Abstract
Chuvash polycythemia results from a homozygous 598C>T mutation in exon 3 of the von Hippel-Lindau (VHL) gene. This disrupts the normoxia pathway for degrading hypoxia inducible factor (HIF)-1α and HIF-2α causing altered expression of HIF-1 and HIF-2 inducible genes. As hypoxia induces expression of proinflammatory cytokines, we hypothesized that there might be an elevation of Th1 cytokines in the setting of Chuvash polycythemia. We analyzed plasma concentrations of Th1 (interleukins-2 and 12, interferon-γ, granulocyte-monocyte colony-stimulating factor, tumor necrosis factor-α) and Th2 cytokines (interleukins-4, 5, 10, and 13) using the Bio-Plex multiplex suspension array system in 34 VHL598C>T homozygotes and 32 VHL wild-type participants from Chuvashia. Concentrations of all the Th1 and Th2 cytokines measured were elevated in the VHL598C>T homozygotes compared with the control wild-type participants, but the ratios of Th1 to Th2 cytokines did not differ by genotype. In parallel, peripheral blood concentrations of CD4 positive T-helper cells and CD4/CD8 ratio were lower in the VHL598C>T homozygotes. In conclusion, the up-regulated hypoxic response in Chuvash polycythemia is associated with increased plasma products of both the Th1 and Th2 pathways, but the balance between the two pathways seems to be preserved.
Introduction
Chuvash polycythemia, a disorder endemic to the Chuvash Republic of the Russian Federation, results from a homozygous mutation of the von Hippel-Lindau gene (VHL598C>T) [1,2]. This mutation disrupts the interaction of VHL protein with hypoxia inducible factor (HIF)-1α and HIF-2α proteins resulting in the accumulation of these proteins and altered expression of HIF-1 and HIF-2-regulated genes [1–3]. For example, plasma concentrations of the products of such HIF-regulated genes as endothelin-1, erythropoietin, plasminogen activator inhibitor-1, transferrin, transferrin receptor, and vascular endothelial growth factor are elevated in patients with Chuvash polycythemia [4,5]. Clinical manifestations of Chuvash polycythemia include increased cardiac valvular abnormalities, hemangiomas, pulmonary arterial hypertension, thrombotic and hemorrhagic events, varicose veins, and shortened lifespan [2,3,6].
Helper T cells were originally subdivided into two subsets, Th1 and Th2, which were distinguished on the basis of the secreted cytokines [7]. The subset balance between Th1 and Th2 cytokines is a critical outcome of the immune response [8]. Th1 cells are involved in promoting cellular immunity by secreting proinflammatory cytokines including interleukins-2 and 12 and granulocyte-monocyte colony-stimulating factor (GM-CSF), a stimulator of hematopoiesis, and inducing macrophages to produce cytokines such as interferon-γ and tumor necrosis factor-α [8–11]. Th2 cells produce anti-inflammatory cytokines including interleukins-4, 5, 10, and 13 [8,10]. Th2 cells target B cells to induce the production of antibodies [8,11–13], although in Th2 deficient mice antibody production can be facilitated by the TFH subset in a non-Th1/Th2-related manner [14]. Th1 and Th2 cytokines also influence vascular endothelial cell functions, including cell growth, differentiation, proliferation, apoptosis, and migration [15].
A number of reports indicate that hypoxia influences the expression of Th-1 and Th-2 cytokines. Hypoxia leads to increased LPS-stimulated expression by murine macrophages of interferon-γ [16] and of tumor necrosis factor-α via expression of HIF-1α [17]. Hypoxia was also reported to increase production by human peripheral blood mononuclear cells (PBMC) of interleukins 2 and 4 and interferon-γ, but not interleukin-10 [18]. However, interleukin-10 expression was up-regulated in hepatocytes exposed to hypoxia [19]. Expression of interferon-γ was reduced in subjects exposed to hypoxia whereas interleukin-4 remained unchanged [20]. Plasma interleukin 10 concentrations were increased in humans exposed to intermittent hypoxia [21]. Cerebral hypoxia-ischemia led to increased plasma concentrations of GM-CSF in an animal model [22]. Exposure of volunteers to high altitude led to an impairment of the homeostatic regulation of Th1/Th2 immune balance with a significant decrease of CD3+ and CD4+ T-cell T lymphocytes and increase in natural killer cells [20].
