Abstract
Separating rat pups from their mothers during the early stages of life is an animal model commonly used to study the development of psychiatric disorders such as anxiety and depression. The present study investigated how soon after the termination of the maternal separation period behavioural and neuroendocrine abnormalities relevant to above-mentioned illnesses would manifest. Sprague Dawley rat pups were subjected to maternal separation (3 hours per day from postnatal day 2 through 14) and their behaviour and HPA axis activity determined 7 days later. We also measured nerve growth factor levels in their hippocampi and assessed the DNA methylation status of the promoter region of exon 17 of the glucocorticoid receptor in this brain region. As early as 7 days after the termination of the adverse event, a change in behaviour was observed that was associated with increased plasma corticosterone release and elevated nerve growth factor levels in the hippocampus. No alteration in the methylation status of the exon 17 glucocorticoid receptor promoter region was observed. Our data indicate that early life adversity may lead to the rapid development of abnormal behaviours and HPA axis dysregulation though no epigenetic changes to the exon 17 glucocorticoid receptor promoter region occurred. We further propose that the observed increased neurotrophin levels reflect compensatory mechanisms that attempt to combat the long-term deleterious effects of maternal separation.
Keywords: maternal separation, HPA axis, neurotrophins, behaviour, DNA methylation
1. Introduction
Clinical studies show that exposure to early adversity such as childhood sexual abuse is associated with an increased risk of developing psychiatric disorders in adulthood (Kessler, 1997; Heim and Nemeroff, 2001; Rinne et al., 2002). Similar to humans, other mammals suffering stressful events at an early age can develop behavioural disturbances in adulthood. For example, postnatally stressed (Daniels et al., 2004a) and young traumatized rats (Uys et al., 2006a) exhibit abnormal behaviour on tests of anxiety when assessed weeks after exposure to stressful events. Further, these behavioural abnormalities in animals were associated with aberrations in the HPA axis, akin to humans (Yehuda et al., 1993).
Maternal separation is one animal model that has been studied extensively to characterize the long-term effects of early life experience on subsequent behaviour in adulthood (Plotsky and Meaney, 1993; Pryce and Feldon, 2003). This model is based on separation of neonatal rat pups from their mothers during a critical period of development, usually during the first two weeks of life. Ladd et al. (1996) found that maternal separation during this period alters stress-induced HPA axis and behavioural responses in adulthood, including increased anxiety-like behaviours and anhedonia. However, decreased anxiety-like behaviours following maternal separation have also been reported (Ecklund and Arborelius, 2006).
The glucocorticoid receptor present in the hippocampus is important in regulating the activity of the HPA axis after stress, and recent studies have shown that removal of the pup from the dam (Enthoven et al., 2008), as well as trauma during adolescence (Uys et al., 2006b), leads to dysregulated corticosterone release with down-regulation of glucocorticoid receptors in the hippocampus. These results therefore suggest that the expression of glucocorticoid receptors may be an integral part of the regulatory mechanisms controlling HPA axis activity.
Alterations in the concentration of nerve growth factor (NGF) occur in conditions in which the HPA axis is dysregulated (Faure et al., 2007; Uys et al., 2006a), suggesting an interaction between neurotrophins and the neuroendocrine system. Binding of NGF to its tyrosine receptor results in the increased expression of the transcription factor NGFI-A (D’Arcangelo and Halegoua, 1993). This transcription factor binds to the exon 17 glucocorticoid receptor promoter region and in doing so induces the activity of the exon 17 GR promoter with subsequent increase in glucocorticoid receptor expression (Szyf et al., 2005). The exon 17 glucocorticoid receptor promoter region contains a CpG island, and the DNA methylation status of this region has been shown to influence gene expression (Weaver et al., 2007). Earlier investigations have shown that the DNA methylation status of this NGFI-A binding site may be influenced by variations in maternal care experienced by rat pups (Weaver et al., 2004; Szyf et al., 2005). We therefore hypothesized that rats subjected to maternal separation develop not only behavioural and neuroendocrine changes but also altered DNA methylation status of the promoter region of exon 17 of the glucocorticoid receptor in the hippocampal region. We chose to do our investigation around weaning as a recent study has demonstrated the presence of abnormalities in the HPA axis at this stage of development (Enthoven et al., 2008).
