Abstract
Mechanisms that preserve endothelial cell (EC) integrity remain elusive, but are critical for new strategies directed against endocrine disorders such as diabetes mellitus (DM). Here we demonstrate in primary cerebral ECs with a clinically relevant model of elevated D-glucose that Akt1 and the post-translational modification and subcellular trafficking of the forkhead transcription factor FoxO3a are critical for early apoptotic membrane signaling and subsequent degradation of nuclear DNA. FoxO3a also directly governs apoptotic mitochondrial signal transduction pathways, since gene knockdown of FoxO3a prevents mitochondrial membrane depolarization as well as the release of cytochrome c. Control of this apoptotic cascade extends to the rapid and progressive activation of caspases. The presence of FoxO3a is necessary for cleaved (active) caspase 1 and 3 expression, since loss of FoxO3a abrogates the induction of caspase activity. Our work identifies Akt1, FoxO3a and closely aligned pathways as key therapeutic targets during impaired glucose tolerance and DM.
Key Words: Akt1, cell demise, diabetes, endothelial
Sub-key Words: (Rat cerebral endothelial cells)
1. Introduction
The endocrine disorder diabetes mellitus (DM) has become a significant health concern for both young and older populations (Donahoe et al., 2007, Erol, 2009, Maiese et al., 2009e). It is predicted that more than 360 million individuals will be afflicted with DM and its debilitating conditions by the year 2030. Individuals with DM can develop a number of disorders that occur as a result of vascular system disease leading to immune dysfunction, cognitive impairment, renal disease, hematological disease, neurodegenerative disease, and cardiovascular disease (Gossai and Lau-Cam, 2009, Guarnieri et al., 2009, Huber et al., 2006, Kuhad et al., 2009, Maiese, 2008, Xu et al., 2004). Loss of vascular endothelial cells (ECs) during DM can occur through apoptotic cell death and oxidative stress since hyperglycemia can lead to increased production of reactive oxygen species (Ceriello et al., 1996, Chong et al., 2007, Yano et al., 2004). Induction of early apoptotic programs that lead to the externalization of phosphatidylserine (PS) residues also can result in phagocytosis by surrounding inflammatory cells and eventual degradation of nuclear DNA (Chong and Maiese, 2008, Kang et al., 2003a, Soares et al., 2008). Furthermore in clinical studies, acute glucose swings in addition to chronic hyperglycemia can trigger oxidative stress mechanisms, illustrating the importance for therapeutic interventions during acute and sustained hyperglycemic episodes to protect the vascular system (Monnier et al., 2006).
Given the susceptibility of ECs during periods of hyperglycemia, elucidation of the underlying cellular mechanisms for vascular diabetic complications becomes vital for the development of new treatment strategies. One novel candidate pathway involves the forkhead transcription factor of the “O” class, FoxO3a, and its upstream control by protein kinase B (Akt1), which have an intricate relationship with cellular development, survival, immune function, and metabolism (Jonsson et al., 2005, Lappas et al., 2009, Lin et al., 1997, Maiese et al., 2008b, Maiese et al., 2009d, Ogg et al., 1997). In addition, polymorphisms of FOXO3a are associated with increased body mass (Kim et al., 2006) and a greater risk of stroke (Kuningas et al., 2007), suggesting an important role for FoxO3a in the development of diabetic complications.
Our work is the first to elucidate a highly novel series of functionally integrated pathways consisting of Akt1, FoxO3a, early and late apoptotic cell injury programs, mitochondrial membrane potential, cytochrome c, and caspases 1 and 3 that drive primary cerebral EC survival during a clinically relevant model of elevated D-glucose. Here we show that post-translational modification and subcellular trafficking of FoxO3a by Akt1 is critical for the induction of both early and late apoptotic injury programs involving cellular membrane PS externalization and nuclear DNA degradation. FoxO3a also is necessary to govern mitochondrial pore permeability and ultimately can lead to the release of cytochrome c. This control of FoxO3a over the apoptotic cascade extends to the rapid and robust activation of caspase 1 and caspase 3 which are known to mediate cellular membrane PS exposure and genomic DNA cleavage (Jessel et al., 2002, Maiese et al., 2005, Mallat et al., 2005, Mari et al., 2004, Takahashi et al., 1999). FoxO3a is necessary for cleaved (active) caspase 1 and 3 expression, since gene knockdown of FoxO3a abrogates the induction of caspase activity. Our work highlights the critical ability of Akt1, FoxO3a and integral apoptotic pathways to control primary cerebral EC injury and identifies this transcription factor as a potential therapeutic target during impaired glucose tolerance and DM.
2. Methods and Methods
2.1 Cerebral microvascular endothelial cell cultures
Per our prior protocols, vascular endothelial cells (ECs) were isolated from male Sprague-Dawley adult rat brain cerebra by using a modified collagenase/dispase-based digestion protocol (Chong et al., 2002, Chong and Maiese, 2007). Briefly, ECs were cultured in endothelial growth media consisting of M199E (M199 with Eagle’s salt) with 20% heat-inactivated fetal bovine serum, 2 mmol/l L-glutamine, 90 µg/ml heparin, and 20 µg/ml EC growth supplement (ICN Biomedicals, Aurora, OH). Cells from the third passage were identified by positive direct immunocytochemistry for factor VIII-related antigen (Chong et al., 2002, Chong and Maiese, 2007) and possessed characteristic spindle-shaped morphology with antigenic properties shown to resemble brain endothelium in vivo (Abbott et al., 1992). All animal experimentation was conducted in accord with accepted standards of humane animal care and NIH guidelines.
2.2 Experimental treatments
Elevated glucose concentrations in ECs was performed by replacing the media with serum-free M199E media with 2 mmol/l L-glutamine and 90 µg/ml heparin containing 20 mM D-glucose and then incubated at 37°C for 48 hours.
2.3 Assessment of cell survival
EC injury was determined by bright field microscopy using a 0.4% trypan blue dye exclusion method 48 hours following treatment with elevated D-glucose per our previous protocols (Chong et al., 2002, Chong and Maiese, 2007). The mean survival was determined by counting eight randomly selected non-overlapping fields with each containing approximately 10–20 cells (viable + non-viable). Each experiment was replicated 6 times independently with different cultures.
2.4 Assessment of DNA fragmentation
Genomic DNA fragmentation was determined by the terminal deoxynucleotidyl transferase nick end labeling (TUNEL) assay (Kang et al., 2003b). Briefly, ECs were fixed in 4% paraformaldehyde/0.2% picric acid/0.05% glutaraldehyde and the 3’-hydroxy ends of cut DNA were labeled with biotinylated dUTP using the enzyme terminal deoxytransferase (Promega, Madison, WI) followed by streptavidin-peroxidase and visualized with 3,3’-diaminobenzidine (Vector Laboratories, Burlingame, CA).
2.5 Assessment of membrane phosphatidylserine (PS) residue externalization
Phosphatidylserine (PS) exposure was assessed through the established use of annexin V. A 30 µg/ml stock solution of annexin V conjugated to phycoerythrin (PE) (R&D Systems, Minneapolis, MN) was diluted to 3 µg/ml in warmed calcium containing binding buffer (10 mmol/L Hepes, pH 7.5, 150 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 1.8 mmol/L CaCl2) (Kang et al., 2003b). Plates were incubated with 500 µl of diluted annexin V for 10 minutes. Images were acquired with "blinded" assessment with a Leitz DMIRB microscope (Leica, McHenry, IL) and a Fuji/Nikon Super CCD (6.1 megapixels) using transmitted light and fluorescent single excitation light at 490 nm and detected emission at 585 nm.
2.6 Expression of phosphorylated Akt1, total Akt1, phosphorylatedFoxO3a, total FoxO3a, and active caspase 1 and 3
ECs were homogenized and each sample (50 µg lane−1) was subjected to SDS-polyacrylamide gel electrophoresis (7.5% Akt1, FoxO3a; 12.5% caspase 1 and 3). After transfer, the membranes were incubated with a rabbit polyclonal antibody against phospho-Akt1 (Ser473, 1:1000, Cell Signaling, Beverly, MA), a rabbit antibody against total Akt1, a rabbit polyclonal antibody against phospho-FoxO3a (1:1000) (p-FoxO3a, Ser253, Cell Signaling, Beverly, MA), a rabbit antibody against total FoxO3a, a rabbit antibody against cleaved (active) caspase 1 (20 kDa) (1:1000), or a rabbit antibody against cleaved (active) caspase 3 (17 kDa) (1:1000) (Cell signaling Technology, Beverly, MA). Following washing, the membranes were incubated with a horseradish peroxidase (HRP) conjugated secondary antibody goat anti-rabbit IgG (1:2000, Zymed Laboratories, Carlsbad, CA). The antibody-reactive bands were revealed by chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ) and band density was performed using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/).
2.7 Gene knockdown of FoxO3a with small interfering RNA (siRNA)
To silence FoxO3a gene expression, the following sequences were synthesized (Ambion, Austin, Texas): the FoxO3a target sequence 5’- AAATCTAACTCATCTGCAAGT -3’, the siRNA sense strand 5’-AUCUAACUCAUCUGCAAGUUU -3’, and the antisense strand 5’-ACUUGCAGAUG AGUUAGAUUU -3’. Transfection of siRNA duplexes was performed with Lipofectamine 2000 reagent according to manufacturer guidelines (Invitrogen, Carlsbad, CA). Experimental assays were performed 72 hours post-transfection. For each siRNA assay, positive controls contain multiple siRNAs including the target siRNA and negative controls are absent of the target siRNA.
2.8 Assessment of mitochondrial membrane potential
The fluorescent probe JC-1 (Molecular Probes, Eugene, OR), a cationic membrane potential indicator, was used to assess the mitochondrial membrane potential. ECs in 35 mm dishes were incubated with 2 µg/ml JC-1 in growth medium at 37 °C for 30 min. The cultures were washed three times using fresh growth medium. Mitochondria were then analyzed immediately under a Leitz DMIRB microscope (Leica, McHenry, IL, USA) with a dual emission fluorescence filter with 515–545 nm for green fluorescence and emission at 585–615 nm for red fluorescence (Kang et al., 2003b).
2.9 Preparation of mitochondria for the analysis of cytochrome c release
After washing once with ice-cold PBS, cells were harvested at 10,000g for 15 min at 4°C and the resulting pellet was re-suspended in buffer A (20 mM HEPES, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.1 phenylmethylsulfonylfluoride) containing 250 mM sucrose and used as the mitochondrial fraction. The supernatant was subjected to ultracentrifugation at 50,000 g for 1 hour at 4°C with the resultant supernatant used as the cytosolic fraction (Kang et al., 2003b).