Given these previous studies suggesting that hypoxia influences the levels of Th1 and Th2 cytokines as well as CD3 and CD4 cells, we hypothesized that the profiles of Th1 and Th2 cytokines and lymphocyte subsets may be altered in Chuvash polycythemia. The purpose of this investigation was to assess plasma levels of Th1 and Th2 1cytokines and lymphocyte subsets in Chuvash polycythemia patients in comparison with normal control subjects.
Results
Of 35 patients with the diagnosis of Chuvash polycythemia studied, 34 were VHL598C>T homozygotes. One person with the diagnosis of Chuvash polycythemia had normal genotype and was assigned to the group of VHL normal alleles. Of the original controls, 31 had normal VHL alleles and four were VHL598C>T heterozygotes. The characteristics of the participants according to VHL genotype are summarized in Table I. There were no significant differences in age or sex between wild-type participants and Chuvash polycythemia homozygotes. Median hemoglobin and hematocrit values for heterozygote subjects fell between the normal allele group values and the homozygote values.
TABLE I.
Demographic and Clinical Characteristics of Participants According to VHL598C>T Status
| Characteristics |
VHL wild type (n = 32) |
VHL598C>T heterozygote (n = 4) |
VHL598C>T homozygote (n = 34) |
P (homozygote vs. wild type) |
|---|---|---|---|---|
| Male (%)a | 15 (47%) | 2 (50%) | 12 (35%) | 0.8 |
| Age (years) | 44 (15–50) | 8 (6–13) | 18 (13–51) | 0.6 |
| BMI (kg/m2) | 20.6 (18.4–24.2) | 14.7 (13.8–19.0) | 19.6 (16.8–22.9) | 0.3 |
| History of smokinga | 6 (19%) | 0 (0%) | 5 (15%) | 0.7 |
| History of alcohol consumptiona | 2 (7%)b | 0 (0%) | 2 (7%)c | 0.9 |
| Systolic blood pressure (mmHg) | 110 (102–120) | 99 (93–112) | 105 (95–116) | 0.032 |
| Diastolic blood pressure (mmHg) | 72 (64–80) | 65 (58–75) | 69 (60–80) | 0.3 |
| Hemoglobin (g/dL) | 13.7 (12.4–14.5) | 14.4 (14.0–15.3) | 18.6 (16.7–20.2) | 0.0001 |
| Hematocrit (%) | 39.4 (36.2–43.1) | 43.0 (40.7–44.2) | 55.7 (51.8–59.2) | 0.0001 |
| MCV (ft) | 90.1 (85.7–95.5) | 85.5 (82.5–87.7) | 87.6 (79.4–91.2) | 0.051 |
| White blood cell (103/mm3) | 5.8 (5.2–6.7) | 5.5 (5.0–8.1) | 5.0 (4.3–5.8) | 0.009 |
| Platelets (103/mm3) | 305 (247–355) | 403 (369–444) | 242 (178–322) | 0.029 |
| Lymphocyte% | 37.3 (35.8–46.2) | 49.3 (46.9–66.1) | 44.0 (39.7–49.9) | 0.2 |
| Lymphocyte counts (103/mm3) | 2.4 (1.9–2.8) | 2.7 (2.4–5.5) | 2.1 (1.8–2.6) | 0.1 |
Results in median and interquartile range, unless otherwise specified.
Number (%).
N = 27.
N = 29.
