Summary
We have investigated the role of the α subunit in the modulation of γ-aminobutyric acid type A (GABAA) receptors by the general anesthetic propofol, using whole-cell patch clamp recordings made from distinct stable fibroblast cell lines which expressed only α1β3γ2 or α6β3γ2 GABAA receptors. At clinically relevant anesthetic concentrations, propofol potentiated submaximal GABA currents in α1β3γ2 receptors to a far greater degree than those in α6β3γ2 receptors. The α subunit influenced the efficacy of propofol for modulation, but not its potency. In contrast, direct gating of the ion channel by propofol, in the absence of GABA, was significantly larger in the α6 than the α1 containing receptors. The potentiation of submaximal GABA by trichloroethanol, and the potentiation and direct gating by methohexital, was also studied, and showed the same relative trends as propofol.
Introduction
GABAA (γ-aminobutyric acid type A) receptors are the major receptors for inhibitory neurotransmission in the mammalian brain. The GABAA receptor is a pentameric complex formed by different glycoprotein subunits (α1–6,β1–4, γ1–4,δ) which combine to form a chloride channel (reviewed by Burt and Kamatchi, 1991; Olsen and Tobin, 1990; Rabow et al., 1995). GABAA receptor subunit expression in the central nervous system is heterogeneous (Laurie et al., 1992a,b; Wisden et al., 1991). For example, the GABAA α6 subunit isoform is localized solely in cerebellar granule cells, whereas α1 is widely expressed throughout the brain (Luddens et al., 1990; Kato, 1990; Mertens et al., 1993; Wisden et al., 1992).
The GABAA receptor complex is modulated allosterically by a wide range of compounds which act at discrete but unknown sites on the receptor (Macdonald and Olsen, 1994). One major group of GABAA receptor modulators is the class of general anesthetics, many of which have been demonstrated to augment GABAA receptor chloride currents at clinically relevant concentrations (e.g. Zimmerman et al., 1994), and are thought to elicit anesthesia by enhancing inhibitory synaptic transmission (Nicoll et al., 1975; Gage and Robertson, 1985). General anesthetics known to potentiate the actions of GABA (γ-aminobutyric acid) include propofol [2,6-diisopropylphenol (PRO); Hales and Lambert (1991); Hara et al. (1994)], steroid anesthetics (Harrison and Simmonds, 1984; Harrison et al., 1987), barbiturates (Study and Barker, 1981), chlormethiazole (Hales and Lambert, 1992), halogenated volatile anesthetics (Wakamori et al., 1991; Jones et al., 1992), and trichloroethanol (TCEt, the active metabolite of chloral hydrate; Lovinger et al., 1993; Peoples and Weight, 1994).
Different combinations of GABAA receptor subunits show variable sensitivity to allosteric modulators (e.g. Horne et al., 1993). The best documented role of specific subunits is for benzodiazepine modulation, where the presence of a γ subunit is required (Pritchett et al., 1989), but benzodiazepine agonist sensitivity is also influenced critically by the type of α subunit isoform. Specifically, the exchange of a single amino acid confers benzodiazepine sensitivity on the normally benzodiazepine-insensitive α6-containing GABAA receptor (Kleingoor et al., 1993).
The role of GABAA receptor subunits in general anesthetic modulation is less clear. For example, modulation of GABAA receptors by PRO is qualitatively independent of the γ subunit (Jones et al., 1995) and can be observed in heteromeric αβ or even in homomeric β1 receptors (Sanna et al., 1995a,b). It was recently shown that the α subunit influences modulation by pentobarbital (Thompson et al., 1996). This study was therefore designed to compare the effects of PRO on GABA-induced chloride currents in receptors containing two different α subunits against a common αβ background. The α6 subunit was chosen as it shares the least homology [along with α4, see Wafford et al. (1996)] with the other α subunit isoforms (Tyndale et al., 1995), and contributes to the differential pharmacology of benzodiazepines (Hadingham et al., 1993). We hypothesized that if differences in PRO modulation were to exist among α subunit isoforms, they might be most apparent in comparisons with α6-containing receptors. Portions of this work were previously presented in abstract form (O’Shea et al., 1996).
MATERIALS AND METHODS
Tissue culture
An Ltk− mouse fibroblast cell line from ATCC (Rockville, MD, U.S.A.) was stably transfected with dexamethasone-inducible GABAA receptor α1β3γ2 or α6β3γ2 cDNA, as previously described in detail (Hadingham et al., 1992). The resultant cell lines were cultured in supplemented Eagle’s minimum essential medium (Sigma, St. Louis, MO, U.S.A.) as previously described.
Electrophysiology
Electrophysiological recordings were performed at room temperature using the whole-cell patch clamp technique. The electrode solution contained (in mM): 147, N-methyl-D-glucamine hydrochloride; 5, CsCl; 5, K2ATP; 5, 4-(2-hydroxyethyl)-l-piperazine ethane sulfonic acid (HEPES); 1, MgCl2; 0.1, CaCl2; and 1.1, ethylene glycol bis(b-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), pH 7.2, osmolarity 315 mosmol. Pipette-to-bath resistance was 4–7 MΩ. During an experiment, cells were constantly perfused with extracellular solution containing (in mM) 145 NaCl, 3 KCl, 1.5 CaC12, 1 MgCl2, 5.5 D-glucose and 10 HEPES, pH 7.4, osmolarity 320–330 mosmol. Cells were voltage-clamped at −60 mV. Since the intracellular and extracellular solutions contained symmetrical chloride concentrations, the chloride reversal potential was ca 0 mV.
