Abstract
The endogenous opioid system plays an important role in the behavioral effects of nicotine. Thus, μ-opioid receptor and the endogenous opioids derived from proenkephalin are involved in the central effects of nicotine. However, the role played by the different endogenous opioid peptides in the acute and chronic effects of nicotine remains to be fully established. Mice lacking β-endorphin were acutely injected with nicotine at different doses to evaluate locomotor, anxiogenic and antinociceptive responses. The rewarding properties of nicotine were evaluated by using the conditioned place-preference paradigm. Mice chronically treated with nicotine were acutely injected with mecamylamine to study the behavioral expression of nicotine withdrawal. Mice lacking β-endorphin exhibited a spontaneous hypoalgesia and hyperlocomotion and a reduction on the anxiogenic and rewarding effects induced by nicotine. Nicotine induced similar antinociception and hypolocomotion in both genotypes and no differences were found in the development of physical dependence. The dissociation between nicotine rewarding properties and physical dependence suggests a differential implication of β-endorphin in these addictive related responses.
Keywords: reward, dependence, antinociception, anxiety, proopiomelanocortin
Introduction
Tobacco is one of the most widely used drugs and is the second major cause of death in the world (World Health Organization, 2008). Nicotine is the main component of tobacco responsible for its addictive properties. Nicotine pharmacological effects are mediated by the activation of nicotinic acetylcholine receptors (nAChRs), which promotes the release of diverse neurotransmitters in the CNS (McGehee et al., 1995; Pontieri et al., 1996). Several neurotransmitter systems are involved in the central effects of nicotine. Indeed, early studies using opioid antagonists have already proposed the involvement of the endogenous opioid system in some of the effects induced by nicotine (Balfour, 1982). In addition, naloxone administration attenuates nicotine antinociceptive (Zarrindast et al., 1997) and rewarding (Zarrindast et al., 2003) effects in mice and precipitates withdrawal in nicotine-dependent mice (Biala et al., 2005) and rats (Malin et al., 1993; Adams and Cicero, 1998). The generation of knockout (KO) mice lacking the different components of the endogenous opioid system has provided new advances in the knowledge of the participation of this system on nicotine effects. Thus, mice lacking the μ-opioid receptor or the preproenkephalin gene showed a decrease of acute nicotine antinociception (Berrendero et al., 2002, 2005). Similarly, nicotine rewarding effects and physical dependence were also attenuated in mutant mice lacking the μ-opioid receptor or the preproenkephalin gene (Berrendero et al., 2002, 2005). These findings support the implication of μ-opioid receptor and the endogenous opioid derived from preproenkephalin in nicotine-induced antinociception, reward and physical dependence.
β-endorphin is an important endogenous opioid peptide derived from proopiomelanocortin (POMC), that shares affinity for both μ and δ-opioid receptors (Corbett et al., 1993, 2006). The role played by β-endorphin on the pharmacological effects of nicotine remains to be clarified. The aim of this study was to evaluate the participation of this endogenous peptide in the acute and chronic responses of nicotine by using mice lacking β-endorphin (Rubinstein et al., 1996). First, acute effects of nicotine on locomotion, anxiety and antinociception were investigated. The rewarding properties of nicotine were evaluated using the conditioned place-preference paradigm. Mice were also chronically treated with nicotine to evaluate the behavioral expression of nicotine withdrawal precipitated by the nicotine antagonist mecamylamine.
Materials and Methods
Animals
The generation of mice lacking β-endorphin has been described previously (Rubinstein et al., 1996). Briefly, F1 mice heterozygous for the gene targeting vector POMCX*4, encoding a truncated POMC prohormone, were obtained from the breeding of a single germ-line penetrant male chimera to C57BL/6N females and crossbred to obtain F2 mice on a 129/sv x C57BL/6N hybrid genetic background. The mice (8-12 weeks old) used in this study were crossed for more than 10 generations to C57BL/6J mice and were therefore congenic for this genetic background. Male mice were housed five per cage in a temperature-controlled room (21 ± 1°C) with a 12 hr light/dark cycle (lights on between 8 A.M. and 8 P.M.). Food and water were available ad libitum. Mice were habituated to their new environment and handled for 1 week before the experimental procedure was started. Behavioral tests and animal care were conducted in accordance with the standard ethical guidelines (National Institutes of Health, 1995; European Communities Directive 86/609 EEC), and approved by the local ethical committee (CEEA-IMAS-UPF).
Drugs
(-)-Nicotine hydrogen tartrate salt [(-)-1-methyl-2(3-pyridyl) pyrrolidine] and mecamylamine hydrochloride (Sigma, Madrid, Spain) were dissolved in physiological saline (0.9%) and administered by subcutaneous route in a volume of 10 ml/kg. All the nicotine doses are expressed as nicotine hydrogen tartrate salt.
Experimental sequence
Mice were first evaluated in the black and white box and one week later in the tail immersion and hot plate tests. Only those mice receiving saline in this first experimental sequence were used for the repeated nicotine administration experiments. One week after the nociceptive tests, the selected mice were evaluated in the conditioned place preference paradigm. Nicotine dependence was induced two weeks after the end of the place conditioning test.
Black and white box
The box consists of a small (15 cm × 20 cm × 25 cm) dimly lit (5 lx) compartment, with black walls and a black floor, connected by a 4 cm long tunnel leading to a larger compartment (30 cm × 20 cm × 25 cm) intensely lit (500 lx) with white walls and white floor. Lines were drawn on the floor of both compartments to allow measurement of locomotor activity by counting the number of squares (5 × 5 cm) crossed. Floor lines separated the lit compartment into three equal zones, from the tunnel to the opposite wall, designated as proximal, median and distal zone. Each animal was placed in the dark compartment facing the tunnel at the beginning of the observation session, which started 5 min after the acute injection of nicotine (0.8 mg/kg). Latency to go for the first time to the lit compartment, time spent, number of squares crossed and number of visits into each zone of the lit compartment were recorded for 5 min.