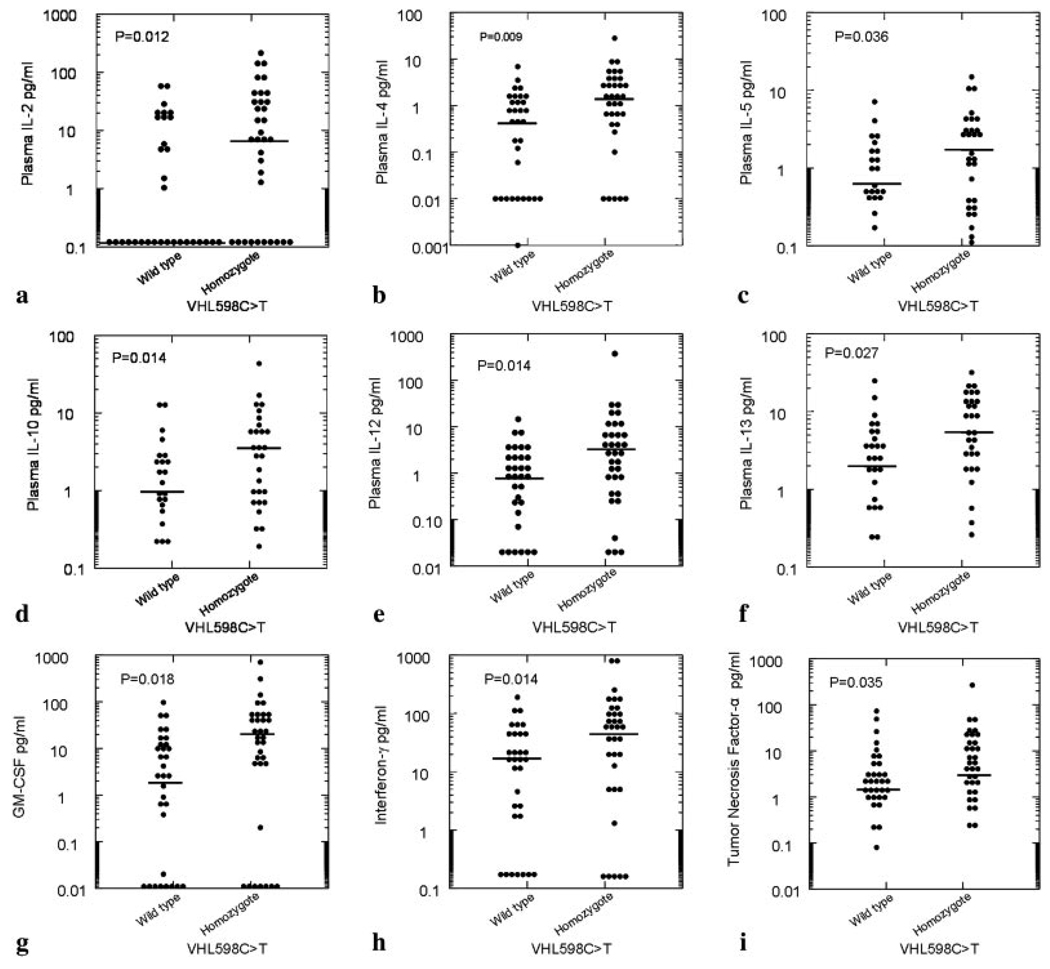
Table II displays the plasma cytokine levels among individuals with normal VHL alleles, VHL598C>T heterozygotes, and VHL598C>T homozygotes. Concentrations of each of the Th1 cytokines and Th2 cytokines measured were higher in the VHL598C>T homozygotes compared with the VHL normal alleles group. These results are graphically shown in Fig. 1. The elevations of cytokines in the VHL598C>T homozygotes paralleled the elevations of circulating concentrations of erythropoietin and vascular endothelial growth factor in the same patients. In contrast, the ratios of Th1 to Th2 cytokines did not differ significantly by genotype.
TABLE II.