Electrophysiology-picospritzer
GABA was applied to the cell under study by brief pressure ejection (2–6 p.s.i., 10–100 msec) from low-resistance micropipettes filled with 20 μM GABA for cells containing α1β3γ2 receptors and 2 μM GABA for cells containing α6β3γ2 receptors. This produced transient inward currents which were standardized by varying the duration of the pressure pipette pulse. For both cell lines, a maximal response was elicited by a 1 sec pulse of GABA from the pressure pipette, which was then decreased to 25–50 msec to achieve transient chloride currents that were ca 20% of the maximum current obtainable by 20 or 2 μM (test response; 19.3 ± 0.89% and 19.2 ± 0.94% for the α1β3γ2 and α6β3γ2 cells, respectively). GABA was applied every 20 sec. Anesthetic agents were applied to the bath until a stable, maximal potentiation of the GABA response was achieved. Drugs were then washed out until the pre-drug current was regained. Using this method, anesthetic equilibrium and recovery typically took 5–10 min. Current responses were low-pass filtered at 5 kHz (−3 dB, Bessel filter 902; Frequency Devices, Inc, MA, U.S.A.), digitized (TLl-125 interface; Axon Instruments, Foster City, CA, U.S.A.), and stored for off-line analysis (AXOBASIC, Axon Instruments).
Electrophysiology-rapid solution changer
GABA and/or anesthetics were rapidly applied to the cell by local perfusion [as described in Koltchine et al. (1996)] using a motor-driven solution exchange device (Bio Logic Rapid Solution Changer RSC-100; Molecular Kinetics, Pullman, WA, U.S.A.). An approximate EC10 GABA test dose was used as the control value (α1β3γ2/PRO: 9.0 ± 2.2% of maximal current; α1β3γ2/methohexital (MTX): 8.4 ± 0.7%; α6β3γ2/PRO: 9.2 ± 0.2%; α6β3γ2/MTX: 10.8 ± 1.4%) for the potentiation experiments. The solution changer was controlled by protocols in the acquisition programs AXOBASIC or pCLAMP5 (Axon Instruments). Laminar flow was maintained by applying all solutions at identical flow rates via a multi-channel infusion pump (Smelting, Wood Dale, IL, U.S.A.). Prior to recording, a blue dye (FD and C Blue No. 1 and FD and C Red No. 40, McCormick, Baltimore, MD, U.S.A.) was used to check the alignment of the solution streams from the rapid solution changer.
Data analysis-electrophysiology
Drug-induced potentiation of a GABA-induced current was defined as the percentage increase of the control GABA response (defined as the average of the pre-drug and post-drug GABA induced currents). Concentration-response data were fitted (KaleidaGraph; Reading, PA, U.S.A.) with the logistic equation: I/Imax = 100*[drug]N/([drug]N+(EC50)N), where I/Imax is the percentage of the maximum obtainable GABA response, EC50 is the concentration producing a half-maximal response, and N is the Hill coefficient. Pooled data are presented as mean ± SEM. Statistical significance was determined by Student’s two-tailed, unpaired t-test.
[3H]Muscimol binding
Binding was performed on homogenized membranes prepared from cells cultured as above. Cells were harvested in ice-cold binding buffer containing (in mM): 20, Tris; 2, ethylenediamine tetraacetic acid (EDTA); 150, KCl, pH 7.4, then centrifuged at 2700g for 10 min at 4°C. The cell pellet was hypotonically lysed in deionized water and centrifuged at 48 000g for 20 min at 4°C. Finally, the pellet was rinsed with binding buffer and stored at −80°C for up to 2 months. Prior to experiments, membranes were thawed on ice and resuspended in assay buffer containing (in mM): 124, NaCl; 1.3, MgS04; 25, HEPES; 2.9, KCl; 1.2, KH2P04; D-glucose, 5.2, pH 7.4. The pellet was then homogenized with a Brinkman polytron (final protein concentration 0.3–0.5 mg/ml by Bradford assay, BSA standard). Membrane homogenates, along with eight concentrations of [3H]muscimol (saturation curve) or GABA plus a fixed concentration of [3H]muscimol (inhibition curve), were incubated in triplicate. After equilibration at room temperature, the assay mixtures were vacuum filtered through GF/B filter paper (Whatman, Clifton, NJ, U.S.A.) using a cell harvester (Brandel model MB-48, Gaithersburg, MD, U.S.A.), and quickly washed 10 times with 0.25 ml assay buffer. Specific binding was determined by subtracting the radioactive counts in the presence of 10 mM GABA from the radioactive counts in buffer alone. Similar results were obtained with a centrifugation assay using binding buffer (data not shown).
Data analysis-binding
Data points are taken from specific binding in individual experiments, and represent the mean of triplicate assays±SEM. Saturation binding data were fit (KaleidaGraph; Reading, PA, U.S.A.) with the equation: B = (Bmax * [L])/([[L] + Kd), where B is the specific binding, [L] is [muscimol], and Kd is the dissociation constant for muscimol. Inhibition data were fit with the equation:
where Bmin is the lowest specific binding, [drug] is the final concentration of GABA, IC50 is the concentration producing a half-maximal displacement of [3H]muscimol. The Ki value for GABA was calculated from: Ki = IC50/(1 + ([L]/Kd).