2.10 Immunocytochemistry for FoxO3a and caspase 3
For immunocytochemical staining of FoxO3a and cleaved caspase 3 (active form), ECs were fixed with 4% paraformaldehyde and permeabilized using 0.2% Triton X-100. Cells were then incubated with rabbit anti-FoxO3a (1:100, Cell Signaling Technology, Beverly, MA) or rabbit anti-cleaved caspase 3 (1:200, Cell Signaling Technology, Beverly, MA) over night at 4°C and then with biotinylated anti-rabbit IgG (1:50, Vector laboratories) for 2 hours followed by Texas Red streptavidin (1:50, Vector laboratories) for 1 hour. Cells were washed in PBS, then stained with DAPI (Sigma, St. Louis, MO) for nuclear identification. FoxO3a and caspase 3 proteins was imaged with fluorescence at the wavelengths of 565 nm (red) and 400 nm (DAPI nuclear staining).
2.11 Subcellular translocation of FoxO3a by western analysis
ECs were initially homogenized. The cytoplasmic and nuclear proteins were subsequently prepared by using NE-PER nuclear and cytoplasmic extraction reagents according to the instructions of the manufacturer (Pierce, Rockford, IL). The expression of FoxO3a in the EC nucleus and cytoplasm was determined by Western analysis. Each sample (50 µg/lane) was subjected to 7.5% SDS-polyacrylamide gel electrophoresis. After transfer, the membranes were incubated with primary rabbit antibody against FoxO3a (1:1000) (Cell Signaling, Beverly, MA). After washing, the membranes were incubated with a horseradish peroxidase conjugated with a secondary antibody (goat anti-rabbit IgG, 1:2000) (Invitrogen, Carlsbad, CA). The antibody-reactive bands were revealed by chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ) and band density was performed using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/).
2.12 Statistical analysis
For each experiment, the mean and standard error were determined. Statistical differences between groups were assessed by means of analysis of variance (ANOVA) from 6 replicate experiments with the post-hoc Dunnett's test. Statistical significance was considered at P<0.05.
3. Results
3.1 Elevated glucose progressively results in EC injury and apoptosis
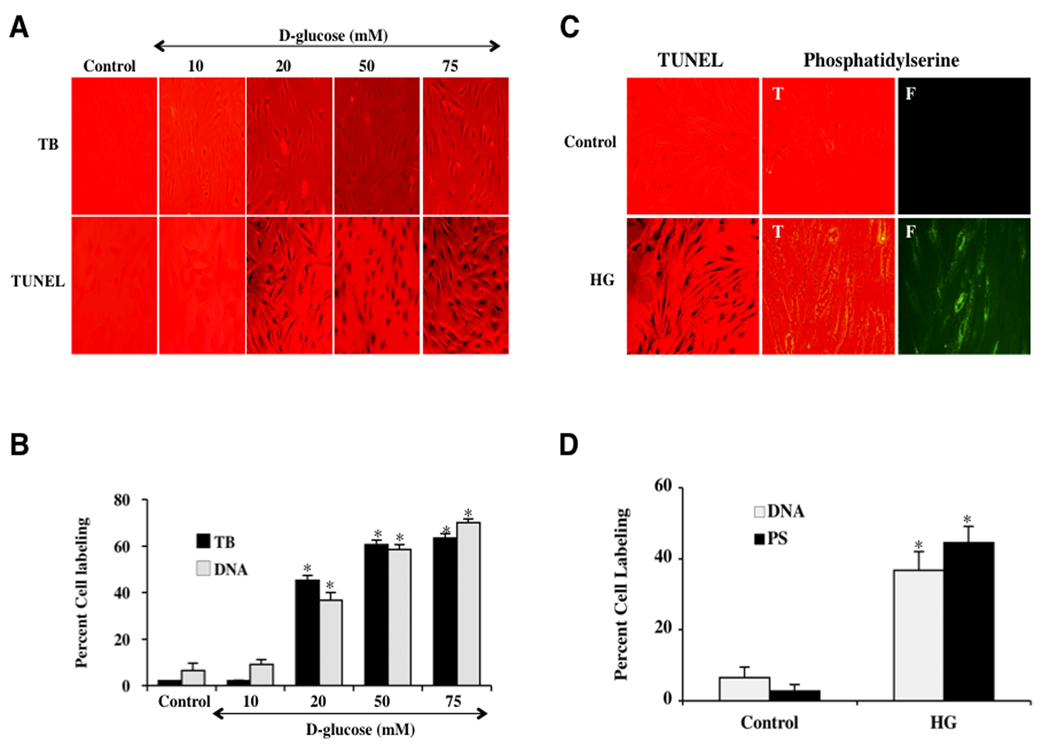
ECs were initially exposed to progressively elevated D-glucose (10, 20, 50 and 75 mM) and cell survival was determined 48 hours later by the trypan blue exclusion method. As shown in Fig. 1A, representative pictures demonstrate that elevated D-glucose treatment (≥20 mM) results in a loss of membrane integrity and staining in a significant number of ECs cells with trypan blue. The quantitative results demonstrate that EC survival was significantly decreased to 55 ± 5% (20 mM), 39 ± 4% (50 mM), and 36 ± 4% (75 mM) following high D-glucose administration when compared with untreated control cultures (98 ± 1%, P<0.01) (Fig. 1B). Next, we examined progressively elevated D-glucose (10, 20, 50 and 75 mM) and apoptosis in ECs. As shown in Figs. 1A and 1B, elevated D-glucose treatment (≥20 mM) results in significant apoptotic DNA fragmentation determined by TUNEL 48 hours later. Since elevated D-glucose (20 mM) exposure resulted in survival rate of approximately 50–60% (a 40–50% EC loss) and apoptotic DNA fragmentation of approximately 40–50%, this concentration of elevated D-glucose was used for the reminder of the experimental paradigms.
Fig. 1. Elevated D-glucose leads to EC injury with early and late apoptotic damage.
(A) Primary cerebral ECs were exposed to elevated D-glucose at the concentration of 10, 20, 50, 75 mM and EC survival (trypan blue, TB) was determined 48 hours later. Representative images illustrate increased trypan blue staining during elevated glucose. (B) Quantification of data demonstrates that EC survival (trypan blue, TB) was significantly decreased to 55 ± 5% (20 mM), 39 ± 4% (50 mM), and 36 ± 4% (75 mM) following administration of elevated (high) D-glucose when compared with untreated control cultures (98 ± 1%, *P <0.01 vs. Control). Each data point represents the mean and SEM from 6 experiments. (A) Primary cerebral ECs were exposed to elevated D-glucose at the concentration of 10, 20, 50, 75 mM and EC apoptotic DNA fragmentation with TUNEL (TUNEL) was determined 48 hours later. Representative images illustrate increased DNA fragmentation during elevated glucose. (B) Quantification of data demonstrates that EC apoptosis (DNA) was significantly increased to 38 ± 4% (20 mM), 59 ± 4% (50 mM), and 62 ± 3% (75 mM) following administration of elevated (high) D-glucose when compared with untreated control cultures (3 ± 1%, *P <0.01 vs. Control). Each data point represents the mean and SEM from 6 experiments. (C) Representative images show that elevated D-glucose (HG = high glucose, 20 mM) leads to apoptotic DNA fragmentation (dark nuclei) with TUNEL stain and membrane PS externalization with transmitted (T) light and corresponding fluorescence (F) assessed by annexin V phycoerythrin (green fluorescence) twenty-four hours following elevated D-glucose. (D) Elevated D-glucose (HG = high glucose, 20 mM) exposure significantly increased DNA fragmentation (DNA) and PS exposure (PS) in ECs (*P<0.01 vs. untreated control). Each data point represents the mean and SEM from 6 experiments.
3.2 Early phosphatidylserine (PS) exposure and subsequent nuclear DNA degradation is present with elevated D-glucose
Following elevated D-glucose (20 mM), apoptotic DNA fragmentation was determined by TUNEL and cell membrane PS exposure was assessed by annexin V 48 hours later. In Fig. 1C, untreated control ECs were without DNA fragmentation or PS externalization. In ECs exposed to elevated D-glucose (20 mM), significant DNA fragmentation (TUNEL) and membrane PS exposure (annexin V) was present. In Fig. 1D, elevated D-glucose (20 mM) lead to a significant increase in percent DNA fragmentation (37 ± 5%) and membrane PS exposure (45 ± 5%) in ECs 48 hours after elevated D-glucose compared to untreated control cultures for DNA (6 ± 3%) and for PS (3 ± 1%) respectively.