Locomotor activity
The locomotor responses induced by nicotine administration (0, 1, and 3 mg/kg, s.c.) were measured by using individual locomotor activity boxes (9 × 20 × 11 cm; Imetronic), as previously described (Castañé et al., 2002). Mice were habituated to the locomotor cages daily during 10 min on three consecutive days. On day 4, mice were placed in the locomotor cages 5 min after drug injection, and horizontal and vertical locomotor activity were recorded for 10 min in a low-luminosity environment (20-25 lux).
Tail-immersion and hot-plate tests
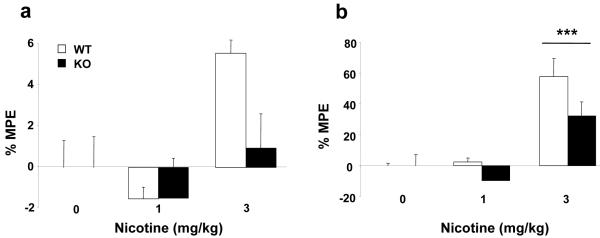
The tail-immersion test was conducted 15 min after nicotine (1 and 3 mg/kg, s.c.) or saline administration, as previously described (Simonin et al., 1998). The water temperature was maintained at 50 ± 0.5°C using a thermo-regulated water-circulating pump (Clifton, North Somerset, UK). The trial was terminated once the animal flicked its tail. In the absence of tail flick, a 10 sec cut-off was used to prevent tissue damage. The hot-plate test was performed 16 min after nicotine (1 and 3 mg/kg, s.c.) or saline injection, as previously described (Simonin et al., 1998). The heated surface of the plate was kept at a temperature of 52 ± 0.1°C (Columbus Instruments, Columbus, OH). The nociceptive behaviour evaluated was the jumping response. In absence of jumps, a 240 sec cut-off was used to prevent tissue damage. The data obtained on both nociceptive models were expressed as percentage of maximum possible effect (MPE %) (see Fig. 2) using the following equation MPE % = (test latency - control latency)/(cut-off time - control latency) × 100.
Figure 2.
Antinociceptive effects of nicotine in β-endorphin knockout (KO) and wild-type (WT) mice. Antinociceptive responses in the tail-immersion (a) and the hot-plate (b) tests were measured 15 and 16 minutes, respectively, after the administration of nicotine (1 and 3 mg/kg s.c.) WT (n = 14), KO (n = 16) and WT (n = 15), KO (n = 18) respectively, or saline WT (n = 13), KO (n = 16). Data is expressed as mean + SEM of the percentage of maximum possible effect (% MPE). *** p < 0.001 vs. saline (Dunnett post-hoc test).
Conditioning place preference
The rewarding effects of nicotine were evaluated by using the conditioning place preference paradigm. The apparatus consisted of two main square conditioning compartments separated by a triangular central division. During the preconditioning phase, each mouse was placed in the middle of the central division and had free access to both compartments of the conditioning apparatus for 18 min, with the time spent in each compartment recorded. Treatments were counterbalanced between compartments to use an unbiased procedure. No initial place preference or aversion for the different compartments was observed in the experiment. For the conditioning phase, mice were treated during 8 days with alternate injections of nicotine (0.5 mg/kg, s.c.) or saline. Mice were confined to the corresponding compartment immediately after injection for 20 min. Nicotine was administered on days 1, 3, 5, and 7, and saline was administered on days 2, 4, 6, and 8. Control animals received saline every day. The test phase was conducted as in the preconditioning phase, i.e., free access to both compartments for 18 min, and the time spent in each compartment was recorded. The time in the central area was proportionally shared and added to the time value of each compartment as reported previously (Maldonado et al., 1997). A score was calculated for each mouse as the difference between test and preconditioning time spent in the drug-paired compartment.
Nicotine dependence and withdrawal
Nicotine dependence was induced by using Alzet osmotic minipumps (Model 2001; Alzet, Cupertino, CA). These minipumps, implanted subcutaneously under brief ether anaesthesia, contained saline or nicotine solutions and delivered a constant subcutaneous flow at a rate of 1 μl/hr. The concentration of nicotine was adjusted to compensate for differences in body weight so average-weighed mice received a dose of 10 mg/kg/day. Nicotine withdrawal syndrome was precipitated 6 days after minipump implantation by injection of the nicotinic receptor antagonist, mecamylamine (1 mg/kg, s.c.). The somatic signs of withdrawal were evaluated immediately after mecamylamine injection during a period of 30 min, as previously reported (Castañé et al., 2002). The number of wet dog shakes, front paw tremors, writhes and scratches was counted. Body tremor, ptosis, teeth chattering, genital licks, and piloerection were scored 1 for appearance or 0 for non-appearance within each 5 min time. The locomotor activity over 5 min periods was rated 0, 1, or 2 (0 for inactivity, 1 for low activity, and 2 for normal activity). A global withdrawal score was calculated for each animal by giving each individual sign a relative weight, as previously reported (Castañé et al., 2002).
Statistical analysis
Results in all experiments were compared by using a between subjects two-way ANOVA (genotype and treatment as factors of variation). Individual treatment effects in each group, mutant and wild-type (WT), and in each treatment (nicotine and control) were analyzed using one-way ANOVA between subjects when appropriate. Post hoc comparisons were made by using Dunnett’s test after significant main effects of treatment by one-way ANOVA. Differences were considered significant if the probability of error was < 5 %.
Results
Nicotine decreased locomotion in wild-type and β-endorphin knockout mice
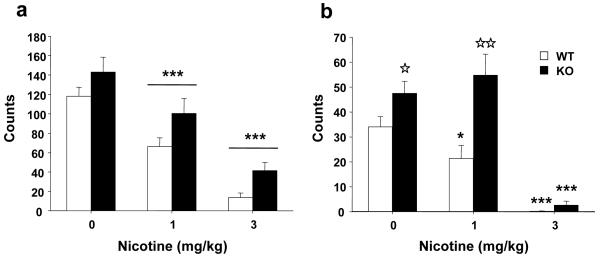
Nicotine (0, 1 and 3 mg/kg, s.c.) dose-dependently decreased horizontal and vertical locomotor activity in both β-endorphin KO and WT mice (Figures 1a-b). In horizontal activity two-way ANOVA revealed significant effects of treatment (F(2,52) = 41.909, p < 0.001) and genotype (F(1,52) = 10.086, p < 0.01), but no significant interaction between these two factors (F(2,52) = 0.088, NS) (Fig. 1a). Subsequent one-way ANOVA for treatment showed a significant effect of nicotine administration (F(2,57) = 36.088, p < 0.001). Post hoc analysis showed a decrease in the horizontal activity after the acute administration of 1 and 3 mg/kg of nicotine (p < 0.001) (Fig. 1a).