Drugs used
Stock solutions of GABA, bicuculline methiodide, TCEt (Sigma), PRO (Aldrich, Milwaukee, WI, U.S.A), MTX sodium (Brevital®, Eli Lilly, Indianapolis, IN, U.S.A.), and midazolam hydrochloride (Versed®, intravenous/intramuscular solution preparation; Roche Pharmaceuticals, Manati, PR, U.S.A.) were diluted into extracellular solution daily before use. PRO was first dissolved into dimethyl sulfoxide (DMSO; Sigma) to form a stock solution of 10 mM PRO which was then dissolved into the extracellular solution to form the final PRO solutions (maximum final concentration of DMSO was 0.05% for a 50 μM PRO solution). Carrier controls were performed with 0.05% DMSO in extracellular medium. No significant effects of this DMSO solution were observed on GABA-induced currents in cells expressing either α1β3γ2 or α6β3γ2 receptors. [3H]muscimol (sp. act. 14.9 and 19.1 Ci/mmol) was obtained from NEN (Wilmington, DE, U.S.A.).
RESULTS
Receptor characteristics
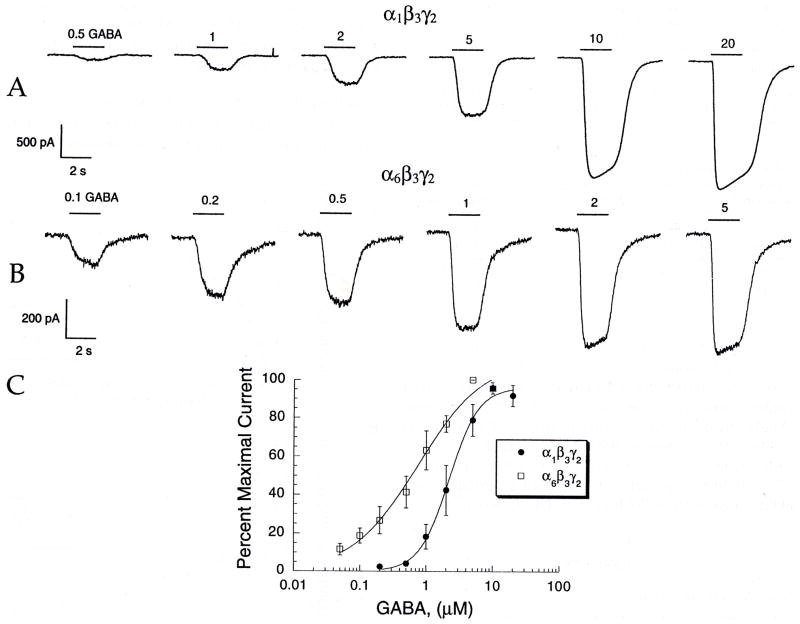
We initially characterized the electrophysiological responses to GABA of the α1β3γ2 and α6β3γ2 combinations. Application of GABA to either cell line by the rapid solution changer elicited concentration-dependent inward currents [Fig. 1(A, B)]. Analysis of the concentration-response data [Fig. 1(C) and Table 1] shows that the α6β3γ2 receptor has a higher apparent affinity for GABA (EC50 0.8 ± 0.1 μM) and a lower Hill coefficient (N= 0.9 ± 0.2) than the α1β3γ2 line (EC50 2.2 ± 0.2 μM, N = 1.9 ± 0.2, respectively; p < 0.05 for both comparisons). These results are in accord with other investigations that compared GABA responses in α1 and α6-containing GABAA receptor combinations (Ducic et al., 1995; Thompson et al., 1996). Also, the α1β3γ2 line produced larger maximal GABA currents than the α6β3γ2 line (2690 ± 400 pA vs 1186 ± 448 PA, p < 0.05). Midazolam (1 μM) potentiated GABA currents in cells containing the α1 subunit, but not in cells containing the α6 subunit, and GABA currents in both receptor subtypes were blocked by 20 μM bicuculline (data not shown).
Fig. 1.
GABA has higher apparent affinity for α6β3γ2 than α1β3γ2. GABA was applied with the rapid solution changer. (A and B) GABA responses from a cell expressing GABAA α1β3γ2 (A) or α6β3γ2 (B) receptors. Bars over current traces indicate the duration of rapid GABA application, with the concentration of applied GABA in μM. (C) Concentration-response curves for GABAA α1β3γ2 or α1β3γ2 receptors. Data points are shown as the normalized means of multiple experiments (5 ≤ n ≤ 7), error bars indicate SEM.
Table 1.
A summary of electrophysiological data using the rapid solution changer (values shown are means ± SEM with number of experiments in parentheses)
| Cell line | EC50 (μM) | Hill coefficient | Maximum (pA) |
|---|---|---|---|
| α1β3γ2 | 2.2 ± 0.2 (6) | 1.9 ± 0.2 (6) | 2690 ± 400 (18) |
| α6β3γ2 | 0.8 ± 0.1 (7) | 0.9 ± 0.2 (7) | 1186 ± 448 (8) |
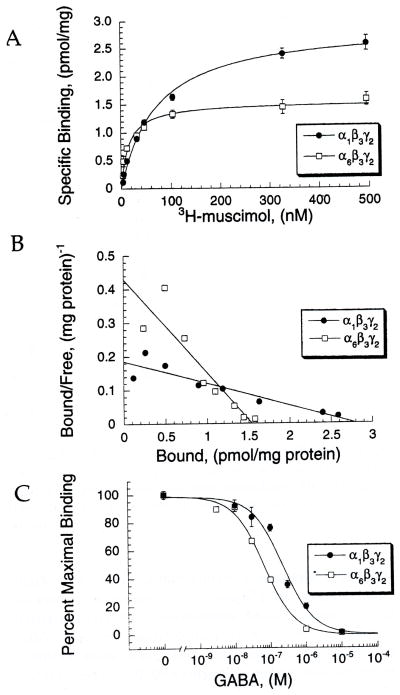
As shown in Fig. 2(A) and Table 2, the α6β3γ2 receptor shows a higher affinity for [3H]muscimol than α1β3γ2 (α6β3γ2 Kd 28 ± 5 nM versus α1β3γ2 Kd 90 ± 23, p <0.05). A Scatchard plot of the data (Fig. 2(B)) illustrates that binding at both receptors was appropriately fit by a one-site model. In displacement assays (Fig. 2(C)), GABA binding affinity to α6 was also higher than at α1-containing receptors, as shown by its lower IC50 (Table 2: α6β3γ2: 45 ± 14 nM vs α1β3γ2: 370 ± 78, p <0.05), and lower Ki value (α6β3γ2: 34 ± 10 versus α1β3γ2: 337 ± 71, p < 0.05).
