Abstract
Neurodegeneration and gliosis are prominent pathological features of subjects with human immunodeficiency virus (HIV) dementia complex (HAD). In these patients, neurodegeneration occurs in uninfected neurons. In addition, these patients develop sensory neuropathy despite the antiretroviral therapy. The HIV protein gp120, which mimics some of the pathological alterations seen in HAD, is retrogradely transported in rodent neurons. However, it is still unclear whether gp120 can also be transported anterogradely and whether axonal transport can occur in the peripheral nervous system (PNS). To determine whether gp120 is transported retrogradely and/or anterogradely, we injected gp120IIIB together with the retrograde tracer fluoro-ruby (FR) or the anterograde tracer 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyamine perchlorate (DiI) into the rat superior colliculi. We discovered that gp120 is retrogradely transported with FR along a direct pathway from the superior colliculus to the retina and anterogradely transported with DiI to several areas of the occipital cortex. To determine whether gp120 is also axonally transported in the peripheral nerves, gp120 and FR were injected into the sciatic nerve. No gp120 immunoreactivity was found in the sciatic nerve or dorsal root ganglia, suggesting that gp120 axonal transport does not occur in the PNS. Gp120 axonal transport may play a role in neuronal injury. Therefore, we examined apoptosis at various time points after gp120 injection. Activated caspase-3 was evident within neurons transporting gp120. These results indicate that axonal transport of gp120 might exacerbate the pathogenesis of HIV-1.
Keywords: DiI, Fluoro-ruby, gp120IIIB, microglia, neuronal apoptosis, retinal ganglion cells, sciatic nerve, superior colliculus
Introduction
Neurological abnormalities occur in a significant number of human immunodeficiency virus (HIV)-infected individuals during the late phase of infection (Janssen et al., 1989). Post mortem brains of these individuals have revealed neuronal loss, simplification of synaptic contacts, astrocytosis, and microglial nodules with multinucleated giant cells (Everall et al., 1993; Masliah et al., 1997). These pathological alterations occur even though HIV-1 productively infects only macrophages and microglia (Budka et al., 1987; Eilbott et al., 1989) and astrocytes can support only limited viral replication (Tornatore et al., 1994). Additionally, markers of neurodegeneration can frequently be detected in brain areas that are distant from sites of viral replication. Given the fact that HIV infection in the brain is relatively anatomically restricted, an unresolved issue is how low viral load in the brain can cause such widespread neuronal dysfunction.
Experimental evidence has shown that viral proteins such as gp120, the activator of HIV transcription Tat, and accessory proteins Nef and Vpr, can cause neuronal cell death in vitro as well as in vivo [reviewed in (Matarrese and Malorni, 2005)]. With regards to gp120, chemokine receptors, especially CXCR4, have been proposed to mediate its neurotoxic effect (Hesselgesser et al., 1998; Kaul and Lipton, 1999; Meucci et al., 1998; Zheng et al., 1999). Consequently, much effort has been directed toward characterizing CXCR4 signal transduction mechanisms responsible for gp120 toxicity. However, far less is known about the role of gp120 trafficking in light of recent studies showing that Tat and gp120 can migrate to different brain areas by axonal transport (Bachis et al., 2006; Bruce-Keller et al., 2003). Intriguingly, Tat internalization and transport occur independently from a receptor-mediated mechanism, whereas gp120, at least the T-tropic strain, requires the chemokine receptor CXCR4 for internalization in neurons (Bachis et al., 2003). Interestingly, compounds that prevent gp120 axonal transport also block gp120 neurotoxicity (Bachis et al., 2006).
Axonal transport is a universal mechanism in the nervous system that allows neurons to communicate between distant sites. Molecules that are transported include endogenous proteins, such as neurotrophic factors. These proteins travel along axons to be transported both anterogradely from cell bodies to the terminals or retrogradely from terminals to the cell bodies. Neurodegeneration may occur if the axonal transport is impaired. Moreover, in infectious diseases, exogenous proteins also utilize axonal transport. These include the prion protein (Borchelt et al., 1994) and the tegument proteins of the herpes virus (Bearer et al., 2000) which can be neurotoxic. Therefore, it is crucial to establish whether a “suicide transport” is one of the mechanisms that account for the widespread neurotoxic activity of gp120. In addition, it is important to establish whether HIV proteins can be both retrogradely and anterogradely transported and whether axonal transport is also operational in the peripheral nervous system (PNS).
The rat visual system is well characterized in terms of anatomical connections. The superior colliculus receives inputs from retinal ganglionic cells (RGC) (Beckstead and Frankfurter, 1983), primary visual cortex, and prefrontal cortex (Sefton and Dreher, 1995). Fibers from the superior colliculus innervate various nuclei of the thalamus and brain stem (Donnelly et al., 1983; Redgrave et al., 1993; Westby et al., 1990). By virtue of these connections, the visual pathway can be used as an experimental model to examine retrograde and anterograde transport of gp120 in the central nervous system (CNS). Conversely, the sciatic nerve, which contains motor and sensory axons of the dorsal root ganglia (DRG), represents a suitable model to study axonal transport in the PNS. The present study was undertaken to demonstrate axonal transport and neurotoxicity of gp120 in the CNS and PNS.
Methods
Animal treatment
All surgical procedures were performed in strict accordance with the Laboratory Animal Welfare Act, the National Institutes of Health Guide for the care and use of laboratory animals, and after approval from the Georgetown University Animal Care and Use Committee. Adult Sprague-Dawley male rats (250 g) were deeply anesthetized with ketamine/xylazine (80 and 10 mg/kg, i.p., respectively). Gp120 (strain IIIB) and heat-inactivated gp120IIIB (Immunodiagnostics, Woburn, MA) were used at 400 ng in 0.1% bovine serum albumin (BSA). Gp120 and the retrograde tracer fluoro-ruby (FR, 10%, Invitrogen Corp., Carlsbad, CA) or anterograde tracer 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyamine perchlorate (DiI, 10%, Molecular Probes) were delivered into both superior colliculi by a microperfusion pump (0.25 μl/min/10min) using a 33 GA cannula. Injections into the superior colliculi (both left and right) were done according to the following coordinates: anteroposterior +6.0 mm; mediolateral ± 1.2 mm; dorsoventral −4.0 mm, from bregma (Paxinos and Watson, 1986). After the injection, the needles were left in place for an additional 5 min to accomplish quantitative diffusion of the volume delivered. Animals were then returned to their cages and allowed to survive for 3 or 18 days. At the appropriate survival times, animals were deeply anesthetized with ketamine/xylazine (80 and 10 mg/kg, i.p., respectively), followed by intracardiac perfusion of 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 (PPB).
Preparation of retinae
Retinae were prepared as previously described (Ahmed et al., 2001). In brief, prior to excision of the eyeball, the center of the insertion of superior rectus muscle into the eye ball was marked with ink. Eyes were bisected at the equator, the lenses were removed, and the posterior segments were fixed with 4% PPB for an additional 30 min. Each retina was then isolated and post-fixed in 4% PPB, for 1 hr. To prepare flat mounts, retinae were dissected from the underlying sclera/choroid, and flattened by four radial cuts.