2. Experimental Procedure
2.1 Animals
A total of 27 Sprague Dawley rats were used in this study. Initially 5 male-female pairs, housed in the Central Research Facility of the University of Stellenbosch (an AAALAC accredited facility), were used to provide off spring for the subsequent experiments. The housing conditions included a 12 hr light/dark cycle with lights on at 06h00, 22°C ambient temperature and humidity of 55%. The animals were fed standard rat chow and tap water ad libitum. All experimental procedures were approved by the Committee for Ethical Animal Research of the University of Stellenbosch.
2.2 Maternal separation procedure
The day pups were first observed was taken as postnatal day (PND) 1. Maternal separation was performed daily beginning PND 2 through PND 14, between 09h30-13h00. Dams were removed from the home cage and placed into individual cages for the duration of the 3 hr separation period, while the entire litter remained in their home cage. The home cage was transferred to a separate room and placed under infrared light to maintain cage temperature between 33-35°C. At the conclusion of the separation period, pups were taken to the colony room where they were joined by their respective dams. Apart from normal cage cleaning, the control group remained undisturbed. Only male rats were used in subsequent experiments.
2.3 Behavioural assessment
The open field was used to assess anxious-like behaviour and locomotor activity of 15 normally reared (control) and 12 separated animals. Behavioural assessment was performed at two different time points spread over two consecutive days. The different time points were PND 19 and 20, which were 5 and 6 days, respectively, after the completion of maternal separation. The first behavioural day consisted of placing the animal in the open field for 10 min. The second behavioural day involved the introduction of a novel object to the center of the arena (inner zone) of the same open field. This served to further investigate the rats’ exploratory behaviour. Rats were videotaped and their behaviour was assessed by means of EthoVision System (Noldus Inc., The Netherlands). Time spent in the inner zone, total distance traveled, and the number of approaches to the novel object was documented.
2.4 Sample collection
Twenty-four hours after the last behavioural assessment, namely PND 21, each animal was brought into a separate room where trunk blood was collected and the plasma obtained for corticosterone determination by using an ELISA kit (Assay Designs Correlate-EIA Corticosterone Enzyme Immunoassay Kit, USA). The hippocampi were quickly dissected from the brain and frozen in liquid nitrogen. Of the group the hippocampi of 12 control and 9 separated rats were analyzed for NGF with an ELISA kit (Promega E-Max ImmunoAssay System, USA), and 3 hippocampi from each group were used for the DNA methylation experiments.
2.5 DNA methylation
The methodology used was validated in a separate study conducted by one of our co-authors during his PhD studies (McEvoy, 2002). We followed the exact steps in this study except, that different primers were used to amplify the gene.
DNA from 3 independent animals of each group was isolated from the hippocampus by using the DNeasy blood & tissue kit (Qiagen) according to the manufacturer’s instructions. Lysis was performed by cutting the tissue in pieces with a surgical blade and incubating these for 3 hr in a waterbath at 56°C with vortexing every half hour. Isolated DNA was further purified by phenol/chloroform extraction and ethanol precipitation.
DNA concentrations were determined by OD measurement by using a Nanodrop ND-1000 spectrophotometer and aliquots of 1 μl sample. DNA quality was measured by gel electrophoresis in 1% agarose.
The methylation status of the exon17 glucocorticoid receptor promoter region was assessed by sequencing PCR amplified bisulfite-modified genomic DNA extracted from the hippocampus (Frommer et al., 1992). Bisulfite modification was done according to protocol with the EZ DNA Methylation-Gold Kit (Zymo Research Corp., Inqaba Biotec). Overall, 1 μg of bisulfite modified DNA samples from PND 21 was used as input.