Fig. 2.
Binding affinity is higher for muscimol and GABA at α6β3γ2 than α1β3γ2. (A) Saturation binding isotherms for α1β3γ2 and α6β3γ2 receptors obtained by using increasing concentrations of [3H]muscimol. Data points show the means ± SEM from a single experiment, with each concentration performed in triplicate. This experiment was performed five times, with similar results. (B) Shows a Scatchard plot from the same experiment from (A). Data points are the means of triplicate measurements. (C) An inhibition curve showing displacement of 10 nM [3H]muscimol with increasing concentrations of GABA. This figure represents the means ± SEM from a single experiment, with each point performed in triplicate. This experiment was performed three times with similar results.
Table 2.
A summary of radioligand binding data using [3H]muscimol (values represent the pooled means±SEM from curve fits of individual experiments with number of experiments in parentheses)
| Cell line | Muscimol Kd (nM) | GABA IC50 (nM) | GABA Ki (nM) |
|---|---|---|---|
| α1β3γ2 | 90 ± 23 (5) | 370 ± 78 (3) | 337 ± 71 (3) |
| α6β3γ2 | 28 ± 5 (5) | 45 ± 14 (3) | 10 ± 10 (3) |
Propofol potentiation of GABA responses
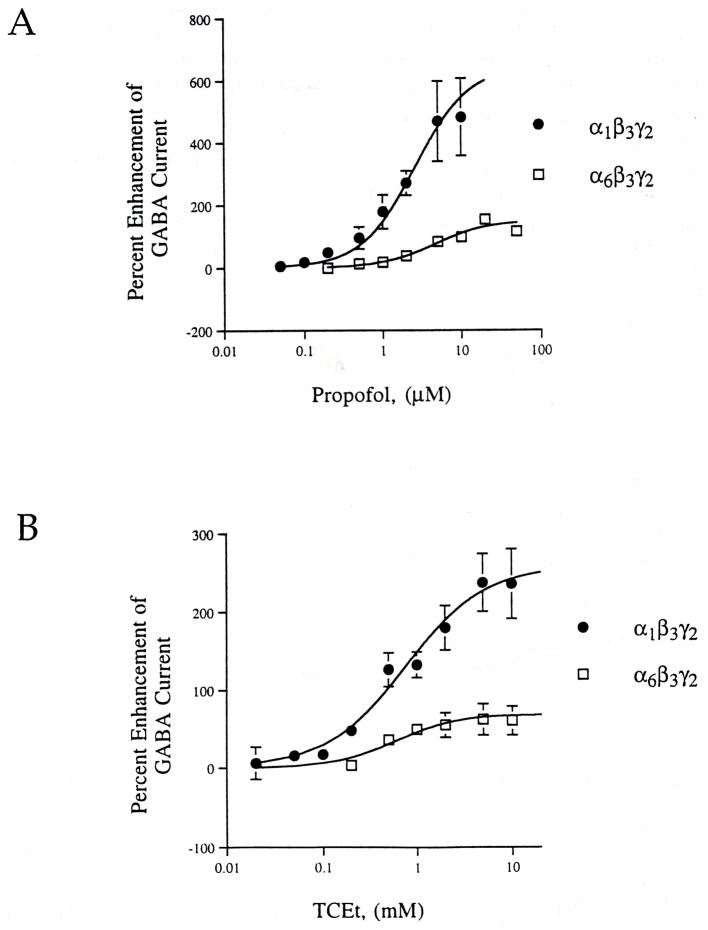
GABA potentiation by PRO was measured electrophysiologically using two separate methods: the picospritzer and rapid solution changer. The picospritzer was used from the initial set of experiments (Fig. 3, Table 3), since this method allowed for a wide range of modulator concentrations to be tested rapidly. However, once the appropriate PRO concentration range was established, later experiments with the rapid solution changer (Fig. 4) provided the means to apply known concentrations of GABA.
Fig. 3.
α subunit affects the efficacy, but not potency of PRO. Submaximal GABA was applied by picospritzer as detailed in Materials and Methods; anesthetics were applied to the bath. (A) PRO (0.05–50 μM) potentiates GABA-induced currents in both α1β3γ2 and α6β3γ2 GABAA receptors. Data points are means ± SEM from pooled experiments (4 ≤ n ≤ 13). (B) TCEt (0.02–10 mM) potentiates GABA-induced currents in both receptor combinations (5 ≤ n ≤ 15; pooled data are shown).
Table 3.