Sciatic nerve injection
Rats were anesthetized as described above. The left sciatic nerve was exposed and firm pressure was applied to the nerve for 30 sec to deliver a crush. FR alone or in combination with gp120 (400 ng in 0.1% BSA in 5μl) or heat-inactivated gp120 (400 ng in 0.1% BSA in 5 μl) was directly injected into the nerve at the crush site. In a group of rats, the uncrushed sciatic nerve was wrapped with gelfoam (Pharmacia & Upjohn Co, Division of Pfizer, New York, NY), prepared in bands of approximately 4×8 mm, and saturated with gp120 (400 ng in 0.1% BSA) or heat-inactivated gp120 (400 ng in 0.1% BSA). Gelfoam was applied to the nerve 2–3 mm proximally to the trifurcation using care not to cause nerve injury or constriction. A group of animals received gp120 2 mm distal to DRG. Animals were euthanized by intracardiac perfusion with 4% PPB 3 days after the injection.
Immunohistochemistry
The brains and sciatic nerves were removed and post-fixed in 4% PPB, then transferred into buffered graded sucrose (10%, 20% and 30%) and serial cross sections (16μm) were prepared. Sections were incubated with 2.5% BSA and 0.01% Tritonx100 prior to immunohistochemistry. Retinae were incubated for 48 hr and brain sections for 24 hr at 4°C with the following antibodies: Anti-gp120 (5 μg/ml; mouse monoclonal, Immunodiagnostics), anti-caspase-3 (1μg/ml; rabbit polyclonal, Cell Signaling Technology Inc., Danvers, MA), anti-neurofilament (rabbit polyclonal 1:400, Millipore, Temecula, CA), anti-glial fibrillary acidic protein (GFAP, 4 μg/ml; guinea pig polyclonal, Advanced ImmunoChemical Inc., Long Beach, CA), and anti-CD11b/OX42 (10 μg/ml; mouse monoclonal, Serotech, Raleigh, NC). The latter visualizes active microglia. Sections were then incubated for 1 hr at room temperature with the corresponding secondary antibodies: anti mouse IgG FITC conjugate (1:100, Sigma, Saint Louis MO), anti mouse IgG AlexaFluor© 594 (1:500, Invitrogen Corp), anti guinea pig Cy3 (1:100, Jackson ImmunoResearch Laboratories Inc., West Grove, PA). After a brief wash with PBS, slides were then mounted using Vectashield mounting medium with 4′,6′-diamidino-2-phenylindole (DAPI, Vector Lab, Burlingame, CA). Control sections were incubated either with primary or secondary antibody only.
Sciatic nerve sections (both coronal and horizontal) were incubated with anti-gp120, anti-neurofilament, or anti GFAP as described above. In addition, sections were incubated with anti-CXCR4 (5 μg/ml; mouse monoclonal 12G5, NIH AIDS Research and Reference Reagent Program, Rockville MD). Secondary antibodies are described above.
Fluoro-Jade
Degenerating neuronal somata and their processes were detected with Fluoro-Jade (Histochem, Jefferson, AK) as originally described (Schmued et al., 1997). Brain sections, prepared as described above, were incubated in each of the following solutions for the time indicated: 100% alcohol, 3 min; 70% alcohol, 1 min; dH2O, 1 min; 0.06% potassium permanganate, 15 min; dH2O, 1 min; 0.001% Fluoro-Jade in 0.09% acetic acid, 30 min; dH2O, 2 × 1 min. Stained sections were allowed to dry at room temperature protected from light, dehydrated with xylene and coverslipped with DPX (Aldrich Chemical Company Inc., Milwaukee, WI).
Histological analysis
Immunofluorescence was analyzed with a Zeiss fluorescence microscope Axioplan2 (Carl Zeiss MicroImaging, Inc., Thornwood, NY) as previously described (Bachis et al., 2006). RGC labeled with FR or gp120 were counted in 72 microscopic fields of retina using a 20× objective and MetaMorph® Imaging software (Universal Imaging Corporation™, Downingtown, PA). Each microscopic field corresponded to an area of 120×160 μm. The selected fields were located at the same distance from the optic disk to prevent variations in RGC density. The number of gp120 and/or caspase-3 positive cells in the visual cortex was assessed in an area of 250μm2 in each section using MetaMorph®. Cells were counted only based on staining, not shape or size.
Confocal Microscopy
Confocal images were acquired with the Pathway Bioimager system (BD Bioscience, Bioimaging Systems, Rockville, MD) which includes full spectrum excitation (mercury lamps), a Nipkow spinning-disk confocal module, and a high resolution cooled CCD camera. DAPI, FITC, and Alexa 594 were excited with narrow band 360/10, 488/10, and 572/20 nm excitation filters, respectively. To ensure pixel registration of the three channels in overlay images, a single polychroic mirror and multiband emission filter were used (83100 and 83101 sets, Chroma Technology Corp., Rockingham, VT). The emitter provides narrow band emission windows (450–480; 510–550; 600–670 nm) to separate emission signals from DAPI, FITC, and Alexa 594, respectively. Slides were imaged with a 20× UAPO, 0.75na Olympus objective or a 40× Fluar, 1.3 na, oil immersion Zeiss objective. Images were captured with camera binning 2×2 (resolution: 0.625 μm/pixel at 20× and 0.3125 μm/pixel at 40×) and saved as 16 bit TIF files. Confocal z stacks were also collected; collapsed z stacks and 3D reconstructions were generated to assess probe location/co-localization.
Western blot analysis
Tissue samples were lysed on 1× ice cold RIPA lysis buffer (Millipore) and processed for immunoblotting. After removal of cellular debris by centrifugation, protein levels in the lysates were measured by the Bradford Coomassie blue colorimetric assay (Bio-Rad Laboratories, Inc., Hercules, CA) and equalized accordingly. A portion of the lysate (30–50 μg of protein) was then fractionated on a 4–12% bis-tris SDS polyacrylamide gels (Invitrogen Corp.). Proteins were transferred to nitrocellulose membranes and the immobilized proteins were incubated overnight at 4°C with a mouse monoclonal antibody to CD68/ED1 (1μg/ml, Serotech). Immunocomplexes were detected with peroxidase-conjugated Affinipure Goat anti-mouse IgG secondary antibody (0.08 μg/ml, Jackson ImmunoResearch Laboratories Inc.) and chemiluminescence reagents (Pierce Biotechnology Inc., Rockford, IL). Membranes were stained with Ponceau S to confirm equal loading and transfer of samples. Semi-quantitative analysis of the relative abundance of CD68/ED1 immunoreactivity was based on the intensity of immunoreactive bands normalized by beta-actin using Quantity One 1-D Analysis Software (Bio-Rad Laboratories, Inc.).
Data analysis
Statistical analysis was done by ANOVA followed by Student’s t test or ANOVA and Scheffe’s test for multiple comparisons (SigmaStat, SPSS Science, San Raphael, CA). p values <0.05 are considered significant.