Plasma Cytokine Concentrations According to VHL Genotype
|
VHL wild type (N = 32) |
VHL598C>T heterozygote (N = 4) |
VHL598C>T homozygote (N = 34) |
P (homozygote vs. wild type) |
|
|---|---|---|---|---|
| Th1 cytokines | ||||
| Interleukin-12 (pg/ml) | 0.9 (0.2–2.5) | 1.6 (0.9–2.2) | 3.0 (0.7–7.1) | 0.014 |
| Interleukin-2 (pg/ml) | 0.1 (0.1–15.6) | 0.1 (0.1–31.5) | 7.5 (0.1–40.5) | 0.012 |
| Interferon-γ (pg/ml) | 15.7 (1.8–44.1) | 23.2 (7.5–55.3) | 57.1 (5.7–100.4) | 0.014 |
| Tumor necrosis factor-α (pg/ml) | 2.1 (1.3–5.6) | 3.3 (1.3–6.1) | 5.4 (1.9–20.6) | 0.035 |
| GM-CSF (pg/ml) | 2.7 (0.02–12.4) | 4.9 (1.3–11.0) | 15.9 (4.7–44.2) | 0.018 |
| Th2 cytokines | ||||
| Interleukin-4 (pg/ml) | 0.5 (0.01–1.5) | 0.6 (0.1–1.5) | 1.5 (0.4–3.2) | 0.009 |
| Interleukin-5 (pg/ml) | 0.5 (0.02–1.3) | 0.3 (0.02–0.8) | 1.2 (0.2–3.0) | 0.036 |
| Interleukin-10 (pg/ml) | 0.7 (0.1–2.3) | 0.5 (0.05–1.3) | 2.2 (0.5–5.8) | 0.014 |
| Interleukin-13 (pg/ml) | 1.9 (0.2–4.2) | 1.7 (0.5–4.4) | 4.4 (1.2–12.8) | 0.027 |
| Ratios | ||||
| Interferon-γ to interleukin-10 | 19 (5–47) | 90 (43–184) | 18 (6–57) | 0.8 |
| Tumor necrosis factor-α to interleukin-10 | 4 (2–17) | 15 (5–29) | 4 (3–7) | 0.4 |
| Interferon-γ to interleukin-4 | 27 (18–42) | 54 (36–437) | 30 (19–39) | 0.9 |
| Tumor necrosis factor-α to interleukin-4 | 7 (4–91) | 6 (4–101) | 6 (3–14) | 0.3 |
| Vascular markers | ||||
| Erythropoietin (pg/ml) | 7.6 (3.5–10.5)a | – | 31.2 (18.2–39.1)b | 0.0006 |
| VEGF (pg/ml) | 50 (19–73)a | – | 86 (73–152)b | 0.012 |
Results in median and interquartile range.
N = 8.
N = 17.
Figure 1.
Density display of plasma cytokine concentrations for 66 participants according VHL genotype. For each cytokine, there is a higher plasma concentration in the VHL598C>T homozygotes. Black lines show the median concentration in each group.
CD4 lymphocyte counts (P = 0.012) and CD4/CD8 ratios (P = 0.015) were lower in the VHL598C>T homozygotes compared with participants with normal VHL alleles. Serum β globulin concentrations were marginally higher in VHL598C>T homozygotes (P = 0.06) (Table III).
TABLE III.
Other Immunologic Tests in Patients and Controls
|
VHL598C>T homozygotes (N = 30) |
VHL normal alleles (N = 30) |
P* | |
|---|---|---|---|
| Serum globulins (g/L) | |||
| α1 globulin | 2.6 (2.3–2.8) | 2.6 (2.4–2.8) | 0.9 |
| α2 globulin | 7.4 (7.0–8.1) | 7.2 (6.3–8.1) | 0.4 |
| β globulin | 10.3 (9.5–11.4) | 9.6 (9.2–10.8) | 0.06 |
| γ globulin | 15.9 (13.5–16.7) | 15.6 (13.5–17.6) | 0.8 |
| Lymphocyte subsets (103/mm3) | |||
| CD3 | 1.3 (1.2–1.5) | 1.5 (1.2–1.7) | 0.09 |
| CD4 | 0.7 (0.5–0.8) | 0.8 (0.7–1.1) | 0.012 |
| CD8 | 0.6 (0.6–0.7) | 0.6 (0.5–0.8) | 0.7 |
| CD4/CD8 ratio | 1.1 (0.9–1.3) | 1.4 (1.1–1.6) | 0.015 |
| CD19 (B-lymphocyte marker) | 0.3 (0.2–0.4) | 0.3 (0.2–0.5) | 0.6 |
| CD16 (NK cell marker) | 0.4 (0.3–0.6) | 0.4 (0.2–0.5) | 0.2 |
| CD95 (apoptosis marker) | 0.07 (0.04–0.13) | 0.06 (0.04–0.09) | 0.2 |
| Other tests | |||
| Circulating immune complexes | 26 (18–50) | 41 (26–65) | 0.2 |
| IgG (g/L) | 14.3 (11.8–18.6) | 16.6 (12.7–22.1) | 0.2 |
| IgA (g/L) | 2.3 (1.8–2.9) | 1.9 (1.7–2.4) | 0.1 |
| IgM (g/L) | 1.8 (1.4–2.2) | 1.7 (1.2–2.1) | 0.3 |
Results in median and interquartile range
Discussion
In this study, we found that both Th1 and Th2 cytokine concentrations were elevated in plasma of patients with Chuvash polycythemia and that CD4 counts in the blood were reduced.