Figure 1.
Locomotor effects of nicotine in β-endorphin knockout (KO) and wild-type (WT) mice. Horizontal (a) and vertical (b) locomotor activity was measured 5 minutes after the acute injection of nicotine (1 and 3 mg/kg s.c.) WT (n = 10), KO (n = 10) and WT (n = 9), KO (n = 10) respectively, or saline WT (n = 10), KO (n = 9). Data are expressed as mean + SEM of photocell counts during a 10 min period. * p < 0.05, *** p < 0.001 vs. saline (Dunnett post-hoc test). ☆ p < 0.05, ☆☆ p < 0.01 WT vs. KO (one-way ANOVA).
KO mice showed an increased spontaneous vertical activity and required a higher dose of nicotine than WT mice to reduce vertical activity (Fig. 1b). Two-way ANOVA showed a significant effect of treatment (F(2,52) = 39.301, p < 0.001), genotype (F(1,52) = 16.668, p < 0.001) and a significant interaction between these two factors (F(2,52) = 5.119, p < 0.01). One-way ANOVAs revealed a significant effect of nicotine in WT (F(2,28) = 18.241, p < 0.001) and KO mice (F(2,28) = 24.767, p < 0.001). Post hoc analysis showed a decrease in the vertical activity of WT (1 and 3 mg/kg, p < 0.05 and p < 0.001 respectively) and KO mice (3 mg/kg, p < 0.001) after acute nicotine. One-way ANOVA for genotype revealed significant differences between WT and KO mice in vertical activity after saline (F(1,18) = 4.698, p < 0.05) and nicotine (1 mg/kg) (F(1,18) = 11.317, p < 0.01) injection. Therefore, KO mice showed a spontaneous hyperactivity that was mainly revealed on the vertical movements, and nicotine reduced locomotion in both genotypes.
Nociception was modified in β-endorphin knockout mice
A trend to decrease antinociceptive responses to the highest dose of nicotine in KO mice was observed in the tail-immersion and hot-plate tests when compared to the antinociceptive responses of this dose of nicotine in WT mice (Fig. 2). In the tail-immersion test (Fig. 2a), two-way ANOVA for MPE values revealed a significant effect of treatment (F(2,86) = 4.138, p < 0.05), but no significant effect of genotype (F(1,86) = 1.177, NS), nor interaction between these two factors (F(2,86) = 1.242, NS). Subsequent one-way ANOVA for treatment showed a significant effect of nicotine in WT and KO mice (F(2,91) = 3.802, p < 0.05). Post hoc analysis did not reveal significant effects. In the absence of β-endorphin the spontaneous nociceptive threshold in the tail-immersion (tail withdrawal latency in seconds) was significantly enhanced (Table 1). Indeed, two-way ANOVA of absolute values revealed a significant effect of treatment (F(2,86) = 4.331, p < 0.05), genotype (F(1,86) = 13.994, p < 0.001), but no significant interaction between these two factors (F(2,86) = 1.406, NS).
Table 1. Antinociceptive effects of acute nicotine in β-endorphin knock-out and wild-type mice.
| Wild-type mice | Knock-out mice | |
|---|---|---|
| Tail-inmersion test | ||
| Saline | 1.37 ± 0.11 | 1.92 ± 0.12 ++ |
| Nicotine (1 mg/kg) | 1.24 ± 0.05 | 1.80 ± 0.15 ++ |
| Nicotine (3 mg/kg) | 1.84 ± 0.21 | 2.00 ± 0.13 ++ |
| Hot-plate test | ||
| Saline | 65.60 ± 2.71 | 78.11 ± 11.73 |
| Nicotine (1 mg/kg) | 69.72 ± 4.74 | 62.34 ± 3.78 |
| Nicotine (3 mg/kg) | 166.24 ± 20.43 *** | 130.07 ± 14.84 *** |
Data (mean ± SEM) obtained in the hot-plate test (jumping response in seconds) and the tail-inmersion test (tail withdrawal latency in seconds) are shown.
p < 0.01 WT vs. KO (one-way ANOVA).
p < 0.001 vs. saline (Dunnett post-hoc test).
In the hot-plate test (Fig. 2b), two-way ANOVA for MPE values revealed a significant effect of treatment (F(2,86) = 28.292, p < 0.001), genotype (F(1,86) = 4.406, p < 0.05), but no interaction between these two factors (F(2,86) = 1.542, NS). Subsequent one-way ANOVA for treatment showed a significant effect of nicotine administration (F(2,91) = 25.956, p < 0.001). Post hoc analysis showed an increase in the nociceptive threshold in mice receiving the highest dose of nicotine (3 mg/kg, p < 0.001). The spontaneous nociceptive threshold at β-endorphin KO mice was similar to WT animals. Indeed, two-way ANOVA of absolute values revealed a significant effect of treatment (F(2,86) = 28.952, p < 0.001), but no significant effect of genotype (F(1,86) = 1.065, NS), nor interaction between these two factors (F(2,86) = 2.019, NS).