A comparison of electrophysiological data from α1β3γ2 and α6β3γ2 using bath-applied PRO or TCEt, picospritzer-applied GABA (values are pooled means ± SEM, 4 ≤ n ≤ 15)
| Anesthetic | Cell line | EC50 | Hill coefficient | Maximum (% enhancement) |
|---|---|---|---|---|
| PRO | α1β3γ2 | 2.6 ± 0.5 μM | 1.3 ± 0.2 | 564 ± 53 |
| α6β 3γ2 | 4.6 ± 1.9 μM | 1.3 ± 0.3 | 136 ± 17 | |
| TCEt | α1β3γ2 | 0.8 ± 0.2 mM | 1.0 ± 0.6 | 258 ± 21 |
| α6β 3γ2 | 0.6 ± 0.2 mM | 1.3 ± 0.3 | 60 ± 3 |
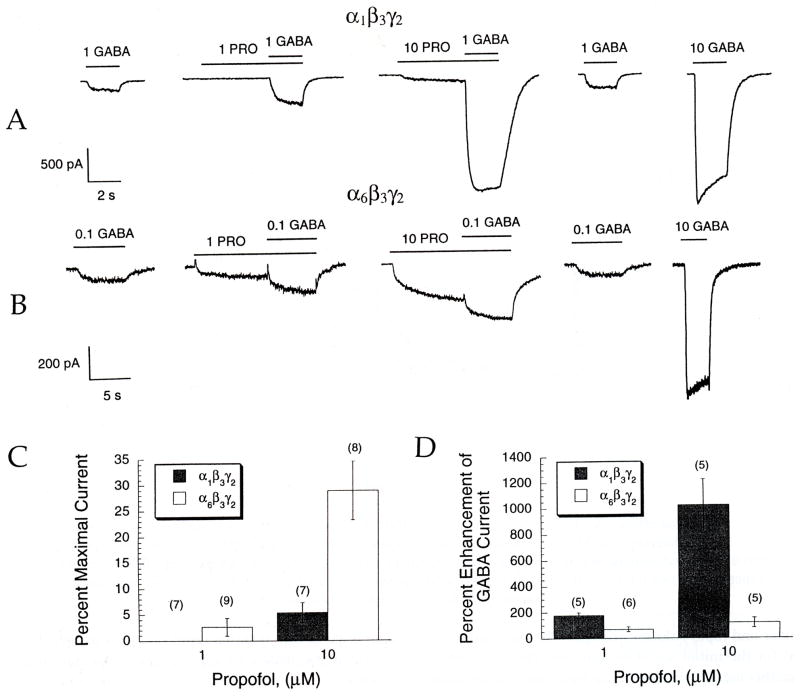
Fig. 4.
PRO causes greater direct gating in α6 than α1, but greater potentiation in α1 than α6-containing receptors. GABA and anesthetics were applied with the rapid solution changer. (A and B) PRO enhances EC10 GABA responses in either (A) α1β3γ2 or (B) α6β3γ2 GABAA receptors. The first trace shows a pre-anesthetic control response to GABA; the last trace shows the response to a maximal concentration of GABA. Bars over current traces indicate GABA and PRO applications, respectively, with the concentration of each drug applied given in μM. In both receptor combinations, pre-application of PRO often elicited a direct inward current in the absence of GABA. (C) PRO directly elicits an inward current in the absence of applied GABA, displayed here as a percentage of the maximal GABA response. Bars show the percentage of direct gating ± SEM from pooled experiments, with the number of experiments given in parentheses. This direct current is significantly greater at 10 μM PRO for the α6β3γ2 receptors (p < 0.05). (D) Enhancement of an EC10 GABA by 1 and 10 μM PRO is significantly greater in the α1β3γ2-containing receptors. The ordinate shows the percentage enhancement of the GABA dose (± SEM) in the presence of PRO compared to the GABA dose alone. (Number of experiments shown in parentheses.)
Significant potentiation of submaximal GABA-induced chloride currents by PRO was first observed at 0.2 μM and 0.5 μM PRO for the α1β3γ2 and α6β3γ2 GABAA receptors, respectively (Fig. 3(A), p < 0.05 for each). Table 3 shows that the magnitude of the maximal potentiation of GABA-induced currents by PRO in these picospritzer experiments was significantly greater in cells expressing the α1 isoform relative to the α6 isoform (maximum: 4. l-fold greater efficacy; p < 0.05). Statistically significant differences between the efficacy for PRO modulation of α1 and α6 receptors were observed at all concentrations > 0.2 μM PRO.
The rapid solution changer was then used to investigate GABAA receptor modulation in greater detail. As shown in Fig. 4, and Table 4, the efficacy of PRO potentiation applied at 10 μM was higher in α1 than at α6-ontaining receptors (1024 ± 203% vs 120 ± 39%; percent enhancement of an EC10 GABA test concentration by α1β3γ2 and α6β3γ2-containing receptors, respectively; p < 0.05).
Table 4.
A comparison of α1β3γ2 and α6β3γ2 using the rapid solution changer to apply GABA or PRO or MTX (values represent means ± SEM pooled from multiple experiments with number of experiments in parentheses)
| Cell line | Concentration (μM) | PRO (% Enhancement) | MTX (% Enhancement) |
|---|---|---|---|
| α1β3γ2 | 1 | 178 ± 21 (5) | 125 ± 16 (6) |
| 10 | 1024 ± 203 (5) | 816 ± 63 (6) | |
| α6β3γ2 | 1 | 70 ± 18 (6) | 124 ± 43 (7) |
| 10 | 120 ± 39 (5) | 309 ± 86 (6) |
Direct activation of GABAA receptors
As Fig. 4 illustrates, some of the single cell responses to PRO show an inward current during pre-equilibration of the anesthetic, prior to co-application with GABA. This direct channel gating by 10 μM PRO was much more pronounced in α6β3γ2 than α1β3γ2-containing receptor (Fig. 4(C): 24.0 ± 6.4% versus 5.3 ± 1.9% of maximal current, respectively; p< 0.05).