PCR amplification was performed in a reaction mixture containing 4 μl bisulfite modified DNA (or 2 μl of a 1/100 dilution of first round PCR product), 5 μl 10 x buffer, 4 μl 25 mM MgCl2, 8 μl 10 mM dNTP’s, 2 μl of each primer (50 pmol/μl), and 0.4 μl HotStarTaq DNA polymerase (Qiagen, Germany) and made up to 50 μl with H2O. Two sets of primers were used in our study. These were as follows:
Round 1: Forward primer:
5′ – TYGTTTTYGTGTTATTTTGTAGTTTTTTTGTTAGTGTGATATA – 3′
Round 1: Reverse primer:
5′ – CTACRACRTCTTATTCCACCCACTTAAAATCC – 3′
Round 2: Forward primer:
5′ – GGATTATTTTTGYGGTTTTGTYGGTTGGTTGTTA – 3′
Round 2: Reverse primer:
5′ – CYACYACTCYAACTCCAAACACTAACTC – 3′
Y (= T or C) and R (= A or G) nucleotides in the above sequences indicate nucleotide positions that may or may not be methylated (ie. C’s that are part of CpG dinucleotides) and this redundancy is used to ensure primer binding to either sequence following bisulfite modification. The sequence of the rat exon17 glucocorticoid receptor promoter region, the positions of the first and second round PCR primers, and the 9 bp NGFI-A binding site are shown in Figure 1.
Figure 1.
The complete sequence of exon17 of the glucocorticoid receptor promoter region (underlined), showing the position of the forward and reverse primers (primer set 1 in italics and primer set 2 in bold), as well as the location of the NGF1-A binding site (boxed).
PCR was performed on a GeneAmp PCR system 2400 machine (Applied Biosystems, South Africa). Fifty cycles were performed, and the 594 bp PCR product run on a 1.5 % low melting point agarose gel (Agarose MS-8, Whitehead Scientific, South Africa), excised from the gel, and purified by using the Wizard SV Gel and PCR Clean-Up System (cat. no. A9282, Promega, U.K.). Samples were sequenced with an ABI prism 3130 xl Genetic Analyzer (Applied Biosystems, U.K.). Round 2 Fw and Rev primers were used for sequencing.
2.6 Statistical analysis
GraphPad Prism 5 was employed for statistical analyses and graphical representations. All data are presented as the means ± S.E.M. Because two groups were compared, the data were subjected to only the Mann-Whitney-U test.
3. Results
3.1 Open field test
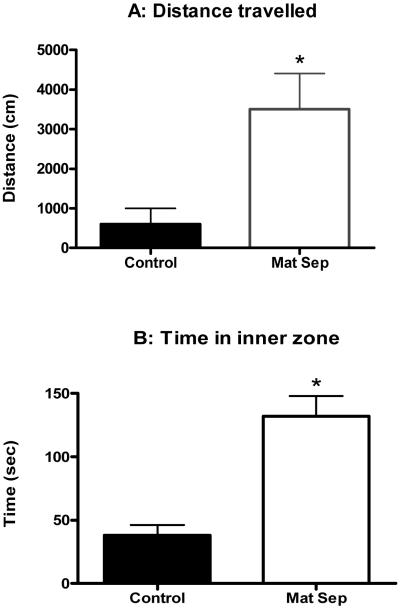
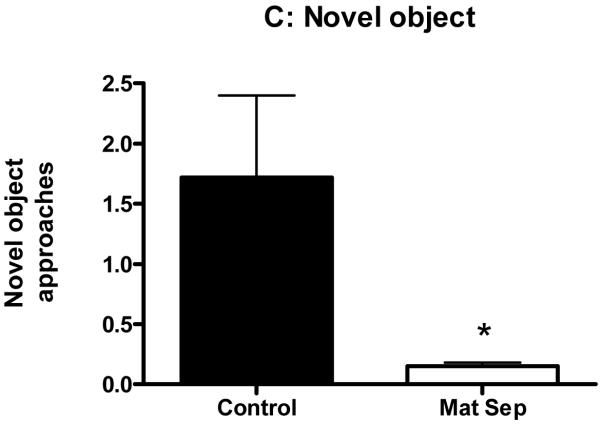
Total distance traveled in the open field was significantly higher for the maternally separated rats (Fig. 2A; p<0.05). Time spent in the inner zone of the open field was significantly higher for the maternally separated rats when compared to their controls (Fig.2B; p<0.05). On the other hand, frequency of approaches to the novel object was significantly lower for the maternally separated rats when compared to their controls (Fig. 2C; p<0.05).
Figure 2.
Normally-reared control and maternally separated rats were placed in the Open Field for 10 min and their behaviour recorded. The data in panel A and B was collected during the first Open Field test, while panel C represents data from the second Open field test with the novel object present. The graphs show significantly greater total distance traveled (A), significantly more time spent in inner zone (B), and significantly less novel object approaches by stressed animals (□) when compared to controls (■). Each value represents mean ± S.E.M. of 15 control and 12 stressed rats.