Nicotine anxiogenic effects were attenuated in β-endorphin knockout mice
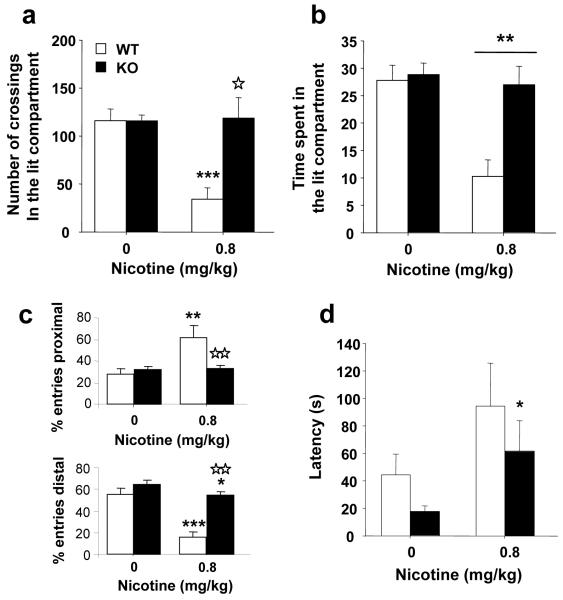
Nicotine (0.8 mg/kg, s.c.) induced anxiety-like responses in WT mice but not in KO animals when tested in the black and white box (Figures 3a-d). First, the administration of nicotine decreased the number of squares crossed in the lit compartment only in WT mice (Fig. 3a). Two-way ANOVA revealed a significant effect of treatment (F(1,35) = 7.272, p < 0.05), genotype (F(1,35) = 5.625, p < 0.05) and interaction between these two factors (F(1,35) = 4.433, p < 0.05). Subsequent one-way ANOVAs for treatment showed a significant effect of nicotine only in WT mice (F(1,18) = 16.258, p < 0.01). One-way ANOVA for genotype showed significant differences between WT and KO mice after nicotine (0.8 mg/kg) (F(1,18) = 6.079, p < 0.05), but not saline injection. In agreement, two-way ANOVA revealed significant effects of treatment (F(1,35) = 9.034, p < 0.01) and genotype (F(1,35) = 5.512, p < 0.05), but no interaction between these two factors (F(1,35) = 2.583, NS) on the time spent in the lit compartment after nicotine administration (Fig. 3b). In addition, nicotine significantly decreased the percentage of visit to the distal zone (40.1%, compared to control group) and increased the visit to the proximal zone (33.0%, compared to control group) in the lit compartment in WT animals. In KO mice nicotine decreased the percentage of visit to the distal zone (10.4%, compared to control group), but did not modify the percentage of visit to the proximal zone (Fig. 3c). For the percentage of visit to the distal zone, two-way ANOVA revealed a significant effect of treatment (F(1,35) = 23.429, p < 0.001), genotype (F(1,35) = 13.509, p < 0.01) and a significant interaction between these two factors (F(1,35) = 4.053, p < 0.05). Subsequent one-way ANOVAs for treatment showed a significant effect of nicotine in WT mice (F(1,18) = 20.737, p < 0.001) and KO mice (F(1,19) = 4.549, p < 0.05). One-way ANOVA for genotype showed significant differences between WT and KO mice after nicotine (0.8 mg/kg) (F(1,18) = 12.816, p < 0.01), but not saline injection. For the percentage of visit to the proximal zone, two-way ANOVA revealed a significant effect of treatment (F(1,35) = 8.349, p < 0.01), genotype (F(1,35) = 6.862, p < 0.05), and interaction between these two factors (F(1,35) = 9.989, p < 0.01). Subsequent one-way ANOVA for treatment showed a significant effect of nicotine only in WT mice (F(1,18) = 10.487, p < 0.01). One-way ANOVA for genotype showed significant differences between WT and KO mice after nicotine (0.8 mg/kg) (F(1,18) = 10.248, p < 0.01), but not saline injection. The anxiogenic effects of nicotine (0.8 mg/kg) were also revealed by the increase in the latency to enter into the lit compartment, and this effect was not abolished in β-endorphin KO mice (Fig. 3d). Two-way ANOVA revealed a significant effect of treatment (F(1,35) = 4.602, p < 0.05), but not effect of genotype (F(1,35) = 0.087, NS), and an interaction between these two factors (F(1,35) = 0.829, NS). Subsequent one-way ANOVAs for treatment showed a significant effect of nicotine in KO mice (F(1,19) = 4.412, p < 0.05). These results suggest that the anxiogenic-like responses induced by nicotine are attenuated in β-endorphin KO mice.
Figure 3.
Anxiogenic effects of nicotine in β-endorphin knockout (KO) and wild-type (WT) mice. Anxiogenic responses were measured in the black and white box 5 minutes after the acute injection of nicotine (0.8 mg/kg s.c.) WT (n = 9), KO (n = 10) or saline WT (n = 10), KO (n = 10). In a number of squares crossed in the lit compartment. In b time spent in the lit compartment. In c percentage of visits to the zones of the lit compartment. In d latency to enter in the lit compartment. Data is expressed as mean + SEM. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. saline (one-way ANOVA).☆ p < 0.05, ☆☆ p < 0.01 WT vs. KO (one-way ANOVA).
The rewarding properties induced by nicotine were abolished in β-endorphin knockout mice
The use of an unbiased procedure was ensured by the similar time spent in the drug paired compartment during the preconditioning phase in the different experimental groups as revealed by one-way ANOVA (F(3,92) = 0.007, NS) (data not shown). Nicotine (0.5 mg/kg) induced rewarding effects in WT but not in β-endorphin KO mice in the place-conditioning paradigm (Fig. 4). Two-way ANOVA showed significant effects of treatment (F(1,82) = 10.997; p < 0.01), no effects of genotype (F(1,82) = 0.138, NS), and a significant interaction between these two factors (F(1,82) = 4.291; p < 0.001). Subsequent one-way ANOVAs revealed that nicotine produced preference for the nicotine-assigned compartment only in WT mice (F(1,39) = 23.614; p < 0.001), whereas no effect was observed in KO mice (F(1,41) = 0.576; NS). These results indicate a reduction of the rewarding properties of nicotine in mice lacking β-endorphin gene.
Figure 4.