Results
Retrograde and anterograde transport of gp120
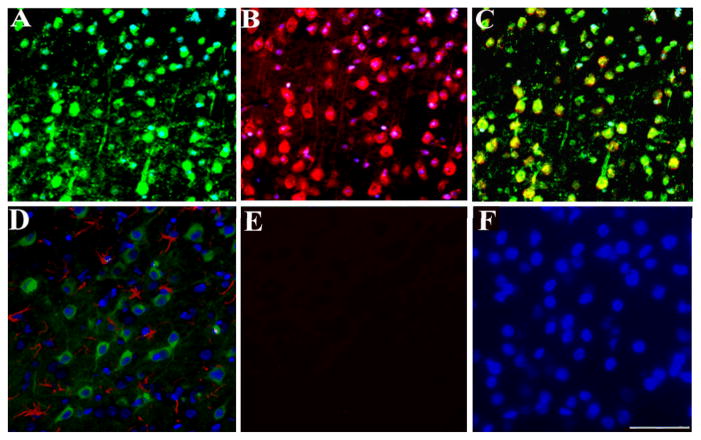
To determine whether gp120 is retrogradely transported in the CNS, rats were microinjected into the superior colliculus with FR together with gp120. FR, a retrogradely transported fluorescent marker, has been shown to produce reasonable and stable retrograde staining of neurons within a few days after injection (Choi et al., 2002). As a control, FR was injected together with heat-inactivated gp120. Rats were euthanized 3 or 18 days after the injection. FR and gp120 immunoreactivity were first analyzed in the RGC which give rise to projections to the superior colliculus. In control rats (FR and heat-inactivated gp120), only FR was detected in RGC (Figs. 1A and B) at any time point. Conversely, in FR and gp120-treated rats, we observed accumulation of gp120 immunoreactivity in RGC at both times examined. In fact, by 3 days, 76% ± 6 of FR positive RCG were also gp120 positive (Figs. 1C and D). By 18 days post-treatment, 48% ± 5 of FR positive RGC exhibited gp120 immunoreactivity (Figs. 1E and F).
Figure 1. gp120 injected into the superior colliculus is retrogradely transported to RGC.
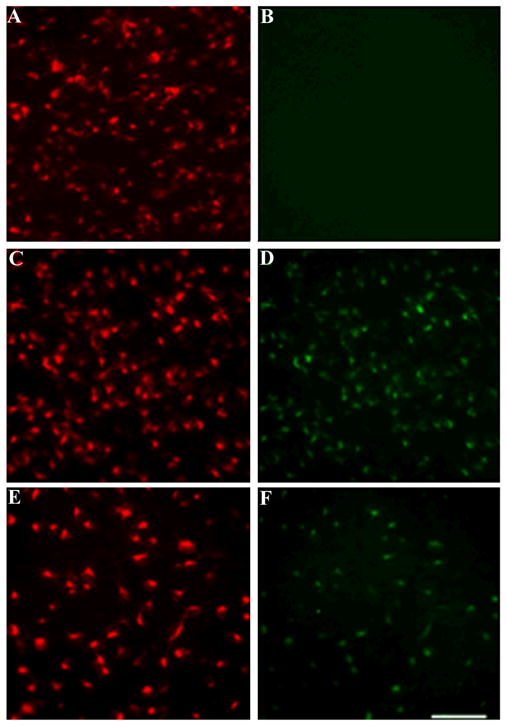
Rats (n=4 each group) were microinjected with FR and heat-inactivated gp120 (control) or FR and gp120 and sacrificed 3 or 18 days later. RGC were analyzed for FR (A, C and E) and gp120 immunoreactivity (B, D and F). A and B, control rats; C and D, rats sacrificed 3 days after the injection of gp120; E and F, rats sacrificed 18 days after gp120 injection. Images are representative of seventy two microscopic fields of retina per animal. By 3 days post-treatment, 35 ± 6 cells per field were gp120 positive (equivalent to ~76% of FR positive cells). By 18 days, 22 ± 7 cells per field were gp120 positive (~48% of FR positive cells). Scale bar = 100μm.
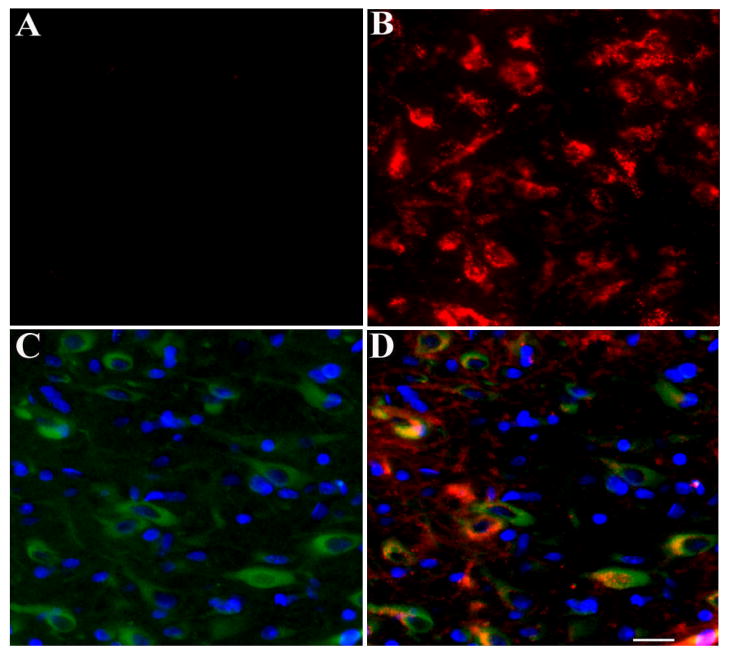
The superior colliculus receives afferents from a number of cortical areas (Sefton and Dreher, 1995) but also projects to the lateral geniculate body (LGB) and other brain areas (Linke et al., 1999; Sefton and Dreher, 1995). LGB, in turn, projects to the visual cortex. Therefore, gp120 can reach the visual cortex by an anterograde transport. Anterograde transport in vivo can be examined with DiI, a non-toxic carbocyanine dye that has been shown to produce reasonable and long-lasting stable anterograde staining of neurons within a few days after injection (Choi et al., 2002). Therefore, gp120 was microinjected into the superior colliculus together with DiI. As a control, rats were also microinjected with DiI and heat-inactivated gp120. Rats were sacrificed 15 days after the injection. In control rats, only DiI was detected in the occipital cortex (Figs. 2A and B). Conversely, in DiI- and gp120-treated rats, we detected gp120 immunoreactivity and DiI in the occipital cortex (Figs. 2C and D). Moreover, several cortical areas distant from the injection site were positive for gp120. These included the cingulated, frontal and visual cortices, the LGB, pontine reticular nucleus and subcoeruleus nucleus (Table 1). Overall, the occipital cortex exhibited more gp120 immunoreactivity than the parietal cortex supporting the well known direct anatomical connection of these areas with the superior colliculus. In addition, gp120 immunoreactivity was confined to neurofilament positive cells (Figs. 3A–C). In fact, no gp120 immunorecativity was detected in GFAP positive cells (Fig. 3D) supporting previous suggestions that gp120 accumulates within neurons (Bachis et al., 2003; Bachis et al., 2006). To examine whether gp120 causes autoproduction of antibodies, sections were stained only with a secondary antibody. No gp120 immunoreactivity was detected in these sections (Figs. 3E and F).