3.3 Phosphorylation of Akt1 and FoxO3a is reduced during elevated D-glucose to allow for FoxO3a nuclear translocation and can be blocked by gene knockdown of FoxO3a
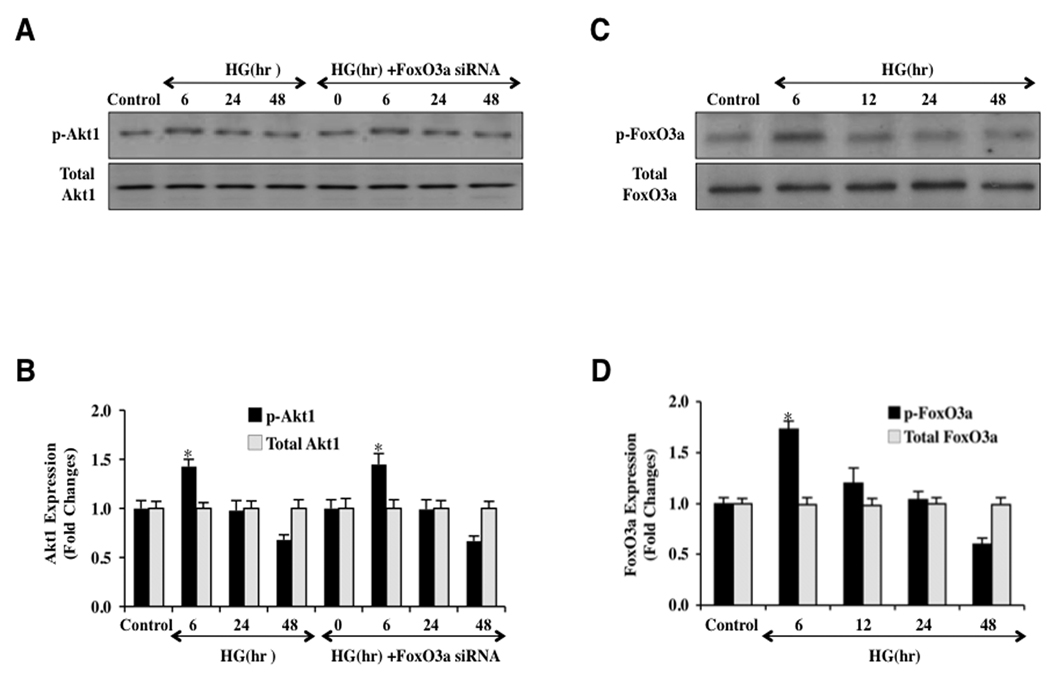
Akt1 is a principal mediator of phosphorylation of FoxO3a that can block activity of this transcription factor (Maiese et al., 2009b, Maiese et al., 2010, Skurk et al., 2004). Absence of phosphorylated (active) Akt1 leads to the loss of post-translational phosphorylation of FoxO3a and prevents association of FoxO3a with 14-3-3 proteins to allow FoxO3a to translocate to the cell nucleus and initiate a “pro-apoptotic” program (Chong and Maiese, 2007, Zhu et al., 2004). We therefore examined the role of elevated D-glucose in ECs during phosphorylation of Akt1 and FoxO3a. Since unphosphorylated FoxO3a can translocate to the cell nucleus, we investigated the phosphorylation of both Akt1 and FoxO3a over a 48 period following elevated D-glucose (20 mM) administration. Western blot assay was performed for phosphorylated Akt1 (p-Akt1), total Akt1, and FoxO3a (p-FoxO3a) at the preferential phosphorylation site for Akt1 of Ser253 as well as for the expression of total FoxO3a (Figs. 2A – 2D). At 6 hours following elevated D-glucose (20 mM) administration, Akt1 phosphorylation and activity was increased, but was subsequently lost and returned to control levels or below at 24 hours and 48 hours after elevated D-glucose (Figs. 2A and 2B). Expression of total Akt1 did not change illustrating that only the phosphorylation status of Akt1 was altered. Gene silencing of FoxO3a did not affect the course of Akt1 phosphorylation during elevated D-glucose as would be expected since FoxO3a is a downstream target of Akt1 (Maiese et al., 2009b, Maiese et al., 2010, Skurk et al., 2004). Interestingly, activity and phosphorylation of FoxO3a directly corresponded to Akt1 activity with loss of Akt1 phosphorylation resulting in the expression of active (unphosphorylated) FoxO3a at 12, 24, and 48 hours after elevated D-glucose (Figs. 2C and 2D). In addition, expression of total FoxO3a remained unchanged, suggesting that the unphosphorylated and active FoxO3a form was present and that the total FoxO3a protein was not degraded (Figs. 2C, 2D).
Fig. 2. Elevated D-glucose results in the eventual loss of phosphorylation of Akt1 and the increased activity of FoxO3a.
In A and B, primary EC protein extracts (50 µ/lane) were immunoblotted with anti-phosphorylated-Akt1 (p-Akt1, Ser473) or anti-total Akt1 at 0 (control), 6, 24, and 48 hours following administration of elevated D-glucose (HG = high glucose, 20 mM). Phosphorylated (active) Akt1 (p-Akt1) expression is initially increased at 6 hours following elevated D-glucose, but subsequently is reduced and returns to control levels at 24 hours and 48 hours after elevated D-glucose (*P<0.01 vs. control). Total Akt1 is not altered. In C and D, primary EC protein extracts (50 µ/lane) were immunoblotted with anti-phosphorylated-FoxO3a (p-FoxO3a, Ser253) or anti-total FoxO3a at 0, 6, 24, and 48 hours following administration of elevated D-glucose (HG = high glucose, 20 mM). Correlating with Akt1 activity, active (unphosphorylated) FoxO3a (p-FoxO3a) expression is increased at 12, 24, and 48 hours after elevated D-glucose but total FoxO3a is not affected (*P<0.01 vs. control).
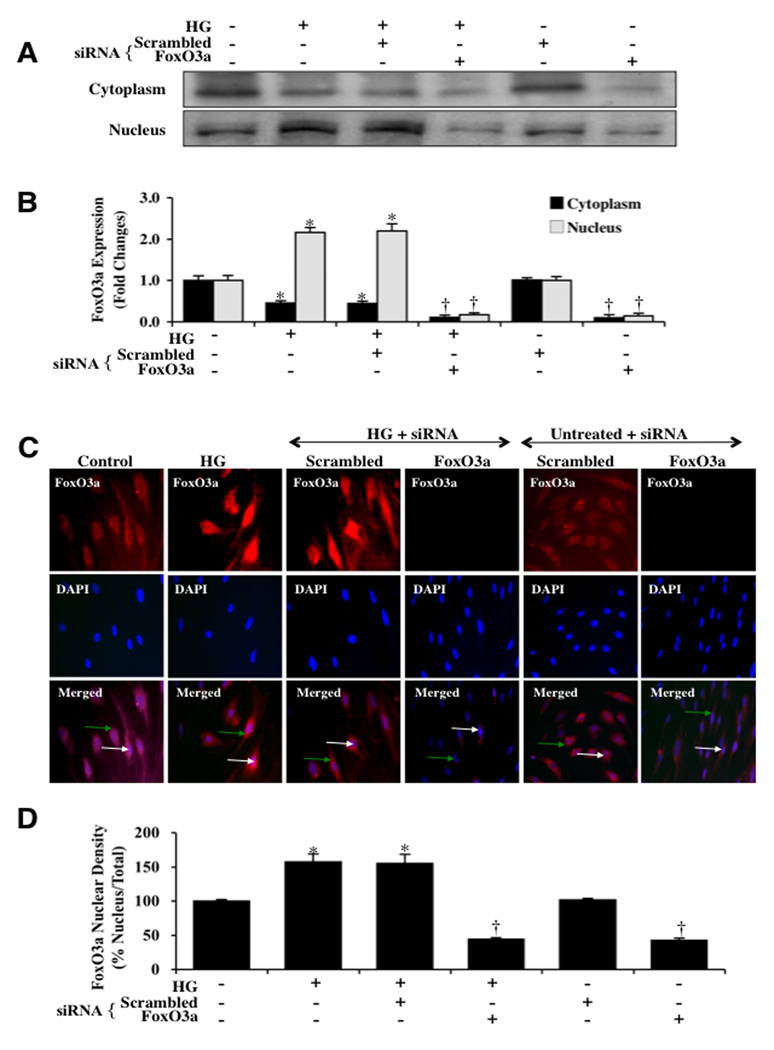
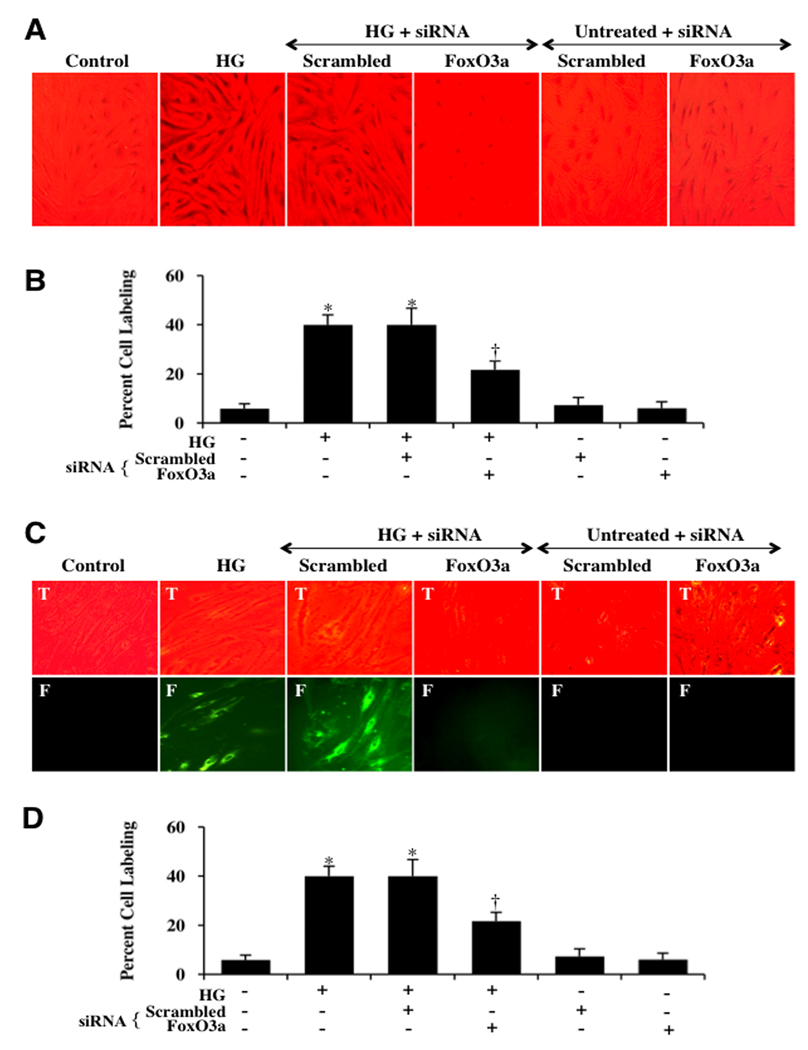
These observations were supported by our studies that show that the active FoxO3a transcription factor translocates from the cell cytosol to the nucleus following 48 hours after high D-glucose administration (20 mM). First, we assessed FoxO3a subcellular translocation from the cell cytoplasm to the nucleus through western analysis. At 48 hours following elevated (high) D-glucose (20 mM), FoxO3a significantly transferred from the cytoplasm to the nucleus. Gene knockdown of FoxO3a prevented this translocation of FoxO3a to the nucleus following elevated D-glucose and non-specific scrambled siRNA did not alter FoxO3a translocation during elevated D-glucose exposure (Figs. 3A and 3B). Next, we also performed in ECs immunofluorescent staining for FoxO3a and DAPI nuclear staining to follow the subcellular translocation of FoxO3a 48 hours after high D-glucose administration (20 mM) (Figs. 3C, 3D). Significant immunofluorescent staining for FoxO3a in the nucleus of ECs is present during elevated D-glucose. This is evident by the inability to detect significant DAPI nuclear staining (blue in color) in cells during merged elevated images since prominent FoxO3a staining is present in the nucleus (Figs. 3C, 3D). In contrast, in untreated control cells, FoxO3a is maintained in the cytoplasm with minimal nuclear staining as shown with DAPI staining (faint blue nuclei in color) in the nucleus in cells in merged images.
Fig. 3. Activation of FoxO3a leads to subcellular trafficking of FoxO3a to the nucleus.