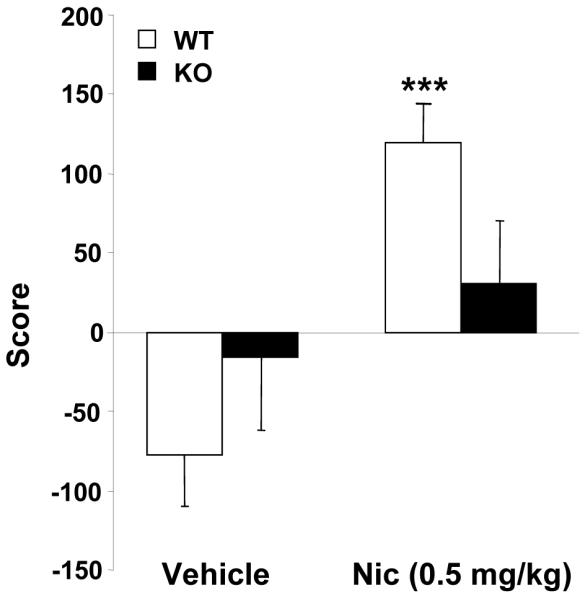
Rewarding effects of nicotine in β-endorphin knockout (KO) and wild-type (WT) mice. WT (n = 19) and KO (n = 21) mice were injected with nicotine (0.5 mg/kg s.c.) immediately before each conditioning session. A control group of WT (n = 21) and KO (n = 21) mice was injected with saline under the same conditions. A score was calculated for each mouse as the difference between test and preconditioning time spent in the drug-paired compartment. Data are expressed as mean score ± SEM of score. *** p < 0.001 vs. saline (one-way ANOVA).
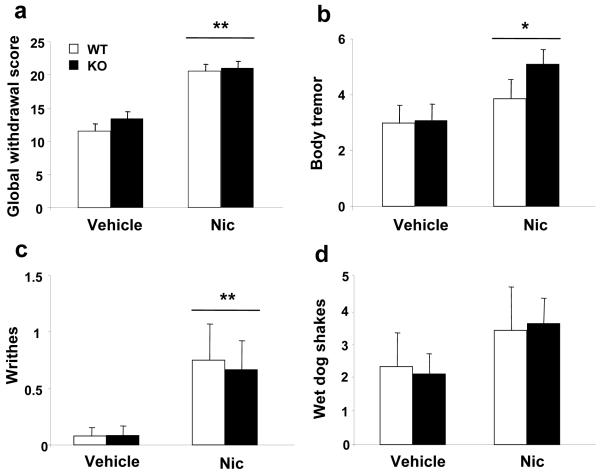
Somatic expression of precipitated nicotine withdrawal was not modified in β-endorphin knockout mice
No somatic signs of withdrawal were observed before mecamylamine (1 mg/kg, s.c.) administration in nicotine-dependent WT and KO mice (data not shown). Mecamylamine injection precipitated a nicotine withdrawal syndrome as revealed by the presence of several somatic signs in nicotine-dependent WT and KO mice (Fig. 5a-d). Two-way ANOVA of the global withdrawal scores showed significant effects of chronic nicotine treatment (F(1,53) = 9.258, p < 0.01), no effects of genotype (F(1,53) = 0.188, NS) nor interaction between these two factors (F(1,53) = 0.066, NS) (Fig. 5a). In agreement, no significant differences between genotypes were found in any of the withdrawal signs evaluated in nicotine dependent and control mice. Indeed, two-way ANOVA for body tremor showed significant effects of nicotine treatment (F(1,53) = 5.918, p < 0.05), no effects of genotype (F(1,53) = 1.105, NS) nor interaction between these two factors (F(1,53) = 0.906, NS) (Fig. 5b). Two-way ANOVA for writhes showed significant effects of chronic nicotine treatment (F(1,53) = 7.117, p < 0.05), no effects of genotype (F(1,53) = 0.027, NS) nor interaction between these two factors (F(1,53) = 0.036, NS) (Fig. 5c). Two-way ANOVA for wet dog shakes showed no significant effects of chronic nicotine treatment (F(1,53) = 1.359, NS), genotype (F(1,53) = 0.000, NS) nor interaction between these two factors (F(1,53) = 0.039, NS) (Fig. 5d).
Figure 5.
Mecamylamine-precipitated nicotine withdrawal in β-endorphin knockout (KO) and wild-type (WT) mice. Abstinence was precipitated after 6 d of nicotine perfusion (10 mg/kg/day) by the acute administration of mecamylamine (1 mg/kg, s.c.) to WT (n = 16) and KO (n = 12) mice. A non-dependent group of WT (n = 13) and KO (n = 12) mice was used as a control. In a global withdrawal score. In b-d representative individual signs of withdrawal. Data is expressed as mean + SEM of the global withdrawal score after the administration of mecamylamine. * p < 0.05, ** p < 0.01 vs. saline (one-way ANOVA).
Discussion
This study reveals that the anxiogenic and rewarding effects of nicotine were attenuated in β-endorphin KO mice while nicotine-induced antinociception, hypolocomotion and physical dependence were maintained in these mutants. A spontaneous hyperlocomotion and an enhanced nociceptive threshold were also revealed in β-endorphin KO mice. These data are in line with previous findings showing the relevance of μ and δ-opioid receptors, the main physiological targets of β-endorphin in the regulation of anxiety (Filliol et al., 2000; Ohinata et al., 2007) and the involvement of μ-opioid in the rewarding properties of nicotine (Berrendero et al., 2002, 2005).
Previous studies have shown a decrease in spontaneous locomotion of KO mice lacking μ-opioid receptor (Matthes et al., 1996; Filliol et al., 2000), whereas mice lacking δ-opioid receptors exhibited a spontaneous hyperlocomotion (Filliol et al., 2000). On the other hand, nicotine-induced hypolocomotion was not modified in mice lacking either μ-opioid receptor or the preproenkephalin gene (Berrendero et al., 2002, 2005). In this study, β-endorphin KO mice displayed spontaneous vertical hyperlocomotion and were less sensitive to nicotine-induced hypolocomotion. Taken into account the previous results obtained in opioid receptor KO mice and the similar high affinity of β-endorphin for μ and δ-opioid receptor (Corbett et al., 1993, 2006), this behavioral phenotype of β-endorphin KO mice seems to be mainly related to the suppression of the effects of this endogenous opioid peptide in δ-opioid receptor.
