder DG, Martin KC, Muzzio IA, Rohrer D, Chruscinski A, Kobilka B, Kandel ER. ERK plays a regulatory role in induction of LTP by theta frequency stimulation and its modulation by beta-adrenergic receptors. Neuron. 1999;24(3):715–726. doi: 10.1016/s0896-6273(00)81124-1. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dudek H, Miranti CK, Greenberg ME. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J Neurosci. 1996;16(17):5425–5436. doi: 10.1523/JNEUROSCI.16-17-05425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi KD, Covey DF, Simpkins JW. Mechanism of Okadaic Acid Induced Neuronal Death and the Effect of Estrogens. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22(5):1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S, Choi J, Yoon J, Huh JW, Kim D. Okadaic acid induces JNK activation, bim overexpression and mitochondrial dysfunction in cultured rat cortical neurons. Neurosci Lett. 2006;394(3):190–195. doi: 10.1016/j.neulet.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Zheng F, Soellner D, Nunez J, Wang H. The basal level of intracellular calcium gates the activation of phosphoinositide 3-kinase-Akt signaling by brain-derived neurotrophic factor in cortical neurons. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 The activation of ERK1/2 and CREB declines following in vitro maturation. Hippocampal neurons were stimulated by 50 mM KCl (a) or 50 uM NMDA/2 uM glycine (b) at DIV 3 and 14. (a). KCl stimulated ERK1/2 phosphorylation at both DIV 3 and 14. (b). NMDA stimulated ERK1/2 phosphorylation at DIV 3, but failed at DIV 14. Samples were collected 15 min after stimulation. The signal of p-ERK1/2 was normalized with total ERK1/2. Data are average +/− SD (n=5 for each group).
Supplementary Fig. 2 Total expression and the phosphorylation of ERK1/2 are significantly up-regulated following in vitro maturation. Primary hippocampal cultures were examined for the level of p-ERK1/2 and total ERK1/2 at DIV 3, 6 and 14. Representative Western blots are shown in (a). The relative levels of total ERK1/2 (T-ERK1/2) were normalized to beta-actin and quantified (n=5 from each group) (b). To obtain the profile for ERK1/2 activation during development, the level of p-ERK1/2 was normalized with total ERK1/2 (c). The relative expression level at DIV 3 was arbitrarily set as 1. Data are average ± S.D. (d) and (e), DIV 14 neurons show more dephosphorylation of p-ERK1/2 when L-VGCC and NMDAR are blocked. Samples were collected from the control and nifedipine/CNQX- or APV/CNQX-treated neurons, and analyzed for the level of p-ERK1/2. The representative Western blots are shown in the upper panels, and quantification in the lower panels (average +/− SD, n=4).