HIF is an essential transcription factor with roles in glucose metabolism, erythropoiesis, and angiogenesis [23,24]. HIF is a heterodimer consisting of α subunits, which are inducible, and β subunits, which are constitutively expressed [25,26]. Under physiological oxygen tension, HIF-α is rapidly degraded via targeting by VHL protein to the ubiquitinprotesome pathway. With hypoxia, VHL protein fails to recognize the HIF-α subunit, resulting in HIF stabilization and accumulation. HIF translocates to the nucleus and influences the transcription of hypoxia-responsive genes [25–27]. Studies have shown that losing VHL function results in constitutive expression of HIF-1α under nonhypoxic conditions [28]. A limitation to this study is that we did not measure relative expressions of HIF-1α and HIF-2β.
Our present findings of increased Th1 and Th2 cytokines associated with VHL598C>T homozygosity are consistent with reports that hypoxia leads to increased expression of interleukin-2 [18], interferon-γ [16,18], tumor necrosis factor-α [17], interleukin-4 [18], and interleukin-10 [19,21]. They are in contrast to reports that reduced HIF-1α expression leads to increased production of inflammatory cytokines by T cells [29] and that interferon-γ was reduced in subjects exposed to hypoxia [20].
Lower CD4 counts in patients with Chuvash polycythemia parallel reports of lower CD4 numbers in humans exposed to high altitude [20] and of increased apoptosis of murine thymocytes and T cells mediated by up regulation of HIF [30,31]. Progression of HIV disease has been associated with increasing plasma interleukin-10 concentration and decreasing CD4 counts [32]. The higher β globulin fraction in patients with Chuvash polycythemia may likely be explained by the fact that transferrin is a prominent protein in this fraction, and the gene for transferrin is up-regulated by HIF-1.
Skewing of the Th1/Th2 cytokine balance can reduce the immune response expected from either of the cytokine subsets [13]. This imbalance has been associated with the pathogenesis of certain immune-mediated diseases such as rheumatoid arthritis and pulmonary sarcoidosis [8,33]. Although plasma levels of both Th1 cytokines and Th2 cytokines were elevated in VHL598C>T homozygotes compared with participants with normal VHL alleles, these elevations were modest and the ratios of interferon-γ or tumor necrosis factor-α to interleukin-10 or interleukin-4 did not differ by genotype.
In conclusion, this analysis demonstrates that there are increases in the cytokine profiles from Th1 and Th2 cells and reduced CD4 counts in patients with Chuvash polycythemia. Improving our knowledge of prototype T-helper cells subset cytokines may help our overall understanding of the pathophyisologic changes associated with this condition. Further investigation of these pathways in Chuvash polycythemia may open insights into the relationship of the hypoxic response to immunity in general.
Materials and Methods
Participants
The Institutional Review Board of Howard University approved the research and all participants provided written informed consent. Of 70 participants, 35 had the diagnosis of Chuvash polycythemia. Thirty-four (22 females, 12 males) of median age 23 years (range 5–62) were homozygous for VHL598C>T. However, one male was wild type, and we counted him in the control group. The control group included 31 Chuvash natives (17 females, 15 males) of median age 32 years (range 5–61) without the diagnosis of Chuvash polycythemia and negative for VHL598C>T. In addition, there were four heterozygous individuals without Chuvash polycythemia whose cytokine profile results are shown but not included in the statistical analysis. The participants were studied in the community under basal circumstances. All participants were from the Chuvash Republic of the Russian Federation. Clinical and demographic characteristics of the patients and controls are shown in Table I.
VHL 598C>T mutation genotyping
Genomic DNA was extracted from peripheral blood collected in EDTA; PCR amplification of VHL exon 3 was performed using the forward primer CCTTGTACTGAGACCCTAG and reverse primer GCTGA GATGAAACAGTGTA. PCR reactions were carried out in 25 µl volumes containing 1.5 mM MgCl2, 200 µM dNTP, 20 nM primers, and 0.5U/reaction Taq DNA polymerase (Roche Applied Science, Indianapolis, IN). PCR (10 µl) product was digested overnight at 37°C with 5U of Fnu4H1 (New England BioLabs, Ipswich, MA) and then separated by electrophoresis on 2.5% agarose gel. The 598 C>T mutation in the VHL gene results in the elimination of the Fnu4H1 restriction site and appearance of uncut 266 bp while wild type VHL allele is cleaved into 188 bp and 78 bp bands.