The relevance of β-endorphin in mouse locomotor activity has been suggested in previous studies showing a correlation between locomotion in a novel environment and β-endorphin levels (Radcliffe and Erwin, 1998). This hyperlocomotion related to the elevated levels of β-endorphin was attenuated by the administration of the opioid antagonist naltrexone (Radcliffe and Erwin, 1998). On the other hand, a reduction of locomotor activity was reported after the central administration of β-endorphin in rats (Spanagel et al., 1991; Krzanowska and Bodnar, 2000). The change in the spontaneous locomotor responses of mice lacking β-endorphin further emphasizes the role played by β-endorphin in the control of locomotion.
The enhancement of the spontaneous nociceptive threshold of β-endorphin KO mice was mainly revealed in the tail-immersion test, in agreement with previous results (Mogil et al., 2000). This enhanced nociceptive threshold might reflect a compensatory regulation of other neurobiological mechanisms involved in pain control. β-endorphin shares affinity for both μ and δ-opioid receptors (Corbett et al., 1993, 2006) and other opioid peptides acting on these receptors or other neurochemical systems involved in pain control might compensate the absence of β-endorphin. In agreement, previous studies have shown that the disruption of peptidergic systems inhibiting pain transmission, such as endorphins (Loh et al, 1976; Tseng et al., 1976) or melanocortin (Vrinten et al., 2001; Beltramo et al., 2003; Bertorelli et al., 2005), might also enhance nociceptive responses due to contra-adaptive mechanisms. The changes on the spontaneous nociceptive threshold cannot be explained by a motor impairment since KO mice presented a hyperlocomotor phenotype. In spite of the change in the basal nociceptive responses of β-endorphin KO mice, nicotine produced antinociception in these mutants in the hot-plate test where the spontaneous nociceptive responses were not significantly modified.
Several studies have reported the involvement of β-endorphin (Areda et al., 2005; Karsi et al., 2005) as well as μ and δ-opioid receptors (Filliol et al., 2000; Ohinata et al., 2007; Wilson and Junor, 2008) in anxiety-like behavior. Thus, mice lacking μ-opioid receptors showed anxiolytic-like responses, whereas δ-opioid receptor KO mice exhibited the opposite phenotype (Filliol et al., 2000; Yoo et al., 2004). In agreement, the administration of the μ-opioid agonist DAMGO induces anxiogenic-like effects in mice (Kudryavtseva et al., 2004). In this study, the anxiogenic-like responses induced by nicotine were attenuated in mice lacking β-endorphin, suggesting the relevance of this endogenous opioid peptide in the emotional effects of nicotine. Since the administration of delta opioid antagonists enhances the anxiogenic-like effects of nicotine in mice (Balerio et al., 2005), the attenuation of nicotine-induced anxiety-like responses in β-endorphin KO mice seems to be due to the absence of β-endorphin effects on μ-opioid receptors. This response was not due to a change in the spontaneous anxiety-like responses of β-endorphin KO mice that were similar to WT mice.
Nicotine rewarding properties are related to its ability to increase dopamine in the mesolimbic system (Risinger and Oakes, 1995; Picciotto et al., 1998; Castañé et al., 2002), similarly to other drugs of abuse (Pontieri et al., 1996; Picciotto et al., 1998). These rewarding properties of nicotine and its effects on dopaminergic transmission are mediated, at least in part, through an activation of μ-opioid receptors (Watkins et al., 2000; Berrendero et al., 2002) produced by the release of endogenous enkephalins (Berrendero et al., 2005). Nicotine administration has also been reported to enhance in vivo (Conte-Devolx et al., 1981; Rosecrans et al., 1985) and in vitro (Marty et al., 1985; Boyadjieva and Sarkar 1997; Houdi et al. 1991) the release of β-endorphin in rodents. Therefore, the release of β-endorphin and enkephalins induced by nicotine seems to be crucial for the manifestations of its rewarding properties.
The severity of mecamylamine precipitated nicotine withdrawal syndrome was not modified in β-endorphin KO mice. μ-opioid receptors are involved in the expression of nicotine physical dependence (Berrendero et al., 2002). Therefore, other endogenous ligands of the μ-opioid receptors, such as the opioid peptides derived from preproenkephalin, seem to be the main responsible for the activation of μ-opioid receptors during nicotine withdrawal, as previously proposed (Houdi et al., 1991; Berrendero et al., 2005). In agreement, nicotinic receptor stimulation has been reported to activate enkephalin release and biosynthesis (Eiden et al., 1984; Houdi et al., 1991) as well as proenkephalin mRNA levels (Suh et al., 1995). The present results revealed a clear dissociation between nicotine rewarding properties and physical dependence in β-endorphin KO mice. The rewarding properties of a drug are independent of its ability to produce physical dependence (Bozarth and Wise, 1984). Accordingly, independent molecular mechanisms have been proposed to be involved in the rewarding properties of nicotine and its capability to develop physical dependence in mice (Besson et al., 2006; Kota et al., 2007). The present results further support the existence of different neurobiological mechanisms underlying nicotine reward and physical dependence.
In summary, the present results demonstrate the important role played by β-endorphin in the effects of nicotine on anxiety and reward. This endogenous opioid peptide also participates in the modulation of the spontaneous locomotion and nociceptive threshold. A dissociation between nicotine rewarding effects and physical dependence was also revealed in mice lacking β-endorphin. Therefore, β-endorphin is selectively involved in the addictive properties of nicotine, together with other endogenous opioid peptides, by mediating the perception of nicotine rewarding properties. This study provides an additional neurobiological substrate to explain the attenuation of nicotine consumption and the subjective pleasure derived from smoking produced by the administration of opioid antagonists in humans (Karras and Kane 1980; Wewers et al. 1998).