Effect of other anesthetics
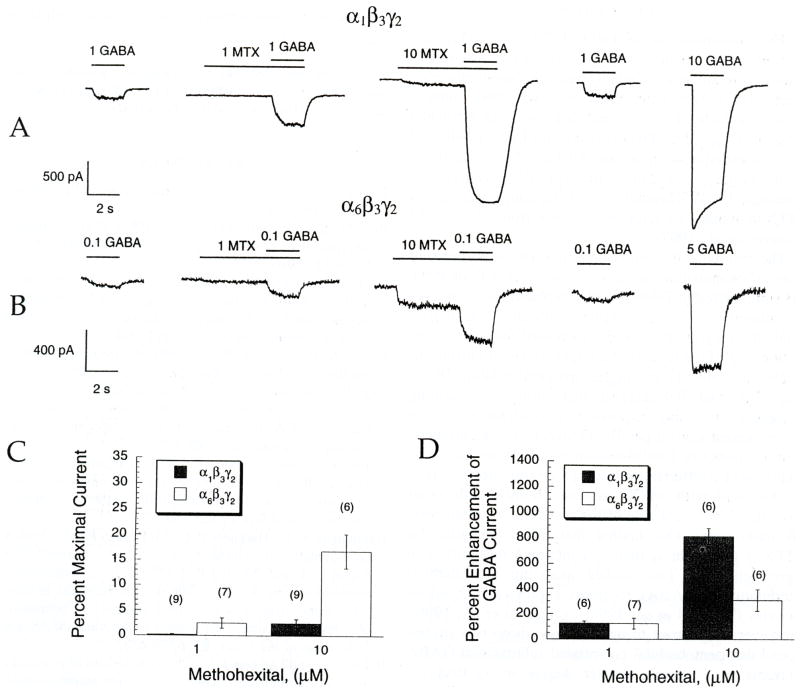
To determine whether these findings applied to other GABA modulators, the barbiturate MTX was also studied (Fig. 5 and Table 4). As with PRO, the efficacy of MTX enhancement of an EC10 GABA test concentration was higher in α1 than α6-containing receptors (Table 4; 816 ± 63% vs 309 ± 86%, respectively; p < 0.05). Figure 5 shows that, in agreement with previously published data studying pentobarbital modulation of GABAA receptors expressed in Xenopus oocytes (Thompson et al., 1996), 10 μM MTX also elicits significantly larger direct current in α6β3γ2 than α1β3γ2 containing receptors (16.7 ± 3.3% versus 2.4 ± 0.8% of maximal current, respectively; p < 0.05). Trichloroethanol was also studied, using picospritzer applied GABA (Fig. 3(B), Table 3). This drug also potentiated submaximal GABA currents, although it was less efficacious than PRO (2.2-fold less efficacious at both receptor subtypes). In a similar fashion to PRO, the efficacy of TCEt potentiation was significantly greater in α1β3γ2 than α6β3γ2-containing receptors p < 0.05).
Fig. 5.
MTX causes greater direct gating in α6 than α1, but greater potentiation in α1 than α6-containing receptors. Experimental design and figure representations are analogous to Fig. 4. (A and B) enhancement of submaximal GABA responses by MTX in cells expressing either (A) α1β3γ2 or (B) α6β3γ2 GABAA receptors. As with PRO, preapplication of MTX often elicited an inward current directly. (C) MTX directly activates an inward current in the absence of applied GABA, shown as a percentage of the maximal GABA response. This direct current is significantly greater at both 1 and 10 μM MTX for the α6β3γ2 receptors (p < 0.05). (D) Enhancement of an EC10 GABA current response by 1 and 10 μM MTX is significantly greater in the α1β3γ2 at 10 μM.
DISCUSSION
The concentrations of PRO, TCEt, and MTX that caused enhancement of GABA-induced currents in this study correlate with clinically relevant anesthetic concentration ranges determined in vivo. PRO induces general anesthesia in rats and dogs with an estimated EC50 of 0.4 μM free PRO (Franks and Lieb, 1994), and TCEt anesthetizes canines and humans at concentrations in the range of 0.2–2 mM (Breimer, 1977; Garrett and Lambert, 1973). Threshold anesthetic concentrations for MTX in humans are estimated to range from 12 to 37 μM (Lauven et al., 1987).
The results of this study underline the importance of subunit composition in the modulatory effects of PRO. As summarized in Tables 3 and 4, replacing an α6 with an α1 subunit in GABAA receptors with an identical β3γ2 subunit background markedly increased the modulatory action of PRO. Although the larger maximal current (as shown in Table 1) and higher maximal binding [Bmax; Fig. 2(A and B)] suggest that more α1-containing receptors are being expressed, normalization of the enhancement against parallel GABA test concentrations demonstrates that the differential enhancement cannot be explained by differences in receptor expression levels.