(A) Equal amounts of cytoplasmic (cytoplasm) or nuclear (nucleus) protein extracts (50 µg/lane) were immunoblotted with anti-total FoxO3a at 48 hours following administration of elevated D-glucose (HG = high glucose, 20 mM). Elevated D-glucose leads to the translocation of FoxO3a from the cytoplasm to the nucleus, but gene knockdown of FoxO3α blocked subcellular translocation of FoxO3a form the cytoplasm to the cell nucleus. Non-specific scrambled siRNA did not alter translocation of FoxO3a during elevated D-glucose. (B) Quantification of the western band intensity was performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image) and demonstrates that significant expression of FoxO3a translocates to the cell nucleus 48 hours following elevated D-glucose (HG = high glucose, 20 mM), but transfection with FoxO3a blocks translocation of FoxO3a to the EC nucleus. Non-specific scrambled siRNA did not alter FoxO3a subcellular translocation and both non-specific siRNA and FoxO3a siRNA alone did not alter FoxO3a translocation in untreated control cells (untreated ECs = Control vs. HG, *P<0.01; FoxO3a siRNA vs. HG, †P<0.01). Each data point represents the mean and SEM from 6 experiments. In C and D, ECs were imaged 48 hours following elevated D-glucose (HG = high glucose, 20 mM) with immunofluorescent staining for FoxO3a (Texas-red streptavidin). Nuclei of ECs were counterstained with DAPI. In merged images, untreated control ECs have visible nuclei (dark blue in color, white arrows) that illustrate absence of FoxO3a in the nucleus. However, merged images after elevated D-glucose show ECs with completely red cytoplasm (green arrows) and minimal visibility of the nucleus with DAPI illustrating translocation of FoxO3a to the nucleus. In contrast, gene knockdown of FoxO3α with transfection of FoxO3a siRNA (siRNA) during elevated D-glucose eliminates the presence and translocation of FoxO3a from the cytoplasm to the cell nucleus during elevated D-glucose with the demonstration of dark blue nuclei (*P<0.01 vs. untreated ECs = Control; †P<0.01 vs. HG). Non-specific scrambled siRNA did not alter total FoxO3a translocation during elevated D-glucose. Quantification of the intensity of FoxO3a nuclear staining was performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image).
We subsequently transfected ECs with FoxO3a siRNA during elevated D-glucose to observe whether nuclear trafficking of FoxO3a could be prevented. Transient gene knockdown of FoxO3a in ECs exposed to elevated D-glucose (20 mM) resulted in markedly reduced translocation of FoxO3a to the cell nucleus with the demonstration of dark blue nuclei (Figs. 3C and 3D). As a control, non-specific scrambled FoxO3a siRNA did not alter FoxO3a translocation during elevated D-glucose exposure, illustrating the specificity for FoxO3a siRNA to block subcellular trafficking of FoxO3a (Figs. 3C and 3D). In Fig. 3D, data analysis illustrated that ECs nuclear staining area is significantly increased (158 ± 11%) following elevated D-glucose. In contrast, ECs transfected with FoxO3a siRNA have significantly reduced nuclear translocation with a reduced nuclear density of 44 ± 2% (P<0.01 vs. high D-glucose) (Fig. 3D).
3.4 Gene knockdown of FoxO3a prevents EC injury during elevated D-glucose
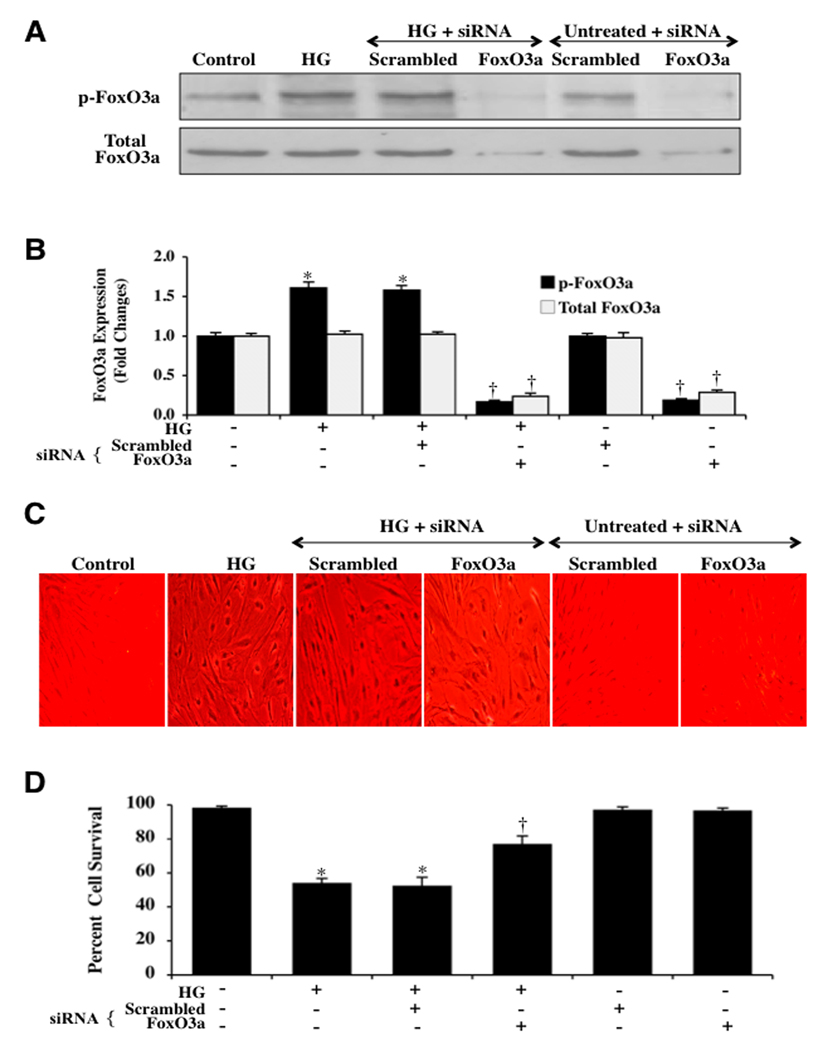
ECs were transfected with FoxO3a siRNA and the expression of total FoxO3a protein was documented with Western blot analysis 6 hours following elevated D-glucose (20 mM) administration (Figs. 4A and 4B). Transient gene knockdown of FoxO3a in either untreated control cells or in cells exposed to elevated D-glucose (high D-glucose, HG) alone resulted in markedly reduced or absent expression of phosphorylated (p-FoxO3a) and total FoxO3a (Figs. 4A and 4B). As a control, non-specific scrambled FoxO3a siRNA did not alter phosphorylated or total FoxO3a expression in untreated control cells or cells exposed to elevated D-glucose, illustrating the specificity for FoxO3a siRNA to block protein expression of total FoxO3a (Figs. 4A and 4B). Representative figures illustrate significant trypan blue staining in ECs 48 hours after elevated (high) D-glucose (20 mM) administration alone or with elevated D-glucose during scrambled (non-specific) siRNA (Fig. 4C). In contrast, significantly reduced trypan blue uptake is present in ECs following elevated D-glucose with FoxO3a siRNA transfection (Fig. 4C), demonstrating that the presence of FoxO3a contributes to EC injury during elevated D-glucose. On further analysis in Fig. 4D, EC survival was increased from 53 ± 3% during elevated D-glucose administration alone to 77 ± 5% (P<0.01) and FoxO3a siRNA 48 hours after elevated D-glucose administration. Transfection with scrambled siRNA did not prevent EC injury during elevated D-glucose.
Fig. 4. Gene knockdown of FoxO3a increases EC survival during elevated D-glucose.
In A and B, EC protein extracts (50 µ/lane) were immunoblotted with anti-phosphorylated-FoxO3a (p-FoxO3a, Ser253) or anti-total FoxO3a at 6 hours following elevated D-glucose (HG = high glucose, 20 mM). Gene knockdown of FoxO3a was performed with transfection of FoxO3a siRNA (siRNA). FoxO3a siRNA significantly reduced expression of total FoxO3a alone or following elevated D-glucose (HG = high glucose, 20 mM) but non-specific scrambled siRNA did not alter total FoxO3a expression (*P<0.01 vs. untreated ECs = Control; †P <0.01 vs. HG). Quantification of the western band intensity was performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image). In C and D, gene knockdown of FoxO3a with FoxO3a siRNA (siRNA) significantly increased EC survival and decreased EC membrane injury assessed by trypan blue staining 48 hours after elevated D-glucose (HG = high glucose, 20 mM). FoxO3a siRNA alone was not toxic and non-specific scrambled siRNA did not protect cells during HG (*P<0.01 vs. untreated ECs = Control; †P <0.01 vs. HG). Each data point represents the mean and SEM from 6 experiments.
3.5 Loss of FoxO3a can block late apoptotic DNA fragmentation and early phosphatidylserine (PS) exposure
We next examined the role of FoxO3a in primary ECs during elevated (high) D-glucose with apoptotic genomic DNA fragmentation and membrane PS exposure through TUNEL and annexin V analysis. In Figs. 5A and 5C, representative figures demonstrate a significant decrease in DNA fragmentation and PS exposure 48 hours following elevated D-glucose (20 mM) transfected with FoxO3a siRNA. Transfection with non-specific scrambled siRNA did not prevent DNA fragmentation or PS exposure in ECs. Quantification of these observations illustrate that transfection of ECs with FoxO3a siRNA during elevated (high) D-glucose decreased DNA fragmentation from 40 ± 4% (elevated D-glucose alone) to 22 ± 4% with FoxO3a siRNA (Fig. 5B) and decreased apoptotic PS exposure from 47 ± 5% (elevated D-glucose alone) to 10 ± 5% with FoxO3a siRNA (Fig. 5D). Transfection with scrambled siRNA did not prevent EC DNA fragmentation or PS exposure during elevated D-glucose when compared to untreated control ECs during elevated D-glucose exposure.
Fig. 5. Gene knockdown of FoxO3a prevents apoptotic DNA fragmentation and phosphatidylserine (PS) exposure in ECs during elevated D-glucose.
In A and C, representative images and quantitative analysis illustrate gene knockdown of FoxO3a with FoxO3a siRNA (siRNA) significantly blocks EC genomic DNA degradation assessed by TUNEL (A) and membrane PS externalization with transmitted (T) light and corresponding fluorescence (F) assessed by annexin V phycoerythrin (green fluorescence) (C) 48 hours after elevated D-glucose (HG = high glucose, 20 mM). Non-specific scrambled siRNA did not alter DNA fragmentation or membrane PS exposure. In B and D, quantification of data illustrates that DNA fragmentation and membrane PS externalization were significantly increased following elevated D-glucose (HG = high glucose, 20 mM) when compared to untreated EC control cultures, but transfection of FoxO3a siRNA prevents DNA fragmentation and membrane PS exposure during elevated D-glucose (*P<0.01 vs. untreated ECs = Control; †P <0.01 vs. HG). FoxO3a siRNA alone was not toxic and non-specific scrambled siRNA did not protect cells during elevated D-glucose. Each data point represents the mean and SEM from 6 experiments.