Acknowledgements
Dr. Trigo was supported by Instituto de Salud Carlos III “Ayudas para contratos posdoctorales de perfeccionamiento”. This work was supported by NIDA (1R01-DA01 6768-0111), Ministerio de Ciencia y Tecnología (SAF2007-64062), Instituto de Salud Carlos III Red de Trastornos Adictivos, Generalitat de Catalunya (2005SGR00131 and ICREA Academia). European Commission (GENADDICT OJ 2004/C164, N005166 and PHECOMP LSHM-CT-2007-037669).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams ML, Cicero TJ. Nitric oxide mediates mecamylamine- and naloxone-precipitated nicotine withdrawal. Eur. J. Pharmacol. 1998;345:R1–2. doi: 10.1016/s0014-2999(98)00089-2. [DOI] [PubMed] [Google Scholar]
- Areda T, Koks S, Philips MA, Vasar E, Karis A, Asser T. Alterations in opioid system of the rat brain after cat odor exposure. Neurosci. Lett. 2005;377:136–139. doi: 10.1016/j.neulet.2004.11.083. [DOI] [PubMed] [Google Scholar]
- Balerio GN, Aso E, Maldonado R. Involvement of the opioid system in the effects induced by nicotine on anxiety-like behaviour in mice. Psychopharmacology. 2005;181:260–269. doi: 10.1007/s00213-005-2238-y. [DOI] [PubMed] [Google Scholar]
- Balfour DJ. The effects of nicotine on brain neurotransmitter systems. Pharmacol. Ther. 1982;16:269–82. doi: 10.1016/0163-7258(82)90058-4. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Campanella M, Tarozzo G, Fredduzzi S, Corradini L, Forlani A, Bertorelli R, Reggiani A. Gene expression profiling of melanocortin system in neuropathic rats supports a role in nociception. Brain Res. Mol. Brain Res. 2003;118:111–118. doi: 10.1016/j.molbrainres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Kieffer BL, Maldonado R. Attenuation of nicotine-induced antinociception, rewarding effects, and dependence in mu-opioid receptor knock-out mice. J. Neurosci. 2002;22:10935–10940. doi: 10.1523/JNEUROSCI.22-24-10935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, Mendizabal V, Robledo P, Galeote L, Bilkei-Gorzo A, Zimmer A, Maldonado R. Nicotine-induced antinociception, rewarding effects, and physical dependence are decreased in mice lacking the preproenkephalin gene. J. Neurosci. 2005;25:1103–1112. doi: 10.1523/JNEUROSCI.3008-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertorelli R, Fredduzzi S, Tarozzo G, Campanella M, Grundy R, Beltramo M, Reggiani A. Endogenous and exogenous melanocortin antagonists induce anti-allodynic effects in a model of rat neuropathic pain. Behav. Brain Res. 2005;157:55–62. doi: 10.1016/j.bbr.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Besson M, David V, Suarez S, Cormier A, Cazala P, Changeux JP, Granon S. Genetic dissociation of two behaviors associated with nicotine addiction: beta-2 containing nicotinic receptors are involved in nicotine reinforcement but not in withdrawal syndrome. Psychopharmacology. 2006;187:189–199. doi: 10.1007/s00213-006-0418-z. [DOI] [PubMed] [Google Scholar]
- Biala G, Budzynska B, Kruk M. Naloxone precipitates nicotine abstinence syndrome and attenuates nicotine-induced antinociception in mice. Pharmacol. Rep. 2005;57:755–760. [PubMed] [Google Scholar]
- Boyadjieva NI, Sarkar DK. The secretory response of hypothalamic beta-endorphin neurons to acute and chronic nicotine treatments and following nicotine withdrawal. Life Sci. 1997;61:PL59–66. doi: 10.1016/s0024-3205(97)00444-x. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Anatomically distinct opiate receptor fields mediate reward and physical dependence. Science. 1984;224:516–517. doi: 10.1126/science.6324347. [DOI] [PubMed] [Google Scholar]
- Castañé A, Valjent E, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptors modifies nicotine behavioural responses, but not nicotine abstinence. Neuropharmacology. 2002;43:857–867. doi: 10.1016/s0028-3908(02)00118-1. [DOI] [PubMed] [Google Scholar]
- Conte-Devolx B, Oliver C, Giraud P, Gillioz P, Castanas E, Lissitzky JC, Boudouresque F, Millet Y. Effect of nicotine on in vivo secretion of melanocorticotropic hormones in the rat. Life Sci. 1981;28:1067–1073. doi: 10.1016/0024-3205(81)90755-4. [DOI] [PubMed] [Google Scholar]
- Corbett AD, Paterson SJ, Kosterlitz HW. Selectivity of ligands for opioid receptors. In: Herz A, editor. Handbook Exp. Pharmacol. 104/1. Berlin Springer-Verlag; New York: 1993. pp. 645–679. [Google Scholar]
- Corbett AD, Henderson G, McKnight AT, Paterson SJ. 75 years of opioid research: the exciting but vain quest for the Holy Grail. Br. J. Pharmacol. 2006;147:153–162. doi: 10.1038/sj.bjp.0706435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden LE, Giraud P, Dave JR, Hotchkiss AJ, Affolter HU. Nicotinic receptor stimulation activates enkephalin release and biosynthesis in adrenal chromaffin cells. Nature. 1984;312:661–663. doi: 10.1038/312661a0. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gavériaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat. Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Houdi AA, Pierzchala K, Marson L, Palkovits M, Van Loon GR. Nicotine-induced alteration in Tyr-Gly-Gly and Met-enkephalin in discrete brain nuclei reflects altered enkephalin neuron activity. Peptides. 1991;12:161–166. doi: 10.1016/0196-9781(91)90183-p. [DOI] [PubMed] [Google Scholar]
- Karras A, Kane JM. Naloxone reduces cigarette smoking. Life Sci. 1980;27:1541–1545. doi: 10.1016/0024-3205(80)90562-7. [DOI] [PubMed] [Google Scholar]