Our data with MTX contrast slightly with other investigations of the role of the α subunit in modulation by barbiturates. Our finding that direct activation by MTX is greater in α6 than α1-containing receptors is in agreement with two studies assessing pentobarbital modulation in GABAA receptors expressed in Xenopus oocytes (Wafford et al., 1996; Thompson et al., 1996). However, in contrast to our findings, those two studies found that pentobarbital potentiated submaximal GABA currents to a slightly greater degree in α6 than α1-icontaining receptors. These differences may be accounted for by the use of different expression systems, barbiturates, and βγ backgrounds between those studies and ours. Use of the rapid solution changer allows for rapid equilibration of the applied GABA concentration, a condition which may be more difficult to achieve in whole oocytes. Nevertheless, from these studies, as well as our own, it is clear that the α subunit plays a significant role in both PRO and barbiturate modulation.
Inspection of Table 3 reveals a difference in the maximal potentiation (efficacy), but not the EC50, for GABA potentiation by PRO between α1β3γ2 or α6β3γ2 GABAA receptors. Thus, differences between the structures of the α1 and α6 subunit isoforms may be important in the extent of allosteric modulation by PRO, but perhaps not direct binding per se, of the drug to the GABAA receptor. Previous work has already shown that modulation by PRO does not require the γ subunit (Jones et al., 1995), and in fact, is even seen in β1 homomers expressed in Xenopus oocytes (Sanna et al., 1995b). Our finding that the α subunit isoform type does not affect the apparent affinity of PRO is consistent with the primary determinants of PRO binding being located on the β subunit, or on components of the α subunit which are highly conserved.
Acknowledgments
Research support was provided by NIH grants GM45129 and GM00623 (NLH), NIDA grant DA07255 (SMO), and NIMH grant MH11504 (MDK).
References
- Breimer DD. Clinical pharmacokinetics of hypnotics. Clinical Pharmacokinetics. 1977;2:93–109. doi: 10.2165/00003088-197702020-00002. [DOI] [PubMed] [Google Scholar]
- Burt DR, Kamatchi GL. GABAA receptor subtypes: from pharmacology to molecular biology. FASEB Journal. 1991;5:2916–2923. doi: 10.1096/fasebj.5.14.1661244. [DOI] [PubMed] [Google Scholar]
- Ducic I, Caruncho HJ, Zhu WJ, Vicini S, Costa E. γ-Aminobutyric acid gating of Cl− channels in recombinant GABAA receptors. Journal of Pharmacology and Experimental Therapeutics. 1995;272:438–445. [PubMed] [Google Scholar]
- Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- Gage PW, Robertson B. Prolongation of inhibitory postsynaptic currents by pentobarbitone, halothane, and ketamine in CA1 pyramidal cells in rat hippocampus. British Journal of Pharmacology. 1985;85:675–681. doi: 10.1111/j.1476-5381.1985.tb10563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett ER, Lambert HJ. Pharmacokinetics of trichloroethanol and metabolites and interconversions among variously referenced pharmacokinetic parameters. Journal of Pharmaceutical Science. 1973;62:550–572. doi: 10.1002/jps.2600620404. [DOI] [PubMed] [Google Scholar]
- Hadingham KL, Harkness PC, McKeman RM, Quirk K, Le Bourdelles B, Horne A, Kemp JA, Barnard EA, Ragan CI, Whiting PJ. Stable expression of mammalian type A γ-aminobutyric acid receptors in mouse cells: demonstration of functional assembly of benzodiazepine-responsive sites. Proceedings of the National Academy of Sciences of the USA. 1992;89:6378–6382. doi: 10.1073/pnas.89.14.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales TG, Lambert JJ. The actions of propofol on inhibitory amino acid receptors of bovine adrenomedullary chromaffin cells and rodent central neurones. British Journal of Pharmacology. 1991;104:619–628. doi: 10.1111/j.1476-5381.1991.tb12479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales TG, Lambert JJ. Modulation of GABAA and glycine receptors by chlormethiazole. European Journal of Pharmacology. 1992;210:239–246. doi: 10.1016/0014-2999(92)90410-6. [DOI] [PubMed] [Google Scholar]
- Hara M, Kai Y, Ikemoto Y. Enhancement by propofol of the γ-aminobutyric acidA response in dissociated hippocampal pyramidal neurons of the rat. Anesthesiology. 1994;81:988–994. doi: 10.1097/00000542-199410000-00026. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Simmonlds MA. Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Research. 1984;323:287–292. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Majewska MD, Harrington JW, Barker JL. Structure-activity relationships for steroid interaction with the γ-aminobutyric acidA receptor complex. Journal of Phunnacology and Experimental Therapeutics. 1987;241:346–353. [PubMed] [Google Scholar]
- Horne AL, Harkness PC, Hadingham KL, Whiting P, Kemp JA. The infuence of the gamma 2L subunit on the modulation of responses to GABAA receptor activation. British Journal of Pharmacology. 1993;108:71l–716. doi: 10.1111/j.1476-5381.1993.tb12866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MV, Brooks PA, Harrison NL. Enhancement of gamma-aminobutyric acid-activated Cl− currents in cultured rat hippocampal neurones by three volatile anesthetics. Joumal of Physiology. 1992;449:279–293. doi: 10.1113/jphysiol.1992.sp019086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MV, Harrison NI, Pritchett DB, Hales TG. Modulation of the GABAA receptor by propofol is independent of the γ subunit. Journal of Pharmacology and Experimental Therapeutics. 1995;274:962–968. [PubMed] [Google Scholar]