Multi-plex analysis of cytokines
Peripheral blood samples were collected into EDTA-containing tubes. Plasma was collected by centrifugation at 1000g for 15 min at RT and aliquots were stored at −80°C until analysis. A human Th1/Th2 multiplex cytokine kit (Bio-Rad catalog #171-A11081 which includes: interleukins-2, 4, 5, 10, 12, and 13, interferon-γ, GM-CSF, and tumor necrosis factor-α) was obtained and the assay performed by using the Bio-plex suspension array system with the manufacturer’s instructions (Bio-Rad, Hercules, CA). The system allows simultaneous identification of cytokines in a 96-well filter plate. All samples were assayed in duplicate. In brief, the appropriate cytokine standards and samples diluted in plasma diluents were added to a 96-well filter plate. The samples were incubated at room temperature for 30 min with antibodies chemically attached to fluorescent-labeled micro beads. After three filter washes, premixed detection antibodies were added to each well and incubated for 30 min. Following three washes, premixed streptavidin-phycoerythrin was added to each well and incubated for 10 min followed by three more washes. Then beads were resuspended with 125 µl of assay buffer, and the cytokines reaction mixture was quantified using the Bio-Plex protein array reader. Data were automatically processed and analyzed by Bio-Plex Manager Software 4.1 using the standard curve produced from recombinant cytokine standard. The minimum levels of detection were 0.12 pg/ml for interleukin-2, 0.03 pg/ml for interleukin-4, 0.21 pg/ml for interleukin-5, 0.16 pg/ml for interleukin-10, 0.25 pg/ml for interleukin-12, 0.08 pg/ml for interleukin-13, 0.07 pg/ml for GM-CSF, 0.13 pg/ml for interferon-γ, and 0.5 pg/ml for tumor necrosis factor-α.
Analysis of vascular markers
Enzyme-linked immunosorbent assay was used to assay circulating levels of erythropoietin (Immulite instrument, Diagnostic Products, Randolph, NJ or R&D Systems Inc., Minneapolis, MN, USA) and of vascular endothelial growth factor (Assay Designs, Ann Arbor, MI or R&D Systems Inc., Minneapolis, MN, USA).
Lymphocyte subsets
Peripheral blood mononuclear cells were isolated from peripheral blood collected into EDTA-containing tubes by centrifugation on Ficoll. About l × 106 PBMCs were incubated in 50 µl with fluorescein isothiocyanate-labeled monoclonal antibodies against CD3, CD4, CD8, CD16, CD19, or CD95 for 45 min at 4°C according to the manufacturers instructions (Sorbent, Moscow, Russia). Then cells were washed twice with Hanks balanced salt solution, resuspended in the same solution and analyzed by FACS (Becton-Dickinson, Franklin Lakes, NH).
Statistical analysis
The Kruskal-Wallis test used to compare results according to VHL genotype. P-values are two-sided. Data were analyzed by STATA 10.0 (College Station, TX).
Acknowledgments
Contract grant sponsor: NHLBI; Contract grant numbers: UH1-HL03679 and RO1 HL079912; Contract grant sponsors: NHLBI and the Office of Research on Minority Health; Contract grant number: R25 HL003679; Contract grant sponsor: NCRR; Contract grant number: MO1-RR10284.
Footnotes
Conflict of interest: Nothing to report.