- Karsi A, Waldbieser GC, Small BC, Wolters WR. Genomic structure of the proopiomelanocortin gene and expression during acute low-water stress in channel catfish. Gen. Comp. Endocrinol. 2005;143:104–112. doi: 10.1016/j.ygcen.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J. Pharmacol. Exp. Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Krzanowska E, Bodnar RJ. Sex differences in locomotor activity following beta-endorphin in the ventrolateral periaqueductal gray. Physiol. Behav. 2000;68:595–598. doi: 10.1016/s0031-9384(99)00242-5. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN, Gerrits MA, Avgustinovich DF, Tenditnik MV, Van Ree JM. Modulation of anxiety-related behaviors by m- and kappa-opioid receptor agonists depends on the social status of mice. Peptides. 2004;25:1355–1363. doi: 10.1016/j.peptides.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Loh HH, Tseng LF, Wei E, Li CH. beta-endorphin is a potent analgesic agent. Proc. Natl. Acad. Sci. USA. 1976;73:2895–2898. doi: 10.1073/pnas.73.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, Saiardi A, Valverde O, Samad TA, Roques BP, Borrelli E. Absence of opiate rewarding effects in mice lacking dopamine D2 receptors. Nature. 1997;388:586–589. doi: 10.1038/41567. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Carter VA, Cunningham JS, Wilson OB. Naloxone precipitates nicotine abstinence syndrome in the rat. Psychopharmacology. 1993;112:339–342. doi: 10.1007/BF02244930. [DOI] [PubMed] [Google Scholar]
- Marty MA, Erwin VG, Cornell K, Zgombick JM. Effects of nicotine on beta-endorphin, alpha MSH, and ACTH secretion by isolated perfused mouse brains and pituitary glands, in vitro. Pharmacol. Biochem. Behav. 1985;22:317–325. doi: 10.1016/0091-3057(85)90397-1. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Grisel JE, Hayward MD, Bales JR, Rubinstein M, Belknap JK, Low MJ. Disparate spinal and supraspinal opioid antinociceptive responses in beta-endorphin-deficient mutant mice. Neuroscience. 2000;101:709–717. doi: 10.1016/s0306-4522(00)00422-x. [DOI] [PubMed] [Google Scholar]
- Ohinata K, Agui S, Yoshikawa M. Soymorphins, novel mu opioid peptides derived from soy beta-conglycinin beta-subunit, have anxiolytic activities. Biosci. Biotechnol. Biochem. 2007;71:2618–2621. doi: 10.1271/bbb.70516. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Radcliffe RA, Erwin VG. Genetic relationship between central beta-endorphin and novelty-induced locomotor activity. Pharmacol. Biochem. Behav. 1998;60:709–718. doi: 10.1016/s0091-3057(98)00045-8. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Oakes RA. Nicotine-induced conditioned place preference and conditioned place aversion in mice. Pharmacol. Biochem. Behav. 1995;51:457–461. doi: 10.1016/0091-3057(95)00007-j. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA, Hendry JS, Hong JS. Biphasic effects of chronic nicotine treatment on hypothalamic immunoreactive beta-endorphin in the mouse. Pharmacol. Biochem. Behav. 1985;23:141–143. doi: 10.1016/0091-3057(85)90141-8. [DOI] [PubMed] [Google Scholar]
- Rubinstein M, Mogil JS, Japón M, Chan EC, Allen RG, Low MJ. Absence of opioid stress-induced analgesia in mice lacking beta-endorphin by site-directed mutagenesis. Proc. Natl. Acad. Sci. USA. 1996;93:3995–4000. doi: 10.1073/pnas.93.9.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, Le Meur M, Roques BP, Maldonado R, Kieffer BL. Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488H and attenuates morphine withdrawal. EMBO J. 1998;17:886–897. doi: 10.1093/emboj/17.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Bals-Kubik R, Shippenberg TS. Beta-endorphin-induced locomotor stimulation and reinforcement are associated with an increase in dopamine release in the nucleus accumbens. Psychopharmacology. 1991;104:51–56. doi: 10.1007/BF02244553. [DOI] [PubMed] [Google Scholar]
- Suh HW, Hudson PM, McMillian MK, Das KP, Wilson BC, Wu GC, Hong JS. Long-term stimulation of nicotinic receptors is required to increase proenkephalin A mRNA levels and the delayed secretion of [Met5]-enkephalin in bovine adrenal medullary chromaffin cells. J. Pharmacol. Exp. Ther. 1995;275:1663–1670. [PubMed] [Google Scholar]
- Tseng LF, Loh HH, Li CH. Beta-Endorphin as a potent analgesic by intravenous injection. Nature. 1976;263:239–240. doi: 10.1038/263239a0. [DOI] [PubMed] [Google Scholar]
- Vrinten DH, Kalkman CJ, Adan RA, Gispen WH. Neuropathic pain: a possible role for the melanocortin system? Eur. J. Pharmacol. 2001;429:61–69. doi: 10.1016/s0014-2999(01)01306-1. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J. Pharmacol. Exp. Ther. 2000;292:1053–1064. [PubMed] [Google Scholar]
- Wewers ME, Dhatt R, Tejwani GA. Naltrexone administration affects ad libitum smoking behavior. Psychopharmacology. 1998;140:185–190. doi: 10.1007/s002130050756. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Junor L. The Role of Amygdalar Mu-Opioid Receptors in Anxiety-Related Responses in Two Rat Models. Neuropsychopharmacology. 2008;33:2957–2968. doi: 10.1038/sj.npp.1301675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization 2008 http://www.who.int/tobacco/health_priority/en/index.html.
- Yoo JH, Lee SY, Loh HH, Ho IK, Jang CG. Altered emotional behaviors and the expression of 5-HT1A and M1 muscarinic receptors in micro-opioid receptor knockout mice. Synapse. 2004;54:72–82. doi: 10.1002/syn.20067. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Pazouki M, Nassiri-Rad S. Involvement of cholinergic and opioid receptor mechanisms in nicotine-induced antinociception. Pharmacol. Toxicol. 1997;81:209–213. doi: 10.1111/j.1600-0773.1997.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Faraji N, Rostami P, Sahraei H, Ghoshouni H. Cross-tolerance between morphine- and nicotine-induced conditioned place preference in mice. Pharmacol Biochem Behav. 2003;74:363–369. doi: 10.1016/s0091-3057(02)01002-x. [DOI] [PubMed] [Google Scholar]