* p<0.05 - significantly different from control, Mann-Whitney U-test.
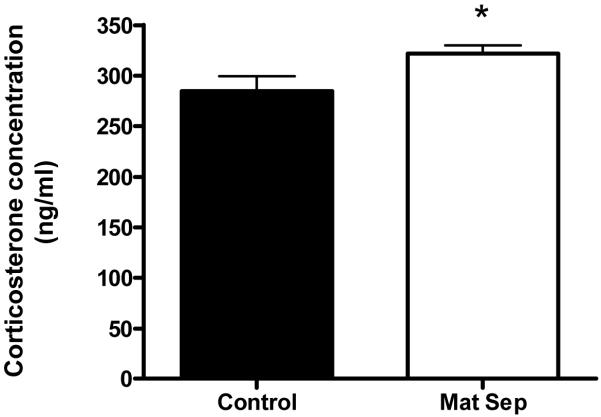
3.2 Corticosterone
Corticosterone levels were significantly increased in maternally separated rats in comparison to the control group (Fig. 3).
Figure 3.
Corticosterone levels of normally-reared control (■) and maternally separated stressed rats (□). Each value represents mean ± S.E.M. of 15 control and 12 stressed rats.
*p<0.05 - significantly different control, Mann-Whitney U-test.
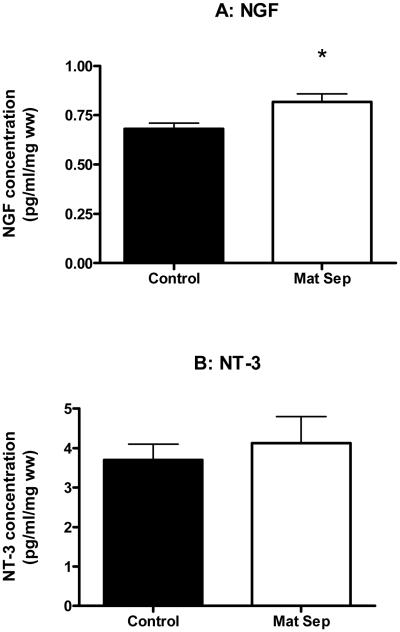
3.3 Neurotrophins
NGF levels in the hippocampus were significantly higher in maternally separated rats than in controls (Fig. 4A), but no significant difference in the levels of NT-3 between the two groups was observed (Fig 4B).
Figure 4.
Graphs showing NGF (A) and NT-3 (B) levels in the hippocampus of normally-reared control (■) and maternally separated (□) rats. Each value represents mean ± S.E.M. of 12 control and 9 stressed rats.
*p<0.05 - significantly different from control, Mann-Whitney U-test.
3.4 Methylation analysis of the exon 17 glucocorticoid receptor promoter region
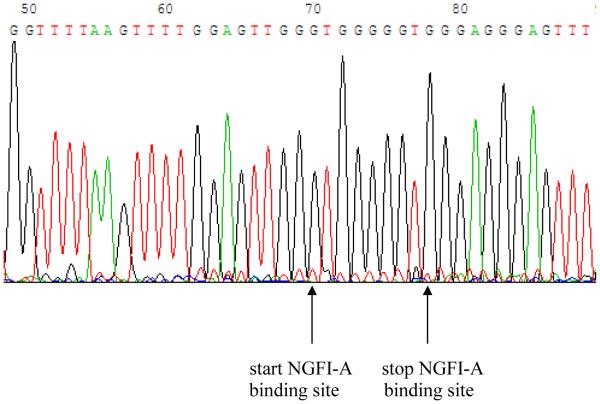
The nested PCR described above was used to successfully amplify a 594 bp product (Fig 5). By using the two second round PCR primers plus an additional internal sequencing primer, we were able to confirm the sequence and characterise the methylation status of 66 CpG sites, including the NGFI-A binding site, within the exon 17 glucocorticoid receptor promoter region. No methylation of the NGF-1A binding site of maternally separated rats occurred, and the sequencing result from maternally separated animals after PND 21 was similar to that of normally reared control animals (Fig 6).