Figure 2. Anterograde transport of gp120.
DiI and heat-inactivated gp120 (control) or DiI and gp120 were microinjected into the superior colliculus. Rats were sacrificed 15 days later and the occipital cortex was analyzed for gp120 immunoreactivity (A and C) and DiI (B and D). A and B, control rats; C and D, gp120-treated rats. C: example of a section stained for gp120 (green) and DAPI nuclear stain (blue). D: example of a section stained for gp120 (green), DiI (red), and DAPI (blue) (yellow=red and green). Images are representative of ten sections per animal (n=4 each group). Scale bar = 20 μm.
Table 1.
Axonal transport of gp120 from the superior colliculus
| Brain Areas | Retrograde transport | Anterograde transport |
|---|---|---|
| Retina | ++++ | |
| Cingulate cortex (Areas 1 & 3) | +++ | |
| Frontal cortex (Aresa 1, 2 &3) | +++ | |
| Parietal cortex (Areas 1 & 2) | + | |
| Pontine reticular nucleus | + | |
| Subcoeruleus nucleus | + | |
| LGN | +++ | |
| Occipital cortex (Area 2) | ++ | ++ |
| Mono/Binocular cortex | ++ | ++ |
Gp120 immunoreactivity was determined in serial sections throughout the brain using a gp120 specific antibody (see Methods). FR and DiI were used to confirm retrograde and anterograde transport, respectively. Brain areas indicated above are identified as described in Paxinos and Watson (1986). + denotes relative abundance of gp120 immunoreactivity in each area.
Figure 3. Transport of gp120 occurs in neurons but not in astrocytes.
Rats were microinjected with gp120 into the superior colliculus. Three days later, rats were sacrificed and gp120 immunoreactivity was analyzed in serial sections from the visual cortex. A, example of a section stained for neurofilament (green) and DAPI (blue). B, example of a section stained for gp120 (red) and DAPI (blue). C, merge of A and B. D, example of a section stained for GFAP (red), DAPI (blue) and gp120 (green). E, example of a section in which the primary antibody for gp120 was omitted. F, section in E was counterstained for DAPI (blue). Images are representative of ten sections per animal. Scale bar= 100μm.
Gp120 is not transported by the sciatic nerve
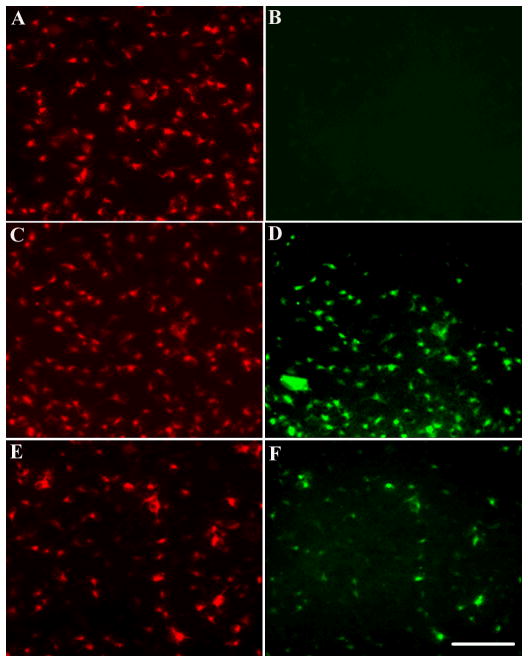
Axonal transport has been historically demonstrated using peripheral nerves. Rat peripheral nerves are sensitive to gp120. In fact, exposure of the sciatic nerve to gp120 produces axonal swelling and edema of the ipsilateral hind paw and weakness of the ipsilateral dorsal flexor muscles (Eliav et al., 1999; Herzberg and Sagen, 2001). Moreover, in DRG cultures, gp120 causes axonal degeneration (Melli et al., 2006). We then examined whether the sciatic nerve is capable of transporting gp120 retrogradely to the dorsal root ganglia. FR and gp120 were injected into the crushed sciatic nerve. FR and gp120 immunoreactivity were then analyzed 3 and 7 days later. FR was detected by 3 days (Figs. 4A and B) as well as at 7 days (data not shown) in both DRG and the sciatic nerve. Instead, gp120 was undetected at 3 (Figs. 4C and D) and 7 days (data not shown), suggesting that only FR is axonally transported. Three additional groups of animals were used to further confirm that gp120 is not transported in the sciatic nerve. One group of rats received an injection of gp120 (400 ng) in the hindpaw. In the other group of animals, a gelfoam containing gp120 (400 ng) was applied to the nerve 2–3 mm proximal to the trifurcation. The third group of animals received gp120 ~2 mm distal to DRG. Gp120 immunoreactivity was then determined at 1 and 3 days after the injection/gelfoam. No gp120 immunoreactivity was observed in any group tested in these experiments (data not shown).
Figure 4. gp120 is not axonally transported by the sciatic nerve.
Rats were injected into the sciatic nerve with FR and gp120. Animals were sacrificed 3 days after the injection. A. FR-labeled DRG; B, FR labeled fibers in the sciatic nerve; C and D, sections containing DRG or fibers of the sciatic nerve, respectively, stained with gp120 antibody. Bar=40 μm.
Neurons transporting gp120 exhibit apoptosis
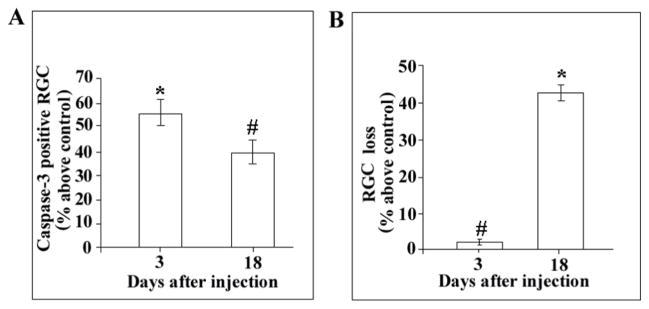
Neurons that internalize gp120 in vitro exhibit apoptosis (Bachis et al., 2003). To examine whether axonally transported gp120 induces apoptosis, we analyzed activated caspase-3 immunoreactivity in RGC of rats microinjected in the superior colliculus with FR and gp120. FR and heat-inactivated gp120 were used as a control. Animals were then sacrificed 3 or 18 days after the injection and apoptosis measured by counting the number of caspase-3/FR positive cells in representative fields of the retina. Control rats did not exhibited caspase-3/FR positive cells (Figs. 5A and B). In contrast, in gp120-treated rats, caspase-3 immunoreactivity was observed in several FR-positive cells at both 3 (Figs. 5C and D) and 18 days (Figs. 5E and F). Analysis by MetaMorph® revealed that gp120 elicited an increase in caspase-3 positive cells in the retina (Fig. 6A). At least 55% and 40% of RGC were caspase-3 positive by 3 and 18 days, respectively (Fig. 6A). The overall survival of RGC was decreased by gp120 because by 18 days at least 45% of neurons were lost (Fig. 6B), suggesting that caspase-3 positive RGC undergo cell death.
Figure 5. Gp120 induces apoptosis.