3.6 Gene knockdown of FoxO3a inhibits mitochondrial depolarization and cytochrome c release
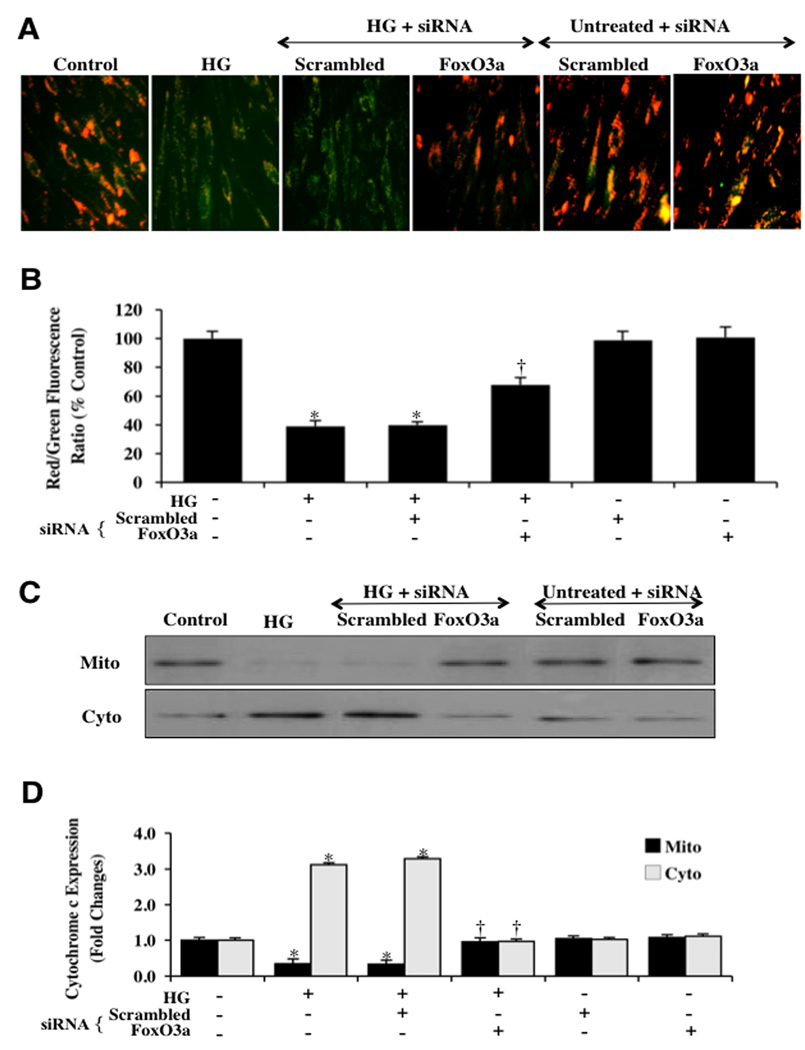
Elevated (high) D-glucose (20 mM) yielded a significant decrease in the mitochondrial red/green fluorescence intensity ratio within 48 hours (39 ± 4%) with labeling of EC mitochondria by the cationic membrane potential indicator JC-1 when compared to untreated control mitochondria (98 ± 4%) (Figs. 6A and 6B), demonstrating that elevated (high) D-glucose results in mitochondrial membrane depolarization. Transfection of FoxO3a siRNA in ECs prior to elevated D-glucose exposure significantly increased the red/green fluorescence intensity of the mitochondria (68 ± 5%), indicating that mitochondrial permeability transition pore membrane potential was markedly improved (Figs 6A and 6B). In contrast, non-specific scrambled siRNA during elevated D-glucose did not prevent mitochondrial membrane depolarization, suggesting that FoxO3a is required for elevated D-glucose to lead to mitochondrial membrane depolarization.
Fig. 6. Gene knockdown of FoxO3a prevents mitochondrial depolarization and the release of cytochrome c in ECs during elevated D-glucose.
(A) elevated D-glucose (HG = high glucose, 20 mM) resulted in a significant decrease in the red/green fluorescence intensity ratio of mitochondria using a cationic membrane potential indicator JC-1 within 48 hours when compared with untreated control ECs, demonstrating that elevated D-glucose leads to mitochondrial membrane depolarization. Gene knockdown of FoxO3a with FoxO3a siRNA (siRNA) during elevated D-glucose significantly increased the red/green fluorescence intensity of mitochondria in ECs, indicating that mitochondrial membrane potential was restored. Non-specific scrambled siRNA did not prevent mitochondrial membrane depolarization during elevated D-glucose and FoxO3a siRNA alone did not alter mitochondrial membrane depolarization in untreated control cells. (B) The relative ratio of red/green fluorescent intensity of mitochondrial staining in both untreated control ECs and ECs exposed to elevated D-glucose (HG = high glucose, 20 mM) or transfected with FoxO3a siRNA was measured in 6 independent experiments with analysis performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image) (untreated ECs = Control vs. HG, *P<0.01; FoxO3a siRNA vs. HG, †P<0.01). (C) Equal amounts of mitochondrial (mito) or cytosol (cyto) protein extracts (50 µg/lane) were immunoblotted demonstrating that transfection of FoxO3a siRNA significantly prevented cytochrome c release from mitochondria 48 hours after elevated D-glucose (HG = high glucose, 20 mM). Non-specific scrambled siRNA did not prevent cytochrome c release during elevated D-glucose. (D) Quantification of the western band intensity was performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image) and demonstrates that significant release of cytochrome c occurs 48 hours following elevated D-glucose (HG = high glucose, 20 mM), but transfection with FoxO3a prevents cytochrome c release from EC mitochondria. Non-specific scrambled siRNA was ineffective during elevated D-glucose to prevent cytochrome c release and both non-specific siRNA and FoxO3a siRNA alone did not alter mitochondrial cytochrome c in untreated control cells (untreated ECs = Control vs. HG, *P<0.01; FoxO3a siRNA vs. HG, †P<0.01). Each data point represents the mean and SEM from 6 experiments
Elevated (high) D-glucose (20 mM) within 48 hours also resulted in a marked release of cytochrome c from the mitochondria to a 3.3 ± 0.2% fold increase when compared to untreated control mitochondria (Figs. 6C and 6D) using western analysis. Non-specific scrambled siRNA did not alter this release of cytochrome c during elevated D-glucose exposure, but transfection of mitochondria with FoxO3a siRNA prevented cytochrome c release to a similar degree that occurs with untreated control EC mitochondria (Figs 6C and 6D).
3.7 FoxO3a modulates caspase 1 and caspase 3 activities during elevated D-glucose exposure
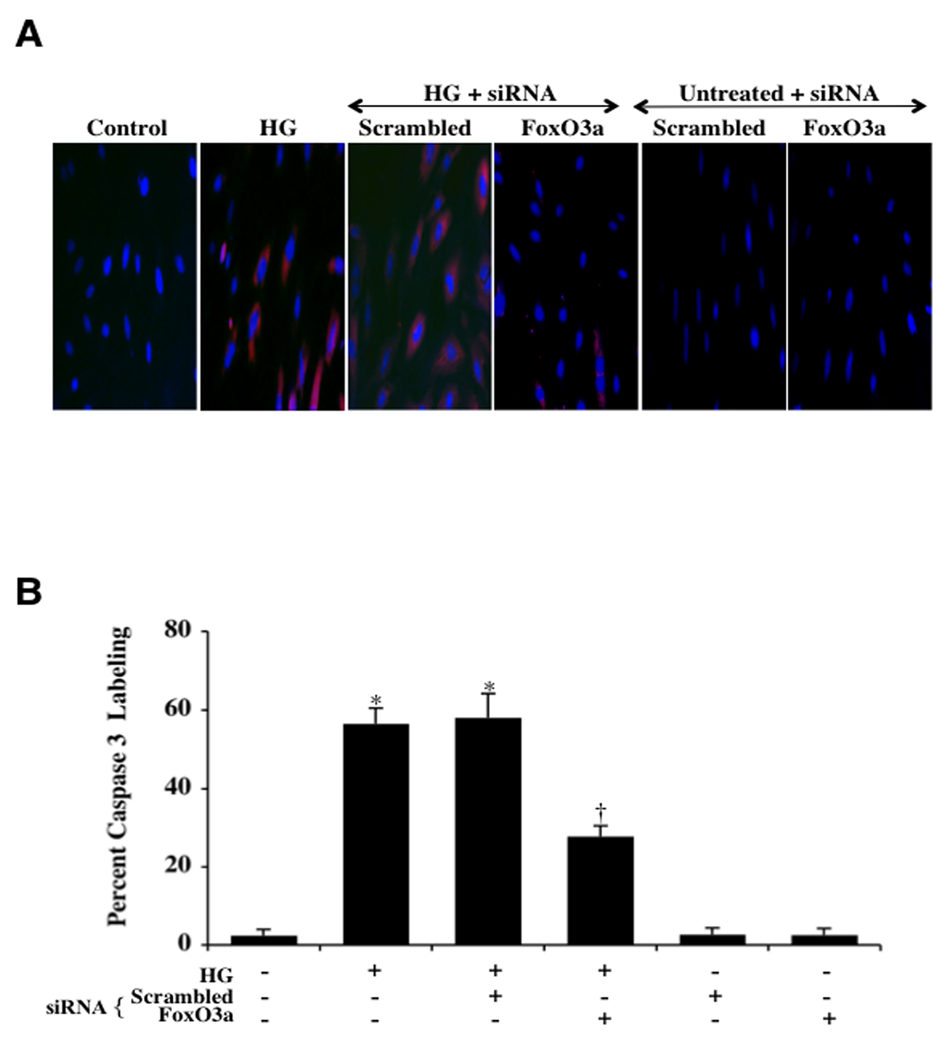
Since mitochondrial function is closely linked to caspase activity (Hao et al., 2009, Leuner et al., 2007, Maiese et al., 2008a), we next investigated the role of the specific caspases 3 and 1 with FoxO3a. In Figs. 7A and 7B, cleaved caspase 3 immunocytochemistry reveals active caspase 3 (blue/red staining) within 6 hours following elevated (high) D-glucose (20 mM) exposure and during transfection of non-specific scrambled siRNA combined with elevated D-glucose administration. In contrast, transfection of FoxO3a siRNA in ECs significantly blocks caspase 3 activity as evidenced by primarily blue immunocytochemical staining and by reducing the percentage of cleaved caspase 3 labeling to 28 ± 3% from 57 ± 4% in ECs exposed to elevated D-glucose alone (Figs. 7A and 7B).