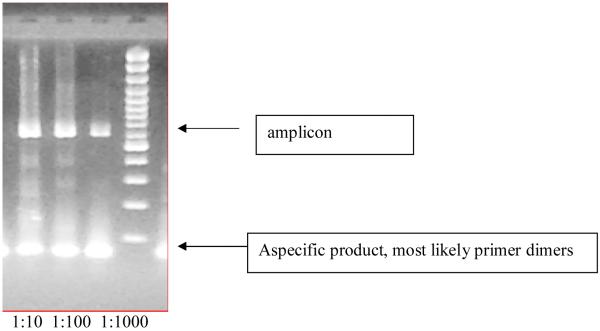
Figure 5.
An example of the predicted 594 bp second round PCR product obtained from the bisulfite modified genomic DNA of the hippocampus of a control rat. The three bands are from the hippocampus of the same animal and represent three different dilutions of the amplicon after the second round of the PCR.
Figure 6.
Part of the sequence of the glucocorticoid receptor promoter region containing the NGFI-A binding site (GCGGGGGCG). No C’s have been converted to T’s, indicating that these cytosine residues were not methylated. This is a representative result from an animal that has been subjected to maternal separation.
4. Discussion
We and many others have used a maternal separation paradigm as a model for studying the consequences of early adversity (Ladd et al., 2000; Daniels et al., 2004a, Slotten et al., 2006). These studies have focused on the long-term effects of maternal separation, including changes in behaviour and neurophysiology. Here we report that within a few days of the termination of the adverse event, a change in behaviour was observed that is associated with increased plasma corticosterone release and elevated NGF levels in the hippocampus, but no alteration in the methylation status of the exon 17 glucocorticoid receptor promoter region.
Both animal and human studies have shown that early adversity results in altered behaviour and neurophysiology. In humans, child abuse is a risk factor for subsequent onset of mental disorders such as depression and post-traumatic stress disorder (Heim and Nemeroff, 2001). Similar studies in which animals were subjected to forms of early life stress also found altered behaviour (Suomi et al., 1992; Daniels et al., 2004a; Marais et al., 2008). The behavioural changes observed in the present study are therefore similar to those in previous investigations. The significant fact of the present findings is these changes manifested soon after the termination of the maternal separation. Our experiments therefore pointed towards a relatively rapid onset of behavioural and neurochemical changes following repeated exposure to adversity. Interestingly, animals subjected to maternal separation displayed a greater degree of locomotor activity and spent more time in the inner zone of the open field. Usually these findings would be suggestive of a relaxed emotional state. However, this increase in activity may also reflect a state of hyperarousal as separated animals were less keen to explore the novel object than controls. Taken together it seems as if separated animals could have been moving anxiously around the open field seeking escape routes or an area of shelter. Maternal separation leading to anxious-like behaviour and hyperactivity has been observed previously (Anand and Scalzo, 2000; Daniels et al., 2004a; Slotten et al., 2006).
The elevated corticosterone levels indicate that the normal function of the HPA axis has been disturbed in separated animals. This is in accordance with reports by others (Ladd et al., 2000; Kalinichev et al., 2002; Huot et al., 2004). A number of mechanisms may be responsible for the malfunctioning of the HPA axis. For example, raised circulating concentrations of corticosterone have been associated with decreased sensitivity of the HPA axis feedback system as reflected by a reduction in glucocorticoid receptor numbers in the hippocampus (Uys et al., 2006b). In addition, glucocorticoid receptors present in other brain regions may also have contributed to the dysregulation of the HPA axis in maternally separated animals. Steroid receptors involved in mediating feedback inhibition of the HPA axis have been identified in other brain structures, including the amygdala, septum and entorhinal cortex (Szyf et al., 2005), forebrain (Boyle et al., 2006), and posterior paraventricular thalamus (Jaferi and Bhatnagar, 2006). Alternatively, the increased secretion of corticosterone could have been due to enhanced release of corticotrophin releasing factor (Daniels et al., 2004b), hypersecretion of ACTH (Daniels et al., 2004a), and/or altered responsiveness of the adrenal gland (Uys et al., 2006a).