References
- 1.Ang SO, Chen H, Gordeuk VR, et al. Endemic polycythemia in Russia: Mutation in the VHL gene. Blood Cells Mol Dis. 2002;28:57–62. doi: 10.1006/bcmd.2002.0488. [DOI] [PubMed] [Google Scholar]
- 2.Gordeuk VR, Sergueeva AI, Miasnikova GY, et al. Congenital disorder of oxygen sensing: Association of the homozygous Chuvash polycythemia VHL mutation with thrombosis and vascular abnormalities but not tumors. Blood. 2004;103:3924–3932. doi: 10.1182/blood-2003-07-2535. [DOI] [PubMed] [Google Scholar]
- 3.Hickey MM, Lam JC, Bezman NA, et al. von Hippel-Lindau mutation in mice recapitulates Chuvash polycythemia via hypoxia-inducible factor-2α signaling and splenic erythropoiesis. J Clin Invest. 2007;117:3879–3889. doi: 10.1172/JCI32614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manalo DJ, Rowan A, Lavoie T, et al. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 5.Greijer AE, van der Groep P, Kemming D, et al. Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1) J Pathol. 2005;206:291–304. doi: 10.1002/path.1778. [DOI] [PubMed] [Google Scholar]
- 6.Bushuev VI, Miasnikova GY, Sergueeva AI, et al. Endothelin-1, vascular endothelial growth factor and systolic pulmonary artery pressure in patients with Chuvash polycythemia. Haematologica. 2006;91:744–749. [PubMed] [Google Scholar]
- 7.Mosmann TR, Cherwinski H, Bond MW, et al. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. 1986. J Immunol. 2005;175:5–14. [PubMed] [Google Scholar]
- 8.Wahlstrom J, Katchar K, Wigzell H, et al. Analysis of intracellular cytokines in CD4+ and CD8+ lung and blood T cells in sarcoidosis. Am J Respir Crit Care Med. 2001;163:115–121. doi: 10.1164/ajrccm.163.1.9906071. [DOI] [PubMed] [Google Scholar]
- 9.Huffman JA, Hull WM, Dranoff G, et al. Pulmonary epithelial cell expression of GM-CSF corrects the alveolar proteinosis in GM-CSF-deficient mice. J Clin Invest. 1996;97:649–655. doi: 10.1172/JCI118461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsiligianni I, Antoniou KM, Kyriakou D, et al. Th1/Th2 cytokine pattern in bronchoalveolar lavage fluid and induced sputum in pulmonary sarcoidosis. BMC Pulm Med. 2005;5:8. doi: 10.1186/1471-2466-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong C, Flavell RA. Th1 and Th2 cells. Curr Opin Hematol. 2001;8:47–51. doi: 10.1097/00062752-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Huaux F, Lardot C, Arras M, et al. Lung fibrosis induced by silica particles in NMRI mice is associated with an upregulation of the p40 subunit of interleukin-12 and Th-2 manifestations. Am J Respir Cell Mol Biol. 1999;20:561–572. doi: 10.1165/ajrcmb.20.4.3342. [DOI] [PubMed] [Google Scholar]
- 13.Borkow G, Bentwich Z. Chronic immune activation associated with chronic helminthic and human immunodeficiency virus infections: Role of hyporesponsiveness and anergy. Clin Microbiol Rev. 2004;17:1012–1030. doi: 10.1128/CMR.17.4.1012-1030.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackay CR. Follicular homing T helper (Th) cells and the Th1/Th2 paradigm. J Exp Med. 2000;192:F31–F34. doi: 10.1084/jem.192.11.f31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naldini A, Pucci A, Bernini C, Carraro F. Regulation of angiogenesis by Th1- and Th2-type cytokines. Curr Pharm Des. 2003;9:511–519. doi: 10.2174/1381612033391423. [DOI] [PubMed] [Google Scholar]
- 16.Murata Y, Ohteki T, Koyasu S, Hamuro J. IFN-γ and pro-inflammatory cytokine production by antigen-presenting cells is dictated by intracellular thiol redox status regulated by oxygen tension. Eur J Immunol. 2002;32:2866–2873. doi: 10.1002/1521-4141(2002010)32:10<2866::AID-IMMU2866>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 17.Kiefmann R, Rifkind JM, Nagababu E, Bhattacharya J. Red blood cells induce hypoxic lung inflammation. Blood. 2008;111:5205–5214. doi: 10.1182/blood-2007-09-113902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naldini A, Carraro F, Silvestri S, Bocci V. Hypoxia affects cytokine production and proliferative responses by human peripheral mononuclear cells. J Cell Physiol. 1997;173:335–342. doi: 10.1002/(SICI)1097-4652(199712)173:3<335::AID-JCP5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 19.Chen W, Qiu JF, Zhang ZQ, et al. Gene expression changes after hypoxic preconditioning in rat hepatocytes. Hepatobiliary Pancreat Dis Int. 2006;5:416–421. [PubMed] [Google Scholar]