Fig. 7. FoxO3a modulates caspase 3 cellular activity during elevated D-glucose.
In A, Ecs were exposed to elevated D-glucose (HG = high glucose, 20 mM) and caspase 3 activation was determined 6 hours after elevated D-glucose exposure through immunocytochemistry with antibodies against cleaved active caspase 3 (17 kDa). Representative images illustrate active caspase 3 staining (red) in cells following elevated D-glucose, but cellular red staining is almost absent during transfection with FoxO3a siRNA. Non-specific scrambled siRNA did not eliminate caspase 3 activity during elevated D-glucose and both non-specific scrambled siRNA and transfection of FoxO3a siRNA alone did not alter caspase 3 activity in untreated control cells. In B, quantification of the western band intensity was performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image) and demonstrates that elevated D-glucose (HG = high glucose, 20 mM) significantly increased the expression of cleaved active caspase 3 when compared to untreated control cells. Yet, the expression of cleaved active caspase 3 was significantly decreased in cells with transfection of FoxO3a siRNA during elevated D-glucose (*P <0.01 vs. untreated ECs = Control; †P <0.01 vs. HG).
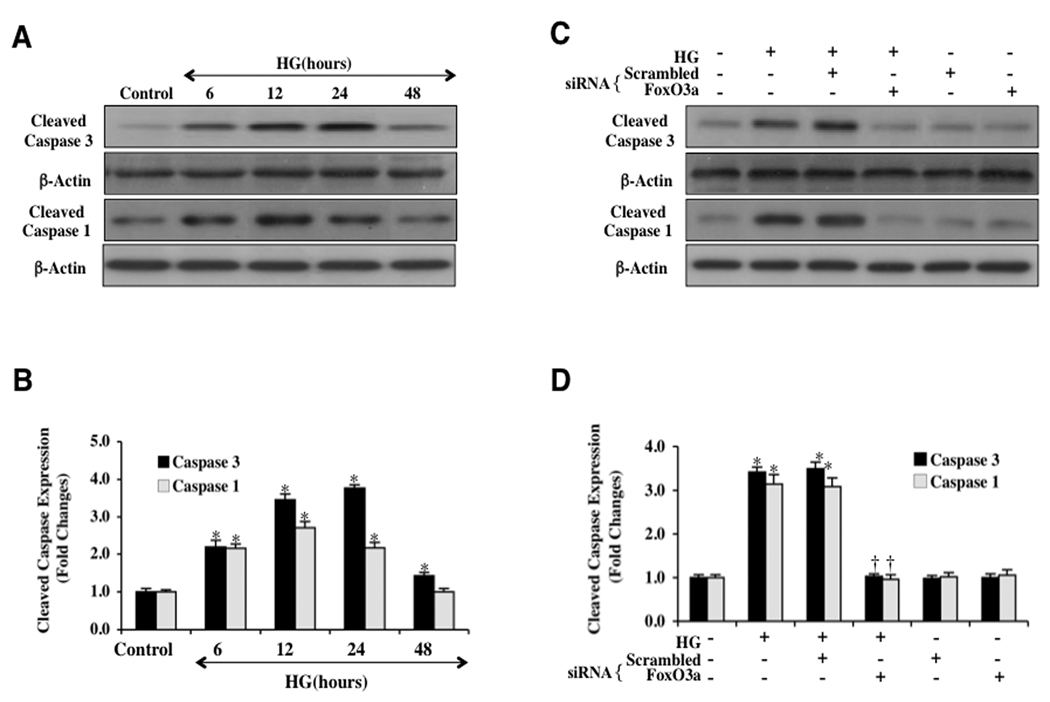
Fig. 8 demonstrates the expression of cleaved (active) caspase 3 and 1 expressions on western analysis. In Fig. 8, western analysis of cleaved caspase 3 (Figs. 8A and 8B) and cleaved caspase 1 (Figs. 8A and 8B) was performed following elevated D-glucose (20 mM) administration in ECs and reveals significant caspase 3 and caspase 1 activity at 12 and 24 hours. We next evaluated the ability of FoxO3a to control caspase activity at the 12 hour period following elevated D-glucose (20 mM) administration in ECs. Expression of cleaved active caspase 3 and caspase 1 (Figs. 8C and 8D) is elevated greater than 3 fold over untreated control EC levels at 12 hours following elevated (high) D-glucose, but transfection with FoxO3a siRNA significantly blocks cleaved active caspase 3 and caspase 1 activities. Non-specific scrambled siRNA was not effective in reducing caspase 3 or caspase 1 activity during elevated (high) D-glucose, further supporting the specific role for FoxO3a to control caspase 3 and caspase 1 activity during elevated D-glucose in ECs (Figs 8C and 8D).
Fig. 8. Caspase 1 and 3 activities are regulated by FoxO3a during elevated D-glucose.
In A, EC protein extracts (50 µg/lane) were immunoblotted with anti-cleaved caspase 3 product (active caspase 3, 17 kDA) and with anti-cleaved caspase 1 product (active caspase 1, 20 kDA) at 6, 12, 24, and 48 hours following elevated D-glucose (HG = high glucose, 20 mM). In B, quantification of the western band intensity was performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image) and illustrates the highest increased caspase 3 and caspase 1 activities at 12 and 24 hours following elevated D-glucose exposure (*P <0.01 vs. untreated ECs = Control). Each data point represents the mean and SEM from 6 experiments. In C, EC protein extracts (50 µg/lane) were immunoblotted with anti-cleaved caspase 3 product (active caspase 3, 17 kDA) and with anti-cleaved caspase 1 product (active caspase 1, 20 kDA) at 12 hours following elevated D-glucose (HG = high glucose, 20 mM). Elevated D-glucose markedly increased cleaved caspase 3 and caspase 1 expression, but gene knockdown of FoxO3a with FoxO3a siRNA (siRNA) significantly blocked cleaved caspase 3 and caspase 1 expression 12 hours after elevated D-glucose. Non-specific scrambled siRNA did not eliminate caspase 3 or caspase 1 activities during elevated D-glucose and both non-specific scrambled siRNA and transfection of FoxO3a siRNA alone did not alter caspase 3 or caspase 1 activities in untreated control cells. In D, quantification of the western band intensity was performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image) and shows that transfection with FoxO3a siRNA (siRNA) prevents cleaved caspase 3 and caspase 1 expression 12 hours after elevated D-glucose exposure, but non-specific scrambled siRNA did not reduce cleaved caspase 3 or caspase 1 expression during elevated D-glucose (*P <0.01 vs. untreated ECs = Control; †P <0.01 vs. HG). FoxO3a siRNA alone in untreated control cells was not toxic and each data point represents the mean and SEM from 6 experiments.
4. Discussion
It is estimated that almost 20 million individuals in the United States and more than 165 million individuals worldwide suffer from DM with increasing incidence that lead to vascular cell injury and multi-system complications (Chan et al., 2009, Maiese, 2008)}. Additional concerns exist with the knowledge that a significant portion of the population has undiagnosed diabetes, impaired glucose tolerance, and hematological complications (Aso et al., 2009, Maiese et al., 2009e). These observations point to the imminent need for improved early diagnosis and the development of new strategies to prevent as well as halt the progression of vascular complications from DM (Maiese et al., 2009e, Rebecchi et al., 2009).
Employing an elevated glucose model for primary ECs, we show that progressively elevated concentrations of D-glucose lead to a significant loss in EC survival and result in apoptotic membrane PS externalization and nuclear DNA fragmentation. A final D-glucose concentration of 20 mM injures approximately 60% of ECs and leads to significant apoptosis, illustrating that primary cerebral ECs are extremely sensitive to elevations in D-glucose to a greater degree than vascular cells from other sources such as human umbilical vein ECs that require elevated D-glucose concentrations at ≥ 25 mM for 3–5 days exposure to generate apoptotic injury (Li et al., 2009). Furthermore, the elevated D-glucose concentrations used in our study are similar to clinical glucose concentrations not only during poorly controlled diabetes (Pagano et al., 1994), but also during early onset diabetes (Ryan et al., 2004) and during expected diurnal variations with diabetes (Monnier et al., 2006, Troisi et al., 2000) known to occur in a range from 15 mM – 25 mM (270 mg/dl – 450 mg/dl). Hyperosmolarity did not play a significant role in EC toxicity, since a mannitol concentration of 20 mM resulted in similar and not significantly different survival rates than untreated control ECs. Furthermore, we performed additional studies with the biologically inactive agent L-glucose and have observed that L-glucose in concentrations of 20 mM did not significantly alter EC survival (data not shown).
The sensitivity of ECs to injury and apoptotic demise during elevated D-glucose exposure is intimately tied to the activity of Akt1 and the post-translational phosphorylation of FoxO3a and its subcellular trafficking. We show that activity and phosphorylation of FoxO3a directly corresponded to Akt1 activity. Loss of Akt1 phosphorylation after 6 hours following elevated D-glucose exposure resulted in active (unphosphorylated) FoxO3a at 24 hours and 48 hours after elevated D-glucose. Elevated D-glucose initially increases the expression of phosphorylated (active) Akt1 and phosphorylated (inactive) p-FoxO3a within 6 following elevated D-glucose exposure, but after this time period expression of phosphorylated (active) Akt1 and phosphorylated (inactive) p-FoxO3a was not present but expression of total Akt1 and total FoxO3a remained unchanged, suggesting that loss of active phosphorylated Akt1 leads to the unphosphorylated and active post-translational form of FoxO3a and that total FoxO3a protein was not destroyed. These observations were further supported by our work that employed both western analysis and immunocytochemistry in ECs that shows that the active (unphosphorylated) FoxO3a transcription factor present at 48 hours following elevated D-glucose exposure translocates from the EC cytosol to the nucleus. Prior studies have illustrated that post-translational modification of FoxO3a that yields an unphosphorylated (active) state prevents the association of FoxO3a with 14-3-3 proteins in the cytosol and allows FoxO3a to shuttle to the nucleus for transcriptional activity (Chong and Maiese, 2007, Zhu et al., 2004).
