Abstract
There is an increasing awareness that adolescent females differ from males in their response to drugs of abuse and consequently in their vulnerability to addiction. One possible component of this vulnerability to drug addiction is the neurobiological impact that reproductive physiology and behaviors have on the mesolimbic dopamine system, a key neural pathway mediating drug addiction. In this review, we examine animal models that address the impact of ovarian cyclicity, sexual affiliation, sexual behavior, and maternal care on the long-term plasticity of the mesolimbic dopamine system. The thesis is that this plasticity in synaptic neurotransmission stemming from an individual’s normal life history contributes to the pathological impact of drugs of abuse on the neurobiology of this system. Hormones released during reproductive cycles have only transient effects on these dopamine systems, whereas reproductive behaviors produce a persistent sensitization of dopamine release and postsynaptic neuronal responsiveness. Puberty itself may not represent a neurobiological risk factor for drug abuse, but attendant behavioral experiences may have a negative impact on females engaging in drug use.
Keywords: Sexual behavior, Pair bonding, Maternal care, Estradiol, Progesterone, Dopamine, Sensitization, Neuronal signaling, Neural Plasticity
1. Introduction
In recent decades there has been an increase in drug use and abuse (both legal and illicit) among adolescent girls and women [233]. Along with this shift in the demographics of drug use is a developing sensitivity to particular experiential variables that co-vary with addiction in females [130]. Because drug use among females may require special approaches to prevention and therapy [164], a good starting point for determining addiction vulnerability in women would be to address life experiences of particular relevance to women. As drug use often begins during adolescence, life history events associated with reproduction may impact the consequences of drug use.
There is growing appreciation of a shared neurobiology between naturally-occurring motivated behaviors, such as reproductive behaviors, and the neural mechanisms of repeated drug use that contribute to addiction vulnerability (e.g. [97]). Two primary features of this shared neurobiology are the importance of limbic and striatal targets of dopamine innervation and cellular mechanisms underlying neural plasticity in response to both drugs of abuse and behavioral experiences [50]. Clearly such processes would occur in both males and females. However, in this review our focus is on females, taking a life-history approach, to evaluate the effects of reproductive cycles, sexual and social experiences, and maternal care on striatal dopamine pathways, as well as on the cellular events relevant to the actions of abused drugs. We provide evidence that synaptic plasticity resulting from a female’s life experiences enhances responsiveness to drugs of abuse in a way than can predispose the individual to addiction.
2. Drugs of abuse affect neuronal plasticity in the nucleus accumbens
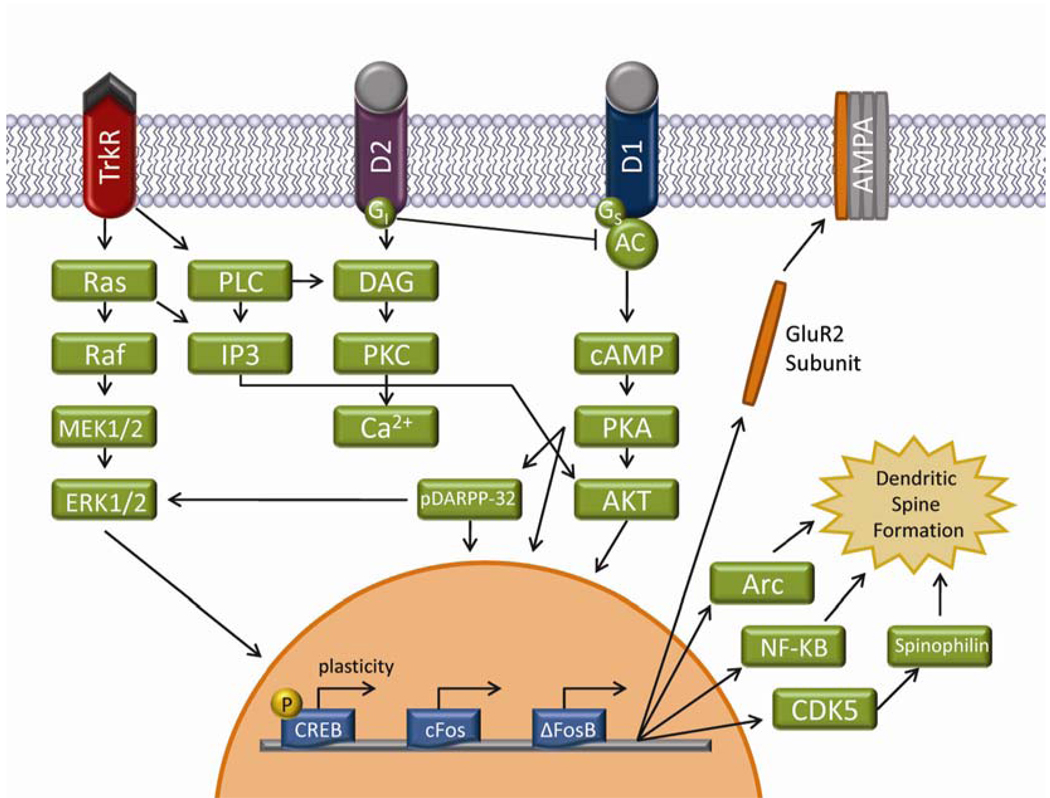
Common to all drugs of abuse is the activation of the mesolimbic dopamine pathway (for more extensive reviews see [39,50,100,146,147]). When dopaminergic projection neurons originating in the ventral tegmental area are activated by drugs of abuse there is a corresponding increase in extracellular dopamine within a number of forebrain regions, most notably for our purposes, the nucleus accumbens. One consequence of repeated exposure to drugs of abuse is a sensitized increase in dopamine neurotransmission, which is postulated to cause consequent morphological and functional changes within medium spiny neurons of the nucleus accumbens. These alterations in neuronal morphology through dynamic dendritic spine remodeling are believed to underlie the persistent behavioral and psychological changes following chronic drug administration [177]. Focusing on dopamine receptor-mediated events, we will provide an overview of some of the molecular mechanisms (Figure 1), which may ultimately lead to the aforementioned changes in dendritic spine morphology and function. For many of these mechanisms, we appreciate that there could be differences between males and females. Unfortunately, the bulk of this literature has been done exclusively in males. We are summarizing this literature with the assumption that these same mechanisms also apply to females.
Figure 1.
Schematic representation of select cell signaling pathways involved in drug addiction. Activation of cell membrane receptors, such as D1 and Trk receptors, results in the propagation of that signal through multiple intracellular signaling cascades. Specifically, the MAPK and adenylate cyclase/PKA signaling cascades have been shown to be activated via dopaminergic signaling. Ultimately, activation of these intracellular signaling cascades results in gene transcription within the nucleus of the cell. Two of the many downstream gene targets of such signaling are CREB and ΔFosB. Whereas CREB phosphorylation is believed to mediate some of the behavioral plasticity resulting from drugs of abuse, the accumulation of the stable transcription factor ΔFosB is postulated to underlie drug induced structural plasticity. Targets of ΔFosB transcription in turn can affect intrinsic membrane excitability through the insertion of GluR2 subunits into AMPA receptors, as well as structural changes through dendritic spine plasticity via cdk5 activation. Importantly, D2 receptor activation counteracts D1 receptor activation [50].
There is a vast literature on experience-based plasticity in dendritic spines [81,124,139,177,187], yet little direct evidence relates this structural plasticity to particular behaviors. Dendritic spine synaptogenesis consequent to drug administration is hypothesized to involve mostly excitatory synapses [177]. Spines themselves also have diverse morphology with varying sizes and shapes, believed to reflect the physiology of the spines, with larger spine heads correlating to stronger synaptic connections [81,187]. Importantly, in the case of chronic drug use, dendritic spines exhibit stability following cessation of stimulation. This atypical stability makes them likely candidates to mediate the long-term underlying cellular plasticity leading to the behavioral effects seen in drug addicts that ultimately cause them to relapse [50]. In fact, previous research has established that exposure to psychostimulants causes an increase in dendritic spine density in nucleus accumbens medium spiny neurons [49,107,121,122,153,174–177].
What are some of the intervening molecular events that can cause changes in dendritic morphology? Transcription factors such as Fos and cyclic AMP response element binding protein (CREB) are likely molecular mediators of dendritic spine formation and long-term cellular plasticity [50]. Multiple cell signaling pathways, including the mitogen activated protein kinase (MAP kinase) pathway, can stimulate transcription factor activity [1]. Entry into the MAP kinase pathway can be achieved via various signaling pathways originating from G-protein coupled receptors, ionotropic glutamate receptors, and growth factor receptors (Figure 1) [1,37,127,131,212]. Binding of these receptors in turn stimulates a cascade of phosphorylation events, including the activation of ERK1/2. Using one example [131], illustrated in Figure 1, increased extracellular dopamine will activate dopamine D1 receptors leading to heightened activation of adenylyl cyclase, an increase in the production of cytosolic cAMP, and activation of protein kinase A (PKA). This increase in PKA activity ultimately increases the activity of multiple downstream targets including Akt (also known as protein kinase B), the 32 kDa dopamine and cAMP dependent phosphoprotein (DARPP-32), as well as directly phosphorylating the transcription factor CREB. When DARPP-32 is activated by phosphorylation it can inhibit activity of protein phosphatase 1. This inhibition of phosphatase activity effectively results in increased phosphorylation of ERK1/2. CREB activation has been shown to be an important component of the behavioral rewarding effects of drugs of abuse, although its direct role in dendritic spine formation has yet to be elucidated. Alternatively, activation of the D2 receptor inhibits adenylyl cyclase, which in turn activates the inositol triphosphate pathway as well as inhibiting the phosphorylation of DARPP-32 (Figure 1). Therefore, D2 receptor stimulation has the effect of counteracting D1 receptor activation.
Whereas an increase in dendritic spine formation has been associated with both D1 and D2 receptor containing medium spiny neurons within the nucleus accumbens, it seems that D1 neurons display more stable changes in dendritic spines [117]. ΔFosB [82], a highly stable transcription factor, is a potential mediator of these long-lasting structural changes, although a molecular connection between dopamine D1 receptors and ΔFosB has not been determined. Unlike other Fos family proteins which are induced transiently following acute activation, ΔFosB accumulates following repeated stimulation and remains elevated for extended periods of time. Putative downstream targets of ΔFosB signaling have been examined by both microarray experiments and overexpression paradigms [5,6,67,80,157,186,215,223,229]. These targets include cyclin dependent kinase 5 (Cdk5) [223], the transcription factor nuclear factor κB (NF-κB) [6], and the GluR2 subunit of the α-amino-3-hydroxy-5-methyl-4-isoxazole (AMPA) receptor [103] (Figure 1). These downstream targets have also been shown to contribute to the long-term cellular and behavioral plasticity following repeated drug use. The importance of both Cdk5 and NF-κB in drug induced dendritic spine formation has been examined following chronic cocaine administration [153,186]. In addition, the insertion of GluR2 subunits into AMPA receptors decreases Ca2+ permeability, potentially affecting the expression of drug-related behaviors [103].
Despite the extensive literature evaluating cell signaling and drug addiction, little is known about how these well characterized pathways respond to natural behavioral experiences. In an effort to better understand the meaning of drug-induced plasticity, we must first appreciate how these pathways respond to natural stimuli. This review will focus on how different components of reproduction in females may act within these well-established signaling pathways to potentially sensitize synaptic neurotransmission in a way that predisposes an individual to addiction.
3. Impact of ovarian hormones on mesolimbic dopamine systems and drug taking
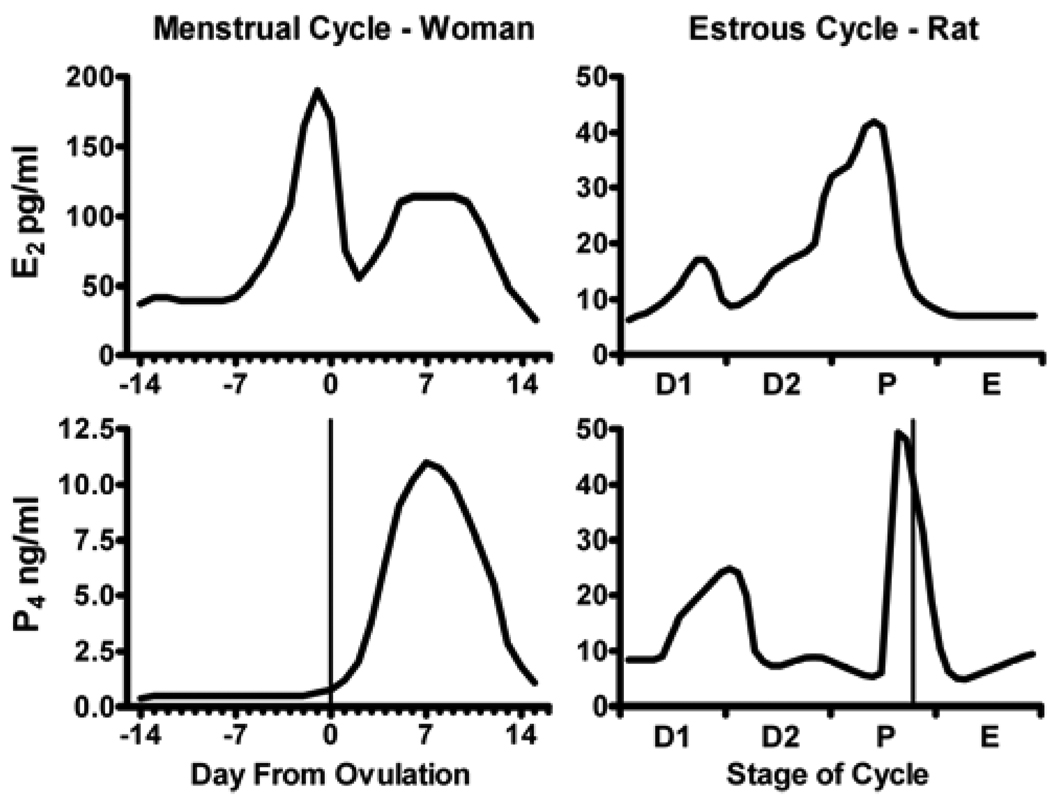
Female rats have been the preponderant species used to model the effects of ovarian hormones on parameters of drug use in human females. The issue with using rodents to model human reproductive cycles is that rats have what is termed an estrous cycle, whereas women have a characteristic menstrual cycle. Hormonal fluctuations across these cycles are depicted in Figure 2. For women, there is a 2 week follicular phase during which estradiol rises, which terminates with ovulation. After ovulation, the corpus luteum that is formed from the ruptured ovarian follicle secretes progesterone for approximately another 2 weeks; this is termed the luteal phase. Thus, the follicular phase (especially the late follicular phase) is characterized by high levels of estradiol, whereas the luteal phase has both elevated estradiol and progesterone.