The hippocampal NGF levels were significantly increased in the maternally separated group of animals when compared to normally reared controls. We initially found an increase in earlier experiments in which animals were subjected to a series of stressors (Faure et al., 2007), but we were unable to replicate this in a subsequent study in which animals were subjected to only the maternal separation paradigm (Marais et al., 2008). Our present results may therefore indicate the attempts by the brain to compensate for the initial stressful insult. This early increase in neurotrophin release subsequently disappears with time as the brain reaches a level of homeostatis. In the case of the earlier study (Faure et al., 2007), the repetitive stressors applied in the experiments presented ongoing onslaughts on the brain, and hence the enhanced release of neurotrophins is observed over a longer time period.
NGF binds to the receptor TrkA and activates the MAPKinase signaling pathway to induce the expression of immediate early genes such as c-jun and NGF1A (D’Arcangelo and Halegoua, 1993, Kendall et al., 1994). The induction of these genes has been observed following exposure to stress (Honkaniemi et al., 1994, Rosen et al., 1998). At the same time, environmental enrichment - which may counter the effects of stress (Mohammed et al., 2002) - also increases both glucocorticoid receptors and NGF1-A gene expression (Olsson et al., 1994). Chronic treatment with glucocorticoids had no effect on immobilization stress-induced increases in NGF1A expression (Umemoto et al., 1997), while the increased expression of NGF1-A cells suppressed the development of neurites in PC12 cells exposed to NGF (Matsumoto et al., 2000). These findings and ours therefore support the hypothesis that stress leads to allostatic load and in order to counter this, increased neurotrophins are released. Our results also reflect the complex interaction between early life stress, glucocorticoids, NGF, and NGF1-A, and underline the need for closer investigation.
Reports by Meaney and colleagues (Szyf et al., 2005; Weaver et al., 2004, Weaver et al., 2007) have suggested that epigenetic alterations may account (at least in part) for behavioural changes induced by early life experiences. These authors showed that the long-term effects of different mothering styles on behavioural outcomes were dependent on the methylation status of DNA, and specifically the promoter region of the glucocorticoid receptor in hippocampus. Unlike their studies, we were unable to show any difference in methylation status of the same region in animals that were maternally separated. The discrepancies between our results and theirs may be due to the different paradigms under study (maternal separation vs mothering style). It may therefore be possible that methylation of the glucocorticoid receptor promoter region is specific for certain patterns of maternal care, but does not hold true for all forms of mothering styles. Some support for such specificity stems from a recent investigation in which no evidence of methylation was observed in the human DNA analogue of the rat NGF1A binding region of normal as well as diseased post mortem tissue (Moser et al., 2007). It is also possible that DNA methylation status is not only condition-dependent but may also be species specific. In our study we used Sprague Dawley rats and not Long Evans hooded rats in which hypo-methylation of DNA has been reported to occur under circumstances of increased maternal attention during early life.
In summary, our study confirmed that maternal separation during early life can lead to behavioural and neurochemical abnormalities. Our data suggest that these changes may manifest rapidly after the separation period. However, the disruption in maternal care during the separation period did not induce epigenetic alterations in the promoter region of the glucocorticoid receptor in the hippocampus. This result, however, does not exclude the possibility of epigenetic modifications of DNA in other parts of the genome in other brain areas.
Acknowledgements
This study was financially supported by grants from the National Institutes of Health (NIH) Fogarty International Center (R01TW008040), the Medical Research Council of South Africa, and the National Research Foundation of South Africa. The authors wish to thank Ms Susan Giegel for editorial services rendered.