However, if FoxO3a is not present and no translocation from the cytoplasm to the cell nucleus occurs, then progressive apoptotic EC injury during elevated D-glucose is prevented. Apoptosis is a dynamic process that consists of both the early exposure of membrane PS residues and the later destruction of genomic DNA (Jessel et al., 2002, Maiese et al., 2008a, Maiese et al., 2005, Mallat et al., 2005). Early externalization of cellular membrane PS residues during apoptosis can occur rapidly within 1–6 hours and becomes a signal for inflammatory cells to dispose of “PS tagged” cells and ultimately lead to cellular nuclear DNA degradation (Chong et al., 2005, Jessel et al., 2002, Kang et al., 2003b, Mallat et al., 2005). As a result, therapeutic strategies designed to preserve EC integrity become dependent upon identifying the mechanisms that can control early cellular membrane PS exposure similar to other disease processes, such as during infectious disorders or immune system modulation (Maiese et al., 2008a, Soares et al., 2008). We now illustrate that both EC apoptotic cellular membrane PS exposure and nuclear DNA fragmentation are controlled by FoxO3a, since gene knockdown of FoxO3a blocks the early and late programs of apoptosis. Furthermore, transfection with non-specific scrambled siRNA did not prevent EC injury or apoptotic program induction, supporting the specificity of FoxO3a to control cell injury and early and late apoptotic programs in ECs. Our studies that identify the ability of FoxO3a to control early and late apoptotic programs are unique, but also are consistent with prior studies supporting a “pro-apoptotic” role for FoxO3a during oxidative stress (Caporali et al., 2008, Nakamura and Sakamoto, 2007), during control of neuronal injury through NAD+ precursors (Chong et al., 2004), in Fas-mediated death pathways (Barthelemy et al., 2004), during oxidative stress (Shang et al., 2009b), and during amyloid toxicity (Shang et al., 2009a). In addition, the cytoprotective capacity of metabotropic receptors (Chong et al., 2006) and some trophic factors (Caporali et al., 2008, Chong and Maiese, 2007) has been attributed to the ability to down-regulate FoxO3a activity.
The preservation of mitochondrial membrane potential also becomes a significant mediator to regulate EC survival during elevated D-glucose. FoxO3a in HCT116 cells has recently been shown to interact with the mitochondrial sirtuin SIRT3 (Jacobs et al., 2008), suggesting that FoxO3a may have an important role in controlling mitochondrial signal transduction pathways. FoxO3a also has been shown to lead to cytochrome c release in neuroblastoma cells and neurons (Chong et al., 2004, Obexer et al., 2007). We now illustrate that FoxO3a has a direct role in modulating mitochondrial membrane potential and subsequent cytochrome c release during the same 48 hour time period that subcellular trafficking of FoxO3a occurs and EC injury ensues. Elevated D-glucose in ECs leads to mitochondrial membrane depolarization and cytochrome c release within 48 hours after elevated D-glucose exposure. Transfection with non-specific scrambled siRNA did not prevent mitochondrial membrane depolarization and cytochrome c release in ECs. Yet, knockdown of FoxO3a in ECs prevents mitochondrial membrane depolarization as well as the release of cytochrome c during elevated D-glucose, illustrating that FoxO3a is required for the initiation of mitochondrial pathways that can lead to apoptotic cell injury in ECs.
Given that FoxO3a is necessary for mitochondrial membrane depolarization and subsequent cytochrome c release in the apoptotic cascade, we investigated whether FoxO3a also controls caspase 3 and caspase 1 activities that are known to oversee both apoptotic DNA degradation and cellular membrane PS exposure (Jessel et al., 2002, Maiese et al., 2005, Mallat et al., 2005, Mari et al., 2004, Takahashi et al., 1999). Prior studies have shown that caspase 3 forms a unique regulatory mechanism that may degrade phosphorylated FoxO3a with the generation of apoptotic “by-products” (Charvet et al., 2003, Chong et al., 2006, Chong et al., 2004). Furthermore, FoxO3a has been associated with increases in caspase 3 and caspase 6 activity in melanoma cells (Gomez-Gutierrez et al., 2006) and FoxO3a relies upon caspase 3 to initiate the proliferation of inflammatory microglial cells during amyloid toxicity (Shang et al., 2009a). We now show that FoxO3a is required for the early and progressive activation of caspase 3 and 1. Elevated D-glucoses in ECs initiates a robust activation of caspase 3 and caspase 1 within 12 hours that rises approximately 3 to 4 fold over untreated control EC levels and then diminishes over a 48 hour period. This is especially evident with cleaved caspase 3 activity assessed through immunocytochemistry that is significantly increased after elevated D-glucose exposure. Furthermore, FoxO3a was necessary for this rapid and marked increase in cleaved caspase 3 and caspase 1 expression following elevated D-glucose that directly correlates with the activity (unphosphorylated) status of FoxO3a, since loss of FoxO3a during gene knockdown abrogated increases in caspase 3 and caspase 1 activities during elevated D-glucose. Interestingly, early activation of FoxO3a may be the most vital during the course of caspase induction as the activity of FoxO3a progressively increases at 12, 24, and 48 hours after elevated D-glucose exposure (Fig. 2C). It is conceivable that early FoxO3a activation works in conjunction with other apoptotic pathways such as the loss of mitochondrial membrane permeability and the release of cytochrome c to lead to marked caspase 3 and caspase 1 activity at 12 and 24 hours after elevated D-glucose exposure (Maiese et al., 2009a, Maiese et al., 2009c, Shang et al., 2009b).
Impaired glucose tolerance and fluctuations in serum glucose in individuals raises significant concerns for the development of diabetic complications in the vascular system (Maiese, 2008, Pagano et al., 1994). Our studies are the first to demonstrate that FoxO3a through control by Akt1 governs both the early and late apoptotic injury programs of PS membrane externalization and nuclear DNA degradation in primary ECs during elevated D-glucose exposure. Furthermore, FoxO3a utilizes a series of integrated pathways in an apoptotic cascade that involves the post-translational modification of FoxO3a and the subcellular trafficking of FoxO3a from the cytosol to the nucleus, the control of mitochondrial membrane permeability, the release of cytochrome c, and the ultimate modulation of rapid caspase 3 and caspase 1 activities. Elucidation of Akt1, FoxO3a and its closely aligned signal transduction pathways as vital components for apoptotic EC injury during models of DM has broad clinical implications and provides new insight for the development of efficacious and safe therapies for the protection of ECs during impaired glucose tolerance and DM.
Acknowledgments
This research was supported by the following grants to Kenneth Maiese: American Diabetes Association, American Heart Association (National), Bugher Foundation Award, Janssen Neuroscience Award, LEARN Foundation Award, MI Life Sciences Challenge Award, Nelson Foundation Award, NIH NIEHS (P30 ES06639), NIH NIA, NIH NINDS, and NIH ARRA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have nothing to disclose.
References
- Abbott NJ, Hughes CC, Revest PA, Greenwood J. Development and characterisation of a rat brain capillary endothelial culture: towards an in vitro blood-brain barrier. J Cell Sci. 1992;103(Pt 1):23–37. doi: 10.1242/jcs.103.1.23. [DOI] [PubMed] [Google Scholar]
- Aso Y, Suganuma R, Wakabayashi S, Hara K, Nakano T, Suetsugu M, Matsumoto S, Nakamachi T, Takebayashi K, Morita K, Inukai T. Anemia is associated with an elevated serum level of high-molecular-weight adiponectin in patients with type 2 diabetes independently of renal dysfunction. Transl Res. 2009;154:175–182. doi: 10.1016/j.trsl.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Barthelemy C, Henderson CE, Pettmann B. Foxo3a induces motoneuron death through the Fas pathway in cooperation with JNK. BMC Neurosci. 2004;5:48. doi: 10.1186/1471-2202-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporali A, Sala-Newby GB, Meloni M, Graiani G, Pani E, Cristofaro B, Newby AC, Madeddu P, Emanueli C. Identification of the prosurvival activity of nerve growth factor on cardiac myocytes. Cell Death Differ. 2008;15:299–311. doi: 10.1038/sj.cdd.4402263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriello A, dello Russo P, Amstad P, Cerutti P. High glucose induces antioxidant enzymes in human endothelial cells in culture. Evidence linking hyperglycemia and oxidative stress. Diabetes. 1996;45:471–477. doi: 10.2337/diab.45.4.471. [DOI] [PubMed] [Google Scholar]
- Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. Jama. 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- Charvet C, Alberti I, Luciano F, Jacquel A, Bernard A, Auberger P, Deckert M. Proteolytic regulation of Forkhead transcription factor FOXO3a by caspase-3-like proteases. Oncogene. 2003;22:4557–4568. doi: 10.1038/sj.onc.1206778. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang J, Li F, Maiese K. mGluRI Targets Microglial Activation and Selectively Prevents Neuronal Cell Engulfment Through Akt and Caspase Dependent Pathways. Curr Neurovasc Res. 2005;2:197–211. doi: 10.2174/1567202054368317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Group I Metabotropic Receptor Neuroprotection Requires Akt and Its Substrates that Govern FOXO3a, Bim, and beta-Catenin During Oxidative Stress. Curr Neurovasc Res. 2006;3:107–117. doi: 10.2174/156720206776875830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J Cereb Blood Flow Metab. 2004;24:728–743. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Maiese K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br J Pharmacol. 2007;150:839–850. doi: 10.1038/sj.bjp.0707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Maiese K. Enhanced Tolerance against Early and Late Apoptotic Oxidative Stress in Mammalian Neurons through Nicotinamidase and Sirtuin Mediated Pathways. Curr Neurovasc Res. 2008;5:159–170. doi: 10.2174/156720208785425666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Shang YC, Maiese K. Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling. Curr Neurovasc Res. 2007;4:194–204. doi: 10.2174/156720207781387150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP, Antman EM. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298:765–775. doi: 10.1001/jama.298.7.765. [DOI] [PubMed] [Google Scholar]