Figure 2.
Plasma concentrations of estradiol (E2) and progesterone (P4) throughout the human menstrual and rat estrous cycles. Values represent the means of measurements taken daily from women and at two-hour intervals from rats. The four-day estrous cycle is broken down into its stages: diestrus-1 (D1), diestrus-2 (D2), proestrus (P), and estrus (E). Vertical lines indicate ovulation. Modified from [207] and [66].
Rats, in contrast, have a 4 day estrous cycle in which there are rising levels of estradiol early in the cycle, with a surge in progesterone on the 4th day. The surge in progesterone is coincident with ovulation. Therefore, with respect to hormonal secretion, rats have a cycle that is a calendar order of magnitude shorter than women (4 days versus 4 weeks). Two approaches have been used in rats to model ovarian hormone effects on parameters of drug use in women. One is to manipulate ovarian hormones within the timing of the rat’s cycle. Here, females are examined at different points of their endogenous cycle, or the ovaries are removed and acute hormone replacement is given to mimic different phases of the cycle. The other approach is to give prolonged estradiol and/or progesterone treatments on the order of weeks. Certainly, under normal circumstances female rats are not exposed to high, chronic levels of either estradiol or progesterone. Still, many investigators, including ourselves, have effectively used this hormonal regimen to tease out questions that are difficult to answer with more acute, physiologically-based approaches. In addition, maintaining the exposure of a female rat to high levels of estradiol or progesterone may be relevant to modeling hormonal exposure during a woman’s menstrual cycle. Nonetheless, in this section we will take a physiologically conservative approach in reviewing the literature on rats, limiting our discussions to steroid treatments that are within the realm of the timing of a 4-day estrous cycle.
There is a rather limited literature in women examining the relation between steroid hormone levels and psychological responses to drugs or their associative cues. The approach in these studies has either been to examine women at different stages of their menstrual cycle or to look at some type of hormone replacement in normally cycling women (see review [57]).
The contributions of estradiol to drug responsiveness have been evaluated by comparing women in the early and late follicular phases of the menstrual cycle. In the late follicular phase small increases were noted in the subjective evaluation of how much the women ‘liked’ a low dose of amphetamine [95]. Two other studies approached this question by supplementing estradiol levels during the early follicular phase. Women given an estradiol patch during the early follicular phase reported more pleasant responses to amphetamine and an increased desire to want more drug compared to the non-treated women [94]. In a similar vein, women who were taking oral contraceptives were given sublingual estradiol during the ‘placebo’ days of the treatment regimen [123]. Compared with controls, the estradiol-treated women showed a greater discriminative ability for the detection of amphetamine and also reported a greater ‘liking’ for the drug.
Comparisons between the follicular and luteal phases of the menstrual cycle are designed to examine the added contribution of progesterone given a background of estradiol exposure. One interesting finding from these studies is that women taking cocaine during the luteal phase had attenuated elevations in heart rate and blood pressure relative to cocaine effects during the follicular phase. This was true for either exposure to cocaine or to cocaine-related cues [56,197,199]. Subjective responses to cocaine or amphetamine were also blunted during the luteal phase. Reports of feeling ‘high’, liking the drug, wanting more of the drug, or craving were all lower during the luteal phase of the cycle [56,96,197,199,218]. Compared to a ‘no hormone’ condition it appears that estradiol augments the subjective components of drug use, whereas progesterone may counteract these effects of estradiol in women.
Analogous studies of estrous cycle effects or specific hormonal treatments on responsiveness to psychostimulants have been conducted in rats. This literature has been effectively reviewed elsewhere [12,32,64], so we will summarize it only briefly.
The simplest approach to testing the hypothesis that ovarian hormones can modulate drug effects is to compare gonadally intact female rats across the estrous cycle with ovariectomized females. Surprisingly, this literature is rather sparse. Ovariectomized female rats show a clear decrease in cocaine stimulated activity compared with intact females [40,110]. For estrous cycle effects we will use two examples. Cocaine-treated female rats had higher activity levels during proestrus and estrus compared with activity measured during either of the diestrus days [192]. In a self-administration study, hormone levels (rather than vaginal cytology) were used to track the estrous cycle [60]. In this case, self-administration of cocaine did not correlate with fluctuations in estradiol levels, though elevations in progesterone were associated with reductions in self-administration.
Estradiol treatments consistently augment locomotor activation in conjunction with either cocaine or amphetamine [58,85,93,165,231,232]. A similar set of results is found with estradiol facilitating self-administration of drugs [7,83,84,90,111,112,128,129,184]. One interesting set of observations is that estradiol may have a nonmonotonic dose-response curve in its interactions with psychostimulants [83,150]. At low doses estradiol may actually inhibit the effects of cocaine. Moderate doses facilitate cocaine’s effects on activity, whereas supraphysiological doses of estradiol may also antagonize cocaine’s actions. Progesterone secretion appears to antagonize these effects of estradiol [7,53,54,231].
The time course of estradiol effects on drug-induced activity typically has had a duration of several days, consistent with an activation of intracellular receptors. Indeed, both estrogen receptors α and β have been implicated in estradiol effects on locomotor activity and self-administration [112,190]. An alternative approach has been to test the effects of cocaine or amphetamine on activity 30 min after estradiol administration [17,148,149]. The rationale behind this treatment is that such a rapid time course for estradiol effects must be mediated by membrane estrogen receptors [12]. In this case, estradiol facilitated amphetamine-induced activity [17], though oddly enough attenuated activity following cocaine [148]. Estradiol appeared to reduce cocaine availability in the brain, which may have mediated cocaine’s diminished effect on activity [149]. In contrast, estradiol enhanced dialysate dopamine levels in response to amphetamine [17,35]. The measurements of dialysate dopamine or cocaine availability explain the behavioral observations, though it is unclear why estradiol should interact differentially with cocaine and amphetamine.
Ovarian hormones have been shown to influence the magnitude of a conditioned place preference to psychostimulants in rats. Relative to gonadally-intact females, ovariectomy decreased cocaine-induced conditioned place preference [185]. Either estradiol alone, or in combination with progesterone, facilitated conditioned place preference to either amphetamine or cocaine [38,185,196]. Preliminary evidence suggests that the effects of estradiol on conditioned place preference are mediated by interactions with estrogen receptor β [196].
Different mechanisms have been explored that potentially could mediate the effects of estradiol on drug action. Consistent with the effects of estradiol on drug-induced activity is the pattern of dopamine release following estradiol treatment. Even in the absence of drugs, basal dopamine is elevated in estrus relative to diestrus [16]. Estradiol administration increases dopamine synthesis/turnover [45,118,163], dopamine release [14,15], and methamphetamine potentiated dopamine release [156]. Part of the basis for the elevated dopamine could be estradiol inhibition of dopamine uptake [52,206]. The inference from these studies that estradiol acts directly on the striatum, is supported by similar effects on dopamine in superfused striatal dissections [51,225]. Further, the observation that catechol estrogens or estradiol conjugated with bovine serum albumen (a membrane impermeable estradiol complex) mimic the effects of estradiol itself points to a membrane action of estradiol on dopamine release [19,20].
Finally, estradiol can modulate postsynaptic mechanisms of dopamine neurotransmission. Dopamine D1 receptor expression is lower during proestrus than diestrus of the female rat’s cycle [119]. Similarly, estradiol potentiates D1 mediated neuronal excitability [47]. In contrast, D2 receptor binding is diminished with estradiol pretreatment [10,42,118], though the neurophysiological consequences of these receptor changes have not been tested. Again, these effects are transient and though they may contribute to the rewarding effects of drugs, they are unlikely to contribute to addiction vulnerability.
4. Sexual experience sensitizes the mesolimbic dopamine system
Adolescence is characterized by the onset of reproductive cyclicity. The potential impact of ovarian hormones on the mesolimbic dopamine system was discussed in the previous section. The presence of reproductive cycles does not require the female to engage in sexual activity, though some 60% of girls in the US have had sexual intercourse by the age of 19 [29]. We know from rodent studies that mechanisms mediating sexual responses are neurobiologically separable from the motivational control of sexual interest. Most notably, lesions of the nucleus accumbens in rats will spare the expression of sexual responsiveness to mounts by a male at the same time that these females resist the male’s mounting attempts [171,172]. In this section, we will review the impact of the motivational consequences of sexual interactions in female rodents on dopamine neurotransmission. The goal here is to identify cellular changes that result from sexual experience which impact the initial response to taking potentially addictive drugs.
Among animals, sexual interactions have evolved in a way that reproductive success is maximized. This pattern of optimal sexual interaction has been well characterized in rodents and is referred to as the ‘species vaginal code’, established by Milton Diamond [48]. Diamond argued that particular patterns of vaginal stimulation by the male’s intromissions were key to inducing pregnancy, establishing that “species-related neural stimuli from the vagina, integrated with other sensory inputs accompanying mating, are crucial for the initiation of neural and endocrine mechanisms supporting pregnancy and pseudopregnancy” [48].
The majority of the work done to dissect these behaviors has been performed in rats and hamsters, and although females of both species express lordosis (a characteristic dorsoflexion of the spine) the two species have rather different mating patterns. For female rats, mating behavior is a very active process that includes darting away from the male and then stopping to permit the male to mount. Flank stimulation provided by the mounting male is a sensory trigger for lordosis in the female. Once the male dismounts, the female terminates lordosis and goes back to darting about the mating area. For female rats, the ability to dart away from the male provides an opportunity for the female to regulate the timing of the male’s mounting attempts, a process termed ‘pacing’ [136].
Mating for female hamsters is not as overtly active as that of female rats. In the presence of a male, a receptive female hamster will enter lordosis for minutes on end, even without any tactile stimulation from the male [151,152]. While in lordosis, the female hamster will move her perineum in the direction of the male’s thrusts in an effort to facilitate intromission [151,152]. In this case, the male determines the pacing of mounting attempts, though the female has considerable control over the extent to which a mounting attempt includes intromission [21,151,152].
Erskine [55] has detailed the ways in which paced mating controlled by the female induces neuroendocrine mechanisms necessary for successful pregnancy. Our view [139] is that pacing in rats or perineal movements in hamsters activate reward circuits in a way that provides sensory feedback to the females, thus optimizing copulatory contacts with the male to maximize reproductive success based on the ‘vaginal code’ for each of these species. The primary way in which the rewarding consequences of sexual behavior in females have been tested is through the conditioned place preference (CPP) paradigm. Not surprisingly, sexual behavior has been shown to induce CPP in both female rats and hamsters [140,158]. For rats, CPP is primarily evident when the female is permitted to pace her sexual interactions with the male [74,91,132,161,162].
Repeated exposure to drugs of abuse also induces CPP in laboratory animals and this CPP can be inhibited by blocking dopamine action using selective dopamine antagonists [209,210], establishing the importance of dopamine action in inducing the rewarding effects of drugs. Although the role of dopamine in inducing CPP with drugs of abuse appears to be relatively clear cut, there are conflicting reports as to the effect on CPP when dopamine transmission has been disrupted during sexual behavior. We evaluated the contribution of dopamine receptor binding to the induction of CPP in female hamsters following repeated sexual bouts. Consistent with findings from the drug literature, systemic injection of selective D2 receptor antagonists resulted in an attenuation of CPP in female hamsters [141]. Horsman and Paredes evaluated [69] the effects of both a non-selective D1/D2 antagonist and a selective D2 antagonist on CPP induced by paced sexual behavior in female rats. In this study, neither dopamine antagonist affected the acquisition of CPP. Because the literature is so limited on this topic, we do not know what might form the basis for these discrepant results.
The regulation of the male’s intromission by female hamsters can be measured indirectly through the male’s ‘hit rate’, which is the proportion of mounts by the male hamster that results in intravaginal intromission. Female hamsters given sexual experience consisting of 6 weekly 10 min tests can improve the hit rate of sexually naive males compared with the performance of males tested with sexually inexperienced females [21]. This increased hit rate remains even after a subsequent 6 week absence from mating with a male, implying a sustained change in the female’s behavior resulting from the initial sexual experience [21]. To evaluate the contribution of nucleus accumbens dopamine to this experience-based change in the female’s behavior, 6-hydroxydopamine (6-OHDA) lesions were made in the nucleus accumbens prior to giving female hamsters sexual experience. After 6-OHDA lesions, the increased hit rate otherwise observed in experienced females was negated, demonstrating that dopamine release in the nucleus accumbens is necessary for the change in female sexual behavior that is observed over time with sexual experience [21].
A more direct relation between sexual behavior and nucleus accumbens dopamine release was established using in vivo microdialysis to monitor extracellular levels of dopamine in female rats and hamsters during sexual behavior [13,92,105,138,143,166]. In sexually receptive female hamsters, increases in extracellular dopamine, as measured by dialysate dopamine concentration, were measured in the nucleus accumbens during mating behavior [138]. This elevation in dialysate dopamine in the nucleus accumbens during mating was dependent upon intromissive stimulation from the male [106].
Similar increases in extracellular dopamine have been demonstrated in receptive female rats by in vivo microdialysis. Initially, it was demonstrated that female rats able to pace their sexual behavior showed the greatest increases in dopamine release in the nucleus accumbens when compared to females who were not responsible for pacing sexual behavior [143]. However, subsequent work has demonstrated that it is not the females pacing behavior per se, but the timing of the copulatory stimuli, regardless of whether the female is responsible for regulating the pacing of sexual contacts [11]. Pfaus et al. [166] took this work a step further, making an effort to dissect the contributions of different components of female sexual behavior to elevations in dialysate dopamine. By evaluating dopamine release during locomotion in a novel environment and during sexual behavior, Pfaus and colleagues [166] demonstrated that increases in extracellular dopamine detected within the nucleus accumbens during mating behavior were a result of contact with the copulating male, and not a side-effect of neuronal stimulation resulting from physical activity or exploration of a novel environment [166].
Jenkins and Becker [92] refined their microdialysis procedure to collect samples every minute during copulation in female rats. By time-locking these samples to particular behaviors, Jenkins and Becker [92] were further able to demonstrate that dopamine release in the nucleus accumbens actually occurs before coital stimulation from the male. This observation supports the hypothesis that dopamine release in the nucleus accumbens is involved in the anticipation of sexual behavior and not necessarily related to the explicit expression of lordosis [92].
‘Sensitization’ is the term used in the drug literature to describe an enhanced dopamine release following repeated exposure to a drug [98,99,167,200]. We were also able to demonstrate that sexual experience sensitized dopamine release in the nucleus accumbens, and that this augmented dopamine release was not accompanied by alterations in lordosis expression [105]. These data suggests that sensitization of the dopamine system is a natural consequence of certain types of repeated behavioral experiences.