- Kato K. Novel GABAA receptor α subunit is expressed only in cerebellar granule cells. Journal of Molecular Biology. 1990;214:619–624. doi: 10.1016/0022-2836(90)90276-r. [DOI] [PubMed] [Google Scholar]
- Kleingoor C, Wieland H4, Korpi ER, Seeburg PH, Kettenmann HL. Current potentiation by diazepam but not GABA sensitivity is determined by a single histidine residue. Neuroreport. 1993;4:187–190. doi: 10.1097/00001756-199302000-00018. [DOI] [PubMed] [Google Scholar]
- Koltchine VV, Ye Q, Fimi SE, Harrison NL. Chimeric GABAA/glycine receptors: expression and barbiturate pharmacology. Neuropharmacology. 1996;35:1445–1456. doi: 10.1016/s0028-3908(96)00088-3. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH, Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain II. Olfactory bulb and cerebellum. Journal of Neuroscience. 1992a;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain III. Embryonic and postnatal development. Journal of Neuroscience. 1992b;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauven PM, Schwilden Hl, Stoeckel H. Threshold hypnotic concentration of methohexitone. European Journal of Clinical Pharmacology. 1987;33:261–265. doi: 10.1007/BF00637559. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Zimmerman SA, Levitin M, Jones MV, Harrison NL. Trichloroethanol potentiates synaptic transmission mediated by γ-aminobutyric acidA receptors in hippocampall neurons. Journal of Pharmacology and Experimental Therapeutics. 1993;264:1097–l103. [PubMed] [Google Scholar]
- Lüddens H, Pritchett DB, Kohler M, Killisch I, Keinanen K, Monyer H, Sprengel R, Seeburg PH. Cerebellar GABAA receptor selective for a behavioural alcohol antagonist. Nature. 1990;346:648–651. doi: 10.1038/346648a0. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Ols’en RW. GABAA receptor channels. Annual Reviews in Neuroscience. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Mertens S, Benke D, Mohler H. GABAA receptor populations with novel subunit combinations and drug binding profiles identified in brain by alpha 5- and delta-subunit-specific immunopurification. Journal of Biological Chemistry. 1993;268:5965–5973. [PubMed] [Google Scholar]
- Nicoll RA, Eccles JC, Oshima T, Rubia C. Prolongation of hippocampal inhibitory post-synaptic potentials by barbiturates. Nature. 1975;258:625–627. doi: 10.1038/258625a0. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Tobin AJ. Molecular biology of GABAA receptors. FASEB Journal. 1990;4:1469–1480. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- O’Shea SM, Krasowski MD, Rick CE, Whiting PJ, Hadingham KL, Czajkowski C, Harrison NL. Subunit-dependent modulation of recombinant GABAA receptors by general anesthetics. Society for Neuroscience Abstracts. 1996;26:511–512. [Google Scholar]
- Peoples RW, Weight FF. Trichloroethanol potentiation of γ-aminobutyric acid-activated chloride current in mouse hippocampal neurons. British Journal of Pharmacology. 1994;113:555–563. doi: 10.1111/j.1476-5381.1994.tb17025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, Seeburg PH. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Rabow LE, Russek SJ, Farb DH. From ion currents to genomic analysis: recent advances in GABAA receptor research. Synapse. 1995;21:189–274. doi: 10.1002/syn.890210302. [DOI] [PubMed] [Google Scholar]
- Sanna E, Mascia MP, Klein RL, Whiting PJ, Biggio G, Harris RA. Actions of the general anesthetic propofol on recombinant human GABAA receptors: influence of receptor subunits. Journal of Pharmacology and Experimental Therapeutics. 1995a;274:353–360. [PubMed] [Google Scholar]
- Sanna E, Garau F, Harris RA. Novel properties of homomeric beta 1 gamma-aminobutyric acid type A receptors: actions of the anesthetics propofol and pentobarbital. Molecular Pharmacology. 1995b;47:213–217. [PubMed] [Google Scholar]
- Study RE, Barker JL. Diazepam and (-)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of γ-aminobutyric acid responses in cultured central neurons. Proceedings of the National Academy of Sciences of the U.S.A. 1981;78:7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SA, Whiting PJ, Wafford KA. Alpha subunits influence the action of pentobarbital on recombinant GABA-A receptors. British Journal of Pharmacology. 1996;117:521–527. doi: 10.1111/j.1476-5381.1996.tb15221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyndale RF, Olsen RW, Tobin AJ. GABAA receptors. In: North RA, editor. The Handbook of Receptors and Channels: Ligand and Voltage-gated Zon Channels. CRC Press; Boca Raton, FL: 1995. pp. 265–290. [Google Scholar]
- Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human γ-aminobutyric acidA receptors containing the α4 subunit. Molecular Pharmacology. 1996;50:670–678. [PubMed] [Google Scholar]
- Wakamori M, lkemoto Y, Akaike N. Effects of two volatile anesthetics and a volatile convulsant on the excitatory and inhibitory amino acid responses in dissociated CNS neurons of the rat. Journal of Neurophysiology. 1991;66:2014–2021. doi: 10.1152/jn.1991.66.6.2014. [DOI] [PubMed] [Google Scholar]
- Wisden W, Gundlach AL, Barnard EA, Seeburg PH, Hunt SP. Distribution of GABAA receptor subunit mRNAs in rat lumbar spinal cord. Brain Research and Molecular Brain Research. 1991;10:179–183. doi: 10.1016/0169-328x(91)90109-b. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. Journal of Neuroscience. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman SA, Jones MV, Harrison NL. Potentiation of gamma-aminobutyric acidA receptor Cl− current correlates with in vivo anesthetic potency. Journal of Pharmacology and Experimental Therapeutics. 1994;270:987–991. [PubMed] [Google Scholar]