Rats were microinjected into the superior colliculus with FR and heat-inactivated gp120 (control) or FR and gp120. Animals were then sacrificed 3 or 18 days after the injection. The retinae were dissected and prepared for FR (A, C, and E) and caspase-3 (B, D and F) staining. Examples of RGC in control (A-B) and gp120-treated rats at 3 (C–D) and 18 days (E–F). Red=FR, green= caspase-3 immunoreactivity. Bar=40 μm.
Figure 6. gp120-treated rats exhibit apoptosis and neuronal loss.
Rats were treated as described in Figure 5. The number of caspase-3 positive RGC (A) and FR labeled cells (B) was counted using MetaMorph®. By 3 and 18 days, at least 25 ± 6 and 18 ± 3 cells per field, respectively, were caspase-3 positive. Data expressed as % above control are the mean ± SEM of 4 animals each group. #p<0.05, *<p0.005 vs control.
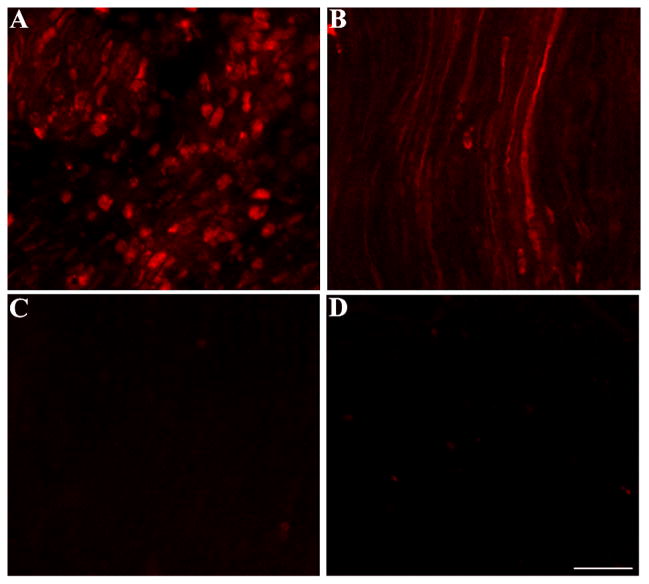
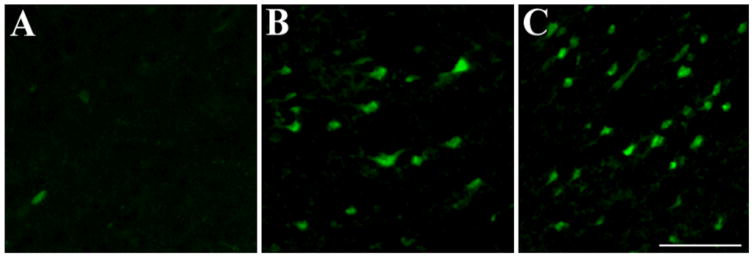
To further establish a correlation between anterograde/retrograde gp120 transport and cell death, we processed sections from the visual cortex for fluoro-Jade, an anionic fluorochrome capable of selectively staining degenerating neurons. In gp120 treated rats, we observed a time-dependent increase in fluoro-Jade positive cells in the visual cortex (Fig. 7) supporting the hypothesis that gp120 can also cause anterograde degeneration.
Figure 7. Anterogradely transported gp120 causes cell loss.
Rats, injected with heat-inactivated gp120 (control) or gp120 into the superior colliculus, were sacrificed 3 or 18 days after the injection. Sections were prepared throughout the visual cortex and stained for fluoro-jade (green). A, control animals; B and C, gp120-treated rats sacrificed 3 or 18 days post injection, respectively. Bar =100μm.
Gp120 activates microglia
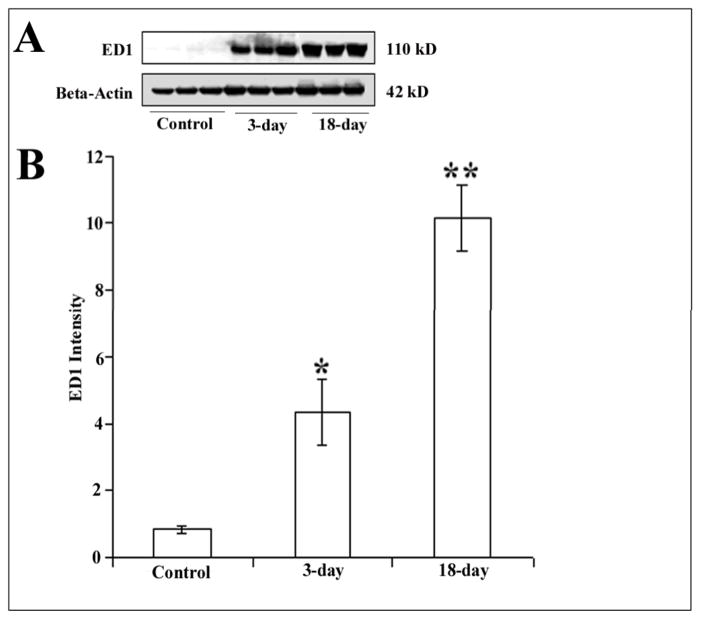
The mechanisms leading to cognitive impairment and dementia in HAD are not completely understood. Recent studies have suggested that infected monocytes cross the blood brain barrier and activate microglia (Everall et al., 2005; Persidsky and Gendelman, 2003). These cells, in turn, secrete cytokines and other neurotoxins which cause neuronal dysfunction, injury, and apoptosis. The ability of gp120 to induce an increase in neuronal apoptosis distal to the injection site prompted us to examine the role of microglia in gp120-mediated neurotoxicity. Activated microglia was determined by measuring ED1 immunoreactivity in cortical lysates from control and gp120-treated rats as well as hystological analyses of CD11 positive cells. Western blot analysis revealed a time-dependent increase in ED1 immunoreactivity in gp120-treated rats (Fig. 8A). In fact, at 18 days post injection more ED1 immunoreactivity was detected than at 3 days (Fig. 8B). Similarly, fewer CD11 positive cells were detected at 3 days than 18 days (data not shown). Interestingly, at 18 days, we observed a number of caspase-3 positive nuclei surrounded by microglia cells (Fig. 9), suggesting that apoptotic neurons might promote microglia cells activation.
Figure 8. gp120 increases ED1 immunoreactivity.
Rats were injected into the superior colliculus with heat-inactivated gp120 or gp120. 3 or 18 days later, rats were euthanized and their visual cortex was dissected. Cortical lysates were prepared for Western blot analysis of ED1 immunoreactivity as described in Methods. A. Representative Western blot analysis of ED1 immunoreactivity in the visual cortex of control and gp120-treated rats (3 and 18 days post-treatment). Beta-Actin was used to control for protein loading. B. Immunoreactive bands were quantified by Quantity One 1-D Analysis Software. Data, expressed as intensity of immunoreactive bands, are the mean ± SEM of six independent samples per group. *p<0.05, **p<0.01 vs control.
Figure 9. Confocal analysis of cortical sections.