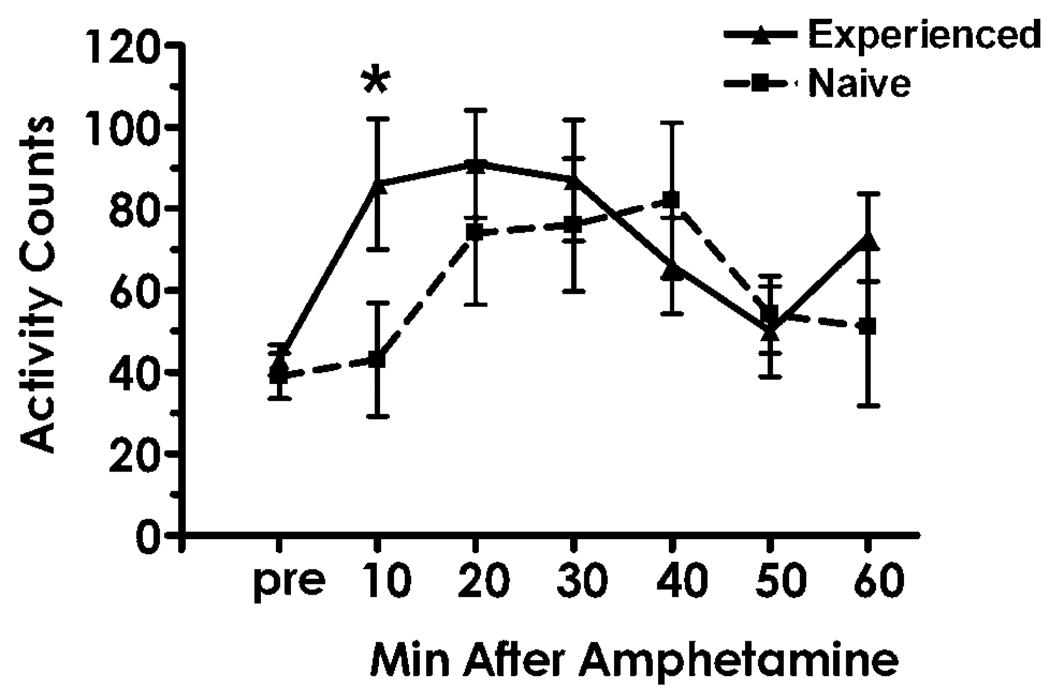
Based on the demonstration that sexual behavior can sensitize the mesolimbic dopamine system in a manner analogous to that of drugs of abuse, we tested whether cross-sensitization of the mesolimbic dopamine response to amphetamine would occur following repeated sexual experiences (Figure 3). When exposed to amphetamine, drug naïve, sexually experienced female hamsters showed a sensitized locomotor response upon their first exposure to amphetamine compared with the response of sexually inexperienced females [24]. Interestingly, the reverse phenomenon has been demonstrated in rats, in that females with previous drug exposure will display increased appetitive behaviors, i.e. hopping and darting towards a copulating male rat, without a change in the expression of lordosis [3]. These data suggest that previous exposure to drugs of abuse enhances the “motivational” control of sexual behavior without affecting the reflexive lordotic components.
Figure 3.
Female sexual experience sensitizes the activity response to amphetamine. When exposed to amphetamine for the first time, sexually experienced female hamsters show a significant increase in activity at the 10 min time point (*) when compared to sexually naïve female hamsters. This activity level remains elevated in sexually experienced females through the first 30 min of the test period (data from [24]).
Thus far, we have established the importance of repeated dopamine release driving the sensitization of the mesolimbic dopamine system on a behavioral and presynaptic level, but what are the neurochemical underpinnings of this sensitization? What changes occur at the synapse of nucleus accumbens neurons that mediate this persistent sensitized response? For the remainder of this section, we will piece together components of intracellular pathways that may contribute to this long-lasting plasticity in neurobiology and behavior.
Mentioned previously, a hallmark of sensitization is an increase in synaptic dopamine concentration in response to fixed stimulus. To date, whether the sensitized synaptic dopamine elevations result from changes in presynaptic release, decreased dopamine reuptake, or some combination of the two has not been tested. What is clear is that this increase in synaptic dopamine concentration enhances postsynaptic dopamine signaling.
It has long been known that drugs of abuse not only sensitize the mesolimbic dopamine system but also alter receptor-mediated intracellular signaling [179,188,189,208,211,213]. In an effort to resolve components of intracellular sensitization that result from sexual experience, we measured cAMP accumulation in homogenates from the nucleus accumbens of female hamsters after in vitro pharmacological stimulation [22]. In this experiment, cAMP accumulation was evaluated following stimulation of the dopamine D1 receptor, activation of the G-protein using Gpp(NH)p, or direct forskolin stimulation of adenylyl cyclase. Homogenates from both sexually naïve and experienced animals showed a dose dependent response to either forskolin or Gpp(NH)p in the nucleus accumbens and caudate, with no differences in response between sexually experienced and inexperienced females. In contrast, preparations taken from sexually experienced female hamsters displayed a significantly enhanced accumulation of cAMP in the nucleus accumbens in response to dopamine when compared to homogenates from sexually-naïve controls. The enhanced cAMP accumulation in the accumbens of sexually-experienced females suggests that in addition to presynaptic dopamine sensitization, repeated sexual behavior may also trigger an increased coupling of the dopamine D1 receptor to its G-protein, which in turn yields an increased efficacy in the activation of cAMP production. These data further the idea that drugs of abuse take advantage of reward circuitry in the brain that is in place to promote naturally occurring behaviors in the animal.
Given the sensitized accumulation of cAMP, it is logical to next evaluate which potential downstream targets are activated, and how this augmented activation may contribute to sensitization and long term changes in the mesolimbic dopamine circuit following sexual experience. When gene expression in the dorsal and ventral striatum of sexually experienced or sexually naïve female hamsters was compared by microarray, a number of gene expression changes were observed, including but not limited to genes involved with neurotransmission, signal transduction, transcriptional regulation, receptors, and cytoskeletal remodeling [23]. In contrast to the effects of repeated exposure to drugs (e.g., [25]), our microarray study found no differences in dopamine receptor expression following sexual experience. Further, our unpublished observations are consistent with the microarray results in that there is no effect of sexual experience on either dopamine D1 or D2 receptor densities (V.J. Watts, B.R. Chemel and R.L. Meisel, unpublished data) or total D1 or D2 receptor protein levels (N.A. Staffend, V.L. Hedges and R.L. Meisel, unpublished data), bolstering the hypothesis that accumulation of cAMP through D1 receptor activation is indeed a result of increased coupling of dopamine D1 receptor to G proteins rather than to receptor changes.
Although a comprehensive view of signaling pathways affected by sexual experience is lacking, there are demonstrations of immediate early gene activation such as c-Fos, as well as accumulation of FosB/ΔFosB in the nucleus accumbens after repeated sexual experience [24,43,139,215]. We demonstrated that sexual behavior induces c-Fos expression in the core of the nucleus accumbens, which is further elevated (i.e. sensitized) if the animal has had prior sexual experience [105]. These results support the contention that sexual experience not only sensitizes dopamine release, but also postsynaptic cellular activation in the nucleus accumbens. Recently, Coria-Avila and Pfaus [43] demonstrated the induction of Fos expression in the nucleus accumbens core of female rats who were conditioned to associate a particular odor or strain of male rat with sexual experience. Unique to these results is the report of neuronal Fos activation in the anticipation, and not receipt, of sexual reward [43], bolstering the hypothesis that dopamine release in the nucleus accumbens core is associated with sexual behavior reflective of the motivational or appetitive state of the female animal.
Most Fos family members are rapidly degraded after transcription. The transient expression of these immediate early genes allows for controlled activation of downstream affectors. ΔFosB is a truncated member of the FosB family of immediate early genes [144] and is unusual because ΔFosB is very stable and accumulates in the brain after chronic stimuli such as drugs of abuse, natural reward and chronic stress (for review see [145]). Due to the transient nature of full length FosB, pan-FosB antibodies are commonly used with confidence for immunohistochemical detection of ΔFosB in brain tissue. Using this approach, sexual experience resulted in a significant accumulation of FosB selectively in the core of the nucleus accumbens of female hamsters when compared to sexually naïve female controls [139]. This accumulation was independent of sexual behavior immediately prior to sacrifice, indicating persistent, long-term accumulation of the ΔFosB isoform.
Because of its stability, ΔFosB is able to produce greater levels of transcriptional activity when compared to more transient Fos family members, and it has been speculated that ΔFosB contributes to long-term behavioral and cellular plasticity associated with previous experience (for review see [137]). In an effort to delineate behavioral consequences of ΔFosB accumulation, transgenic and viral vector methods have been implemented to manipulate the level of ΔFosB gene expression [67,80,157,215]. As discussed previously, sexual experience is known to induce conditioned place preference in female rodents, and these data are taken to indicate rewarding consequences of sex behavior. Previous work has demonstrated that sexual pairings once a week for 5–6 weeks is sufficient to induce conditioned place preference in female hamsters [80,140,141] as well as provide adequate experience enabling the female to increase the hit rate of a sexually naïve male partner [24]. However, 2 weeks of sexual experience is inadequate to produce conditioned place preference or to affect copulatory interactions with a male [80]. When ΔFosB was overexpressed by an adeno-associated virus in the nucleus accumbens, female hamsters showed significant conditioned place preference after only 2 weeks of sexual pairing. These female hamsters overexpressing ΔFosB when given 2 weeks of sexual pairings were also able to significantly increase the hit rate of males compared to males mated with GFP-transfected control females [80]. These results suggest that ΔFosB is augmenting the consequences of sexual behavior thereby reinforcing the effects of behavioral experience at earlier stages of behavioral acquisition. A possible action of ΔFosB in this regard is through sustained downstream transcription of gene products important for neuronal plasticity.
In addition to intracellular and behavioral modifications, sexual experience has the ability to induce plastic changes of the neuronal architecture, perhaps altering synaptic connectivity in various brain regions [139]. After 6 weeks of sexual experience, significant increases in dendritic spine density were noted in Golgi stained sections from the nucleus accumbens of sexually experienced female hamsters, a characteristic commonly resulting from a sensitizing dosing regimen of many drugs of abuse [104,122,194].
Taken together, it is clear that previous sexual experience has the ability to sensitize the mesolimbic dopamine system in a manner analogous to that of drugs of abuse. Although there are many gaps in our knowledge of the specific signaling molecules and intracellular pathways involved, it is apparent that repeated sexual behavior leads to increased dopamine release in the nucleus accumbens of female rodents and this increased dopamine release has significant postsynaptic effects on signaling, transcription, and synaptic morphology.
5. Activation of the mesolimbic dopamine system by pair bonding in voles
Social interactions can be viewed more broadly than simply sexual behavior, as other types of intersexual social interactions activate the mesolimbic dopamine pathway. Most notable among these social interactions is pair bonding in prairie voles [89]. Pair bonds are defined as long-term, selective and affiliative social attachments. Voles are microtine rodents, which display an array of social systems, ranging from highly social to solitary. Prairie voles exhibit selective pair bonding and monogamous affiliation with an established partner, as well as biparental care in a shared nest. In the field, pair bonds in prairie voles are characterized by the preferential association with a single partner and the failure to take on a new mate upon the death of that partner [70–72,169]. In the laboratory, the formation of a pair bond is first assessed by the presence of a partner preference. In this paradigm, a presumably pair-bonded vole is given a choice to spend its time with either its partner or a conspecific stranger. Importantly, mating facilitates partner preference between an individual male and female [34,87,193]. Short-term cohabitation (which would produce a pair bond in mated animals) in the absence of mating is insufficient to produce a partner preference [87,219,222], however, long-term cohabitation with a male (in the absence of mating) can induce partner preference [219]. In this section we will focus on the importance of the mesolimbic dopamine system in pair bond formation in the prairie vole. In addition to discussions of synaptic dopamine neurotransmission and the interaction of dopamine with the neuropeptide oxytocin in the female vole, we will also examine this pair bond model in terms of its relevance to susceptibility to drug addiction.
One question that has been asked is whether mating in the female prairie vole is sufficient to activate the mesolimbic dopamine system. Stimuli from the male are required for the display of female sexual behavior in prairie voles [33]. An in vivo microdialysis experiment in estrous female prairie voles found an increase in extracellular dopamine levels within the nucleus accumbens during mating [73]. Dopamine was increased 50% over baseline in the first 15 min sample following introduction of the male and remained elevated at 30% for the following 3 hrs [73]. Non-mated non-estrous females given oil vehicle did not show an increase in extracellular dopamine levels, indicating that the increased dopamine is not due to nonspecific social interactions, but instead to the sexual interaction itself. This finding is reminiscent of dopamine changes during mating in non-pair bonding species, such as female rats [143,166] and hamsters [106,138], indicating that an increase in extracellular dopamine does not fully explain the formation of a pair bond. What may be significant here is the protracted maintenance of the extracellular dopamine levels in voles as these elevations can be more transient in other species [106].
Both monogamy and pair bonding are rarely found in nature. As sexual behavior can stimulate dopamine release in the nucleus accumbens of promiscuous species, how is it that dopamine release underlies pair bonding in the vole? In this regard, a pharmacological approach was taken to determine the significance of dopamine in the formation of partner preferences. In one study, the nonspecific dopamine agonist, apomorphine, was administered to estrous females, which were then tested in a partner preference test after cohabitation with a male in the absence of mating. Administration of varying doses of apomorphine was able to produce a partner preference in the absence of mating [216]. In contrast, haloperidol, a general dopamine antagonist, blocked the formation of a partner preference in females that were allowed to mate for 24 hr with a male. To further elucidate the dopamine receptor subtype of importance, Wang et al. [216] repeated the experiment with specific D1 and D2 receptor antagonists. The D1 receptor antagonist SCH23390 did not affect the vole’s ability to form a partner preference. However, administration of the D2 receptor antagonist, eticlopride, blocked mating induced partner preference regardless of whether the drug was administered systemically or directly into the nucleus accumbens. It appears that the D2 antagonist, while not affecting mating, specifically disrupted the formation of a partner preference, which parallels the effect of D2 antagonism on conditioned place preference in female hamsters. The importance of D2 receptor activation was further validated by a formation of partner preferences in the absence of mating following treatment with the D2 agonist quinpirole given either systemically or by direct infusion into the nucleus accumbens. Again, this effect was not seen with the D1 agonist SKF38393 [216].