- 20.Facco M, Zilli C, Siviero M, et al. Modulation of immune response by the acute and chronic exposure to high altitude. Med Sci Sports Exerc. 2005;37:768–774. doi: 10.1249/01.mss.0000162688.54089.ce. [DOI] [PubMed] [Google Scholar]
- 21.Wang JS, Lin HY, Cheng ML, et al. Chronic intermittent hypoxia modulates eosinophil- and neutrophil-platelet aggregation and inflammatory cytokine secretion caused by strenuous exercise in men. J Appl Physiol. 2007;103:305–314. doi: 10.1152/japplphysiol.00226.2007. [DOI] [PubMed] [Google Scholar]
- 22.Clarkson AN, Liu H, Schiborra F, et al. Angiogenesis as a predictive marker of neurological outcome following hypoxia-ischemia. Brain Res. 2007;1171:111–121. doi: 10.1016/j.brainres.2007.06.100. [DOI] [PubMed] [Google Scholar]
- 23.Maxwell PH. Hypoxia-inducible factor as a physiological regulator. Exp Physiol. 2005;90:791–797. doi: 10.1113/expphysiol.2005.030924. [DOI] [PubMed] [Google Scholar]
- 24.Bruick RK. Oxygen sensing in the hypoxic response pathway: Regulation of the hypoxia-inducible transcription factor. Genes Dev. 2003;17:2614–2623. doi: 10.1101/gad.1145503. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Zhao Q, Mooney SM, Lee FS. Sequence determinants in hypoxia-inducible factor-1α for hydroxylation by the prolyl hydroxylases PHD1, PHD2, and PHD3. J Biol Chem. 2002;277:39792–39800. doi: 10.1074/jbc.M206955200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knowles HJ, Mole DR, Ratcliffe PJ, Harris AL. Normoxic stabilization of hypoxia-inducible factor-1α by modulation of the labile iron pool in differentiating U937 macrophages: Effect of natural resistance-associated macrophage protein 1. Cancer Res. 2006;66:2600–2607. doi: 10.1158/0008-5472.CAN-05-2351. [DOI] [PubMed] [Google Scholar]
- 27.Olenyuk BZ, Zhang GJ, Klco JM, et al. Inhibition of vascular endothelial growth factor with a sequence-specific hypoxia response element antagonist. Proc Natl Acad Sci USA. 2004;101:16768–16773. doi: 10.1073/pnas.0407617101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semenza GL. HIF-1 and human disease: One highly involved factor. Genes Dev. 2000;14:1983–1991. [PubMed] [Google Scholar]
- 29.Lukashev D, Klebanov B, Kojima H, et al. Cutting edge: Hypoxia-inducible factor 1α and its activation-inducible short isoform I. 1 negatively regulate functions of CD4+ and CD8+ T lymphocytes. J Immunol. 2006;177:4962–4965. doi: 10.4049/jimmunol.177.8.4962. [DOI] [PubMed] [Google Scholar]
- 30.Biju MP, Neumann AK, Bensinger SJ, et al. Vhlh gene deletion induces Hif-1-mediated cell death in thymocytes. Mol Cell Biol. 2004;24:9038–9047. doi: 10.1128/MCB.24.20.9038-9047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carraro F, Pucci A, Pellegrini M, et al. p66Shc is involved in promoting HIF-1α accumulation and cell death in hypoxic T cells. J Cell Physiol. 2007;211:439–447. doi: 10.1002/jcp.20951. [DOI] [PubMed] [Google Scholar]
- 32.Srikanth P, Castillo RC, Sridharan G, et al. Increase in plasma IL-10 levels and rapid loss of CD4+ T cells among HIV-infected individuals in south India. Int J STD AIDS. 2000;11:49–51. doi: 10.1258/0956462001914904. [DOI] [PubMed] [Google Scholar]
- 33.Skapenko A, Wendler J, Lipsky PE, et al. Altered memory T cell differentiation in patients with early rheumatoid arthritis. J Immunol. 1999;163:491–499. [PubMed] [Google Scholar]