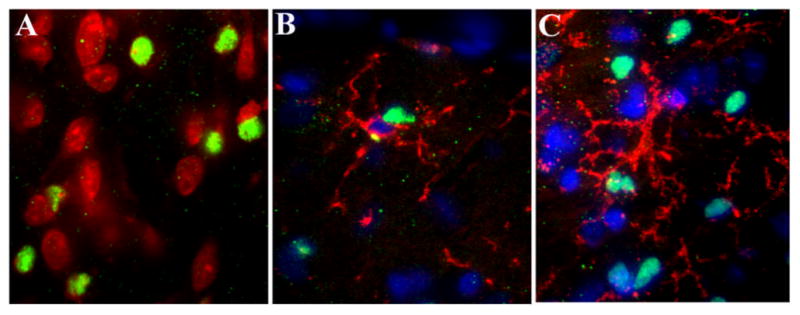
Rats were treated as described in Fig. 7. Coronal sections through the visual cortex were prepared, stained for Nissl, caspase-3, cd11b and analyzed by confocal imaging as described in Methods. A, example of a section of gp120-treated rats stained for Nissl (red) and caspase-3 (green). B and C, examples of coronal sections stained for caspase-3 (green), CD11b/OX42 (red) and counterstained with DAPI (blue) of rats euthanized 3 or 18 days after injection, respectively. Note that microglia cells have their processes encircling the outside of caspase-3 positive cells. Mag 40X.
Discussion
One of the characteristics of HAD is neuronal loss. Productive HIV-1 infection does not occur in neurons; rather, it is confined to macrophages and related microglia (Lee et al., 1993; Persidsky and Gendelman, 2003). This apparent discrepancy has led to the consensus that indirect mechanisms may account for the toxic property of HIV. Indeed, experimental data have suggested that HIV damages neurons through toxic substances released from infected cells. These include cytokines and excitatory amino acids [reviewed in (Kaul et al., 2001)]. In this work, we present evidence that the HIV envelope protein gp120 is transported retrogradely and anterogradely to the soma of CNS neurons. Neurons transporting gp120 exhibit caspase-3, a marker of apoptosis, and die. Therefore, we propose that gp120 neuronal endocytosis and trafficking may contribute to neuronal loss observed in HAD. This suggestion may help explain the initial process of HIV neurotoxicity by invoking a direct neurotoxic effect of gp120.
The axonal transport of gp120 may involve a series of complex events far from being fully characterized. We have previously shown that gp120IIIB is internalized by neurons through a CXCR4-dependent mechanism (Bachis et al., 2006). CXCR4 is the co-receptor that mediates productive infection of the T-tropic strain of HIV (Blauvelt et al., 1997). CXCR4 is a G-protein coupled receptor that undergoes spontaneous and ligand-induced internalization (Orsini et al., 1999; Tarasova et al., 1998). While internalization of CXCR4 plays a physiological role in the regulation of membrane-associated receptors, the same internalization process may be equally crucial to explain the neurotoxic effect of gp120. In fact, blocking gp120 neuronal internalization with AMD3100 (Bachis et al., 2006), an inhibitor of CXCR4 (Donzella et al., 1998) or brain-derived neurotrophic factor, a neurotrophin that down-regulates CXCR4 (Nosheny et al., 2007), rescues neurons from cell death. Thus, we propose that gp120 accumulates in microglia or other non-neuronal cells until it is released from these cells. Gp120 is then internalized by neurons in a CXCR4-dependent manner and is axonally transported to the neuronal cell body, where it activates apoptosis. These events may occur independently from the presence of productive viral infection.
Several lines of independent investigations support the notion that a ligand-tyrosine kinase receptor complex physically translocates from nerve terminal to cell bodies to mediate a retrograde signal that could lead to cell survival or death. For instance, in the case of the neurotrophins, and in particular of nerve growth factor (NGF), axonal transport plays a crucial role in the survival of sympathetic and sensory neurons (Hendry et al., 1974; Johnson et al., 1987). Cellular events involved in this transport include NGF binding to TrkA receptor, which in its phosphorylated state, in turn is retrogradely transported toward the cell body (Riccio et al., 1997; Watson et al., 1999). In the case of gp120 however, axonal transport leads to cell death. Both survival and cell death processes are highly dependent upon the microtubular system because they are blocked by colchicine, a compound that impairs the polymerization of microtubules (Brown, 2003). In fact, we have recently shown that colchicine blocks the neurotoxic effect of gp120 in the nigro-striatal system (Bachis et al., 2006). In this work, we have provided additional evidence that gp120 axonal transport delivers a neurotoxic signal to different neuronal populations. In fact, RGC, as well as a number of cortical neurons that were gp120 positive, exhibited caspase-3 immunoreactivity and died a few days after gp120 injections. Thus, our data suggest that axonal transport of gp120 might be a crucial event involved in the neurotoxic activity of HIV. Nevertheless, more experiments are needed to prove this hypothesis because a direct link between gp120 and caspase-3 in human brain has not yet been established.
In addition to motor and memory impairments, AIDS patients exhibit chronic pain, and at least 1/3 of these patients develop sensory neuropathy (Cornblath and McArthur, 1988; Simpson and Tagliati, 1995). The underlying cause of this pathology is still under investigation. Interestingly, a number of studies have failed to show a correlation between HIV-1 RNA levels and severity of neurological symptoms. On the other hand, experimental data have suggested that gp120 may be the cause of chronic pain seen in HIV patients. In fact, intrathecal injection of gp120 has been shown to induce thermal hyperalgesia and mechanical allodynia (Herzberg and Sagen, 2001; Milligan et al., 2001), two characteristics of a chronic neuropathy-like pain syndrome commonly seen in AIDS patients. Allodynia and other neuropathological effects of HIV may be related to the ability of gp120 to promote activation of immune responses, including the release of proinflammatory cytokines from activated astrocytes and/or microglia in the dorsal spinal cord (Milligan et al., 2001). On the other hand, T-tropic gp120 has been shown to promote DRG axonal damage (Melli et al., 2006). These considerations lead to the hypothesis that T-tropic gp120 could be retrogradely transported in peripheral nerves and cause axonal damage by apoptosis similar to the scenario that we observed in the brain. In this work, we have failed to detect gp120 transport through the sciatic nerve. Analysis of CXCR4 immunoreactivity has revealed that this receptor is expressed by Schwann cells and not by nerve fibers (supplement material). Our previous results have shown that only neurons internalize gp120 (Bachis et al., 2006). Therefore, gp120 may be transported into DRG only when applied to axons as suggested by recent studies using in vitro cultures of DRG (Melli et al., 2006). However, delivering gp120 to DRG sensory axons in vivo can be challenging. We have utilized injection of gp120 in the paw as a non-invasive method to deliver gp120 in DRG motor axons. We have failed to detect gp120 immunoreactivity in DRG. Nevertheless, we cannot exclude that at the site of injection gp120 could diffuse out and not be able to bind to axonal CXCR4 receptors. Future experiments are needed to test this hypothesis.