The involvement of the neuropeptide oxytocin and its interaction with dopamine within the mesolimbic system was studied in the context of the regulation of pair bonding [224]. Monogamous prairie voles have higher densities of both oxytocin receptor oxytocin receptor gene expression in the nucleus accumbens than do promiscuous voles [228]. This species difference alludes to the importance of oxytocin receptor activation in pair bond formation. Indeed, extracellular concentrations of oxytocin are increased in the nucleus accumbens of female prairie voles during unrestricted interactions with a male [182]. Unilateral 6-OHDA lesions in the nucleus accumbens of female prairie voles resulted in diminished binding of the dopamine transporter, while leaving oxytocin receptor binding unaffected [227]. This indicates that the oxytocin receptors are likely located on postsynaptic targets within the nucleus accumbens, rather than on dopaminergic terminals originating from the ventral tegmental area. . The studies reviewed here focus on voles, though it is important to note that the distribution of oxytocin immunoreactive fibers within the nucleus accumbens is conserved in voles, mice, and rats [182].
The interaction between dopamine and oxytocin systems has been demonstrated to critical for pair bonding in female prairie voles. Intracerebroventricular (icv) infusions of oxytocin in female voles increase social contact with males, as well as facilitate partner preference in the absence of mating [41,221]. Similar icv infusions of an oxytocin receptor antagonist block the formation of a partner preference even after extensive mating [87]. Indeed, direct nucleus accumbens infusions of an oxytocin receptor antagonist block partner preference [227], identifying the nucleus accumbens as a site of action for the ventricular treatments. In the absence of mating, blocking oxytocin receptor activation during extended cohabitation with a male also prevented the formation of a partner preference [227].
A study by Ross et al. [183] used adeno-associated viral vectors to overexpress oxytocin receptor in the nucleus accumbens of both adult monogamous female prairie voles and nonmonogamous female meadow voles to examine how oxytocin receptor density in the accumbens was related to social behaviors. Overexpression of the oxytocin receptor in the already monogamous prairie vole resulted in accelerated partner preference after cohabitation with a male. Interestingly, when the oxytocin receptor was overexpressed in the promiscuous meadow vole, there was no effect on partner preference. This study suggests that oxytocin receptor expression within the nucleus accumbens is not sufficient to produce a partner preference in nonmonogamous species [183]. Still, species difference in oxytocin neurotransmission may impact whether a species shows a monogamous or polygamous mating strategy.
The oxytocin results taken together with the data presented earlier regarding dopamine D2 receptor activation indicate the involvement of both systems in pair bonding, but do not necessarily demonstrate an interaction between these systems. The interaction of oxytocin with dopamine was demonstrated by co-administration of the D2 receptor agonist quinpirole with either the D2 receptor antagonist eticlopride or an oxytocin antagonist into the shell of the nucleus accumbens [125]. These combined treatments blocked quinpirole-induced partner preference. Complementing these results, co-administration of oxytocin with either an oxytocin or D2 receptor antagonist into the nucleus accumbens shell blocked oxytocin-induced partner preference. These data indicate that oxytocin and dopamine D2 receptors indeed interact to facilitate the development of partner preference in female prairie voles [125]. One interpretation is that the oxytocin-dopamine interaction is unique in the monogamous pair bonding vole, when compared to other promiscuous species, a theory partially supported by the species-specific distribution of oxytocin receptors within the nucleus accumbens [88,227]. A similar oxytocin-D2 interaction, as well as receptor distribution, is found in the medial prefrontal cortex of female prairie voles, another area within the mesolimbic dopamine pathway that could be important for pair bond formation [198].
There exist two hypotheses for mechanisms of oxytocin action in pair bond formation after mating. First, oxytocin transmission within brain areas of high oxytocin receptor density (e.g., nucleus accumbens and prefrontal cortex) is known to play an important role in reward processing and acts to reinforce the association with the partner male. Another hypothesis is that while D2 receptors may mediate the maintenance of the pair bond, oxytocin acts on social recognition, which is critical for pair bond formation. Indeed, the administration of a low dose of oxytocin facilitates social recognition in rats [170]. Rodents usually recognize previously encountered conspecifics by olfactory recognition cues. Olfactory cues are certainly important in voles, as olfactory bulb removal inhibits the formation of a partner preference and estrus induction in female prairie voles [220]. Additionally, oxytocin knockout mice have been shown to exhibit social amnesia [62], a behavior that can be rescued by direct infusion of oxytocin into the medial amygdala [61]. The inability to recognize individuals in these knockout mice is not due to problems involving learning and memory or olfactory processing because these animals perform similarly to wild types in the Morris water maze and habituate to nonsocial odors. In addition, Fos induction does not differ in brain regions involved in olfactory processing between knockouts and wild types after a social encounter, however knockouts fail to show Fos induction in the medial amygdala [62]. The medial amygdala receives both direct and indirect projections from the accessory olfactory bulbs and mediates socially significant olfactory cues [142], as shown by lesions of the vomeronasal organ inhibiting pair bond formation in female prairie voles [44]. Further experimentation is needed to fully elucidate the specific details underlying the interaction between dopamine and oxytocin within the nucleus accumbens, but the importance of both neuromodulators is undeniable in the formation of pair bonds in female prairie voles.
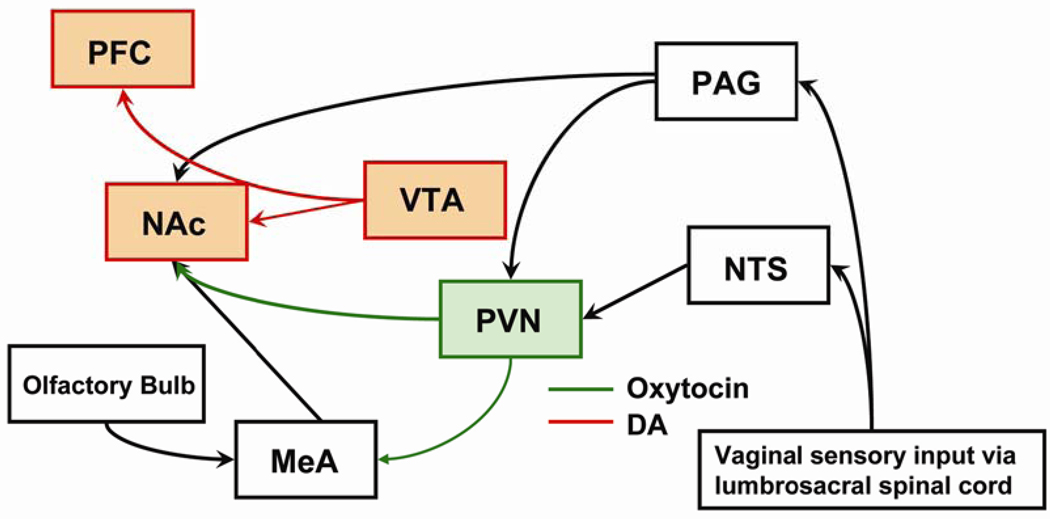
Young et al. [226] proposed a neural circuitry model of social bonding in monogamous prairie voles (Figure 4). In short, sensory information from the vagina traveling via spinal projections from the lumbrosacral spinal cord converges onto both the nucleus tractus solitarius and the midbrain periaqueductal gray, which in turn project to the nucleus accumbens. Olfactory information is processed by the accessory olfactory bulb, which then projects to the oxytocin receptor rich medial amygdala, where oxytocin facilitates social recognition. The subsequent activation of the mesolimbic reward pathway resulting from copulation results in a surge in dopamine release in both the nucleus accumbens and the prefrontal cortex. The nucleus accumbens may act as a coincidence detector, so that when dopaminergic and peptidergic signals occur simultaneously a selective pair bond can result.
Figure 4.
Schematic representation of the proposed circuitry for pair bonding in female prairie voles. Various sensory stimuli ultimately result in dopaminergic and peptidergic release at the level of the nucleus accumbens, thereby positioning the nucleus accumbens in an optimal anatomical location to act as a coincidence detector for pair bond formation [226]. DA, dopamine; MeA, medial amygdala; NAc, nucleus accumbens; NTS, nucleus tractus solitarius; PAG, periaqueductal gray; PFC, prefrontal cortex; PVN, paraventricular nucleus; VTA, ventral tegmental area.
The neural circuitry critical to the expression of pair bonding in the female vole certainly overlaps pathways that underlie addiction. Most notable in this shared circuitry is the placement of the mesolimbic dopamine pathway as a final common output to both the regulation of pair bonding and functional consequences of drugs of abuse. The beauty of the intrinsic neurobiology of the vole model system is that it is uniquely placed to uncover possible contributions of oxytocin to mechanisms mediating addiction vulnerability. Although a few studies on dopamine-oxytocin interactions with respect to drugs of abuse have been done in male rats [108,109], clearly this research area deserves more study.
6. Activation of the mesolimbic dopamine system by maternal behavior
Maternal care is a highly motivated behavior, where the dam engages in many pup-directed behaviors including licking and grooming, nursing, nest building, and retrieving pups to the nest site [65,155,201,202]. While it is clear this highly motivated state that allows the immediate care of offspring is brought about by both the hormonal events of pregnancy and parturition, there is also substantial evidence that similar to drugs of abuse, activation of the mesolimbic dopamine system is key to the expression of this behavior [204]. This section of the review will focus on research that has investigated the rewarding properties of pups, the presynaptic facilitation of dopamine release during maternal behavior, and the importance of post-synaptic D1 and D2 receptor activation.
The onset of maternal care behaviors is rapid at birth, but can also be induced in virgin female rats following 6 to 8 days of repeated exposure to pups [65,180]. Although it was initially reported that postpartum lactating females behave in a manner indistinguishable from pup-sensitized virgin females, Bridges et al. [26] showed that lactating females performed superiorly to pup-sensitized females in a T-maze pup retrieval task. To further investigate the mechanism underlying this difference in behavior, Stern and Mackinnon [202] attached a T-maze to the dam’s home cage and tested a variety of conditions, comparing normal postpartum females, thelectomized (nipple removal) females, hormonally-facilitated maternal behavior, and non-hormonal conditions (pup-sensitized) in their ability to choose the most rewarding stimulus (the pup). Over the 5 days of testing, one arm of the T-maze would hold a previously suckled pup and the other a pup-sized toy, with arm assignments randomly chosen each test day. Interestingly, nearly every postpartum, thelectomized and hormonally primed female retrieved the pup and returned it to the nest (while ignoring the toy). In contrast, only half of the pup-sensitized females were able to perform this task. This study implicated the obvious importance of hormones in the specific rewarding maternal behavior of pup retrieval [202].
The rewarding value of pups for maternal female rats has been further investigated using traditional paradigms. In one such behavioral test, a pup ‘self-administration’ procedure, dams would readily bar press to gain access to pups, suggesting that the pups were highly rewarding to these females [116]. An unusual approach was taken in a study examining cocaine self administration throughout the reproductive cycle of female rats [79]. Female rats were able to self administer cocaine in their home cage with pups present. The highest intake of cocaine was seen during estrus and the first trimester of pregnancy, whereas intake rapidly dropped at parturition and remained at a decreased level throughout the first 8 days of lactation [79]. This decrease in cocaine self administration at the time of parturition suggested that pups are such a highly valued natural stimulus that they are preferred over cocaine. The idea that female rats would prefer pups over cocaine was supported in a functional magnetic resonance imaging study [63]. In this study, both nursing dams and cocaine-exposed virgin females had activation of the mesolimbic dopamine system. When the lactating dams were exposed to cocaine, there was suppression of activation within this reward system [63], demonstrating that for the dam nursing is more rewarding than cocaine.
A condition place preference paradigm, where dams chose between a pup-paired or cocaine-paired environment, was utilized to test whether females would exhibit a place preference to a pup-conditioned environment following parturition [133]. The place preference for pups was maintained for a short time after parturition, but decreased over time such that dams eventually changed their preference to the cocaine-paired environment [134,135]. This pattern of preference (pup preferring in early postpartum and cocaine preferring in late post partum) was maintained even when the incentive salience of the cocaine-paired condition was increased by both route of cocaine administration and the duration of the conditioning session [191]. Mattson and Morrell [135] went on to investigate the differential distribution of the immediate early gene c-Fos and cocaine and amphetamine-regulated transcript expression in these cocaine- and pup-preferring dams. They found dams that prefer the cocaine associated compartments had a greater increase in c-Fos immunoreactivity in both the nucleus accumbens core and shell than either dams that preferred the pup associated compartments or unconditioned (control) dams. Interestingly, when these animals were tested for locomotor sensitization, only the cocaine-preferring dams showed a sensitized response following the cocaine conditioning compared to pup-preferring dams [191].
To determine if pup reward shares circuitry with drugs of abuse, several laboratories have examined how different aspects of maternal care affect extracellular dopamine within the nucleus accumbens. Consistent with this hypothesis, a microdialysis experiment showed that the reuniting of separated pups with the dam significantly increased dopamine and its metabolites within the nucleus accumbens [76]. Further, 6-OHDA lesions of either the dopaminergic ventral tegmental projection neurons or the nucleus accumbens terminals impaired the maternal pup retrieval [78]. Apparently this treatment only produced a partial disruption of maternal retrieval, as it was overridden by extending the time of maternal separation from the pups to 3–6 hours [77]. Champagne et al. [36] further examined nucleus accumbens dopamine during maternal licking/grooming using in vivo voltammetry within the nucleus accumbens shell. It was found that there was an increase in extracellular dopamine while the dam was in a nursing posture and immediately before the onset of a licking/grooming bout. Dopamine levels remained at an elevated level throughout the bout, but then decreased immediately before the termination of the behavior. Additionally, the amplitude of the dopamine response was correlated to whether dams had been characterized as either low or high licking/grooming dams, with high licking/grooming dams having an augmented dopamine signal compared to low licking/grooming dams [36]. These observations were supported by a microdialysis experiment that compared normal Sprague-Dawley rats with Flinders sensitive rats, which display low levels of maternal behavior [113]. The Flinders rats were found to be deficient in affiliative maternal behaviors, and these decreased maternal behaviors correlated with a decrease in dopamine during microdialysis. One interpretation of these results is that the Flinders rats do not properly interact with pups because they do not find them appropriately rewarding [113].
Previous maternal and reproductive experiences also effect extracellular dopamine concentrations within the nucleus accumbens shell, as well as dopamine dependent behaviors. A study by Felicio et al. [59] examined the impact of prior parity on dopaminergic activity. Dams with two prior pregnancies exhibited significantly higher striatal dopamine levels compared to dams with only one prior pregnancy, suggesting that previous maternal experience had the ability to sensitize striatal dopamine levels [59]. Another study used nulliparous (no prior births) and multiparous (multiple prior births) female rats given pup exposure along with microdialysis during a period that elicits little to no maternal behavior in any females [2]. Despite the lack of observable maternal behavior at this time, an increase in dopamine was still observed during the first 8 min of pup exposure, with multiparous females having higher dopamine levels than nulliparous females. This effect on dopamine quickly dissipated in the following 8 min test sample. Still, the experience-dependent increase in dopamine supports the idea that nucleus accumbens dopamine plays a part in mediating the rewarding consequences of pup exposure in females with previous maternal experience [2].