References
- Anand KJ, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Biol Neonate. 2000;77(2):69–82. doi: 10.1159/000014197. [DOI] [PubMed] [Google Scholar]
- Boyle MP, Kolber BJ, Vogt SK, Wozniak DF, Muglia LJ. Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J Neurosci. 2006;26:1971–1978. doi: 10.1523/JNEUROSCI.2173-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels WM, Pietersen CY, Carstens ME, Stein DJ. Maternal Separation in Rats Leads to Anxiety-Like Behavior and a Blunted ACTH Response and Altered Neurotransmitter Levels in Response to a Subsequent Stressor. Metab Brain Dis. 2004(a);19:3–14. doi: 10.1023/b:mebr.0000027412.19664.b3. [DOI] [PubMed] [Google Scholar]
- Daniels WMU, Richter L, Stein DJ. The effects of intra-amygdala CRF injections on rat behaviour and HPA axis function after stress. Metab. Brain Dis. 2004(b);19(1/2):25–33. doi: 10.1023/b:mebr.0000027413.42946.61. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Halegoua S. A branched signaling pathway for nerve growth factor is revealed by Src-, Ras-, and Raf-mediated gene inductions. Mol Cell Biol. 1993;13(6):3146–3155. doi: 10.1128/mcb.13.6.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecklund MB, Arborelius L. Twice daily long maternal separations in Wistar rats decreases anxiety-like behaviour in females but does not affect males. Behav Brain Res. 2006;172:278–85. doi: 10.1016/j.bbr.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Enthoven L, de Kloet ER, Oitzl MS. Differential development of stress system (re)activity at weaning dependent on time of disruption of maternal care. Brain Res. 2008;1217:62–69. doi: 10.1016/j.brainres.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Faure J, Uys JDK, Marais L, Stein DJ, Daniels WMU. Early maternal separation alters the response to traumatization resulting in increased levels of hippocampal neurotrophic factors. Metab Brain Dis. 2007;22(2):183–195. doi: 10.1007/s11011-007-9048-3. [DOI] [PubMed] [Google Scholar]
- Frommer M, McDonald LE, Millar DS, Collis CM, Watt F, Grigg GW, Molloy PL, Paul CL. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci. 1992;89:1827–1831. doi: 10.1073/pnas.89.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Honkaniemi J, Kononen J, Kainu T, Pyykönen I, Pelto-Huikko M. Induction of multiple immediate early genes in rat hypothalamic paraventricular nucleus after stress. Mol Brain Res. 1994;25:234–241. doi: 10.1016/0169-328x(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Huot RL, Gonzalez ME, Ladd CO, Thrivikraman KV, Plotsky PM. Foster litters prevent hypothalamic-pituitary-adrenal axis sensitization mediated by neonatal maternal separation. Psychoneuroendocrinol. 2004;29(2):279–89. doi: 10.1016/s0306-4530(03)00028-3. [DOI] [PubMed] [Google Scholar]
- Jaferi A, Bhatnagar S. Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamo–pituitary–adrenal activity in animals that habituate to repeated stress. Endocrin. 2006;147:4917–4930. doi: 10.1210/en.2005-1393. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone responses and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol Biochem Behav. 2002;73(1):131–40. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- Kendall G, Ensor E, BrarRai A, Winter J, Latchman DS. Nerve growth factor induces expression of immediate-early genes NGFIA (Egr-1) and NGFI-B (nur 77) in adult rat dorsal root ganglion neurons. Mol Brain Res. 1994;25:73–79. doi: 10.1016/0169-328x(94)90280-1. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:91–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meany MJ, Plotsky PM. Long-term behavioural and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Owens MJ, Nemeroff CB. Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal deprivation. Endocrinol. 1996;137:1212–218. doi: 10.1210/endo.137.4.8625891. [DOI] [PubMed] [Google Scholar]
- Marais L, Van Rensburg SJ, Van Zyl JM, Stein DJ, Daniels WMU. Maternal separation of rat pups increases the risk of developing depressive-like behavior after subsequent chronic stress by altering corticosteorne and neurotrophin levels in the hippocampus. Neurochem Res. 2008;61(1):106–12. doi: 10.1016/j.neures.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Akiyama N, Kizaka-Kondoh S, Noda M. Transient over-expression of NGF1-A gene suppresses NGF-induced neurite outgrowth in PC12 cells. Neuroreport. 2000;11(5):1001–5. doi: 10.1097/00001756-200004070-00021. [DOI] [PubMed] [Google Scholar]