- Erol A. Unraveling the Molecular Mechanisms Behind the Metabolic Basis of Sporadic Alzheimer's Disease. J Alzheimers Dis. 2009 doi: 10.3233/JAD-2009-1047. [DOI] [PubMed] [Google Scholar]
- Gomez-Gutierrez JG, Souza V, Hao HY, Montes de Oca-Luna R, Dong YB, Zhou HS, McMasters KM. Adenovirus-mediated gene transfer of FKHRL1 triple mutant efficiently induces apoptosis in melanoma cells. Cancer Biol Ther. 2006;5:875–883. doi: 10.4161/cbt.5.7.2911. [DOI] [PubMed] [Google Scholar]
- Gossai D, Lau-Cam CA. The effects of taurine, taurine homologs and hypotaurine on cell and membrane antioxidative system alterations caused by type 2 diabetes in rat erythrocytes. Adv Exp Med Biol. 2009;643:359–368. doi: 10.1007/978-0-387-75681-3_37. [DOI] [PubMed] [Google Scholar]
- Guarnieri G, Zanetti M, Vinci P, Cattin MR, Barazzoni R. Insulin resistance in chronic uremia. J Ren Nutr. 2009;19:20–24. doi: 10.1053/j.jrn.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Hao J, Shen W, Tian C, Liu Z, Ren J, Luo C, Long J, Sharman E, Liu J. Mitochondrial nutrients improve immune dysfunction in the type 2 diabetic Goto-Kakizaki rats. J Cell Mol Med. 2009;13:701–711. doi: 10.1111/j.1582-4934.2008.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JD, VanGilder RL, Houser KA. Streptozotocin-induced diabetes progressively increases blood-brain barrier permeability in specific brain regions in rats. Am J Physiol Heart Circ Physiol. 2006;291:H2660–H2668. doi: 10.1152/ajpheart.00489.2006. [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Pennington JD, Bisht KS, Aykin-Burns N, Kim HS, Mishra M, Sun L, Nguyen P, Ahn BH, Leclerc J, Deng CX, Spitz DR, Gius D. SIRT3 interacts with the daf-16 homolog FOXO3a in the mitochondria, as well as increases FOXO3a dependent gene expression. Int J Biol Sci. 2008;4:291–299. doi: 10.7150/ijbs.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessel R, Haertel S, Socaciu C, Tykhonova S, Diehl HA. Kinetics of apoptotic markers in exogeneously induced apoptosis of EL4 cells. J Cell Mol Med. 2002;6:82–92. doi: 10.1111/j.1582-4934.2002.tb00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson H, Allen P, Peng SL. Inflammatory arthritis requires Foxo3a to prevent Fas ligand-induced neutrophil apoptosis. Nat Med. 2005;11:666–671. doi: 10.1038/nm1248. [DOI] [PubMed] [Google Scholar]
- Kang JQ, Chong ZZ, Maiese K. Akt1 protects against inflammatory microglial activation through maintenance of membrane asymmetry and modulation of cysteine protease activity. J Neurosci Res. 2003a;74:37–51. doi: 10.1002/jnr.10740. [DOI] [PubMed] [Google Scholar]
- Kang JQ, Chong ZZ, Maiese K. Critical role for Akt1 in the modulation of apoptotic phosphatidylserine exposure and microglial activation. Mol Pharmacol. 2003b;64:557–569. doi: 10.1124/mol.64.3.557. [DOI] [PubMed] [Google Scholar]
- Kim JR, Jung HS, Bae SW, Kim JH, Park BL, Choi YH, Cho HY, Cheong HS, Shin HD. Polymorphisms in FOXO gene family and association analysis with BMI. Obesity (Silver Spring) 2006;14:188–193. doi: 10.1038/oby.2006.24. [DOI] [PubMed] [Google Scholar]
- Kuhad A, Bishnoi M, Tiwari V, Chopra K. Suppression of NF-kappabeta signaling pathway by tocotrienol can prevent diabetes associated cognitive deficits. Pharmacol Biochem Behav. 2009;92:251–259. doi: 10.1016/j.pbb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Kuningas M, Magi R, Westendorp RG, Slagboom PE, Remm M, van Heemst D. Haplotypes in the human Foxo1a and Foxo3a genes; impact on disease and mortality at old age. Eur J Hum Genet. 2007;15:294–301. doi: 10.1038/sj.ejhg.5201766. [DOI] [PubMed] [Google Scholar]
- Lappas M, Lim R, Riley C, Rice GE, Permezel M. Localisation and expression of FoxO1 proteins in human gestational tissues. Placenta. 2009;30:256–262. doi: 10.1016/j.placenta.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Leuner K, Hauptmann S, Abdel-Kader R, Scherping I, Keil U, Strosznajder JB, Eckert A, Muller WE. Mitochondrial dysfunction: the first domino in brain aging and Alzheimer's disease? Antioxid Redox Signal. 2007;9:1659–1675. doi: 10.1089/ars.2007.1763. [DOI] [PubMed] [Google Scholar]
- Li Y, Wu H, Khardori R, Song YH, Lu YW, Geng YJ. Insulin-like growth factor-1 receptor activation prevents high glucose-induced mitochondrial dysfunction, cytochrome-c release and apoptosis. Biochem Biophys Res Commun. 2009;384:259–264. doi: 10.1016/j.bbrc.2009.04.113. [DOI] [PubMed] [Google Scholar]
- Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- Maiese K. Triple play: Promoting neurovascular longevity with nicotinamide, WNT, and erythropoietin in diabetes mellitus. Biomed Pharmacother. 2008;62:218–232. doi: 10.1016/j.biopha.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong Z, Hou J, Shang Y. The “O” Class: Crafting clinical care with FoxO transcription factors. Adv Exp Med Biol. 2009a;665:242–260. doi: 10.1007/978-1-4419-1599-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Hou J, Shang YC. New strategies for Alzheimer's disease and cognitive impairment. Oxid Med Cell Longev. 2009b;2:279–290. doi: 10.4161/oxim.2.5.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Hou J, Shang YC. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009c;14:3446–3485. doi: 10.3390/molecules14093446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Hou J, Shang YC. Oxidative stress: Biomarkers and novel therapeutic pathways. Exp Gerontol. 2010;45:217–234. doi: 10.1016/j.exger.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Li F, Shang YC. Erythropoietin: Elucidating new cellular targets that broaden therapeutic strategies. Prog Neurobiol. 2008a;85:194–213. doi: 10.1016/j.pneurobio.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med. 2008b;14:219–227. doi: 10.1016/j.molmed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC, Hou J. A "FOXO" in sight: targeting Foxo proteins from conception to cancer. Med Res Rev. 2009d;29:395–418. doi: 10.1002/med.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Hou J, Chong ZZ, Shang YC. Erythropoietin, forkhead proteins, and oxidative injury: biomarkers and biology. ScientificWorldJournal. 2009e;9:1072–1104. doi: 10.1100/tsw.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. Jama. 2005;293:90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat M, Marin-Teva JL, Cheret C. Phagocytosis in the developing CNS: more than clearing the corpses. Curr Opin Neurobiol. 2005;15:101–107. doi: 10.1016/j.conb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Mari C, Karabiyikoglu M, Goris ML, Tait JF, Yenari MA, Blankenberg FG. Detection of focal hypoxic-ischemic injury and neuronal stress in a rodent model of unilateral MCA occlusion/reperfusion using radiolabeled annexin V. Eur J Nucl Med Mol Imaging. 2004;31:733–739. doi: 10.1007/s00259-004-1473-5. [DOI] [PubMed] [Google Scholar]
- Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Sakamoto K. Forkhead transcription factor FOXO subfamily is essential for reactive oxygen species-induced apoptosis. Mol Cell Endocrinol. 2007;281(1–2):47–55. doi: 10.1016/j.mce.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Obexer P, Geiger K, Ambros PF, Meister B, Ausserlechner MJ. FKHRL1-mediated expression of Noxa and Bim induces apoptosis via the mitochondria in neuroblastoma cells. Cell Death Differ. 2007;14:534–547. doi: 10.1038/sj.cdd.4402017. [DOI] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Pagano G, Bargero G, Vuolo A, Bruno G. Prevalence and clinical features of known type 2 diabetes in the elderly: a population-based study. Diabet Med. 1994;11:475–479. doi: 10.1111/j.1464-5491.1994.tb00309.x. [DOI] [PubMed] [Google Scholar]
- Rebecchi KR, Wenke JL, Go EP, Desaire H. Label-free quantitation: a new glycoproteomics approach. J Am Soc Mass Spectrom. 2009;20:1048–1059. doi: 10.1016/j.jasms.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Ryan EA, Imes S, Wallace C. Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care. 2004;27:1028–1032. doi: 10.2337/diacare.27.5.1028. [DOI] [PubMed] [Google Scholar]
- Shang YC, Chong ZZ, Hou J, Maiese K. The forkhead transcription factor FoxO3a controls microglial inflammatory activation and eventual apoptotic injury through caspase 3. Curr Neurovasc Res. 2009a;6:20–31. doi: 10.2174/156720209787466064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang YC, Chong ZZ, Hou J, Maiese K. FoxO3a Governs Early Microglial Proliferation and Employs Mitochondrial Depolarization with Caspase 3, 8, and 9 Cleavage During Oxidant Induced Apoptosis. Curr Neurovasc Res. 2009b;6:223–238. doi: 10.2174/156720209789630302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurk C, Maatz H, Kim HS, Yang J, Abid MR, Aird WC, Walsh K. The Akt-regulated forkhead transcription factor FOXO3a controls endothelial cell viability throughv modulation of the caspase-8 inhibitor FLIP. J Biol Chem. 2004;279:1513–1525. doi: 10.1074/jbc.M304736200. [DOI] [PubMed] [Google Scholar]
- Soares MM, King SW, Thorpe PE. Targeting inside-out phosphatidylserine as a therapeutic strategy for viral diseases. Nat Med. 2008;14:1357–1362. doi: 10.1038/nm.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Nakamura S, Asano K, Kinouchi M, Ishida-Yamamoto A, Iizuka H. Fas antigen modulates ultraviolet B-induced apoptosis of SVHK cells: sequential activation of caspases 8, 3, and 1 in the apoptotic process. Exp Cell Res. 1999;249:291–298. doi: 10.1006/excr.1999.4476. [DOI] [PubMed] [Google Scholar]
- Troisi RJ, Cowie CC, Harris MI. Diurnal variation in fasting plasma glucose: implications for diagnosis of diabetes in patients examined in the afternoon. Jama. 2000;284:3157–3159. doi: 10.1001/jama.284.24.3157. [DOI] [PubMed] [Google Scholar]
- Xu WL, Qiu CX, Wahlin A, Winblad B, Fratiglioni L. Diabetes mellitus and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Neurology. 2004;63:1181–1186. doi: 10.1212/01.wnl.0000140291.86406.d1. [DOI] [PubMed] [Google Scholar]
- Yano M, Hasegawa G, Ishii M, Yamasaki M, Fukui M, Nakamura N, Yoshikawa T. Short-term exposure of high glucose concentration induces generation of reactive oxygen species in endothelial cells: implication for the oxidative stress associated with postprandial hyperglycemia. Redox Rep. 2004;9:111–116. doi: 10.1179/135100004225004779. [DOI] [PubMed] [Google Scholar]
- Zhu W, Bijur GN, Styles NA, Li X. Regulation of FOXO3a by brain-derived neurotrophic factor in differentiated human SH-SY5Y neuroblastoma cells. Brain Res Mol Brain Res. 2004;126:45–56. doi: 10.1016/j.molbrainres.2004.03.019. [DOI] [PubMed] [Google Scholar]