To further evaluate how previous reproductive experience can affect dopamine neurotransmission, Byrnes et al. [31] examined the effects of dopamine agonists on the striatal dependent behaviors, prepulse inhibition and oral stereotypy, in nulliparous females and in multiparous females that had previously weaned their litters. Apomorphine, a mixed D1/D2 receptor agonist disrupted prepulse inhibition and oral stereotypy in multiparous, but not nulliparous females. Consistent with these behavioral effects, multiparous females had higher striatal dopamine content compared to nulliparous females [31]. These results suggest that prior pregnancies can sensitize striatal dopamine systems, potentially making these females more susceptible to drugs of abuse.
If previous maternal experience has the ability to sensitize dopamine release, it could then be possible for maternal behavior to cross sensitize to drugs of abuse. To test this possibility nulliparous and multiparous female rats were given amphetamine and tested for locomotor activity [86]. Compared to nulliparous females, multiparous females showed higher locomotor activity following amphetamine administration. These results demonstrate that prior reproductive experience can cross sensitize to amphetamine [86].
Several pharmacological approaches have been taken to understand the role of dopamine in the nucleus accumbens on maternal behavior. Administration of a mixed D1/D2 receptor antagonist directly into the nucleus accumbens disrupted normal maternal behavior in rats [101] and the biparental female prairie vole [126]. To further parse out which dopamine receptor subtype could be mediating these effects, a D1 receptor antagonist was directly injected in the nucleus accumbens of female rats. This manipulation decreased pup retrieval (a D2 antagonist was ineffective) only if applied to the nucleus accumbens as opposed to other neural sites (i.e., the ventral pallidum or medial preoptic area) [154].
Surgical termination of pregnancy is a procedure that produces a short-latency onset of maternal behavior [181]. Direct injection of a D1, but not a D2, agonist into the nucleus accumbens of pregnancy terminated rats facilitated the maternal response to donor pups [204], implicating D1 receptor activation for the onset of maternal behavior. While these studies demonstrate the importance of D1 receptor activation in the onset of maternal care, another aspect of maternal behavior termed maternal memory, has also been shown to be mediated by dopamine receptor activation in the nucleus accumbens [27,28]. Maternal memory is the long term retention of maternal behavior following partuition and is believed to be important in maintaining high levels of maternal care that remains following the immediate post-partum period. Indeed, the long-term effects of pup removal at parturition results in almost the complete loss of maternal behavior so that the dam acts in a similar manner to virgin females [28]. Research showing that lesions of the entirety of the nucleus accumbens or the shell alone flanking the time of pup exposure is sufficient to disrupt maternal memory [115,120], but not other maternal behaviors such as licking and nursing [120].
The separate administration of both D1 and D2 receptor antagonists throughout parturition has been found to significantly attenuate maternal behaviors tested at the time of parturition [30], as well as the maintenance of maternal care [195]. Females treated with either a D1 or D2 antagonist following parturition failed to group pups in the nest and crouch over them appropriately, however only the D2 receptor antagonist affected the retention of maternal behavior and maternal memory when these same dams were tested 7 days following parturition [30]. Parada et al. [160] has also shown that a D2 antagonist microinfusion into the nucleus accumbens shell produces deficits in maternal memory when tested following the 7 day latency between pup exposures, without any effect of the D1 antagonists. They surprisingly found that the combined D1/D2 antagonist infusion showed an additive effect, which would suggest that blocking both receptor subtypes is necessary to completely disrupt maternal behavior [160]. Both D1 and D2 antagonists have also been employed to examine ongoing maternal behavior in lactating rats. A mixed D1/D2 antagonist, haloperidol, significantly reduced pup retrieval, pup grouping, and nest building, with similar results observed from the D2 receptor blocker pimozide and the D1 antagonist SKF-83566 [195].
The results of these studies highlight the importance of nucleus accumbens dopamine neurotransmission in maternal behavior, and its responsiveness to drugs of abuse. In addition to maternal behavior itself, prior parity also seems to sensitize dopamine systems in a way that make females more vulnerable to the effects of drugs. A valuable addition to this literature would be studies comparing the commonalities of the molecular mechanisms underlying the effects of maternal behavior and reproductive experience with mechanisms of drug induced plasticity, for example as outlined in Figure 1.
7. Conclusions
We began this review with the thesis that life experiences, particularly those related to reproduction, can affect the mesolimbic dopamine system in a way that makes females susceptible to the addictive properties of drugs. This idea stems from the proposition that the mesolimbic dopamine system retains a high degree of synaptic plasticity that is functionally related to an individual’s changing needs. Activation by a variety of drug classes usurps this plasticity, yielding persisting sensitized cellular and behavioral responses to these drugs. If under certain conditions, naturally-occurring behaviors promote a similar sensitization of these dopaminergic circuits, a given pattern of drug use becomes more potent in accelerating the stages leading from drug use to abuse. In this regard, there should be parity in the effects of drugs and motivated behaviors on the long-term sensitization of dopamine neurotransmission and its functional consequences. That is, repeated drug administration should impact the expression of normal motivated behaviors just as behavioral experience can affect an individual’s responsiveness to drugs. In fact, animal studies have convincingly demonstrated that a variety of drugs that people abuse can affect different parameters of female sexual behavior (reviewed by [75]). We have provided an overview of mechanisms through which different components of a female’s reproductive experiences can alter dopamine neurotransmission in a way that the system shows a sensitized response to drugs even upon the initial exposure. Our focus has been on reproductive events in females, though it is important to note that there is a rich literature in rats demonstrating that male sexual experience sensitizes the mesolimbic dopamine system [8,9,68,159,168,205] in much the same way as we have described for females.
Common to all stages of reproduction in females reviewed here is an activation of the mesolimbic dopamine system. Ovarian hormone levels impact dopamine neurotransmission in the nucleus accumbens in a way that can acutely impact the rewarding and psychomotor potency of drugs. These effects are dynamic, changing with the integrated fluctuations of estradiol and progesterone across an individual’s cycle. In contrast, reproductive behaviors and prior pregnancies produce a sensitization of dopaminergic neurotransmission, which leads to a long-lasting potentiation of receptor mediated signaling. It is thought that transcriptional activation consequent to these signaling events induces the formation and stabilization of dendritic spines that presumably impact neuronal excitability [50]. Though this plasticity is biologically adaptive as our examples of sexual behavior, social affiliation, and maternal behavior demonstrate, an undesirable outcome is that it can potentiate cellular responses to drugs.
A number of voices have expressed the view that mesolimbic dopamine pathways are common ground for both the neurobiological control of motivated behaviors and the rewarding and addictive properties of drugs (e.g., [97,102,173]). By extension, the argument, which is eminently reasonable, is that addiction is therefore a pathology of motivation. The question commonly raised is why aren't motivated behaviors addictive [214]? One simple answer to this question is that they indeed are addictive, if we consider extremes of sexual behavior, shopping, eating, or gambling [4,46,178,230]. Nonetheless, the conventional answer is that our normal life experiences do not activate the mesolimbic system to the extent that potent pharmacological agents do, so addiction represents crossing a neurobiological threshold that is not reached as a result of simple life experiences [214].
Perhaps more illuminating is the observation that not all drug users become addicts. Drug use in people goes through a series of stages in which drugs initially produce a subjective rewarding experience [97]. With repeated drug use, tolerance to the pleasant effects of drugs develops, causing people to take higher doses to achieve the same subjective experience. It is this escalation of drug use that presumably leads to the neurobiological changes underlying addiction [97]. These changes in the addicted brain are extremely resistant to therapeutic intervention, and even when treatment is effective, people are vulnerable to relapse [203]. Even so, we are left with the questions of why there are individual differences in vulnerability to addiction and why once an individual is addicted that treatment is ineffective.
Plasticity in the mesolimbic dopamine system in response to natural behavioral experiences speaks to these essential issues related to drug addiction. One factor that certainly contributes to addiction vulnerability is genetics [114]. Early life experiences have also been identified as critical variables, especially with respect to an individual’s negative or stressful life experiences [114]. An additional factor that we have highlighted here is an individual’s normal behavioral life history. Our hypothesis is that these experiences affect neurotransmission in the mesolimbic dopamine system in a biologically advantageous manner; though an unfortunate consequence is that drug use in this case may accelerate the individual through the stages of addiction more quickly.
What then is the basis for the resistance to therapeutic intervention? Reproductive behaviors are fully developed in the absence of explicit associative processes. The incentive properties of relevant cues for the behaviors are well-established prior to an individual’s initial experience. Female rats engage in effective copulatory interactions with males when paired for the first time, assuming appropriate physiological conditions are met. Similarly, parturient female rats effectively care for their newborn pups. As we have discussed, these behavioral ‘reflexes’ are controlled by a mesolimbic dopamine circuitry that has the special property of responding to biologically-meaningful incentive stimuli. In the case of drugs, Belin et al. [18] articulated a learning-based neural model in which repeated stimulation of the ventral striatum produces a shift in neural processing from the nucleus accumbens shell to the accumbens core and dorsal striatum that is matched by behavioral associations that become less malleable, producing what they term an ‘incentive habit’. This neural plasticity is exemplified by dendritic spine studies in which cocaine administration is sufficient for spine induction in the shell, whereas behaviorally sensitizing cocaine treatments are required for spine formation in the nucleus accumbens core [122]. Because sensitization following sexual experience preferentially targets the core of the nucleus accumbens, such experience may facilitate this drug-mediated shift in neural processing. Collectively, these learned responses to drugs acquire the characteristics of natural motivated behaviors. The unusual strength of these associations [18], as with the motivated behaviors in our examples, makes addicted patterns of behavior extremely difficult to extinguish (e.g., [217]).
In sum, we propose that female reproductive processes, broadly considered, can produce enduring effects on synaptic dopamine neurotransmission in the nucleus accumbens. Under normal conditions, the neural consequences of these behavioral experiences serve important adaptive functions by presumably increasing reproductive success. Developing our mechanistic understanding of mesolimbic dopamine systems with respect to motivated behaviors that are part of an individual’s natural life is certainly important in its own right. An added benefit of this research is that it will impact new developments in the prevention and treatment of addiction [214].
Acknowledgements
We wish to thank Caroline Loftus for her excellent assistance with bibliographic and figure preparation. Support was provided by NIH grants DA 13680 (RLM) and DA 026255 (VLH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams JP, Roberson ED, English JD, Selcher JC, Sweatt JD. MAPK regulation of gene expression in the central nervous system. Acta Neurobiol Exp (Wars) 2000;60:377–394. doi: 10.55782/ane-2000-1357. [DOI] [PubMed] [Google Scholar]
- 2.Afonso VM, Grella SL, Chatterjee D, Fleming AS. Previous maternal experience affects accumbal dopaminergic responses to pup-stimuli. Brain Res. 2008;1198:115–123. doi: 10.1016/j.brainres.2007.12.042. [DOI] [PubMed] [Google Scholar]
- 3.Afonso VM, Mueller D, Stewart J, Pfaus JG. Amphetamine pretreatment facilitates appetitive sexual behaviors in the female rat. Psychopharmacology (Berl) 2009;205:35–43. doi: 10.1007/s00213-009-1511-x. [DOI] [PubMed] [Google Scholar]
- 4.Albrecht U, Kirschner NE, Grusser SM. Diagnostic instruments for behavioural addiction: an overview. Psychosoc Med. 2007;4 Doc11. [PMC free article] [PubMed] [Google Scholar]
- 5.Alibhai IN, Green TA, Potashkin JA, Nestler EJ. Regulation of fosB and DeltafosB mRNA expression: in vivo and in vitro studies. Brain Res. 2007;1143:22–33. doi: 10.1016/j.brainres.2007.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ang E, Chen J, Zagouras P, Magna H, Holland J, Schaeffer E, Nestler EJ. Induction of nuclear factor-kappaB in nucleus accumbens by chronic cocaine administration. J Neurochem. 2001;79:221–224. doi: 10.1046/j.1471-4159.2001.00563.x. [DOI] [PubMed] [Google Scholar]
- 7.Anker JJ, Larson EB, Gliddon LA, Carroll ME. Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp Clin Psychopharmacol. 2007;15:472–480. doi: 10.1037/1064-1297.15.5.472. [DOI] [PubMed] [Google Scholar]