The role of microglia and inflammatory responses in the neuropathology of HAD has been suggested. Infected microglia may alter neuronal function and survival through multiple mechanisms. These cells are a significant source of neurotoxins that may contribute to HAD. These toxic factors include, but are not limited to, chemokines (Albright et al., 2003), eicosanoids (Genis et al., 1992), excitatory amino acids (Jiang et al., 2001), kinurenic acid (Kerr et al., 1997) and viral proteins (Kaul et al., 2001; Kolson and Gonzalez-Scarano, 2001; Nath et al., 1999). In this study, we have shown that gp120 increases ED1 immunoreactivity and the number of CD11b positive cells, suggesting an activation of microglia by the viral protein. However, activated microglia was mainly seen following rather than preceding apoptosis. Moreover, most of CD11 positive cells appeared to be “ameboid” microglia surrounding apoptotic neurons rather than resting “microglia”. The former type of microglia is believed to be involved in the elimination of apoptotic cells (Fiedorowicz et al., 2008). Thus, it appears that gp120 injection promotes a change from resting state to an active state of the microglia migrating toward dying neurons. We propose that gp120 first induces neuronal apoptosis which, in turn, triggers a microglia response. This cascade of events, if unchallenged, would ultimately lead to widespread apoptosis of a larger population of neurons and likely contribute to the neuropathogenicity of HIV-1.
Supplementary Material
Acknowledgments
Supported by HHS grants NS040670 and NS051656. Special thank to NIH AIDS Research Reference Reagent Program for the CXCR4 antibody and to Diane Leader for editorial assistance.
References
- Ahmed FA, Chaudhary P, Sharma SC. Effects of increased intraocular pressure on rat retinal ganglion cells. Int J Dev Neurosci. 2001;19:209–218. doi: 10.1016/s0736-5748(00)00073-3. [DOI] [PubMed] [Google Scholar]
- Albright AV, Soldan SS, Gonzalez-Scarano F. Pathogenesis of human immunodeficiency virus-induced neurological disease. J Neurovirol. 2003;9:222–7. doi: 10.1080/13550280390194073. [DOI] [PubMed] [Google Scholar]
- Bachis A, Major EO, Mocchetti I. Brain-derived neurotrophic factor inhibits human immunodeficiency virus-1/gp120-mediated cerebellar granule cell death by preventing gp120 internalization. J Neurosci. 2003;23:5715–5722. doi: 10.1523/JNEUROSCI.23-13-05715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Aden SA, Nosheny RL, Andrews PM, Mocchetti I. Axonal transport of Human Immunodeficiency Virus Type 1 envelope glycoprotein 120 is found in association with neuronal apoptosis. J Neurosci. 2006;26:6771–6780. doi: 10.1523/JNEUROSCI.1054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer EL, Breakefield XO, Schuback D, Reese TS, LaVail JH. Retrograde axonal transport of herpes simplex virus: Evidence for a single mechanism and a role for tegument. Proc Natl Acad Sci USA. 2000;97:8146–8150. doi: 10.1073/pnas.97.14.8146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead RM, Frankfurter A. A direct projection from the retina to the intermediate gray layer of the superior colliculus demonstrated by anterograde transport of horseradish peroxidase in monkey, cat and rat. Exp Brain Res. 1983;52:261–8. doi: 10.1007/BF00236635. [DOI] [PubMed] [Google Scholar]
- Blauvelt A, Asada H, Saville MW, Klaus-Kovtun V, Altman DJ, Yarchoan R, Katz SI. Productive infection of dendritic cells by HIV-1 and their ability to capture virus are mediated through separate pathways. J Clin Invest. 1997;100:2043–53. doi: 10.1172/JCI119737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchelt DR, Koliatsos VE, Guarnieri M, Pardo CA, Sisodia SS, Price DL. Rapid anterograde axonal transport of the cellular prion glycoprotein in the peripheral and central nervous systems. J Biol Chem. 1994;269:14711–14714. [PubMed] [Google Scholar]
- Brown A. Axonal transport of membranous and nonmembranous cargoes: a unified perspective. J Cell Biol. 2003:817–821. doi: 10.1083/jcb.200212017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Chauhan A, Dimayuga FO, Gee J, Keller JN, Nath A. Synaptic transport of human immunodeficiency virus-Tat protein causes neurotoxicity and gliosis in rat brain. J Neurosci. 2003;23:8417–8422. doi: 10.1523/JNEUROSCI.23-23-08417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budka H, Costanzi G, Cristina S, Lechi A, Parravicini C, Trabattoni R, Vago L. Brain pathology induced by infection with the human immunodeficiency virus (HIV). A histological, immunocytochemical, and electron microscopical study of 100 autopsy cases. Acta Neuropathol. 1987;75:185–98. doi: 10.1007/BF00687080. [DOI] [PubMed] [Google Scholar]
- Choi D, Li D, Raisman G. Fluorescent retrograde neuronal tracers that label the rat facial nucleus: a comparison of Fast Blue, Fluoro-ruby, Fluoro-emerald, Fluoro-Gold and DiI. J Neurosci Meths. 2002;117:167–172. doi: 10.1016/s0165-0270(02)00098-5. [DOI] [PubMed] [Google Scholar]
- Cornblath DR, McArthur JC. Predominantly sensory neuropathy in patients with AIDS and AIDS-related complex. Neurology. 1988;38:794–6. doi: 10.1212/wnl.38.5.794. [DOI] [PubMed] [Google Scholar]
- Donnelly JF, Thompson SM, Robertson RT. Organization of projections from the superior colliculus to the thalamic lateral posterior nucleus in the rat. Brain Res. 1983;288:315–9. doi: 10.1016/0006-8993(83)90109-9. [DOI] [PubMed] [Google Scholar]
- Donzella GA, Schols D, Lin SW, Este JA, Nagashima KA, Maddon PJ, Allaway GP, Sakmar TP, Henson G, De Clercq E, Moore JP. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–7. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- Eilbott DJ, Peress N, Burger H, LaNeve D, Orenstein J, Gendelman HE, Seidman R, Weiser B. Human immunodeficiency virus type 1 in spinal cords of acquired immunodeficiency syndrome patients with myelopathy: expression and replication in macrophages. Proc Natl Acad Sci U S A. 1989;86:3337–41. doi: 10.1073/pnas.86.9.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliav E, Herzberg U, Ruda MA, Bennett GJ. Neuropathic pain from an experimental neuritis of the rat sciatic nerve. Pain. 1999;83:169–182. doi: 10.1016/s0304-3959(99)00102-5. [DOI] [PubMed] [Google Scholar]
- Everall I, Luthert P, Lantos P. A review of neuronal damage in human immunodeficiency virus infection: its assessment, possible mechanism and relationship to dementia. J Neuropathol Exp Neurol. 1993;52:561–566. doi: 10.1097/00005072-199311000-00002. [DOI] [PubMed] [Google Scholar]
- Everall IP, Hansen LA, Masliah E. The shifting patterns of HIV encephalitis neuropathology. Neurotox Res. 2005;8:51–61. doi: 10.1007/BF03033819. [DOI] [PubMed] [Google Scholar]
- Fiedorowicz A, Figiel I, Zaremba M, Dzwonek K, Oderfeld-Nowak B. The ameboid phenotype of NG2 (+) cells in the region of apoptotic dentate granule neurons in trimethyltin intoxicated mice shares antigen properties with microglia/macrophages. Glia. 2008;56:209–222. doi: 10.1002/glia.20605. [DOI] [PubMed] [Google Scholar]