- McEvoy C. PhD thesis. Flinders University of South Australia; 2002. Characterisation of tumour associated HLA class I genetic and epigenetic gene alterations. [Google Scholar]
- Mohammed AH, Zhu SW, Darmopil S, Hjerling-Leffler J, Ernfors P, Winblad B, Diamond MC, Eriksson PS, Bogdanovic N. Environmental enrichment and the brain. Prog Brain Res. 2002;138:109–133. doi: 10.1016/S0079-6123(02)38074-9. [DOI] [PubMed] [Google Scholar]
- Moser D, Molitor A, Kumsta R, Tatschner T, Riederer P, Meyer J. The glucocorticoid receptor gene exon 1-F promoter is not methylated at the NGF1-A binding site in human hippocampus. World J Biol Psychiatry. 2007;8(4):262–8. doi: 10.1080/15622970701429862. [DOI] [PubMed] [Google Scholar]
- Olsson T, Mohammed AH, Donaldson LF, Hendriksson BG, Seckl JR. Glucocorticoid receptor and NGFl-A gene expression are induced in the hippocampus after environmental enrichment in adult rats. Brain Res Mol Brain Res. 1994;23:349–353. doi: 10.1016/0169-328x(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meany MJ. Early, postnatal experience alters hypothalamic corticotrophin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Feldon J. Long-term neurobehavioral impact of the postnatal environment in rats: manipulations, effects, and mediating mechanisms. Neurosci Biobehav Rev. 2003;27:57–71. doi: 10.1016/s0149-7634(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Rinne T, de Kloet ER, Wouters L, Goekoop JG, DeRijk RH, van den Brink W. Hyperresponsiveness of the hypothalamic-pituitary-adrenal axis to combined dexamethasone/corticotropin-releasing hormone challenge in female borderline personality disorder subjects with a history of sustained childhood abuse. Biol Psychiatry. 2002;52(11):1102–12. doi: 10.1016/s0006-3223(02)01395-1. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Fanselow MS, Young SL, Sitcoske M, Maren S. Immediate-early gene expression in the amygdala following footshock stress and contextual fear conditioning. Brain Res. 1998;796:132–142. doi: 10.1016/s0006-8993(98)00294-7. [DOI] [PubMed] [Google Scholar]
- Slotten HA, Kalinichev M, Hagen JJ, Marsden CA, Fona KC. Long-lasting changes in behavioural and neuroendocrine indices in the rat following neonatal maternal separation: gender dependent effects. Brain Res. 2006;1097:123–132. doi: 10.1016/j.brainres.2006.04.066. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Rasmussen KLR, Higley JD. Primate models of behavioral and physiological change in adolescence. In: McAnarney K, Kreipe RE, Orr DP, Gormerci GD, editors. Textbook of adolescent medicine. Saunders; Philadelphia: 1992. pp. 135–140. [Google Scholar]
- Szyf M, Weaver IC, Champagne FA, Diorio J, Meaney MJ. Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Front Neuroendocrinol. 2005;26(3-4):139–62. doi: 10.1016/j.yfrne.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Umemoto S, Kawai Y, Ueyama T, Senba E. Chronic glucocorticoid administration as well as repeated stress affects the subsequent acute immobilization stress-induced expression of immediate early genes but not that of NGFI-A. Neuroscience. 1997;80:763–773. doi: 10.1016/s0306-4522(97)00050-x. [DOI] [PubMed] [Google Scholar]
- Uys JDK, Marais L, Faure J, Prevoo D, Swart P, Mohammed AH, Stein DJ, Daniels WM. Developmental Trauma is Associated with Behavioral Hyperarousal, Altered HPA Axis Activity, and Decreased Hippocampal Neurotrophin Expression in the Adult Rat. Ann NY Acad Sci. 2006(a);1071:542–546. doi: 10.1196/annals.1364.060. [DOI] [PubMed] [Google Scholar]
- Uys JDK, Muller CF, Marais L, Harvey BH, Stein DJ, Daniels WMU. Early life trauma decreases glucoroticoid receptors in rat dentate gyrus upon restress: reversal by escitalopram. Neuroscience. 2006(b);137:619–625. doi: 10.1016/j.neuroscience.2005.08.089. [DOI] [PubMed] [Google Scholar]
- Weaver IC, D’Alessio AC, Brown SE, Hellstrom IC, Dymov S, Sharma S, Szyf M, Meaney MJ. The transcription factor nerve growth factor-inducible protein mediates epigenetic programming: altering epigenetic marks by immediate-early genes. J Neurosci. 2007;27:1756–1768. doi: 10.1523/JNEUROSCI.4164-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Diorio J, Seckl JR, Szyf M, Meaney MJ. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: Characterization of intracellular mediators and potential genomic target sites. Ann NY Acad Sci. 2004;1024:182–212. doi: 10.1196/annals.1321.099. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, Mason JW. Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am J Psychiatry. 1993;150:83–86. doi: 10.1176/ajp.150.1.83. [DOI] [PubMed] [Google Scholar]