- 8.Balfour ME, Brown JL, Yu L, Coolen LM. Potential contributions of efferents from medial prefrontal cortex to neural activation following sexual behavior in the male rat. Neuroscience. 2006;137:1259–1276. doi: 10.1016/j.neuroscience.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Balfour ME, Yu L, Coolen LM. Sexual behavior and sex-associated environmental cues activate the mesolimbic system in male rats. Neuropsychopharmacology. 2004;29:718–730. doi: 10.1038/sj.npp.1300350. [DOI] [PubMed] [Google Scholar]
- 10.Bazzett TJ, Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 1994;637:163–172. doi: 10.1016/0006-8993(94)91229-7. [DOI] [PubMed] [Google Scholar]
- 11.Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann N Y Acad Sci. 2001;937:172–187. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- 12.Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker JB, Rudick CN, Jenkins WJ. The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. J Neurosci. 2001;21:3236–3241. doi: 10.1523/JNEUROSCI.21-09-03236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker JB. Direct effect of 17 beta-estradiol on striatum: sex differences in dopamine release. Synapse. 1990;5:157–164. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- 15.Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett. 1990;118:169–171. doi: 10.1016/0304-3940(90)90618-j. [DOI] [PubMed] [Google Scholar]
- 16.Becker JB, Cha JH. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav Brain Res. 1989;35:117–125. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- 17.Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav. 1999;64:53–57. doi: 10.1016/s0091-3057(99)00091-x. [DOI] [PubMed] [Google Scholar]
- 18.Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 19.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boulware MI, Mermelstein PG. The influence of estradiol on nervous system function. Drug News Perspect. 2005;18:631–637. doi: 10.1358/dnp.2005.18.10.959578. [DOI] [PubMed] [Google Scholar]
- 21.Bradley KC, Haas AR, Meisel RL. 6-Hydroxydopamine lesions in female hamsters (Mesocricetus auratus) abolish the sensitized effects of sexual experience on copulatory interactions with males. Behav Neurosci. 2005;119:224–232. doi: 10.1037/0735-7044.119.1.224. [DOI] [PubMed] [Google Scholar]
- 22.Bradley KC, Mullins AJ, Meisel RL, Watts VJ. Sexual experience alters D1 receptor-mediated cyclic AMP production in the nucleus accumbens of female Syrian hamsters. Synapse. 2004;53:20–27. doi: 10.1002/syn.20030. [DOI] [PubMed] [Google Scholar]
- 23.Bradley KC, Boulware MB, Jiang H, Doerge RW, Meisel RL, Mermelstein PG. Changes in gene expression within the nucleus accumbens and striatum following sexual experience. Genes Brain Behav. 2005;4:31–44. doi: 10.1111/j.1601-183X.2004.00093.x. [DOI] [PubMed] [Google Scholar]
- 24.Bradley KC, Meisel RL. Sexual behavior induction of c-Fos in the nucleus accumbens and amphetamine-stimulated locomotor activity are sensitized by previous sexual experience in female Syrian hamsters. J Neurosci. 2001;21:2123–2130. doi: 10.1523/JNEUROSCI.21-06-02123.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briand LA, Flagel SB, Garcia-Fuster MJ, Watson SJ, Akil H, Sarter M, Robinson TE. Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited,access to self-administered cocaine. Neuropsychopharmacology. 2008;33:2969–2980. doi: 10.1038/npp.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bridges R, Zarrow MX, Gandelman R, Denenberg VH. Differences in maternal responsiveness between lactating and sensitized rats. Dev Psychobiol. 1972;5:123–127. doi: 10.1002/dev.420050205. [DOI] [PubMed] [Google Scholar]
- 27.Bridges RS, Scanlan VF. Maternal memory in adult, nulliparous rats: effects of testing interval on the retention of maternal behavior. Dev Psychobiol. 2005;46:13–18. doi: 10.1002/dev.20038. [DOI] [PubMed] [Google Scholar]
- 28.Bridges RS. Long-term effects of pregnancy and parturition upon maternal responsiveness in the rat. Physiol Behav. 1975;14:245–249. doi: 10.1016/0031-9384(75)90028-1. [DOI] [PubMed] [Google Scholar]
- 29.Brooks-Gunn J, Furstenberg FF., Jr Adolescent sexual behavior. Am Psychol. 1989;44:249–257. [PubMed] [Google Scholar]
- 30.Byrnes EM, Rigero BA, Bridges RS. Dopamine antagonists during parturition disrupt maternal care and the retention of maternal behavior in rats. Pharmacol Biochem Behav. 2002;73:869–875. doi: 10.1016/s0091-3057(02)00941-3. [DOI] [PubMed] [Google Scholar]
- 31.Byrnes EM, Byrnes JJ, Bridges RS. Increased sensitivity of dopamine systems following reproductive experience in rats. Pharmacol Biochem Behav. 2001;68:481–489. doi: 10.1016/s0091-3057(01)00449-x. [DOI] [PubMed] [Google Scholar]
- 32.Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Carter CS, Witt DM, Schneider J, Harris ZL, Volkening D. Male stimuli are necessary for female sexual behavior and uterine growth in prairie voles (Microtus ochrogaster) Horm Behav. 1987;21:74–82. doi: 10.1016/0018-506x(87)90032-8. [DOI] [PubMed] [Google Scholar]
- 34.Carter CS, Getz LL. Monogamy and the prairie vole. Sci Am. 1993;268:100–106. doi: 10.1038/scientificamerican0693-100. [DOI] [PubMed] [Google Scholar]
- 35.Castner SA, Xiao L, Becker JB. Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res. 1993;610:127–134. doi: 10.1016/0006-8993(93)91225-h. [DOI] [PubMed] [Google Scholar]
- 36.Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24:4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 38.Chen HH, Yang YK, Yeh TL, Cherng CF, Hsu HC, Hsiao SY, Yu L. Methamphetamine-induced conditioned place preference is facilitated by estradiol pretreatment in female mice. Chin J Physiol. 2003;46:169–174. [PubMed] [Google Scholar]
- 39.Chen JC, Chen PC, Chiang YC. Molecular mechanisms of psychostimulant addiction. Chang Gung Med J. 2009;32:148–154. [PubMed] [Google Scholar]
- 40.Chin J, Sternin O, Wu HB, Burrell S, Lu D, Jenab S, Perrotti LI, Quinones-Jenab V. Endogenous gonadal hormones modulate behavioral and neurochemical responses to acute and chronic cocaine administration. Brain Res. 2002;945:123–130. doi: 10.1016/s0006-8993(02)02807-x. [DOI] [PubMed] [Google Scholar]
- 41.Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- 42.Clopton J, Gordon JH. In vivo effects of estrogen and 2-hydroxyestradiol on D-2 dopamine receptor agonist affinity states in rat striatum. J Neural Transm. 1986;66:13–20. doi: 10.1007/BF01262954. [DOI] [PubMed] [Google Scholar]
- 43.Coria-Avila GA, Pfaus JG. Neuronal activation by stimuli that predict sexual reward in female rats. Neuroscience. 2007;148:623–632. doi: 10.1016/j.neuroscience.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 44.Curtis JT, Liu Y, Wang Z. Lesions of the vomeronasal organ disrupt mating-induced pair bonding in female prairie voles (Microtus ochrogaster) Brain Res. 2001;901:167–174. doi: 10.1016/s0006-8993(01)02343-5. [DOI] [PubMed] [Google Scholar]
- 45.D'Astous M, Gajjar TM, Dluzen DE, Di Paolo T. Dopamine transporter as a marker of neuroprotection in methamphetamine-lesioned mice treated acutely with estradiol. Neuroendocrinology. 2004;79:296–304. doi: 10.1159/000079664. [DOI] [PubMed] [Google Scholar]
- 46.Davis C, Carter JC. Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite. 2009;53:1–8. doi: 10.1016/j.appet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 47.Demotes-Mainard J, Arnauld E, Vincent JD. Estrogens Modulate the Responsiveness of in vivo Recorded Striatal Neurons to lontophoretic Application of Dopamine in Rats: Role of D and D2 Receptor Activation. J Neuroendocrinol. 1990;2:825–832. doi: 10.1111/j.1365-2826.1990.tb00647.x. [DOI] [PubMed] [Google Scholar]
- 48.Diamond M. Intromission pattern and species vaginal code in relation to induction of pseudopregnancy. Science. 1970;169:995–997. doi: 10.1126/science.169.3949.995. [DOI] [PubMed] [Google Scholar]
- 49.Diaz Heijtz R, Kolb B, Forssberg H. Can a therapeutic dose of amphetamine during pre-adolescence modify the pattern of synaptic organization in the brain? Eur J Neurosci. 2003;18:3394–3399. doi: 10.1046/j.0953-816x.2003.03067.x. [DOI] [PubMed] [Google Scholar]
- 50.Dietz DM, Dietz KC, Nestler EJ, Russo SJ. Molecular mechanisms of psychostimulant-induced structural plasticity. Pharmacopsychiatry. 2009;42 Suppl 1:S69–S78. doi: 10.1055/s-0029-1202847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Disshon KA, Dluzen DE. Use of in vitro superfusion to assess the dynamics of striatal dopamine clearance: influence of estrogen. Brain Res. 1999;842:399–407. doi: 10.1016/s0006-8993(99)01863-6. [DOI] [PubMed] [Google Scholar]
- 52.Disshon KA, Boja JW, Dluzen DE. Inhibition of striatal dopamine transporter activity by 17beta-estradiol. Eur J Pharmacol. 1998;345:207–211. doi: 10.1016/s0014-2999(98)00008-9. [DOI] [PubMed] [Google Scholar]
- 53.Dluzen DE, Ramirez VD. Modulatory effects of progesterone upon dopamine release from the corpus striatum of ovariectomized estrogen-treated rats are stereo-specific. Brain Res. 1991;538:176–179. doi: 10.1016/0006-8993(91)90395-c. [DOI] [PubMed] [Google Scholar]
- 54.Dluzen DE, Ramirez VD. In vitro progesterone modulation of amphetamine-stimulated dopamine release from the corpus striatum of ovariectomized estrogen-treated female rats: response characteristics. Brain Res. 1990;517:117–122. doi: 10.1016/0006-8993(90)91016-a. [DOI] [PubMed] [Google Scholar]
- 55.Erskine MS, Lehmann ML, Cameron NM, Polston EK. Co-regulation of female sexual behavior and pregnancy induction: an exploratory synthesis. Behav Brain Res. 2004;153:295–315. doi: 10.1016/j.bbr.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 56.Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- 57.Evans SM. The role of estradiol and progesterone in modulating the subjective effects of stimulants in humans. Exp Clin Psychopharmacol. 2007;15:418–426. doi: 10.1037/1064-1297.15.5.418. [DOI] [PubMed] [Google Scholar]
- 58.Febo M, Jimenez-Rivera CA, Segarra AC. Estrogen and opioids interact to modulate the locomotor response to cocaine in the female rat. Brain Res. 2002;943:151–161. doi: 10.1016/s0006-8993(02)02748-8. [DOI] [PubMed] [Google Scholar]
- 59.Felicio LF, Florio JC, Sider LH, Cruz-Casallas PE, Bridges RS. Reproductive experience increases striatal and hypothalamic dopamine levels in pregnant rats. Brain Res Bull. 1996;40:253–256. doi: 10.1016/0361-9230(96)00008-1. [DOI] [PubMed] [Google Scholar]
- 60.Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89:183–189. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 63.Ferris CF, Kulkarni P, Sullivan JM, Jr, Harder JA, Messenger TL, Febo M. Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci. 2005;25:149–156. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Festa ED, Quinones-Jenab V. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Horm Behav. 2004;46:509–519. doi: 10.1016/j.yhbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 65.Fleming AS, Rosenblatt JS. Maternal behavior in the virgin and lactating rat. J Comp Physiol Psychol. 1974;86:957–972. doi: 10.1037/h0036414. [DOI] [PubMed] [Google Scholar]
- 66.Freeman ME. The Neuroendocrine Control of the Ovarian Cycle of the Rat. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Second Edition. Vol. 2. New York: Raven Press, Ltd; 1994. pp. 613–658. [Google Scholar]
- 67.Freet CS, Steffen C, Nestler EJ, Grigson PS. Overexpression of DeltaFosB is associated with attenuated cocaine-induced suppression of saccharin intake in mice. Behav Neurosci. 2009;123:397–407. doi: 10.1037/a0015033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frohmader KS, Wiskerke J, Wise RA, Lehman MN, Coolen LM. Methamphetamine acts on subpopulations of neurons regulating sexual behavior in male rats. Neuroscience. doi: 10.1016/j.neuroscience.2009.12.070. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garcia Horsman P, Paredes RG. Dopamine antagonists do not block conditioned place preference induced by paced mating behavior in female rats. Behav Neurosci. 2004;118:356–364. doi: 10.1037/0735-7044.118.2.356. [DOI] [PubMed] [Google Scholar]
- 70.Getz LL, Carter CS, Gavish L. The mating system of the prairie vole, Microtus ochrogaster: Field and laboratory evidence for pair-bonding. Behavioral Ecology and Sociobiology. 1981;8:189–194. [Google Scholar]
- 71.Getz LL, Carter CS. Prairie-vole partnerships. Am Sci. 1996;84:56–62. [Google Scholar]
- 72.Getz LL, Hofmann JE. Social organizations in free-living prairie voles, Microtus ochrogaster. Behavioral Ecology and Sociobiology. 1986;18:275–282. [Google Scholar]
- 73.Gingrich B, Liu Y, Cascio C, Wang Z, Insel TR. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster) Behav Neurosci. 2000;114:173–183. doi: 10.1037//0735-7044.114.1.173. [DOI] [PubMed] [Google Scholar]
- 74.Gonzalez-Flores O, Camacho FJ, Dominguez-Salazar E, Ramirez-Orduna JM, Beyer C, Paredes RG. Progestins and place preference conditioning after paced mating. Horm Behav. 2004;46:151–157. doi: 10.1016/j.yhbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 75.Guarraci FA. "Sex, drugs and the brain": The interaction between drugs of abuse and sexual behavior in the female rat. Horm Behav. 2009 doi: 10.1016/j.yhbeh.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 76.Hansen S, Bergvall AH, Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol Biochem Behav. 1993;45:673–676. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- 77.Hansen S. Maternal behavior of female rats with 6-OHDA lesions in the ventral striatum: characterization of the pup retrieval deficit. Physiol Behav. 1994;55:615–620. doi: 10.1016/0031-9384(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 78.Hansen S, Harthon C, Wallin E, Lofberg L, Svensson K. The effects of 6-OHDA- induced dopamine depletions in the ventral or dorsal striatum on maternal and sexual behavior in the female rat. Pharmacol Biochem Behav. 1991;39:71–77. doi: 10.1016/0091-3057(91)90399-m. [DOI] [PubMed] [Google Scholar]
- 79.Hecht GS, Spear NE, Spear LP. Changes in progressive ratio responding for intravenous cocaine throughout the reproductive process in female rats. Dev Psychobiol. 1999;35:136–145. [PubMed] [Google Scholar]
- 80.Hedges VL, Chakravarty S, Nestler EJ, Meisel RL. Delta FosB overexpression in the nucleus accumbens enhances sexual reward in female Syrian hamsters. Genes Brain Behav. 2009;8:442–449. doi: 10.1111/j.1601-183X.2009.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 82.Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 83.Hu M, Becker JB. Acquisition of cocaine self-administration in ovariectomized female rats: effect of estradiol dose or chronic estradiol administration. Drug Alcohol Depend. 2008;94:56–62. doi: 10.1016/j.drugalcdep.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- 85.Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23:693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hucke EE, Cruz-Casallas PE, Sider LH, Felicio LF. Reproductive experience modulates dopamine-related behavioral responses. Pharmacol Biochem Behav. 2001;68:575–582. doi: 10.1016/s0091-3057(01)00458-0. [DOI] [PubMed] [Google Scholar]