- Genis P, Jett M, Bernton EW, Boyle T, Gelbard HA, Dzenko K, Keane RW, Resnick L, Mizrachi Y, Volsky DJ, et al. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: implications for the neuropathogenesis of HIV disease. J Exp Med. 1992;176:1703–18. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry IA, Stockel K, Thoenen H, Iversen LL. The retrograde axonal transport of nerve growth factor. Brain Res. 1974;68:103–21. doi: 10.1016/0006-8993(74)90536-8. [DOI] [PubMed] [Google Scholar]
- Herzberg U, Sagen J. Peripheral nerve exposure to HIV viral envelope protein gp120 induces neuropathic pain and spinal gliosis. J Neuroimmunol. 2001;116:29–39. doi: 10.1016/s0165-5728(01)00288-0. [DOI] [PubMed] [Google Scholar]
- Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- Janssen RS, Cornblath DR, Epstein LG, McArthur J, Price RW. Human immunodeficiency virus (HIV) infection and the nervous system: report from the American Academy of Neurology AIDS Task Force. Neurology. 1989;39:119–22. doi: 10.1212/wnl.39.1.119. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, Piggee C, Heyes MP, Murphy C, Quearry B, Bauer M, Zheng J, Gendelman HE, Markey SP. Glutamate is a mediator of neurotoxicity in secretions of activated HIV-1-infected macrophages. J Neuroimmunol. 2001;117:97–107. doi: 10.1016/s0165-5728(01)00315-0. [DOI] [PubMed] [Google Scholar]
- Johnson E, Taniuchi M, Clark H, Springer J, Koh S, Tayrien M, Loy R. Demonstration of the retrograde transport of nerve growth factor receptor in the peripheral and central nervous system. J Neurosci. 1987:923–929. doi: 10.1523/JNEUROSCI.07-03-00923.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 1999;96:8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–94. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kerr SJ, Armati PJ, Pemberton LA, Smythe G, Tattam B, Brew BJ. Kynurenine pathway inhibition reduces neurotoxicity of HIV-1-infected macrophages. Neurology. 1997;49:1671–81. doi: 10.1212/wnl.49.6.1671. [DOI] [PubMed] [Google Scholar]
- Kolson DL, Gonzalez-Scarano F. HIV-associated neuropathies: role of HIV-1, CMV, and other viruses. J Peripher Nerv Syst. 2001;6:2–7. doi: 10.1046/j.1529-8027.2001.006001002.x. [DOI] [PubMed] [Google Scholar]
- Lee SC, Hatch WC, Liu W, Kress Y, Lyman WD, Dickson DW. Productive infection of human fetal microglia by HIV-1. Am J Pathol. 1993;143:1032–9. [PMC free article] [PubMed] [Google Scholar]
- Linke R, De Lima AD, Schwegler H, Pape HC. Direct synaptic connections of axons from superior colliculus with identified thalamo-amygdaloid projection neurons in the rat: possible substrates of a subcortical visual pathway to the amygdala. J Comp Neurol. 1999;403:158–70. [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Matarrese P, Malorni W. Human immunodeficiency virus (HIV)-1 proteins and cytoskeleton: partners in viral life and host cell death. Cell Death Differ. 2005;12:932–941. doi: 10.1038/sj.cdd.4401582. [DOI] [PubMed] [Google Scholar]
- Melli G, Keswani SC, Fischer A, Chen W, Hoke A. Spatially distinct and functionally independent mechanisms of axonal degeneration in a model of HIV-associated sensory neuropathy. Brain. 2006;129:1330–8. doi: 10.1093/brain/awl058. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, O’Connor KA, Nguyen KT, Armstrong CB, Twinning C, Gaykema RPA, Holguin A, Martin D, Maier SF, Watkins LR. Intrathecal HIV envelope protein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J Neurosci. 2001;21:2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Conant K, Chen P, Scott C, Major EO. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J Biol Chem. 1999;274:17098–102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Amhed F, Yakovlev AG, Meyer EM, Ren K, Tessarollo L, Mocchetti I. Brain-derived neurotrophic factor prevents the nigrostriatal degeneration induced by human immunodeficiency virus-1 glycoprotein 120 in vivo. Eur J Neurosci. 2007;25:2275–2284. doi: 10.1111/j.1460-9568.2007.05506.x. [DOI] [PubMed] [Google Scholar]
- Orsini MJ, Parent J-L, Mundell SJ, Benovic JL. Trafficking of the HIV Coreceptor CXCR4. Role of arrestins and identificaton of residues in the C-terminas tail that medaite receptor internalization. J Biol Chem. 1999;274:31076–31086. doi: 10.1074/jbc.274.43.31076. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat and Brain in Stereotaxic Coordinates. Vol. 2. Academic Press; Sydney: 1986. [Google Scholar]
- Persidsky Y, Gendelman HE. Mononuclear phagocyte immunity and the neuropathogenesis of HIV-1 infection. J Leukoc Biol. 2003;74:691–701. doi: 10.1189/jlb.0503205. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Westby GW, Dean P. Functional architecture of rodent superior colliculus: relevance of multiple output channels. Prog Brain Res. 1993;95:69–77. doi: 10.1016/s0079-6123(08)60358-1. [DOI] [PubMed] [Google Scholar]
- Riccio A, Pierchala BA, Ciarallo CL, Ginty DD. An NGF-TrkA-mediated retrograde signal to transcription factor CREB in sympathetic neurons. Science. 1997:1097–1100. doi: 10.1126/science.277.5329.1097. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Albertson C, Slikker W., Jr Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- Sefton AJ, Dreher B. Visual system. In: Paxinos G, editor. The rat nervous system. Vol. 2. Academic Press; San Diego, CA: 1995. pp. 833–898. [Google Scholar]
- Simpson DM, Tagliati M. Nucleoside analogue-associated peripheral neuropathy in human immunodeficiency virus infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:153–61. [PubMed] [Google Scholar]
- Tarasova NI, Stauber RH, Michejda CJ. Spontaneous and ligand-induced trafficking of CXC-chemokine receptor 4. J Biol Chem. 1998;273:15883–6. doi: 10.1074/jbc.273.26.15883. [DOI] [PubMed] [Google Scholar]
- Tornatore C, Chandra R, Berger JR, Major EO. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology. 1994;44:481–487. doi: 10.1212/wnl.44.3_part_1.481. [DOI] [PubMed] [Google Scholar]
- Watson FL, Heerssen DB, Moheban MZ, Lin MZ, Sauvageot CM, Bhattacharyya A, Pomeroy SL, Segal RA. Rapid nuclear responses to target-derived neurotrophins require retrograde transport of ligand-receptor complex. J Neurosci. 1999:7889–7900. doi: 10.1523/JNEUROSCI.19-18-07889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westby GW, Keay KA, Redgrave P, Dean P, Bannister M. Output pathways from the rat superior colliculus mediating approach and avoidance have different sensory properties. Exp Brain Res. 1990;81:626–38. doi: 10.1007/BF02423513. [DOI] [PubMed] [Google Scholar]
- Zheng J, Thylin MR, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, Zeng YC, Gelbard HA, Shepard RB, Swartz JM, Gendelman HE. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.