- 87.Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- 88.Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Insel TR. Is social attachment an addictive disorder? Physiol Behav. 2003;79:351–357. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- 90.Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- 91.Jenkins WJ, Becker JB. Female rats develop conditioned place preferences for sex at their preferred interval. Horm Behav. 2003;43:503–507. doi: 10.1016/s0018-506x(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 92.Jenkins WJ, Becker JB. Dynamic increases in dopamine during paced copulation in the female rat. Eur J Neurosci. 2003;18:1997–2001. doi: 10.1046/j.1460-9568.2003.02923.x. [DOI] [PubMed] [Google Scholar]
- 93.Joyce JN, Montero E, Van Hartesveldt C. Dopamine-mediated behaviors: characteristics of modulation by estrogen. Pharmacol Biochem Behav. 1984;21:791–800. doi: 10.1016/s0091-3057(84)80021-0. [DOI] [PubMed] [Google Scholar]
- 94.Justice AJ, de Wit H. Acute effects of estradiol pretreatment on the response to d- amphetamine in women. Neuroendocrinology. 2000;71:51–59. doi: 10.1159/000054520. [DOI] [PubMed] [Google Scholar]
- 95.Justice AJ, De Wit H. Acute effects of d-amphetamine during the early and late follicular phases of the menstrual cycle in women. Pharmacol Biochem Behav. 2000;66:509–515. doi: 10.1016/s0091-3057(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 96.Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- 97.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 98.Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. J Neurosci. 1993;13:266–275. doi: 10.1523/JNEUROSCI.13-01-00266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- 100.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 101.Keer SE, Stern JM. Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol Behav. 1999;67:659–669. doi: 10.1016/s0031-9384(99)00116-x. [DOI] [PubMed] [Google Scholar]
- 102.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kelz MB, Chen J, Carlezon WA, Jr, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, Tkatch T, Baranauskas G, Surmeier DJ, Neve RL, Duman RS, Picciotto MR, Nestler EJ. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- 104.Kim Y, Teylan MA, Baron M, Sands A, Nairn AC, Greengard P. Methylphenidate-induced dendritic spine formation and DeltaFosB expression in nucleus accumbens. Proc Natl Acad Sci U S A. 2009;106:2915–2920. doi: 10.1073/pnas.0813179106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kohlert JG, Meisel RL. Sexual experience sensitizes mating-related nucleus accumbens dopamine responses of female Syrian hamsters. Behav Brain Res. 1999;99:45–52. doi: 10.1016/s0166-4328(98)00068-0. [DOI] [PubMed] [Google Scholar]
- 106.Kohlert JG, Rowe RK, Meisel RL. Intromissive stimulation from the male increases extracellular dopamine release from fluoro-gold-identified neurons within the midbrain of female hamsters. Horm Behav. 1997;32:143–154. doi: 10.1006/hbeh.1997.1415. [DOI] [PubMed] [Google Scholar]
- 107.Kolb B, Gorny G, Li Y, Samaha AN, Robinson TE. Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proc Natl Acad Sci U S A. 2003;100:10523–10528. doi: 10.1073/pnas.1834271100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kovacs GL, Sarnyai Z, Barbarczi E, Szabo G, Telegdy G. The role of oxytocin-dopamine interactions in cocaine-induced locomotor hyperactivity. Neuropharmacology. 1990;29:365–368. doi: 10.1016/0028-3908(90)90095-9. [DOI] [PubMed] [Google Scholar]
- 109.Kovacs GL, Sarnyai Z, Szabo G. Oxytocin and addiction: a review. Psychoneuroendocrinology. 1998;23:945–962. doi: 10.1016/s0306-4530(98)00064-x. [DOI] [PubMed] [Google Scholar]
- 110.Kuhn CM, Walker QD, Kaplan KA, Li ST. Sex steroids and stimulant sensitivity. Ann N Y Acad Sci. 2001;937:188–201. doi: 10.1111/j.1749-6632.2001.tb03565.x. [DOI] [PubMed] [Google Scholar]
- 111.Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol. 2007;15:461–471. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- 112.Larson EB, Carroll ME. Estrogen receptor beta, but not alpha, mediates estrogen's effect on cocaine-induced reinstatement of extinguished cocaine-seeking behavior in ovariectomized female rats. Neuropsychopharmacology. 2007;32:1334–1345. doi: 10.1038/sj.npp.1301249. [DOI] [PubMed] [Google Scholar]
- 113.Lavi-Avnon Y, Weller A, Finberg JP, Gispan-Herman I, Kinor N, Stern Y, Schroeder M, Gelber V, Bergman SY, Overstreet DH, Yadid G. The reward system and maternal behavior in an animal model of depression: a microdialysis study. Psychopharmacology (Berl) 2008;196:281–291. doi: 10.1007/s00213-007-0961-2. [DOI] [PubMed] [Google Scholar]
- 114.Le Moal M. Drug abuse: vulnerability and transition to addiction. Pharmacopsychiatry. 2009;42 Suppl 1:S42–S55. doi: 10.1055/s-0029-1216355. [DOI] [PubMed] [Google Scholar]
- 115.Lee A, Li M, Watchus J, Fleming AS. Neuroanatomical basis of maternal memory in postpartum rats: selective role for the nucleus accumbens. Behav Neurosci. 1999;113:523–538. doi: 10.1037//0735-7044.113.3.523. [DOI] [PubMed] [Google Scholar]
- 116.Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 2000;108:215–231. doi: 10.1016/s0166-4328(99)00170-9. [DOI] [PubMed] [Google Scholar]
- 117.Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci U S A. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Levesque D, Di Paolo T. Rapid conversion of high into low striatal D2-dopamine receptor agonist binding states after an acute physiological dose of 17 beta-estradiol. Neurosci Lett. 1988;88:113–118. doi: 10.1016/0304-3940(88)90324-2. [DOI] [PubMed] [Google Scholar]
- 119.Levesque D, Gagnon S, Di Paolo T. Striatal D1 dopamine receptor density fluctuates during the rat estrous cycle. Neurosci Lett. 1989;98:345–350. doi: 10.1016/0304-3940(89)90426-6. [DOI] [PubMed] [Google Scholar]
- 120.Li M, Fleming AS. Differential involvement of nucleus accumbens shell and core subregions in maternal memory in postpartum female rats. Behav Neurosci. 2003;117:426–445. doi: 10.1037/0735-7044.117.3.426. [DOI] [PubMed] [Google Scholar]
- 121.Li Y, Kolb B, Robinson TE. The location of persistent amphetamine-induced changes in the density of dendritic spines on medium spiny neurons in the nucleus accumbens and caudate-putamen. Neuropsychopharmacology. 2003;28:1082–1085. doi: 10.1038/sj.npp.1300115. [DOI] [PubMed] [Google Scholar]
- 122.Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- 123.Lile JA, Kendall SL, Babalonis S, Martin CA, Kelly TH. Evaluation of estradiol administration on the discriminative-stimulus and subject-rated effects of d-amphetamine in healthy pre-menopausal women. Pharmacol Biochem Behav. 2007;87:258–266. doi: 10.1016/j.pbb.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lippman J, Dunaevsky A. Dendritic spine morphogenesis and plasticity. J Neurobiol. 2005;64:47–57. doi: 10.1002/neu.20149. [DOI] [PubMed] [Google Scholar]
- 125.Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- 126.Lonstein JS. Effects of dopamine receptor antagonism with haloperidol on nurturing behavior in the biparental prairie vole. Pharmacol Biochem Behav. 2002;74:11–19. doi: 10.1016/s0091-3057(02)00952-8. [DOI] [PubMed] [Google Scholar]
- 127.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 128.Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl) 2008;197:237–246. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- 129.Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav. 2001;68:641–646. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 130.Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- 131.Marinissen MJ, Gutkind JS. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol Sci. 2001;22:368–376. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- 132.Martinez I, Paredes RG. Only self-paced mating is rewarding in rats of both sexes. Horm Behav. 2001;40:510–517. doi: 10.1006/hbeh.2001.1712. [DOI] [PubMed] [Google Scholar]
- 133.Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci. 2001;115:683–694. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- 134.Mattson BJ, Williams SE, Rosenblatt JS, Morrell JI. Preferences for cocaine- or pup-associated chambers differentiates otherwise behaviorally identical postpartum maternal rats. Psychopharmacology (Berl) 2003;167:1–8. doi: 10.1007/s00213-002-1351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mattson BJ, Morrell JI. Preference for cocaine- versus pup-associated cues differentially activates neurons expressing either Fos or cocaine- and amphetamineregulated transcript in lactating, maternal rodents. Neuroscience. 2005;135:315–328. doi: 10.1016/j.neuroscience.2005.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McClintock MK, Adler NT. The Role of the Female During Copulation in Wild and Domestic Norway Rats (Rattus Norvegicus) Behaviour. 1978;67 [Google Scholar]
- 137.McClung CA, Nestler EJ. Neuroplasticity mediated by altered gene expression. Neuropsychopharmacology. 2008;33:3–17. doi: 10.1038/sj.npp.1301544. [DOI] [PubMed] [Google Scholar]
- 138.Meisel RL, Camp DM, Robinson TE. A microdialysis study of ventral striatal dopamine during sexual behavior in female Syrian hamsters. Behav Brain Res. 1993;55:151–157. doi: 10.1016/0166-4328(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 139.Meisel RL, Mullins AJ. Sexual experience in female rodents: cellular mechanisms and functional consequences. Brain Res. 2006;1126:56–65. doi: 10.1016/j.brainres.2006.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Meisel RL, Joppa MA. Conditioned place preference in female hamsters following aggressive or sexual encounters. Physiol Behav. 1994;56:1115–1118. doi: 10.1016/0031-9384(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 141.Meisel RL, Joppa MA, Rowe RK. Dopamine receptor antagonists attenuate conditioned place preference following sexual behavior in female Syrian hamsters. Eur J Pharmacol. 1996;309:21–24. doi: 10.1016/0014-2999(96)00389-5. [DOI] [PubMed] [Google Scholar]
- 142.Meredith M, Westberry JM. Distinctive responses in the medial amygdala to same-species and different-species pheromones. J Neurosci. 2004;24:5719–5725. doi: 10.1523/JNEUROSCI.1139-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mermelstein PG, Becker JB. Increased extracellular dopamine in the nucleus accumbens and striatum of the female rat during paced copulatory behavior. Behav Neurosci. 1995;109:354–365. doi: 10.1037//0735-7044.109.2.354. [DOI] [PubMed] [Google Scholar]
- 144.Nakabeppu Y, Nathans D. A naturally occurring truncated form of FosB that inhibits Fos/Jun transcriptional activity. Cell. 1991;64:751–759. doi: 10.1016/0092-8674(91)90504-r. [DOI] [PubMed] [Google Scholar]
- 145.Nestler EJ. Review. Transcriptional mechanisms of addiction: role of DeltaFosB. Philos Trans R Soc Lond B Biol Sci. 2008;363:3245–3255. doi: 10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nestler EJ. The neurobiology of cocaine addiction. Sci Pract Perspect. 2005;3:4–10. doi: 10.1151/spp05314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Niehaus JL, Cruz-Bermudez ND, Kauer JA. Plasticity of addiction: a mesolimbic dopamine short-circuit? Am J Addict. 2009;18:259–271. doi: 10.1080/10550490902925946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Niyomchai T, Jenab S, Festa ED, Akhavan A, Quinones-Jenab V. Effects of short- and long-term estrogen and progesterone replacement on behavioral responses and c-fos mRNA levels in female rats after acute cocaine administration. Brain Res. 2006;1126:193–199. doi: 10.1016/j.brainres.2006.07.099. [DOI] [PubMed] [Google Scholar]
- 149.Niyomchai T, Akhavan A, Festa ED, Lin SN, Lamm L, Foltz R, Quinones-Jenab V. Estrogen and progesterone affect cocaine pharmacokinetics in female rats. Brain Res Bull. 2006;68:310–314. doi: 10.1016/j.brainresbull.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 150.Niyomchai T, Weirstall K, Jenab S, Quinones-Jenab V. Coadministration of estrogen and progesterone differentially affects locomotor responses to cocaine in rats. Ethn Dis. 2008;18 S2-51-53. [PubMed] [Google Scholar]
- 151.Noble RG. Limited coital stimulation facilitates sexual responses of the female hamster. Physiol Behav. 1979;23:1007–1010. doi: 10.1016/0031-9384(79)90289-0. [DOI] [PubMed] [Google Scholar]
- 152.Noble RG. The sexual responses of the female hamster: a descriptive analysis. Physiol Behav. 1979;23:1001–1005. doi: 10.1016/0031-9384(79)90288-9. [DOI] [PubMed] [Google Scholar]
- 153.Norrholm SD, Bibb JA, Nestler EJ, Ouimet CC, Taylor JR, Greengard P. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM, Smith CD. The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav Neurosci. 2005;119:1588–1604. doi: 10.1037/0735-7044.119.6.1588. [DOI] [PubMed] [Google Scholar]
- 155.Numan M, Fleming AS, Levy F. Maternal Behaviour. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Third Edition. Boston: Elsevier; 2006. pp. 1921–1993. [Google Scholar]
- 156.Ohtani H, Nomoto M, Douchi T. Chronic estrogen treatment replaces striatal dopaminergic function in ovariectomized rats. Brain Res. 2001;900:163–168. doi: 10.1016/s0006-8993(01)02276-4. [DOI] [PubMed] [Google Scholar]
- 157.Olausson P, Jentsch JD, Tronson N, Neve RL, Nestler EJ, Taylor JR. DeltaFosB in the nucleus accumbens regulates food-reinforced instrumental behavior and motivation. J Neurosci. 2006;26:9196–9204. doi: 10.1523/JNEUROSCI.1124-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Oldenburger WP, Everitt BJ, de Jonge FH. Conditioned place preference induced by sexual interaction in female rats. Horm Behav. 1992;26:214–228. doi: 10.1016/0018-506x(92)90043-u. [DOI] [PubMed] [Google Scholar]
- 159.Olivier JD, de Jong TR, Jos Dederen P, van Oorschot R, Heeren D, Pattij T, Waldinger MD, Coolen LM, Cools AR, Olivier B, Veening JG. Effects of acute and chronic apomorphine on sex behavior and copulation-induced neural activation in the male rat. Eur J Pharmacol. 2007;576:61–76. doi: 10.1016/j.ejphar.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 160.Parada M, King S, Li M, Fleming AS. The roles of accumbal dopamine D1 and D2 receptors in maternal memory in rats. Behav Neurosci. 2008;122:368–376. doi: 10.1037/0735-7044.122.2.368. [DOI] [PubMed] [Google Scholar]
- 161.Paredes RG, Alonso A. Sexual behavior regulated (paced) by the female induces conditioned place preference. Behav Neurosci. 1997;111:123–128. doi: 10.1037//0735-7044.111.1.123. [DOI] [PubMed] [Google Scholar]
- 162.Paredes RG, Vazquez B. What do female rats like about sex? Paced mating. Behav Brain Res. 1999;105:117–127. doi: 10.1016/s0166-4328(99)00087-x. [DOI] [PubMed] [Google Scholar]
- 163.Pasqualini C, Olivier V, Guibert B, Frain O, Leviel V. Acute stimulatory effect of estradiol on striatal dopamine synthesis. J Neurochem. 1995;65:1651–1657. doi: 10.1046/j.1471-4159.1995.65041651.x. [DOI] [PubMed] [Google Scholar]
- 164.Pelissier B, Jones N. A review of gender differences among substance abusers. Crime Delinquency. 2005;51:343–372. [Google Scholar]
- 165.Perrotti LI, Russo SJ, Fletcher H, Chin J, Webb T, Jenab S, Quinones-Jenab V. Ovarian hormones modulate cocaine-induced locomotor and stereotypic activity. Ann N Y Acad Sci. 2001;937:202–216. doi: 10.1111/j.1749-6632.2001.tb03566.x. [DOI] [PubMed] [Google Scholar]
- 166.Pfaus JG, Damsma G, Wenkstern D, Fibiger HC. Sexual activity increases dopamine transmission in the nucleus accumbens and striatum of female rats. Brain Res. 1995;693:21–30. doi: 10.1016/0006-8993(95)00679-k. [DOI] [PubMed] [Google Scholar]
- 167.Pierce RC, Kalivas PW. Amphetamine produces sensitized increases in locomotion and extracellular dopamine preferentially in the nucleus accumbens shell of rats administered repeated cocaine. J Pharmacol Exp Ther. 1995;275:1019–1029. [PubMed] [Google Scholar]
- 168.Pitchers KK, Balfour ME, Lehman MN, Richtand NM, Yu L, Coolen LM. Neuroplasticity in the Mesolimbic System Induced by Natural Reward and Subsequent Reward Abstinence. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pizzuto T. Female prairie voles (Microtus ochrogaster) fail to form a new pair after loss of mate. Behavioural Processes. 1998;43:79–86. doi: 10.1016/s0376-6357(97)00091-0. [DOI] [PubMed] [Google Scholar]
- 170.Popik P, Vetulani J, van Ree JM. Low doses of oxytocin facilitate social recognition in rats. Psychopharmacology (Berl) 1992;106:71–74. doi: 10.1007/BF02253591. [DOI] [PubMed] [Google Scholar]
- 171.Rivas FJ, Mir D. Accumbens lesion in female rats increases mount rejection without modifying lordosis. Rev Esp Fisiol. 1991;47:1–6. [PubMed] [Google Scholar]
- 172.Rivas FJ, Mir D. Effects of nucleus accumbens lesion on female rat sexual receptivity and proceptivity in a partner preference paradigm. Behav Brain Res. 1990;41:239–249. doi: 10.1016/0166-4328(90)90111-q. [DOI] [PubMed] [Google Scholar]
- 173.Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- 174.Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- 176.Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 177.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47 Suppl 1:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 178.Roller CG. Sexually compulsive/addictive behaviors in women: a women's healthcare issue. J Midwifery Womens Health. 2007;52:486–491. doi: 10.1016/j.jmwh.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 179.Roseboom PH, Hewlett GH, Gnegy ME. Repeated amphetamine administration alters the interaction between D1-stimulated adenylyl cyclase activity and calmodulin in rat striatum. J Pharmacol Exp Ther. 1990;255:197–203. [PubMed] [Google Scholar]
- 180.Rosenblatt JS. Nonhormonal basis of maternal behavior in the rat. Science. 1967;156:1512–1514. doi: 10.1126/science.156.3781.1512. [DOI] [PubMed] [Google Scholar]
- 181.Rosenblatt JS, Siegel HI. Hysterectomy-induced maternal behavior during pregnancy in the rat. J Comp Physiol Psychol. 1975;89:685–700. doi: 10.1037/h0077052. [DOI] [PubMed] [Google Scholar]
- 182.Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Roth ME, Casimir AG, Carroll ME. Influence of estrogen in the acquisition of intravenously self-administered heroin in female rats. Pharmacol Biochem Behav. 2002;72:313–318. doi: 10.1016/s0091-3057(01)00777-8. [DOI] [PubMed] [Google Scholar]
- 185.Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraish M, Jenab S, Quinones-Jenab V. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 2003;120:523–533. doi: 10.1016/s0306-4522(03)00317-8. [DOI] [PubMed] [Google Scholar]
- 186.Russo SJ, Wilkinson MB, Mazei-Robison MS, Dietz DM, Maze I, Krishnan V, Renthal W, Graham A, Birnbaum SG, Green TA, Robison B, Lesselyong A, Perrotti LI, Bolanos CA, Kumar A, Clark MS, Neumaier JF, Neve RL, Bhakar AL, Barker PA, Nestler EJ. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J Neurosci. 2009;29:3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sala C, Cambianica I, Rossi F. Molecular mechanisms of dendritic spine development and maintenance. Acta Neurobiol Exp (Wars) 2008;68:289–304. doi: 10.55782/ane-2008-1696. [DOI] [PubMed] [Google Scholar]
- 188.Sala M, Braida D, Colombo M, Groppetti A, Sacco S, Gori E, Parenti M. Behavioral and biochemical evidence of opioidergic involvement in cocaine sensitization. J Pharmacol Exp Ther. 1995;274:450–457. [PubMed] [Google Scholar]
- 189.Schoffelmeer AN, Voorn P, Jonker AJ, Wardeh G, Nestby P, Vanderschuren LJ, De Vries TJ, Mulder AH, Tjon GH. Morphine-induced increase in D-1 receptor regulated signal transduction in rat striatal neurons and its facilitation by glucocorticoid receptor activation: possible role in behavioral sensitization. Neurochem Res. 1996;21:1417–1423. doi: 10.1007/BF02532383. [DOI] [PubMed] [Google Scholar]
- 190.Schultz KN, von Esenwein SA, Hu M, Bennett AL, Kennedy RT, Musatov S, Toran-Allerand CD, Kaplitt MG, Young LJ, Becker JB. Viral vector-mediated overexpression of estrogen receptor-alpha in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J Neurosci. 2009;29:1897–1903. doi: 10.1523/JNEUROSCI.4647-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Seip KM, Morrell JI. Increasing the incentive salience of cocaine challenges preference for pup- over cocaine-associated stimuli during early postpartum: place preference and locomotor analyses in the lactating female rat. Psychopharmacology (Berl) 2007;194:309–319. doi: 10.1007/s00213-007-0841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J Pharmacol Exp Ther. 2000;293:879–886. [PubMed] [Google Scholar]
- 193.Shapiro LE, Dewsbury DA. Differences in affiliative behavior, pair bonding, and vaginal cytology in two species of vole (Microtus ochrogaster and M. montanus) J Comp Psychol. 1990;104:268–274. doi: 10.1037/0735-7036.104.3.268. [DOI] [PubMed] [Google Scholar]
- 194.Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Silva MR, Bernardi MM, Felicio LF. Effects of dopamine receptor antagonists on ongoing maternal behavior in rats. Pharmacol Biochem Behav. 2001;68:461–468. doi: 10.1016/s0091-3057(01)00471-3. [DOI] [PubMed] [Google Scholar]
- 196.Silverman JL, Koenig JI. Evidence for the involvement of ERbeta and RGS9-2 in 17- beta estradiol enhancement of amphetamine-induced place preference behavior. Horm Behav. 2007;52:146–155. doi: 10.1016/j.yhbeh.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sinha R, Fox H, Hong KI, Sofuoglu M, Morgan PT, Bergquist KT. Sex steroid hormones, stress response, and drug craving in cocaine-dependent women: implications for relapse susceptibility. Exp Clin Psychopharmacol. 2007;15:445–452. doi: 10.1037/1064-1297.15.5.445. [DOI] [PubMed] [Google Scholar]
- 198.Smeltzer MD, Curtis JT, Aragona BJ, Wang Z. Dopamine, oxytocin,and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci Lett. 2006;394:146–151. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 199.Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- 200.Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the ventral striatum. Brain Res. 1991;559:29–36. doi: 10.1016/0006-8993(91)90283-2. [DOI] [PubMed] [Google Scholar]
- 201.Stern JM, Lonstein JS. Neural mediation of nursing and related maternal behaviors. Prog Brain Res. 2001;133:263–278. doi: 10.1016/s0079-6123(01)33020-0. [DOI] [PubMed] [Google Scholar]
- 202.Stern JM, Mackinnon DA. Postpartum, hormonal, and nonhormonal induction of maternal behavior in rats: effects on T-maze retrieval of pups. Horm Behav. 1976;7:305–316. doi: 10.1016/0018-506x(76)90036-2. [DOI] [PubMed] [Google Scholar]
- 203.Stewart J. Review. Psychological and neural mechanisms of relapse. Philos Trans R Soc Lond B Biol Sci. 2008;363:3147–3158. doi: 10.1098/rstb.2008.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Stolzenberg DS, McKenna JB, Keough S, Hancock R, Numan MJ, Numan M. Dopamine D1 receptor stimulation of the nucleus accumbens or the medial preoptic area promotes the onset of maternal behavior in pregnancy-terminated rats. Behav Neurosci. 2007;121:907–919. doi: 10.1037/0735-7044.121.5.907. [DOI] [PubMed] [Google Scholar]
- 205.Tenk CM, Wilson H, Zhang Q, Pitchers KK, Coolen LM. Sexual reward in male rats: effects of sexual experience on conditioned place preferences associated with ejaculation and intromissions. Horm Behav. 2009;55:93–97. doi: 10.1016/j.yhbeh.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Thompson TL. Attenuation of dopamine uptake in vivo following priming with estradiol benzoate. Brain Res. 1999;834:164–167. doi: 10.1016/s0006-8993(99)01508-5. [DOI] [PubMed] [Google Scholar]
- 207.Thorneycroft IH, Mishell DR, Jr, Stone SC, Kharma KM, Nakamura RM. The relation of serum 17-hydroxyprogesterone and estradiol-17-beta levels during the human menstrual cycle. Am J Obstet Gynecol. 1971;111:947–951. doi: 10.1016/0002-9378(71)90951-3. [DOI] [PubMed] [Google Scholar]
- 208.Tjon GH, De Vries TJ, Ronken E, Hogenboom F, Wardeh G, Mulder AH, Schoffelmeer AN. Repeated and chronic morphine administration causes differential long- lasting changes in dopaminergic neurotransmission in rat striatum without changing its delta- and kappa-opioid receptor regulation. Eur J Pharmacol. 1994;252:205–212. doi: 10.1016/0014-2999(94)90598-3. [DOI] [PubMed] [Google Scholar]
- 209.Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 210.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 211.Unterwald EM, Fillmore J, Kreek MJ. Chronic repeated cocaine administration increases dopamine D1 receptor-mediated signal transduction. Eur J Pharmacol. 1996;318:31–35. doi: 10.1016/s0014-2999(96)00841-2. [DOI] [PubMed] [Google Scholar]
- 212.Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Van Vliet BJ, Van Rijswijk AL, Wardeh G, Mulder AH, Schoffelmeer AN. Adaptive changes in the number of Gs- and Gi-proteins underlie adenylyl cyclase sensitization in morphine-treated rat striatal neurons. Eur J Pharmacol. 1993;245:23–29. doi: 10.1016/0922-4106(93)90165-6. [DOI] [PubMed] [Google Scholar]
- 214.Volkow ND, Li TK. Drug addiction: the neurobiology of behaviour gone awry. Nat Rev Neurosci. 2004;5:963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- 215.Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A, Graham DL, Green TA, Kirk A, Iniguez SD, Perrotti LI, Barrot M, DiLeone RJ, Nestler EJ, Bolanos-Guzman CA. The influence of DeltaFosB in the nucleus accumbens on natural reward-related behavior. J Neurosci. 2008;28:10272–10277. doi: 10.1523/JNEUROSCI.1531-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang Z, Yu G, Cascio C, Liu Y, Gingrich B, Insel TR. Dopamine D2 receptor-mediated regulation of partner preferences in female prairie voles (Microtus ochrogaster): a mechanism for pair bonding? Behav Neurosci. 1999;113:602–611. doi: 10.1037//0735-7044.113.3.602. [DOI] [PubMed] [Google Scholar]
- 217.Weiss F, Martin-Fardon R, Ciccocioppo R, Kerr TM, Smith DL, Ben-Shahar O. Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacology. 2001;25:361–372. doi: 10.1016/S0893-133X(01)00238-X. [DOI] [PubMed] [Google Scholar]
- 218.White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- 219.Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm Behav. 1992;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- 220.Williams JR, Slotnick BM, Kirkpatrick BW, Carter CS. Olfactory bulb removal affects partner preference development and estrus induction in female prairie voles. Physiol Behav. 1992;52:635–639. doi: 10.1016/0031-9384(92)90390-n. [DOI] [PubMed] [Google Scholar]
- 221.Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J Neuroendocrinol. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 222.Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- 223.Winstanley CA, LaPlant Q, Theobald DE, Green TA, Bachtell RK, Perrotti LI, DiLeone RJ, Russo SJ, Garth WJ, Self DW, Nestler EJ. DeltaFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J Neurosci. 2007;27:10497–10507. doi: 10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Witt DM, Winslow JT, Insel TR. Enhanced social interactions in rats following chronic, centrally infused oxytocin. Pharmacol Biochem Behav. 1992;43:855–861. doi: 10.1016/0091-3057(92)90418-f. [DOI] [PubMed] [Google Scholar]
- 225.Xiao L, Becker JB. Effects of estrogen agonists on amphetamine-stimulated striatal dopamine release. Synapse. 1998;29:379–391. doi: 10.1002/(SICI)1098-2396(199808)29:4<379::AID-SYN10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 226.Young LJ, Murphy Young AZ, Hammock EA. Anatomy and neurochemistry of the pair bond. J Comp Neurol. 2005;493:51–57. doi: 10.1002/cne.20771. [DOI] [PubMed] [Google Scholar]
- 227.Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- 228.Young LJ, Huot B, Nilsen R, Wang Z, Insel TR. Species differences in central oxytocin receptor gene expression: comparative analysis of promoter sequences. J Neuroendocrinol. 1996;8:777–783. doi: 10.1046/j.1365-2826.1996.05188.x. [DOI] [PubMed] [Google Scholar]
- 229.Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, Shaw-Lutchman T, Berton O, Sim-Selley LJ, Dileone RJ, Kumar A, Nestler EJ. An essential role for DeltaFosB in the nucleus accumbens in morphine action. Nat Neurosci. 2006;9:205–211. doi: 10.1038/nn1636. [DOI] [PubMed] [Google Scholar]
- 230.Zack M, Poulos CX. Parallel roles for dopamine in pathological gambling and psychostimulant addiction. Curr Drug Abuse Rev. 2009;2:11–25. doi: 10.2174/1874473710902010011. [DOI] [PubMed] [Google Scholar]
- 231.Zhang D, Yang S, Yang C, Jin G, Zhen X. Estrogen regulates responses of dopamine neurons in the ventral tegmental area to cocaine. Psychopharmacology (Berl) 2008;199:625–635. doi: 10.1007/s00213-008-1188-6. [DOI] [PubMed] [Google Scholar]
- 232.Zhen X, Goswami S, Abdali SA, Frankfurt M, Friedman E. Estrogen-modulated frontal cortical CaMKII activity and behavioral supersensitization induced by prolonged cocaine treatment in female rats. Psychopharmacology (Berl) 2007;191:323–331. doi: 10.1007/s00213-006-0648-0. [DOI] [PubMed] [Google Scholar]
- 233.Zilberman M, Tavares H, el-Guebaly N. Gender similarities and differences: the prevalence and course of alcohol- and other substance-related disorders. J Addict Dis. 2003;22:61–74. doi: 10.1300/j069v22n04_06. [DOI] [PubMed] [Google Scholar]